Abstract
Objective
This study evaluates the long-term trends in Multiple Myeloma (MM) incidence, mortality, and Age-Period-Cohort (APC) effects in the BRICS nations (Brazil, Russia, India, China, and South Africa).
Methods
Data on age-standardized incidence rate (ASIR), age-standardized mortality rate (ASMR), and 95% uncertainty intervals (UIs), were obtained from the Global Burden of Disease Study 2021. Joinpoint regression model was used to estimate the average annual percentage change (AAPC) and annual percentage change (APC) trends from 1992 to 2021, and the Age-Period-Cohort model evaluated nonlinear impacts of age, period, and cohort effects. Projections to 2046 were calculated using Bayesian APC modeling.
Results
From 1992 to 2021, MM incidence and death cases in the BRICS nations increased nearly four to fivefold, with ASIR and ASMR nearly doubling. China and India had lower ASIR and ASMR than other BRICS countries despite accounting for over half of cases and deaths. South Africa consistently had the highest ASIR and ASMR in both 1992 and 2021. China experienced a significant increase in ASIR (AAPC 4.92%, p < 0.001) and ASMR (AAPC 4.07%, p < 0.001) over the past three decades. MM incidence and mortality increased with aging, and the age effect on MM was more pronounced among individuals aged greater than 40 years. Birth cohorts’ impact on MM varied greatly between BRICS, with China suffering the largest risk increase among those born after the 1970s. Projections indicate ASIR and ASMR will reach 2.44 and 1.82 per 100,000 by 2046, continuing to rise across the BRICS nations.
Conclusions
MM burden is rapidly increasing in the BRICS, closely tied to population aging. Targeted strategies addressing each country’s unique challenges are essential as the burden continues to grow.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-025-22688-2.
Keywords: Multiple myeloma (MM), Incidence, Mortality, BRICS, Age-period-cohort analysis
Introduction
Multiple myeloma (MM) is a hematological malignancy characterized by the proliferation of clonal plasma cells within the bone marrow, leading to anemia, bone destruction, hypercalcemia, and renal failure [1]. The global incidence of MM is 4.5–6 cases per 100,000 individuals annually [2]. From 1990 to 2016, the incidence increased by 126% globally, with growth rates ranging from 106 to 192% across all Socio-Demographic Index (SDI) quintiles: income per capita, educational attainment, and fertility rate indicators [3]. The disease is more prevalent in individuals over 60 years old, males, Black individuals, and those with a family history of MM [4, 5]. In the United States, about 3–5% of individuals over 65 years old, with a life-long incidence rate of 1% per year [6, 7]. The age-standardized incidence rate (ASIR) and age-standardized mortality rate (ASMR) have risen in all SDI quintiles except the low SDI quintile, with the most significant increases observed in middle SDI countries, and the highest ASIR and ASMR found in high SDI countries [3].
The acronym “BRICS” refers to five countries including Brazil, Russia, India, China, and South Africa, representing emerging economies characterized by rapid economic growth and large populations, collectively accounting for 42% of the world’s population and about 25% of the global economy [8, 9]. Despite rapid socioeconomic development and improvements in healthy life expectancy (HALE), BRICS countries face substantial challenges, including environmental pollution, unequal distribution of healthcare resources, and an increasing burden of noncommunicable diseases [10, 11]. Cancer incidence has increased by 28% over the past three decades, while 71% of all deaths in the middle- and low-income countries are related to non-communicable diseases. The BRICS nations shoulder a disproportionate share of this burden, particularly for cancers linked to aging and lifestyle transitions. However, epidemiological research on MM in these emerging economies remain lacking. Given the particular demographic change and healthcare challenges within BRICS nations, it may be particularly important for trends and future forecasts on MM in these countries to provide important evidence for the adaptation of public health strategies.
This research uses data from the Global Burden of Disease Study (GBD) to analyze the long-term trends of MM incidence and mortality across BRICS countries from 1992 to 2021, with projections extending to 2046. By evaluating age, period, and cohort effects on MM epidemiology using age-period-cohort (APC) model, we aim to enhance understanding of MM trends in developing regions and support the formulation of targeted strategies for MM prevention, diagnosis, and management.
Methods and materials
Data source
This study utilized data on MM in BRICS nations, derived from the GBD 2021 study (https://vizhub.healthdata.org/gbd-results/). The GBD 2021 database, managed by the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, provides a comprehensive analysis of the prevalence, incidence, mortality rates, disability-adjusted life years (DALYs), years lived with disability (YLDs), and years of life lost (YLLs) across 371 diseases in 204 countries and territories from 1990 to 2021. Data cleaning and bias adjustments followed GBD 2021 methodology [12]. Data quality verification involved age-sex splitting, systematic outlier detection based on the median absolute deviation and spatiotemporal smoothing using Gaussian Process Regression (ST-GPR). Known biases due to differential case definitions or measurement were systematically corrected through Bayesian meta-regression with regularization and trimming (MR-BRT).
In this study, “Global”, “Brazil”, “Russia”, “India”, “China”, and “South Africa” were chosen from the database as the location, “multiple myeloma” for the cause, and “incidence” and “death” for measures, to explore the demographic and social impact of age, period, and cohort on MM in BRICS. ASIR, ASMR, and 95% uncertainty intervals (UIs) were calculated on the basis of GBD 2021 global age-standard population. The absolute numbers and crude rates of MM incidence and mortality were also extracted by sex and age. Data on multiple myeloma (MM) from 5,119 sources were included in the Global Burden of Disease (GBD) 2021 study (https://ghdx.healthdata.org/gbd-2021/sources). Among BRICS, South Africa contributed the fewest sources (20) and the smallest number of metadata rows (31440).
