Abstract
Introduction
Infants have the highest incidence of meningococcal meningitis (MM) among all age groups in China. Infants receive their first and second doses of serogroup A meningococcal polysaccharide vaccine at 6 and 9 months of age.
Methods
We extracted data on MM cases among 0–11-month-old children reported during 2006–2023 from the National Notifiable Diseases Registry System and the National Meningococcal Disease Surveillance System and conducted an epidemiological and clinical analysis.
Results
During the study period, 721 infant MM cases were reported. Incidence decreased from 7.31 cases per million to 2.74 per million, while the all-age incidence declined from 1.27 cases per million to 0.06 per million. Among 210 cases with serogrouping results, five serogroups (A, B, C, W, Y) and non-groupable strains were detected. Serogroup A cases decreased from 36.36% to 1.87% during the study period, while serogroup B increased from 14.55% to 67.29%. Fever, nausea, and/or vomiting were common symptoms across all serogroups. The frequencies of petechiae and/or purpura in serogroup A (73%) and C (92%) were substantially higher than in other serogroups. Among serogroup B cases, 26.42% developed petechiae and/or purpura, 26.42% exhibited neck stiffness, and 13.21% had positive Kernig’s and/or Brudzinski’s signs.
Conclusions
The incidence of MM in infants has significantly decreased but remains higher than incidence across all age groups. Serogroup B cases were the most common. Atypical symptoms in infant cases challenge timely diagnosis. We suggest eligible infants receive meningococcal vaccination timely, and the development of serogroup B meningococcal vaccines should be accelerated.
Keywords: Meningococcal meningitis, Infants, Serogroups
Meningococcal meningitis (MM) is an acute respiratory-transmitted infectious disease caused by Neisseria meningitidis (N. meningitidis) that is characterized by high fatality and serious sequelae in survivors (1–2). From the 1980s to 2007, meningococcal vaccine was a non-program vaccine in China. In 2007, two meningococcal polysaccharide vaccines were introduced into the National Immunization Program (NIP) in a schedule that has been adhered to since then. Children receive serogroup A meningococcal polysaccharide vaccine at 6 months and 9 months of age and serogroups A and C meningococcal polysaccharide vaccine at 3 years and 6 years of age. In addition to these program vaccines, three non-program meningococcal vaccines are available as voluntary, family-pay vaccines: serogroups A and C meningococcal conjugate vaccine, ACYW135 meningococcal polysaccharide vaccine, and ACYW135 meningococcal conjugate vaccine. Due to widespread vaccine use, the incidence of MM decreased from 110 per million in 1985 to 0.91 per million in 2007, and further declined to 0.06 per million in 2023 (3). However, the risk of infection and progression to severe disease in younger children remains elevated compared to other age groups, partly due to their immature immune systems and changes in pathogenic N. meningitidis serogroups (4–5). The National Meningococcal Disease Surveillance System was established in 2006, enabling access to more detailed epidemiological and clinical data on MM cases. In this study, we conducted a descriptive analysis of nationwide surveillance data for infant cases from 2006 to 2023. Our findings provide substantial evidence supporting further research on disease burden in young infants and vaccination strategies for eligible children.
METHODS
Epidemiological data on MM cases were obtained from the National Notifiable Diseases Registry System (NNDRS). Clinical and serogrouping data were obtained from the National Meningococcal Disease Surveillance System (NMDSS). All hospitals across China report and register MM cases through these two systems.
We analyzed all reported MM cases in children 0–11 months old with illness onset between January 1, 2006 and December 31, 2023. This analysis comprehensively included both clinically diagnosed cases (diagnosed with MM based on clinical symptoms and signs without laboratory etiological detection) and laboratory-confirmed cases (with positive etiological detection for N. meningitidis), as classified by hospitals. N. meningitidis serogrouping results were verified by provincial centers for disease control and prevention (CDCs). All cases in this study were successfully matched when merging the two systems using unique notifiable disease case identifiers.
