Abstract
Background/Aims
This study aimed to evaluate the performance of the Model for End-Stage Liver Disease (MELD) 3.0 for predicting mortality and liver-related complications compared with the Child-Pugh classification, albumin-bilirubin (ALBI) grade, the MELD, and the MELD sodium (MELDNa) score.
Methods
We evaluated a multicenter retrospective cohort of incorporated patients with cirrhosis between 2013 and 2019. We conducted comparisons of the area under the receiver operating characteristic curve (AUROC) of the MELD3.0 and other models for predicting 3-month mortality. Additionally, we assessed the risk of cirrhosis-related complications according to the MELD3.0 score.
Results
A total of 3,314 patients were included. The mean age was 55.9±11.3 years, and 70.2% of the patients were male. Within the initial 3 months, 220 patients (6.6%) died, and the MELD3.0 had the best predictive performance among the tested models, with an AUROC of 0.851, outperforming the Child-Pugh classification, ALBI grade, MELD, and MELDNa. A high MELD3.0 score was associated with an increased risk of mortality. Compared with that of the group with a MELD3.0 score <10 points, the adjusted hazard ratio of the group with a score of 10–20 points was 2.176, and that for the group with a score of ≥20 points was 4.892. Each 1-point increase in the MELD3.0 score increased the risk of cirrhosis-related complications by 1.033-fold. The risk of hepatorenal syndrome showed the highest increase, with an adjusted hazard ratio of 1.149, followed by hepatic encephalopathy and ascites.
Conclusions
The MELD3.0 demonstrated robust prognostic performance in predicting mortality in patients with cirrhosis. Moreover, the MELD3.0 score was linked to cirrhosis-related complications, particularly those involving kidney function, such as hepatorenal syndrome and ascites.
Keywords: Liver cirrhosis, Ascites, Hepatic encephalopathy, Hepatorenal syndrome
INTRODUCTION
Evaluating liver function in patients with liver cirrhosis is important, as prognosis depends on the residual liver function. Various endeavors have been made to develop dependable models for predicting prognosis including short-term survival in patients with liver cirrhosis. The Child-Pugh classification has been extensively utilized to evaluate liver function across all etiologies of liver diseases.1 However, two of its five constituent factors, encephalopathy and ascites, can be subjectively interpreted. To overcome the limitation of Child-Pugh classification, which is the arbitrary definition of disease severity, albumin-bilirubin (ALBI) grade was suggested.2 This measure uses only albumin and bilirubin to assess liver function, and its usefulness was validated in classifying prognosis across a wide range of populations. Several issues have been raised with the ALBI grade: it was originally developed for patients with hepatocellular carcinoma (HCC), the calculation is complex, and the patient distribution is skewed, with only a few patients classified as ALBI grade 3.3 In addition, the European Association introduced the Chronic Liver Failure model, a Chinese group developed the Chinese Group on the Study of Severe Hepatitis B model, and an American group established the North American Consortium for the Study of End-stage Liver Disease.4-6 However, all these models, due to their complexity and potential subjectivity, have limitations for wide applicability.7
The Model for End-Stage Liver Disease (MELD) has emerged as a widely applicable model for predicting short-term survival in patients with cirrhosis. It has been adopted as a liver allocation model for patients awaiting liver transplantation because of its objectivity and enhanced usability, which result from the inclusion of serum creatinine, bilirubin, and prothrombin time.8-10 Since the inception of the original MELD model, several modified versions have been proposed to improve accuracy.11 In 2016, MELD sodium (MELDNa) was introduced, as low serum sodium concentration was independently associated with increased risk of mortality, because lower sodium concentration reflects the severity of liver cirrhosis complication including ascites.12,13 However, the predictive power for short-term survival of the MELD and MELDNa declined, with the c-statistics decreasing from 0.80 to 0.71. This is probably because both the age and comorbidity burden of patients with liver cirrhosis are increasing over time, alongside a noticeable shift in etiology.14 MELD3.0, the most recent model of MELD, was introduced in 2021. MELD3.0 included sex and serum albumin as additional variables, accounted for relevant interactions among variables, and capped the upper value of serum creatinine at 3 mg/dL.15 As MELD3.0 has been recently proposed, thorough external validations across regions and populations are necessary to establish it as a primary surrogate marker for predicting prognosis in patients with cirrhosis.
We aimed to compare the prognostic ability of MELD3.0 with other liver function assessment models in terms of survival rates among patients with cirrhosis and to investigate its role in predicting cirrhosis-related complications.
MATERIALS AND METHODS
1. Study design and population
This multicenter, retrospective cohort study recruited patients with liver cirrhosis who had visited one of the eight affiliated hospitals of College of Medicine, The Catholic University of Korea, Republic of Korea, from January 2013 to December 2019. All consecutive patients were identified in the electronic clinical data warehouse, and clinical and laboratory data were retrieved from the electronic medical record system.
A total of 6,667 patients aged 19 to 80 years with liver cirrhosis at baseline were enrolled. Liver cirrhosis was defined using a combination of claimed history of liver cirrhosis (K74), based on the International Statistical Classification of Diseases and Related Health Problems-10 (ICD-10). We excluded (1) 1,832 patients who were diagnosed with malignancies, including HCC, before or within 30 days of enrollment and (2) 1,521 patients with incomplete clinical data. A total of 3,314 patients were included in the final analysis. The index date for our current analysis was the date of liver cirrhosis diagnosis. All patients were observed until they reached 1 of the following endpoints: death, their last hospital visit, or the end date of the study, which was December 2023. Approval for this study was obtained from the Institutional Review Board of The Catholic University of Korea (IRB number: DC23WIDI0053). The study was conducted in accordance with both the Declarations of Helsinki and Istanbul. Informed consent was not required due to the utilization of de-identified data.