Joinpoint regression analysis
To evaluate the temporal trends in MM incidence and mortality during this the study period, the joinpoint regression analysis was performed to break the secular trend line into multiple statistically significant sections through model fitting. Annual percent changes (APCs) were calculated for each identified trend segment. Positive values indicate an increasing trend, while negative values signify a decreasing trend. Direction and magnitude changes in incidence and mortality were derived using the Average Annual Percent Changes (AAPCs) and 95% confidence intervals (CIs) for the most recent decades. An AAPC or APC greater than 0, with a 95% CI entirely above 0, indicates a significant increasing trend. An AAPC or APC below 0, with a 95% CI entirely below 0, indicates a significant decreasing trend. If the AAPC or APC includes 0 within its 95% CI, the rate is stable over time.
Age-period-cohort analysis
Age-period-cohort (APC) analysis was conducted to assess the effect of age, period, and cohort on the incidence and mortality of MM among males and females across the BRICS countries. The main problem with APC modeling is collinearity because cohort effects are intrinsically associated with period and age (cohort = period - age). We applied the intrinsic estimator algorithm, which has been proven to provide estimable, unbiased, efficient, and asymptotic normal parameters. The model is expressed as:
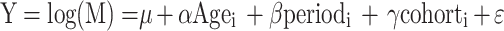 |
where α, β, and γ represent the age, period, and cohort effects, respectively. Specifically, α denotes the risk associated with a given age group, β reflects temporal variations in risk, γ captures cohort-specific risk patterns, and i for the age group (i = 20–24, 25–29, 30–34,.,85–89, 90–95). µ is the intercept, and ε represents the random error term [13].
In the APC model, net drift represents the overall annual percentage change, while local drifts indicate annual percentage changes for each age group. The annual change of MM incidence and mortality was calculated for 15 age groups (20–24, 25–29, 30–34,…,85–89, 90–95) with 5-year intervals. The diagnostic period was divided into six consecutive time periods from 1992 to 1996 to 2017–2021, with 2002–2006 as the reference period. Incidence, mortality, and population counts were based on the mid-year of the six time points (1994, 1999,…, 2019) instead of the average of the 5-year periods, to represent each specific period.
Bayesian age-period-cohort model (BAPC)
A Bayesian age-period-cohort (BAPC) model was employed to predict the progression of MM mortality and incidence through 2046, based on the historical records. The BAPC analysis assumed that the effects of age, period, and cohort that are adjacent in time are similar, using a second-order random walk to smooth priors for age, period, and cohort effects. This approach was used to project posterior incidence and mortality rates. To avoid the mixing and convergence issues typically associated with Markov chain Monte Carlo (MCMC) sampling techniques traditionally used in Bayesian approaches, integrated nested Laplace approximations (INLA) were introduced in the BAPC model to approximate the marginal posterior distributions, offering better coverage and precision compared to other prediction methods [14].
Statistical analysis
Statistical descriptions and analyses of the incidence and mortality cases were conducted using the R software (4.2.1). The joinpoint regression analysis was performed with the Joinpoint Regression Program from the Surveillance Research Program of the US National Cancer Institute. Estimated parameters were obtained through the APC web tool (https://analysistools.cancer.gov/apc/) provided by the National Cancer Institute. Wald chi-square tests were used to assess the significance of the functions and estimable parameters. The BAPC analysis was performed using R-package BAPC [15] were generated with the R-package ggplot2 [16]. All statistical tests were two-sided, with p < 0.05 considered statistically significant.
Results
Time trends of MM incidence and mortality in BRICS
Tables 1 and 2, and Fig. 1 illustrate the trends in MM incidence and mortality across BRICS countries from 1992 to 2021. During this period, the number of global MM incident cases increased substantially, from 59,535 (95% UI, 55,562 − 63,702) in 1992 to 148,755 (95% UI, 131,780 − 162,049) in 2021. Similarly, global deaths increased from 50,567 (95% UI, 47,086 − 54,583) to 116,360 (95% UI, 103,079–128,471). In 1992, the global MM ASIR and ASMR were 1.5006 per 100,000 and 1.3109 per 100,000, respectively. By 2021, these rates had shown a modest increase to 1.7401 per 100,000 for ASIR and 1.3749 per 100,000 for ASMR.
Table 1.
