Abstract
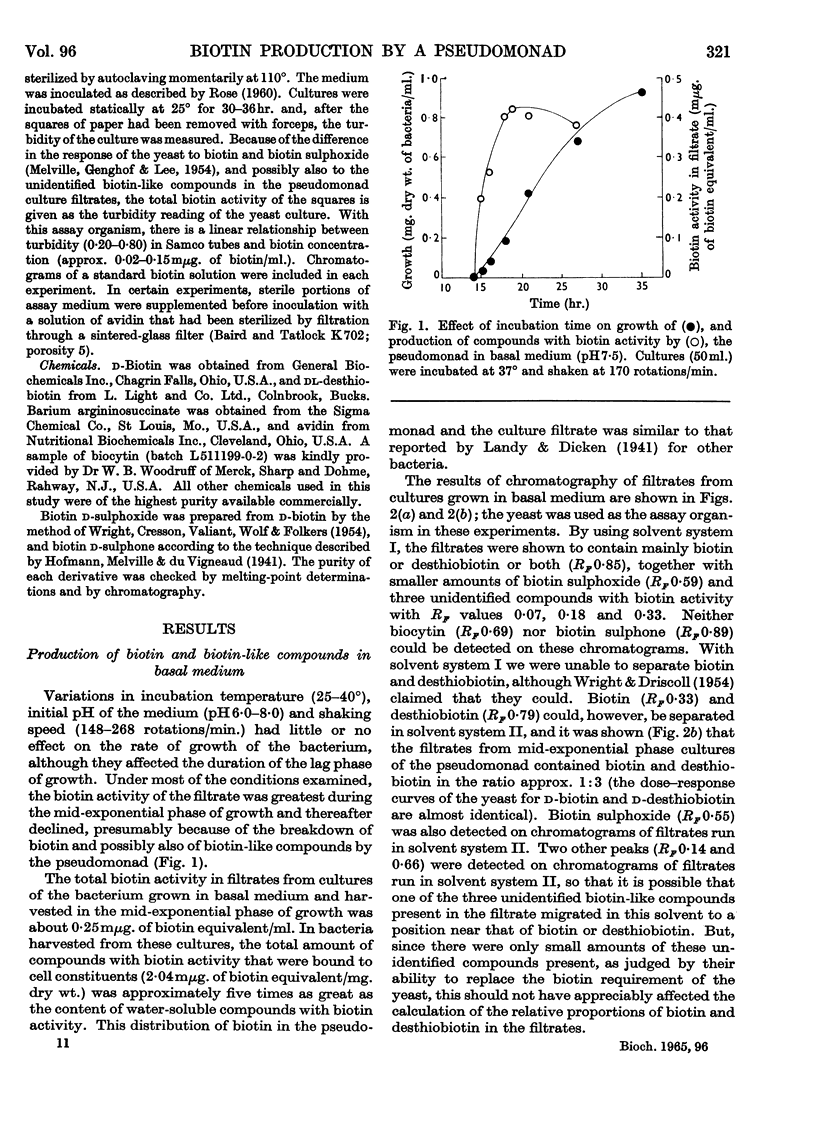
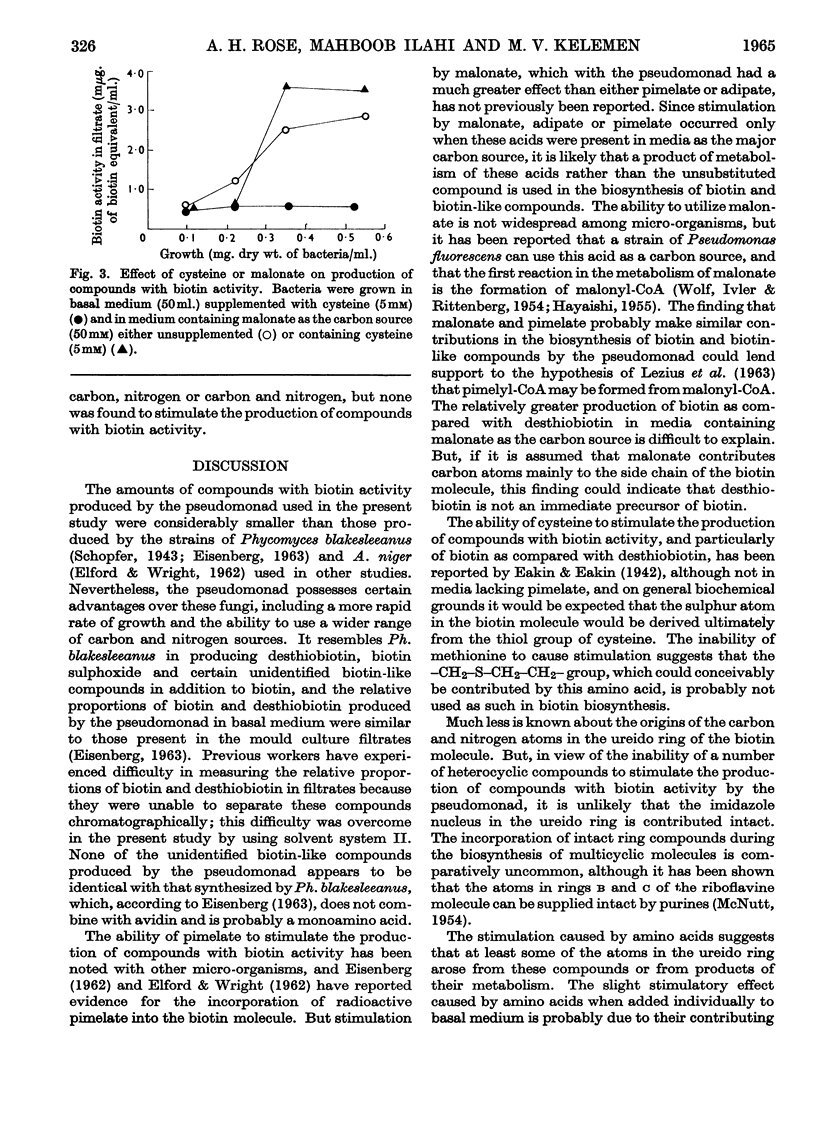
1. Filtrates from cultures of a strain of Pseudomonas aeruginosa, grown in a basal glucose–ammonium chloride–vitamins–salts medium, possessed biotin activity as detected by microbiological assays. Exponential-phase culture filtrates contained biotin and desthiobiotin in the approximate ratio 1:3, with smaller amounts of biotin sulphoxide and three unidentified compounds with biotin activity. 2. The addition of malonate, adipate or pimelate to the basal medium stimulated the production of compounds with biotin activity; this effect was enhanced when these compounds were included in the medium as the major carbon source. Succinate, glutarate, suberate, fumarate or oxaloacetate did not stimulate the production of compounds with biotin activity. The ratio of biotin to desthiobiotin in filtrates from cultures grown in medium containing malonate as the carbon source was about 1:1. Experiments in which mixtures of malonate and pimelate were included in the medium as the carbon sources showed that these acids probably make a similar contribution in biotin biosynthesis. 3. A number of heterocyclic compounds, including several containing the ureido group (–NH–CO–NH–), were included in the basal medium but none of them stimulated the production of compounds with biotin activity to any marked degree. 4. Several amino acids, particularly cysteine (or cystine) and lysine, when added individually as supplements to the basal medium, stimulated the production of compounds with biotin activity. Filtrates from cultures grown in medium supplemented with cysteine contained approximately equal proportions of biotin and desthiobiotin. A much greater stimulation in the production of compounds with biotin activity was obtained when certain amino acids were included in the medium as the major source of nitrogen or carbon and nitrogen; ornithine, citrulline and argininosuccinate had the most marked effect. The ratio of biotin to desthiobiotin in filtrates from these cultures was usually greater than in filtrates from cultures grown in basal medium. 