ABSTRACT
The insect microbiome is comprised of extracellular microbial communities that colonize the host surfaces and endosymbionts that reside inside host cells and tissues. Both of these communities participate in essential aspects of host biology, including the immune response and interactions with pathogens. In recent years, our knowledge about the role of the insect microbiome in infection has increased tremendously. While many studies have highlighted the microbiome’s protective effect against various natural enemies of insects, unexpected discoveries have shown that some members of the microbiota can facilitate pathogenic infections. Here, we summarize studies in the fruit fly, Drosophila melanogaster, that have substantially progressed our understanding of host-pathogen-microbiome interactions during infection. We summarize studies on the protective mechanisms of Drosophila gut microbiota, highlight examples of microbiome exploitation by pathogens, and detail the mechanisms of endosymbiont-mediated host protection. In addition, we delve into a previously neglected topic in Drosophila microbiome research—the crosstalk between endosymbionts and gut microbiota. Finally, we address how endosymbionts and gut microbiota remain resilient to host immune responses and stably colonize the host during infection. By examining how the microbiome is influenced by and reciprocally affects infection outcomes, this review provides timely and cohesive coverage of the roles of Drosophila endosymbionts and gut microbiota during infections.
KEYWORDS: gut microbiota, infectious disease, endosymbionts, host-microbe interactions, Drosophila
INTRODUCTION
Occupying the interface between host and environment, host-associated microbes play essential roles in interactions with pathogens and influence disease progression (1–4). Considering that most entry sites for pathogens into the host organism are colonized with microbiota (5), pathogen-commensal interactions are an inevitable and fundamental aspect of the disease.
The importance of such interactions is exemplified by colonization resistance—a concept of protection of the host from pathogens by commensal microbes (6–9). Colonization resistance is a widely observed phenomenon in many organisms and can be direct or indirect. Direct colonization resistance occurs when intestinal microbiota directly suppresses the pathogen via nutrient competition or secretion of antimicrobial molecules, like bacteriocins or organic acids (10–16). Indirect colonization resistance occurs when commensals protect the host by modulating the intestinal immune responses to increase the resistance to infection. One mechanism of modulation is the induction of the expression of intestinal anti-microbial C-type lectins (17–20). The protective role of commensals is well studied, and a plethora of underlying mechanisms have been identified. However, studies of different systems have implied that microbiota may take on a pathogenic rather than protective role, where they promote intestinal infections (1, 21–23).
Commensals and their derived signals and nutrients can be hijacked by various enteric pathogens to promote disease and coordinate the expression of the virulence repertoire (1, 4).
The metabolic interplay between microbiota and bacterial pathogens is frequently identified as an underlying cause of altered virulence of a bacterial community. For example, Salmonella enterica ser. Typhimurium feeds on microbiota-derived hydrogen to invade the gut ecosystem (24). Pathogens can also make use of host-derived metabolites liberated by commensals to modulate pathogenesis. For instance, Bacteroides thetaiotaomicron releases fucose and sialic acid from host glycans and produces high levels of succinate, which are necessary for pathogen expansion in the mammalian intestine and are sensed by enterohemorrhagic Escherichia coli (EHEC), S. Typhimurium, and Clostridium (25–27). Moreover, a recent study showed that B. thetaiotaomicron, through the digestion of dietary pectin, releases galacturonic acid which is used by EHEC and Citrobacter rodentium in the gut as a carbon source, aiding pathogens’ initial expansion (28). Beyond providing nutrients to pathogens, commensal-derived metabolites can signal the regulation of virulence factor production and ultimately affect the progression of disease. For example, microbiota-produced ethanolamine is used as a nitrogen source and a regulator of virulence genes by EHEC, S. Typhimurium, and Listeria monocytogenes (29–32). Similar effects were reported for a number of other metabolites, like succinate, acetate, butyrate, and taurocholate (33–38).
Beyond these few exceptions, we are still largely in the dark when it comes to the molecular mechanisms of how commensals facilitate infections.
Given the complexity of animal microbiota, one of the main challenges is to identify the microbiota member or community implicated in positive or negative interactions with a particular pathogen. Since the vast majority of mammalian microbiota remains uncultivable and unamenable to genetic manipulation, functional validation of suspected interactions is not experimentally feasible, and many studies remain correlative.
The fruit fly, Drosophila melanogaster, with its extensive genetic toolkit, evolutionary-conserved innate immune defense, and genetically tractable microbiome, represents an ideal model to address an ambitious question of the mechanistic role of commensals in facilitating intestinal infections (39–44).
Several distinct attributes of Drosophila microbiota stand behind the successful use of the fruit fly model in microbiome research. Genetic tractability and cultivability of the Drosophila microbiota members combined with the simple taxonomic composition allow functional studies of the molecular mechanisms of commensal-host interactions (41, 45–47). The investigation into the host component of these interactions is facilitated by a wealth of genetic, genomic, and molecular resources available in Drosophila (48, 49). Another particular advantage of the fruit fly model is the simplicity of generating and maintaining germ-free, or axenic, animals. Moreover, gnotobiotic animals colonized with a defined microbiota can be generated easily (45, 49).
Due to these exceptional features, Drosophila models have been widely utilized to investigate the effect of host-associated microbes on host physiology, including interactions with pathogens (23, 47, 49–54).
This review aims to provide timely and unified coverage of the role of Drosophila gut microbiota and endosymbionts in infection.
COMPOSITION AND ESTABLISHMENT OF DROSOPHILA MICROBIOTA
In laboratory and field settings, Drosophila melanogaster is colonized by relatively simple microbial communities comprising 2–30 species, belonging to the Proteobacteria and Firmicutes phyla. These communities are represented by two dominant families Acetobacteraceae and Lactobacillaceae, and by minor families Enterococceae and Enterobacteriaceae (55–60). The following species are the most consistently associated with flies across studies: Lactiplantibacillus plantarum, Levilactobacillus brevis, Acetobacter pomorum, A. pasteurianus, and Enterococcus faecalis (42, 46, 47, 49, 61, 62). This community, rich in lactic acid and acetic acid bacteria, reflects the fermentative substrates consumed by flies (42, 63). The Drosophila microbiota composition is significantly affected by the fly diet; the continuous ingestion of microbes from the food is crucial for the establishment and maintenance of intestinal commensals in Drosophila. The majority of fruit fly intestinal commensals cannot stably colonize the gut and must be regularly reintroduced through re-ingested food (61, 64, 65).
Newly emerged flies initially harbor a low number of microbes in their gut. However, within the first day of their adult life, these flies acquire microbiota by consuming bacteria from food contaminated with their parents’ feces (61, 64). Moreover, female flies pass on their microbiota to their offspring by depositing microbes on the eggshells. Upon hatching, larvae become colonized by ingesting the bacteria-rich eggshell and feeding on the microbe-laden food (62, 65). These interactions among D. melanogaster, microbiota, and nutrition likely contribute to the significant variability in microbiota composition and density observed among individual flies raised in the same culture vial (66, 67). The bacterial load can differ by up to one logarithmic unit between flies cohabiting in the same environment (61). Furthermore, flies that are frequently transferred to sterile food, preventing re-ingestion of microbes with the diet, can lose their microbiota and become germ-free (64, 68). Further importance of the food substrate in Drosophila-microbiota interactions is illustrated by the finding that the diet, rather than the host, is the major force driving the evolution of symbiotic properties of the prominent fly commensal L. plantarum (69).
While the transitory association between Drosophila and its commensals holds true for bacterial isolates from Drosophila laboratory stocks, some of the bacteria isolated from wild-caught D. melanogaster can stably persist and proliferate in the gut. Such stable association confers fitness advantages for both partners in an ecological context (68). Recent studies started to address the mechanisms of microbial stable colonization using L. plantarum isolated from a wild-caught fly, which persists in the Drosophila foregut. Dodge et al. discovered a precise, spatially defined, physical niche within the adult Drosophila foregut, including the proventriculus, the crop, and the crop duct that is specifically colonized by wild strains of Lactobacillus (70). Subsequent work demonstrated that L. plantarum colonizes its niche through host-specific serine-rich repeat protein adhesins encoded by genes carried on a colonization island (71, 72). Beyond uncovering the basis of niche-specific colonization, this work together with the other studies highlights the importance of intra-strain variation in conferring the host phenotypes (67, 68, 73, 74). Differences among microbial strains are often neglected and could explain some of the conflicting results in the field (75).
BENEFICIAL AND DETRIMENTAL ROLE OF DROSOPHILA MICROBIOTA DURING INFECTIONS
Drosophila microbiota affects essentially every aspect of the host physiology, including development, behavior, lifespan, and disease resistance. While these topics were reviewed previously (46, 47, 54, 62, 76), here we would like to focus specifically on the microbiome’s role during infection—an emerging topic that received little attention in previous reviews.
Consistent with the well-described protective role of host microbiota in different organisms against pathogens and parasites, some Drosophila-associated commensals exhibit a defensive role. For instance, Blum et al. (64) demonstrated that L. plantarum improved Drosophila survival in the presence of pathogens Pseudomonas aeruginosa or Serratia marcescens, but the mechanism of this protection has not been explored. A recent study proposed that gut microbiota may protect the host from invasive microbes through environmental acidification. Specifically, the production of lactic acid by L. plantarum through lactate dehydrogenase creates an acidic environment that inhibits the growth of invasive pathogens (77). Lactic acid produced by L. plantarum was also implicated in the inhibition of and fly protection against a newly described fungus: Diaporthe FY (78). Acetobacter pomorum might similarly protect flies via acetic acid-mediated inhibition of fungi (79). This mechanism could potentially explain the increased survival likelihood of microbiota-colonized as compared to axenic Drosophila larvae after Candida albicans infection (80). Thus, acid-mediated pathogen inhibition by the microbiota emerges as a gatekeeper against invading pathogens in Drosophila (81) (Fig. 1).
Fig 1.
