Abstract
Endogenously expressed small noncoding microRNAs (miRNAs) play an important role in posttranscriptionally regulating gene expression by binding to mRNAs with complementary sequences. miRNA–mRNA interactions allow for cellular flexibility to fine tune gene expression by controlling translation in response to a multitude of signaling events. Disease states or perturbations in cellular homeostasis can lead to aberrant miRNA expression. The discovery of stable miRNAs in circulation generated enormous interest in exploring their utility as potential noninvasive biomarkers. Additionally, selectively inhibiting or supplementing an miRNA contributing to pathogenesis is being pursued as a therapeutic strategy for a variety of disorders. Studies from rodent models of pain and patients have now implicated a role for miRNAs in mediating various aspects of pain processing. These non-coding RNAs can provide mechanistic insights into the pathways modulated and could serve as therapeutic targets. Here we discuss the challenges associated with miRNA research and the promises ahead in this vastly unexplored avenue in pain biology.
Keywords: MicroRNA, biomarker, exosomes, pain
Introduction
MicroRNAs (miRNAs) are single-stranded small noncoding RNAs that consist of approximately 22 nucleotides (nt) and play a crucial role in fine tuning gene expression1. miRNAs regulate a wide range of biological processes, and dysregulation of individual or entire families of miRNAs is associated with the pathogenesis of an array of human diseases2–4. Modulation of gene expression is achieved by binding of the miRNA to one or more complementary sequences in the 3′ untranslated region (3′UTR) of specific target mRNAs. This mRNA recognition is mediated by 2 to 7 nt usually found in the 5′ end of the miRNAs called the seed sequence. Binding of miRNA can result in either translational repression or mRNA degradation depending on the degree of complementarity between mRNA and miRNA. Because the required sequence complementarity is relatively short, a single miRNA can target many genes and mRNAs can harbor multiple miRNA binding sites in their 3′UTR. Hence it is estimated that ~60% of genes in humans are regulated by miRNAs5. It is now well established that miRNAs play key regulatory roles in nearly every aspect of biology, including physiological and pathological processes.
History and biogenesis
miRNAs, the fine tuning regulators of the transcriptional milieu, are found in all plants and multicellular eukaryotes. In plants, miRNAs bind to mRNA targets with near complete complementarity, while many animal miRNAs show only partial complementarity to their cognate mRNAs6. In 1993 while studying larval developmental stages in Caenorhabditis elegans (C. elegans), a model organism for developmental genetics and neurobiology, the Ambros laboratory discovered regulation of the lin-41 mRNA by lin-4 miRNA7. The Ruvkun laboratory characterized the lin-4:lin-41 RNA interaction by sequential nucleotide deletion and identified a strongly complementary sequence in the 5′ region of the miRNA that could bind several stretches of sequence in the lin-14 3′UTR. This region of sequence complementarity of 6 to 8 bases in the 5′ portion of lin-4 miRNA was eventually termed the seed sequence as computational studies revealed that many miRNAs were at least partially complementary to sequences in the untranslated regions of mRNAs1. In a study measuring the effects of increasing lin-4 miRNA during development, a second target of lin-4 was identified, lin-14, which showed decreased protein levels in parallel with lin-4 expression8. Interestingly, lin-14 mRNA levels remained constant, indicating that lin-4 was able to translationally repress lin-14 mRNA without causing its degradation. While lin-4 has no human homologue, the second miRNA discovered, let-7 plays an important role in developmental timing in C. elegans and is found in humans and other higher species9.
