Abstract
Insecticide resistance is a major public health concern. Biopesticides, derived from naturally occurring substances such as plant extracts and fungal metabolites, are utilized as natural control agents against mosquito vectors. This study focuses on biopesticides to reduce chemical insecticide use in Penang Island, Malaysia. Ipomoea cairica Linnaeus Sweet (Solanales: Convolvulaceae) leaf extracts, Metarhizium anisopliae s.l. (Metsch) Sorok (Ascomycota: Clavicipitaceae) strain Meta–G4, and synergistic effects of both agents were demonstrated against Aedes albopictus Skuse (Diptera: Culicidae) and Aedes aegypti Linnaeus (Diptera: Culicidae) using larvicidal bioassays. Before assessing synergism, the compatibility of both agents was performed to minimize inhibition of mycelial growth on potato dextrose agar. The results showed that Ae. aegypti field strains (urban and suburban) are significantly more susceptible to I. cairica leaf extracts compared to Ae. albopictus field strains (urban and suburban) due to the lower lethal concentrations (LC50 and LC95). Aedes albopictus suburban field strain is significantly more susceptible than other strains when tested with M. anisopliae due to the lower LC50. The combination of M. anisopliae (1 × 106 conidia/ml) with I. cairica leaf extracts (350 ppm) achieved the lowest LT50 and LT95 against Ae. aegypti urban field strain. This study indicates that the synergistic combination of both agents exhibits significant larvicidal efficacy and holds promise for future biological control strategies targeting Aedes populations.
Keywords: Biocontrol, mosquitoes, plant extract, fungus, synergistic effects
Introduction
In Malaysia, dengue continues to be a major public health concern, with chemical insecticides serving as the primary approach for controlling the mosquito vectors responsible for diseases such as chikungunya, dengue, Zika, and yellow fever (Rasli et al. 2021). Pyrethroids and organophosphates are commonly employed in vector control initiatives carried out by the Ministry of Health, private pest control companies, and local communities in Malaysia. However, the prolonged and excessive reliance on these classes of insecticides has resulted in overuse and improper application, ultimately leading to resistance developing in the mosquito vectors (Akhir et al. 2021). Therefore, scientists are directing their efforts toward developing biological control methods to curb the spread of dengue fever without posing threats to the environment and public health (Frentiu et al. 2014). Numerous research has examined the potential of phytochemicals as biopesticides in the biological management of Aedes mosquitoes, spurred by the identification of compounds that are mosquitocidal in plant extracts (Ravi et al. 2018, Lim et al. 2023, Bharathithasan et al. 2024). The phytochemical extracts derived from Azolla pinnata have exhibited considerable larvicidal efficacy against the larvae of Aedes aegypti (Ravi et al. 2018) encompassing a variety of essential phytochemicals, including flavonoids, tannins, alkaloids, saponins, phenolic compounds, and terpenoids, which collectively enhance its biological activity (Bharathithasan et al. 2024). The hexane extract derived from the leaves of Ocimum americanum encompasses a range of terpenes, including sesquiterpenes, monoterpenes, and triterpenes, which have exhibited significant larvicidal efficacy against both Aedes albopictus and Ae. aegypti (Lim et al. 2023). Plant constituents with proven larvicidal and adulticidal properties include phenolics, terpenoids, flavonoids, and alkaloids (Elumalai et al. 2012), which may reduce the general reliance on chemical pesticides and increase the efficiency of plant pesticides in the biological control of vectors (Rodzay and Zuharah 2021).
Ipomoea cairica (Linnaeus) Sweet, a perennial herb from the Convolvulaceae family, order Solanales, within the clades Tracheophytes, Angiosperms, Eudicots, and Asterids, originated from tropical Africa or Asia, has become one of the numerous plants under research for its potential medicinal, agricultural, and ecological applications, including its use in biopesticides and traditional medicine (Austin and Huaman 1996). It is commonly referred to as morning glory, a fast-growing plant that thrives in tropical regions and is often cultivated for its ornamental value. The plant is rich in a variety of secondary metabolites, which have been linked to multiple biological activities, including larvicidal, anticancer, antimicrobial, antinociceptive, and cytotoxic properties. Its rapid growth and widespread presence make it a notable species in tropical ecosystems (Valverde et al. 2022). The crude extracts from I. cairica leaves exhibited the greatest larvicidal activity in comparison to the flower and stem extracts (AhbiRami et al. 2014a).
In the 1990s, promising advancements in fungus-based insect control emerged as global interest grew in reducing the use of chemical pesticides to minimize environmental contamination (Singh and Prakash 2014). The entomopathogenic fungus Metarhizium anisopliae s.l. (Metsch) Sorok (Ascomycota: Clavicipitaceae) is widely applied as a biological pesticide to control arthropod pests in agriculture (Bitencourt et al. 2023). Metarhizium anisopliae spores initially attach to the host’s cuticle, then germinate and form an appressorium, which generates a penetration peg. This peg uses mechanical force and enzymes to breach the cuticle of insects (Vestergaard et al. 1999). Blastospores and conidia of M. anisopliae showed strong potential as larvicidal agents against Ae. aegypti larvae (Bitencourt et al. 2023) and caused high larval mortality of Anopheles gambiae and Anopheles stephensi (Bukhari et al. 2010).
Synergism refers to interactions between elements that yield a combined effect greater than the sum of individual effects (Benelli et al. 2017)). The activity of entomopathogenic fungus combined with essential oils has been documented against various arthropods, including Lutzomyia longipalpis (Figueredo et al. 2020) and Ae. aegypti mosquitoes (Gomes et al. 2015). The combination of M. anisopliae and I. cairica shows a strong synergistic effect, supporting the potential of a single formulation that enhances and prolongs control over dengue vectors (Zuharah et al. 2018).
Although some authors have used I. cairica and M. anisopliae as biopesticides against Ae. albopictus and Ae. aegypti larvae (AhbiRami et al. 2014a, Ishak et al. 2014, Zuharah et al. 2018, Valverde et al. 2022), the synergistic impact resulting from the integration of these two biocontrol agents has not been previously examined. Therefore, the objective of this study was to elucidate the effectiveness of I. cairica leaf plant extracts, M. anisopliae fungus (Meta-G4), as well as the synergistic interactions between these agents, which are anticipated to yield a prolonged control effect against the larvae of Ae. albopictus and Ae. aegypti.
