Abstract
Viruses are highly dynamic macromolecular assemblies. They undergo large-scale changes in structure and organization at nearly every stage of their infectious cycles from virion assembly to maturation, receptor docking, cell entry, uncoating and genome delivery. Understanding structural transformations and dynamics across the virus infectious cycle is an expansive area for research that that can also provide insight into mechanisms for blocking infection, replication, and transmission. Additionally, the processes viruses carry out serve as excellent model systems for analogous cellular processes, but in more accessible form. Capturing and analyzing these dynamic events poses a major challenge for many structural biological approaches due to the size and complexity of the assemblies and the heterogeneity and transience of the functional states that are populated. Here we examine the process of protein-mediated membrane fusion, which is carried out by specialized machinery on enveloped virus surfaces leading to delivery of the viral genome. Application of two complementary methods, cryo-electron tomography and structural mass spectrometry enable dynamic intermediate states in intact fusion systems to be imaged and probed, providing a new understanding of the mechanisms and machinery that drive this fundamental biological process.
Keywords: CryoEM structural biology, dynamics and function, integrative structural biology, membranes, virology
Finding a captivating biological question with room to grow
I was introduced to the concept that viruses can undergo major conformational changes while I was a graduate student in the lab of Bertrand Garcia-Moreno E., where my thesis work centered on precise measurement of electrostatic interactions, dielectric constants, and pH-linked effects in proteins (Lee et al., 2002a, 2002b). We were focused on small, single-domain proteins such as staphylococcal nuclease, but Bertrand often talked about how changes in charge state and buried ionizable residues with shifted pKa values were found in large, dynamic viral proteins that had evolved to exploit pH-linked effects and environmental signals in order to trigger conformational changes during infection.
Later, during my postdoctoral training with Jack Johnson, I had the opportunity to apply biophysical approaches to study the elaborate, cooperative structural transformations that many bacteriophages undergo during their DNA packaging and maturation events. During this critical stage of assembly, hundreds of protein subunits in the icosahedral capsids reorganize through rotations and translations, expanding like nanoscale Hoberman spheres (Figure 1)(Hoberman, 1988). The increases in volume and stability of the bacteriophage capsids transform the capsid such that the mature assembly is able to encapsulate DNA packaged to pressures rivaling bottled champagne (Roos et al., 2012).
Figure 1.

Viruses undergo dramatic conformation changes throughout their infectious cycles. (a) One example of this is seen in bacteriophage capsid maturation, where hundreds of protein subunits organized in an icosahedral shell reorganize while maintaining capsid integrity to produce an expanded mature capsid with thinner but more stable walls—due to greater inter-subunit contacts and fewer holes in the lattice—and greatly increased internal volume. A cross-section of HK97 bacteriophage is shown. Figure adapted from Wikoff et al. (2006)). This structural transformation is highly cooperative and is highly reminiscent of the expansion of (b) a mechanical Hoberman sphere, a “reversibly expandable, double-curved truss structure” Figure adapted from USA Patent US4942700A (Hoberman, 1988).
In establishing my independent research program, I searched for a biophysical mechanism and biological system where research at the intersection of structure, dynamics, and infectious disease could address long-standing questions of broad biological significance. I chose to dive into the study of protein-mediated membrane fusion since it is a fundamental biological process that takes place at pivotal stages of enveloped virus infection as well as during cellular vesicle trafficking, neuronal signaling, mitochondrial maintenance, and in cell–cell fusion events that take place during gamete fertilization, placenta morphogenesis, and muscle formation to name just a few examples (Martens and McMahon, 2008; Rey and Lok, 2018). Intriguingly, while the fundamental physical process that must be carried out is the same, namely fusion proteins draw two membranes together, initiate lipid merging, and break down the membrane barrier separating previously membrane-bound compartments, allowing their contents to mix, the machinery to carry out this process has evolved multiple times based upon many distinct protein folds and complexes. In addition, the environmental triggers that activate fusion machinery leading to the conformational changes that drive membrane remodeling are diverse, ranging from pH-induced activation following endocytic uptake into cells, to binding of one or more cellular receptors, to proteolytic cleavage of viral subunits, as well as combinations of these (White et al., 2023). How do diverse machines carry out what appears to be similar physical process of fusing two membranes into one, and do they traverse similar intermediate states and follow similar reaction pathways?
To address these questions, one would like to image and understand how fusion protein conformational change is coupled to membrane reorganization and to identify what stages of membrane bilayer deformation are populated leading to lipid mixing and fusion. While it had long been speculated that an aqueous fusion pore is ultimately formed following fusion, it had not been clear what its architecture might be and what steps of membrane remodeling lead up to its formation. The prevailing hypothesis that emerged from a rich body of theoretical and modeling studies suggested that fusion initiates when fusion proteins induce localized dimple-like contacts, leading to point-like interfaces that result in merging of the proximal outer leaflets forming a “hemifused stalk” (Figure 2) (Kozlov and Markin, 1983; Kozlovsky et al., 2002; Chernomordik and Kozlov, 2005). It had been proposed that the stalk then converts into a widened hemifused bilayer, or diaphragm, composed of the two distal or inner leaflets, which subsequently disintegrates to open a fusion pore allowing content transfer (Chernomordik et al., 1995; Kozlovsky et al., 2002; Nikolaus et al., 2011).
Figure 2.
A common pathway has been proposed for protein-mediated membrane fusion leading to fusion pore formation via a “hemifused” intermediate.