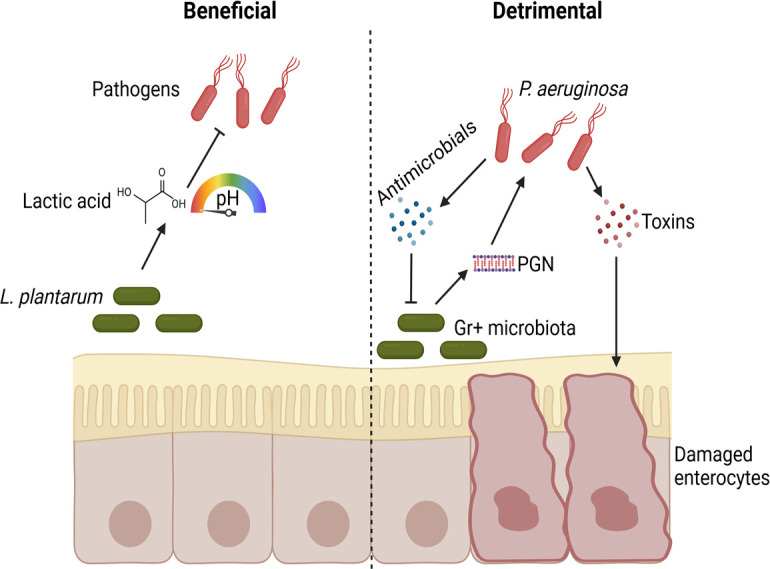
Examples of beneficial and detrimental effects of the microbiota during infection in Drosophila. Beneficial: L. plantarum produces lactic acid, which acidifies the environment and specific regions of the Drosophila gut, inhibiting pathogen growth. Detrimental: peptidoglycan (PGN) released by Gram-positive microbiota activates toxin and antimicrobial production in P. aeruginosa, leading to epithelial damage and suppression of the microbiota. Created in BioRender. Iatsenko, I. (2025) https://BioRender.com/b61j017
In addition to gut microbiota, Drosophila surface-associated microbes can protect the flies against fungal infections. While the Drosophila surface microbiota remains underexplored, several studies have demonstrated that fly external surfaces serve as battlegrounds for microbiota-pathogen competition. Hong et al. showed that surface bacteria could defend flies against fungal infections. L. plantarum specifically was shown to antagonize fungal spore germination and significantly delay fungal infection of axenic flies (82). Intriguingly, fungus that lacks defensin-like antimicrobial gene BbAMP1 and is thus not able to inhibit insect surface microbiota was impaired in virulence in gnotobiotic but not in axenic flies (83). This finding suggests that the ability of fungi to compete with microbiota is essential for virulence. In line with this, the entomopathogenic fungus Metarhizium robertsii, engineered to express the antibacterial moricin gene, showed a substantially enhanced ability to kill insects. This effect was due to the ability of fungus to suppress insect cuticular bacteria and to disrupt the gut microbiome. Specifically, an overgrowth and translocation to the hemolymph of the opportunistic pathogens of Providencia species was detected and shown to contribute to insect death (84). A very similar scenario was described in mosquitoes: fungal infection with Beauveria bassiana caused dysbiosis of mosquito gut microbiota. In particular, overgrowth of the opportunistic pathogenic bacterium Serratia marcescens in the midgut and translocation to the hemocoel were identified as leading causes of mosquito death (85). These two examples with fungal infections illustrate that microbiota might be exploited by some pathogens rather than serve as a protective barrier. Cases of infection facilitation in flies by microbiota were also observed with bacterial pathogens. For example, Pseudomonas aeruginosa virulence was increased by the microbiota in flies, resulting in increased death of microbiota-colonized versus axenic flies. Mechanistically, P. aeruginosa senses peptidoglycans shed by gram-positive bacteria and responds to this cue through the enhanced production of virulence factors (86) (Fig. 1). The pathogenesis of Vibrio cholerae was also increased by microbiota in Drosophila models (52). Specifically, Fast et al. found that Vibrio cholerae T6SS contributes indirectly to Drosophila death. T6SS-dependent killing of the host required the presence of gut commensal Acetobacter pasteurianus (87). Subsequent work showed that V. cholerae causes damage to the midgut epithelium and prevents compensatory epithelial renewal. This destruction is dependent on microbiota, as elimination of the intestinal commensals restores epithelial renewal capacity in infected intestines (88). Collectively, these findings suggest that microbiota facilitate V. cholerae infection by enabling T6SS-dependent inhibition of epithelial renewal.
Given that Drosophila microbiota modulates several immune and repair signaling pathways in the gut, including the generation of reactive oxygen species (ROS), immune deficiency (IMD) pathway, Janus kinase/Signal transducers and activators of transcription (JAK/STAT) pathway, and c-Jun NH2-terminal kinase (JNK) pathway (43, 44, 76, 89), it is very likely that microbiota impact the outcome of infections by regulating these pathways. For instance, the altered susceptibility of axenic flies to infections could be due to dampened immune or repair pathway activation. Such an indirect role of microbiota in infection via alteration of the host is well established in other models but still needs to be investigated in Drosophila.
THE ROLE OF DIET IN DROSOPHILA INTERACTIONS WITH MICROBIOTA AND PATHOGENS
Diet is a well-recognized factor affecting host physiology and host interactions with pathogens and microbiota (90, 91). The diet composition was shown to have a major impact on the structure and abundance of fly microbiome (92, 93). For instance, an increase in yeast concentration led to a substantial increase in the total abundance of gut microbes but decreased their alpha diversity (94). Another study associated a yeast-rich diet with a high abundance of Enterobacteriaceae (63). By contrast, studies that explored the effect of high-sugar diets on the microbiome reported an increase in microbiota diversity (95) and a high prevalence of Providencia species (63). Raising flies on a diet supplemented with casein shifted the microbiota composition to predominantly Lactobacillus species (95)
Several studies that investigated the effect of a high-fat diet on microbiota reported an overall increase in gut microbiota abundance when flies were fed fat-rich food (96–98). The dominant species, however, differ among studies. Wang et al. found that a high-fat diet significantly increased the abundance of Acetobacter malorum in the gut (96), while von Frieling detected significant enrichment of orders Enterobacteriales and Caulobacterales upon high-fat diet feeding (97).
Diet composition is also known to affect Drosophila’s susceptibility to infections (99). For example, flies fed high-sugar diets are more susceptible to infections by the Gram-negative pathogens Providencia rettgeri and Serratia marcescens (100, 101). Yeast-rich diets are often associated with the increased survival of insects after infection (102), while protein shortage has been reported to negatively affect survival after infections (103, 104). These dietary interventions were shown to affect the susceptibility to infections by altering the host defenses. The potential role of microbiota in mediating dietary effects on infection outcomes was mostly neglected. However, given that diet influences microbiota and host susceptibility to infections, diet, besides its direct impact on the host, might affect infection susceptibility indirectly by altering host microbiota. The link between diet-induced changes in fly microbiome and susceptibility to infections remains to be demonstrated. Furthermore, the impact of diet on microbiota complicates comparisons between different studies that utilized different media. For example, microbiota was reported to protect flies against (64) but also support the infection with the pathogen P. aeruginosa (86). Such discrepancies could be attributed to diet-driven variations in fly microbiota between labs. Therefore, the importance of diet in Drosophila-microbe interactions should not be underestimated, and precise descriptions of diet composition and microbial strains should always be reported.
DEFENSIVE ENDOSYMBIONTS OF FRUIT FLIES
In addition to microbial communities colonizing the gut and external surfaces, insects frequently harbor endosymbionts: symbiotic microbes that live inside host cells or tissues. Wolbachia and Spiroplasma are the only known endosymbionts of Drosophila (59, 105, 106). Wolbachia are intracellular Alphaproteobacteria that are transmitted vertically through host eggs and cause reproductive manipulations (107, 108). Spiroplasma are gram-positive, helical bacteria devoid of cell walls, which belong to an ancient lineage of host-associated Mollicutes (105, 109, 110). Spiroplasma live extracellularly in the host hemolymph where they feed on host lipids (111, 112). One of the most prominent phenotypes conferred by Wolbachia and Spiroplasma is host protection against natural enemies (113–115). wMel, the Wolbachia strain present in Drosophila melanogaster, provides strong protection against multiple RNA viruses, which could offer a fitness benefit in nature (116–118). Wolbachia’s antiviral properties are exploited in the control of dengue and Zika virus transmission by mosquito vectors (119). The release of Wolbachia-infected Aedes aegypti mosquitoes was shown to reduce the number of dengue cases in affected areas of the world (120, 121). Although the molecular mechanisms of Wolbachia-mediated antiviral protection remain unknown, the following mechanisms have been proposed: immune priming (122), increased ROS production (123), and competition for resources between symbiont and pathogen (124, 125). In addition, several Wolbachia strains with different degrees of protection have been isolated. Generally, strains that reached higher titers in the host conferred higher antiviral protection (126, 127), demonstrating a correlation between Wolbachia abundance and protection. However, further mechanistic studies of Wolbachia-mediated protection remain challenging as this endosymbiont cannot be cultured outside of host cells or genetically manipulated. Although Wolbachia-mediated antiviral protection attracted a lot of attention, the potential role of Wolbachia in interactions with other types of pathogens, including fungi and bacteria, has been little studied. A recent study demonstrated that Wolbachia can confer protection against several but not all tested fungal and yeast pathogens. Host sex, genetics, and pathogen species were identified as significant determinants of each infection outcome (128). Further research is required to understand the variable ability of Wolbachia to inhibit fungal pathogens and the mechanistic basis of antifungal protection. Regarding antibacterial protection, several studies reported no significant effect of Wolbachia on Drosophila survival or ability to control pathogen growth after systemic infections with different bacteria (129, 130). One study, however, demonstrated that Wolbachia-carrying flies exhibited reduced mortality after enteric but not systemic infection with Pseudomonas aeruginosa (131). Thus, the route of infection is an important determinant of Wolbachia antibacterial protection in Drosophila. It will be important to investigate how such protection is achieved and how common it is among different endosymbionts.
Another endosymbiont of Drosophila, Spiroplasma poulsonii, has attracted research interest for its ability to protect flies against parasites such as nematodes and wasps (132). The protective effect against nematodes was initially observed in Drosophila neotestacea, a species commonly infected by the generalist nematode Howardula aoronymphium. Flies carrying a specific strain of S. poulsonii exhibit resistance to nematode infections, quickly outcompeting their symbiont-free counterparts in natural populations throughout North America (133). Notably, only the indigenous S. poulsonii strain from D. neotestacea demonstrated efficacy in shielding flies from nematode invasion. Strains originating from other hosts failed to provide protection, indicating variations in the defensive capabilities of S. poulsonii strains.