Materials and Methods
Mosquito Colonies
Ovitraps were placed in Desa Permai Indah (Hamna low-cost flat, urban residential area), Penang (5°21′N, 100°18′E), and Taman Indah (Batu Maung low-cost flat, suburban residential area), Penang (5°17′N, 100°17′E). The eggs and larvae of Ae. aegypti and Ae. albopictus were collected, brought back to the laboratory, and reared in enamel trays filled with dechlorinated tap water until the adult stage under laboratory conditions at a temperature of 28 ± 2 °C and a relative humidity (RH) of 70% to 85%, with a 12 h light/12 h dark photoperiod. The temperature and humidity in the laboratory were measured using a hydro-thermometer (Extech Instruments, 445703, China). Dog biscuits (Pedigree, Malaysia), beef liver, yeast (Mauri-pan, Malaysia) and milk powder (Nestle, Malaysia) were fed to the larvae in a 2:1:1:1 weight ratio; the mixture was ground into a fine powder and given to the larvae at a dose of 1 mg daily. A 10% sucrose solution was provided to the emerged adult mosquitoes (Ahbirami et al. 2014a). One to two days after their emergence, the adult female species were identified using characteristic morphological keys for Ae. aegypti and Ae. albopictus (Rueda 2004). Morphological identification of the screened adult female mosquitoes was performed using a VISOPTIC-2D Stereo Microscope (Visoptic, TZ5620, Carlssoon Technologies (Malaysia) Sdn Bhd), and the mosquitoes were placed into separate cages. The screened adult female mosquitoes (F0 generation) were fed on blood from a restrained rat kept in a resting cage for 4 h. The Animal Ethics Committee of Universiti Sains Malaysia has accepted and approved the use of rats for this study (USM/IACUC/2017/(110)(891)). Two days after blood feeding, a plastic cup lined with filter paper was placed in the cage to serve as an oviposition substrate. The mosquito culture was reared under laboratory conditions at a temperature of 28 ± 2 °C, with a 12 h light/12 h dark photoperiod and RH of 70% to 85% (Ahbirami et al. 2014b). The obtained eggs were hatched in an enamel plate to produce first-generation (F1) larvae. Late third- or early fourth-instar F1 larvae from 6 mosquito strains were used: Ae. albopictus (urban field, suburban field, laboratory strains) and Ae. aegypti (urban field, suburban field, laboratory strains). The laboratory strains of Ae. aegypti and Ae. albopictus, obtained from the Vector Control Research Unit (VCRU) at Universiti Sains Malaysia, located in Penang. These strains, which were initially collected from Penang (Rahim et al. 2017), have been continuously cultured since the 1960s for more than 600 generations.
Ipomoea cairica Leaf Extract Preparation
Ipomoea cairica leaf parts were chosen for this study, as opposed to stems, due to the higher efficacy demonstrated by the leaf acetone extract (AhbiRami et al. 2014a). Acetone (CAS NO: 67-64-1, Company: Modern-Lab Chemicals Sdn Bhd) was chosen as the extraction solvent for I. cairica leaf parts due to its higher larvicidal activity compared to methanol, as well as its lower polarity (5.1) and viscosity (0.32), which enhance the diffusion and yield of active compounds. The selection is supported by previous findings that lower polarity solvents increase efficacy, and that acetone removes compounds like steroids and alkaloids (Mulla and Su 1999, Ghosh et al. 2012, AhbiRami et al. 2014a, Khayyat and Roselin 2018). Fresh leaves of I. cairica were collected from Teluk Kumbar, Penang, Malaysia (5°17′N, 100°15′E). The plant was identified using the key to the taxa of I. cairica based on cotyledon characteristics (Ogunwenmo 2003) and verified by the Herbarium of the School of Biological Sciences, Universiti Sains Malaysia (voucher number: 11866). After collection, I. cairica plant leaves were cleaned with distilled water to prevent any potential trace contamination (Ishak et al. 2014). Next, I. cairica leaves were shade-dried at room temperature (28 ± 3 °C) for 7 to 10 d. The dried parts were crushed into powder from a stainless-steel electrical blender (ELBA, EBG-F1534GR, Elba (M) Sdn Bhd). A Soxhlet apparatus (DURAN 500 ml set, 55/44, condenser 28 cm, SL.Ext 2007, My Laboratories and Services) was employed to extract 40 g of finely powdered leaves with acetone (2,000 ml, Qualigens, Favorit). The leaf powder was mixed with clean pebbles in a cellulose thimble (43 mm × 123 mm, Favourite) to ensure proper solvent flow. The apparatus was set to acetone’s boiling point of 56 °C (Amer and Paxton 1956). The extraction was completed in about 3 h when the solvent in the siphon appeared nearly colorless. Fresh batches of leaf powders were used for each repetition of this method (Ahbirami et al. 2014a, 2014b).
The solvents were extracted from the obtained plant extracts using a rotary evaporator (IKA, Germany, RV 8, Interscience Sdn Bhd). The speed was set at 100 rpm, and the temperature of the water bath was adjusted to 56 °C, which is the boiling point of acetone. Next, the concentrated crude extract was heated in an electric oven (Incucell 111, Type ECO Line MC000222, Labmallx) to 37 °C for 2 d to eliminate its solvent one more time. The measured and collected crude extract was stored in a Petri plate at 4 °C to be used later (Thomas et al. 2004). A stock solution (10,000 ppm) was prepared by dissolving 1 g of the crude extract in 100 ml of acetone. A 1,000 ppm solution was then made by diluting 50 ml of the stock solution in 450 ml of distilled water. Further dilutions, ranging from 10 to 500 ppm, were made as required (Ahbirami et al. 2014a).
M. anisopliae (Meta-G4) Preparation
In this study, 14-d-old M. anisopliae (Meta-G4) was used due to its peak viability. After a duration of 14 d, the viability of spores experiences a decline, presumably attributable to the exhaustion of carbon and nitrogen reserves, demonstrating a decrease in spore viability. Consequently, a 14-d incubation period is deemed optimal for maximizing germination rates and ensuring suitability for commercial biocontrol applications (Daryaei et al. 2016, Halim et al. 2021). Meta-G4 conidia obtained from Felda Tenang, Setiu, Terengganu (5°32.079′N, 102°31.626′E), agricultural soils yielded isolate Meta-G4 of M. anisopliae var. anisopliae were subcultured on fresh potato dextrose agar (PDA) (Sigma–Aldrich) plates using agar-cultured stock samples, and the culture was kept under laboratory conditions at a temperature of 28 ± 2°C, 78 ± 10% RH, and a photoperiod of 12 h light and 12 h dark. Utilizing 5 PDA plates cultured with Meta-G4, the conidia were collected by gently scraping the surface of the 14-d-old culture with an inoculation loop. To detach the spores from the surface, the conidia were suspended in sterilized distilled water containing 0.01% Tween-20 (Himedia, FC-BIOS Sdn Bhd), which served as a surfactant to facilitate spore release (Zuharah et al. 2021).
Compatibility of Ipomoea cairica Leaf Extract and M. anisopliae (Meta-G4)
Ipomoea cairica leaf extracts and PDA were dissolved in distilled water to create a 10 ppm solution, which was autoclaved (Hirayama Autoclave, HVA-110, Biohaus Store Sdn Bhd) at 121 °C and 15 psi for 20 min. After sterilization, the mixture was poured into disposable Petri dishes (Brandon, 90 × 15 mm, Modern-Lab Chemicals Sdn Bhd) and allowed to solidify. The Petri dishes were inoculated using micropipette (Eppendorf Research plus G, single-channel, variable 0.5 to 10 µL, 3120000020, Modern-Lab Chemicals Sdn Bhd) with 1 µL of M. anisopliae spore suspension (1 × 106 conidia/ml) containing 0.01% Tween-20 obtained from a 14-d-old M. anisopliae culture after the medium had solidified (Zuharah et al. 2018). This experiment was repeated separately for the combinations of M. anisopliae concentrations from 1 × 106 conidia/ml with 20 to 500 ppm of I. cairica leaf extracts that were mixed with PDA medium. A total of 56 plates were used for these combinations. Each treatment was in 4 replicates. The experiment was repeated 3 times. A control treatment was set up using a potato agar medium with sterilized distilled water as a reference for comparison. One microliter of M. anisopliae spore suspension (1 × 106 conidia/ml) from a 14-d-old M. anisopliae culture was used to inoculate the plate. The infected plates were kept in an incubator (Hirayama Autoclave, HVA-110, Biohaus Store Sdn Bhd) under laboratory conditions at a temperature of 28 ± 2 °C, 78 ± 10% RH and a photoperiod of 12 h light and 12 h dark. Fungal growth was monitored daily until the control plates were fully covered with mycelium, and the radial growth of mycelium was measured in millimeters using an electronic vernier caliper (Prado, HL-MEASURE-01, Modern-Lab Chemicals Sdn Bhd) (Sharma and Chandel 2016).