miRNAs are transcribed as 150- to 200-nt precursor sequences (pri-miRNAs) that are capped and polyadenylated and reside in the nucleus in a double-stranded (ds) stem loop shape1 (Figure 1). RNA-mediated interference machinery facilitates miRNA processing by forming the microprocessor. This complex contains two essential proteins, the dsRNA binding protein DGCR8 (DiGeorge syndrome chromosome region) and the RNase III enzyme Drosha10, that process pri-miRNAs into 60- to 70-nt short hairpins with a phosphorylated 5′ end and a short overhang on the 3′ end that allows the hairpin to be recognized by the RNA-induced silencing complex (RISC) once exported by exportin 5 to the cytoplasm11. The RISC complex contains the essential RNA-binding proteins argonaut (Ago), the TAR RNA binding protein (TRBP), and the enzyme DICER. Pre-miRNAs contain both the passenger strand and the guide strand that contains the mature miRNA. In a yet undiscovered mechanism, the RISC complex deciphers between the guide strand that will be loaded onto Ago to silence its cognate mRNA, and the passenger strand, which will be destroyed by the “slicer” enzyme of the RISC complex1,12,13.
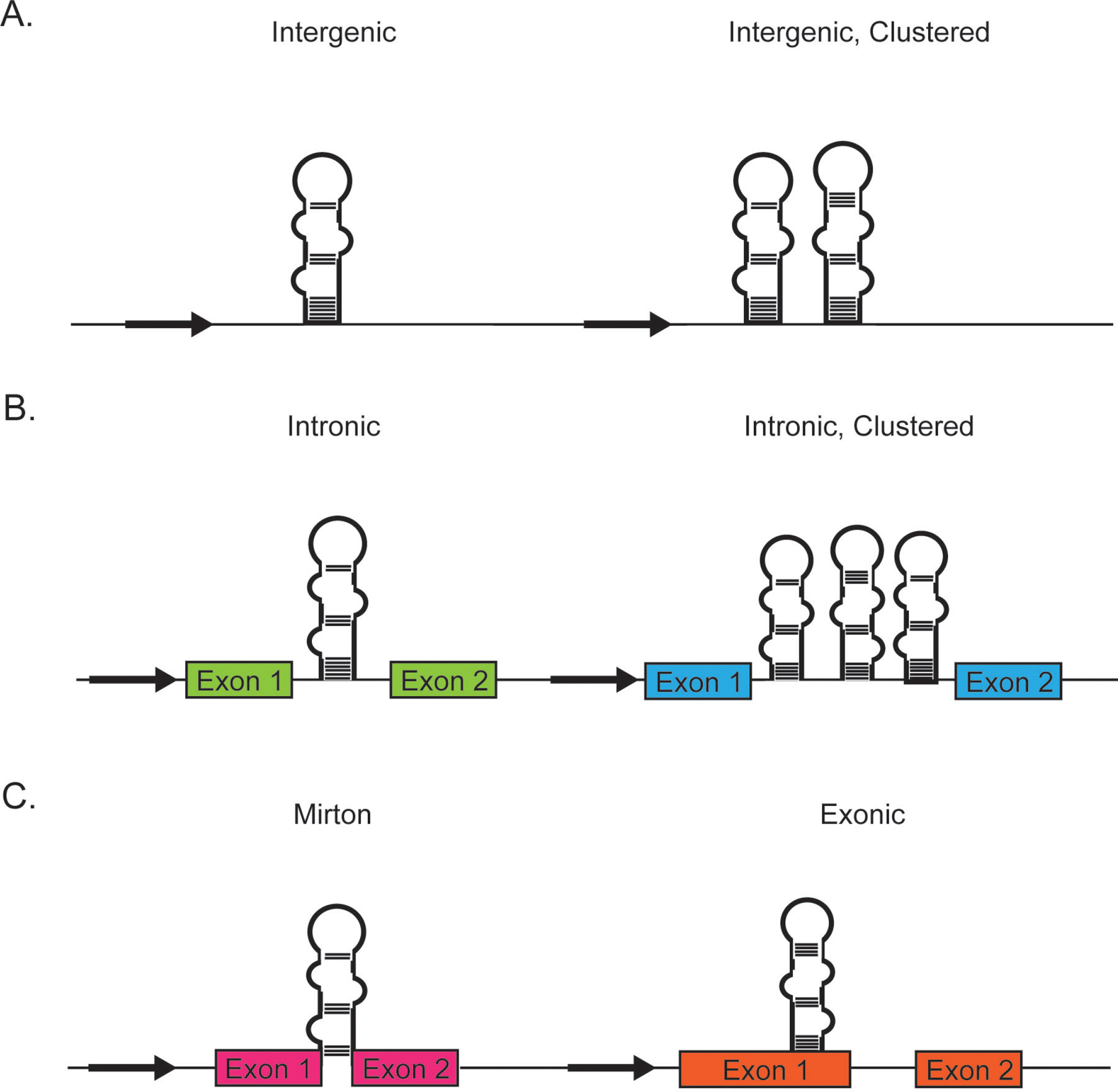
Figure 1. Genomic location of miRNAs.