Moreover, multiple strains of S. poulsonii derived from D. hydei, D. melanogaster, and D. neotestacea have been documented to protect against parasitoid wasps from diverse lineages (134, 135). The toxins generated by S. poulsonii play a pivotal role in host defense against both nematodes and wasps (132, 136–138). Specifically, Spiroplasma produces ribosome-inactivating proteins (RIP) that target nematode 28S rRNA, disrupting an essential adenine base in a crucial loop structure necessary for translation initiation. This action irreversibly hampers protein synthesis, instigates apoptosis, and ultimately leads to necrosis. In vitro experiments with purified Spiroplasma RIP toxin showed depurination of nematode rRNA, while nematodes within Spiroplasma-infected flies exhibited pronounced signs of RIP-induced rRNA modification (137, 139). Apart from toxin-mediated parasite eradication, the competition between Spiroplasma and wasps for host lipids has been identified as a significant fly defense mechanism (140). Recently, we expanded the known defensive spectrum of Spiroplasma beyond wasps and nematodes by revealing a previously unappreciated role of Spiroplasma in host protection against bacterial and fungal pathogens (141). We identified Transferrin-mediated iron sequestration (142) and enhanced melanization (143) induced by Spiroplasma as a key mechanism underlying protection. Beyond protection, we discovered that symbiont-harboring flies were more susceptible to systemic infection with a specific pathogen, Pseudomonas entomophila (141). A negative effect of Spiroplasma on fly survival was also reported for infections with E. carotovora and Enterobacter cloacae (112). These cases illustrate that symbionts, similar to some gut commensals, might facilitate certain infections. Thus, specific pathogens might benefit from the symbiont-induced alterations in host physiology. Given that flies can simultaneously harbor both Spiroplasma and Wolbachia, it will be important to investigate how the two endosymbionts together affect the host’s ability to fight infections. One study that attempted to address this question reported that flies carrying both Wolbachia and Spiroplasma, and those containing single symbionts only had similar survival rates after infection with P. luminescens or Escherichia coli bacteria (144). Future studies should expand the spectrum of tested pathogens and include cases where the protective effect of either symbiont is known. For example, whether the antiviral effect of Wolbachia will be affected by Spiroplasma should be investigated. Considering recent advances in Spiroplasma transformation and in vitro culture (145, 146), with further methodology developments, this endosymbiont holds great promise of becoming fully genetically tractable. Combined with the power of fruit fly genetics, a Drosophila-Spiroplasma model might offer unique genetic manipulation opportunities and the ability to study both partners involved in endosymbiosis.
CROSSTALK BETWEEN ENDOSYMBIONTS AND GUT MICROBIOTA
The Drosophila holobiont contains two groups of microbes: endosymbionts (Wolbachia and Spiroplasma) residing in host cells and tissues, and extracellular host-associated microbes colonizing the gut and other host surfaces. While endosymbionts and extracellular microbes collectively form the host’s microbiota, they are often treated and studied as distinct entities. Historically, the research on host-microbiota interactions has been focused on binary interactions either between host and symbiont or between host and microbiota. While such a reductionist focus provided important insights into the role of symbionts and microbiota in host physiology, it neglected interactions between endosymbionts and microbiota and how these interactions impact the host and interacting microbes (147). A holistic approach that covers the interactions between symbionts, host, and the remaining microbiota is challenging as it requires a model where the elimination of endosymbionts is possible without affecting the microbiota and vice versa. The fruit fly is one such model that has been used to study the interactions between endosymbionts and gut microbiota.
Several studies investigated the impact of Wolbachia on gut microbiota in Drosophila and other insects. In most cases, the presence of Wolbachia was correlated with reduced taxonomic diversity of microbiota and changes in the abundance of certain microbiota members. For example, Ye et al. reported that the presence of Wolbachia not only reduced microbiome diversity in the fly gut without affecting the total bacterial quantity but also led to the increase in abundance of Leuconostocaceae and Acetobacteraceae families (148). Recent work similarly reported that Wolbachia promotes extracellular microbiome growth. Specifically, colony-forming units of Acetobacter and Lactobacillus species in the presence of Wolbachia were 7.07-fold and 9.78-fold higher compared to symbiont-free flies (149). Another study, however, reported the opposite effect and found that Wolbachia bacteria reduce the proportion of Acetobacteraceae and specifically A. pasteurianus levels in gnotobiotic organisms (150). The abundance of Proteobacteria, especially Acetobacter, was reduced in the Wolbachia-infected Drosophila nigrosparsa, while those of Bacteroidetes and Actinobacteria were significantly increased (151). While the reduced diversity of resident bacteria in the presence of Wolbachia is consistent across studies and insects, including Nilaparvata lugens (152), Aedes aegypti (153, 154), Sogatella furcifera (155), and D. melanogaster (156), abundance even of the same microbiota species is differently affected across studies. Such discrepancies could be due to variables that differed among studies, including environment, host, and symbiont genetics. Thus, more controlled investigations are needed to conclusively establish the relationships between Wolbachia and intestinal microbial communities as well as the mechanisms that regulate them.
A few publications also aimed to address a reciprocal question: what is the effect of microbiota on Wolbachia abundance? Again, there was no clear consensus as the presence of gut microbiota was shown to both increase (149) and decrease (148) Wolbachia densities.
In addition, relationships between the two Drosophila endosymbionts, Wolbachia and Spiroplasma have been explored. In D. melanogaster, coinfection with the endosymbiont bacterium Spiroplasma reduced Wolbachia density, while Spiroplasma numbers remained unaffected by the presence of Wolbachia (157). In a different Drosophila species, D. neotestacea, Wolbachia abundance did not differ significantly between flies that bore or lacked Spiroplasma. Spiroplasma quantity, however, was increased in the presence of Wolbachia, indicating that Wolbachia promotes Spiroplasma populations. However, this effect is not reciprocated by Spiroplasma (158).
The increasing appreciation of symbiont-microbiota crosstalk in host physiology is evident in recent publications. Nevertheless, this field remains underexplored, with our understanding of the intricate interactions still limited. Many unanswered questions underscore the need for further exploration. Could crosstalk between endosymbionts or endosymbionts and microbiota explain some of the phenotypes conferred by microbiota or endosymbionts? Can symbionts affect the host physiology by modulating microbiota and vice versa? For example, can the protective effect of Wolbachia specifically against oral but not systemic Pseudomonas infection (131) be mediated by the Wolbachia-triggered changes in gut microbiota? Can symbionts and microbiota synergize in certain effects, like in host protection, by providing different mechanisms of protection? These are some of the exciting questions that await further investigation.
HOST-SYMBIONT HOMEOSTASIS DURING INFECTION
The host-associated microbial communities are frequently exposed to defense responses induced by pathogens. Immune defense mechanisms are often non-specific and target conserved molecular patterns present in both pathogens and symbionts, raising the question of how symbionts endure such immune responses and stably colonize the host (159, 160). In the case of many endosymbionts, spatial separation of symbionts and immune responses could explain this phenomenon. Endosymbionts live inside host cells, tissues, or specialized symbiotic organs called bacteriomes which protection the host immune effectors (161, 162). How extracellular endosymbionts, like S. poulsonii, that reside in the host hemolymph endure the action of host immune effectors remains to be investigated. Intestinal microbial communities similarly reside in an open ecosystem offering no physical barriers against the host immune molecules. Although the Drosophila gut is compartmentalized into regions, some of which are not immune responsive (163), we found that gut bacteria localize in regions with strong immune activity (164), suggesting that they do not simply avoid immune defenses. Consistent with our findings, the symbiotic niche in the Drosophila gut—the proventriculus—is a gut region preferentially colonized by microbiota (70) despite undergoing a strong immune response (163, 165). Thus, gut symbionts colonizing the niche should have mechanisms to withstand the host’s immune defenses. In our recent papers, we provided the first insights into these mechanisms. Our first finding was that Drosophila microbiota composition and abundance were not significantly affected by the host immune responses triggered by intestinal infection. One microbiome member, L. plantarum, even increased in abundance after infection (164). We used L. plantarum as a model to investigate the mechanisms of commensal resilience in an inflamed gut environment. Given that antimicrobial peptides (AMPs) are the major immune effectors in Drosophila (40), we pursued a hypothesis that intrinsic resistance to AMPs allows L. plantarum to stay in the gut during infection. Consistent with this hypothesis, in vitro experiments confirmed L. plantarum resistance to several AMPs and antibiotics resembling AMP action. In a genetic screen, we identified several L. plantarum mutants sensitive to AMPs. The identified mutants were impaired in different processes like peptidoglycan O-acetylation, teichoic acid D-alanylation, or synthesis of lysyl-phosphatidylglycerol (164, 166). All of these disruptions led to increased negative cell surface charge and higher affinity to cationic AMPs. Our subsequent in vivo experiments demonstrated that in wild-type flies, AMP-sensitive mutants were eliminated from the gut following infection. However, in AMP-deficient flies, these mutants persisted, indicating that the ability to resist host AMPs is crucial for the resilience of commensals in an infected gut environment (164, 166). Given a similar finding in human commensal Bacteroidetes (167), resistance to host AMPs might be a common mechanism of microbiota persistence during infection. These results, together with the fact that resistance to host AMPs is one of the major virulence factors of several pathogens (168, 169), suggest that host-symbiont and host-pathogen interactions are mediated by the same molecular principles (170, 171).
We found that some Drosophila gut commensals, like Acetobacter sp., are susceptible to AMPs in vitro. These findings agree with in vivo results demonstrating that the abundance of Acetobacter sp. is increased in ∆AMP mutant flies, supporting the crucial role of AMPs in controlling Acetobacter species (172). Although AMPs play a significant role in shaping the Drosophila microbiota, it has been demonstrated that the microbiota itself is the primary factor driving the evolution of Drosophila AMPs (173, 174).
In addition, non-inherited mechanisms might contribute to microbiota resilience. For example, microbiota exhibit rapid transcriptional reprogramming in response to host immune activation. Given that such transcriptional response includes upregulation of stress response-related genes, adaptation on a transcriptional level might be part of the microbiota resilience program (175). Furthermore, considering that Drosophila commensals under homeostatic conditions induce mild AMP response as compared to the pathogens (163, 165), exposure to such sub-lethal concentrations of AMPs might prime microbiota and increase tolerance to the high AMP concentration produced during infection (176). Exposure to low pH in the acidic region of the gut might also prime microbiota and increase the resistance to AMPs, as was demonstrated in Vibrio fischeri (177).
Besides AMPs, fruit flies produce additional immune effectors during infection. Specifically, reactive oxygen species (ROS) (178, 179) and iron-sequestration molecules (142) produced target commensals as well as pathogens. While the effect of ROS and iron limitation on microbiota was investigated in different systems (180–182), it remains to be studied how Drosophila microbiota is affected by and withstands these defense reactions.