At the time it was not possible to directly test these hypothetical models, though extensive experimentation using fluorescence spectroscopy and microscopy could track the kinetics of lipid and content mixing. These early findings provided indirect support for the proposed stages of membrane reorganization but lacked the sufficient resolution needed to visualize detailed membrane structure and individual fusion proteins. Indeed, even super-resolution light microscopy can only resolve fluorescent probes at ~20 nanometer separation (Bond et al., 2022), whereas the phosphate headgroup layers for the leaflets of a membrane bilayer are typically spaced ~3–4 nm apart. Moreover, at the time, mechanisms of protein conformational change and their action on membranes were largely inferred from stable, inert pre- and post-fusion structures of ectodomain fragments that lacked key structural elements such as transmembrane anchors, membrane, and cytoplasmic domains that modulate fusion protein activity. The protein states that drive fusion reactions, however, are generally refractory to classical structure determination due to their transience and flexible, dynamic nature. Methods capable of capturing and imaging protein-mediated fusion reactions for intact fusion systems at molecular resolution were needed in order to reveal fusion mechanisms and to characterize critical intermediate states.
Choosing effective techniques to address the question
As it turns out, around the time I was learning about conformational changes in viruses, imaging techniques that were beautifully suited to address the questions of how viral machinery mediates membrane fusion were coming online and ready for application. Cryo-electron microscopy (cryo-EM) has revolutionized structural biology by enabling all manner of biological systems to be imaged in their native hydration states without requiring crystallization and fixation. Structure determination of complex macromolecular assemblies was unleashed from the constraints of crystallization. While structures of ribosomes, icosahedral viruses, and large macromolecular assemblies demonstrated the power of single particle analysis approaches, this approach generally involves stringent classification of individual images of each macromolecule or complex, sorting out conformational heterogeneity and variation, followed by averaging over tens and hundreds of thousand images of the most uniform computationally purified samples (Cheng, 2015; Cheng et al., 2015; Chari and Stark, 2023). The resulting high-resolution structures in some cases, can be based upon a small percentage of the total input dataset. Tomographic approaches by comparison offer a means to obtain 3D structures of complex biological objects that may not be amenable to global averaging. With cryo-electron tomography (cryo-ET), one images the sample over a range of axial angular tilts, then reconstructs the individual images into a 3D tomogram without relying on averaging over populations of conformationally homogeneous targets (Ognjenovic et al., 2019).
One common theme that has motivated our research and that seems to have been borne out by experiments is that it is difficult to infer how viral machinery or any biological machinery functions when examining just fragments or isolated components. When excised from their native membrane-bound contexts, the fusion proteins can lose stability, alter their conformation, and shift their dynamic behavior and activity. The ability to analyze whole viruses and intact, functional fusion systems using tomography has been critical to the effort to apply cryo-EM to analyze membrane fusion reactions. Indeed, viruses were some of the first complex biological objects visualized using cryo-ET, starting with herpes simplex, vaccinia, and later influenza A virus, HIV-1, SIV, and others (Figure 3) (Li, 2022). For the first time, using cryo-ET, the native architecture of whole intact viral particles could be resolved with molecular detail. Cryo-ET is the only structural technique available that can be used to image the 3D organization, abundance, and disposition of viral surface proteins, as well as to visualize internal structural assemblies such as matrix and capsid layers, nucleoprotein complexes, and membrane fine structure, including local curvature and leaflet integrity. Cryo-EM/ET can even be used to directly image lipid nanodomains (Cornell et al., 2020; Heberle et al., 2020). Of note, cryo-ET is also an especially powerful technique for studying biological processes such as membrane fusion because one can trigger a reaction and then flash-freeze the specimen, trapping intermediates and allowing the 3D organization of the fusion complexes to be visualized. It is thus perfectly suited to reveal how viral machinery drives membrane fusion.
Figure 3.
Cryo-electron tomography reveals virus ultrastructure with resolution of individual fusion glycoproteins (blue arrows), membrane organization, and internal viral contents. (a) Central section for influenza A virus, (b) paraformaldehyde fixed SARS-CoV-2, and (c) HIV-1 virus particles. The differences in fusion protein abundance and distribution are clearly evident. (cryo-ET by Drs. Long Gui, Nancy Hom, and Vidya Mangala Prasad).
Selecting a biological system to focus on
With an exciting biological question and a suitable experimental technique in hand, next it was necessary to choose a tractable system to study. As a virus and as a model system for membrane fusion, the influenza virus has been studied intensively for decades. Influenza hemagglutinin (HA), the virus’ receptor binding and membrane fusion protein, an archetypal largely helical, trimeric class-I fusion protein, was the best characterized system where crystal structures were available for pre- and post-fusion conformations of the isolated ectodomain, and considerable biophysical information was available from numerous techniques such as fluorescence spectroscopy and microscopy, NMR, negative stain EM, and even thin-section TEM of virus entering into cells (Figure 4a) (Matlin et al., 1981). Cryo-ET also had been shown to be effective for visualizing the architecture of whole influenza virions by Alasdair Steven’s and later Peter Rosenthal’s labs (Harris et al., 2006; Calder et al., 2010).
Figure 4.

Advances in resolution of influenza virus-membrane interactions. (a) Some of the earliest glimpses of influenza’s membrane fusion event were captured for the virus inside of endosomes using heavy metal-stained, thin-section TEM of fixed cells (Matlin et al., 1981). The pathway of virus entry could be tracked, but direct resolution of protein and membranes and their structures was not possible with this approach. With the application of cryo-ET, a virus interacting with liposomes in vitro could be visualized under native buffer conditions in vitrified ice. Over the past several years, technical advances have increased resolution and data quality as is evident from the sharper images of proteins as well as membrane fine structure including resolution of leaflets in cryo-electron tomograms. For example (b) shows a central section through a tomogram of influenza virus interacting with a liposome that was collected using a cryo-EM configuration with a side-entry sample holder and charge-coupled device (CCD) (Lee, 2010), while (c) shows a tomogram collected on a contemporary microscope with a more sensitive, fast frame readout direct electron detector, an energy filter to filter out inelastically scattered electrons, and a more stable specimen stage (cryo-ET image provided by Dr. Vidya Mangala Prasad).