Data processing and analyses were performed with Excel (version 2016, Microsoft Office, Washington, USA), SAS (version 9.4, SAS Institute Inc., Cary, NC, USA), and R (version 4.2.1, R Foundation for Statistical Computing, Vienna, Austria). We calculated infants’ ages in months by subtracting birth dates from the onset dates. Subjects were divided into four age groups based on vaccination schedule: 0–2 months, 3–5 months, 6–9 months, and 10–11 months. We used Fisher’s exact test to compare clinical manifestations between cases with different serogroups; two-sided P<0.05 was considered statistically significant.
RESULTS
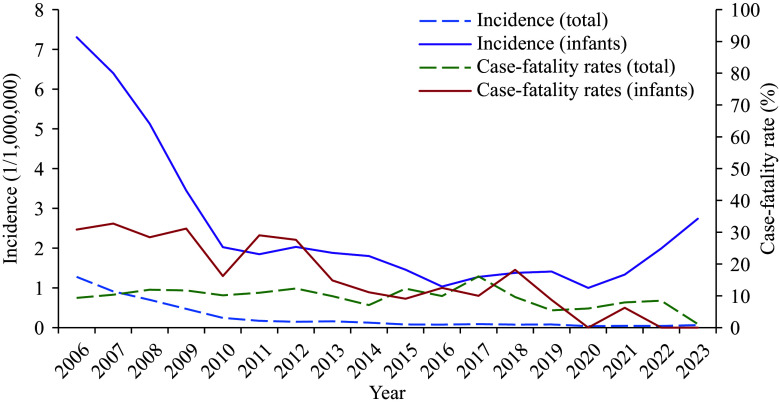
During the study period of 2006–2023, 721 infant MM cases were reported in China. The incidence decreased from 7.31 per million in 2006 to 2.74 per million in 2023. Following the introduction of meningococcal vaccines to the NIP, there was a rapid decline in incidence between 2006 and 2010. Since 2010, incidence has fluctuated between 1 per million and 2.74 per million. Throughout the study period, 160 fatal infant MM cases were reported, with the fatality rate ranging from 32.71% in 2007 to 0% in 2022–2023 (Figure 1).
Figure 1.
Incidence and case-fatality rates of reported MM in all ages and in infants in China, 2006–2023.
Abbreviation: MM=meningococcal meningitis.
The distribution of cases across time periods showed 422 cases during 2006–2010, 137 during 2011–2015, and 162 during 2016–2023, accounting for 8.78% (422/4,806), 14.89% (137/920), and 22.98% (162/705) of all cases reported across all age groups, respectively. Among infant cases, the proportion of those aged 0–5 months increased from 39.81% during 2006–2010 to 73.46% during 2016–2023 (Table 1).
Table 1. Characteristics of Neisseria meningitidis cases under 1 year of age in China, 2006–2023.
| Variable | Year | Total | ||
| 2006–2010 | 2011–2015 | 2016–2023 | ||
| Number of cases in all age groups | 4 806 | 920 | 705 | 6 431 |
| Cases aged 0–11 months, n (%) | 422 (8.78) | 137 (14.89) | 162 (22.98) | 721 (11.21) |
| Gender, n (%) | ||||
| Male | 245 (58.06) | 89 (64.96) | 94 (58.02) | 429 (59.50) |
| Female | 177 (41.94) | 48 (35.04) | 68 (41.98) | 292 (40.50) |
| Age in months | ||||
| 0–2 | 57 (13.51) | 36 (26.28) | 70 (43.21) | 163 (22.61) |
| 3–5 | 111 (26.30) | 43 (31.39) | 49 (30.25) | 203 (28.16) |
| 6–9 | 177 (41.94) | 49 (35.77) | 28 (17.28) | 254 (35.23) |
| 10–11 | 77 (18.25) | 9 (6.57) | 15 (9.26) | 101 (14.01) |
Of the 210 cases with available N. meningitidis serogrouping results, five serogroups (A, B, C, W, Y) and non-groupable strains were identified (Table 2). Serogroup A predominated from 2006 to 2010, accounting for 36.36% (20/55) of cases. Its prevalence subsequently declined to less than 10% during 2011–2015 and 2016–2023, while serogroup B emerged as the most common serogroup (67.29%, 72/107) during these later periods (Table 2, Figure 2A). Among the 160 fatal cases, 20 had serogrouping results, with serogroup B accounting for 55% (11 cases), followed by serogroup A (5 cases), serogroup C (2 cases), and non-groupable strains (2 cases).
