Abstract

Employing 9-BBN and pinacol boronic ester as surrogate stoppers, pseudorotaxanes presenting terminal alkenes can be transformed into organoborane or organoboronate rotaxanes, respectively. Various boron-based reactions can then be used to replace these temporary stoppers with bulky derivatives of aromatic bromides, α-bromo ketones, or 1-bromo-1-alkynes, facilitating the selective formation of sp3–sp2, sp3–sp3, and sp3–sp carbon–carbon single bonds, respectively.
The interlocked structures and switchable properties of rotaxanes have made them functional materials with applications in such fields as sensing,1 catalysis,2 and gelation.3 Over the past three decades, many strategies have been developed for their synthesis, including threading-followed-by-stoppering,4 slippage,5 clipping,6 threading-followed-by-swelling,7 threading-followed-by-ring-shrinking,8 and active-template synthesis.9 Among them, threading-followed-by-stoppering has emerged as the most popular, owing to its relative synthetic ease and structural flexibility. Nevertheless, the range of stoppering reactions is limited by the need for solvents and/or reagents that ensure the stability of the pseudorotaxane structures.
Previously reported stoppering reactions allow the introduction of diverse functionalities—including ester,10 amide,11 imine,12 and triazole13 groups—as links between the linear component’s termini and the stopper units in the resulting rotaxanes. Despite the importance to organic chemistry of reactions that form carbon–carbon single bonds, and the potential to use their high stability to facilitate the postsynthesis of rotaxanes, few methods are available for interlocking pseudorotaxanes through the formation of C–C single bonds (Figure 1).14 This situation is due to many of the reactions leading to C–C single bond formation involving strong nucleophiles or bases, or polar solvents, which tend to destabilize most pseudorotaxanes.
Figure 1.

Reactions that interlock surrogate rotaxanes through the formation of C–C bonds in the (a–c) presence and (d, e) absence of transition metals.
Successful examples of stoppering with C–C single bond formation have typically involved the preformation of an interlocked intermediate (i.e., a surrogate rotaxane) that prevents the macrocycle from dethreading under the potentially harsher conditions required for C–C bond formation.15 By complexing a transition metal ion catalyst to the interlocked intermediates, rotaxanes with newly formed C–C single bond linkages can be synthesized through Tsuji–Trost allylation16 (Figure 1a), alkyne hydroruthenation followed by allene insertion17 (Figure 1b), and I2-promoted coupling of cis-alkyne–Pt–alkyne complexes18 (Figure 1c). In examples where no transition metal ion is needed, Wittig reactions19 (Figure 1d) and malonate alkylations4b (Figure 1e) allow the indirect (hydrogenation of the formed C=C double bond) and direct production of C–C single bonds, respectively, from triphenylphosphonium- and trialkylanilinium-stopped surrogate rotaxanes, respectively.
New practical and robust methods for interlocking the components of pseudorotaxanes are always welcome, especially if they allow for the efficient formation of rotaxanes with different types of C–C single bond linkages. Herein, we report the use of 9-borabicyclo[3.3.1]nonane (9-BBN) and bis(pinacolato)diboron (B2pin2) as surrogate stoppers for the efficient interlocking of pseudorotaxanes presenting alkene termini, and the reactions of the resulting organoborane and organoboronic ester rotaxanes with bulky versions of an aromatic bromide, an α-bromo ketone, and a 1-bromo-1-alkyne, in the presence or absence of transition metals, to form rotaxanes with sp3–sp2-, sp3–sp3-, and sp3–sp-hybridized C–C single bond linkages, respectively.20
A single type of stoppering reaction would not always be practical for every host–guest recognition system because the noncovalent interactions that stabilize their pseudorotaxanes would respond differently under the reaction conditions. Therefore, any practical general method for interlocking would best be tested using relatively weakly associated recognition systems—and particularly those more sensitive to the reaction conditions (i.e., to the presence of nucleophiles, anions, and extreme solvent polarity). Here, we chose the monopyridinium/bis-p-xylyl[26]crown-6 (BPX26C6)21 and urea/Na+/BPX26C622 recognition systems. In theory, concerted additions, which release no molecular fragments into the reaction mixture, would minimize disturbance from external chemical additives to the pseudorotaxane complexes. Therefore, we examined the addition of bulky 9-BBN to terminal alkenes in the preparation of surrogate rotaxanes. We prepared the semidumbbell-shaped pyridinium salt 1·TFPB and the urea 2, both presenting alkene termini, through N- and O-alkylations of the pyridine 3 and the urea 4, respectively, with 6-bromo-1-hexene (Scheme 1).