Characteristics of MM incidence and its trends across BRICS countries from 1992 to 2021
| 1992 | 2021 | 1992–2021 | |||||
|---|---|---|---|---|---|---|---|
| Case | ASIR/100,000 | Case | ASIR/100,000 | AAPC (95%CI) | P | ||
| Brazil | Both | 1293.60 | 1.3441 | 5332.10 | 2.1137 | 1.65 (1.17, 2.15) | 0.000 |
| Male | 622.96 | 1.3829 | 2652.98 | 2.3500 | 1.90 (1.44, 2.36) | 0.000 | |
| Female | 670.64 | 1.3096 | 2679.12 | 1.9266 | 1.36 (0.97, 1.75) | 0.000 | |
| Russia | Both | 2219.85 | 1.1589 | 4349.70 | 1.8061 | 1.63 (1.10, 2.16) | 0.000 |
| Male | 987.25 | 1.4109 | 1769.06 | 1.8862 | 1.09 (0.74, 1.45) | 0.000 | |
| Female | 1232.60 | 1.0342 | 2580.64 | 1.7540 | 1.78 (1.57, 1.99) | 0.000 | |
| India | Both | 2910.64 | 0.5990 | 12588.15 | 1.0647 | 2.04 (1.83, 2.45) | 0.000 |
| Male | 1739.52 | 0.6967 | 7320.84 | 1.2928 | 2.16 (1.91, 2.41) | 0.000 | |
| Female | 1171.12 | 0.4976 | 5267.31 | 0.8599 | 1.93 (1.69, 2.18) | 0.000 | |
| China | Both | 1862.12 | 0.2054 | 17249.51 | 0.8112 | 4.92 (4.28, 5.57) | 0.000 |
| Male | 1026.47 | 0.2379 | 10771.75 | 1.0632 | 5.31 (5.00, 5.62) | 0.000 | |
| Female | 835.65 | 0.1799 | 6477.76 | 0.5893 | 4.15 (3.72, 4.58) | 0.000 | |
| South Africa | Both | 327.36 | 1.4680 | 1025.51 | 2.1768 | 1.36 (0.95, 1.78) | 0.000 |
| Male | 162.43 | 1.7036 | 506.58 | 2.5572 | 1.40 (0.88, 1.93) | 0.000 | |
| Female | 164.93 | 1.2948 | 518.92 | 1.9183 | 1.34 (1.08, 1.59) | 0.000 | |
Table 2.
Characteristics of MM mortality and its trends across BRICS countries from 1992 to 2021
| 1992 | 2021 | 1992–2021 | |||||
|---|---|---|---|---|---|---|---|
| Deaths | ASMR/100,000 | Deaths | ASMR/100,000 | AAPC (95%CI) | P | ||
| Brazil | Both | 1175.21 | 1.2556 | 4517.35 | 1.8099 | 1.37 (0.83, 1.91) | 0.000 |
| Male | 573.02 | 1.3129 | 2285.11 | 2.0705 | 1.56 (1.15, 1.97) | 0.000 | |
| Female | 602.19 | 1.2048 | 2232.24 | 1.6057 | 1.04 (0.64, 1.45) | 0.000 | |
| Russia | Both | 1857.43 | 0.9745 | 3325.89 | 1.3701 | 1.14 (0.86, 1.42) | 0.000 |
| Male | 821.85 | 1.2270 | 1341.87 | 1.4610 | 0.66 (0.34, 0.97) | 0.000 | |
| Female | 1035.58 | 0.8577 | 1984.03 | 1.3140 | 1.43 (1.22, 1.63) | 0.000 | |
| India | Both | 2830.82 | 0.6071 | 11634.79 | 1.0138 | 1.83 (1.60, 2.06) | 0.000 |
| Male | 1688.70 | 0.7082 | 6748.77 | 1.2404 | 1.99 (1.65, 2.33) | 0.000 | |
| Female | 1142.13 | 0.5034 | 4886.02 | 0.8154 | 1.72 (1.43, 1.99) | 0.000 | |
| China | Both | 1741.45 | 0.1994 | 12984.05 | 0.6168 | 4.07 (3.21, 4.93) | 0.000 |
| Male | 944.01 | 0.2309 | 7792.86 | 0.7915 | 4.34 (4.04, 4.65) | 0.000 | |
| Female | 797.44 | 0.1761 | 5191.19 | 0.4707 | 3.48 (2.95, 4.01) | 0.000 | |
| South Africa | Both | 310.41 | 1.4321 | 925.95 | 2.0330 | 1.21 (0.78, 1.64) | 0.000 |
| Male | 152.33 | 1.6672 | 453.13 | 2.4127 | 1.27 (0.85, 1.69) | 0.000 | |
| Female | 158.08 | 1.2638 | 472.82 | 1.7843 | 1.25 (1.00, 1.51) | 0.000 | |
Fig. 1.
Total incidence and death cases, incidence rate, death rate, ASIR and ASMR of MM across BRICS countries between 1992 and 2021. Note: ASIR: the age-standardized incidence rate; ASMR: age-standardized mortality rate; MM: Multiple Myeloma; BRICS: Brazil, Russia, India, China, and South Africa
In 2021, the BRICS nations collectively accounted for 27.3% of global MM incidence cases and 28.7% of global deaths. Specifically, within the BRICS consortium, 40,545 MM cases were recorded in 2021, representing a fivefold increase compared to 1992. Meanwhile, the number of deaths increased fourfold, reaching 33,388 in 2021. The ASIR experienced a significant increase, doubling from 0.5059 per 100,000 in 1992 to 1.0534 per 100,000 in 2021, while the ASMR followed a similar upward trajectory, rising from 0.4810 to 0.8808 per 100,000 over the same period across the BRICS countries. India had the highest incidence and mortality cases among the BRICS nations in 1992. However, by 2021, China had surpassed India in both metrics. Despite accounting for over half of the MM cases and deaths within the BRICS, China and India reported lower ASIR and ASMR compared to the other countries. Throughout the study period, China consistently maintained the lowest ASIR (0.2054 to 0.8112 per 100,000) and ASMR (0.1994 to 0.6168 per 100,000). Conversely, South Africa, despite having the fewest cases and deaths, consistently exhibited the highest ASIR and ASMR within the BRICS, leading in both 1992 and 2021.
