Abstract
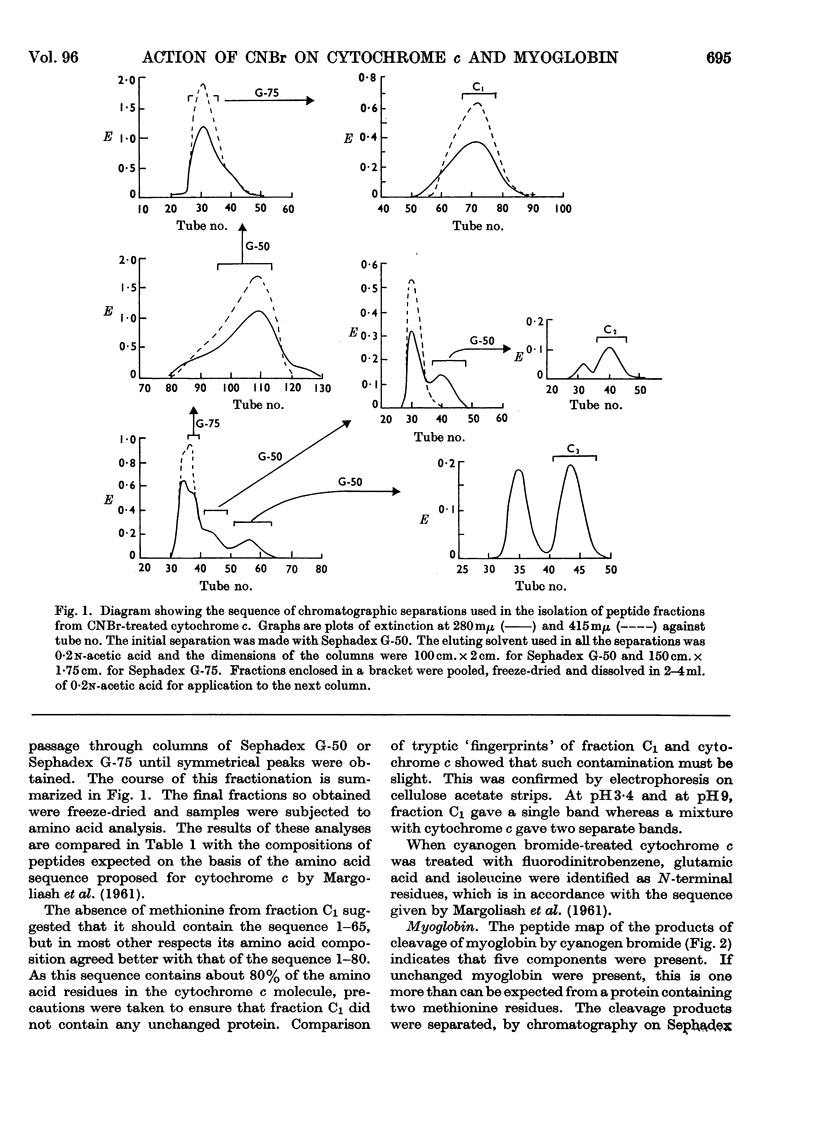
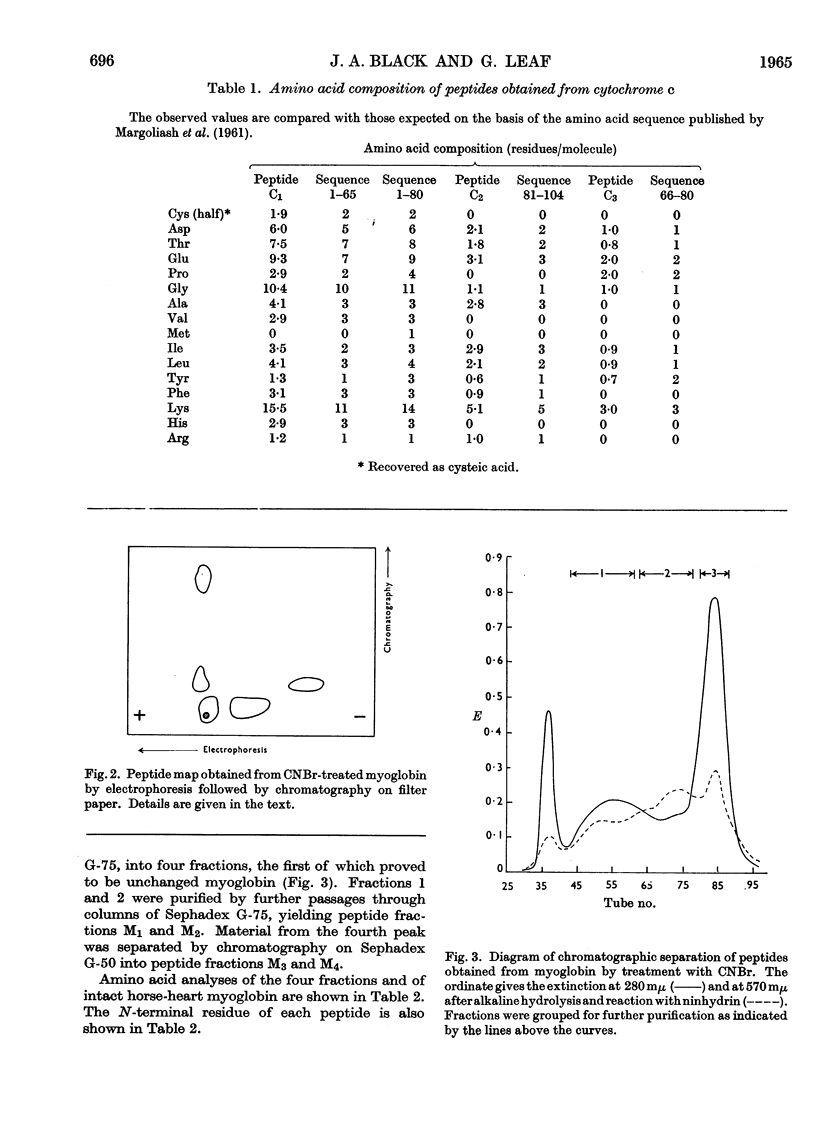
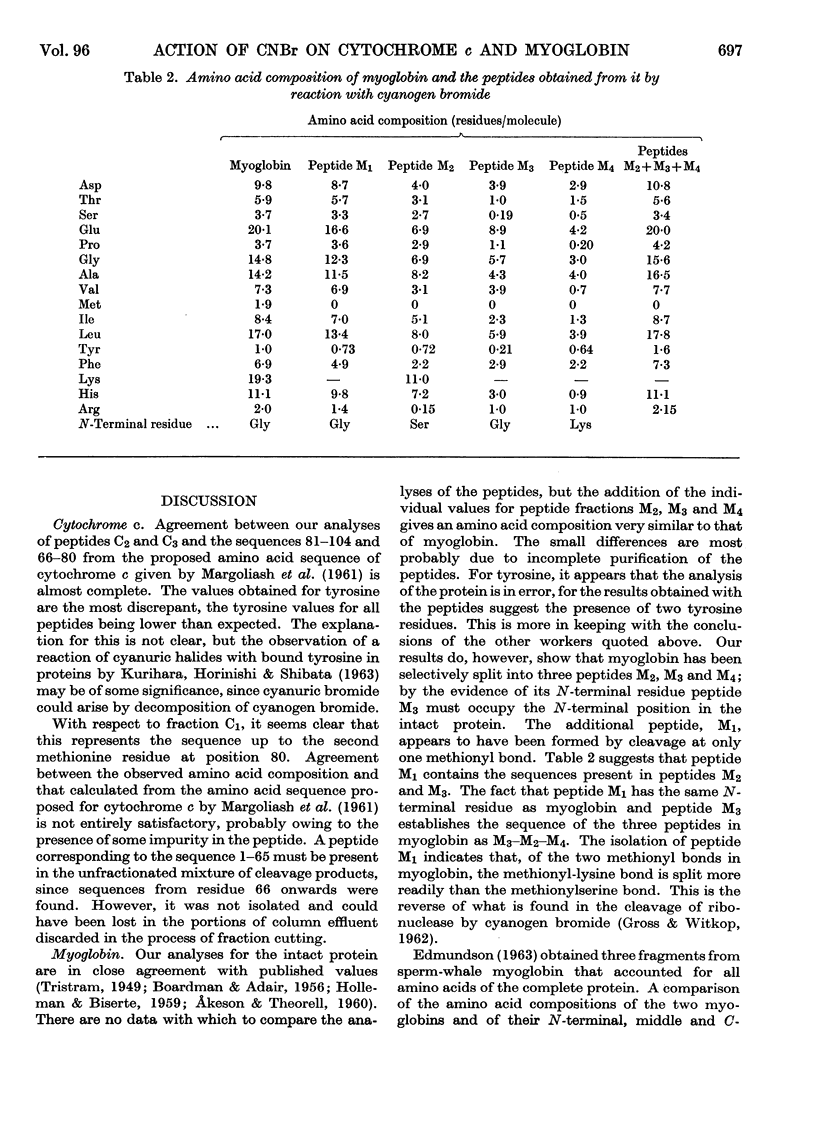
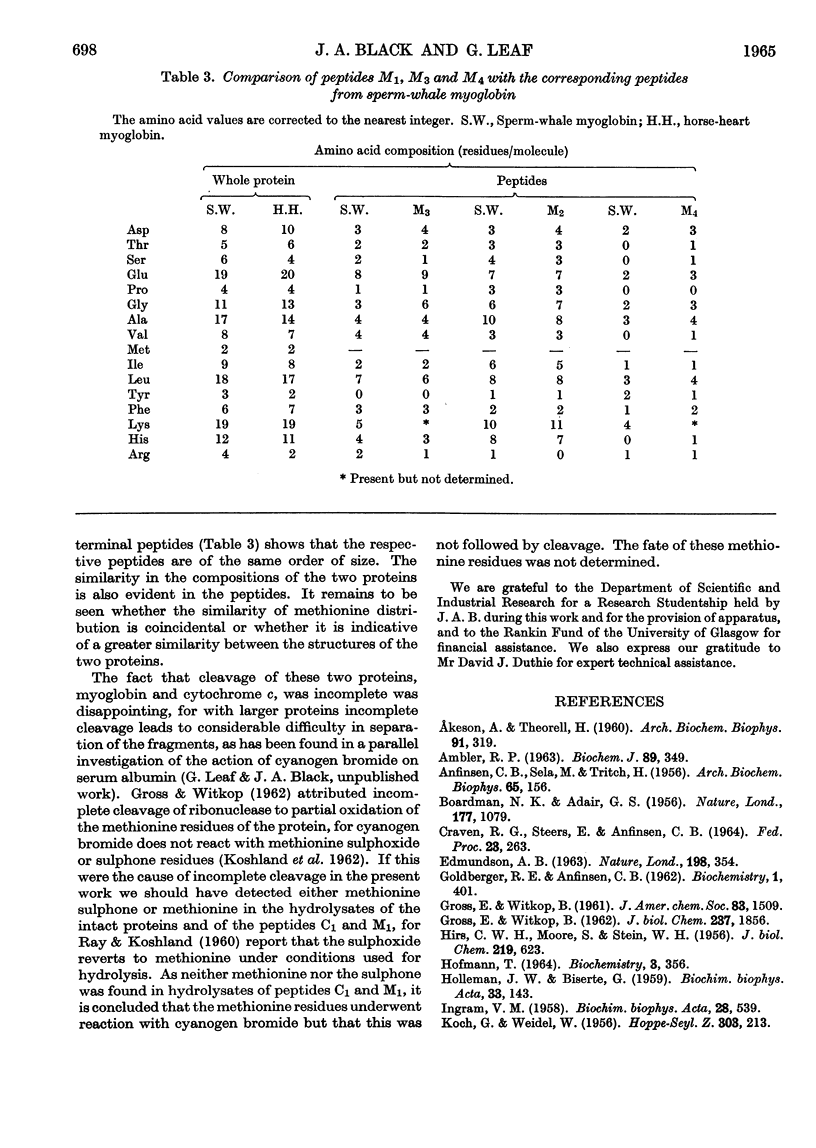
1. The effects of cyanogen bromide on horse-heart cytochrome c and horse-heart myoglobin have been investigated. Cytochrome c yielded four fragments, of which two were haemopeptides. The two colourless peptides had amino acid compositions corresponding to those that are expected, on the basis of the sequence proposed for horse-heart cytochrome c by Margoliash, Smith, Kreil & Tuppy (1961), from cleavage at both methionine residues. Of the two haemopeptides, one was isolated and shown to be that derived from cleavage at only one methionine residue, that nearer to the C-terminus of the peptide chain. 2. Myoglobin also gave four peptides, three of which accounted for the total amino acid content of the intact protein. The fourth fragment arose by cleavage at a single methionine residue, that nearer the C-terminus. Characterization of this fourth fragment made it possible to deduce the order of arrangement of the fragments in the intact molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKESON A., THEORELL H. On the microheterogeneity of horse myoglobin. Arch Biochem Biophys. 