Scheme 1. Synthesis of Semidumbbell-Shaped Threadlike Species Presenting Alkene Termini.
We added 9-BBN dimer (0.22 M) to an equimolar (0.4 M) solution of threadlike salt 1·TFPB and macrocycle BPX26C6 in toluene-d8 and then heated the mixture at 60 °C (Scheme 2). By diluting aliquots of the solution over time, 1H NMR spectroscopy (toluene-d8) revealed the diminution of the alkene signals (Figure 2a) and the growth of those for the interlocked macrocycle; these changes were complete after 16 h, suggesting successful formation of the rotaxane 5·TFPB through hydroboration of the alkene terminus of the pseudorotaxane BPX26C6⊃1·TFPB. Based on integration of signals of the aromatic protons of the interlocked (δ = 6.52) and free (br, δ = 6.62–6.85) macrocycles, we estimated the reaction yield to be approximately 80%. Because alkyl-9-BBN compounds are generally highly air-sensitive,23 we applied the crude rotaxane 5·TFPB directly in the next step, rather than purifying it. Using Pd(dppf)Cl2 to catalyze the coupling of the crude interlocked organoborane 5·TFPB and 1-bromo-3,5-di-tert-butylbenzene (6) in DMF,24 we obtained the stopper-exchanged product, the rotaxane 7·TFPB, in an overall yield (for sequential hydroboration/Suzuki coupling) of 70% after column chromatography. Thus, using 9-BBN to make an interlocked organoborane surrogate, followed by Suzuki coupling to generate a C–C (sp3–sp2-hybridized) bond, is a feasible and reasonably efficient method for linking a pseudorotaxane’s terminal alkene and a bulky aromatic bromide.
Scheme 2. Synthesis of 9-BBN-Stoppered Rotaxanes, and Their Exchange with New Stoppers to Form sp3–sp2-Hybridized C–C Bond Linkages.
Figure 2.

1H NMR spectra (400 MHz, 298 K) of (a) the sample obtained after mixing 1·TFPB (0.4 M), BPX26C6 (0.4 M), and 9-BBN dimer (0.22 M) in toluene and heating at 60 °C for 16 h (toluene-d8); (b) the sample obtained after mixing 1·TFPB (0.4 M) and 9-BBN dimer (0.22 M) and heating at 60 °C for 16 h (toluene-d8); (c) the sample obtained after mixing 1 equiv of BPX26C6 with the solution in (b) and heating at 60 °C for 16 h (toluene-d8); (d) the rotaxane 7·TFPB (CD2Cl2); and (e) the rotaxane 9 (CD2Cl2). Asterisks: signals from residual solvent. Hashtag: signals from the TFPB– anion.
We obtained single crystals suitable for X-ray crystallography after slowly evaporating a solution of [2]rotaxane 7·TFPB in CH2Cl2/hexanes (1:2). The solid state structure25 (Figure 3) confirms the [2]rotaxane geometry of 7·TFPB, with the interlocked macrocycle encircling the pyridinium unit. This structure is stabilized by multiple C–H···O hydrogen bonds between the hydrogen atoms adjacent to the pyridinium nitrogen and the macrocycle’s oxygen atoms, as well as by an aromatic stacking interaction between the pyridinium unit and a xylene ring (centroid–centroid distance: 3.587 Å).
Figure 3.