Table 2. Distribution of serogroups of N. meningitidis in cases under 1 year of age in China, 2006–2023.
| Variable | Serogroups of of N. meningitidis | Total | |||||
|
Serogroup A
n (%) |
Serogroup B
n (%) |
Serogroup C
n (%) |
Serogroup W
n (%) |
Serogroup Y
n (%) |
Non-groupable | ||
| Abbreviation: N. meningitidis=Neisseria meningitidis. | |||||||
|
N. meningitides had serogrouping results |
26 (12.38) | 106 (50.48) | 12 (5.71) | 2 (0.95) | 2 (0.95) | 62 (29.52) | 210 (100) |
| Years | |||||||
| 2006–2010 | 20 (36.36) | 8 (14.55) | 8 (14.55) | 0 (0) | 0 (0) | 19 (34.55) | 55 (100) |
| 2011–2015 | 4 (8.33) | 26 (54.17) | 1 (2.08) | 1 (2.08) | 0 (0) | 16 (33.33) | 48 (100) |
| 2016–2023 | 2 (1.87) | 72 (67.29) | 3 (2.80) | 1 (0.93) | 2 (1.87) | 27 (25.23) | 107 (100) |
| Age in months | |||||||
| 0–2 | 1 (1.32) | 47 (61.84) | 2 (2.63) | 1 (1.32) | 1 (1.32) | 24 (31.58) | 76 (100) |
| 3–5 | 11 (15.49) | 36 (50.70) | 4 (5.63) | 1 (1.41) | 1 (1.41) | 18 (25.35) | 72 (100) |
| 6–9 | 9 (20.45) | 20 (45.45) | 3 (6.82) | 0 (0) | 0 (0) | 12 (27.27) | 44 (100) |
| 10–11 | 5 (26.32) | 3 (15.79) | 3 (15.79) | 0 (0) | 0 (0) | 8 (42.11) | 19 (100) |
Figure 2.
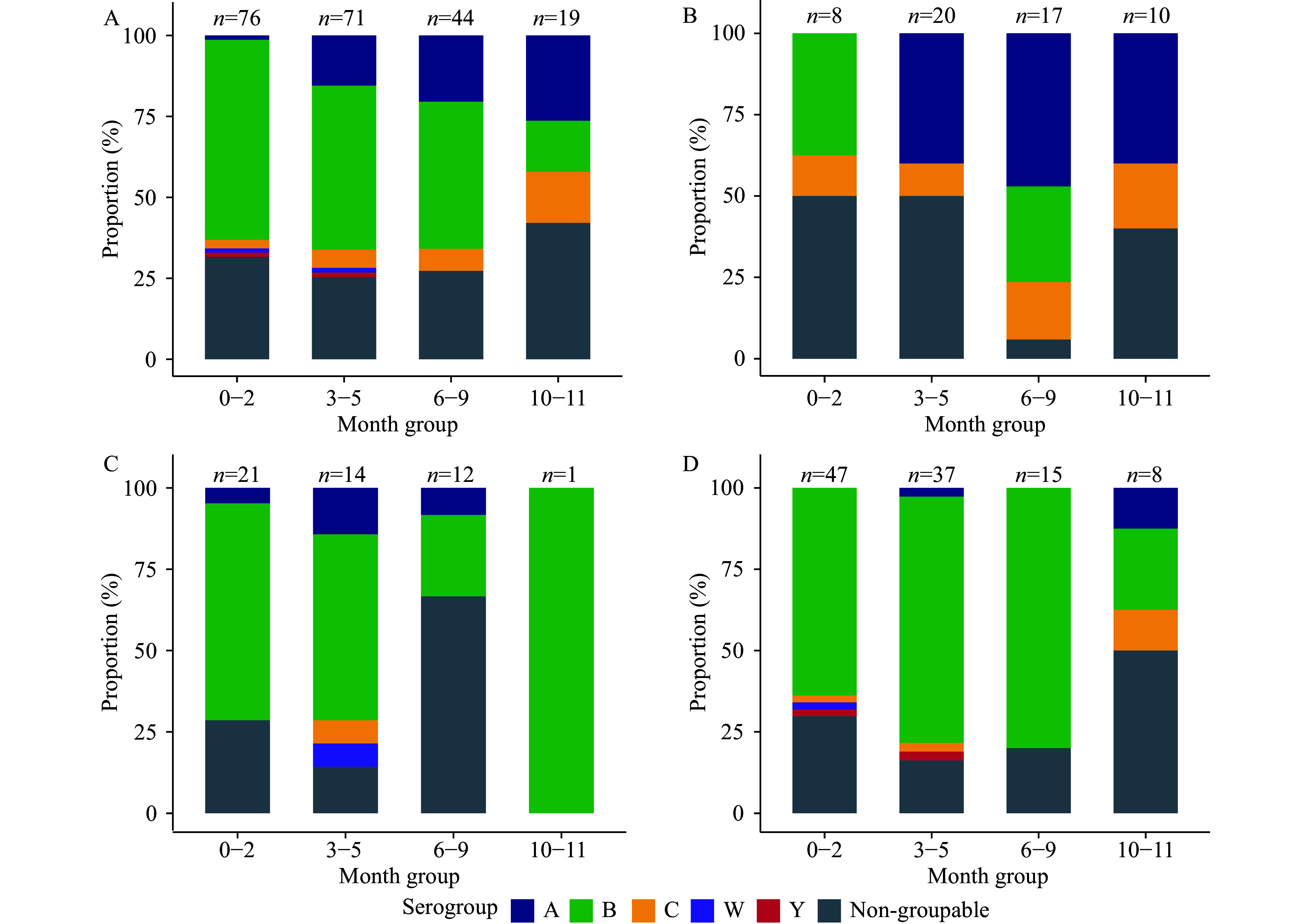
Serogroup distribution of 210 Neisseria meningitidis cases by age group during the entire study period of (A) 2006–2023; (B) 2006–2010; (C) 2011–2015; and (D) 2016–2023.