1960 Dec;91:319–325. doi: 10.1016/0003-9861(60)90507-5. [DOI] [PubMed] [Google Scholar]
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANFINSEN C. B., SELA M., TRITCH H. A method for the specific proteolytic cleavage of protein chains. Arch Biochem Biophys. 1956 Nov;65(1):156–163. doi: 10.1016/0003-9861(56)90184-9. [DOI] [PubMed] [Google Scholar]
- BOARDMAN N. K., ADAIR G. S. Isolation of two myoglobins from horseheart extracts and the determination of the molecular weight of the main component. Nature. 1956 Jun 9;177(4519):1078–1079. doi: 10.1038/1771078a0. [DOI] [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- HIRS C. H., MOORE S., STEIN W. H. Peptides obtained by tryptic hydrolysis of performic acid-oxidized ribonuclease. J Biol Chem. 1956 Apr;219(2):623–642. [PubMed] [Google Scholar]
- HOFMANN T. THE PURIFICATION AND PROPERTIES OF FRAGMENTS OF TRYPSINOGEN OBTAINED BY CYANOGEN BROMIDE CLEAVAGE. Biochemistry. 1964 Mar;3:356–364. doi: 10.1021/bi00891a010. [DOI] [PubMed] [Google Scholar]
- HOLLEMAN J. W., BISERTE G. Données nouvelles sur la structure de la myoglobine de cheval. Biochim Biophys Acta. 1959 May;33(1):143–149. doi: 10.1016/0006-3002(59)90507-4. [DOI] [PubMed] [Google Scholar]
- INGRAM V. M. Abnormal human haemoglobins. I. The comparison of normal human and sickle-cell haemoglobins by fingerprinting. Biochim Biophys Acta. 1958 Jun;28(3):539–545. doi: 10.1016/0006-3002(58)90516-x. [DOI] [PubMed] [Google Scholar]
- KOCH G., WEIDEL W. Uber die Receptorsubstanz für den Phagen T5. IV. Eine einfache Methode zur quantitativen Bestimmung von Aminosäuren und ihre Anwendung für Vergleiche zwischen Receptorsubstanz und mutativem Abwandlungsprodukt. Hoppe Seylers Z Physiol Chem. 1956 Mar 17;303(3-6):213–223. [PubMed] [Google Scholar]
- KOSHLAND D. E., Jr, STRUMEYER D. H., RAY W. J., Jr Amino acids involved in the action of chymotrypsin. Brookhaven Symp Biol. 1962 Dec;15:101–133. [PubMed] [Google Scholar]
- LEVY A. L. A paper chromatographic method for the quantitative estimation of amino-acids. Nature. 1954 Jul 17;174(4420):126–127. doi: 10.1038/174126a0. [DOI] [PubMed] [Google Scholar]