A) Individual miRNAs or polycistronic miRNAs encoded in intergenic region are regulated by an miRNA-specfic promoter. They are located in genomic regions distinct from where transcription for protein coding genes occur. B) Individual or clustered miRNAs can be located between exon boundaries in intronic regions of DNA encoding for proteins or long noncoding RNAs. Typically they are transcribed from the same promoter as their host genes. C) miRNAs encoded in a short intron of a gene (mirtrons) or within a gene (exonic) are rare and often bypass nuclear processing.
Genomic location of miRNAs
Based on the sequences flanking miRNAs, genomic location of miRNAs can be broadly divided into intergenic, intronic, or exonic (Figure 2). The majority of miRNAs are encoded in intergenic regions existing as independent transcription units, with miRNA-specific promoters. Intronic miRNAs processed from the introns of the host gene are transcribed from the same promoter as their host genes. These introns can be that of a protein coding gene or within long noncoding RNAs. Synchronized expression of intronic miRNAs along with the genes harboring them has been widely observed14,15. Exonic miRNAs are far rarer and often overlap an exon and an intron of a noncoding gene. These miRNAs are also thought to be transcribed by their host gene promoter but their maturation is often independent of the host gene function. miRNAs can be transcribed in a cluster (polycistronic) with a shared promoter. Similar to single or monocistronic miRNAs, these clusters can originate from an intergenic or intronic location. miRNAs derived from short intronic hairpins are referred to as mirtrons. Such miRNA precursors bypass Drosha/microprocessor processing because these are already pre-miRNA with splice sites on either side. Both pre-miRNA and mirtron hairpins are exported from the nucleus by exportin-5 and cleaved by Dicer to generate ~22-nt RNA duplexes16. Interestingly, more than 50% of miRNA genes are located at fragile sites and cancer susceptibility loci17 and miRNAs are frequently amplified or downregulated in cancer. Thus the genomic location and transcription levels indicate tight integration of miRNAs in cellular regulatory circuits.
Figure 2. miRNA biogenesis.
Primary miRNAs are transcribed from DNA as capped and polyadenylated hairpins that are processed by Drosha/DGCR8 nuclear complex into pre- miRNAs. After export from the nucleus by exportin 5, the pre-miRNA is recognized by DICER, an enzyme that produces short double-stranded intermediates with 5′ phosphates and 3′ overhangs. The miRNA encoded on the guide strand is loaded into argonaut (Ago), and the passenger strand is either degraded or loaded into Ago as a mature miRNA/Ago complex called an miRNP.