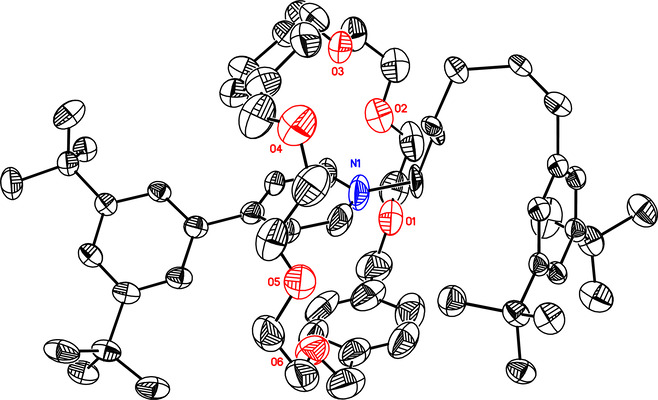
ORTEP representation of the [2]rotaxane 7·TFPB with 50% probability atomic displacement parameters. Anions, hydrogen atoms, and solvent molecules have been omitted for clarity.
Although the successful synthesis of rotaxane 7·TFPB from surrogate rotaxane 5·TFPB in polar DMF implied that 9-BBN is an appropriate stopper for interlocking BPX26C6, we examined whether 9-BBN was possibly a slippage stopper for BPX26C6 in 5·TFPB. First, we heated a toluene-d8 solution of threadlike salt 1·TFPB (0.4 M) and 9-BBN dimer (0.22 M) at 60 °C for 16 h. The 1H NMR spectrum of the sample obtained after diluting an aliquot of this mixture revealed (Figure 2b) no alkene signals, suggesting that the hydroboration had been successful. Next, we added BPX26C6 (0.4 M) to the reaction mixture and heated it to 60 °C for 16 h. No signals for complexation appeared in the 1H NMR spectrum (Figure 2c), suggesting that the rotaxane 5·TFPB had indeed been constructed through threading-followed-by-stoppering with 9-BBN functioning as a real stopper for BPX26C6 under the reaction conditions.
We applied the same synthetic method to efficiently interlock [(BPX26C6·Na)⊃2], a pseudorotaxane that is highly sensitive to anions and solvent polarity. Using 9-BBN dimer (0.22 M) to hydroborate the alkene terminus of the pseudorotaxane formed from an equimolar (0.4 M) mixture of the threadlike urea 2, BPX26C6, and NaTFPB, followed by Suzuki coupling between the resulting surrogate rotaxane 8 and the stopper 6 in the presence of Pd(dppf)Cl2, we isolated the rotaxane 9 with a C–C (sp3–sp2-hybridized) single bond linking the pseudorotaxane’s terminus and the stopper, in 67% yield (Scheme 2).
Having confirmed that hydroboration of a pseudorotaxane’s terminal C=C double bond with 9-BBN and subsequent Pd-catalyzed coupling between the resulting organoborane surrogate rotaxane and a bulky aromatic bromide could afford a rotaxane with a new sp3–sp2-hybridized C–C single bond, we turned our attention to a transition-metal-free method for C–C single bond formation. Brown et al. demonstrated that organoboranes can react with α-bromo esters under basic conditions;26 we suspected that an organoborane surrogate rotaxane should react similarly with a bulky α-bromoacetophenone to afford a rotaxane with an sp3–sp3-hybridized C–C bond linkage, without the need for a transition metal. We prepared the surrogate pyridinium-based rotaxane 5·TFPB and mixed the crude product with α-bromo ketone 10 in the presence of a base, potassium 2,6-di-tert-butylphenolate (11), in THF, affording rotaxane 12·TFPB in 39% yield after chromatography (Scheme 3). Similarly, reacting the crude surrogate urea-based rotaxane 8 with the α-bromo ketone 10 in the presence of base 11 afforded the rotaxane 13 in 50% yield. Therefore, depending on the synthetic design, the 9-BBN stoppers of the surrogate rotaxanes 5·TFPB and 8 can be replaced by other bulky stoppers to form sp3–sp2- and sp3–sp3-hybridized C–C bond linkages in the presence and absence, respectively, of a transition metal catalyst.
Scheme 3. Forming sp3–sp3-Hybridized C–C Bonds through Reactions of the 9-BBN-Stoppered Surrogate Rotaxanes.