This experiment exhibited the lowest inhibitory growth percentage in M. anisopliae compatibility (Meta-G4) with I. cairica leaf extracts, which was selected to explore the synergistic effect of I. cairica and M. anisopliae using the bioassay technique in the next study.
Larvicidal Bioassay Using Ipomoea cairica Leaf Extracts, M. anisopliae (Meta-G4), and a Combination of I. cairica Leaf Extracts with M. anisopliae
Larval bioassay using I. cairica leaf extracts was performed using the conventional approach for testing larval susceptibility (WHO 2005a). Twenty-five larvae each of Ae. albopictus and Ae. aegypti, in their late third- or early fourth instars, were placed into 350 ml plastic containers containing 250 ml of the test medium (distilled water and plant extracts solution). One ml of 10% acetone was added to 250 ml of distilled water to create the control set (AhbiRami et al. 2014a). To ensure consistency among the test solutions, which could have had residual solvent, the solvent was also added to the control containers. The larvae were exposed to 13 concentrations (10 to 500 ppm) to determine LC50 and LC95 at 24 h.
Larval bioassay using culture of M. anisopliae was carried out in accordance with the standard (WHO 2005a). Larvicidal bioassays were conducted using a larval susceptibility test method in 350 ml plastic containers containing 25 late third- or early fourth-instar larvae of Ae. albopictus and Ae. aegypti, along with 250 ml of test media (distilled water and M. anisopliae). Five concentrations of M. anisopliae (1 × 106 to 1 × 1010 conidia/ml) containing 0.01% Tween-20 at a 14 d age was used to find the value of LC50 and LC95 at 24 h. The control contains 250 ml of pure water. The experiment was conducted with 4 replicates per concentration across 3 trials.
For the combination of I. cairica leaf extracts with 14-d-old M. anisopliae, 1 × 106 conidia/ml of M. anisopliae + 350 ppm of I. cairica leaf extracts were used to find the value of lethal times (LT50 and LT95) because it exhibited the lowest inhibitory mycelial growth percentage. Twenty-five late third- or early fourth-instar larvae were placed into a 350 ml plastic container filled with 250 ml of the test medium mixture (I. cairica leaf extracts and M. anisopliae).
Larval mortality was recorded at 24 h. The experiment was conducted with 4 replicates and repeated 3 times. During the above experiments, no larval food was given (Ishak et al. 2014). The experiments were conducted at 28 ± 2 °C room temperature, 78 ± 10% RH, and a photoperiod of 12 h light and 12 h dark (AhbiRami et al. 2014a). After being probed with a needle in the siphon or cervical region, motionless larvae were considered dead (WHO 2005b, Oliveira et al. 2010).
Statistical Analysis
The data were analyzed using log-probit technique with SPSS version 26.0 software (IBM SPSS Statistics version 26.0, IBM Corporation, U.S) to find the value of LC50 and LC95 of I. cairica leaf extracts and M. anisopliae (Meta-G4), LT50 and LT95 of the combination of I. cairica leaf extracts and M. anisopliae (Meta-G4) at 95% confidence limit. A factorial analysis of variance (ANOVA) was carried out with concentration, and Aedes larval species were set as fixed factor variables to identify significant differences in the parameters of larvae mortality. Prior to analysis, the statistical normality of the mortality data was verified using the Shapiro–Wilk test. The data were converted using the natural logarithm (ln[yt1]) due to deviations from homogeneity of variance (Blaustein et al. 2005).
The method employed to calculate the mycelial growth inhibition percentage followed the protocol described by Vincent (1947).
where, I is the percentage of mycelia growth inhibition, C is the Mycelial growth of fungus in control (mm), and T is the Mycelial growth of fungus in treatment (mm).
One-way ANOVA was used to statistically analyze the findings of the larvicidal bioassay using 14-d-old M. anisopliae (Meta-G4) at different concentrations of M. anisopliae (Meta-G4). The compatibility between M. anisopliae and I. cairica leaf extracts was subjected to a statistical analysis (Meta-G4), and the significance of treatment changes in several studies was assessed using a Completely Randomized Design (Gomez and Gomez 1983). However, the data on larval mortality from the larval bioassay, which was produced from the combined activity of M. anisopliae (Meta-G4) and I. cairica leaf extracts against Aedes larvae, were analyzed using two-way ANOVA to determine the efficacy of the synergism. The novel formulation was tested on both Ae. albopictus and Ae. aegypti larvae, with the significant effects of the combined agents’ post-treatment days acting as factors. Before the statistical analysis, the normality of all the data was assessed using the Shapiro–Wilk test and the results showed that all the data were normal at a significance level of P > 0.05. The significance level for the statistical analysis was set at P < 0.05.
Results
Compatibility of Ipomoea cairica Leaf Extracts and M. anisopliae (Meta-G4) at 1 × 106 conidia/ml
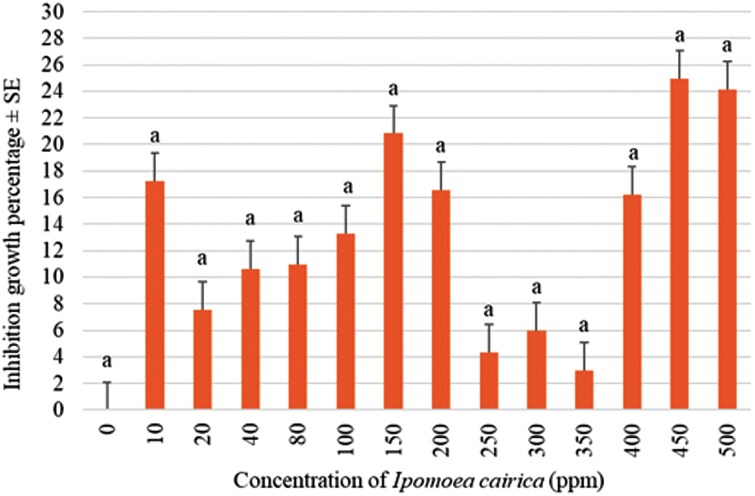
In the compatibility test, 14-d-old M. anisopliae at 1 × 10⁶ conidia/ml and 350 ppm I. cairica leaf extract resulted in the lowest mycelial inhibition percentage, showing no statistically significant difference compared to other concentrations (F = 0.868, df = 13, P > 0.01; Fig. 1). This indicates that the combination of 1 × 10⁶ conidia/ml of M. anisopliae and 350 ppm of I. cairica leaf extract showed the highest compatibility, inhibiting fungal growth by 2.97 ± 3.43%.
Fig. 1.
Mycelial inhibition growth percentage in compatibility of Metarhizium anisopliae (Meta-G4) at 1 × 10⁶ conidia/ml with various concentrations of Ipomoea cairica leaf extracts. The same lowercase letter indicates no significant differences by Tukey’s test (ANOVA, P < 0.05). Error bars represent the standard error.