miRNA nomenclature
Established in 2002, miRBase (formerly known as MicroRNA Registry18) is the online searchable database for all published miRNA sequences and associated annotations. According to miRBase (http://www.mirbase.org/), the numbering of miRNA genes is sequential, reflecting the order of their discovery19. miRNAs are named with the prefix “mir” followed by a dash and a number. For example, in hsa-miR-25, “hsa” represents the species (“hsa” is for Homo sapiens, “mmu” for Mus musculus, and “rno” for Rattus norvegicus). This is followed by “miR” (with capital “R”) for mature miRNA or “mir” (with small “r”) for pri-miRNA, pre-miRNA, or genomic locus. Two mature miRNAs are generated from either the 3′ or 5′ arm of the pre-miRNA hairpin sequence. Based on their relative abundance, one was designated with an asterisk following the name to indicate lower expression. Thus the abundant form is referred to as miR, while the less abundant sequence produced from the opposite arm of the hairpin is called miR* (rno-miR-25* is a less abundant form compared with rno-miR-25). This convention was recently replaced by miRBase with rno-miR-25-5p (from the 5′ arm) and rno-miR-25-3p (from the 3′ arm). In addition to reflecting the origin from both arms of the predicted hairpin, this change accounts for the fact that both 5p and 3p versions may be expressed equally depending on the cell types20. miRNAs with nearly identical sequences are annotated with an additional lower case letter. For example, miR-181a would be closely related to miR-181b. Though transcribed from different loci or different regions in the genome, some pre-miRNAs can produce identical mature miRNAs. These are indicated with an additional dash-number suffix. Examples mentioned by miRBase are the pre-miRNAs hsa-mir-194-1 and hsa-mir-194-2.
Release 20 (June 2013) of miRBase contains 24,521 entries representing hairpin precursor miRNAs, expressing 30,424 mature miRNA products, in 206 species. The number reported for H. sapiens includes 1872 precursors and 2578 mature miRNAs. In addition to this comprehensive “encyclopedia” on miRNAs, other databases have been developed to consolidate information on verified mRNA targets of individual miRNAs (miRTarBase) and relationships between noncoding RNAs and disease states, including the mammalian noncoding RNA-disease database (MNDR) (www.rna-society.org/mndr/), the Human MiRNA Disease Database (HMDD) (http://202.38.126.151/hmdd/mirna/md/), and miR2Disease (http://www.mir2disease.org/)21–23.
miRNA target determination
The functional consequence of miRNA binding is defined by the genes targeted and the effect a miRNA has on regulating translation of the target mRNAs, thus influencing gene expression. A given mRNA can harbor either one or more miRNA target binding sites in its 3′UTR. miRNAs regulate gene expression by inhibition of translation initiation, translation elongation or by targeting mRNAs for degradation24,25. miRNAs predominantly bind to the 3′UTR of target mRNAs, often resulting in imperfect base heteroduplexes between the seed sequence and the mRNA sequence. However, the influence of the UTR sequence in providing the “sequence context” for miRNA–mRNA interactions has been observed. Reporter assays have demonstrated that an identical site can mediate repression in some UTRs but not in others26,27. Thus seed matches are not always sufficient for repression, indicating that additional features of the UTR context are crucial to miRNA regulation and in determining the specificity of targeting. For example AU-rich nucleotide composition near the site can be associated with weaker mRNA secondary structure in the vicinity of the site and thus increased accessibility to the seed sequence. Target locations are not evenly distributed throughout the whole UTR and there is a strong preference for targets to be located in close vicinity of the stop codon and the polyadenylation sites. This includes positioning within the 3′ UTR at least 15 nt from the stop codon. mRNA enters the ribosome ~15 nt downstream of the decoding site and presumably, this will result in the removal of any silencing complex. This will block rebinding until the ribosome dissociated from mRNA. This interference by the ribosome could also explain why the 5’UTR and open reading frame harbor very few miRNA target binding sites. Silencing complexes bound to these regions would be displaced by the translation machinery as it moves along from the cap-binding complex through the open reading frame. Genome-wide analyses indicate that more binding sites were selectively maintained near the ends than in the central region of the 3’ UTR especially for mRNAs with long (>1300 nt) 3’UTR1,28,29. Increased site accessibility or proximity to translation machinery could explain the increased efficacy of sites falling near the ends of long UTRs. One explanation that has been put forth for the presence of larger number of miRNA binding sites near either ends of the 3’UTR and their increased efficacy is that, when mRNAs form the circularized structures with the poly(A) tail interacting with the 5’ cap, sites located in the middle of long 3’UTRs would be furthest from the translational machinery1.