2. Data collection
The baseline demographic characteristics of the patients in our study encompassed sex and age. Laboratory assessments comprised variables necessary for MELD calculation including creatinine, bilirubin, prothrombin time, serum sodium, platelet count, aspartate aminotransferase, and alanine aminotransferase levels. Based on the gathered data, we assessed several scoring systems including Child-Pugh class, ALBI, MELD, MELDNa, and MELD3.0. The ALBI score was derived from the formula, (log10 bilirubin [µmol/ L]×0.66)+ (albumin [g/L]×−0.0852), yielding ALBI grades 1, 2, and 3 as follows: ALBI score ≤−2.60 (ALBI grade 1), >−2.60 to ≤−1.39 (ALBI grade 2), and >−1.39 (ALBI grade 3).2 The MELD score was calculated using the formula 6.43+9.57×loge creatinine (mg/dL) +3.78×loge bilirubin (mg/dL)+11.2×loge INR.8 The MELDNa score was determined as MELD+1.32×(137-Na)–[0.033×MELD×(137-Na)].16 The MELD3.0 was computed as 1.33 (if female) +4.56×loge (bilirubin)+0.82×(137-Na)–0.24×(137-Na) ×loge (bilirubin)+9.09×loge (INR)+11.14×loge (creatinine)+1.85×(3.5-albumin)–1.83×(3.5-albumin) ×loge (creatinine)+6.15 Bilirubin, international normalized ratio (INR), and creatinine values less than 1.0 were adjusted to 1.0. Sodium levels were confined within the range of 125 to 137 mmol/L, while albumin levels ranged from 1.5 to 3.5 g/dL.
Ascites were defined based on imaging findings or a history of paracentesis. Hepatic encephalopathy was diagnosed in individuals who were hospitalized for this condition and received treatment with lactulose enemas and rifaximin. Gastrointestinal variceal bleeding was defined as endoscopic variceal ligation or obliteration. Hepatorenal syndrome was identified by a medication history including terlipressin and albumin replacement for more than 5 days. HCC was identified based on a history of claimed HCC ICD-10 code, C220.
3. Study aims
The primary outcome of this study was to assess the performance of the Child-Pugh class, ALBI grade, MELD, MELDNa, and MELD3.0 in predicting survival, particularly within the 3-month window after enrollment. Additionally, the secondary aim was to explore the clinical relevance of MELD3.0 concerning cirrhosis-related complications, including the emergence or exacerbation of ascites, gastrointestinal variceal bleeding, hepatorenal syndrome, and hepatic encephalopathy.
4. Statistical analysis
Categorical variables are presented as frequencies and percentages, while continuous variables are expressed as means and standard deviations. The chi-square test or Fisher exact test, and Student t-test were employed for analyzing categorical and continuous variables, respectively. Patients were stratified into 10-point intervals to depict changes in patient distribution based on the MELD model. We reported the number of patients at enrollment, the number of deaths, and the proportion of deaths at 3 months.
The MELD scores were grouped into 10-point intervals for patient classification (e.g., 6–9, 10–19, 20–29, 30–39, ≥40). Patients who moved to a higher score category were labeled as “up-categorized,” indicating potentially increased clinical risk. Conversely, those who moved to a lower score category were designated as “down-categorized,” suggesting an improvement in prognosis. This terminology highlights clinically meaningful changes in patient status. This classification was proposed by Kim et al. in their study on developing the MELD3.0 model.15
To compare the performance of the Child-Pugh class, ALBI grade, MELD, MELDNa, and MELD3.0 in predicting survival, we performed receiver operating characteristic curve analyses and calculated the area under the receiver operating characteristic curve (AUROC). Comparison between MELD3.0 and other models was performed using the DeLong test. Cox proportional hazard models were utilized to predict mortality and cirrhosis-related complications during our study period; outcomes were summarized as hazard ratios (HRs) and 95% confidence intervals (CIs). For the outcome with low incidence, we applied Firth Correction. Kaplan-Meier curves and log-rank tests were employed to assess cirrhosis-related complications according to the MELD3.0 score. Statistical significance was defined as p-values <0.05. Statistical analyses were performed using R (4.3.3 packages timeROC, survival; R Foundation for Statistical Computing, Vienna, Austria) and SAS (9.4 SAS Institute Inc., Cary, NC, USA).
RESULTS
1. Baseline characteristics of the study population
A total of 3,314 patients was included in this study, and patient’s baseline characteristics are summarized in Table 1. The mean age of the patients was 55.9±11.3 years, and 2,326 (70.2%) were male. Of the patients, 1,354 were diagnosed with liver cirrhosis because of alcohol, and 701 were infected with hepatitis B virus. The prevalence of patients with cirrhosis-related complications was 21.8%. Gastro-esophageal varix bleeding was the most common complication (18.6%) followed by ascites (5.2%) and hepatic encephalopathy (1.8%). The mean score of Child-Pugh was 7.4±1.6, with 35.0% of class A, 53.7% of class B, and 11.4% of class C. Likewise, the mean score of ALBI was –1.5±0.7 with 7.5% of patients with grade 1, 42.9% of grade 2, and 49.7% of grade 3, respectively. The mean values of MELD, MELDNa, and MELD3.0 were 14.7±6.3, 15.7±7.1, and 16.8±7.1, respectively.