Next, we replaced 9-BBN with a bulky boronic ester so that we could isolate the surrogate rotaxanes as air-stable products. Here, we applied organocatalytic diboration27 to interlock a pseudorotaxane presenting an alkene terminus. After mixing the threadlike salt 1·TFPB (0.25 M), BPX26C6 (0.25 M), bis(pinacolato)diboron (B2pin2, 0.50 M), Cs2CO3 (2 equiv), MeOH (1 M), and TBA·TFPB (0.25 M) in CH2Cl2, we isolated the rotaxane 14·TFPB in 45% yield (Scheme 4).28 Similarly, we reacted threadlike urea 2 (0.25 M), NaTFPB (0.75 M), BPX26C6 (0.75 M), bis(pinacolato)diboron (B2pin2, 0.50 M), MeOH (0.75 M), and 1,8-bis(dimethylamino)naphthalene (proton sponge, 0.125 M) in CH2Cl2 and obtained a 75% yield of the rotaxane 15.
Scheme 4. Synthesis of Bis(Boronate) Surrogate Rotaxanes and Their Exchange with New Stoppers to Form sp3–sp-Hybridized C–C Bond Linkages.
Although alkyl pinacol boronates are generally much less reactive than alkyl boranes when used as substrates in cross-couplings, the reactivity of the primary organoboronate in a 1,2-bis(boronate) is enhanced by vincinal O–B chelation, which increases the Lewis acidity29 and allows the reactions to proceed efficiently under relatively benign conditions. By reacting the rotaxane 14·TFPB (0.2 M) with a mixture of CuCN (60 mM), lithium methoxide (0.6 M), and the 1-bromo-1-alkyne 16 (0.24 M),30 we obtained the pyridinium-based rotaxane 17·TFPB (44% yield),31 featuring a newly generated sp3–sp-hybridized C–C single bond between the erstwhile threadlike component and the stopper. We removed the remaining boronic ester group in 17·TFPB through NaBO3·H2O-mediated oxidation, providing alcohol rotaxane 18·TFPB in 89% yield. Applying the same transformation to the urea-based rotaxane 15 afforded the boronic ester rotaxane 19 (65% yield), which we then converted to the alcohol rotaxane 20 using NaBO3·H2O, also in 89% yield.
Using 9-BBN and pinacol boronic ester to form surrogate stoppers, we have transformed pseudorotaxanes presenting alkene termini into rotaxanes by creating C–C single bond linkages to proper stoppers. This approach might be generally useful because terminal alkenes are readily prepared synthetically and because boron-based reactions allow the installation of C–C single bonds, with sp3–sp3, sp3–sp2, and sp3–sp hybridization, through distinct stopper exchange processes, leaving room for further installation of various functionalities on the threadlike components. We believe that such stoppering methods will aid in the design and synthesis of interlocked (macro)molecules and switches.
Acknowledgments
We thank the National Science and Technology Council (NSTC-113-2123-M-002-004) for financial support.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.5c01233.
Synthetic procedures, characterization data and NMR spectra of the rotaxanes (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Kraemer J.; Grimm L. M.; Zhong C.; Hirtz M.; Biedermann F. A supramolecular cucurbit[8]uril-based rotaxane chemosensor for the optical tryptophan detection in human serum and urine. Nat. Commun. 2023, 14, 518. 10.1038/s41467-023-36057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wilmore J. T.; Beer P. D. Exploiting the mechanical bond effect for enhanced molecular recognition and sensing. Adv. Mater. 2024, 36, 2309098. 10.1002/adma.202309098. [DOI] [PubMed] [Google Scholar]