miRNA detection, quantification, and functional studies
Several approaches are available to profile and quantify miRNA expression in tissues, cells, and bodily fluids. Because the expression of the pri- and pre-miRNA transcript does not correspond linearly to the expression level of mature miRNAs, only quantification of mature miRNAs will accurately indicate their presence and regulation30. However, if the goal is to identify miRNAs transcribed, different methods can be employed. Methods for genome-wide analysis of miRNA expression include microarrays, RNA sequencing, and quantitative PCR in array formats13. All three methods enable analysis of large numbers of miRNAs in parallel and can be used either for detection or for investigation of differential expression in diseased states. Data obtained from microarray require further confirmation and the study is limited to known miRNAs represented in the chip. Array-based qPCR technologies such as the Taqman low-density array (TLDA) and Open array cards allow for profiling of ~750 miRNAs. Although the profiling is limited to a definite number of known miRNAs, use of TLDA can eliminate the qPCR confirmatory step. There is the additional option of custom designing TLDA and Open array cards; hence these platforms are more flexible. With the advent of next-generation sequencing, total RNA or small RNA sequencing offers a complete overview of all RNAs including pri-miRNA and pre-miRNA. This method offers the advantage of potential identification of novel miRNAs and enables determination of various stages of biosynthesis for miRNAs of interest.
Once the miRNAs of interest have been identified, online databases listing miRNA sequences and annotations20 for bioinformatically predicted31 as well as experimentally validated32 miRNA targets can be used to explore the functionality of miRNAs of interest. Computational methods of inferring miRNA functions have been invaluable in providing insights into miRNA function22. Integrating heterogeneous data sources and pathway analysis of individual miRNAs or disease-specific miRNA signatures has aided in inferring miRNA regulatory modules, thus advancing miRNA functional annotation.
Reporter assays are commonly used to confirm miRNA binding to its target mRNA. One approach to perform this assay is to clone the 3′UTR of interest harboring one or more of the miRNA binding sites downstream of the luciferase reporter gene. A decrease in luciferase activity upon cotransfection of miRNA with the luciferase-3′UTR reporter plasmid indicates binding of the miRNA to the UTR which regulates the expression of luciferase gene. Mutating the miRNA binding site in the UTR or utilizing an miRNA inhibitor to block miRNA function can be employed to further confirm the miRNA interaction with its target mRNA. Transfection of miRNAs into cells endogenously expressing target genes followed by qPCR and Western blot analysis will confirm whether the miRNA is mediating its effect via translational repression or mRNA degradation. Because individual mRNAs can harbor binding sites for different miRNAs, the resulting regulatory network is complex and can be functionally redundant. Pull-down assays for proteins involved in miRNA processing such as Ago have been used to identify target mRNA:miRNA interactions. Biochemical techniques such as high-throughput sequencing of RNA isolated by cross-linking immunoprecipitation (HITS-CLIP)33 and photoactivatable-ribonucleoside-enhanced crosslinking and immunoprecipitation (PAR-CLIP)34, are commonly used to identify RNA sequences that bind to Ago proteins. Additional genome-wide approaches pursued for target identification include transcriptional profiling of specific miRNA-knockdown or miRNA-overexpressing cells and control cells. Though these studies are valuable in providing a global view of target gene expression changes, it is difficult to determine whether the resulting changes are mediated directly or indirectly by the miRNA of interest.