FUTURE DIRECTIONS
Despite recent advances in our understanding of the role of endosymbionts and microbiota in Drosophila interactions with pathogens, there are still many outstanding questions that remain to be addressed. Specifically, in many cases, the mechanisms of Wolbachia-conferred host protection remain unknown due to genetic intractability and uncultivability of Wolbachia. Although Wolbachia can be propagated in insect cell lines (183), genetic manipulation in cell culture is technically challenging, necessitating the development of axenic (cell-free) culture. In contrast to other endosymbionts, like Spiroplasma, that were axenically cultured, Wolbachia has a strongly degenerated genome and lacks almost all biosynthetic pathways to produce amino acids de novo and has retained only incomplete pathways for the synthesis of vitamins and cofactors (108, 184). Consistent with intracellular lifestyle and strong dependence on the host, Wolbachia requires fastidious growth conditions in terms of nutritional requirements and physicochemical environment (temperature, pH, and oxygen levels). Optimization of these culture conditions in combination with novel culture techniques (e.g., microfluidics) is necessary to advance mechanistic studies of Wolbachia-host interactions.
Another aspect that deserves particular attention is the need to move beyond the bacteria-centric view of Drosophila microbiota and expand our studies to non-bacterial components of the fruit fly microbiota. For example, various yeast species are commonly isolated from the Drosophila gut and food substrates and can affect the host in multiple ways, ranging from nutrient provisioning to behavior modulation (63, 185, 186). Similar important functions were reported for the fungal microbiota of fruit flies (187). The role of these fungal and yeast communities during infections has not been investigated yet. Furthermore, the most abundant inhabitants of the animal gut, bacteriophages, remain uncharacterized in Drosophila. Besides a single metagenome-based study (188) reporting the presence of sequences from potentially novel bacteriophages that could target major gut bacteria of D. melanogaster—including Lactobacillus, Acetobacter, and Gluconobacter—the phages of the Drosophila gut microbiota await their discovery. An enticing hypothesis that remains untested is whether phages could affect Drosophila physiology, including infection outcome, by modulating the microbiota. Another important aspect that needs to be considered is the role of interspecies interactions within the microbiota of the host. Studies that investigated pathogen-microbiota interactions in Drosophila primarily used gnotobiotic animals colonized with a single specific gut microbe. While successful in many cases, such a reductionist approach likely overlooked the contribution of interactions between microbiota members to infection outcomes (79, 189). Finally, the observations that male and female flies exhibit differences in microbiota composition (190, 191), that the microbiota influences infection outcomes, and that there is sexual dimorphism in infection susceptibility (192) raise an intriguing possibility that sex differences in microbiota communities contribute to sex dimorphism in infection susceptibility. Overall, the Drosophila model of pathogen-microbiome interactions offers many exciting avenues for future investigations.
ACKNOWLEDGMENTS
We thank Camilla Regalia for proofreading the manuscript. We would like to apologize to those colleagues whose work could not be cited due to space restrictions.
This work was supported by the Max Planck Society, grants IA 81/2-1 and IA81/3-1 from the Deutsche Forschungsgemeinschaft (DFG) to I.I., an Exploration grant from the Boehringer Ingelheim Foundation to I.I., and postdoctoral fellowship from the Alexander von Humboldt Foundation to Y.Y.
Biographies

Yi Yu received his Ph.D. from the University Medical Center Groningen, University of Groningen, the Netherlands in 2021. He has been a postdoctoral fellow at the Max Planck Institute for Infection Biology, Berlin, Germany since October 2021. He was awarded a 2-year Humboldt Research Fellowship for the period 2023–2025. In his PhD projects, he was interested in the host (Drosophila melanogaster)-gut microbiome interactions, and in his current position, he is extending his interest to study host-gut microbiome-pathogen interactions. He has been working in the field of host-gut microbiome-pathogen interactions for 3 years.

Igor Iatsenko earned a BS in Biology and an MS in Microbiology at Taras Shevchenko National University of Kyiv (Ukraine). In 2014, he completed his PhD on C. elegans-microbe interactions in Ralf Sommer’s lab at the Max Planck Institute for Biology. In 2019, he completed his postdoctoral training on Drosophila-microbe interactions in Bruno Lemaitre’s lab at École polytechnique fédérale de Lausanne (EPFL). In January 2020, he started his independent research program as Max Planck Research Group Leader at the Max Planck Institute for Infection Biology. He began his research career in 2006 as a student research assistant at Zabolotny Institute of Microbiology and Virology where he worked on plant growth-promoting bacteria. His current research focuses on understanding the molecular interplay between the host, pathogens, and symbionts during infection.
Contributor Information
Igor Iatsenko, Email: iatsenko@mpiib-berlin.mpg.de.
Karen M. Ottemann, University of California at Santa Cruz Department of Microbiology and Environmental Toxicology, Santa Cruz, California, USA
REFERENCES
- 1. Cameron EA, Sperandio V. 2015. Frenemies: signaling and nutritional integration in pathogen-microbiota-host interactions. Cell Host Microbe 18:275–284. doi: 10.1016/j.chom.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belkaid Y, Hand TW. 2014. Role of the microbiota in immunity and inflammation. Cell 157:121–141. doi: 10.1016/j.cell.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsolis RM, Bäumler AJ. 2020. Gastrointestinal host-pathogen interaction in the age of microbiome research. Curr Opin Microbiol 53:78–89. doi: 10.1016/j.mib.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 4. Bäumler AJ, Sperandio V. 2016. Interactions between the microbiota and pathogenic bacteria in the gut. Nature New Biol 535:85–93. doi: 10.1038/nature18849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cho I, Blaser MJ. 2012. The human microbiome: at the interface of health and disease. Nat Rev Genet 13:260–270. doi: 10.1038/nrg3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamada N, Chen GY, Inohara N, Núñez G. 2013. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 14:685–690. doi: 10.1038/ni.2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pickard JM, Zeng MY, Caruso R, Núñez G. 2017. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev 279:70–89. doi: 10.1111/imr.12567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ducarmon QR, Zwittink RD, Hornung BVH, van Schaik W, Young VB, Kuijper EJ. 2019. Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol Mol Biol Rev 83:e00007-19. doi: 10.1128/MMBR.00007-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buffie CG, Pamer EG. 2013. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 13:790–801. doi: 10.1038/nri3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roe AJ, McLaggan D, Davidson I, O’Byrne C, Booth IR. 1998. Perturbation of anion balance during inhibition of growth of Escherichia coli by weak acids. J Bacteriol 180:767–772. doi: 10.1128/JB.180.4.767-772.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sorbara MT, Pamer EG. 2019. Interbacterial mechanisms of colonization resistance and the strategies pathogens use to overcome them. Mucosal Immunol 12:1–9. doi: 10.1038/s41385-018-0053-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deriu E, Liu JZ, Pezeshki M, Edwards RA, Ochoa RJ, Contreras H, Libby SJ, Fang FC, Raffatellu M. 2013. Probiotic bacteria reduce Salmonella Typhimurium intestinal colonization by competing for iron. Cell Host & Microbe 14:26–37. doi: 10.1016/j.chom.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donia MS, Cimermancic P, Schulze CJ, Wieland Brown LC, Martin J, Mitreva M, Clardy J, Linington RG, Fischbach MA. 2014. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell 158:1402–1414. doi: 10.1016/j.cell.2014.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kommineni S, Bretl DJ, Lam V, Chakraborty R, Hayward M, Simpson P, Cao Y, Bousounis P, Kristich CJ, Salzman NH. 2015. Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature New Biol 526:719–722. doi: 10.1038/nature15524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Microbiome diversity protects against pathogens by nutrient blocking. Science. Available from: https://www.science.org/doi/10.1126/science.adj3502. Retrieved 17 Dec 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iatsenko I, Yim JJ, Schroeder FC, Sommer RJ. 2014. B. subtilis GS67 protects C. elegans from Gram-positive pathogens via fengycin-mediated microbial antagonism. Curr Biol 24:2720–2727. doi: 10.1016/j.cub.2014.09.055 [DOI] [PubMed] [Google Scholar]