The influenza virus was also an advantageous system for in vitro studies because the trigger that activates its HA fusion machinery is low pH, rather than receptor binding as is often employed by other viruses. Influenza encounters this activation signal following endocytic uptake into cells when endosomal maturation leads to staged drops in pH to ~5, which triggers HA’s membrane fusion activity, leading to genome delivery into the cytosol. In vitro, it was relatively straightforward to use liposomes and vesicles as a stand-in for the endosomal membrane and to acidify the buffer to mimic endosomal pH conditions (Figure 4b,c). Such an experimental setup also offered considerable control over target membrane lipid composition, pH, temperature, and timing, enabling us to test the influence of those variables on the fusion mechanism.
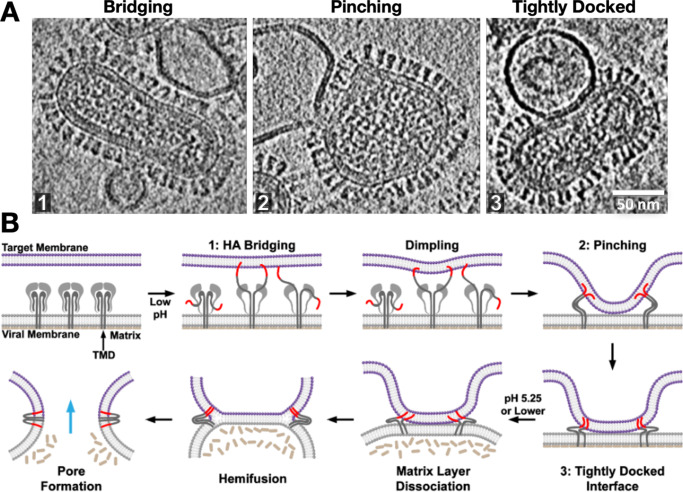
With this approach, we and others were able to capture snapshots of the intermediate states that the influenza virus traverses across the fusion pathway (Lee, 2010; Calder and Rosenthal, 2016; Chlanda et al., 2016; Gui et al., 2016; Benhaim et al., 2020). Moreover, by carrying out a time course and tracking the ebb and flow of intermediate state populations as the reaction proceeds, it was possible to infer the sequence that the reaction intermediates followed and reconstruct the pathway leading from fusion protein activation to stages of membrane remodeling all the way to fusion pore opening (Gui et al., 2016). These studies demonstrated that the initial stages of membrane remodeling, driven by hairpin refolding of a localized set of HA trimers, were almost entirely focused on the target membrane, resulting in the formation of tightly curved dimples that are drawn down to the relatively unperturbed virus surface. These studies demonstrated the role of the internal, membrane-associated viral matrix layer in butressing the viral envelope during these early stages of membrane reorganization. The matrix layer also exhibits pH-dependent activity, dissociating from the viral membrane at a pH below HA’s activation pH (Fontana and Steven, 2013; Gui et al., 2016). Thus, the internal superstructure helps to regulate viral membrane plasticity and progression along the fusion pathway. Indeed, the fusion machinery for influenza virus, and it is likely for many enveloped viruses that bear structural membrane-associated matrix layers, is embodied by not just the fusion protein itself, but the fusion protein, the viral membrane, and matrix proteins working in concert.
Strikingly, we observed that following dimple formation, rather than observing transitions to the previously proposed hemifusion stalk organization (Figure 2), the target and virus membranes became tightly docked over extended areas in which the proximal leaflets were apparently so closely apposed that they often appeared superimposed (Figure 5). This lipid configuration has also been observed in SNARE-mediated and mitofusin-mediated fusion complexes (Mirjanian et al., 2010; Hernandez et al., 2012). Interestingly simulations suggest that headgroups can line up along a single layer, appearing as a single leaflet, while acyl chains are splayed across the two membranes (Stevens et al., 2003; Kasson et al., 2010) or alternatively, the largely dehydrated headgroups from proximal leaflets may interact with each other to in essence “solvate” each other through dipolar coupling, reducing the dehydration penalty that is typically associated with bringing two phospholipid layers into close proximity (Witkowska et al., 2021). Tightly docked membrane contacts increased in abundance with the addition of fusion-promoting lipids and rose to be the dominant intermediate after dimple formation during influenza virus fusion (Gui et al., 2016). These intermediates then dissipated as post-fusion complexes rose, suggesting they lie on the fusion pathway. Surprisingly, in our hands, one of the main membrane fusion intermediates that had been previously investigated, the hemifusion diaphragm, was observed to be exceedingly rare, perhaps indicative of its transience and instability in the influenza virus system (Gui et al., 2016), while other groups were able to arrest fusion in hemifused states using HA fusion peptide mutants (Chlanda et al., 2016).
Figure 5.
Snapshots and inferred pathway of influenza virus HA-mediated membrane fusion with liposomes visualized with cryo-ET. (a) Several intermediate membrane remodeling intermediates can be captured by initiating fusion reactions in vitro and flash-freezing the reaction (Figure adapted from (Benhaim and Lee, 2020), original data in (Gui et al., 2016)). (b) By carrying out a time course and monitoring the population kinetics of these intermediates, the sequence and pathway for HA-mediated fusion were inferred. These experiments highlighted the centrality of the tightly docked membranes following target membrane apposition to the virus surface through localized dimples but did not capture classical hemifused “stalks.” Cryo-ET also demonstrated that the internal matrix structural layer works in concert with HA to mediate fusion.