- a Kwamen C.; Niemeyer J. Functional rotaxanes in catalysis. Chem.—Eur. J. 2021, 27, 175–186. 10.1002/chem.202002876. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tseng I.-C.; Zhang M.-X.; Kang S.-L.; Chiu S.-H. An anion-switchable dual-function rotaxane catalyst. Angew. Chem., Int. Ed. 2023, 62, e202309889 10.1002/anie.202309889. [DOI] [PubMed] [Google Scholar]; c Puigcerver J.; Zamora-Gallego J. M.; Marin-Luna M.; Martinez-Cuezva A.; Berna J. Urea-based [2]rotaxanes as effective phase-transfer organocatalysts: hydrogen-bonding cooperative activation enabled by the mechanical bond. J. Am. Chem. Soc. 2024, 146, 22887–22892. 10.1021/jacs.4c06630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ghiassinejad S.; Kumar Sharma A.; Fustin C.-A.; van Ruymbeke E. Effect of ring mobility on the dynamics of the slide-ring gels. Chem. Mater. 2024, 36, 8311–8322. 10.1021/acs.chemmater.4c01235. [DOI] [Google Scholar]; b Oh J.; Liu G.; Kim H.; Hertzog J. E.; Nitta N.; Rowan S. J. Exploring the impact of ring mobility on the macroscopic properties of doubly threaded slide-ring gel networks. Angew. Chem., Int. Ed. 2024, 63, e202411172 10.1002/anie.202411172. [DOI] [PubMed] [Google Scholar]
- a Arun A.; Docker A.; Min Tay H.; Beer P. D. Squaramide-based heteroditopic [2]rotaxanes for sodium halide ion-pair recognition. Chem.—Eur. J. 2023, 29, e202301446 10.1002/chem.202301446. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chang C.-T.; Chiu S.-H. Sequential nucleophilic substitution of pseudorotaxanes forms rotaxanes with various linking functionalities and recycling of the surrogate stopper. Chem. Commun. 2024, 60, 8704–8707. 10.1039/D4CC02975E. [DOI] [PubMed] [Google Scholar]
- a Vogel J.; Chen Y.; Fadler R. E.; Flood A. H.; von Delius M. Steric control over the threading of pyrophosphonates with one or two cyanostar macrocycles during pseudorotaxane formation. Chem.—Eur. J. 2023, 29, e202300899 10.1002/chem.202300899. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jaiswal M.; Dasgupta S. Tuning stopper size in multiresponsive [2]rotaxanes for fluoride anion selective metastability. Org. Lett. 2024, 26, 6776–6781. 10.1021/acs.orglett.4c02544. [DOI] [PubMed] [Google Scholar]
- a Razi S. S.; Marin-Luna M.; Alajarin M.; Martinez-Cuezva A.; Berna J. Conjugated bis(enaminones) as effective templates for rotaxane assembly and their post-synthetic modifications. Commun. Chem. 2024, 7, 170. 10.1038/s42004-024-01258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chen S.; Katsonis N.; Leigh D. A.; Patanapongpibul M.; Ryabchun A.; Zhang L. Changing liquid crystal helical pitch with a reversible rotaxane switch. Angew. Chem., Int. Ed. 2024, 63, e202401291 10.1002/anie.202401291. [DOI] [PubMed] [Google Scholar]
- Chiu C.-W.; Lai C.-C.; Chiu S.-H. Threading-followed-by-swelling: A new protocol for rotaxane synthesis. J. Am. Chem. Soc. 2007, 129, 3500–3501. 10.1021/ja069362i. [DOI] [PubMed] [Google Scholar]
- Hsueh S.-Y.; Ko J.-L.; Lai C.-C.; Liu Y.-H.; Peng S.-M.; Chiu S.-H. A metal-free “threading-followed-by-shrinking” protocol for rotaxane synthesis. Angew. Chem., Int. Ed. 2011, 50, 6643–6647. 10.1002/anie.201101524. [DOI] [PubMed] [Google Scholar]
- a Jamagne R.; Power M. J.; Zhang Z.-H.; Zango G.; Gibber B.; Leigh D. A. Active template synthesis. Chem. Soc. Rev. 2024, 53, 10216–10252. 10.1039/D4CS00430B. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Saady A.; Malcolm G. K.; Fitzpatrick M. P.; Pairault N.; Tizzard G. J.; Mohammed S.; Tavassoli A.; Goldup S. M. A platform approach to cleavable macrocycles for the controlled disassembly of mechanically caged molecules. Angew. Chem., Int. Ed. 2024, 63, e202400344 10.1002/anie.202400344. [DOI] [PubMed] [Google Scholar]