Larvicidal Bioassay using Ipomoea cairica Leaf Extracts
By comparing the same species of the field strains, the susceptibility was the highest for Ae. aegypti urban field strain (LC50 = 476.16 ppm) and Ae. albopictus urban field strain (LC50 = 884.70 ppm) among Ae. aegypti and Ae. albopictus field strains, respectively. Therefore, Ae. aegypti and Ae. albopictus (urban and suburban) field strain showed significant differences in LC50 and LC95 (F = 70.844, df = 5, P < 0.01; Table 1). This indicated that Ae. aegypti field strain (urban and suburban) is significantly more susceptible than Ae. albopictus field strain (urban and suburban) toward different concentrations of I. cairica leaf extracts and are significantly more effective in controlling Ae. aegypti field strains compared to Ae. albopictus field strains (F = 9.709, df = 65, P < 0.01; Table 1).
Table 1.
Susceptibility by larvae of Aedes aegypti and Aedes albopictus to Ipomoea cairica leaf extracts at 24 h based on LC50 and LC95 through log-probit analysis
| Parameters | Urban | Suburban | Laboratory |
|---|---|---|---|
| Aedes aegypti strain | |||
| LC50 (ppm) (95% CI) |
476.16 (433.77 to 534.53) |
493.70 (459.59 to 539.38) |
532.71 (492.49 to 586.09) |
| LC95 (ppm) (95% CI) |
852.99 (753.73 to 1001.26) |
792.49 (716.38 to 902.80) |
921.34 (829.73 to 1048.87) |
| Regression equation | 0.025x – 0.707 | 0.023x – 1.476 | 0.020x – 0.810 |
| χ 2 | 125.29 | 100.40 | 68.47 |
| P-value | <0.001 | <0.001 | 0.084 |
| Aedes albopictus strain | |||
| LC50 (ppm) (95% CI) |
884.70 (722.57 to 1256.72) |
896.67 (708.57 to 1390.44) |
422.05 (387.52 to 469.83) |
| LC95 (ppm) (95% CI) |
1,389.40 (1,085.58 to 2,100.75) |
1,459.22 (1,098.14 to 2,427.74) |
602.28 (535.98 to 733.69) |
| Regression equation | 0.005x – 0.263 | 0.005x – 0.151 | 0.033x – 2.697 |
| χ 2 | 81.36 | 111.30 | 413.31 |
| P-value | <0.001 | <0.001 | <0.001 |
ppm = parts per million; LC50 = lethal concentration that killed 50% of the treated population; LC95 = lethal concentration that killed 95% of the treated population; 95% CI = 95% of confidence interval; χ2 = Chi-square, P < 0.05.
After 24 h of exposure to I. cairica leaf extracts, the most common abnormalities observed in larvae were the presence of extract content in the midgut and darkened cuticles, as shown in Fig. 2. Untreated larvae appear in Fig. 2A, while Fig. 2B shows the dark midgut of larvae that ingested the extracts.
Fig. 2.
Morphological effects of Ipomoea cairica leaf extracts on Aedes mosquito larvae: A) untreated larvae, B) larvae with darkened abdomen after 24 h of exposure.
Larvicidal Bioassay Using M. anisopliae (Meta-G4)
By comparing the same species of the field strains, the susceptibility was the highest for Ae. aegypti urban field strain (7.68 × 108 conidia/ml) and Ae. albopictus suburban field strain (6.66 × 108 conidia/ml) among Ae. aegypti and Ae. albopictus field strain, respectively. Thus, Ae. aegypti and Ae. albopictus (urban and suburban) field strain showed significant differences in LC50 and LC95 (F = 2.724, df = 5, P < 0.01; Table 2). The result demonstrated that Ae. albopictus suburban field strain is significantly more susceptible than Ae. aegypti field strain (urban and suburban) toward different concentrations of M. anisopliae and are significantly more prominent in controlling Ae. albopictus field strains compared to Ae. aegypti field strains (F = 2.833, df = 20, P < 0.01; Table 2).
Table 2.
Susceptibility by larvae of Aedes aegypti and Aedes albopictus to Metarhizium anisopliae (Meta-G4) at 24 h based on LC50 and LC95 through log-probit analysis
| Parameters | Urban | Suburban | Laboratory |
|---|---|---|---|
| Aedes aegypti strain | |||
| LC50 (ppm) (95% CI) | 7.68 × 108 (6.73 × 108 to 1.25 × 109) |
2.50 × 109 (1.75 × 109 to 3.39 × 109) |
4.65 × 108 (2.90 × 108 to 1.55 × 109) |
| LC95 (ppm) (95% CI) | 1.22 × 109 (1.12 × 109 to 2.11 × 109) |
4.43 × 109 (2.98 × 109 to 7.40 × 109) |
7.67 × 108 (4.66 × 108 to 2.64 × 109) |
| Regression equation | 1.03 × 10−8x − 0.04 | 4.10 × 10−9x + 1.64 | 3.09 × 10−8x − 0.11 |
| χ² | 20.81 | 52.53 | 13.27 |
| P-value | <0.001 | 0.001 | <0.001 |
| Aedes albopictus strain | |||
| LC50 (ppm) (95% CI) | 1.38 × 109 (9.98 × 108 to 1.65 × 109) |
6.66 × 108 (4.79 × 108 to 7.50 × 108) |
1.72 × 109 (1.43 × 109 to 2.11 × 109) |
| LC95 (ppm) (95% CI) | 2.30 × 109 (1.45 × 109 to 2.58 × 109) |
1.08 × 109 (9.98 × 108 to 1.19 × 109) |
2.87 × 109 (2.35 × 109 to 3.13 × 109) |
| Regression equation | 3.81 × 10−9x + 0.29 | 7.76 × 10−9x + 0.29 | 5.23 × 10−9x + 0.32 |
| χ 2 | 51.80 | 40.08 | 48.74 |
| P-value | <0.001 | <0.001 | <0.001 |
LC50 = lethal concentration that killed 50% of the treated population; LC95 = lethal concentration that killed 95% of the treated population; 95% CI = 95% of confidence interval; χ2 = Chi-square, P < 0.05.
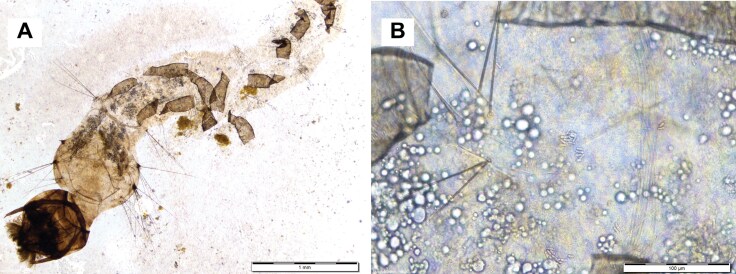
Figure 3A illustrates the impact of M. anisopliae on the muscular layers of the midgut, leading to changes in thickness, including the disappearance of muscle layer supports. Intestinal cells were destroyed because of fungal spore invasion. Following the ingestion of M. anisopliae, the intestinal cells displayed severe cytoplasmic vacuolization along with basal membrane disruption, rough endoplasmic reticulum dilatation, and fragmentation. The treatment caused significant damage to the epithelial cells of the muscle, cuticle, intestinal tissue, and midgut, with the latter group suffering the most. It also caused the detachment of the midgut cells from their basal membrane.
Fig. 3.
Morphological defects induced by Metarhizium anisopliae (Meta-G4) against Aedes mosquito larvae: A) Degradation of the intestinal cells in the gut lumen of mosquito larvae, B) accumulation and colonization of conidia spores of M. anisopliae in the gut lumen of mosquito larvae.
