Abstract
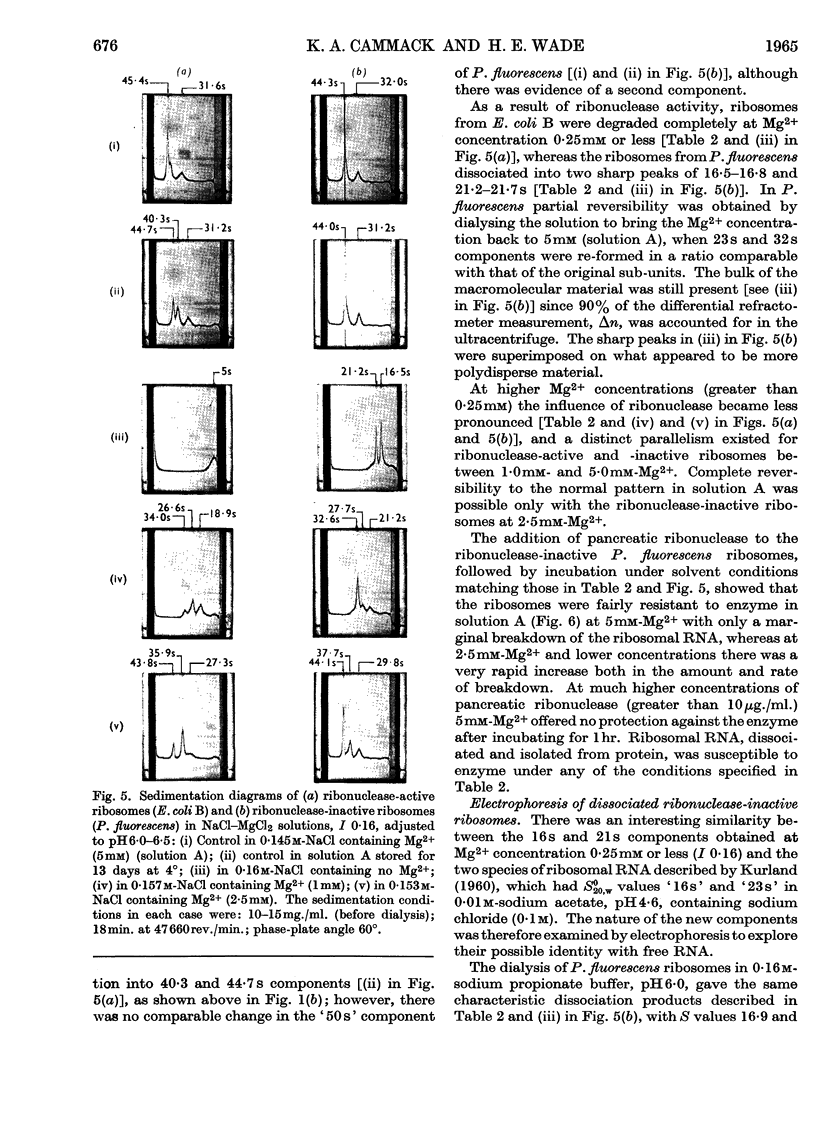
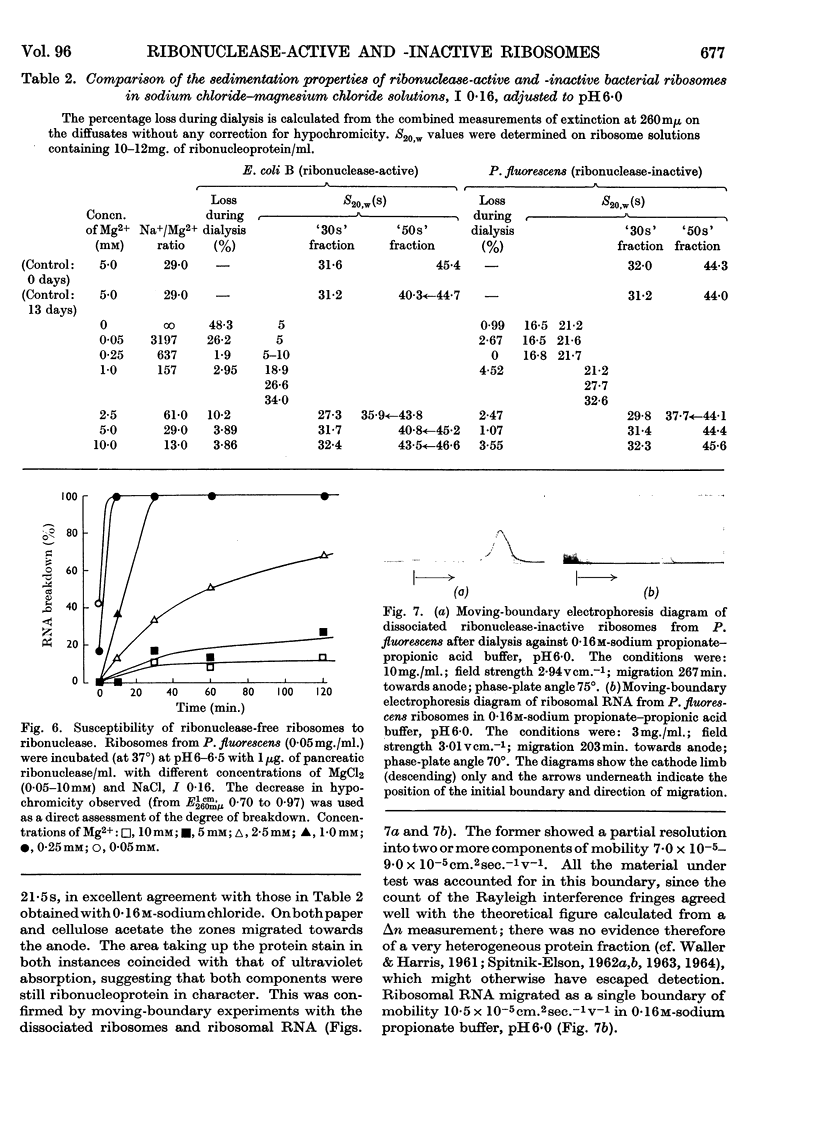
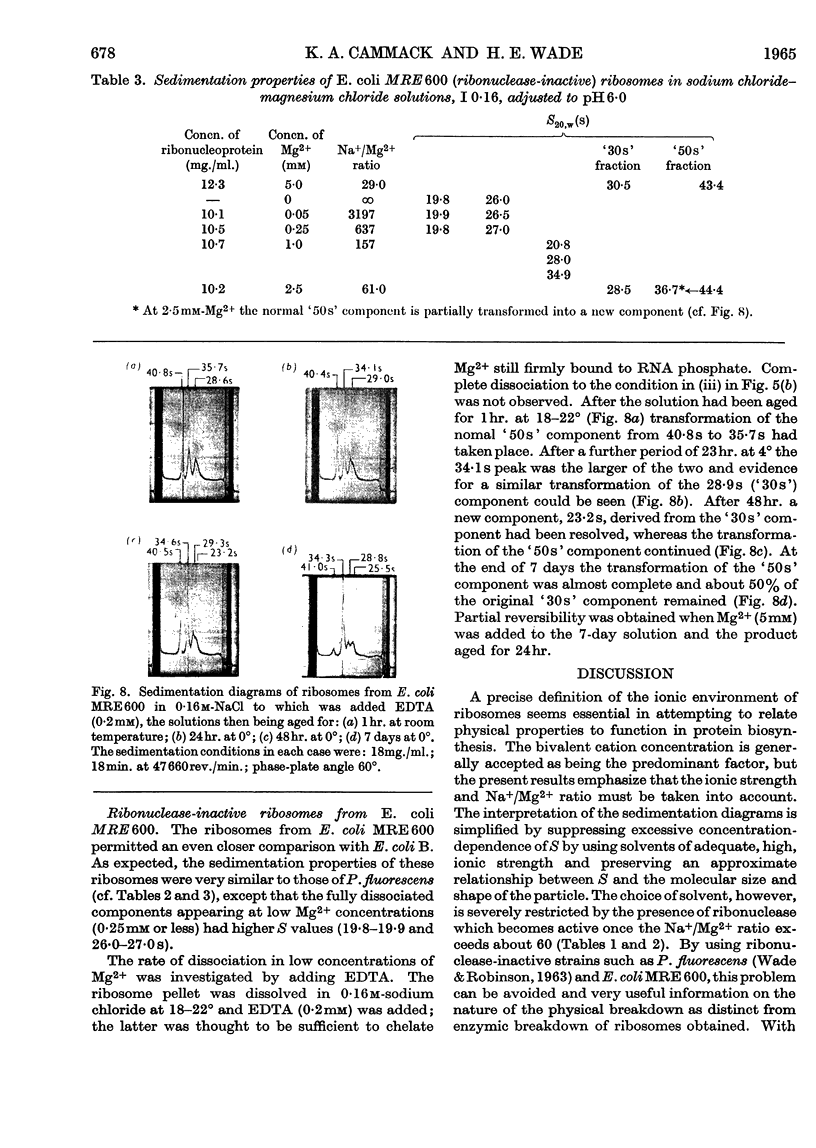
1. The `30s' and `50s' ribosomes from ribonuclease-active (Escherichia coli B) and -inactive (Pseudomonas fluorescens and Escherichia coli MRE600) bacteria have been studied in the ultracentrifuge. Charge anomalies were largely overcome by using sodium chloride–magnesium chloride solution, I 0·16, made 0–50mm with respect to Mg2+. 2. Differentiation of enzymic and physical breakdown at Mg2+ concentrations less than 5mm was made by comparing the properties of E. coli B and P. fluorescens ribosomes. 3. Ribonuclease-active ribosomes alone showed a transformation of `50s' into 40–43s components. This was combined with the release of a small amount of `5s' material which may be covalently bound soluble RNA. Other transformations of the `50s' into 34–37s components were observed in both ribonuclease-active and -inactive ribosomes at 1·0–2·5mm-Mg2+, and also with E. coli MRE600 when EDTA (0·2mm) was added to a solution in 0·16m-sodium chloride. 4. Degradation of ribonuclease-active E. coli B ribosomes at Mg2+ concentration 0·25mm or less was coincident with the formation of 16s and 21s ribonucleoprotein in P. fluorescens, and this suggested that complete dissociation of RNA from protein was not an essential prelude to breakdown of the RNA by the enzyme. 5. As high Cs+/Mg2+ ratios cause ribosomal degradation great care is necessary in the interpretation of equilibrium-density-gradient experiments in which high concentrations of caesium chloride or similar salts are used. 6. The importance of the RNA moiety in understanding the response of ribosomes to their ionic environment is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOGDANOVA E. S., GAVRILOVA L. P., DVOIRKIN G. A., KISELEV N. A., SPIRIN A. S. [Studies on the macromolecular structure of high polymer (ribosomal) ribonucleic acid from Escherichia coli]. Biokhimiia. 1962 May-Jun;27:387–402. [PubMed] [Google Scholar]