- 17. Sassone-Corsi M, Raffatellu M. 2015. No vacancy: how beneficial microbes cooperate with immunity to provide colonization resistance to pathogens. J Immunol 194:4081–4087. doi: 10.4049/jimmunol.1403169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cash HL, Whitham CV, Behrendt CL, Hooper LV, Frierson JM, Hooper LV, Dermody TS, Pfeiffer JK. 2006. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313:1126–1130. doi: 10.1126/science.1127119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gensollen T, Iyer SS, Kasper DL, Blumberg RS. 2016. How colonization by microbiota in early life shapes the immune system. Science 352:539–544. doi: 10.1126/science.aad9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng D, Liwinski T, Elinav E. 2020. Interaction between microbiota and immunity in health and disease. Cell Res 30:492–506. doi: 10.1038/s41422-020-0332-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rolhion N, Chassaing B. 2016. When pathogenic bacteria meet the intestinal microbiota. Philos Trans R Soc Lond B Biol Sci 371:20150504. doi: 10.1098/rstb.2015.0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Venturi V, da Silva DP. 2012. Incoming pathogens team up with harmless “resident” bacteria. Trends Microbiol 20:160–164. doi: 10.1016/j.tim.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 23. Stevens EJ, Bates KA, King KC. 2021. Host microbiota can facilitate pathogen infection. PLoS Pathog 17:e1009514. doi: 10.1371/journal.ppat.1009514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maier L, Vyas R, Cordova CD, Lindsay H, Schmidt TSB, Brugiroux S, Periaswamy B, Bauer R, Sturm A, Schreiber F, von Mering C, Robinson MD, Stecher B, Hardt W-D. 2013. Microbiota-derived hydrogen fuels Salmonella typhimurium invasion of the gut ecosystem. Cell Host Microbe 14:641–651. doi: 10.1016/j.chom.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 25. Ferreyra JA, Wu KJ, Hryckowian AJ, Bouley DM, Weimer BC, Sonnenburg JL. 2014. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe 16:770–777. doi: 10.1016/j.chom.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, Naidu N, Choudhury B, Weimer BC, Monack DM, Sonnenburg JL. 2013. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature New Biol 502:96–99. doi: 10.1038/nature12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, Sperandio V. 2012. Fucose sensing regulates bacterial intestinal colonization. Nature New Biol 492:113–117. doi: 10.1038/nature11623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jimenez AG, Ellermann M, Abbott W, Sperandio V. 2020. Diet-derived galacturonic acid regulates virulence and intestinal colonization in enterohaemorrhagic Escherichia coli and Citrobacter rodentium . Nat Microbiol 5:368–378. doi: 10.1038/s41564-019-0641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, Sterzenbach T, Tsolis RM, Roth JR, Bäumler AJ. 2011. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc Natl Acad Sci U S A 108:17480–17485. doi: 10.1073/pnas.1107857108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kendall MM, Gruber CC, Parker CT, Sperandio V. 2012. Ethanolamine controls expression of genes encoding components involved in interkingdom signaling and virulence in enterohemorrhagic Escherichia coli O157:H7. MBio 3:mBio doi: 10.1128/mBio.00050-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bertin Y, Girardeau JP, Chaucheyras-Durand F, Lyan B, Pujos-Guillot E, Harel J, Martin C. 2011. Enterohaemorrhagic Escherichia coli gains a competitive advantage by using ethanolamine as a nitrogen source in the bovine intestinal content. Environ Microbiol 13:365–377. doi: 10.1111/j.1462-2920.2010.02334.x [DOI] [PubMed] [Google Scholar]
- 32. Joseph B, Przybilla K, Stühler C, Schauer K, Slaghuis J, Fuchs TM, Goebel W. 2006. Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening. J Bacteriol 188:556–568. doi: 10.1128/JB.188.2.556-568.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lawhon SD, Maurer R, Suyemoto M, Altier C. 2002. Intestinal short-chain fatty acids alter Salmonella Typhimurium invasion gene expression and virulence through BarA/SirA. Mol Microbiol 46:1451–1464. doi: 10.1046/j.1365-2958.2002.03268.x [DOI] [PubMed] [Google Scholar]
- 34. Curtis MM, Hu Z, Klimko C, Narayanan S, Deberardinis R, Sperandio V. 2014. The gut commensal bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape. Cell Host Microbe 16:759–769. doi: 10.1016/j.chom.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Theriot CM, Koenigsknecht MJ, Carlson PE Jr, Hatton GE, Nelson AM, Li B, Huffnagle GB, Z Li J, Young VB. 2014. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun 5:1–10. doi: 10.1038/ncomms4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takao M, Yen H, Tobe T. 2014. LeuO enhances butyrate-induced virulence expression through a positive regulatory loop in enterohaemorrhagic Escherichia coli . Mol Microbiol 93:1302–1313. doi: 10.1111/mmi.12737 [DOI] [PubMed] [Google Scholar]
- 37. Tobe T, Nakanishi N, Sugimoto N. 2011. Activation of motility by sensing short-chain fatty acids via two steps in a flagellar gene regulatory cascade in enterohemorrhagic Escherichia coli. Infect Immun 79:1016–1024. doi: 10.1128/IAI.00927-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakanishi N, Tashiro K, Kuhara S, Hayashi T, Sugimoto N, Tobe T. 2009. Regulation of virulence by butyrate sensing in enterohaemorrhagic Escherichia coli . Microbiology (Reading) 155:521–530. doi: 10.1099/mic.0.023499-0 [DOI] [PubMed] [Google Scholar]
- 39. Wangler MF, Yamamoto S, Bellen HJ. 2015. Fruit flies in biomedical research. Genetics 199:639–653. doi: 10.1534/genetics.114.171785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Westlake H, Hanson MA, Lemaitre B. 2024. The Drosophila immunity handbook. EPFL Press. Available from: https://infoscience.epfl.ch/handle/20.500.14299/241083 [Google Scholar]
- 41. Douglas AE. 2019. Simple animal models for microbiome research. Nat Rev Microbiol 17:764–775. doi: 10.1038/s41579-019-0242-1 [DOI] [PubMed] [Google Scholar]
- 42. Broderick NA, Lemaitre B. 2012. Gut-associated microbes of Drosophila melanogaster. Gut Microbes 3:307–321. doi: 10.4161/gmic.19896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Buchon N, Silverman N, Cherry S. 2014. Immunity in Drosophila melanogaster--from microbial recognition to whole-organism physiology. Nat Rev Immunol 14:796–810. doi: 10.1038/nri3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Buchon N, Broderick NA, Lemaitre B. 2013. Gut homeostasis in a microbial world: insights from Drosophila melanogaster . Nat Rev Microbiol 11:615–626. doi: 10.1038/nrmicro3074 [DOI] [PubMed] [Google Scholar]
- 45. Douglas AE. 2018. The Drosophila model for microbiome research. Lab Anim 47:157–164. doi: 10.1038/s41684-018-0065-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grenier T, Leulier F. 2020. How commensal microbes shape the physiology of Drosophila melanogaster. Curr Opin Insect Sci 41:92–99. doi: 10.1016/j.cois.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 47. Lesperance DN, Broderick NA. 2020. Microbiomes as modulators of Drosophila melanogaster homeostasis and disease. Curr Opin Insect Sci 39:84–90. doi: 10.1016/j.cois.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hales KG, Korey CA, Larracuente AM, Roberts DM. 2015. Genetics on the fly: a primer on the drosophila model system. Genetics 201:815–842. doi: 10.1534/genetics.115.183392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ludington WB, Ja WW. 2020. Drosophila as a model for the gut microbiome. PLoS Pathog 16:e1008398. doi: 10.1371/journal.ppat.1008398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuraishi T, Hori A, Kurata S. 2013. Host-microbe interactions in the gut of Drosophila melanogaster . Front Physiol 4:375. doi: 10.3389/fphys.2013.00375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Trinder M, Daisley BA, Dube JS, Reid G. 2017. Drosophila melanogaster as a high-throughput model for host-microbiota interactions. Front Microbiol 8:751. doi: 10.3389/fmicb.2017.00751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Davoodi S, Foley E. 2020. Host-microbe-pathogen interactions: a review of Vibrio cholerae pathogenesis in Drosophila. Front Immunol 10. doi: 10.3389/fimmu.2019.03128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hrdina A, Iatsenko I. 2022. The roles of metals in insect-microbe interactions and immunity. Curr Opin Insect Sci 49:71–77. doi: 10.1016/j.cois.2021.12.004 [DOI] [PubMed] [Google Scholar]
- 54. Arias-Rojas A, Iatsenko I. 2022. The role of microbiota in Drosophila melanogaster aging. Front Aging 3:1–10. doi: 10.3389/fragi.2022.909509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Staubach F, Baines JF, Künzel S, Bik EM, Petrov DA. 2013. Host species and environmental effects on bacterial communities associated with Drosophila in the laboratory and in the natural environment. PLoS ONE 8:e70749. doi: 10.1371/journal.pone.0070749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wong AC-N, Chaston JM, Douglas AE. 2013. The inconstant gut microbiota of Drosophila species revealed by 16S rRNA gene analysis. ISME J 7:1922–1932. doi: 10.1038/ismej.2013.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wong CNA, Ng P, Douglas AE. 2011. Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ Microbiol 13:1889–1900. doi: 10.1111/j.1462-2920.2011.02511.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Adair KL, Wilson M, Bost A, Douglas AE. 2018. Microbial community assembly in wild populations of the fruit fly Drosophila melanogaster. ISME J 12:959–972. doi: 10.1038/s41396-017-0020-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Douglas AE. 2022. Insects and their beneficial microbes. Princeton University Press. Available from: https://www.jstor.org/stable/j.ctv24q54q5 [Google Scholar]
- 60. Martino ME, Ma D, Leulier F. 2017. Microbial influence on Drosophila biology. Curr Opin Microbiol 38:165–170. doi: 10.1016/j.mib.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 61. Broderick NA, Buchon N, Lemaitre B. 2014. Microbiota-induced changes in Drosophila melanogaster host gene expression and gut morphology microbiota-induced changes in Drosophila melanogaster . MBio. doi: 10.1128/mBio.01117-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Erkosar B, Leulier F, Kasper LH, Just W. 2014. Transient adult microbiota, gut homeostasis and longevity: novel insights from the Drosophila model. FEBS Lett 588:4250–4257. doi: 10.1016/j.febslet.2014.06.041 [DOI] [PubMed] [Google Scholar]
- 63. Chandler JA, Morgan Lang J, Bhatnagar S, Eisen JA, Kopp A. 2011. Bacterial communities of diverse Drosophila species: ecological context of a host–microbe model system. PLoS Genet 7:e1002272. doi: 10.1371/journal.pgen.1002272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Blum JE, Fischer CN, Miles J, Handelsman J. 2013. Frequent replenishment sustains the beneficial microbiome of Drosophila melanogaster. MBio 4:e00860–13. doi: 10.1128/mBio.00860-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Storelli G, Strigini M, Grenier T, Bozonnet L, Schwarzer M, Daniel C, Matos R, Leulier F. 2018. Drosophila perpetuates nutritional mutualism by promoting the fitness of its intestinal symbiont Lactobacillus plantarum. Cell Metab 27:362–377. doi: 10.1016/j.cmet.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang Y, Staubach F. 2018. Individual variation of natural D.melanogaster-associated bacterial communities. FEMS Microbiol Lett 365:fny017. doi: 10.1093/femsle/fny017 [DOI] [PubMed] [Google Scholar]