In infants aged 6–9 months, the proportion of serogroup A cases decreased from 47% during 2006–2015 to 0% during 2016–2023, while serogroup C cases similarly declined from 17.65% to 0%. Conversely, the proportion of serogroup B increased substantially from 29.41% to 80%. A similar pattern was observed in the 10–11-month age group. Cases involving serogroups W and Y were observed exclusively in infants ≤5 months of age (Figure 2B, 2C, 2D).
Table 3 shows the clinical manifestations of MM cases with N. meningitidis serogrouping results. Fever was the most common symptom across all serogroups (ranging from 88.46% in serogroup A to 100% in serogroups C/W/Y); more than half of cases presented with nausea and/or vomiting. Beyond these general symptoms, serogroups A (73.08%) and C (91.67%) were characterized by a significantly higher proportion of petechiae or purpura compared to other serogroups (P<0.001); in contrast, only 26.42% of serogroup B cases exhibited these manifestations. Seizures, consciousness disorders, and bulging fontanelle were observed in fewer than 50% of cases. Opisthotonus was uncommon but occurred in 11.54% of serogroup A cases. Meningeal signs were present across all serogroups, with nuchal rigidity occurring in over 25% of cases regardless of serogroup. Kernig’s and/or Brudzinski’s signs were observed primarily in serogroups A and B, occurring in 11.54% to 13.21% of these cases.