Joinpoint regression of MM in BRICS
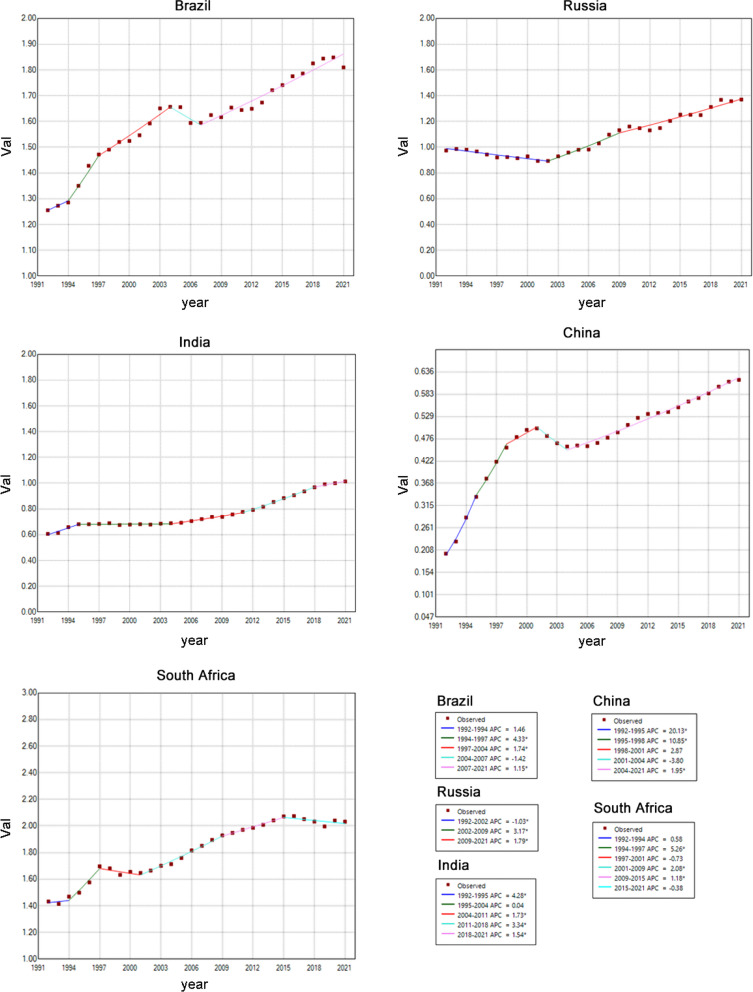
We analyzed the age-standardized trends of ASIR and ASMR for MM in BRICS countries from 1992 to 2021 using joinpoint regression analysis. As shown in Tables 1 and 2, the overall trends of both ASIR and ASMR for MM increased during this period. China experienced a significant increase in ASIR (AAPC 4.92%, 95% CI: 4.28–5.57%, p < 0.001) and ASMR (AAPC 4.07%, 95% CI: 3.21–4.93%, p < 0.001).
From 1992 to 2021, China’s ASIR for MM exhibited a marked upward trend during 1992–1995 (APC 20.42%, 95% CI: 17.09–23.85%, p < 0.001), while Brazil experienced the most substantial decrease during 1992–2002 (APC − 1.27%, 95% CI: -1.62% to -0.92%, p < 0.001). ASIR varied significantly across BRICS (Fig. 2 and Table 1), with China showing both the highest and lowest APCs of ASIR. In Russia, the ASIR of MM initially declined before later rising. India consistently showed an increasing ASIR, with the highest APC during 1992–1995 (max APC = 3.87%, 95% CI: 2.55–5.21%, p < 0.001). South Africa exhibited an upward trend in the early years, with no significant changes observed after 2015.
Fig. 2.
Trends in ASIR of MM across the BRICS countries from 1992 to 2021. Note: ASIR: the age-standardized incidence rate; MM: Multiple Myeloma; BRICS: Brazil, Russia, India, China, and South Africa
ASMR trends largely mirrored those of ASIR, with China showing the most significant increase during 1992–1995 (APC 20.13%, 95% CI: 15.57–24.86%, p < 0.001) and a non-significant decrease during 2001–2004 (APC − 3.8%, 95% CI: -7.82–0.40%, p = 0.07). In India, ASMR consistently increased, with the highest APC during 1992–1995 (APC 4.28%, 95% CI: 2.80–5.77%, p < 0.001) (Fig. 3 and Table 2).
Fig. 3.