- 67. Obadia B, Güvener ZT, Zhang V, Ceja-Navarro JA, Brodie EL, Ja WW, Ludington WB. 2017. Probabilistic invasion underlies natural gut microbiome stability. Curr Biol 27:1999–2006. doi: 10.1016/j.cub.2017.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pais IS, Valente RS, Sporniak M, Teixeira L. 2018. Drosophila melanogaster establishes a species-specific mutualistic interaction with stable gut-colonizing bacteria. PLOS Biol 16:e2005710. doi: 10.1371/journal.pbio.2005710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Martino ME, Joncour P, Leenay R, Gervais H, Shah M, Hughes S, Gillet B, Beisel C, Leulier F. 2018. Bacterial adaptation to the host’s diet is a key evolutionary force shaping Drosophila-Lactobacillus symbiosis. Cell Host & Microbe 24:109–119. doi: 10.1016/j.chom.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dodge R, Jones EW, Zhu H, Obadia B, Martinez DJ, Wang C, Aranda-Díaz A, Aumiller K, Liu Z, Voltolini M, Brodie EL, Huang KC, Carlson JM, Sivak DA, Spradling AC, Ludington WB. 2023. A symbiotic physical niche in Drosophila melanogaster regulates stable association of a multi-species gut microbiota. Nat Commun 14:1557. doi: 10.1038/s41467-023-36942-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gutiérrez-García K, Aumiller K, Dodge R, Obadia B, Deng A, Agrawal S, Yuan X, Wolff R, Zhu H, Hsia R-C, Garud N, Ludington WB. 2024. A conserved bacterial genetic basis for commensal-host specificity. Science 386:1117–1122. doi: 10.1126/science.adp7748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rahimi-Midani A, Iatsenko I. 2025. Colonization island directs L. plantarum to its niche. Cell Host & Microbe 33:168–170. doi: 10.1016/j.chom.2025.01.005 [DOI] [PubMed] [Google Scholar]
- 73. Morgan SJ, Chaston JM. 2023. Flagellar genes are associated with the colonization persistence phenotype of the Drosophila melanogaster microbiota. Microbiol Spectr 11:e0458522. doi: 10.1128/spectrum.04585-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang Y, Baumdicker F, Schweiger P, Kuenzel S, Staubach F. 2021. Horizontal gene transfer-mediated bacterial strain variation affects host fitness in Drosophila. BMC Biol 19:187. doi: 10.1186/s12915-021-01124-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Douglas AE. 2018. Contradictory results in microbiome science exemplified by recent Drosophila research. MBio 9:e01758-18. doi: 10.1128/mBio.01758-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tafesh-Edwards G, Eleftherianos I. 2023. The role of Drosophila microbiota in gut homeostasis and immunity. Gut Microbes 15:2208503. doi: 10.1080/19490976.2023.2208503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Barron AJ, Agrawal S, Lesperance DNA, Doucette J, Calle S, Broderick NA. 2024. Microbiome-derived acidity protects against microbial invasion in Drosophila . Cell Rep 43:114087. doi: 10.1016/j.celrep.2024.114087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Su W, Liu J, Bai P, Ma B, Liu W. 2019. Pathogenic fungi-induced susceptibility is mitigated by mutual Lactobacillus plantarum in the Drosophila melanogaster model. BMC Microbiol 19:302. doi: 10.1186/s12866-019-1686-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fischer CN, Trautman EP, Crawford JM, Stabb EV, Handelsman J, Broderick NA. 2017. Metabolite exchange between microbiome members produces compounds that influence Drosophila behavior. Elife 6:e18855. doi: 10.7554/eLife.18855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Glittenberg MT, Kounatidis I, Christensen D, Kostov M, Kimber S, Roberts I, Ligoxygakis P. 2011. Pathogen and host factors are needed to provoke a systemic host response to gastrointestinal infection of Drosophila larvae by Candida albicans. Dis Model Mech 4:515–525. doi: 10.1242/dmm.006627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yang JL, Zhu H, Sadh P, Aumiller K, Guvener ZT, Ludington WB. 2025. Commensal acidification of specific gut regions produces a protective priority effect against enteropathogenic bacterial infection. bioRxiv:2025.02.12.637843. doi: 10.1101/2025.02.12.637843 [DOI] [Google Scholar]
- 82. Hong S, Sun Y, Sun D, Wang C. 2022. Microbiome assembly on Drosophila body surfaces benefits the flies to combat fungal infections. iScience 25:104408. doi: 10.1016/j.isci.2022.104408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hong S, Sun Y, Chen H, Wang C. 2023. Suppression of the insect cuticular microbiomes by a fungal defensin to facilitate parasite infection. ISME J 17:1–11. doi: 10.1038/s41396-022-01323-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hong S, Gao H, Chen H, Wang C. 2024. Engineered fungus containing a caterpillar gene kills insects rapidly by disrupting their ecto- and endo-microbiomes. Commun Biol 7:955. doi: 10.1038/s42003-024-06670-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wei G, Lai Y, Wang G, Chen H, Li F, Wang S. 2017. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc Natl Acad Sci U S A 114:5994–5999. doi: 10.1073/pnas.1703546114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Korgaonkar A, Trivedi U, Rumbaugh KP, Whiteley M. 2013. Community surveillance enhances Pseudomonas aeruginosa virulence during polymicrobial infection. Proc Natl Acad Sci U S A 110:1059–1064. doi: 10.1073/pnas.1214550110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fast D, Kostiuk B, Foley E, Pukatzki S. 2018. Commensal pathogen competition impacts host viability. Proc Natl Acad Sci USA 115:7099–7104. doi: 10.1073/pnas.1802165115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fast D, Petkau K, Ferguson M, Shin M, Galenza A, Kostiuk B, Pukatzki S, Foley E. 2020. Vibrio cholerae-symbiont interactions inhibit intestinal repair in Drosophila. Cell Rep 30:1088–1100. doi: 10.1016/j.celrep.2019.12.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. 2009. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev 23:2333–2344. doi: 10.1101/gad.1827009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Harris EV, de Roode JC, Gerardo NM. 2019. Diet-microbiome-disease: Investigating diet’s influence on infectious disease resistance through alteration of the gut microbiome. PLoS Pathog 15:e1007891. doi: 10.1371/journal.ppat.1007891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ignatiou A, Pitsouli C. 2024. Host–diet–microbiota interplay in intestinal nutrition and health. FEBS Lett 598:2482–2517. doi: 10.1002/1873-3468.14966 [DOI] [PubMed] [Google Scholar]
- 92. Obadia B, Keebaugh ES, Yamada R, Ludington WB, Ja WW. 2018. Diet influences host–microbiota associations in Drosophila. Proc Natl Acad Sci USA 115. doi: 10.1073/pnas.1804948115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lesperance DNA, Broderick NA. 2020. Meta-analysis of diets used in Drosophila microbiome research and introduction of the Drosophila dietary composition calculator (DDCC). G3 (Bethesda) 10:2207–2211. doi: 10.1534/g3.120.401235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Erkosar B, Yashiro E, Zajitschek F, Friberg U, Maklakov AA, van der Meer JR, Kawecki TJ. 2018. Host diet mediates a negative relationship between abundance and diversity of Drosophila gut microbiota. Ecol Evol 8:9491–9502. doi: 10.1002/ece3.4444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Galenza A, Hutchinson J, Campbell SD, Hazes B, Foley E. 2016. Glucose modulates Drosophila longevity and immunity independent of the microbiota. Biol Open 5:165–173. doi: 10.1242/bio.015016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang J, Gu J, Yi J, Li J, Li W, Zhai Z. 2024. High-fat diets induce inflammatory IMD/NFκB signaling via gut microbiota remodeling in Drosophila. Front Cell Infect Microbiol 14. doi: 10.3389/fcimb.2024.1347716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. von Frieling J, Faisal MN, Sporn F, Pfefferkorn R, Nolte SS, Sommer F, Rosenstiel P, Roeder T. 2020. A high-fat diet induces a microbiota-dependent increase in stem cell activity in the Drosophila intestine. PLoS Genet 16:e1008789. doi: 10.1371/journal.pgen.1008789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fink C, Staubach F, Kuenzel S, Baines JF, Roeder T. 2013. Noninvasive analysis of microbiome dynamics in the fruit fly Drosophila melanogaster. Appl Environ Microbiol 79:6984–6988. doi: 10.1128/AEM.01903-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ponton F, Morimoto J, Robinson K, Kumar SS, Cotter SC, Wilson K, Simpson SJ. 2020. Macronutrients modulate survival to infection and immunity in Drosophila. J Anim Ecol 89:460–470. doi: 10.1111/1365-2656.13126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Unckless RL, Rottschaefer SM, Lazzaro BP. 2015. The complex contributions of genetics and nutrition to immunity in Drosophila melanogaster. PLoS Genet 11:e1005030. doi: 10.1371/journal.pgen.1005030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Darby AM, Okoro DO, Aredas S, Frank AM, Pearson WH, Dionne MS, Lazzaro BP. 2024. High sugar diets can increase susceptibility to bacterial infection in Drosophila melanogaster. PLoS Pathog 20:e1012447. doi: 10.1371/journal.ppat.1012447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rai KE, Yin H, Bengo ALC, Cheek M, Courville R, Bagheri E, Ramezan R, Behseta S, Shahrestani P. 2023. Immune defense in Drosophila melanogaster depends on diet, sex, and mating status. PLOS ONE 18:e0268415. doi: 10.1371/journal.pone.0268415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Miller CVL, Cotter SC. 2018. Resistance and tolerance: the role of nutrients on pathogen dynamics and infection outcomes in an insect host. J Anim Ecol 87:500–510. doi: 10.1111/1365-2656.12763 [DOI] [PubMed] [Google Scholar]
- 104. Kutzer MAM, Armitage SAO. 2016. The effect of diet and time after bacterial infection on fecundity, resistance, and tolerance in Drosophila melanogaster. Ecol Evol 6:4229–4242. doi: 10.1002/ece3.2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Haselkorn TS. 2010. The Spiroplasma heritable bacterial endosymbiont of Drosophila. Fly (Austin) 4:80–87. doi: 10.4161/fly.4.1.10883 [DOI] [PubMed] [Google Scholar]
- 106. Mateos M, Castrezana SJ, Nankivell BJ, Estes AM, Markow TA, Moran NA. 2006. Heritable endosymbionts of Drosophila . Genetics 174:363–376. doi: 10.1534/genetics.106.058818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Porter J, Sullivan W. 2023. The cellular lives of Wolbachia. Nat Rev Microbiol 21:750–766. doi: 10.1038/s41579-023-00918-x [DOI] [PubMed] [Google Scholar]
- 108. Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751. doi: 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- 109. Anbutsu H, Fukatsu T. 2011. Spiroplasma as a model insect endosymbiont. Environ Microbiol Rep 3:144–153. doi: 10.1111/j.1758-2229.2010.00240.x [DOI] [PubMed] [Google Scholar]
- 110. Gerth M, Martinez-Montoya H, Ramirez P, Masson F, Griffin JS, Aramayo R, Siozios S, Lemaitre B, Mateos M, Hurst GDD. 2021. Rapid molecular evolution of Spiroplasma symbionts of Drosophila. Microb Genom 7:1–15. doi: 10.1099/mgen.0.000503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Herren JK, Paredes JC, Schüpfer F, Arafah K, Bulet P, Lemaitre B. 2014. Insect endosymbiont proliferation is limited by lipid availability. Elife 3:e02964. doi: 10.7554/eLife.02964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Herren JK, Lemaitre B. 2011. Spiroplasma and host immunity: activation of humoral immune responses increases endosymbiont load and susceptibility to certain Gram-negative bacterial pathogens in Drosophila melanogaster . Cell Microbiol 13:1385–1396. doi: 10.1111/j.1462-5822.2011.01627.x [DOI] [PubMed] [Google Scholar]