- MILLS G. L. Observations on the application of fluorodinitrobenzene to the quantitative analysis of proteins. Biochem J. 1952 Mar;50(5):707–712. doi: 10.1042/bj0500707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIEZ K. A., MORRIS L. A modified procedure for the automatic analysis of amino acids. Anal Biochem. 1960 Nov;1:187–201. doi: 10.1016/0003-2697(60)90045-2. [DOI] [PubMed] [Google Scholar]
- RAY W. J., Jr, KOSHLAND D. E., Jr Comparative structural studies of phosphoglucomutase and chymotrypsin. Brookhaven Symp Biol. 1960 Nov;13:135–150. [PubMed] [Google Scholar]
- SPACKMAN D. H., STEIN W. H., MOORE S. The disulfide bonds of ribonuclease. J Biol Chem. 1960 Mar;235:648–659. [PubMed] [Google Scholar]
- Sanger F. The free amino groups of insulin. Biochem J. 1945;39(5):507–515. doi: 10.1042/bj0390507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSH K. A., KAUFFMAN D. L., KUMAR K. S., NEURATH H. ON THE STRUCTURE AND FUNCTION OF BOVINE TRYPSINOGEN AND TRYPSIN. Proc Natl Acad Sci U S A. 1964 Feb;51:301–308. doi: 10.1073/pnas.51.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]