Trends in ASMR of MM across the BRICS countries from 1992 to 2021. Note: ASMR: the age-standardized mortality rate; MM: Multiple Myeloma; BRICS: Brazil, Russia, India, China, and South Africa
Net drift and local drift in different age groups
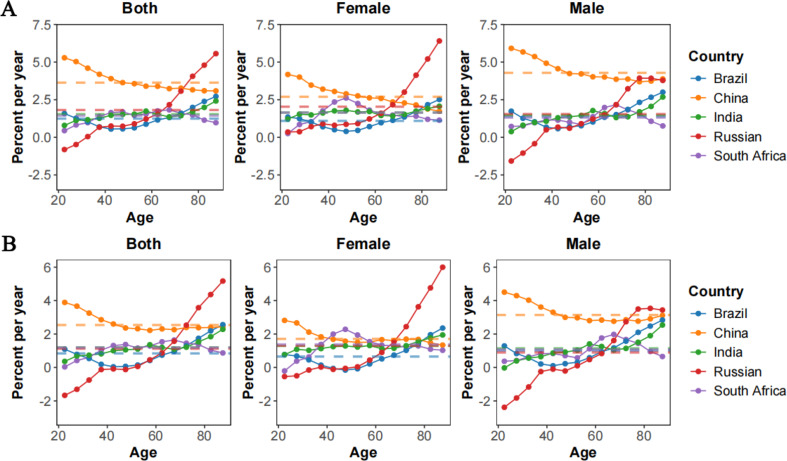
Figure 4 provides a detailed analysis of the net and local drifts in MM incidence and mortality across BRICS nations, as defined by annual percentage changes. The net drift, depicted by dotted lines in Fig. 4, corresponds to the annual changes in age-adjusted rates, while the local drift, depicted by curves, represents changes in age-specific rates for different age groups in each country.
Fig. 4.
Local drift with net drift values for MM incidence (A) and mortality (B) and gender difference in BRICS from 1992 to 2021. Note: MM: Multiple Myeloma; BRICS: Brazil, Russia, India, China, and South Africa
For MM incidence, China exhibited the fastest annual increase (3.63%, 95% CI: 3.47–3.80%) among all BRICS countries. The overall net drift was comparable in Brazil, India, South Africa, and Russia (Supplementary Table S1), indicating a substantial increase in MM incidence over the study period. In China, incidence increased more sharply in males (4.29%, 95% CI: 4.06–4.51%) compared to females (2.70%, 95% CI: 2.45–2.95%). Notably, younger age groups in Russia were the exception to the rising trend observed across all other age groups. In China, local drifts for those aged 20 to 44 exceeded the net drift, suggesting a trend toward earlier onset of MM. Conversely, Russia appeared a notable rise in local drift among individuals aged 66 to 95 (Fig. 4 and Supplementary Table S1).
Regarding MM mortality, China again led with the highest annual increase (2.54%, 95% CI: 2.36–2.72%). The net drifts were comparable in Russia, India, and South Africa, while Brazil recorded the lowest net drift (0.84%, 95% CI: 0.51–1.18%) (Supplementary Table S2). Local drifts across China, South Africa, Brazil, and India were all above zero, indicating worsening MM mortality. Russia experienced the most significant reduction in mortality among those aged 20 to 40, whereas the fastest increases were seen in Chinese teenagers and elderly Russians (Fig. 4 and Supplementary Table S2). The rate of mortality increased in Russian females over 75 was notably lower than in males.
APC effects in BRICS
In Figs. 5 and 6, the estimates of age, period and cohort effects on MM incidence and mortality, revealing fluctuating trends among BRICS populations. The age effect showed a general upward trend in MM incidence and mortality with advancing age across BRICS countries, with the most pronounced increases observed in Brazil and South Africa, where the age effect accelerates significantly in the older population. In contrast, Russia exhibits a notable decrease in MM incidence and mortality among the elderly, suggesting a significant reduction in MM risk and mortality in this age group.
Fig. 5.
The age, period, and cohort effects on MM incidence across BRICS countries. Note: MM: Multiple Myeloma; BRICS: Brazil, Russia, India, China, and South Africa
Fig. 6.
The age, period, and cohort effects on MM mortality across BRICS countries. Note: MM: Multiple Myeloma; BRICS: Brazil, Russia, India, China, and South Africa
Improvements in MM incidence and mortality related to the period effect was observed only in the early period in Russia and the late period in South Africa. In Russia, the period effect decreased before 2002 but increased rapidly after 2006. In South Africa, the trends remained nearly stable throughout the study period. In India, the trends stabilized from 1992 to 2006 but began rising again after 2006. In China, the period effects showed improvement during 2002–2006, while the rest of the past two decades exhibited an adverse trend.
In China, the incidence and mortality rate ratios surged in cohorts born after 1952. In contrast, Russia saw a mild decline in these ratios among birth cohorts from 1982 onward. In India, South Africa, and Brazil, the rate ratios of MM incidence and mortality experienced mild increases over time. However, favourable improvements were observed in older cohorts across India, South Africa, China, and Brazil.
BAPC of MM in BRICS
To predict future trends in the disease burden of MM across BRICS in the coming years, BAPC analysis was conducted, with the results summarized in Fig. 7. Generally, the ASIR and ASMR in 2046 are predicted to be 2.44 per 100,000 and 1.82 per 100,000, respectively, continuing to rise across the BRICS nations. In Fig. 7, the ASIR and ASMR of South Africa are expected to show a continuous downward trend, while the rates in the other BRICS countries are projected to rise. Compared to 2021, India’s ASIR and ASMR are predicted to increase by 54.1% and 34.8%, respectively, by 2046. In contrast, Brazil’s ASMR is expected to see the smallest increase, at just 2.7%.
Fig. 7.