- a Legigan T.; Riss-Yaw B.; Clavel C.; Coutrot F. Active esters as pseudostoppers for slippage synthesis of [2]pseudorotaxane building blocks: A straightforward route to multi-interlocked molecular machines. Chem.—Eur. J. 2016, 22, 8835–8847. 10.1002/chem.201601286. [DOI] [PubMed] [Google Scholar]; b Becharguia N.; Wasielewski E.; Abidi R.; Nierengarten I.; Nierengarten J.-F. Stepwise functionalization of a pillar[5]arene-containing [2]rotaxane with pentafluorophenyl ester stoppers. Chem.—Eur. J. 2024, 30, e202303501 10.1002/chem.202303501. [DOI] [PubMed] [Google Scholar]
- a Kwon T.-w.; Song B.; Nam K. W.; Stoddart J. F. Mechanochemical enhancement of the structural stability of pseudorotaxane intermediates in the synthesis of rotaxanes. J. Am. Chem. Soc. 2022, 144, 12595–12601. 10.1021/jacs.2c00515. [DOI] [PubMed] [Google Scholar]; b Becharguia N.; Nierengarten I.; Strub J.-M.; Cianferani S.; Remy M.; Wasielewski E.; Abidi R.; Nierengarten J.-F. Solution and solvent-free stopper exchange reactions for the preparation of pillar[5]arene-containing [2]- and [3]rotaxanes. Chem.—Eur. J. 2024, 30, e202304131 10.1002/chem.202400246. [DOI] [PubMed] [Google Scholar]
- For stability reasons, the imine functionalities have generally been reduced to amino groups; see:; a Rowan S. J.; Stoddart J. F. Thermodynamic synthesis of rotaxanes by imine exchange. Org. Lett. 1999, 1, 1913–1916. 10.1021/ol991047w. [DOI] [Google Scholar]; b Liu H.; Hu Z.; Ji X. Characterization by gel permeation chromatography of the molecular weight of supramolecular polymers generated by forming polyrotaxanes through the introduction of external stoppers. Chem.—Eur. J. 2024, 30, e202400099 10.1002/chem.202400099. [DOI] [PubMed] [Google Scholar]
- a Saady A.; Goldup S. M. Triazole formation and the click concept in the synthesis of interlocked molecules. Chem. 2023, 9, 2110–2127. 10.1016/j.chempr.2023.07.001. [DOI] [Google Scholar]; b Grzelczak R. A.; Basak T.; Trzaskowski B.; Kinzhybalo V.; Szyszko B. Multimodal molecular motion in the rotaxanes and catenanes incorporating flexible calix[n]phyrin stations. Angew. Chem., Int. Ed. 2025, 64, e202413579 10.1002/anie.202413579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Bruns C. J.; Stoddart J. F.. The Nature of the Mechanical Bond: From Molecules to Machines; Wiley: Hoboken, USA, 2016. [Google Scholar]; b Waelès P.; Gauthier M.; Coutrot F. Challenges and opportunities in the post-synthetic modification of interlocked molecules. Angew. Chem., Int. Ed. 2021, 60, 16778–16799. 10.1002/anie.202007496. [DOI] [PubMed] [Google Scholar]
- For pseudorotaxanes assembled primarily via hydrophobic effects, Suzuki coupling can be directly applied for interlocking through C–C bond formation; see:Terao J.; Tang A.; Michels J. J.; Krivokapic A.; Anderson H. L. Synthesis of poly(para-phenylenevinylene) rotaxanes by aqueous suzuki coupling. Chem. Commun. 2004, 56–57. 10.1039/b311762f. [DOI] [PubMed] [Google Scholar]
- Kihara N.; Motoda S.; Yokozawa T.; Takata T. End-cap exchange of rotaxane by the Tsuji-Trost allylation reaction. Org. Lett. 2005, 7, 1199–1202. 10.1021/ol047639i. [DOI] [PubMed] [Google Scholar]
- Sasabe H.; Kihara N.; Mizuno K.; Ogawa A.; Takata T. Rotaxane synthesized by end-capping via hydroruthenation of axle terminal acetylene and its derivation to η3-allylruthenium complex-containing rotaxane. Chem. Lett. 2006, 35, 212–213. 10.1246/cl.2006.212. [DOI] [Google Scholar]
- Hu Y.; Wang W.; Yao R.; Wang X.-Q.; Wang Y.-X.; Sun B.; Chen L.-J.; Zhang Y.; Zhao X.-L.; Xu L.; Tan H.-W.; Yu Y.; Li X.; Yang H.-B. Facile synthesis of diverse rotaxanes via successive supramolecular transformations. Mater. Chem. Front. 2019, 3, 2397–2402. 10.1039/C9QM00430K. [DOI] [Google Scholar]