5-Aminovalerate also caused some stimulation when used as the nitrogen source, but urea was inactive. The effect of binary mixtures of certain amino acids was also examined. 5. The results are discussed in relation to the possible role of the stimulatory compounds during biotin biosynthesis.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAMPBELL L. L., Jr, WILLIAMS O. B. Observations on the biotin requirement of thermophilic bacteria. J Bacteriol. 1953 Feb;65(2):146–147. doi: 10.1128/jb.65.2.146-147.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DU Vigneaud V., Dittmer K., Hague E., Long B. THE GROWTH-STIMULATING EFFECT OF BIOTIN FOR THE DIPHTHERIA BACILLUS IN THE ABSENCE OF PIMELIC ACID. Science. 1942 Aug 21;96(2486):186–187. doi: 10.1126/science.96.2486.186. [DOI] [PubMed] [Google Scholar]
- EISENBERG M. A. BIOTIN BIOSYNTHESIS. I. BIOTIN YIELDS AND BIOTIN VITAMERS IN CULTURES OF PHYCOMYCES BLAKESLEEANUS. J Bacteriol. 1963 Oct;86:673–680. doi: 10.1128/jb.86.4.673-680.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENBERG M. A. The incorporation of 1,7 C14 pimelic acid into biotin vitamers. Biochem Biophys Res Commun. 1962 Aug 31;8:437–441. doi: 10.1016/0006-291x(62)90292-9. [DOI] [PubMed] [Google Scholar]
- Eakin R. E., Eakin E. A. A BIOSYNTHESIS OF BIOTIN. Science. 1942 Aug 21;96(2486):187–188. doi: 10.1126/science.96.2486.187. [DOI] [PubMed] [Google Scholar]
- GREEN N. M. AVIDIN. 3. THE NATURE OF THE BIOTIN-BINDING SITE. Biochem J. 1963 Dec;89:599–609. doi: 10.1042/bj0890599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYAISHI O. Enzymatic decarboxylation of malonic acid. J Biol Chem. 1955 Jul;215(1):125–136. [PubMed] [Google Scholar]