Generalizing studies to identify conserved properties and fundamental physical aspects of the biological process
Visualization of membrane fusion intermediates at the level of detail described above would not have been achievable using any other biophysical or imaging approach. Cryo-ET analysis of numerous protein-mediated membrane fusion reactions with different fusion protein systems have now revealed striking commonalities, even for viruses from different families that employ protein fusogens from distinct structural folds organized on the virus surface in drastically different ways (White et al., 2023; Kephart et al., 2024). For example, class-II fusion proteins, such as those in flavi- and alphaviruses as well as in some eukaryotic gamete and cell fusion systems, are based upon an entirely different fold from the class-I fusogens (Rey and Lok, 2018). Despite this basic difference, these fusogens also have a membrane anchor and a fusion loop that is initially sequestered, only to become unmasked in response to a trigger. Once unmasked, the fusion loop inserts into a target membrane. Like in class-I fusion proteins, a switch to a folded-back hairpin is believed to colocalize the membrane-active elements, drawing the membranes together.
Perhaps not surprisingly, initial stages of the membrane fusion reaction in which the target membranes are engaged differ rather significantly between systems such as influenza HA and alphavirus E1-mediated reactions that have been described to date (Cao and Zhang, 2013; Calder and Rosenthal, 2016; Chlanda et al., 2016; Gui et al., 2016; Mangala Prasad et al., 2022). However, once the fusion proteins fold back to draw the membranes into apposition, common membrane and fusion protein configurations are observed. Namely, an increasing abundance of experimental observation seems to indicate that tightly docked membranes with juxtaposed or even comingling proximal leaflets are populated by nearly all fusion systems observed to date.
Later stages of fusion reveal some differences, particularly in the propensity for the fusion complexes to transition between the states we have observed, such as between tightly docked membranes, hemifusion diaphragms, and open fusion pores. For example, while hemifusion was a rare, transiently populated intermediate with influenza virus, for alphavirus fusion, large hemifused diaphragms that spanned nearly the viral particle diameter were relatively abundant at intermediate time points, and then dissipated as the reaction proceeded (Mangala Prasad et al., 2022). The diaphragms were seen to disintegrate, opening fusion pores that enabled nucleocapsid delivery. The greater instability of hemifusion diaphragms among class-I viral fusion systems may, in part, be due to the ability of transmembrane and fusion peptide domains to form tight complexes in the folded-back hairpin state, which likely applies force to the membranes and destabilizes local lipid packing. By contrast, such a coupling of the two membrane active motifs, fusion loop and transmembrane domain, in class-II fusion proteins does not seem to be generally observed.
What do the cryo-ET snapshots miss?
One notable limitation of the “time-resolved” cryo-ET approach is it mainly reveals the metastable states that accumulate in population over the course of a fusion reaction, but the transient transition states are sparingly and fleetingly populated at any given time and thus difficult to capture and characterize in detail. To have a hope of analyzing these transient intermediates will require more extensive sampling of larger populations. Computational modeling, molecular dynamics and simulation coupled to quantitative analysis of cryo-ET data can also provide highly detailed insights into the transitions between membrane configurations and the forces and energetics that shape membrane remodeling during fusion.
On a related note, the essential dimension of time is missing from the cryo-ET snapshots. Population kinetics can show how populations of viruses progress along a reaction pathway, taking seconds to minutes to reach completion, but each individual reaction thus far can only be monitored using fluorescence light microscopy and tracking the exchange of lipid probes and fluorescent contents, and this appears to occur on the millisecond-second time scales (Floyd et al., 2008; Blijleven et al., 2016).
Turning to complementary methods to probe protein dynamics and conformational change
As informative as the cryo-ET analysis of protein-mediated membrane fusion has been, particularly for revealing membrane remodeling states and how fusion proteins engage with the membranes, complementary methods are needed to probe how the fusion protein machinery itself is acting during the fusion process. Cryo-ET at present does not provide sufficient resolution to reveal how proteins are refolding beyond highlighting large-scale changes in structure as the fusogens drive membrane remodeling, though coupled with modeling and simulations, quasiatomic models can be compared with and docked into the tomograms.
Given the dynamic nature of the fusion machinery, our lab sought to develop experimental methods that would enable conformational changes to be monitored in the protein machinery itself. Various approaches to investigate viral protein dynamics have been described in recent years including, for example, single-molecule FRET and molecular dynamics simulations (Risselada and Grubmuller, 2021; Groves et al., 2023). We pursued a structural mass spectrometry approach called hydrogen/deuterium-exchange mass spectrometry (HDX-MS) (Hodge et al., 2020). HDX-MS probes the degree to which backbone amides are involved in secondary structure and indirectly in deeply buried regions of protein (Venkatakrishnan et al., 2024). HDX-MS does not provide 3D structural information but rather enables us to track local protein dynamics along segments of polypeptide sequence. It is particularly effective for probing conformational changes, effects of ligand binding, and for examining how proteins respond to changes in the environment.