- CANNON M., KRUG R., GILBERT W. THE BINDING OF S-RNA BY ESCHERICHIA COLI RIBOSOMES. J Mol Biol. 1963 Oct;7:360–378. doi: 10.1016/s0022-2836(63)80030-3. [DOI] [PubMed] [Google Scholar]
- CHAO F. C. Dissociation of macromolecular ribonucleoprotein of yeast. Arch Biochem Biophys. 1957 Aug;70(2):426–431. doi: 10.1016/0003-9861(57)90130-3. [DOI] [PubMed] [Google Scholar]
- CHAO F. C., SCHACHMAN H. K. The isolation and characterization of a macro-molecular ribonucleoprotein from yeast. Arch Biochem Biophys. 1956 Mar;61(1):220–230. doi: 10.1016/0003-9861(56)90334-4. [DOI] [PubMed] [Google Scholar]
- COX R. A., LITTAUER U. Z. Ribonucleic acid from Escherichia coli. III. The influence of ionic strength and temperature on hydrodynamic and optical properties. Biochim Biophys Acta. 1962 Aug 20;61:197–208. [PubMed] [Google Scholar]
- COX R. A., LITTAUER U. Z. Secondary structure of ribonucleic acid in solution. Nature. 1959 Sep 12;184(Suppl 11):818–819. doi: 10.1038/184818a0. [DOI] [PubMed] [Google Scholar]
- ELSON D. A ribonucleic acid particle released from ribosomes by salt. Biochim Biophys Acta. 1961 Oct 14;53:232–234. doi: 10.1016/0006-3002(61)90818-6. [DOI] [PubMed] [Google Scholar]
- ELSON D. Latent enzymic activity of a ribonucleoprotein isolated from Escherichia coli. Biochim Biophys Acta. 1959 Dec;36:372–386. doi: 10.1016/0006-3002(59)90179-9. [DOI] [PubMed] [Google Scholar]
- ELSON D. Latent ribonuclease activity in a ribonucleoprotein. Biochim Biophys Acta. 1958 Jan;27(1):216–217. doi: 10.1016/0006-3002(58)90320-2. [DOI] [PubMed] [Google Scholar]