Table 3. Clinical manifestations of MM cases under 1 year of age by serogroup.
| Symptom | Serogroup A (N=26) | Serogroup B (N=106) | Serogroup C (N=12) | Serogroup W (N=2) | Serogroup Y (N=2) | Non-groupable (N=62) | P* |
| Note: N/A means data missing or unreported. Abbreviation: MM=meningococcal meningitis. * Hypothesis testing was performed using Fisher’s exact test. | |||||||
| Fever (n, %) | |||||||
| Yes | 23 (88.46) | 102 (96.23) | 12 (100.00) | 2 (100.00) | 2 (100.00) | 59 (95.16) | 0.486 |
| No | 3 (11.54) | 4 (3.77) | 0 (0) | 0 (0) | 0 (0) | 3 (4.84) | |
| N/A | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Nausea and/or vomiting (n, %) | |||||||
| Yes | 14 (53.85) | 40 (37.74) | 7 (58.33) | 1 (50.00) | 1 (50.00) | 36 (58.06) | 0.240 |
| No | 7 (26.92) | 48 (45.28) | 4 (33.33) | 1 (50.00) | 1 (50.00) | 15 (24.19) | |
| N/A | 5 (19.23) | 18 (16.98) | 1 (8.33) | 0 (0) | 0 (0) | 11 (17.74) | |
| Seizure (n, %) | |||||||
| Yes | 8 (30.77) | 32 (30.19) | 4 (33.33) | 0 (0) | 1 (50.00) | 27 (43.55) | 0.604 |
| No | 14 (53.85) | 67 (63.21) | 7 (58.33) | 2 (100.00) | 1 (50.00) | 31 (50.00) | |
| N/A | 4 (15.38) | 7 (6.60) | 1 (8.33) | 0 (0) | 0 (0) | 4 (6.45) | |
| Petechiae and/or purpura (n, %) | |||||||
| Yes | 19 (73.08) | 28 (26.42) | 11 (91.67) | 1 (50.00) | 0 (0) | 24 (38.71) | <0.001 |
| No | 1 (3.85) | 16 (15.09) | 0 (0) | 0 (0) | 0 (0) | 7 (11.29) | |
| N/A | 6 (23.08) | 62 (58.49) | 1 (8.33) | 1 (50.00) | 2 (100.00) | 31 (50.00) | |
| Consciousness disorder (n, %) | |||||||
| Yes | 11 (42.31) | 33 (31.13) | 6 (50.00) | 2 (100.00) | 0 (0) | 20 (32.26) | 0.434 |
| No | 14 (53.85) | 59 (55.66) | 6 (50.00) | 0 (0) | 2 (100.00) | 32 (51.61) | |
| N/A | 1 (3.85) | 14 (13.21) | 0 (0) | 0 (0) | 0 (0) | 10 (16.13) | |
| Opisthotonus (n, %) | |||||||
| Yes | 3 (11.54) | 5 (4.72) | 0 (0) | 0 (0) | 0 (0) | 4 (6.45) | 0.414 |
| No | 18 (69.23) | 90 (84.91) | 9 (75.00) | 2 (100.00) | 2 (100.00) | 54 (87.10) | |
| N/A | 5 (19.23) | 11 (10.38) | 3 (25.00) | 0 (0) | 0 (0) | 4 (6.45) | |
| Bulging fontanelle (n, %) | |||||||
| Yes | 8 (30.77) | 41 (38.68) | 1 (8.33) | 1 (50.00) | 1 (50.00) | 19 (30.65) | 0.118 |
| No | 15 (57.69) | 64 (60.38) | 11 (91.67) | 1 (50.00) | 1 (50.00) | 41 (66.13) | |
| N/A | 3 (11.54) | 1 (0.94) | 0 (0) | 0 (0) | 0 (0) | 2 (3.23) | |
| Nuchal rigidity (n, %) | |||||||
| Yes | 8 (30.77) | 28 (26.42) | 4 (33.33) | 1 (50.00) | 1 (50.00) | 26 (41.94) | 0.165 |
| No | 16 (61.54) | 72 (67.92) | 5 (41.67) | 1 (50.00) | 1 (50.00) | 34 (54.84) | |
| N/A | 2 (7.69) | 6 (5.66) | 3 (25.00) | 0 (0) | 0 (0) | 2 (3.23) | |
| Kernig and/or Brudzinski signs (n, %) | |||||||
| Yes | 3 (11.54) | 14 (13.21) | 0 (0) | 0 (0) | 0 (0) | 8 (12.90) | 0.628 |
| No | 16 (61.54) | 80 (75.47) | 10 (83.33) | 2 (100.00) | 2 (100.00) | 48 (77.42) | |
| N/A | 7 (26.92) | 12 (11.32) | 2 (16.67) | 0 (0) | 0 (0) | 6 (9.68) | |
DISCUSSION
In this study, we found that the incidence of MM in infants significantly decreased following the introduction of meningococcal polysaccharide vaccines into the NIP, but remained elevated compared to all-age incidence. The fatality rate has consistently fluctuated around 10% at the end of the study period, which remains a critical public health concern despite the current low endemic status of MM nationwide.