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | Total (n=3,314) |
|---|---|
| Demographic characteristics | |
| Age, yr | 55.9±11.3 |
| Male sex | 2,326 (70.2) |
| Etiology of liver disease | |
| HBV | 701 (21.1) |
| HCV | 199 (6.0) |
| Alcohol | 1,354 (40.9) |
| Others | 1,060 (32.0) |
| Laboratory findings | |
| Platelets, 103/μL | 102.8±62.3 |
| AST, IU/L | 143.4±442.2 |
| ALT, IU/L | 70.5±218.3 |
| Prothrombin time, INR | 1.6±0.9 |
| Albumin, g/dL | 2.9±0.7 |
| Total bilirubin, mg/dL | 4.1±5.5 |
| Creatinine, mg/dL | 1.0±0.9 |
| Sodium, mmol/L | 136.7±8.0 |
| Cirrhosis-related complication | 723 (21.8) |
| Ascites | 171 (5.2) |
| Gastroesophageal varix bleeding | 616 (18.6) |
| Hepatorenal syndrome | 1 (0.03) |
| Hepatic encephalopathy | 59 (1.8) |
| Models | |
| Child-Pugh score | 7.4±1.6 |
| Child-Pugh class | |
| Class A | 1,159 (35.0) |
| Class B | 1,779 (53.7) |
| Class C | 376 (11.4) |
| ALBI score | –1.5±0.7 |
| ALBI grade | |
| Grade 1 | 248 (7.5) |
| Grade 2 | 1,420 (42.9) |
| Grade 3 | 1,646 (49.7) |
| MELD | 14.7±6.3 |
| MELDNa | 15.7±7.1 |
| MELD3.0 | 16.8±7.1 |
Data are presented as mean±SD or number (%).
HBV, hepatitis B virus; HCV, hepatitis C virus; AST, alanine aminotransferase; ALT, aspartate aminotransferase; INR, international normalized ratio; ALBI, albumin-bilirubin; MELD, Model for End-Stage Liver Disease; MELDNa, MELD sodium; MELD3.0, MELD 3.0.
2. Reclassification of patients according to MELD, MELDNa, and MELD3.0 score and mortality at 3 months
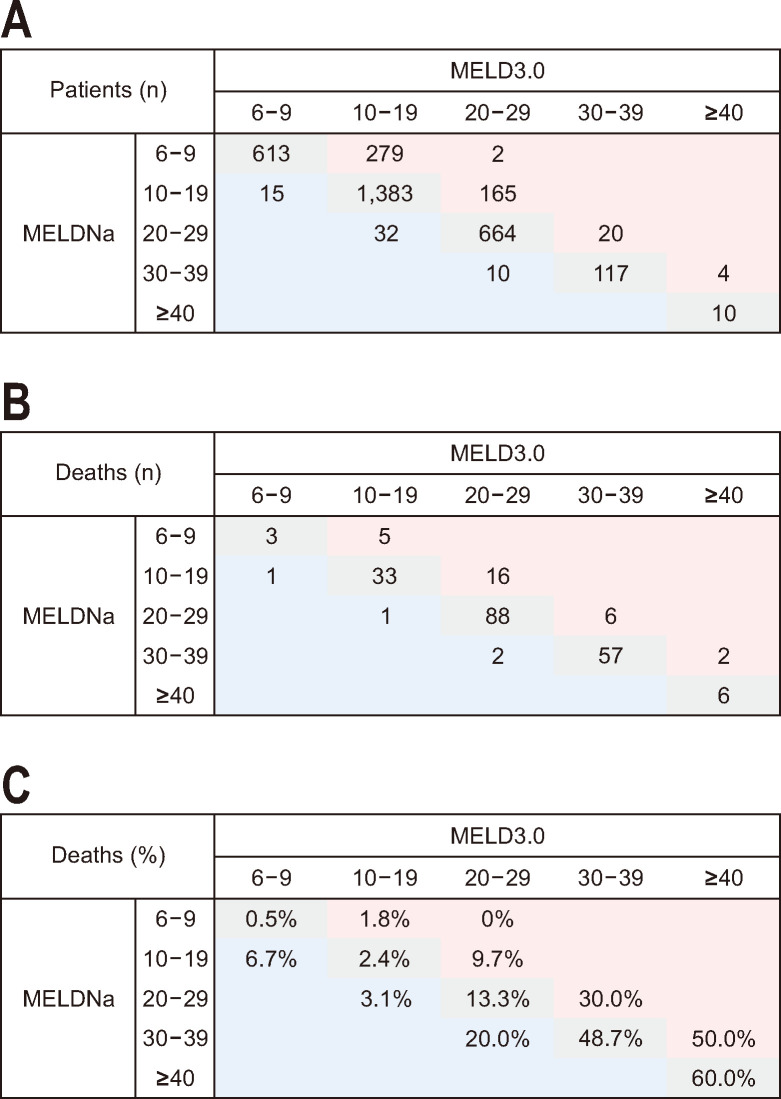
Fig. 1 shows the distribution of patients according to the score of MELD, MELDNa, and MELD3.0 and mortality at 3 months. In our study, 220 patients (6.6%) were dead at 3 months. Both MELDNa and MELD3.0 scores exhibited a positively skewed distribution, with 69.1% of patients having scores below 20 for both MELDNa and MELD3.0. Of 3,314 patients, 84.1% remained in the same score categories in both MELDNa and MELD3.0. Up-categorized patients comprised 14.2% of the total, while 1.7% were down-categorized.
Fig. 1.
Reclassification of patients with liver cirrhosis between MELDNa and MELD3.0 scores. (A) the number of patients, (B) the number of deaths at 3 months and (C) the proportion of death at 3 months (B divided by A). Red-demarcated areas indicate higher score categories in MELD3.0 and blue-demarcated areas the lower score categories in MELD3.0 than MELDNa. MELDNa, Model for End-Stage Liver Disease (MELD) sodium; MELD3.0, MELD 3.0.
Higher score categories of both MELDNa and MELD3.0 exhibited an increase in the 3-month mortality rate. Out of the 220 deceased patients, 187 (85.0%) remained in the same category, while 29 (13.2%) were up-categorized in MELD3.0 compared to MELDNa, and four (1.8%) were down-categorized. The 3-month mortality rates for the patients in the same category, up-categorized, and down-categorized were 6.7%, 7.0%, and 6.1%, respectively. Up-categorized patients exhibited a higher 3-month mortality rate (7.0%) compared to those in the same score category (6.7%) or with down-categorized status (6.1%).