- ELSON D., TAL M. Biochemical differences in ribonucleo-proteins. Biochim Biophys Acta. 1959 Nov;36:281–282. doi: 10.1016/0006-3002(59)90107-6. [DOI] [PubMed] [Google Scholar]
- ELSON D. THE RIBOSOMAL TRANSFER RNA: IDENTIFICATION, ISOLATION, LOCATION AND PROPERTIES. Biochim Biophys Acta. 1964 Mar 23;80:379–390. doi: 10.1016/0926-6550(64)90140-9. [DOI] [PubMed] [Google Scholar]
- HAMILTON M. G., PETERMANN M. L. Ultracentrifugal studies on ribonucleoprotein from rat liver microsomes. J Biol Chem. 1959 Jun;234(6):1441–1446. [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- LITTAUER U. Z., EISENBERG H. Ribonucleic acid from Escherichia coli; preparation, characterization and physical properties. Biochim Biophys Acta. 1959 Apr;32:320–337. doi: 10.1016/0006-3002(59)90604-3. [DOI] [PubMed] [Google Scholar]
- MESELSON M., NOMURA M., BRENNER S., DAVERN C., SCHLESSINGER D. CONSERVATION OF RIBOSOMES DURING BACTERIAL GROWTH. J Mol Biol. 1964 Sep;9:696–711. doi: 10.1016/s0022-2836(64)80176-5. [DOI] [PubMed] [Google Scholar]
- PETERMANN M. L. Ribonucleoprotein from a rat tumor, the Jensen sarcoma. I. The effect of magnesium binding on ultracentrifugal and electrophoretic properties. J Biol Chem. 1960 Jul;235:1998–2003. [PubMed] [Google Scholar]
- Rodgers A. The exchange properties of magnesium in Escherichia coli ribosomes. Biochem J. 1964 Mar;90(3):548–555. doi: 10.1042/bj0900548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPITNIK-ELSON P. FRACTIONATION OF THE RIBOSOMAL PROTEIN OF ESCHERICHIA COLI BY ION-EXCHANGE CHROMATOGRAPHY ON CARBOXYMETHYL CELLULOSE. Biochim Biophys Acta. 1964 Apr 27;80:594–600. doi: 10.1016/0926-6550(64)90304-4. [DOI] [PubMed] [Google Scholar]