Both in vitro and in vivo inhibition or overexpression of miRNAs can be achieved using a variety of synthetic mimics, anti-miRNA oligonucleotide (AMO), expression constructs, and viral vectors. AMOs, originally called antagomirs, are synthetic DNA/LNA (locked nucleic acid) hybrids with complementarity to at least 15 nt of the endogenous miRNA35 (see Text Box 1). LNAs, which incorporate stability by the addition of a 2′O methyl or 2’O to 4’C bond to a synthetic RNA, are also used to design miRNA mimics. A miRNA sponge is a plasmid-based technology encoding multiple miRNA binding sites that are available to sequester the miRNA from the endogenous mRNA pool when the construct is expressed36. Either viral or non-viral gene transfer vectors can be used to deliver the miRNA sponge expression cassette into cells to inhibit miRNA function37. Genetic gain- and loss-of-function approaches to specific miRNAs or miRNA clusters have been successful. Overexpression, however, can lead to nonphysiological levels of miRNA and potentially to biologically irrelevant regulation of mRNAs. Genetic deletion of miRNAs present in introns or as a part of polycistronic transcript should be designed so that the mRNA of the gene harboring intronic miRNAs or other miRNAs in the polycistronic cluster is not affected. The major limiting factor in genetic deletion studies is the lack of phenotypic effect. The redundancy in miRNA function coupled with compensation could hamper the elucidation of functional consequences of miRNA that was knocked out. miRNA-deficient embryonic stem cells38 and conditional knockout mice39 are freely available and can serve as a valuable resource in advancing our understanding of miRNA functions in vivo.
Box 1. miRNA-based tools.
Viral vectors - a viral-based approach to constitutively express a miRNA by transducing cells with viral particles encoding the miRNA. Several virus types are currently being used. These include Adeno-associated virus (AAV), Herpes Simplex virus (HSV), or Lentivirus (LV).
Anti-miRNA oligonucleotide (AMO) - a synthetic oligonucleotide designed with high specificity and significant complementarity to its miRNA target to form a double-stranded duplex that blocks the miRNA function.
Antagomir - original AMO designed with 100% complementarity, containing RNA with 2′O methyl modification, phosphorothioate linkages, and 3’cholesterol to increase cellular uptake.
LNA (locked nucleic acid) - a nucleic acid that contains at least one type of stabilizing element such as a 2′O methyl group added to the ribose, an extra bond between the 2′O and 4′C of the ribose ring, or phosphorothioate linkages between nts.
miRNA mimic - synthetic, double-stranded LNA-based oligonucleotide with a guide strand that is identical to the miRNA and a passenger strand containing stabilization and uptake elements such as cholesterol.
miRNA sponge - a vector-based approach using DNA encoding multiple miRNA binding sites downstream of a promoter that effectively reduces available miRNA when expressed in a cell.
Circulating miRNAs
Recent identification of stable miRNAs in bodily fluids40–42 generated a lot of interest in exploring their utility as novel noninvasive biomarkers. Cells employ multiple distinct processes to release miRNAs into circulation. miRNAs are found enclosed within membranous vesicles (exosomes, shedding vesicles, and apoptotic bodies), in association with high-density lipoprotein, and bound by RNA-binding proteins (Ago 2 or nucleophosmin 1) (Figure 3). Thus, the term circulating miRNAs encompasses all miRNAs found in bodily fluids including those associated with RNA binding proteins, cholesterols or encapsulated in exosomes43,44. Although it is still unclear whether all circulating miRNAs are Ago bound and/or associated in exosomes, miRNAs found in plasma were reportedly resistant to endogenous RNases40,45. Extracellular vesicles are broadly classified into shedding microvesicles, exosomes, and apoptotic bodies based on their distinct mode of biogenesis. Exosomes are secreted microvesicles (30–100 nm in diameter) that form from the early endosomal pathway and their membranes originate from invaginations of the cell membrane (Figure 3). Intraluminal budding results in multivesicular bodies, and the fusion of these multivesicular bodies with the plasma membrane results in the release of the exosomes into the extracellular space46,47. Thus, exosomal cell membranes and their contents are reflective of the cell from which they are secreted. Upon their release from cells, these vesicles can impact both adjacent and distant cells48,49. Exosomes carry numerous proteins, lipids, and nucleic acids, including mRNA, miRNA, and long noncoding RNAs50,51. Vesiclepedia (http://www.microvesicles.org) is a manually curated compendium that contains molecular data identified in all classes of extracellular vesicles52 whereas ExoCarta (http://www.exocarta.org), a database for molecular data (proteins, RNA, and lipids) identified in exosomes, catalogs only exosomal studies as reported by the authors53.