When comparing MELD3.0 with MELD, 77.2% of patients stayed in the same score category, 21.8% were up-categorized, and 1.0% were down-categorized (Supplementary Fig. 1). Among the 2,558 patients with the same score category, the 3-month mortality rate was 6.1%. For the 724 up-categorized patients, this rate was higher at 8.8%, while it decreased to 3.1% in the 32 down-categorized patients.
When comparing MELD with MELDNa, 91.5% of patients maintained the same score category, 8.4% of patients were up-categorized, and no patients were down-categorized (Supplementary Fig. 2). Among the patients with the same score category, 6.0% died; 13.6% of patients up-categorized died.
3. Performance of MELD3.0 and other models
The predictive performance of Child-Pugh class, ALBI grade, and MELD models for 3-month mortality was summarized in Table 2 and Fig. 2. The MELD3.0 score demonstrated superior predictive performance for 3-month mortality (AUROC, 0.851; 95% CI, 0.822 to 0.880) compared to the Child-Pugh class (AUROC, 0.754; 95% CI, 0.725 to 0.783; p<0.001) and ALBI grade (AUROC, 0.704; 95% CI, 0.680 to 0.728; p<0.001). In comparison to both MELD (AUROC, 0.846; 95% CI, 0.816 to 0.876; p=0.235) and MELDNa (AUROC, 0.849; 95% CI, 0.819 to 0.878; p=0.584), MELD3.0 exhibited improved predictive accuracy for 3-month mortality, although the difference was not significant (Fig. 2A). Among male patients, MELD3.0 exhibited the highest AUROC of the models. Conversely, for female patients, MELDNa demonstrated the highest AUROC for predicting 3-month mortality (Supplementary Table 1, Fig. 2B and C). According to the etiology of liver disease, the AUROC for 3-month mortality was highest with MELDNa in cases of chronic viral hepatitis and alcohol-related liver disease, while MELD3.0 had the highest AUROC for other etiologies, without statistical significance (Supplementary Table 2). When predicting 6- and 12-month mortality, MELD3.0 displayed the most robust performance in terms of AUROC. Nevertheless, statistical significance was not observed compared with MELD and MELDNa (Table 2, Supplementary Fig. 3).
Table 2.
The AUROC of Models in Predicting Mortality in Patients with Liver Cirrhosis
| Model | 3-mo mortality | 6-mo mortality | 9-mo mortality | 12-mo mortality | 15-mo mortality | C-Index | p-value for C-Index |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUROC (95% CI) | p-value* | AUROC (95% CI) | p-value* | AUROC (95% CI) | p-value* | AUROC (95% CI) | p-value* | AUROC (95% CI) | p-value* | |||||||
| Child-Pugh class | 0.754 (0.725–0.783) | <0.001 | 0.720 (0.694–0.747) | <0.001 | 0.710 (0.687–0.733) | <0.001 | 0.694 (0.671–0.718) | <0.001 | 0.680 (0.666–0.711) | <0.001 | 0.64 | <0.001 | ||||
| ALBI grade | 0.704 (0.680–0.728) | <0.001 | 0.680 (0.656–0.704) | <0.001 | 0.673 (0.651–0.696) | <0.001 | 0.668 (0.646–0.690) | <0.001 | 0.662 (0.640–0.683) | <0.001 | 0.622 | <0.001 | ||||
| MELD | 0.846 (0.816–0.876) | 0.235 | 0.789 (0.760–0.818) | 0.057 | 0.766 (0.740–0.791) | 0.002 | 0.747 (0.721–0.773) | 0.001 | 0.740 (0.715–0.764) | 0.001 | 0.685 | 0.531 | ||||
| MELDNa | 0.849 (0.819–0.878) | 0.584 | 0.793 (0.765–0.822) | 0.256 | 0.772 (0.746–0.797) | 0.024 | 0.754 (0.727–0.780) | 0.007 | 0.744 (0.720–0.769) | 0.010 | 0.689 | 0.701 | ||||
| MELD3.0 | 0.851 (0.822–0.880) | (Ref) | 0.798 (0.770–0.826) | (Ref) | 0.779 (0.754–0.804) | (Ref) | 0.764 (0.739–0.789) | (Ref) | 0.753 (0.729–0.777) | (Ref) | 0.693 | (Ref) | ||||
AUROC, area under the receiver operating characteristic curve; CI, confidence interval; ALBI, albumin-bilirubin; MELD, Model for End-Stage Liver Disease; MELDNa, MELD sodium; MELD3.0, MELD 3.0.
*Each model was compared with MELD3.0, and p-values were calculated using the DeLong method.
Fig. 2.
Comparison of the models predicting 3-months mortality in (A) the entire cohort, (B) the male cohort, and (C) the female cohort. ALBI, albumin-bilirubin; MELD, Model for End-Stage Liver Disease; MELDNa, MELD sodium; MELD3.0, MELD 3.0.
4. Association of models and overall mortality
During the median follow up of 11,247 person-years in our study cohort, 1,041 patients died, representing an annual mortality rate of 92.6 per 1,000 person-years. Using multivariable Cox regression analysis, elevated Child-Pugh class (class B adjusted HR, 2.133; 95% CI, 1.836 to 2.478; p<0.001 and class C adjusted HR, 4.841; 95% CI, 3.985 to 5.880; p<0.001), ALBI grade (grade 2 adjusted HR, 2.041; 95% CI, 1.436 to 2.901; p<0.001 and grade 3 adjusted HR, 4.203; 95% CI, 2.974 to 5.940; p<0.001), MELD group (score 10 to <20 adjusted HR, 2.225; 95% CI, 1.870 to 2.647; p<0.001 and score ≥20 adjusted HR, 4.859; 95% CI, 4.006 to 5.894; p<0.001), MELDNa group (score 10 to <20 adjusted HR, 2.077; 95% CI, 1.739 to 2.481; p<0.001 and score ≥20 adjusted HR, 4.420; 95% CI, 3.676 to 5.315; p<0.001), and MELD3.0 (score 10 to <20 adjusted HR, 2.176; 95% CI, 1.758 to 2.693; p<0.001 and score ≥20 adjusted HR, 4.892; 95% CI, 3.941 to 6.072; p<0.001) were independently associated with mortality. This risk remained regardless of age, sex, and underlying etiology of liver disease (Table 3).