We and others have used HDX-MS to monitor receptor and environment-triggered conformational changes in viral fusion proteins such as HIV-1 Env, SARS-CoV-2 S, and influenza HA (Figure 6). This method can be applied to both soluble, recombinant proteins, as well as viral fusion proteins in their native membrane-displayed contexts (Davenport et al., 2013; Guttman et al., 2014, 2015; Garcia et al., 2015; Lim et al., 2017; Benhaim et al., 2020; Hodge et al., 2022, 2023; Mangala Prasad et al., 2022b; Braet et al., 2023; Calvaresi et al., 2023). In one study, we applied a pulse deuteration approach to compare pH-triggered refolding of influenza HA in soluble ectodomain form and on intact virions (Benhaim et al., 2020). By labeling the samples with pulses of buffer made with D2O at specific time points following pH-triggered HA fusion activation, it was possible to track the regions of HA that changed conformation and to monitor how their local structural dynamic behavior changed as they transitioned from pre- to post-fusion conformations.
Figure 6.
Fusion proteins in native contexts, such as on whole virions, function differently than isolated, soluble ectodomains that are typically the subject of structural studies. We used pulse deuteration HDX-MS to monitor activation and conformational change of influenza HA refolding pathway (a) on intact influenza virus (b) revealing the existence of a transient but highly populated dynamic intermediate state with exposed fusion peptides and dynamic B-loop motifs, in contrast to soluble HA ectodomain (c) that converted directly from pre- to post-fusion states (Benhaim et al., 2020). Pulse deuteration HDX-MS is thus a powerful means to take snapshots as the proteins traverse their pathways of conformational change and track the states of each peptide segment throughout the protein populates over time (insets on the right).
Early in their study, HA and other viral fusion proteins were described as undergoing a spring-loaded transition from pre- to post-fusion states (Carr and Kim, 1993). Indeed, when one examines isolated fusion protein ectodomains, they often do behave in a two-state fashion, transitioning from a pre- to post-fusion conformation as our pulse HDX-MS analysis of HA demonstrated (Figure 6) (Benhaim et al., 2020). However, when HA is activated in its native context on viral particles, with transmembrane anchor embedded in the viral membrane and cytoplasmic tail present where it can interact with the internal matrix layer, the fusion assembly populates highly dynamic intermediate conformational ensembles with unrestrained fusion peptide domains that can persist for extended periods of time before the assembly ultimately converts to the post-fusion conformation. This is in striking contrast to what was observed for soluble HA ectodomains. The stabilization of dynamic intermediate conformations with fusion peptides freely deployed likely facilitates virus engagement with target membranes.
HDX-MS has also proven uniquely capable of providing structural and dynamic insights into how a given fusion protein assembly differs among strains of a given virus (Davenport et al., 2013; Garcia et al., 2015; Guttman et al., 2015; Lim et al., 2017, 2021; Sharma et al., 2019; Hodge et al., 2022, 2023; Braet et al., 2023; Calvaresi et al., 2023). A motivating hypothesis for us was that much of the structural basis for antigenic, phenotypic and functional variation between viral strains would be most evident at the level of structural dynamics, when the virus entry and fusion machines are fluctuating with thermal energy under native conditions. Indeed, the same machines that mediate the pivotal first steps of virus infection of cells, receptor binding, and membrane fusion, are also the primary target for neutralizing antibodies and thus subject to intense immune selective pressures. They undergo rapid diversification and immune escape while maintaining conserved receptor binding and fusion activities. HIV-1 offers an extreme example of such diversification, yet to date, structural analysis has been largely focused on a few well-behaved, stable HIV-1 Env glycoprotein variants. Combined HDX-MS and antibody binding studies of HIV-1 Env trimers revealed that differences in antigenic phenotype can be traced back to differences in local epitope dynamic fluctuations in the fusion protein assemblies as well as to differences in large-scale conformational dynamic switching where a polypeptide segment and associated structural domain may sample conformational states with different backbone amide protection and deuteration propensities on a relatively slow time scale (Weis et al., 2006; Hodge et al., 2022, 2023; Venkatakrishnan et al., 2024). Likewise, HDX-MS has revealed differences in fusion protein activation dynamics between strains in influenza HA, coronavirus spike proteins, and among dengue virus serotypes (Lim et al., 2017; Sharma et al., 2019; Braet et al., 2023; Calvaresi et al., 2023; Chen et al., 2023; Garcia et al., 2023). Structural dynamics thus embody cryptic determinants of virus phenotype and provide a unique window into strain-specific variation that is difficult to access through most structural approaches.
Outlook
One important question that looms over the studies described above is: do these processes operate in cells and in vivo in ways that track with the in vitro studies? Addressing this is becoming more feasible with the availability of cellular tomography methods that utilize focused ion beam milling to produce thin “lamella” suitable for cryo-ET, and recent studies have begun to catch glimpses of virus processes, including membrane fusion deep inside of cells (Graham and Zhang, 2023; Hong et al., 2023; Zimmermann and Chlanda, 2023; Zhou and Lok, 2024). While methods are being developed for in-cell HDX-MS, the method is inherently a bulk population measurement, and it is not feasible to extract information for the subset of fusion proteins that may be mediating fusion at a given time against the overwhelming background of the fusion proteins present in total. Nevertheless, looking ahead, it seems clear that through a combination of in vitro and in cell methods, coupled with computational modeling to connect experimentally observed snapshots, the mechanisms that drive the essential and ubiquitous biophysical and biological process of protein-mediated membrane fusion will come into ever sharper focus.
Acknowledgments
The aim of this Perspectives review is to describe one researcher’s scientific trajectory rather than to provide a comprehensive overview of protein-mediated membrane fusion or enveloped virus structural biology, which have been reviewed in a number of excellent recent articles (Li, 2022; White et al., 2023; Winter and Chlanda, 2023; Jahn et al., 2024; Kephart et al., 2024; Zhou and Lok, 2024). Studies of viral glycoprotein structure, dynamics, and membrane fusion in the Lee lab are supported by R01-AI165808, R01-AI140868, and R01-AI179697.