- Jacoby G. A. The induction and repression of amino acid oxidation in Pseudomonas fluorescens. Biochem J. 1964 Jul;92(1):1–8. doi: 10.1042/bj0920001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORZENOVSKY M., WERKMAN C. H. Conversion of citrulline to ornithine by cell-free extracts of Streptococcus lactis. Arch Biochem Biophys. 1953 Sep;46(1):174–185. doi: 10.1016/0003-9861(53)90180-5. [DOI] [PubMed] [Google Scholar]
- LICHSTEIN H. C. The biotin requirements of the genus Propionibacterium. Arch Biochem Biophys. 1955 Oct;58(2):423–430. doi: 10.1016/0003-9861(55)90142-9. [DOI] [PubMed] [Google Scholar]
- Leonian L. H., Lilly V. G. Conversion of Desthiobiotin Into Biotin or Biotinlike Substances by Some Microorganisms. J Bacteriol. 1945 Mar;49(3):291–297. doi: 10.1128/jb.49.3.291-297.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEISTER A. Enzymatic transamination reactions involving arginine and ornithine. J Biol Chem. 1954 Feb;206(2):587–596. [PubMed] [Google Scholar]
- MELVILLE D. B., GENGHOF D. S., LEE J. M. Biological properties of biotin d- and l-sulfoxides. J Biol Chem. 1954 Jun;208(2):503–512. [PubMed] [Google Scholar]
- McNUTT W. S. The direct contribution of adenine to the biogenesis of riboflavin by Eremothecium ashbyii. J Biol Chem. 1954 Oct;210(2):511–519. [PubMed] [Google Scholar]
- NICKERSON W. J., ROSE A. H. Secretion of nicotinic acid by biotin-dependent yeasts. J Bacteriol. 1956 Sep;72(3):324–328. doi: 10.1128/jb.72.3.324-328.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE A. H. Excretion of nicotinic acid by biotin-deficient Saccharomyces cerevisiae. J Gen Microbiol. 1960 Aug;23:143–152. doi: 10.1099/00221287-23-1-143. [DOI] [PubMed] [Google Scholar]
- SCHWEET R. S., HOLDEN J. T., LOWY P. H. The metabolism of lysine in Neurospora. J Biol Chem. 1954 Dec;211(2):517–529. [PubMed] [Google Scholar]
- WALKER J. B., MYERS J. The formation of arginosuccinic acid from arginine and fumarate. J Biol Chem. 1953 Jul;203(1):143–152. [PubMed] [Google Scholar]
- WOLFE J. B., IVLER D., RITTENBERG S. C. Malonate decarboxylation by Pseudomonas fluorescens. I. Observations with dry cells and cell-free preparations. J Biol Chem. 1954 Aug;209(2):867–873. [PubMed] [Google Scholar]