Table 3.
Risks of Overall Mortality in Patients with Liver Cirrhosis According to the Models
| Model | Unadjusted HR (95% CI) |
p-value | Adjusted HR*
(95% CI) |
p-value | Adjusted HR†
(95% CI) |
p-value | Adjusted HR‡
(95% CI) |
p-value |
|---|---|---|---|---|---|---|---|---|
| Child-Pugh class | ||||||||
| Class A | (Ref) | (Ref) | (Ref) | (Ref) | ||||
| Class B | 2.040 (1.758–2.368) | <0.001 | 2.160 (1.860–2.509) | <0.001 | 2.164 (1.864–2.514) | <0.001 | 2.133 (1.836–2.478) | <0.001 |
| Class C | 4.334 (3.580–5.247) | <0.001 | 4.974 (4.096–6.039) | <0.001 | 4.979 (4.101–6.046) | <0.001 | 4.841 (3.985–5.880) | <0.001 |
| ALBI grade | ||||||||
| Grade 1 | (Ref) | (Ref) | (Ref) | (Ref) | ||||
| Grade 2 | 2.125 (1.496–3.020) | <0.001 | 2.104 (1.481–2.989) | <0.001 | 2.100 (1.478–2.983) | <0.001 | 2.041 (1.436–2.901) | <0.001 |
| Grade 3 | 4.144 (2.935–5.852) | <0.001 | 4.374 (3.096–6.179) | <0.001 | 4.370 (3.094–6.173) | <0.001 | 4.203 (2.974–5.940) | <0.001 |
| MELD | ||||||||
| <10 | (Ref) | (Ref) | (Ref) | (Ref) | ||||
| 10 to <20 | 2.148 (1.806–2.554) | <0.001 | 2.251(1.892–2.677) | <0.001 | 2.249 (1.890–2.675) | <0.001 | 2.225 (1.870–2.647) | <0.001 |
| ≥20 | 4.449 (3.675–5.386) | <0.001 | 4.901(4.042–5.942) | <0.001 | 4.894 (4.035–5.934) | <0.001 | 4.859 (4.006–5.894) | <0.001 |
| MELDNa | ||||||||
| <10 | (Ref) | (Ref) | (Ref) | (Ref) | ||||
| 10 to <20 | 2.008 (1.682–2.397) | <0.001 | 2.095 (1.754–2.502) | <0.001 | 2.094 (1.754–2.501) | <0.001 | 2.077 (1.739–2.481) | <0.001 |
| ≥20 | 4.064 (3.387–4.877) | <0.001 | 4.487 (3.733–5.392) | <0.001 | 4.483 (3.729–5.389) | <0.001 | 4.420 (3.676–5.315) | <0.001 |
| MELD3.0 | ||||||||
| <10 | (Ref) | (Ref) | (Ref) | (Ref) | ||||
| 10 to <20 | 2.149 (1.737–2.658) | <0.001 | 2.163 (1.749–2.676) | <0.001 | 2.191 (1.771–2.711) | <0.001 | 2.176 (1.758–2.693) | <0.001 |
| ≥20 | 4.560 (3.679–5.652) | <0.001 | 4.913 (3.961–6.094) | <0.001 | 4.984 (4.016–6.185) | <0.001 | 4.892 (3.941–6.072) | <0.001 |
HR, hazard ratio; CI, confidence interval; ALBI, albumin-bilirubin; MELD, Model for End-Stage Liver Disease; MELDNa, MELD sodium; MELD3.0, MELD 3.0.
*Adjusted for age; †Adjusted for age and sex; ‡Adjusted for age, sex, and etiology of liver disease.
In both male and female patients, higher values in each model were associated with an elevated risk of mortality (Supplementary Table 3). In the male cohort, MELD3.0 (score 10 to <20 adjusted HR, 2.054; 95% CI, 1.616 to 2.611; p<0.001 and score ≥20 adjusted HR, 4.691; 95% CI, 3.679 to 5.982; p<0.001) was independently associated with mortality after adjustment for age and etiology of liver disease. Similarly, in the female cohort, higher class of MELD3.0 was associated with an increased risk of overall mortality (score 10 to <20 adjusted HR, 2.659; 95% CI, 1.649 to 4.287; p<0.001 and score ≥20 adjusted HR, 5.678; 95% CI, 3.499 to 9.214; p<0.001).
5. Associations of MELD3.0 and liver cirrhosis-related complication
Patients were categorized into three groups based on MELD3.0 score: 19.0% of patients had MELD3.0 scores below 10, 51.1% had scores between 10 and 20, and 29.9% had scores of 20 or higher. The incidence rate of liver cirrhosis-related complications within 3 months was compared among the three groups (Table 4). The most common cirrhosis-related complication was ascites (20.4%), followed by gastroesophageal varix bleeding (5.1%). The highest incidence rate of cirrhosis-related complications occurred in patients with MELD3.0 scores of 20 or higher (32.6%), followed by those with MELD3.0 scores between 10 and 20 (26.5%) and, finally, those with MELD3.0 scores below 10 (15.6%). As depicted in Table 5, the risk of cirrhosis-related complications was independently associated with an elevated MELD3.0 score after adjustment, with an adjusted HR of 1.033 (95% CI, 1.025 to 1.041; p<0.001). As the MELD3.0 score increased, the risk of hepatorenal syndrome showed the largest increase (adjusted HR, 1.149; 95% CI, 1.111 to 1.188; p<0.001), followed by that of hepatic encephalopathy (adjusted HR, 1.077; 95% CI, 1.063 to 1.090; p<0.001) and then that of ascites (adjusted HR, 1.040; 95% CI, 1.028 to 1.053; p<0.001) (Fig. 3).