Trends in MM incidence and mortality in BRICS with observed (dashed lines) and predicted (solid lines) data from 1992 to 2046. Note: MM: Multiple Myeloma; BRICS: Brazil, Russia, India, China, and South Africa
Discussion
This study highlights the epidemiological landscape of MM across the BRICS nations, showing disparities in incidence and mortality from 1992 to 2021. BRICS countries accounted for a substantial proportion of global MM cases and deaths. During this period, MM incidence and mortality cases in the BRICS nations increased nearly four to fivefold, with the ASMR and ASIR of MM nearly doubled, considerably higher than the global rates. Factors driving this rapid increase warrant further investigation. Age-period-cohort analysis showed that MM incidence and mortality rose with age, especially among those over 40. Higher MM risks were observed in recent decades, particularly in China’s later birth cohorts (post-1970s). Both global and BRICS data indicated a growing MM burden since 1992, projected to continue increasing.
Our study shows that China and India, despite having over half of BRICS MM cases and deaths due to large populations, have lower ASIR and ASMR than other BRICS nations, aligning with global data indicating lower MM incidence in Asians [17]. Over the past three decades, South Africa consistently recorded the highest ASIR and ASMR among the BRICS. Given that 80% of South Africa’s population is Black, which corresponds to global patterns showing a higher MM incidence among Blacks [18], underscoring the influence of sociocultural and environmental factors [19]. Recent evidence suggests that MM incidence rates are increasing in some Asian countries such as South Korea and Taiwan [20, 21]. Our findings also showed a rapid rise in the incidence and mortality of MM both in China and India, likely connected with aging populations, changing lifestyles, and healthcare practices [20, 21]. The limited availability of new therapies in South Africa’s public health sector reflects broader challenges faced by many emerging economies in providing advanced care for MM patients [22]. Racial and economic disparities in BRICS countries may affect health equity [23, 24]. COVID-19 did not significantly impact MM ASIR and ASMR in 2020–2021, consistent with research showing stable age-standardized MM death rates during the pandemic [25]. Though some studies report a decline in incidence due to disruptions in diagnosis and treatment [26–28], highlighting the need for further data to confirm these trends.
APC analysis shows that MM incidence and mortality increase with age across BRICS countries, rising after age 40 and peaking between 70 and 80. This trend is consistent with both developed (such as the US, Canada, and New Zealand) and developing nations [29–32]. However, Russia shows a less pronounced increase with age. Notably, Brazil and South Africa showed a continuously increasing MM risk among individuals in their 80s and older, likely influenced by socioeconomic constraints and higher rates of delayed MM diagnosis [5, 33]. Limited access to modern therapies like ASCT and bortezomib in public hospitals further impacts treatment outcomes [34]. Moreover, the elderly have poorer tolerance to anti-tumor therapies and are more susceptible to treatment-induced toxicities, leading to poorer prognoses [35].
MM is rare in the pediatric population, with less than 0.3% of cases diagnosed before age 30 [36], consistent with our findings of low MM incidence and mortality in younger generations. However, rising trends were observed in China and Brazil, particularly in China’s later birth cohorts (post-1970s), especially in males. Improvements in medical infrastructure and reporting accuracy in China may contribute to the higher reported rates of MM in newer generations [37]. This could potentially inflate the perceived risk in newly born populations, especially with the expansion of health insurance since the mid-20th century [38]. Higher APC effects on incidence and mortality were observed in Chinese males. In addition to genetic and hormonal factors, gender disparities in lifestyle and occupational exposure, such as radiation, chemicals, air and water pollution [39], likely contribute to the rising MM risk among males [40]. A similar trend has been reported in other Asian countries, such as Korea [20], Taiwan [21], and Singapore [19].
The predicted trends for MM incidence and mortality showed an upward trajectory across BRICS countries, correlating with rising life expectancy in these nations and suggesting an escalating burden of MM as populations age. South Africa is an exception, with HALE limited due to economic challenges, institutional reforms, and the ongoing public health crisis of HIV/AIDS [41, 42]. Limited medical resources are a key challenge. In other BRICS countries, especially India, where life expectancy has risen [12, 43], the predicted MM burden also shows a notable increase. Addressing this anticipated challenge requires targeted and tailored policy interventions, including enhanced early diagnosis programs, strengthened healthcare infrastructure, equitable access to advanced therapeutic options, and public health strategies focused on managing risk factors related to aging and changing lifestyles.
This study has several limitations. First, the GBD study relies on secondary data from various administrative records, which may introduce biases when comparing data across regions. Second, APC analyses primarily rely on raw data. In low-resource countries with weaker healthcare infrastructure, limited diagnostic and screening capabilities may lead to an underestimation of MM incidence and mortality. The scarcity of data from South Africa in GBD2021 further increases uncertainty in trend interpretation. Finally, the analysis does not differentiate between MM subtypes, such as MGUS, smoldering multiple myeloma (SMM), and active multiple myeloma (aMM), each of which may have distinct pathogenesis and environmental risk factors. Further research should investigate or incorporate existing studies on these MM subtypes.
Conclusion
In conclusion, this study revealed a nearly four- to fivefold increase in MM incidence and mortality in the BRICS nations over the past 30 years. The burden of MM varies across these countries, with significant disparities influenced by aging populations, socioeconomic factors, and access to healthcare. APC analysis highlights a pronounced age and cohort effect, projecting continued increases in incidence and mortality by 2046. Addressing this growing burden requires targeted, country-specific interventions tailored to distinct epidemiological and healthcare challenges within each BRICS nation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We extend our gratitude to the Institute for Health Metrics and Evaluation (University of Washington) and the GBD Collaborators, along with all the personnel who facilitated access to the essential data for our study. We also sincerely appreciate the Institute for Health Metrics and Evaluation (IHME) for their generosity in providing the GBD data utilized in this research.