Figure 3 distinctly revealed the extensive colonization of M. anisopliae spores in the digestive canal through ingestion. The production of lipase enzymes by the fungus is mainly responsible for the changes seen in the midgut of Aedes larvae infected with it. These enzymes aid in the breakdown of adipose tissues and have an impact on the plasma membrane of adipose cells, causing lysis and overlapping that creates a variety of sizable holes inside the tissue. The invasion of spores into the midgut has been shown to cause a loss of functional specialization in the larvae’s fat cells.
From the results of log-probit analysis, Ae. aegypti urban field strain had the significantly lowest LT50 (0.2 d) and showed significant differences among the species (F = 14.247, df = 30, P < 0.01; Table 3). This implied that Ae. aegypti urban field strain was significantly susceptible to larvicidal effects from the combination of I. cairica leaf extracts and M. anisopliae (Meta-G4).
Table 3.
Susceptibility by larvae of Aedes aegypti and Aedes albopictus to combination of Ipomoea cairica leaf extracts (350 ppm) and Metarhizium anisopliae (Meta-G4) (1 × 106 conidia/ml) at 24 hours based on LT50 and LT95 through log-probit analysis
| Parameters | Urban | Suburban | Laboratory |
| Aedes aegypti strain | |||
| LT50 (ppm) (95% CI) | 0.2 (0.2 to 1.0) | 1.8 (1.7 to 1.9) | 1.3(1.2 to 1.4) |
| LT95 (ppm) (95% CI) | 2.0 (1.7 to 2.7) | 3.0 (2.9 to 3.1) | 2.4 (2.3 to 2.6) |
| Regression equation | 3.0x + 83.9 | 12.0x + 34.1 | 8.4x + 54.6 |
| χ2 | 146.98 | 113.78 | 37.44 |
| P-value | <0.001 | <0.001 | <0.001 |
| Aedes albopictus strain | |||
| LT50 (ppm) (95% CI) | 1.8 (1.6 to 1.9) | 0.9 (0.9 to 1.0) | 1.2 (1.1 to 1.3) |
| LT95 (ppm) (95% CI) | 3.2 (3.0 to 3.4) | 1.7 (1.6 to 1.8) | 2.8 (2.7 to 3.0) |
| Regression equation | 11.9x + 34.0 | 4.7x + 74.9 | 8.0x + 56.0 |
| χ 2 | 78.37 | 8.43 | 7.66 |
| P-value | <0.001 | <0.001 | <0.001 |
LT50 = lethal time that killed 50% of the treated population; LT95 = lethal time that killed 95% of the treated population; 95%CI = 95% of confidence interval; P-value = probability value; χ2 = Chi-square, P < 0.05.
Discussion
In the present study of the inhibition growth percentage in the compatibility of M. anisopliae (Meta-G4) with I. cairica leaf extracts, the most compatible and showed a substantial synergistic interaction is M. anisopliae aged 14 d old at the concentration of 1 × 106 conidia/ml and I. cairica leaf extracts at the concentration of 350 ppm because it shows the least inhibition growth percentage. This finding aligns with Zuharah et al. (2018), who reported optimal fungal growth with 14-d-old M. anisopliae (6 × 10⁶ conidia/ml) combined with I. cairica leaf extracts (350 ppm).
Applications of larvicides derived from plant extracts have been demonstrated to be an effective mosquito control method (Ghosh et al. 2012). As a novel perspective bioactive agent in vector control management, I. cairica, also known as morning glory, is a shrub that grows abundantly throughout Malaysia (AhbiRami et al. 2014a, Thiagaletchumi et al. 2014). Another primary strategy for managing mosquito populations is the entomopathogenic fungus M. anisopliae (Frentiu et al. 2014). However, the efficiency of this fungus is limited by its low environmental stability, spore viability, delayed lethal activities, and the number of spores accessible to meet the need for effective mosquito control (Kamareddine et al. 2013). Due to these challenging circumstances, M. anisopliae may be more successful when it interacts in an integrated manner with other natural agents—a process known as synergism. If the 2 agents demonstrate synergism, this could enhance the potency of each component, as the combined effect of the mixture may be stronger than the sum of their individual effects (Bhan et al. 2013).
The current investigation shows that I. cairica leaf extracts achieve significant larval mortality within 24 h. The result aligned with AhbiRami et al. (2014a), who reported that the acetone extract from I. cairica leaves showed the highest larvicidal efficacy against Ae. aegypti and Ae. albopictus, with LC₅₀ values of 101.94 and 105.59 ppm, and LC₉₅ values of 447.78 and 321.56 ppm, respectively, surpassing the flower and stem extracts. In this study, Aedes larvae treated with I. cairica leaf extracts exhibited morphological deformities, including darkened abdomens—early indicators of toxic effects before death. This finding aligns with Rodzay and Zuharah (2021), who also observed abnormalities in Aedes larvae exposed to I. cairica extracts. Such effects arise from plant secondary metabolites, which plants produce to defend against herbivorous insects. These compounds disrupt insect physiology, impacting various receptors and causing issues like acetylcholinesterase (AChE) inhibition, mitochondrial respiration interference, and potential damage to the midgut and malpighian tubules (Rattan 2010, Ghosh et al. 2012).
Ipomoea cairica contains alkaloids with natural larvicidal properties effective against mosquitoes (Lin et al. 2008, Kennedy and Wightman 2011, Talontsi et al. 2011). Common pesticide alkaloids, such as nicotine, anabasine, and ryanodine, disrupt insect nerve impulses by inhibiting AChE, leading to paralysis and death (Ghosh et al. 2012, Mann and Kaufman 2012). Saponins in I. cairica leaves also weaken insect cuticle membranes, causing developmental delays, reduced food intake, and increased mortality (Hostettmann and Marston 2005). Furthermore, the flavonoids in I. cairica inhibit AChE activity, likely contributing to their larvicidal action (Wuillda et al. 2019). These flavonoids, such as keranjin, regulate growth, inhibit feeding, and act as oviposition deterrents (Perumalsamy et al. 2015, Harith et al. 2018). Toxic flavonoids like rotenone disrupt cellular respiration by blocking the mitochondrial electron transport chain, impairing larvae’s swimming ability and preventing them from surfacing for oxygen (Hollingworth et al. 1994, Castrique 2004, Bhattacharya et al. 2014, Ileke and Ogungbite 2015).
In this study, M. anisopliae (Meta-G4) showed the strongest larvicidal effect against the Ae. albopictus suburban field strain, with the lowest LC₅₀ and LC₉₅ values. This result aligns with findings by Sani et al. (2016) and Benserradj and Mihoubi (2014), who reported LC₅₀ values of 3.8 × 10⁸ and 3.9 × 10⁸ conidia/mL, respectively, for Cx. quinquefasciatus fourth-instar larvae treated with M. anisopliae. From this study, observations revealed M. anisopliae conidia spores accumulate in the larval gut lumen, degrading intestinal cells and colonizing the gut. Spores of M. anisopliae enter the larval body via the mouth or siphon, adhering to internal surfaces and germinating, which releases endotoxins that damage larval tissues (Hegedus and Khachatourians 1995). Mortality follows as mycotoxins disrupt the intestines. Infection by conidia suspension prolongs death as germination on the cuticle takes time, with hyphae eventually penetrating the hemolymph, leading to mosquito death within 7 to 14 d, depending on dosage, formulation, and fungal strain (Scholte et al. 2008). Under normal conditions, larval intestines digest food within 1 to 1.5 h (Aly and Mulla 1986, Meritt et al. 1992). Within 24 h of exposure, spores swell in the gut and exuviae, penetrating tissues within a day and releasing cuticle-degrading enzymes such as lipase, protease, and chitinase, leading to hemolymph absorption and destruxin production, ultimately causing larval death. After death, larvae harden as mycelium covers the body (Prayogo 2005). The fungus M. anisopliae synthesizes destruxin, a cyclic peptide toxin with insecticidal forms A, B, and E, causing muscle paralysis and impairing larval feeding and movement (Augusto and Marilene 2010).