Table 4.
Risk of Cirrhosis-Related Complications According to MELD3.0 within the Enrollment of the First 3 Months
| MELD3.0 | p-value | |||
|---|---|---|---|---|
| <10 (n=628) | 10 to <20 (n=1,694) | ≥ 20 (n=992) | ||
| Cirrhosis-related complication, No. (%) | 98 (15.6) | 449 (26.5) | 323 (32.6) | <0.001 |
| New onset or uncontrolled ascites | 75 (11.9) | 346 (20.4) | 155 (25.9) | <0.001 |
| Gastroesophageal varix bleeding | 27 (4.3) | 102 (6.0) | 41 (4.1) | 0.058 |
| Hepatorenal syndrome | 0 (0.0) | 2 (0.1) | 18 (1.8) | <0.001 |
| Hepatic encephalopathy | 3 (0.5) | 45 (2.7) | 58 (5.9) | <0.001 |
MELD3.0, Model for End-Stage Liver Disease 3.0.
Table 5.
Risk of Cirrhosis-Related Complications According to MELD3.0
| Unadjusted HR (95% CI) |
p-value | Adjusted HR* (95% CI) |
p-value | Adjusted HR† (95% CI) |
p-value | Adjusted HR‡ (95% CI) |
p-value | |
|---|---|---|---|---|---|---|---|---|
| Cirrhosis-related complication |
1.038 (1.030–1.046) | <0.001 | 1.039 (1.031–1.047) | <0.001 | 1.039 (1.031–1.048) | <0.001 | 1.033 (1.025–1.041) | <0.001 |
| New onset or uncontrolled ascites |
1.041 (1.029–1.053) | <0.001 | 1.041 (1.029–1.053) | <0.001 | 1.041 (1.029–1.053) | <0.001 | 1.040 (1.028–1.053) | <0.001 |
| Gastroesophageal varix bleeding | 1.008 (0.997–1.019) | 0.178 | 1.005 (0.994–1.017) | 0.361 | 1.006 (0.995–1.018) | 0.270 | 1.006 (0.995–1.017) | 0.301 |
| Hepatorenal syndrome§ | 1.143 (1.106–1.180) | <0.001 | 1.145 (1.108–1.184) | <0.001 | 1.148 (1.110–1.186) | <0.001 | 1.149 (1.111–1.188) | <0.001 |
| Hepatic encephalopathy | 1.068 (1.054–1.081) | <0.001 | 1.078 (1.064–1.091) | <0.001 | 1.077 (1.064–1.091) | <0.001 | 1.077 (1.063–1.090) | <0.001 |
MELD3.0, Model for End-Stage Liver Disease 3.0; HR, hazard ratio; CI, confidence interval.
*Adjusted for age; †Adjusted for age and sex; ‡Adjusted for age, sex, and etiology of liver disease; §Firth correction was applied for analyzing hepatorenal syndrome.
Fig. 3.
Cumulative risk of cirrhosis-related complications according to the MELD3.0 score; (A) ascites, (B) gastroesophageal varix bleeding, (C) hepatorenal syndrome, and (D) hepatic encephalopathy. MELD3.0, Model for End-Stage Liver Disease 3.0.
DISCUSSION
In this comprehensive multicenter cohort study, we found that MELD3.0 demonstrated the highest accuracy, with an AUROC of 0.851, in predicting 3-month mortality for patients with liver cirrhosis. Previously utilized models such as Child-Pugh class, ALBI grade, MELD, and MELDNa also demonstrated acceptable predictive performance. Moreover, for every 1-point increase in the MELD3.0 score, the risk of cirrhosis-related complications increased by 1.033-fold. Specifically, the risk of hepatorenal syndrome, ascites, and hepatic encephalopathy increased with higher MELD3.0 score.
MELD3.0 improved accuracy with following methods: (1) incorporation of two new variables—serum albumin and female sex—with a notable inclusion of 1.33 points for female sex; (2) reduction in the upper limit for serum creatinine from 4.0 to 3.0 mg/dL; and (3) inclusion of two interactions between albumin and creatinine and between bilirubin and sodium.15 MELD3.0, the most recently developed model, exhibited greater predictive potential for short-term mortality compared to both MELD and MELDNa. However, external validation results have been variable, and concerns have been raised regarding the lack of model calibration reporting, potential biases of reclassification methods, and the use of a lower limit of 1 for bilirubin, creatinine, and INR, which may affect the accuracy and generalizability of MELD3.0.17 Yoo et al.18 also reported that MELD3.0 outperformed MELD or MELDNa in predicting prognosis. Their study, which encompassed 2,153 Korean patients with liver cirrhosis from two hospitals, revealed an integrated time-dependent AUC of 0.759 for MELD3.0 in predicting 3-month transplant-free survival, compared to 0.748 for MELD and 0.761 for MELDNa. On the other hand, Díaz et al.19 reported that for 2,124 patients with alcoholic hepatitis, MELD3.0 (AUROC, 0.744) exhibited superior predictive capability for 3-month mortality compared to MELDNa (AUROC, 0.721) but was comparable to MELD (AUROC, 0.731). In a Chinese study involving 576 patients with alcohol-related liver disease, the AUC of MELD3.0 (0.713) was lower than that of MELD (0.740) or MELDNa (0.758) for predicting 3-month mortality.20 Also, MELD3.0 was not superior to MELDNa in a multinational study of 519 patients waiting liver transplantation.21 In our comprehensive cohort of 3,314 individuals with various etiologies, we compared several assessment methods for liver function other than MELD, including Child-Pugh class and ALBI. MELD3.0 demonstrated better performance in predicting 3-, 6-, and 12-month mortality in patients with cirrhosis compared to previous models including Child-Pugh class, ALBI grade, MELD, and MELDNa. Indeed, the AUROC of MELD3.0 for predicting 3-month mortality in patients with liver cirrhosis was 0.851, which is higher than that of previous studies (AUROC, 0.744 to 0.776).18-20,22 In our study, MELD3.0 was an effective method for evaluating liver function and predicting related prognosis in patients with liver cirrhosis.
