Abstract
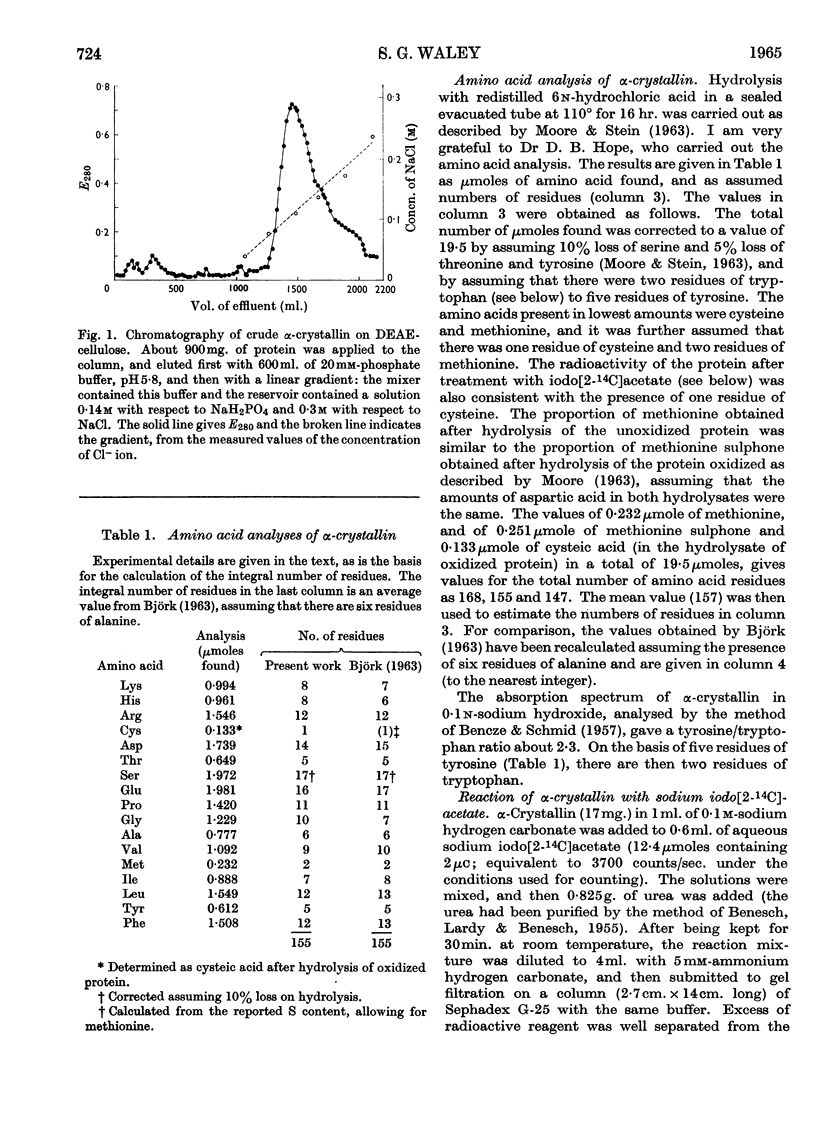
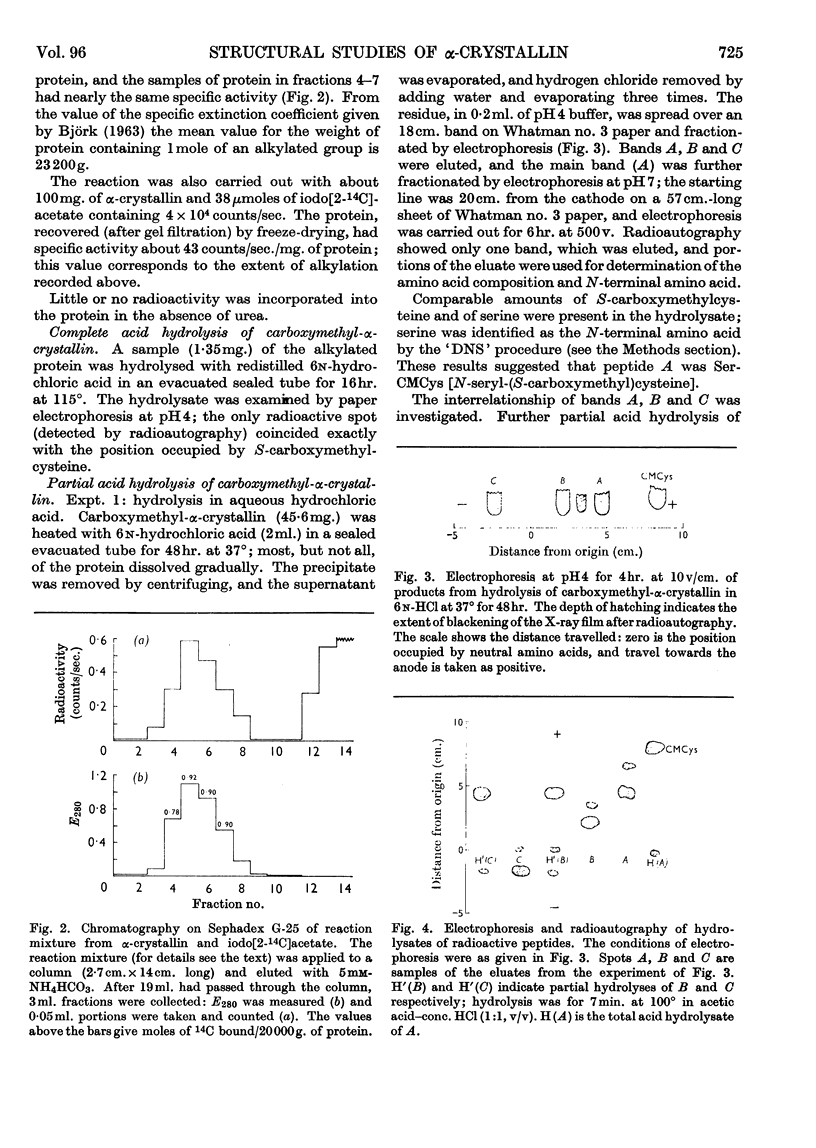
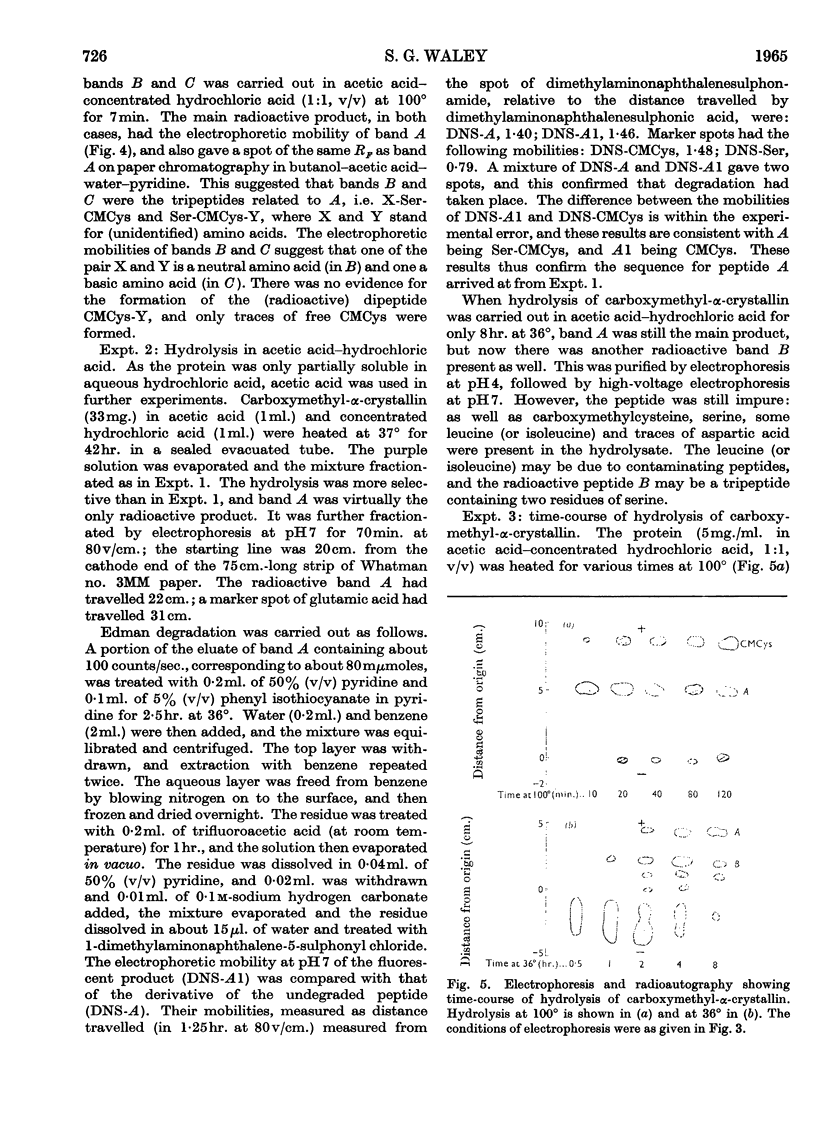
1. α-Crystallin has been isolated from the cortex of ox lens by isoelectric precipitation followed by chromatography on DEAE-cellulose. The amino acid composition is in agreement with that reported for α-crystallin prepared by a different method. There is one thiol group/20000g. of protein (20000 is the order of magnitude of the sub-unit molecular weight), and disulphide bonds are absent. 2. The thiol group has been alkylated with radioactive iodoacetate in the presence of urea. 3. Partial acid hydrolysis of the alkylated protein gives, according to the conditions, mainly three radioactive peptides or nearly exclusively one radioactive dipeptide. The dipeptide is N-seryl-(S-carboxymethyl)cysteine, Ser-CMCys. The two other peptides are probably the tripeptides related to Ser-CMCys. 4. The simplest interpretation of these results is that the sequence around the cysteine residue is a common structural feature of the sub-units of α-crystallin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENESCH R. E., LARDY H. A., BENESCH R. The sulfhydryl groups of crystalline proteins. I. Some albumins, enzymes, and hemoglobins. J Biol Chem. 1955 Oct;216(2):663–676. [PubMed] [Google Scholar]
- BLOEMENDAL H., BONT W. S., JONGKIND J. F., WISSE J. H. Splitting and recombination of alpha-crystallin. Exp Eye Res. 1962 Jun;1:300–305. doi: 10.1016/s0014-4835(62)80015-3. [DOI] [PubMed] [Google Scholar]
- BLOEMENDAL H., ten CATE Isolation and properties of alpha-crystallin from the bovine lens. Arch Biochem Biophys. 1959 Oct;84:512–527. doi: 10.1016/0003-9861(59)90612-5. [DOI] [PubMed] [Google Scholar]