In the current investigation, the combination of M. anisopliae (Meta-G4) and I. cairica leaf extracts demonstrated a strong lethal effect with low LT50 and LT95 on Ae. aegypti and Ae. albopictus larvae. Gomes et al. (2015) observed that the combination of neem oil and M. anisopliae fungus led to a significantly greater reduction in larval survival compared to the effects of individual treatments. Another study demonstrated that essential oils and entomopathogenic fungi showed insecticidal activity against the cowpea bruchid, Callosobruchus maculatus, with a synergistic effect on mortality (Garima et al. 2021).
In conclusion, plant extracts and fungus are being used to control mosquitoes during their larval stage. Numerous compounds that are produced by plants have growth-regulating, therapeutic, insecticidal, and repelling characteristics. Ipomoea cairica leaf extracts do not accumulate, do not pose any long-term risks to the environment, biodegradable, less harmful, and prevent mosquitoes from developing resistance. Additionally, M. anisopliae, an entomopathogenic fungus with low environmental risk that is employed in pest management, functions as a generalist biocontrol agent that targets termites, cattle ticks, and mosquitoes. Insect pests find it difficult to fight against entomopathogenic fungi like M. anisopliae. M. anisopliae (Meta-G4) and I. cairica leaf extracts have been applied in combination to improve the efficacy of treatment against Ae. aegypti and Ae. albopictus. Significant improvements were observed with this synergistic method, especially when there was a larger proportion of I. cairica (350 ppm) and a smaller proportion of M. anisopliae spores (1 × 106 conidia/ml). By altering the larvae of Aedes antioxidant defense mechanism, the subsequent synergism demonstrated the strongest interaction, requiring less time and concentration to produce significant death. As a result, the combined action of I. cairica and M. anisopliae spores exhibits potential as an important component in the integrated management of Aedes larvae.
Disclosure
All authors have thoroughly reviewed and unanimously concurred with the content presented in this manuscript. There are no conflicts of interest encompassing financial interests, relationships, or affiliations pertinent to the subject matter discussed in this paper.
Acknowledgments
We would like to thank the staff at the School of Biological Sciences for their help throughout the project.
Contributor Information
Kin Hoong Ng, School of Biological Sciences, Universiti Sains Malaysia, Malaysia, Penang, Malaysia.
Wan Fatma Zuharah, School of Biological Sciences, Universiti Sains Malaysia, Malaysia, Penang, Malaysia.
Author contributions
Kin Hoong Ng (Conceptualization [equal], Data curation [lead], Formal analysis [equal], Investigation [lead], Methodology [equal], Project administration [equal], Writing—original draft [lead]), and Wan Fatma Zuharah (Conceptualization [lead], Data curation [equal], Formal analysis [equal], Funding acquisition [lead], Investigation [equal], Methodology [lead], Project administration [equal], Resources [lead], Supervision [lead], Visualization [lead], Writing—review & editing [lead])
Ethics approval and consent to participate
All procedures performed in the study are in accordance with the ethical standards of the institutional and/or national research committee. We further declare that no animal was harmed during the study.
Consent for publication
Informed consent was obtained from all the individual participants included in the study.
Funding
This project was funded by the Fundamental Research Grant Scheme, Ministry of Higher Education Malaysia (FRGS/1/2023/STG03/USM/02/4) under the grant received by Wan Fatma Zuharah.
Conflicts of interest. The authors declare that there is no conflict of interest.
References
- AhbiRami R, Zuharah WF, Thiagaletchumi M, et al. 2014a. Larvicidal efficacy of different plant parts of railway creeper, Ipomoea cairica extract against dengue vector mosquitoes, Aedes albopictus (Diptera: Culicidae) and Aedes aegypti (Diptera: Culicidae). J. Insect Sci. 14:1–6. https://doi.org/ 10.1093/jisesa/ieu042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AhbiRami R, Zuharah WF, Yahaya ZS, et al. 2014b. Oviposition deterring and oviciding potentials of Ipomoea cairica L. leaf extract against dengue vectors. Trop. Biomed. 31:456–465. https://doi.org/PMID: 25382472 [PubMed] [Google Scholar]
- Akhir MAM, Wajidi MFF, Lavoue S, et al. 2021. Molecular insight into the knockdown resistance (kdr) in the voltage gated sodium channel (vgsc) gene of the main dengue vector, Aedes aegypti (Diptera: Culicidae) and the discovery of novel regional specific point mutation A1007G in malaysia. Res. Sq. 15:1–30. https://doi.org/ 10.1186/s13071-022-05192-z [DOI] [Google Scholar]
- Aly C, Mulla MS.. 1986. Orientation and ingestion rates of larval Anopheles albimanus in response to floating particles. Entomol. Exp. Appl. 42:83–90. https://doi.org/ 10.1111/j.1570-7458.1986.tb02191.x [DOI] [Google Scholar]
- Amer HH, Paxton RR, Winkle MV.. 1956. Methanol–ethanol acetone: vapour liquid equilibria. Ind. Eng. Chem. 48:142–146. https://doi.org/ 10.1021/ie50553a041 [DOI] [Google Scholar]
- Augusto S, Marilene H.. 2010. Metarhizium anisopliae enzymes and toxins. Toxicon 56:1267–1274. https://doi.org/ 10.1093/jb/mvj159 [DOI] [PubMed] [Google Scholar]
- Austin DF, Huaman Z.. 1996. A synopsis of Ipomoea (Convolvulaceae) in the Americas. Taxon. 45:3–38. https://doi.org/ 10.2307/1222581 [DOI] [Google Scholar]
- Benserradj O, Mihoubi I.. 2014. Larvicidal activity of entomopathogenic fungi Metarhizium anisopliae against mosquito larvae in Algeria. Int. J. Curr. Microbiol. Appl. Sci. 3:54–62. [Google Scholar]
- Benelli G, Pavela R, Iannarelli R, et al. 2017. Synergized mixtures of Apiaceae essential oils and related plant–borne compounds: larvicidal effectiveness on the filariasis vector Culex quinquefasciatus Say. Ind. Crop. Prod. 96:186–195. https://doi.org/ 10.1016/j.indcrop.2016.11.059 [DOI] [Google Scholar]
- Bhan S, Shrankhla LM, Srivastava CN.. 2013. Larvicidal toxicity of temephos and entomopathogenic fungus, Aspergillus flavus and their synergistic activity against malaria vector, Anopheles stephensi. J. Entomol. Zool. Stud. 1:55–60. [Google Scholar]