Author contributions
Z.H., Y.Y.Q., Z.X.Q., W.J.L., and F.Y.J. designed the study protocol and provided overall guidance; Z.H., Y.Y.Q., and Z.X.Q. analyzed the research study; Y.Y.Q. and W.T.H. collected the data; Z.H., Z.X.Q., X.H.J. and W.J.L. validation and visualisation the data; Z.H., W.J.L., and F.Y.J. wrote and revised the manuscript. All authors have read and approved the manuscript.
Funding
This study was supported by Natural Science Foundation of Jiangsu Province (grant no. BK20220197), Jiangsu Funding Program for Excellent Postdoctoral Talent (grant no. 323721), Nanjing Medical Science and Technology Development Project (grant no. YKK22163), Nanjing Postdoctoral Research Funding Program (grant no. 323721, 310162), Science and Technology Development Foundation of Nanjing Medical University (grant no. NMUB20210063, NMUB20220015), Young Talent Support Project of Children’s Hospital of Nanjing Medical University (grant no. TJGC2021014).
Data availability
All data used in this study can be freely accessed at the GBD 2021 online database (http://ghdx.healthdata.org/gbd-results-tool).
Declarations
Ethics approval and consent to participate
This study was granted an exemption by the institutional review board, as it relied solely on publicly accessible data that did not include any confidential or personally identifiable patient information.
Informed consent
Informed consent was not needed.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Heng Zhang, Yuqian Yao and Xiaoqian Zhang contributed equally to this work and should be considered co-first authors.
Contributor Information
Jiali Wang, Email: jlwang@njmu.edu.cn.
Yongjun Fang, Email: fyj322@189.cn.
References
- 1.Sv R, Ma D, Mv APJBGM. M, International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15. [DOI] [PubMed]
- 2.Sj JSST. G. What is multiple myeloma? JAMA. 2022;327.
- 3.Aj C, C A, Mp ABHBIB. C, Global burden of multiple myeloma: A systematic analysis for the global burden of disease study 2016. JAMA Oncol. 2018;4. [DOI] [PMC free article] [PubMed]
- 4.O LS, K MMHBP. Y, J W, Multiple myeloma and physical activity: A scoping review. BMJ Open. 2015;5. [DOI] [PMC free article] [PubMed]
- 5.Mp C, Mm O, Drm S, Dlb S. Epidemiology of multiple myeloma in 17 Latin American countries: an update. Cancer Med. 2018;7. [DOI] [PMC free article] [PubMed]
- 6.O L, Ra K, Rm P, Ja K, Ne C, Rb H et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood. 2009;113. [DOI] [PMC free article] [PubMed]
- 7.Ra K, Tm T, Sv R, Dr L, Mf P Jr. O, Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354. [DOI] [PubMed]
- 8.C RMDM et al. T, S S, W L, S R,. An assessment of progress towards universal health coverage in brazil, russia, India, China, and south africa (BRICS). Lancet (London, England). 2014;384. [DOI] [PMC free article] [PubMed]
- 9.A H, F F. The BRICS countries: A new force in global health? Bull World Health Organ. 2014;92. [DOI] [PMC free article] [PubMed]
- 10.P R, A P, K B, T H. Health system outcomes in BRICS countries and their association with the economic context. Front Public Health. 2020;8. [DOI] [PMC free article] [PubMed]
- 11.Sl SA, D L-A, N B. M, L M, J M, BRICS and global health. Bull World Health Organ. 2014;92. [DOI] [PMC free article] [PubMed]
- 12.GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the global burden of disease study 2021. Lancet (lond Engl). 2024;403:2133–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The intrinsic estimator for age. -period‐cohort analysis: what it is and how to use It1| american journal of sociology: vol 113, no 6. https://www.journals.uchicago.edu/doi/10.1086/587154. Accessed 13 Mar 2025.