- FRANCOIS J., RABAEY M., WIEME R. J. New method for fractionation of lens proteins. AMA Arch Ophthalmol. 1954 Apr;53(4):481–486. doi: 10.1001/archopht.1955.00930010483004. [DOI] [PubMed] [Google Scholar]
- GRASSMANN W., HANNIG K., PLOCKL M. Eine Methode zur quantitativen Bestimmung der Aminosäurezusammensetzung von Eiweisshydrolysaten durch Kombination von Elektrophorese und Chromatographie. Hoppe Seylers Z Physiol Chem. 1955;299(5-6):258–276. [PubMed] [Google Scholar]
- KINOSHITA J. H., MEROLA L. O. The distribution of glutathione and protein sulfhydryl groups in calf and cattle lenses. Am J Ophthalmol. 1958 Jul;46(1 Pt 2):36–42. doi: 10.1016/0002-9394(58)90032-1. [DOI] [PubMed] [Google Scholar]
- MANSKI W. J., HALBERT S. P., AUERBACH T. P. Immunochemical analyses of lens protein separations. Arch Biochem Biophys. 1961 Mar;92:512–524. doi: 10.1016/0003-9861(61)90392-7. [DOI] [PubMed] [Google Scholar]
- MILSTEIN C., SANGER F. An amino acid sequence in the active centre of phosphoglucomutase. Biochem J. 1961 Jun;79:456–469. doi: 10.1042/bj0790456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAUGHTON M. A., SANGER F., HARTLEY B. S., SHAW D. C. The amino acid sequence around the reactive serine residue of some proteolytic enzymes. Biochem J. 1960 Oct;77:149–163. doi: 10.1042/bj0770149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON G. G., ABRAHAM E. P. Degradation, structure and some derivatives of cephalosporin N. Biochem J. 1954 Sep;58(1):103–111. doi: 10.1042/bj0580103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OREKHOVICH W. N., FIRFAROVA K. F. Des protéines du cristallin de l'oeil. Bull Soc Chim Biol (Paris) 1959;41(2-3):209–225. [PubMed] [Google Scholar]