- Bharathithasan M, Kotra V, Abbas SA, et al. 2024. Review on biologically active natural insecticides from Malaysian tropical plants against Aedes aegypti and Aedes albopictus. Arab J. Chem. 17:1–10. https://doi.org/ 10.1016/j.arabjc.2023.105345 [DOI] [Google Scholar]
- Bhattacharya K, Chandra G.. 2014. Phagodeterrence, larvicidal and oviposition deterrence activity of Tragia involucrate L (Euphorbiaceae) root extractives against vector of lymphatic filariasis Culex quinquefasciatus (Diptera: Culicidae). Asian Pac. J. Trop. Dis. 4:226–232. https://doi.org/ 10.1016/S2222-1808(14)60444-8 [DOI] [Google Scholar]
- Bitencourt RDOB, Santos–Mallet JRD, Lowenberger C, et al. 2023. A novel model of pathogenesis of Metarhizium anisopliae propagules through the midguts of Aedes aegypti larvae. Insects 14:1–12. https://doi.org/ 10.3390/insects14040328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein L, Blaustein J, Chase J.. 2005. Chemical detection of the predator Notonecta irrorata by ovipositing Culex mosquitoes. J. Vector Ecol. 30:299–301. [PubMed] [Google Scholar]
- Bukhari T, Middelman A, Koenraadt CJM, et al. 2010. Factors affecting fungus-induced larval mortality in Anopheles gambiae and Anopheles stephensi. Malar. J. 9:1–15. https://doi.org/ 10.1186/1475-2875-9-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrique E. Rotenone. Molecule of the Month. 2004. 6p. [Google Scholar]
- Daryaei A, Jones EE, Ghazalibiglar H, et al. 2016. Effects of temperature, light and incubation period on production, germination and bioactivity of Trichoderma atroviride. J. Appl. Microbiol. 120:999–1009. https://doi.org/ 10.1111/jam.13076 [DOI] [PubMed] [Google Scholar]
- Elumalai K, Dhanasekaran S, Anandan A, et al. 2012. Larvicidal, ovicidal and pupicidal activity of Eranthemumroseum (Vahl) R. BR. against malarial vector mosquito, Anopheles stephensi (liston) (Diptera: Culicidae). Int. J. Curr. Agric. Sci. 2:28–33. [Google Scholar]
- Figueredo LA, Luna RLN, Miranda DEO, et al. 2020. Beauveria bassiana (Hypocreales: Cordycipitaceae) reduces the survival time of Lutzomyia longipalpis (Diptera: Psychodidae), the main vector of the visceral leishmaniasis agent in the Americas. J. Med. Entomol. 57:2025–2029. https://doi.org/ 10.1093/jme/tjaa131 [DOI] [PubMed] [Google Scholar]
- Frentiu FD, Walker T, O’ Neill SL.. 2014. Biological control of dengue and Wolbachia-based strategies. In: Gubler, DJ, editor. Dengue and Dengue Hemorrhagic Fever, 2nd ed., CABI, 537–547. https://doi.org/ 10.1079/9781845939649.0537 [DOI] [Google Scholar]
- Garima G, Khan R, Seal D.. 2021. Cowpea Weevil, Callosobruchus Maculatus (Insecta: Coleoptera: Bruchidae). EDIS 2021. https://doi.org/ 10.32473/edis-in1338-2021 [DOI] [Google Scholar]
- Ghosh A, Chowdury N, Chandra G.. 2012. Plants extract as potential mosquito larvicides. Indian J. Med. Res. 135:581–598. https://doi.org/PMCID: PMC3401688 [PMC free article] [PubMed] [Google Scholar]
- Gomes SA, Paula AR, Ribeiro A, et al. 2015. Neem oil increases the efficiency of the entomopathogenic fungus Metarhizium anisopliae for the control of Aedes aegypti (Diptera: Culicidae) larvae. Parasit. Vectors. 8:669. https://doi.org/ 10.1186/s13071-015-1280-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez KA, Gomez A.. 1983. Statistical procedure for agricultural research. 2nd edn.John Wiley and Sons; 357–427. [Google Scholar]
- Halim NA, Jalinas J, Zakaria A, et al. 2021. Effects of nutrient additives and incubation period on sporulation and viability of the entomopathogenic fungus, Metarhizium anisopliae (Hypocreales: Clavicipitaceae). Malays. J. Microbiol. 17:97–102. https://doi.org/ 10.21161/mjm.200749 [DOI] [Google Scholar]
- Harith SS, Mohd SNAS, Aziz NA, et al. 2018. Phytochemical screening and larvicidal activity of Murraya koenigii leaves extracts against mosquito larvae. Malays. J. Anal. Sci. 22:471–476. https://doi.org/ 10.17576/mjas-2018-2203-14 [DOI] [Google Scholar]
- Hegedus DD, Khachatourians GG.. 1995. The impact of biotechnology on hyphomycetous fungal insect biocontrol agents. Biotechnol. Adv. 13:455–490. https://doi.org/ 10.1016/0734-9750(95)02006-o [DOI] [PubMed] [Google Scholar]
- Hollingworth R, Ahmmadsahib K, Gedelhak G, et al. 1994. New inhibitors of complex I of the mitochondrial electron transport chain with activity as pesticides. Biochem. Soc. Trans. 22:230–233. https://doi.org/ 10.1042/bst0220230 [DOI] [PubMed] [Google Scholar]
- Hostettmann K, Marston A.. 2005. Chemistry and pharmacology of natural products: Saponins. Cambridge University Press, 548p. https://doi.org/ 10.1021/ja9553056 [DOI] [Google Scholar]
- Ileke KD, Ogungbite OC.. 2015. Alstonia boonei De Wild oil extract in the management of mosquito (Anopheles gambiae), a vector of malaria disease. J. Coast Life Med. 3:557–563. https://doi.org/ 10.12980/JCLM.3.2015J5-74 [DOI] [Google Scholar]
- Ishak AR, Dom NC, Hussain H, et al. 2014. Biolarvacidal potential of Ipomoea cairica extracts against key dengue vectors. Procedia – Soc. Behav. Sci. 153:180–188. https://doi.org/ 10.1016/j.sbspro.2014.10.052 [DOI] [Google Scholar]
- Kamareddine L, Fan Y, Osta MA, et al. 2013. Expression of Trypsin Modulating Oostatic Factor (TMOF) in an entomopathogenic fungus increases its virulence towards Anopheles gambiae and reduces fecundity in the target mosquito. Parasit. Vectors 6:1–5. https://doi.org/ 10.1186/1756-3305-6-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DO, Wightman EL.. 2011. Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2:32–50. https://doi.org/ 10.3945/an.110.000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayyat SA, Roselin LS.. 2018. Recent progress in photochemical reaction on main components of some essential oils. J. Saudi Chem. Soc. 22:855–875. https://doi.org/ 10.1016/j.jscs.2018.01.008 [DOI] [Google Scholar]
- Lim H, Lee SY, Ho LY, et al. 2023. Mosquito larvicidal activity and cytotoxicity of the extracts of aromatic plants from Malaysia. Insects 14:512–518. https://doi.org/ 10.3390/insects14060512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RJ, Chen CY, Lo WL.. 2008. Cytotoxic activity of Ipomoea cairica. Nat. Prod Res. 22:747–753. https://doi.org/ 10.1080/14786410701628739 [DOI] [PubMed] [Google Scholar]
- Mann RS, Kaufman PE.. 2012. Natural product pesticides: their development, delivery and use against insect vectors. Mini–Rev Org Chem. 9:185–202. https://doi.org/ 10.2174/157019312800604733 [DOI] [Google Scholar]