Open peer review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/qrd.2025.3.
Author contribution
The author conceived of and wrote this Perspectives review.
Competing interests
The author declares that he has no competing financial interests.
References
- Benhaim MA and Lee KK (2020) New biophysical approaches reveal the dynamics and mechanics of type i viral fusion machinery and their interplay with membranes. Viruses 12(4). 10.3390/v12040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhaim MA, Mangala Prasad V, Garcia NK, Guttman M and Lee KK (2020) Structural monitoring of a transient intermediate in the hemagglutinin fusion machinery on influenza virions. Science Advances 6(18), eaaz8822. 10.1126/sciadv.aaz8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blijleven JS, Boonstra S, Onck PR, van der Giessen E and van Oijen AM (2016) Mechanisms of influenza viral membrane fusion. Seminars in Cell and Developmental Biology 60, 78–88. 10.1016/j.semcdb.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Bond C, Santiago-Ruiz AN, Tang Q and Lakadamyali M (2022) Technological advances in super-resolution microscopy to study cellular processes. Molecular Cell 82(2), 315–332. 10.1016/j.molcel.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braet SM, Buckley TSC, Venkatakrishnan V, Dam KA, Bjorkman PJ and Anand GS (2023) Timeline of changes in spike conformational dynamics in emergent SARS-CoV-2 variants reveal progressive stabilization of trimer stalk with altered NTD dynamics. Elife 12, 1–21. 10.7554/eLife.82584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder LJ and Rosenthal PB (2016) Cryomicroscopy provides structural snapshots of influenza virus membrane fusion. Nature Structural and Molecular Biology 23(9), 853–858. 10.1038/nsmb.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder LJ, Wasilewski S, Berriman JA and Rosenthal PB (2010) Structural organization of a filamentous influenza A virus. Proceedings of the National Academy of Sciences U S A 107(23), 10685–10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvaresi V, Wrobel AG, Toporowska J, Hammerschmid D, Doores KJ, Bradshaw RT, Parsons RB, Benton DJ, Roustan C, Reading E, Malim MH, Gamblin SJ and Politis A (2023) Structural dynamics in the evolution of SARS-CoV-2 spike glycoprotein. Nature Communications 14(1), 1421. 10.1038/s41467-023-36745-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S and Zhang W (2013) Characterization of an early-stage fusion intermediate of Sindbis virus using cryoelectron microscopy. Proceedings of the National Academy of Sciences U S A 110(33), 13362–13367. 10.1073/pnas.1301911110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CM and Kim PS (1993) A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell 73(4), 823–832. [DOI] [PubMed] [Google Scholar]
- Chari A and Stark H (2023) Prospects and limitations of high-resolution single-particle cryo-electron microscopy. Annual Review of Biophysics 52, 391–411. 10.1146/annurev-biophys-111622-091300. [DOI] [PubMed] [Google Scholar]
- Chen C, Zhu R, Hodge EA, Diaz-Salinas MA, Nguyen A, Munro JB and Lee KK (2023) hACE2-induced allosteric activation in SARS-CoV versus SARS-CoV-2 spike assemblies revealed by structural dynamics. ACS Infectious Diseases 9(6), 1180–1189. 10.1021/acsinfecdis.3c00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y (2015) Single-particle cryo-EM at crystallographic resolution. Cell 161(3), 450–457. 10.1016/j.cell.2015.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Grigorieff N, Penczek PA and Walz T (2015) A primer to single-particle cryo-electron microscopy. Cell 161(3), 438–449. 10.1016/j.cell.2015.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L, Chanturiya A, Green J and Zimmerberg J (1995) The hemifusion intermediate and its conversion to complete fusion: Regulation by membrane composition. Biophysical Journal 69(3), 922–929. 10.1016/S0006-3495(95)79966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV and Kozlov MM (2005) Membrane hemifusion: Crossing a chasm in two leaps. Cell 123(3), 375–382. [DOI] [PubMed] [Google Scholar]
- Chlanda P, Mekhedov E, Waters H, Schwartz CL, Fischer ER, Ryham RJ, Cohen FS, Blank PS and Zimmerberg J (2016) The hemifusion structure induced by influenza virus haemagglutinin is determined by physical properties of the target membranes. Nature Microbiology 1(6), 16050. 10.1038/nmicrobiol.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell CE, Mileant A, Thakkar N, Lee KK and Keller SL (2020) Direct imaging of liquid domains in membranes by cryo-electron tomography. Proceedings of the National Academy of Sciences U S A 117(33), 19713–19719. 10.1073/pnas.2002245117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport TM, Guttman M, Guo W, Cleveland B, Kahn M, Hu SL and Lee KK (2013) Isolate-specific differences in the conformational dynamics and antigenicity of HIV-1 gp120. Journal of Virology 87, 10855–10873. 10.1128/JVI.01535-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd DL, Ragains JR, Skehel JJ, Harrison SC and van Oijen AM (2008) Single-particle kinetics of influenza virus membrane fusion. Proceedings of the National Academy of Sciences U S A 105(40), 15382–15387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana J and Steven AC (2013) At low pH, influenza virus matrix protein M1 undergoes a conformational change prior to dissociating from the membrane. Journal of Virology 87(10), 5621–5628. 10.1128/JVI.00276-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia NK, Guttman M, Ebner JL and Lee KK (2015) Dynamic changes during acid-induced activation of influenza hemagglutinin. Structure 23(4), 665–676. 10.1016/j.str.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia NK, Kephart SM, Benhaim MA, Matsui T, Mileant A, Guttman M and Lee KK (2023) Structural dynamics reveal subtype-specific activation and inhibition of influenza virus hemagglutinin. Journal of Biological Chemistry 299(6), 104765. 10.1016/j.jbc.2023.104765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M and Zhang P (2023) Cryo-electron tomography to study viral infection. Biochemical Society Transactions 51(4), 1701–1711. 10.1042/BST20230103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves D, Hepp C, Kapanidis AN and Robb NC (2023) Single-molecule FRET for virology: 20 years of insight into protein structure and dynamics. Quarterly Reviews in Biophysics 56, e3. 10.1017/S0033583523000021. [DOI] [PubMed] [Google Scholar]