- 113. Łukasik P, Kolasa MR. 2024. With a little help from my friends: the roles of microbial symbionts in insect populations and communities. Philos Trans R Soc Lond B Biol Sci 379:20230122. doi: 10.1098/rstb.2023.0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gerardo NM, Parker BJ. 2014. Mechanisms of symbiont-conferred protection against natural enemies: an ecological and evolutionary framework. Curr Opin Insect Sci 4:8–14. doi: 10.1016/j.cois.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 115. Brownlie JC, Johnson KN. 2009. Symbiont-mediated protection in insect hosts. Trends Microbiol 17:348–354. doi: 10.1016/j.tim.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 116. Teixeira L, Ferreira A, Ashburner M. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster . PLOS Biol 6:e2. doi: 10.1371/journal.pbio.1000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chrostek E, Martins N, Marialva MS, Teixeira L. 2021. Wolbachia-conferred antiviral protection is determined by developmental temperature. MBio 12:e0292320. doi: 10.1128/mBio.02923-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hedges LM, Brownlie JC, O’Neill SL, Johnson KN. 2008. Wolbachia and virus protection in insects. Science 322:702. doi: 10.1126/science.1162418 [DOI] [PubMed] [Google Scholar]
- 119. Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O’Neill SL. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium . Cell 139:1268–1278. doi: 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- 120. Chrostek E, Hurst GDD, McGraw EA. 2020. Infectious diseases: antiviral Wolbachia limits dengue in Malaysia. Curr Biol 30:R30–R32. doi: 10.1016/j.cub.2019.11.046 [DOI] [PubMed] [Google Scholar]
- 121. Utarini A, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, Ansari MR, Supriyati E, Wardana DS, Meitika Y, Ernesia I, Nurhayati I, Prabowo E, Andari B, Green BR, Hodgson L, Cutcher Z, Rancès E, Ryan PA, O’Neill SL, Dufault SM, Tanamas SK, Jewell NP, Anders KL, Simmons CP, AWED Study Group . 2021. Efficacy of Wolbachia-infected mosquito deployments for the control of Dengue. N Engl J Med 384:2177–2186. doi: 10.1056/NEJMoa2030243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Rancès E, Ye YH, Woolfit M, McGraw EA, O’Neill SL. 2012. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog 8:e1002548. doi: 10.1371/journal.ppat.1002548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, Xi Z. 2012. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A 109:E23–31. doi: 10.1073/pnas.1116932108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Caragata EP, Rancès E, Hedges LM, Gofton AW, Johnson KN, O’Neill SL, McGraw EA. 2013. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog 9:e1003459. doi: 10.1371/journal.ppat.1003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Molloy JC, Sommer U, Viant MR, Sinkins SP. 2016. Wolbachia modulates lipid metabolism in Aedes albopictus mosquito cells. Appl Environ Microbiol 82:3109–3120. doi: 10.1128/AEM.00275-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Martinez J, Longdon B, Bauer S, Chan Y-S, Miller WJ, Bourtzis K, Teixeira L, Jiggins FM. 2014. Symbionts commonly provide broad spectrum resistance to viruses in insects: a comparative analysis of Wolbachia strains. PLoS Pathog 10:e1004369. doi: 10.1371/journal.ppat.1004369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chrostek E, Marialva MSP, Esteves SS, Weinert LA, Martinez J, Jiggins FM, Teixeira L. 2013. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLOS Genet 9:e1003896. doi: 10.1371/journal.pgen.1003896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Perlmutter JI, Atadurdyyeva A, Schedl ME, Unckless RL. 2023. Wolbachia enhances the survival of Drosophila infected with fungal pathogens. bioRxiv. doi: 10.1101/2023.09.30.560320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wong ZS, Hedges LM, Brownlie JC, Johnson KN. 2011. Wolbachia-mediated antibacterial protection and immune gene regulation in Drosophila. PLoS One 6:e25430. doi: 10.1371/journal.pone.0025430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rottschaefer SM, Lazzaro BP. 2012. No effect of Wolbachia on resistance to intracellular infection by pathogenic bacteria in Drosophila melanogaster. PLoS One 7:e40500. doi: 10.1371/journal.pone.0040500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Gupta V, Vasanthakrishnan RB, Siva-Jothy J, Monteith KM, Brown SP, Vale PF. 2017. The route of infection determines Wolbachia antibacterial protection in Drosophila . Proc Biol Sci 284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ballinger MJ, Perlman SJ. 2019. The defensive Spiroplasma . Curr Opin Insect Sci 32:36–41. doi: 10.1016/j.cois.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 133. Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. 2010. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329:212–215. doi: 10.1126/science.1188235 [DOI] [PubMed] [Google Scholar]
- 134. Xie J, Tiner B, Vilchez I, Mateos M. 2011. Effect of the Drosophila endosymbiont Spiroplasma on parasitoid wasp development and on the reproductive fitness of wasp-attacked fly survivors. Evol Ecol 53:1065–1079. doi: 10.1007/s10682-010-9453-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Xie J, Butler S, Sanchez G, Mateos M. 2014. Male killing Spiroplasma protects Drosophila melanogaster against two parasitoid wasps. Heredity (Edinb) 112:399–408. doi: 10.1038/hdy.2013.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Oliver KM, Perlman SJ. 2020. Toxin-mediated protection against natural enemies by insect defensive symbionts. Adv In Insect Phys 58:277–316. doi: 10.1016/bs.aiip.2020.03.005 [DOI] [Google Scholar]
- 137. Ballinger MJ, Perlman SJ. 2017. Generality of toxins in defensive symbiosis: ribosome-inactivating proteins and defense against parasitic wasps in Drosophila . PLoS Pathog 13:e1006431. doi: 10.1371/journal.ppat.1006431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Moore LD, Ballinger MJ. 2023. The toxins of vertically transmitted Spiroplasma. Front Microbiol 14:1148263. doi: 10.3389/fmicb.2023.1148263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hamilton PT, Peng F, Boulanger MJ, Perlman SJ. 2016. A ribosome-inactivating protein in a Drosophila defensive symbiont. Proc Natl Acad Sci U S A 113:350–355. doi: 10.1073/pnas.1518648113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Paredes JC, Herren JK, Schüpfer F, Lemaitre B. 2016. The role of lipid competition for endosymbiont-mediated protection against parasitoid wasps in Drosophila. MBio 7:e01006-16. doi: 10.1128/mBio.01006-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hrdina A, Serra Canales M, Arias-Rojas A, Frahm D, Iatsenko I. 2024. The endosymbiont Spiroplasma poulsonii increases Drosophila melanogaster resistance to pathogens by enhancing iron sequestration and melanization. MBio 15:e0093624. doi: 10.1128/mbio.00936-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Iatsenko I, Marra A, Boquete JP, Peña J, Lemaitre B. 2020. Iron sequestration by transferrin 1 mediates nutritional immunity in Drosophila melanogaster. Proc Natl Acad Sci U S A 117:7317–7325. doi: 10.1073/pnas.1914830117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Dudzic JP, Hanson MA, Iatsenko I, Kondo S, Lemaitre B. 2019. More than black or white: melanization and toll share regulatory serine proteases in Drosophila . Cell Rep 27:1050–1061. doi: 10.1016/j.celrep.2019.03.101 [DOI] [PubMed] [Google Scholar]
- 144. Shokal U, Yadav S, Atri J, Accetta J, Kenney E, Banks K, Katakam A, Jaenike J, Eleftherianos I. 2016. Effects of co-occurring Wolbachia and Spiroplasma endosymbionts on the Drosophila immune response against insect pathogenic and non-pathogenic bacteria. BMC Microbiol 16:16. doi: 10.1186/s12866-016-0634-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Masson F, Schüpfer F, Jollivet C, Lemaitre B. 2020. Transformation of the Drosophila sex-manipulative endosymbiont Spiroplasma poulsonii and persisting hurdles for functional genetic studies. Appl Environ Microbiol 86:e00835-20. doi: 10.1128/AEM.00835-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Masson F, Calderon Copete S, Schüpfer F, Garcia-Arraez G, Lemaitre B. 2018. In vitro culture of the insect endosymbiont Spiroplasma poulsonii highlights bacterial genes involved in host-symbiont interaction. MBio 9:mBio doi: 10.1128/mBio.00024-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Brinker P, Fontaine MC, Beukeboom LW, Falcao Salles J. 2019. Host, symbionts, and the microbiome: the missing tripartite interaction. Trends Microbiol 27:480–488. doi: 10.1016/j.tim.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 148. Ye YH, Seleznev A, Flores HA, Woolfit M, McGraw EA. 2017. Gut microbiota in Drosophila melanogaster interacts with Wolbachia but does not contribute to Wolbachia-mediated antiviral protection. J Invertebr Pathol 143:18–25. doi: 10.1016/j.jip.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 149. Imchen M, Kaur R, McGarry A, Bordenstein SR. 2024. The holobiont’s extracellular microbiome modulates the adaptation of an intracellular endosymbiont. Microbiology. doi: 10.1101/2024.09.10.611936 [DOI] [Google Scholar]
- 150. Simhadri RK, Fast EM, Guo R, Schultz MJ, Vaisman N, Ortiz L, Bybee J, Slatko BE, Frydman HM. 2017. The gut commensal microbiome of Drosophila melanogaster is modified by the endosymbiont Wolbachia. mSphere 2. doi: 10.1128/mSphere.00287-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Detcharoen M, Jiggins FM, Schlick-Steiner BC, Steiner FM. 2023. Wolbachia endosymbiotic bacteria alter the gut microbiome in the fly Drosophila nigrosparsa. J Invertebr Pathol 198:107915. doi: 10.1016/j.jip.2023.107915 [DOI] [PubMed] [Google Scholar]
- 152. Li T-P, Zha S-S, Zhou C-Y, Gong J-T, Zhu Y-X, Zhang X, Xi Z, Hong X-Y. 2020. Newly introduced Cardinium endosymbiont reduces microbial diversity in the rice brown planthopper Nilaparvata lugens. FEMS Microbiol Ecol 96:fiaa194. doi: 10.1093/femsec/fiaa194 [DOI] [PubMed] [Google Scholar]
- 153. Audsley MD, Seleznev A, Joubert DA, Woolfit M, O’Neill SL, McGraw EA. 2018. Wolbachia infection alters the relative abundance of resident bacteria in adult Aedes aegypti mosquitoes, but not larvae. Mol Ecol 27:297–309. doi: 10.1111/mec.14436 [DOI] [PubMed] [Google Scholar]