- Merritt RW, Dadd RH, Walker ED.. 1992. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu. Rev. Entomol. 37:349–376. https://doi.org/ 10.1146/annurev.en.37.010192.002025 [DOI] [PubMed] [Google Scholar]
- Mulla MR, Su T.. 1999. Oviposition bioassay responses of Culex tarsalis and Culex quinquefasciatus to neem products containing azadirachtin. Entomol. Exp. Appl. 9:1337–1345. [Google Scholar]
- Ogunwenmo KO. 2003. Cotyledon morphology: an aid in identification of Ipomoeataxa (Convolvulaceae). Feddes Repert. 114:198–203. https://doi.org/ 10.1002/fedr.200390022 [DOI] [Google Scholar]
- Oliveira PV, Ferreira JC, Moura JFS, et al. 2010. Larvicidal activity of 94 extracts from ten plant species of northeastern of Brazil against Aedes aegypti L. (Diptera: Culicidae). Parasitol. Res. 107:403–407. https://doi.org/ 10.1007/s00436-010-1880-4 [DOI] [PubMed] [Google Scholar]
- Perumalsamy H, Jang MJ, Kim JR, et al. 2015. Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species. Parasit Vectors. 8:1–14. https://doi.org/ 10.1186/s13071-015-0848-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prayogo Y. 2005. Potensi, kendala dan upaya mempertahankan keefektifan cendawan entomopatogen untuk mengendalikan hama tanaman pangan. Buletin Palawija. 10:53–65. https://doi.org/ 10.21082/bulpalawija.v0n10.2005.p53-65 [DOI] [Google Scholar]
- Rahim J, Ahmad AH, Ahmad H, et al. 2017. Adulticidal susceptibility evaluation of Aedes albopictus using new diagnostic doses in Penang Island, Malaysia. JAMCA 33:200–208. https://doi.org/ 10.2987/16-6607R.1 [DOI] [PubMed] [Google Scholar]
- Rasli R, Cheong YL, Ibrahim MKC, et al. 2021. Insecticide resistance in dengue vectors from hotspots in Selangor, Malaysia. PLoS Negl.Trop. Dis. 15:1–15. https://doi.org/ 10.1371/journal.pntd.0009205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan RS. 2010. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 29:913–920. https://doi.org/ 10.1016/j.cropro.2010.05.008 [DOI] [Google Scholar]
- Ravi R, Zulkrnin NSH, Rozhan NN, et al. 2018. Chemical composition and larvicidal activities of Azolla pinnata extracts against Aedes (Diptera: Culicidae). PLoS One 13:1–18. https://doi.org/ 10.1371/journal.pone.0206982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodzay R, Zuharah WF.. 2021. The determination of effective concentration of acethonilic Ipomoea cairica leaves extract against laboratory and field strains of Aedes albopictus and Aedes aegypti mosquito larvae. Trop. Biomed. 38:446–452. https://doi.org/ 10.47665/tb.38.3.087 [DOI] [PubMed] [Google Scholar]
- Rueda LM. 2004. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with dengue virus transmission. Zootaxa 589:1–60. https://doi.org/ 10.11646/zootaxa.589.1.1 [DOI] [Google Scholar]
- Sani I, Yusuf U, Umar KM.. 2016. Larvicidal efficacy of entomopathogenic fungus, Metarhizium anisopliae against Culex quinquefasciatus (Say) (Diptera: Culicidae). Ann. Exp. Biol. 4:17–21. [Google Scholar]
- Scholte EJ, Bart GJK, Willem T.. 2008. An entomophatogenic fungus (Metarhizium anisopliae) for control of the adult African malaria vector Anopheles. Entomol. Ber. 68:21–26. https://doi.org/ 10.1126/science.1108639 [DOI] [Google Scholar]
- Sharma M, Chandel S.. 2016. In vitro evaluation of compatibility of indigenous Trichoderma harzianum with botanicals. Int. J. Sci. Environ. Technol. 5:1131–1136. [Google Scholar]
- Singh G, Prakash S.. 2014. New prospective on fungal pathogens for mosquitoes and vectors control technology. J. Mosq. Res. 4:36–52. https://doi.org/ 10.5376/jmr.2014.04.0007 [DOI] [Google Scholar]
- Talontsi FM, Matasyob JC, Ngoumfo RM, et al. 2011. Mosquito larvicidal activity of alkaloids from Zanthoxylum lemairei against the malaria vector Anopheles gambiae. Pestic. Biochem. Physiol. 99:82–85. https://doi.org/ 10.1016/j.pestbp.2010.11.003 [DOI] [Google Scholar]
- Thiagaletchumi M, Zuharah WF, Rami RA, et al. 2014. Assessment of residual bio=efficacy and persistence of Ipomoea cairica plant extract against Culex quinquefasciatus Say mosquito. Trop. Biomed. 31:466–476. https://doi.org/PMID: 25382473 [PubMed] [Google Scholar]
- Thomas TG, Rao S, Lal S.. 2004. Mosquito larvicidal properties of essential oil of an indigenous plant, Ipomoea carica Linn. Jpn. J. Infect. Dis. 57:176–177. [PubMed] [Google Scholar]
- Valverde VA, Rodriguez GR, Vargas SA.. 2022. Bioguided phytochemical study of Ipomoea cairica extracts with larvicidal activity against Aedes aegypti. Molecules 27:1–13. https://doi.org/ 10.3390/molecules27041348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard S, Butt TM, Bresciani J, et al. 1999. Light and electron microscopy studies of the infection of the western flower thrips Frankliniella occidentalis (Thysanoptera: Thripidae) by the entomopathogenic fungus Metarhizium anisopliae. J. Invertebr. Pathol. 73:25–33. https://doi.org/ 10.1006/jipa.1998.4802 [DOI] [PubMed] [Google Scholar]
- Vincent JM. 1947. Distortion of fungal hyphae in the presence of certain inhibitors. Nature 159:850. https://doi.org/ 10.1038/159850b0 [DOI] [PubMed] [Google Scholar]
- WHO. 2005a. Regional framework for an integrated vector management strategy for the Southeast Asia region. SEA-VBC-86, p. 1–13. SEA-CD-239 [Google Scholar]
- WHO. 2005b. Guidelines for laboratory and field testing of mosquito larvicides. Geneva, World Health Organization, p. 8–11. https://www.who.int/publications/i/item/WHO-CDS-WHOPES-GCDPP-2005.13 [Google Scholar]
- Wuillda ACJDS, Martins RCC, Costa FDN.. 2019. Larvicidal activity of secondary plant metabolites in Aedes aegypti control: An overview of the previous 6 years. Nat. Prod. Commun. 14:1–11. https://doi.org/ 10.1177/1934578X19862893 [DOI] [Google Scholar]
- Zuharah WF, Rodzay RMD, Azmi WA, et al. 2018. The integrated vector management of the synergism formulation between Ipomoea cairica plant extract and Metarhizium anisopliae Meta–G4 on Aedes mosquitoes. Serangga 23:76–83. [Google Scholar]
- Zuharah WF, Rohaiyu MR, Azmi WA, et al. 2021. Pathogenicity of entomopathogenic fungus, Metarhizium anisopliae MET–GRA4 isolate on dengue vectors, Aedes albopictus and Aedes aegypti mosquito larvae (Diptera: Culicidae). J. Asia-Pac. Entomol. 24:24–29. https://doi.org/ 10.1016/j.aspen.2021.04.008 [DOI] [Google Scholar]