One of the most notable features of MELD3.0 is that it differentiates between sexes. Kim et al.15 addressed sex disparity by adding 1.3 points to female patients, who were found to have an increased risk of mortality compared to male patients even with same MELD scores. This might be associated with their relatively lower creatinine values compared to males, even when renal function, expressed as estimated glomerular filtration rate, is similar.23,24 The inherent limitations of serum creatinine—being influenced by age, sex, muscle mass, and protein intake—might have resulted in lower MELD scores in previous models, potentially reducing the accuracy of prognostic assessments. By accounting for sex disparity, which probably originated from serum creatinine, MELD3.0 demonstrated better performance than MELD or MELDNa. On the other hand, in our study, MELD3.0 did not provide a substantial improvement over MELDNa in female patients. Among female patients, given additional 1.3 points may lead to an overestimation of the MELD score, potentially affecting the accuracy of MELD3.0. Furthermore, the inclusion of serum albumin as an additional variable in MELD3.0 might not be effective as estrogen reduces albumin synthesis, which may diminish the discriminative power of MELD3.0.25 Future studies exploring sex-specific calibration of scoring systems or the inclusion of alternative biomarkers less influenced by sex-based physiological factors may improve the equity and accuracy of prognostic models.
Previous studies suggested the association of MELD3.0 and various liver-related complications. In Díaz et al.,19 MELD3.0 was a reliable prediction model for 1-month renal replacement therapy. Buckholz et al.26 reported that MELD3.0 can serve as a predictive tool for mortality after variceal bleeding. In a study reported by Verma et al.,27 MELD3.0 was associated with the increased risk of acute on chronic liver failure. In our study, MELD3.0 was an independent risk factor for cirrhosis-related complications mainly associated with kidney dysfunction such as ascites, hepatorenal syndrome, and hepatic encephalopathy.28 The incidence of cirrhosis-related complications, reflecting the disease burden, was highest in ascites within the first 3 months of enrollment. Nevertheless, the risk of hepatorenal syndrome exhibited the most significant increase, with a 1.149-fold increase for each one-point increment of the MELD3.0 score. Hepatorenal syndrome is a severe complication of cirrhosis with high mortality, driven by mechanisms such as circulatory dysfunction and systemic inflammation. The survival rate of patients with hepatorenal syndrome ranges from 27% to 59%, even with appropriate management involving vasoconstrictors.29 Serum creatinine, a key component of the MELD3.0, serves as a valuable biomarker for the early prediction of hepatorenal syndrome.30 Patients with elevated MELD3.0, particularly those with concurrent increases in creatinine, should be considered at heightened risk for hepatorenal syndrome, and careful monitoring with short-term interval of renal function in these patients is advisable. Our findings could provide insight into the role of MELD3.0 as both a surrogate marker for liver cirrhosis severity and a prognostic factor for cirrhosis-related complications, in particular, hepatorenal syndrome.
One of the strengths of our study was the use of real-world data, including those of 3,314 patients from multiple centers. These patients with liver cirrhosis had various etiologies, including chronic viral hepatitis, alcoholic hepatitis, and others. This multidimensional evaluation provides a broader perspective on liver function and patient prognosis. We also demonstrated that MELD3.0 was an effective surrogate for predicting cirrhosis-related complications, particularly those involving kidney function, such as ascites and hepatorenal syndrome. We compared MELD3.0 with other assessment tools, including not only MELD models but also ALBI and Child-Pugh scores. However, there are some limitations. First, due to the retrospective nature of our study, there is a possibility of biases and confounders influencing the results. Therefore, we tried to minimize potential biases by employing multivariable analysis. Also, the results were consistent in various subgroup analyses. Second, the score or grade of each model can be influenced by changes in patient condition over time, although this likely had little effect as we utilized results from a single timepoint. We used findings at the time of enrollment, which may reflect the patient's overall condition outside the hospital. Lastly, other potential confounding factors, such as comorbidities or treatment history, which might affect survival or cirrhosis-related complications, were not considered in this study. Further studies addressing these factors are needed.
In conclusion, our study highlights that MELD3.0 exhibits good prognostic performance in predicting mortality and cirrhosis-related complications in patients with cirrhosis. Patients with a MELD3.0 score of 20 or higher had a 4.892 times greater risk of overall mortality compared to those with a MELD3.0 score of 10 or lower. Each 1-point increase in MELD3.0 increases the risk of cirrhosis-related complications by 1.033-fold. Based on these insights, we advocate for a thorough evaluation of liver function, including assessment of the MELD3.0 score, in patients with cirrhosis. This comprehensive approach can facilitate appropriate treatment strategies aimed at preventing cirrhosis-related complications and improving survival.
ACKNOWLEDGEMENTS
This work was supported by Clinical Research Institute Grant funded by Daejeon St. Mary’s Hospital, The Catholic University of Korea (2023).
We thank Hana Kim (Biostatistician, Medical Excellence) for her assistance with statistical analysis.
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl240584.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: M.J.S. Data acquisition: all authors. Data analysis and interpretation: M.J.S., J.L. Drafting of the manuscript: M.J.S., J.L. Critical revision of the manuscript for important intellectual content: J.H.K., A.L., J.W.H., S.K.L., H.Y., H.N., H.L.L., D.S.S., S.W.L., H.Y.K., J.H.K., C.W.K., U.I.C., S.W.N., S.H.K., P.S.S., J.W.J., S.H.B., J.Y.C., S.K.Y. Statistical analysis: M.J.S., J.L. Obtained funding: M.J.S. Administrative, technical, or material support; study supervision: M.J.S. Approval of final manuscript: all authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon request from the corresponding author (M.J.S.). The data are not publicly available as they contain information that could compromise the privacy of research participants.