- Gui L, Ebner JL, Mileant A, Williams JA and Lee KK (2016) Visualization and sequencing of membrane remodeling leading to influenza virus fusion. Journal of Virology. 10.1128/JVI.00240-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M, Cupo A, Julien JP, Sanders RW, Wilson IA, Moore JP and Lee KK (2015) Antibody potency relates to the ability to recognize the closed, pre-fusion form of HIV Env. Nature Communications 6, 6144. 10.1038/ncomms7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M, Garcia NK, Cupo A, Matsui T, Julien JP, Sanders RW, Wilson IA, Moore JP and Lee KK (2014) CD4-induced activation in a soluble HIV-1 Env trimer. Structure 22(7), 974–984. 10.1016/j.str.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, Cardone G, Winkler DC, Heymann JB, Brecher M, White JM and Steven AC (2006) Influenza virus pleiomorphy characterized by cryoelectron tomography. Proceedings of the National Academy of Sciences U S A 103(50), 19123–19127. 10.1073/pnas.0607614103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberle FA, Doktorova M, Scott HL, Skinkle AD, Waxham MN and Levental I (2020) Direct label-free imaging of nanodomains in biomimetic and biological membranes by cryogenic electron microscopy. Proceedings of the National Academy of Sciences U S A 117(33), 19943–19952. 10.1073/pnas.2002200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JM, Stein A, Behrmann E, Riedel D, Cypionka A, Farsi Z, Walla PJ, Raunser S and Jahn R (2012) Membrane fusion intermediates via directional and full assembly of the SNARE complex. Science 336(6088), 1581–1584. 10.1126/science.1221976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoberman C (1988) Reversibly expandable doubly-curved truss structure. USA.
- Hodge EA, Benhaim MA and Lee KK (2020) Bridging protein structure, dynamics, and function using hydrogen/deuterium-exchange mass spectrometry. Protein Science 29(4), 843–855. 10.1002/pro.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge EA, Chatterjee A, Chen C, Naika GS, Laohajaratsang M, Mangala Prasad V and Lee KK (2023) An HIV-1 broadly neutralizing antibody overcomes structural and dynamic variation through highly focused epitope targeting. NPJ Viruses 1(1), 2. 10.1038/s44298-023-00002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge EA, Naika GS, Kephart SM, Nguyen A, Zhu R, Benhaim MA, Guo W, Moore JP, Hu SL, Sanders RW and Lee KK (2022) Structural dynamics reveal isolate-specific differences at neutralization epitopes on HIV Env. iScience 25(6), 104449. 10.1016/j.isci.2022.104449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Song Y, Zhang Z and Li S (2023) Cryo-electron tomography: The resolution revolution and a surge of in situ virological discoveries. Annual Review of Biophysics 52, 339–360. 10.1146/annurev-biophys-092022-100958. [DOI] [PubMed] [Google Scholar]
- Jahn R, Cafiso DC and Tamm LK (2024) Mechanisms of SNARE proteins in membrane fusion. Nature Reviews Molecular Cell Biology 25(2), 101–118. 10.1038/s41580-023-00668-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasson PM, Lindahl E and Pande VS (2010) Atomic-resolution simulations predict a transition state for vesicle fusion defined by contact of a few lipid tails. PLoS Computational Biology 6(6), e1000829. 10.1371/journal.pcbi.1000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kephart SM, Hom N and Lee KK (2024) Visualizing intermediate stages of viral membrane fusion by cryo-electron tomography. Trends in Biochemical Sciences 49(10), 916–931. 10.1016/j.tibs.2024.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov MM and Markin VS (1983) [Possible mechanism of membrane fusion]. Biofizika 28(2), 242–247. [PubMed] [Google Scholar]
- Kozlovsky Y, Chernomordik LV and Kozlov MM (2002) Lipid intermediates in membrane fusion: formation, structure, and decay of hemifusion diaphragm. Biophysical Journal 83(5), 2634–2651. 10.1016/S0006-3495(02)75274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK (2010) Architecture of a nascent viral fusion pore. EMBO Journal 29(7), 1299–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK, Fitch CA and Garcia-Moreno EB (2002a) Distance dependence and salt sensitivity of pairwise, coulombic interactions in a protein. Protein Science 11(5), 1004–1016. 10.1110/ps.4700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK, Fitch CA, Lecomte JT and Garcia-Moreno EB (2002b) Electrostatic effects in highly charged proteins: Salt sensitivity of pKa values of histidines in staphylococcal nuclease. Biochemistry 41(17), 5656–5667. 10.1021/bi0119417. [DOI] [PubMed] [Google Scholar]
- Li S (2022) Cryo-electron tomography of enveloped viruses. Trends in Biochemical Sciences 47(2), 173–186. 10.1016/j.tibs.2021.08.005. [DOI] [PubMed] [Google Scholar]