Infants may be at increased risk for meningococcal disease for several reasons. One primary factor is their immature immune systems, which increases their general susceptibility to infections (6). This vulnerability is particularly pronounced in unvaccinated infants aged 0–5 months. Our findings show that the proportion of cases in infants ≤5 months of age among all infant cases increased from 40% during 2006–2010 to 73% during 2016–2023, while the proportion of cases in infants 6–11 months of age decreased from 60% to 27%. This shift could be attributed to the nationwide free meningococcal vaccination program that provides doses of serogroup A polysaccharide vaccine at 6 months and 9 months of age. Transmission patterns of N. meningitidis may provide another explanation for elevated risk in infants. Previous studies (7–8) have found that adolescents have the highest meningococcal carriage rates. Their social activity patterns and frequent interactions with family members contribute to a significantly higher risk of transmitting N. meningitidis to susceptible infants and older individuals. This may explain why serogroup A and C cases among infants aged 0–5 months who have not yet been vaccinated have decreased.
Since 2016, there has been a slight increase in MM incidence among infants. The observed changes in serogroup distribution may partially explain this trend. During 2016–2023, serogroup B emerged as the most common serogroup, while serogroups A and C were only sporadically reported due to widespread vaccination among children aged 6 months and above. Serogroups W and Y were detected in infants aged 0–2 and 3–5 months. This pattern is consistent with evidence from the United States, Canada, and Europe (9–11), where serogroup B accounts for 60%–70% of infant cases, followed by serogroups W, C, and Y. However, no licensed serogroup B meningococcal vaccines are currently available in China, and our findings strongly support the need for vaccines against serogroup B meningococcal disease.
In young children, clinical manifestations of MM may be subtle and non-specific, initially presenting with fever, irritability, and lethargy, which can be difficult to differentiate from common viral infections (12). Seizures may occur at disease onset in some cases. Additionally, neck stiffness is rare in children under 2 years of age, while bulging anterior fontanelle may occur in infants under 18 months (13–14). Our study found that only 26.42% of serogroup B cases developed petechiae or purpura, 26% exhibited neck stiffness, and 13% showed positive Kernig’s and Brudzinski’s signs. This variability complicates early diagnosis in infants, highlighting the need to recognize that symptoms such as fever, irritability, and feeding difficulties may indicate early stages of severe infection (15). Enhanced monitoring, combined with rapid diagnostic tools, can improve early detection; however, vaccination remains the most fundamental preventive measure against MM.
This study was subject to some limitations. The dataset for our analysis originated from a passive surveillance system, which is inherently vulnerable to underreporting. We identified only 210 N. meningitidis isolates with serogrouping results during the study period, constraining the size of the dataset available for analysis. Nonetheless, given the comprehensive nationwide scope of the surveillance system, we believe that these findings are representative of the national epidemiological and clinical characteristics of meningococcal meningitis in China.
In conclusion, the disease burden of MM among infants remains a cause for concern. There has been a diversification of meningococcal serogroups nationwide, with serogroup B becoming predominant. Our findings highlight the importance of vaccination against meningococcal disease and underscore the need for serogroup B meningococcal vaccine development in China.
Conflicts of interest
No conflicts of interest.