- PAPACONSTANTINOU J., RESNIK R. A., SAITO E. Biochemistry of bovine lens proteins. I. Isolation and characterization of adult alpha-crystallins. Biochim Biophys Acta. 1962 Jul 2;60:205–216. doi: 10.1016/0006-3002(62)90396-7. [DOI] [PubMed] [Google Scholar]
- SELA M., WHITE F. H., Jr, ANFINSEN C. B. The reductive cleavage of disulfide bonds and its application to problems of protein structure. Biochim Biophys Acta. 1959 Feb;31(2):417–426. doi: 10.1016/0006-3002(59)90016-2. [DOI] [PubMed] [Google Scholar]
- SPECTOR A. METHODS OF ISOLATION OF ALPHA, BETA, AND GAMMA CRYSTALLINS AND THEIR SUBGROUPS. Invest Ophthalmol. 1964 Apr;3:182–193. [PubMed] [Google Scholar]
- THOMANN H. Amperometrische Bestimmungen des Sulfhydrylgruppengehaltes tierischer Linsen. Albrecht Von Graefes Arch Ophthalmol. 1958;160(3):219–225. [PubMed] [Google Scholar]
- WALEY S. G. Acidic peptides of the lens. Biochem J. 1956 Dec;64(4):715–726. doi: 10.1042/bj0640715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALEY S. G., WATSON J. The action of trypsin on polylysine. Biochem J. 1953 Sep;55(2):328–337. doi: 10.1042/bj0550328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD D. C., BURGESS L. An electrophoretic study of soluble lens proteins from different species. Am J Ophthalmol. 1961 Feb;51:305–314. [PubMed] [Google Scholar]
- Waley S. G. Metabolism of amino acids in the lens. Biochem J. 1964 Jun;91(3):576–583. doi: 10.1042/bj0910576. [DOI] [PMC free article] [PubMed] [Google Scholar]