- 154. Pascar J, Middleton H, Dorus S. 2023. Aedes aegypti microbiome composition covaries with the density of Wolbachia infection. Microbiome 11:255. doi: 10.1186/s40168-023-01678-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Li T-P, Zhou C-Y, Wang M-K, Zha S-S, Chen J, Bing X-L, Hoffmann AA, Hong X-Y. 2022. Endosymbionts reduce microbiome diversity and modify host metabolism and fecundity in the planthopper Sogatella furcifera. mSystems 7:e0151621. doi: 10.1128/msystems.01516-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Henry LP, Fernandez M, Wolf S, Abhyankar V, Ayroles JF. 2024. Wolbachia impacts microbiome diversity and fitness-associated traits for Drosophila melanogaster in a seasonally fluctuating environment. Ecol Evol 14:e70004. doi: 10.1002/ece3.70004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Goto S, Anbutsu H, Fukatsu T. 2006. Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Appl Environ Microbiol 72:4805–4810. doi: 10.1128/AEM.00416-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Fromont C, Adair KL, Douglas AE. 2019. Correlation and causation between the microbiome, Wolbachia and host functional traits in natural populations of drosophilid flies. Mol Ecol 28:1826–1841. doi: 10.1111/mec.15041 [DOI] [PubMed] [Google Scholar]
- 159. Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. 2017. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol 15:630–638. doi: 10.1038/nrmicro.2017.58 [DOI] [PubMed] [Google Scholar]
- 160. Cipelli M, da Silva EM, Câmara NOS. 2023. Gut microbiota resilience mechanisms against pathogen infection and its role in inflammatory bowel disease. Curr Clin Micro Rpt 10:187–197. doi: 10.1007/s40588-023-00207-4 [DOI] [Google Scholar]
- 161. Ferrarini MG, Dell’Aglio E, Vallier A, Balmand S, Vincent-Monégat C, Hughes S, Gillet B, Parisot N, Zaidman-Rémy A, Vieira C, Heddi A, Rebollo R. 2022. Efficient compartmentalization in insect bacteriomes protects symbiotic bacteria from host immune system. Microbiome 10:156. doi: 10.1186/s40168-022-01334-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ratzka C, Gross R, Feldhaar H. 2012. Endosymbiont tolerance and control within insect hosts. Insects 3:553–572. doi: 10.3390/insects3020553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bosco-Drayon V, Poidevin M, Boneca IG, Narbonne-Reveau K, Royet J, Charroux B. 2012. Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune responses to infectious bacteria and tolerance to microbiota. Cell Host & Microbe 12:153–165. doi: 10.1016/j.chom.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 164. Arias-Rojas A, Frahm D, Hurwitz R, Brinkmann V, Iatsenko I. 2023. Resistance to host antimicrobial peptides mediates resilience of gut commensals during infection and aging in Drosophila. Proc Natl Acad Sci U S A 120:e2305649120. doi: 10.1073/pnas.2305649120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Iatsenko I, Boquete J-P, Lemaitre B. 2018. Microbiota-derived lactate activates production of reactive oxygen species by the intestinal NADPH oxidase nox and shortens Drosophila lifespan. Immunity 49:929–942. doi: 10.1016/j.immuni.2018.09.017 [DOI] [PubMed] [Google Scholar]
- 166. Arias-Rojas A, Arifah AQ, Angelidou G, Alshaar B, Schombel U, Forest E, Frahm D, Brinkmann V, Paczia N, Beisel CL, Gisch N, Iatsenko I. 2024. MprF-mediated immune evasion is necessary for Lactiplantibacillus plantarum resilience in the Drosophila gut during inflammation. PLoS Pathog 20:e1012462. doi: 10.1371/journal.ppat.1012462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Cullen TW, Schofield WB, Barry NA, Putnam EE, Rundell EA, Trent MS, Degnan PH, Booth CJ, Yu H, Goodman AL. 2015. Gut microbiota. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science 347:170–175. doi: 10.1126/science.1260580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Moule MG, Monack DM, Schneider DS. 2010. Reciprocal analysis of Francisella novicida infections of a Drosophila melanogaster model reveal host-pathogen conflicts mediated by reactive oxygen and imd-regulated innate immune response. PLoS Pathog 6:e1001065. doi: 10.1371/journal.ppat.1001065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Shaka M, Arias-Rojas A, Hrdina A, Frahm D, Iatsenko I. 2022. Lipopolysaccharide -mediated resistance to host antimicrobial peptides and hemocyte-derived reactive-oxygen species are the major Providencia alcalifaciens virulence factors in Drosophila melanogaster. PLoS Pathog 18:e1010825. doi: 10.1371/journal.ppat.1010825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bosch TCG, Blaser MJ, Ruby E, McFall-Ngai M. 2024. A new lexicon in the age of microbiome research. Phil Trans R Soc B 379. doi: 10.1098/rstb.2023.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Chu H, Mazmanian SK. 2013. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol 14:668–675. doi: 10.1038/ni.2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Marra A, Hanson MA, Kondo S, Erkosar B, Lemaitre B. 2021. Drosophila antimicrobial peptides and lysozymes regulate gut microbiota composition and abundance. MBio 12:e0082421. doi: 10.1128/mBio.00824-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Hanson MA. 2024. When the microbiome shapes the host: immune evolution implications for infectious disease. Philos Trans R Soc Lond B Biol Sci 379:20230061. doi: 10.1098/rstb.2023.0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Hanson MA, Grollmus L, Lemaitre B. 2023. Ecology-relevant bacteria drive the evolution of host antimicrobial peptides in Drosophila. Science 381:eadg5725. doi: 10.1126/science.adg5725 [DOI] [PubMed] [Google Scholar]
- 175. Becattini S, Sorbara MT, Kim SG, Littmann EL, Dong Q, Walsh G, Wright R, Amoretti L, Fontana E, Hohl TM, Pamer EG. 2021. Rapid transcriptional and metabolic adaptation of intestinal microbes to host immune activation. Cell Host Microbe 29:378–393. doi: 10.1016/j.chom.2021.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Rodríguez-Rojas A, Baeder DY, Johnston P, Regoes RR, Rolff J. 2021. Bacteria primed by antimicrobial peptides develop tolerance and persist. PLoS Pathog 17:e1009443. doi: 10.1371/journal.ppat.1009443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Chen F, Krasity BC, Peyer SM, Koehler S, Ruby EG, Zhang X, McFall-Ngai MJ. 2017. Bactericidal permeability-increasing proteins shape host-microbe interactions. MBio 8. doi: 10.1128/mBio.00040-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ryu J-H, Ha E-M, Oh C-T, Seol J-H, Brey PT, Jin I, Lee DG, Kim J, Lee D, Lee W-J. 2006. An essential complementary role of NF-kappaB pathway to microbicidal oxidants in Drosophila gut immunity. EMBO J 25:3693–3701. doi: 10.1038/sj.emboj.7601233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Ha EM, Oh CT, Bae YS, Lee WJ. 2005. A direct role for dual oxidase in Drosophila gut immunity. Science 310:847–850. doi: 10.1126/science.1117311 [DOI] [PubMed] [Google Scholar]
- 180. Guo L, Tang J, Tang M, Luo S, Zhou X. 2023. Reactive oxygen species are regulated by immune deficiency and Toll pathways in determining the host specificity of honeybee gut bacteria. Proc Natl Acad Sci USA 120. doi: 10.1073/pnas.2219634120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Spiga L, Fansler RT, Perera YR, Shealy NG, Munneke MJ, David HE, Torres TP, Lemoff A, Ran X, Richardson KL, Pudlo N, Martens EC, Folta-Stogniew E, Yang ZJ, Skaar EP, Byndloss MX, Chazin WJ, Zhu W. 2023. Iron acquisition by a commensal bacterium modifies host nutritional immunity during Salmonella infection. Cell Host & Microbe 31:1639–1654. doi: 10.1016/j.chom.2023.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Zhu W, Winter MG, Spiga L, Hughes ER, Chanin R, Mulgaonkar A, Pennington J, Maas M, Behrendt CL, Kim J, Sun X, Beiting DP, Hooper LV, Winter SE. 2020. Xenosiderophore utilization promotes bacteroides thetaiotaomicron resilience during colitis. Cell Host Microbe 27:376–388. doi: 10.1016/j.chom.2020.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. O’Neill SL, Sinkins SP, Andreadis TG, Braig HR. 1997. Cultivation of in an cell line. Insect Mol Biol 6:33–39. doi: 10.1046/j.1365-2583.1997.00157.x [DOI] [PubMed] [Google Scholar]
- 184. Kaur R, Shropshire JD, Cross KL, Leigh B, Mansueto AJ, Stewart V, Bordenstein SR, Bordenstein SR. 2021. Living in the endosymbiotic world of Wolbachia: a centennial review. Cell Host & Microbe 29:879–893. doi: 10.1016/j.chom.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Mure A, Sugiura Y, Maeda R, Honda K, Sakurai N, Takahashi Y, Watada M, Katoh T, Gotoh A, Gotoh Y, Taniguchi I, Nakamura K, Hayashi T, Katayama T, Uemura T, Hattori Y. 2023. Identification of key yeast species and microbe-microbe interactions impacting larval growth of Drosophila in the wild. Elife 12:RP90148. doi: 10.7554/eLife.90148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Chandler JA, Eisen JA, Kopp A. 2012. Yeast communities of diverse Drosophila species: comparison of two symbiont groups in the same hosts. Appl Environ Microbiol 78:7327–7336. doi: 10.1128/AEM.01741-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Bozkurt B, Terlemez G, Sezgin E. 2023. Basidiomycota species in Drosophila gut are associated with host fat metabolism. Sci Rep 13:13807. doi: 10.1038/s41598-023-41027-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Ansari MH, Staubach F, Alacatli N, Obbard DJ. 2024. A diverse gut virome from Drosophila melanogaster. bioRxiv. doi: 10.1101/2024.03.19.585549 [DOI] [Google Scholar]
- 189. Gould AL, Zhang V, Lamberti L, Jones EW, Obadia B, Korasidis N, Gavryushkin A, Carlson JM, Beerenwinkel N, Ludington WB. 2018. Microbiome interactions shape host fitness. Proc Natl Acad Sci USA 115. doi: 10.1073/pnas.1809349115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Han G, Lee HJ, Jeong SE, Jeon CO, Hyun S. 2017. Comparative analysis of Drosophila melanogaster gut microbiota with respect to host strain, sex, and age. Microb Ecol 74:207–216. doi: 10.1007/s00248-016-0925-3 [DOI] [PubMed] [Google Scholar]
- 191. Leech T, McDowall L, Hopkins KP, Sait SM, Harrison XA, Bretman A. 2021. Social environment drives sex and age-specific variation in Drosophila melanogaster microbiome composition and predicted function. Mol Ecol 30:5831–5843. doi: 10.1111/mec.16149 [DOI] [PubMed] [Google Scholar]
- 192. Belmonte RL, Corbally MK, Duneau DF, Regan JC. 2019. Sexual dimorphisms in innate immunity and responses to infection in Drosophila melanogaster. Front Immunol 10:3075. doi: 10.3389/fimmu.2019.03075 [DOI] [PMC free article] [PubMed] [Google Scholar]