Ethical statement
The data used in this study were obtained from the national surveillance system for notifiable diseases. As all data were fully anonymized prior to download and analysis, ethical approval and informed consent were not required.
Funding Statement
Supported by Beijing Natural Science Foundation and - Haidian Original Innovation Joint Fund (L232072)
References
- 1.Linder KA, Malani PN Meningococcal meningitis. JAMA. 2019;321(10):1014. doi: 10.1001/jama.2019.0772. [DOI] [PubMed] [Google Scholar]
- 2.Olbrich KJ, Müller D, Schumacher S, Beck E, Meszaros K, Koerber F Systematic review of invasive meningococcal disease: sequelae and quality of life impact on patients and their caregivers. Infect Dis Ther. 2018;7(4):421–38. doi: 10.1007/s40121-018-0213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinese Preventive Medicine Association Experts’ consensus on immunization with meningococcal vaccines in China. Chin J Epidemiology. 2019;40(2):123–8. doi: 10.3760/cma.j.issn.0254-6450.2019.02.001. [DOI] [Google Scholar]
- 4.Pace D, Gauci C, Barbara C The epidemiology of invasive meningococcal disease and the utility of vaccination in Malta. Eur J Clin Microbiol Infect Dis. 2020;39(10):1885–97. doi: 10.1007/s10096-020-03914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacNeil JR, Bennett N, Farley MM, Harrison LH, Lynfield R, Nichols M, et al Epidemiology of infant meningococcal disease in the United States, 2006-2012. Pediatrics. 2015;135(2):e305–11. doi: 10.1542/peds.2014-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, et al Meningococcal vaccination: recommendations of the advisory committee on immunization practices, United States, 2020. MMWR Recomm Rep. 2020;69(9):1–41. doi: 10.15585/mmwr.rr6909a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yue MM, Xu J, Yu JX, Shao ZJ Carriage prevalence of Neisseria meningitidis in China, 2005-2022: a systematic review and meta-analysis. BMC Infect Dis. 2022;22(1):594. doi: 10.1186/s12879-022-07586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen H, May M, Bowen L, Hickman M, Trotter CL Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–61. doi: 10.1016/S1473-3099(10)70251-6. [DOI] [PubMed] [Google Scholar]
- 9.MacNeil JR, Blain AE, Wang X, Cohn AC Current epidemiology and trends in meningococcal disease-United States, 1996-2015. Clin Infect Dis. 2018;66(8):1276–81. doi: 10.1093/cid/cix993. [DOI] [PubMed] [Google Scholar]
- 10.Saboui M, Tsang RS, MacTavish R, Agarwal A, Li YA, Salvadori MI, et al Epidemiology of invasive meningococcal disease in Canada, 2012-2019. Can Commun Dis Rep. 2022;48(5):228–36. doi: 10.14745/ccdr.v48i05a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladhani SN, Flood JS, Ramsay ME, Campbell H, Gray SJ, Kaczmarski EB, et al Invasive meningococcal disease in England and Wales: implications for the introduction of new vaccines. Vaccine. 2012;30(24):3710–6. doi: 10.1016/j.vaccine.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Abu Raya B, Sadarangani M Meningococcal vaccination in pregnancy. Hum Vaccin Immunother. 2018;14(5):1188–96. doi: 10.1080/21645515.2018.1445447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine 2012;30 Suppl 2:B3-9. http://dx.doi.org/10.1016/j.vaccine.2011.12.062.
- 14.Dass Hazarika R, Deka NM, Khyriem AB, Lyngdoh WV, Barman H, Duwarah SG, et al Invasive meningococcal infection: analysis of 110 cases from a tertiary care centre in North East India. Indian J Pediatr. 2013;80(5):359–64. doi: 10.1007/s12098-012-0855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson MJ, Ninis N, Perera R, Mayon-White R, Phillips C, Bailey L, et al Clinical recognition of meningococcal disease in children and adolescents. Lancet. 2006;367(9508):397–403. doi: 10.1016/S0140-6736(06)67932-4. [DOI] [PubMed] [Google Scholar]