REFERENCES
- 1.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 2.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudo M. Newly developed modified ALBI grade shows better prognostic and predictive value for hepatocellular carcinoma. Liver Cancer. 2021;11:1–8. doi: 10.1159/000521374.a4b789c1e51b4f2c8050d106bf7542ac [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 5.Wu T, Li J, Shao L, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67:2181–2191. doi: 10.1136/gutjnl-2017-314641. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj JS, O'Leary JG, Reddy KR, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250–256. doi: 10.1002/hep.27077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo J, Li J, Li P, et al. Acute-on-chronic liver failure: far to go-a review. Crit Care. 2023;27:259. doi: 10.1186/s13054-023-04540-4.7f9b046837a4485c90c0be95611b418a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 9.Barber K, Madden S, Allen J, et al. Elective liver transplant list mortality: development of a United Kingdom end-stage liver disease score. Transplantation. 2011;92:469–476. doi: 10.1097/TP.0b013e318225db4d. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Hwang S, Ahn CS, et al. Absence of influence of the Korean MELD score-based liver allocation system on pretransplant MELD score in patients undergoing living donor liver transplantation. Ann Liver Transplant. 2021;1:10–17. doi: 10.52604/alt.21.0003. [DOI] [Google Scholar]
- 11.Tejedor M, Selzner N, Berenguer M. Are MELD and MELDNa still reliable tools to predict mortality on the liver transplant waiting list? Transplantation. 2022;106:2122–2136. doi: 10.1097/TP.0000000000004163. [DOI] [PubMed] [Google Scholar]
- 12.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Lee JS, Lee SH, et al. The association between the serum sodium level and the severity of complications in liver cirrhosis. Korean J Intern Med. 2009;24:106–112. doi: 10.3904/kjim.2009.24.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godfrey EL, Malik TH, Lai JC, et al. The decreasing predictive power of MELD in an era of changing etiology of liver disease. Am J Transplant. 2019;19:3299–3307. doi: 10.1111/ajt.15559. [DOI] [PubMed] [Google Scholar]
- 15.Kim WR, Mannalithara A, Heimbach JK, et al. MELD 3.0: the Model for End-Stage Liver Disease updated for the modern era. Gastroenterology. 2021;161:1887–1895. doi: 10.1053/j.gastro.2021.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulligan D, Hirose R. OPTN/UNOS Liver and Intestinal Organ Transplantation Committee Meeting Summary. Organ Procurement and Transplantation Network; Richmond: 2014. [Google Scholar]
- 17.Goudsmit BF, Putter H, Van Hoek B. The Model for End-Stage Liver Disease 3.0: an update without proven accuracy. Gastroenterology. 2022;162:1781–1782. doi: 10.1053/j.gastro.2021.09.047. [DOI] [PubMed] [Google Scholar]
- 18.Yoo JJ, Chang JI, Moon JE, Sinn DH, Kim SG, Kim YS. Validation of MELD 3.0 scoring system in East Asian patients with cirrhosis awaiting liver transplantation. Liver Transpl. 2023;29:1029–1040. doi: 10.1097/LVT.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 19.Díaz LA, Fuentes-López E, Ayares G, et al. MELD 3.0 adequately predicts mortality and renal replacement therapy requirements in patients with alcohol-associated hepatitis. JHEP Rep. 2023;5:100727. doi: 10.1016/j.jhepr.2023.100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duan F, Liu C, Zhai H, Quan M, Cheng J, Yang S. The Model for End-Stage Liver Disease 3.0 is not superior to the Model for End-Stage Liver Disease-Na in predicting survival: a retrospective cohort study. Hepatol Commun. 2023;7:e0250. doi: 10.1097/HC9.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tejedor M, Bellón JM, Fernández de la Varga M, et al. Validation of MELD3.0 in 2 centers from different continents. Hepatol Commun. 2024;8:e0504. doi: 10.1097/HC9.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim J, Kim JH, Kim SE, et al. Validation of MELD 3.0 in patients with alcoholic liver cirrhosis using prospective KACLiF cohort. J Gastroenterol Hepatol. 2024;39:1932–1938. doi: 10.1111/jgh.16591. [DOI] [PubMed] [Google Scholar]
- 23.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pottel H, Delanaye P, Cavalier E. Exploring renal function assessment: creatinine, cystatin C, and estimated glomerular filtration rate focused on the European Kidney Function Consortium equation. Ann Lab Med. 2024;44:135–143. doi: 10.3343/alm.2023.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May FE, Ryffel GU, Weber R, Westley BR. Estrogen dramatically decreases albumin mRNA levels and albumin synthesis in Xenopus laevis liver. J Biol Chem. 1982;257:13919–13923. doi: 10.1016/S0021-9258(19)45320-9. [DOI] [PubMed] [Google Scholar]
- 26.Buckholz A, Wong R, Curry MP, et al. MELD, MELD 3.0, versus Child score to predict mortality after acute variceal hemorrhage: a multicenter US cohort. Hepatol Commun. 2023;7:e0258. doi: 10.1097/HC9.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma N, Roy A, Valsan A, et al. Liver dysfunction and systemic inflammation drive organ failures in acute decompensation of cirrhosis: a multicentric study. Am J Gastroenterol. 2025;120:182–193. doi: 10.14309/ajg.0000000000003115. [DOI] [PubMed] [Google Scholar]
- 28.European Association for the Study of the Liver, author. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Ginès P, Solà E, Angeli P, Wong F, Nadim MK, Kamath PS. Hepatorenal syndrome. Nat Rev Dis Primers. 2018;4:23. doi: 10.1038/s41572-018-0022-7. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Zhou Z, Xu C, et al. Establishment and evaluation of an early prediction model of hepatorenal syndrome in patients with decompensated hepatitis B cirrhosis. BMC Gastroenterol. 2023;23:1. doi: 10.1186/s12876-022-02618-x.755b01a455324312a0714e02b051b33b [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.