- Chiu S.-H.; Rowan S. J.; Cantrill S. J.; Stoddart J. F.; White A. J. P.; Williams D. J. Post-assembly processing of [2]rotaxanes. Chem.—Eur. J. 2002, 8, 5170–5183. . [DOI] [PubMed] [Google Scholar]
- Recently, boron has been used as a bridging unit to assemble hosts and guests into pseudorotaxane-like structures; see:; a Hicguet M.; Verrieux L.; Mongin O.; Roisnel T.; Berree F.; Fihey A.; Le Guennic B.; Trolez Y. Threading a linear molecule through a macrocycle thanks to boron: Optical properties of the threaded species and synthesis of a rotaxane. Angew. Chem., Int. Ed. 2024, 63, e202318297 10.1002/anie.202318297. [DOI] [PubMed] [Google Scholar]; b Yu J.; Gaedke M.; Das S.; Stares D. L.; Schalley C. A.; Schaufelberger F. Boronic ester-templated pre-rotaxanes as versatile intermediates for rotaxane endo-functionalisation. Chem. Sci. 2024, 15, 19443–19451. 10.1039/D4SC04879B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P.-N.; Lin C.-F.; Liu Y.-H.; Lai C.-C.; Peng S.-M.; Chiu S.-H. [3]Pseudorotaxane-like complexes formed between bipyridinium dications and bis-p-xylyl[26]crown-6. Org. Lett. 2006, 8, 435–438. 10.1021/ol052679n. [DOI] [PubMed] [Google Scholar]
- Inthasot A.; Tung S.-T.; Chiu S.-H. Using alkali metal ions to template the synthesis of interlocked molecules. Acc. Chem. Res. 2018, 51, 1324–1337. 10.1021/acs.accounts.8b00071. [DOI] [PubMed] [Google Scholar]
- Molander G. A.; Yun C.-S. Cross-coupling reactions of primary alkylboronic acids with aryl triflates and aryl halides. Tetrahedron 2002, 58, 1465–1470. 10.1016/S0040-4020(02)00009-1. [DOI] [Google Scholar]
- Kreeger S.; Sarrels B.; Lucero C. The first total syntheses of lorneic acids C and D and improved synthetic routes to lorneic acids A and J. Tetrahedron 2024, 163, 134139. 10.1016/j.tet.2024.134139. [DOI] [Google Scholar]
- CCDC-2434497.
- Brown H. C.; Nambu H. Reaction of organoboranes with ethyl 4-bromocrotonate under the influence of potassium 2,6-di-t-butylphenoxide. A convenient procedure for a four-carbon-atom homologation. J. Am. Chem. Soc. 1970, 92, 1761–1763. 10.1021/ja00709a059. [DOI] [Google Scholar]
- Bonet A.; Sole C.; Gulyas H.; Fernandez E. Asymmetric organocatalytic diboration of alkenes. Org. Biomol. Chem. 2012, 10, 6621–6623. 10.1039/c2ob26079d. [DOI] [PubMed] [Google Scholar]
- The successful synthesis of a rotaxane from a mixture of 1·TFPB, BPX26C6, and pinacolborane in the presence of an Ir-catalyst suggested that Bpin is indeed a proper stopper for BPX26C6 (see the Supporting Information).
- Mlynarski S. N.; Schuster C. H.; Morken J. P. Asymmetric synthesis from terminal alkenes by cascades of diboration and cross-coupling. Nature 2014, 505, 386–390. 10.1038/nature12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N.; Kong Z.; Wang J. Z.; Lovinger G. J.; Morken J. P. Copper-catalyzed coupling of alkyl vicinal bis(boronic esters) to an array of electrophiles. J. Am. Chem. Soc. 2022, 144, 17815–17823. 10.1021/jacs.2c02817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Because of purification challenges, we determined the yield based on the pure fraction obtained from column chromatography. When we used crude 17·TFPB directly in the subsequent NaBO3·H2O-mediated oxidation, the overall two-step yield of the rotaxane 18·TFPB from 14·TFPB was 62%, suggesting that the actual yield of 17·TFPB was significantly higher than 44%.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.