- 14.Knoll M, Furkel J, Debus J, Abdollahi A, Karch A, Stock C. An R package for an integrated evaluation of statistical approaches to cancer incidence projection. BMC Med Res Methodol. 2020;20:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rue H, Martino S, Chopin N. Approximate bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. 10.1111/j.1467-9868.2008.00700.x
- 16.Ginestet C. ggplot2: elegant graphics for data analysis. J Royal Stat Soc Ser A: Stat Soc. 2011;174:245–6. [Google Scholar]
- 17.Q LZ. Y, G W, L W, Y H, K H, Measuring the global, regional, and National burden of multiple myeloma from 1990 to 2019. BMC Cancer. 2021;21. [DOI] [PMC free article] [PubMed]
- 18.Ow MBSR. B. Myeloma and race: A review of the literature. Cancer Metastasis Rev. 2003;22. [DOI] [PubMed]
- 19.Jh KK, Js L, Ck K, Ss M. Y, K S, Clinical profiles of multiple myeloma in asia-an Asian myeloma network study. Am J Hematol. 2014;89. [DOI] [PubMed]
- 20.Jh L, Ds L, Jj L, Yh C, Jy J, Dy J et al. Multiple myeloma in Korea: past, present, and future perspectives. Experience of the Korean multiple myeloma working party. Int J Hematol. 2010;92. [DOI] [PubMed]
- 21.Sy H, M Y, Jl T, Wc L, W T, Al C et al. Epidemiology of multiple myeloma in Taiwan: increasing incidence for the past 25 years and higher prevalence of extramedullary myeloma in patients younger than 55 years. Cancer. 2007;110. [DOI] [PubMed]
- 22.Lm M. S C, T W. Cost utility and budget impact analysis of dexamethasone compared with bortezomib and Lenalidomide for the treatment of second line multiple myeloma from a South African public health perspective. Cost effectiveness and resource allocation: C/E; 2022. p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rd SA, M FPAAS. T, R M, Racial disparity in utilization of therapeutic modalities among multiple myeloma patients: A SEER-medicare analysis. Cancer Med. 2017;6. [DOI] [PMC free article] [PubMed]
- 24.Ma F, Tm W. Racial disparities in treatment use for multiple myeloma. Cancer. 2017;123. [DOI] [PMC free article] [PubMed]
- 25.Fedeli U, Barbiellini Amidei C, Han X, Jemal A. Changes in mortality associated with different hematologic malignancies during the pandemic in the united States. Int J Cancer. 2024;154:1703–8. [DOI] [PubMed] [Google Scholar]
- 26.Mousavi SE, Ilaghi M, Aslani A, Yekta Z, Nejadghaderi SA. A population-based study on incidence trends of myeloma in the united States over 2000–2020. Sci Rep. 2023;13:20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Lopez J, Hernandez-Ibarburu G, Alonso R, Sanchez-Pina JM, Zamanillo I, Lopez-Muñoz N, et al. Impact of COVID-19 in patients with multiple myeloma based on a global data network. Blood Cancer J. 2021;11:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carmichael J, Seymour F, McIlroy G, Tayabali S, Amerikanou R, Feyler S, et al. Delayed diagnosis resulting in increased disease burden in multiple myeloma: the legacy of the COVID-19 pandemic. Blood Cancer J. 2023;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mj S, Im BC. M. Trends in myeloma incidence, mortality and survival in new Zealand (1985–2016). Cancer Epidemiol. 2019;60. [DOI] [PubMed]
- 30.Turesson I, Bjorkholm M, Blimark CH, Kristinsson S, Velez R, Landgren O. Rapidly changing myeloma epidemiology in the general population: increased incidence, older patients, and longer survival. Eur J Haematol. 2018. 10.1111/ejh.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Y Z, D N, E Y. J H, J W, X H, Secular trends in the burden of multiple myeloma from 1990 to 2019 and its projection until 2044 in China. Front Public Health. 2022;10. [DOI] [PMC free article] [PubMed]
- 32.Dt Z, A P, L AL, Tr ZHA. R. Multiple myeloma incidence and mortality trends in the united States, 1999–2020. Sci Rep. 2024;14. [DOI] [PMC free article] [PubMed]
- 33.Lh C, I M. N R. Profile and outcome of multiple myeloma with and without HIV treated at a tertiary hospital in KwaZulu-natal, South Africa. PLoS ONE. 2023;18. [DOI] [PMC free article] [PubMed]
- 34.Tarín-Arzaga L, Arredondo-Campos D, Martínez-Pacheco V, Martínez-González O, Ramírez-López A, Gómez-De León A, et al. Impact of the affordability of novel agents in patients with multiple myeloma: Real-world data of current clinical practice in Mexico. Cancer. 2018;124:1946–53. [DOI] [PubMed] [Google Scholar]
- 35.Wildes TM, Rosko A, Tuchman SA. Multiple myeloma in the older adult: better prospects, more challenges. J Clin Oncol. 2014;32:2531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ra JB, Pr K. G. Multiple myeloma in patients younger than 30 years. Report of 10 cases and review of the literature. Arch Intern Med. 1996;156. [DOI] [PubMed]
- 37.C JLWLLMXZ. C, J M, Incidence and mortality of multiple myeloma in China, 2006–2016: an analysis of the global burden of disease study 2016. J Hematol Oncol. 2019;12. [DOI] [PMC free article] [PubMed]
- 38.Cohen AK, Syme SL, Education. A missed opportunity for public health intervention. Am J Public Health. 2013;103:997–1001. [DOI] [PMC free article] [PubMed]
- 39.van de Donk NWCJ, Pawlyn C, Yong KL. Multiple myeloma. Lancet (London England). 2021;397:410–27. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Mauzerall DL, Zhu T, Liang S, Ezzati M, Remais JV. Environmental health in China: progress towards clean air and safe water. Lancet (London England). 2010;375:1110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosn J, Taiwo B, Seedat S, Autran B, Katlama C, Hiv. Lancet (London England). 2018;392:685–97. [DOI] [PubMed]
- 42.GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022–2050: A forecasting analysis for the global burden of disease study 2021. Lancet (London England). 2024;403:2204–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pk DJ, Mr S, Pk T. S. Statistical modeling and estimating number of healthy life years lost and healthy life expectancy in India, 2000–2019. Aging medicine (Milton (NSW)). 2023;6. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study can be freely accessed at the GBD 2021 online database (http://ghdx.healthdata.org/gbd-results-tool).