- Lim XX, Chandramohan A, Lim XY, Bag N, Sharma KK, Wirawan M, Wohland T, Lok SM and Anand GS (2017) Conformational changes in intact dengue virus reveal serotype-specific expansion. Nature Communications 8, 14339. 10.1038/ncomms14339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim XX, Shu B, Zhang S, AWK Tan, Ng TS, Lim XN, Chew VS, Shi J, Screaton GR, Lok SM and Anand GS (2021) Human antibody C10 neutralizes by diminishing Zika but enhancing dengue virus dynamics. Cell 184(25), 6067–6080.e13 Placeholder Text. 10.1016/j.cell.2021.11.009. [DOI] [PubMed] [Google Scholar]
- Mangala Prasad V, Blijleven JS, Smit JM and Lee KK (2022) Visualization of conformational changes and membrane remodeling leading to genome delivery by viral class-II fusion machinery. Nature Communications 13(1). 10.1038/s41467-022-32431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangala Prasad V, Leaman DP, Lovendahl KN, Croft JT, Benhaim MA, Hodge EA, Zwick MB and Lee KK (2022b) Cryo-ET of Env on intact HIV virions reveals structural variation and positioning on the Gag lattice. Cell 185(4), 641–653 e617. 10.1016/j.cell.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens S and McMahon HT (2008) Mechanisms of membrane fusion: Disparate players and common principles. Nature Reviews Molecular Cell Biology 9(7), 543–556. 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- Matlin KS, Reggio H, Helenius A and Simons K (1981) Infectious entry pathway of influenza virus in a canine kidney cell line. Journal of Cell Biology 91(3 Pt 1), 601–613. 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirjanian D, Dickey AN, Hoh JH, Woolf TB and Stevens MJ (2010) Splaying of aliphatic tails plays a central role in barrier crossing during liposome fusion. Journal of Physiscal Chemistry B 114(34), 11061–11068. 10.1021/jp1055182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaus J, Warner JM, O’Shaughnessy B and Herrmann A (2011) The pathway to membrane fusion through hemifusion. Current Topics in Membranes 68, 1–32. 10.1016/B978-0-12-385891-7.00001-5. [DOI] [PubMed] [Google Scholar]
- Ognjenovic J, Grisshammer R and Subramaniam S (2019) Frontiers in cryo electron microscopy of complex macromolecular assemblies. Annual Review of Biomedical Engineering 21, 395–415. 10.1146/annurev-bioeng-060418-052453. [DOI] [PubMed] [Google Scholar]
- Rey FA and Lok SM (2018) Common features of enveloped viruses and implications for immunogen design for next-generation vaccines. Cell 172(6), 1319–1334. 10.1016/j.cell.2018.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risselada HJ and Grubmuller H (2021) How proteins open fusion pores: insights from molecular simulations. European Biophysics Journal 50(2), 279–293. 10.1007/s00249-020-01484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos WH, Gertsman I, May ER, Brooks CL 3rd, Johnson JE and Wuite GJ (2012) Mechanics of bacteriophage maturation. Proceedings of the National Academy of Sciences U S A 109(7), 2342–2347. 10.1073/pnas.1109590109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma KK, Lim XX, Tantirimudalige SN, Gupta A, Marzinek JK, Holdbrook D, XYE Lim, Bond PJ, Anand GS and Wohland T (2019) Infectivity of dengue virus serotypes 1 and 2 Is correlated with E-protein intrinsic dynamics but not to envelope conformations. Structure 27(4), 618–630 e614. 10.1016/j.str.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Stevens MJ, Hoh JH and Woolf TB (2003) Insights into the molecular mechanism of membrane fusion from simulation: evidence for the association of splayed tails. Physical Review Letters 91(18), 188102. 10.1103/PhysRevLett.91.188102. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan V, Braet SM and Anand GS (2024) Dynamics, allostery, and stabilities of whole virus particles by amide hydrogen/deuterium exchange mass spectrometry (HDXMS). Current Opinion in Structural Biology 86, 102787. 10.1016/j.sbi.2024.102787. [DOI] [PubMed] [Google Scholar]
- Weis DD, Wales TE, Engen JR, Hotchko M and Ten Eyck LF (2006) Identification and characterization of EX1 kinetics in H/D exchange mass spectrometry by peak width analysis. Journal of the American Society of Mass Spectrometry 17(11), 1498–1509. [DOI] [PubMed] [Google Scholar]
- White JM, Ward AE, Odongo L and Tamm LK (2023) Viral membrane fusion: A dance between proteins and lipids. Annual Review of Virology 10(1), 139–161. 10.1146/annurev-virology-111821-093413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikoff WR, Conway JF, Tang J, Lee KK, Gan L, Cheng N, Duda RL, Hendrix RW, Steven AC and Johnson JE (2006) Time-resolved molecular dynamics of bacteriophage HK97 capsid maturation interpreted by electron cryo-microscopy and X-ray crystallography. Journal of Structural Biology 153(3), 300–306. 10.1016/j.jsb.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Winter SL and Chlanda P (2023) The art of viral membrane fusion and penetration. Subcellular Biochemistry 106, 113–152. 10.1007/978-3-031-40086-5_4. [DOI] [PubMed] [Google Scholar]
- Witkowska A, Heinz LP, Grubmuller H and Jahn R (2021) Tight docking of membranes before fusion represents a metastable state with unique properties. Nature Communications 12(1), 3606. 10.1038/s41467-021-23722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q and Lok SM (2024) Visualizing the virus world inside the cell by cryo-electron tomography. Journal of Virology 98(12), e0108523. 10.1128/jvi.01085-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann L and Chlanda P (2023) Cryo-electron tomography of viral infection – from applications to biosafety. Current Opinion in Virology 61, 101338. 10.1016/j.coviro.2023.101338. [DOI] [PubMed] [Google Scholar]