- SPITNIK-ELSON P. Fractionation of ribosomal protein from Escherichia coli by ammonium sulfate precipitation. Biochim Biophys Acta. 1963 Jul 2;74:105–112. doi: 10.1016/0006-3002(63)91334-9. [DOI] [PubMed] [Google Scholar]
- SPITNIK-ELSON P. The solubilization of ribosomal protein from Escherichia coli. Biochim Biophys Acta. 1962 May 14;55:741–747. doi: 10.1016/0006-3002(62)90852-1. [DOI] [PubMed] [Google Scholar]
- TAL M., ELSON D. THE LOCATION OF RIBONUCLEASE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Sep 17;76:40–47. [PubMed] [Google Scholar]
- TS'O P. O., BONNER J., VINOGRAD J. Structure and properties of microsomal nucleoprotein particles from pea seedlings. Biochim Biophys Acta. 1958 Dec;30(3):570–582. doi: 10.1016/0006-3002(58)90104-5. [DOI] [PubMed] [Google Scholar]
- TS'O P. O., VINOGRAD J. Studies on ribosomes from reticulocytes. Biochim Biophys Acta. 1961 Apr 29;49:113–129. doi: 10.1016/0006-3002(61)90875-7. [DOI] [PubMed] [Google Scholar]
- WADE H. E., ROBINSON H. K. ABSENCE OF RIBONUCLEASE FROM THE RIBOSOMES OF PSEUDOMONAS FLUORESCENS. Nature. 1963 Nov 16;200:661–663. doi: 10.1038/200661a0. [DOI] [PubMed] [Google Scholar]
- WADE H. E. The autodegradation of ribonucleoprotein in Escherichia coli. Biochem J. 1961 Mar;78:457–472. doi: 10.1042/bj0780457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLER D. L., HOROWITZ J. A 30-S RIBONUCLEOPROTEIN PARTICLE DERIVED FROM 50-S RIBOSOMES OF ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Jun 22;87:361–364. doi: 10.1016/0926-6550(64)90239-7. [DOI] [PubMed] [Google Scholar]
- Wade H. E., Robinson H. K. The distribution of ribosomal ribonucleic acids among subcellular fractions from bacteria and the adverse effect of the membrane fraction on the stability of ribosomes. Biochem J. 1965 Sep;96(3):753–765. doi: 10.1042/bj0960753. [DOI] [PMC free article] [PubMed] [Google Scholar]