Figure 3. Circulating miRNAs and exosome biogenesis.

Circulating miRNAs are secreted by cells in association with RNA binding proteins such as Ago, high density lipoproteins, or enclosed in microvesicles. Microvesicles include lipid-bound vesicles that are released during apoptosis (pink), shedding vesicles released by direct membrane budding (orange), or exosomes (blue) released from the multivesicular bodies (MVBs). Exosomes, the smallest of the microvesicles (30–100 nm), form within MVBs originating from the early endosomal pathway. Exosomes contain miRNAs, mRNAs, proteins, and lipids, the composition of which is reflective of the secreting cell. However, not all miRNAs present within the secreting cells are packaged into exosomes, and the mechanisms determining what is included in the exosomes for secretion is not well understood. After secretion, exosomes enter the blood stream and are transferred into recipient cells, releasing functional contents. Mechanisms similar to receptor-ligand mediated recognition has been postulated to guide exosomal uptake by recepient cells. It has been shown that extracellular Ago-bound miRNAs influence cellular function through receptor binding and hypothesized that HDL-associated miRNAs are secreted and taken up in a receptor-dependent mechanism.
Exosomes deliver these biomolecules including miRNAs to recipient cells with functional targeting capabilities50. Exosomes contain only a subset of all miRNAs present in the secreting cell and are reflective of the cellular environment and state. How specific miRNAs are selected to be packaged into exosomes and whether mRNAs and miRNAs are sorted together into the exosomes are areas of extensive investigation, but it is clear that RNAs are not randomly loaded into exosomes. Specific mechanisms exist for their recognition, packaging, and secretion54. Specific proteins such as hnRNPA2B1, along with cis-acting elements in the RNA sequence, control the sorting of RNAs into exosomes54. Additionally, exosome release is inhibited by small molecules that inactivate neutral sphingomyelinase 2, nSMase2, an essential enzyme of the ceramide pathway55.
Defining the source and destination of secreted miRNAs has been challenging. Their presence in circulation indicates that they can be transported long distance, and the communication mediated by exosomes will be defined by the contents. Exosomes can deliver their cargo by fusing with the cell membrane, thereby directly releasing the contents into the cytoplasm of the recipient cells. Classical adhesion molecules involved in cell–cell interactions such as integrins and intercellular adhesion molecules, along with exosomal-membrane-rich molecules such as TIM-binding phosphatidylserines, carbohydrate/lectin receptors, and heparan sulfateproteoglycans, have been implicated in mediating the binding of exosomes to the surface of recipient cells. In addition to direct fusion, exosomes can be internalized by endocytic pathways employing clathrin-dynamin-caveolae-dependent endocytosis, pinocytosis, or phagocytosis54.
Though secreted miRNAs can be transported between distant locations via circulation, how an miRNA taken up by a cell might achieve a sufficient concentration to inhibit its targets is not known. It is unclear whether the differentially expressed miRNAs participate in the disease process or simply serve as markers of disease progression. Though circulating miRNAs are ubiquitous in all body fluids, they exhibit fluid-specific expression profile signatures that can be used to distinguish individuals with disease from healthy controls40,56. Thus there is increased interest in exploring the utility of the biomolecular signature of exosomes, including miRNA profiles, in tumor biology; cardiovascular, immunological, and neurological disorders; and stem cell biology, both as clinical biomarkers and therapeutic tools57,58.
A recent review focusing on circulating miRNAs as potential biomarkers of human cancers highlighted approximately 100 miRNAs that may allow earlier diagnosis and represent potential therapeutic avenues57. Additionally, miRNA signatures in cerebrospinal fluid might be useful for diagnosing brain diseases such as Alzheimer’s disease59,60. A study profiling circulating miRNAs after nerve transplantation indicated that three miRNAs were specifically upregulated in the whole blood and in the serum of immunosuppressed animals that received an allograft. Nerve transplants are commonly supplemented with immunosuppressive drugs to enhance reinnervation through the allograft. These miRNAs are not expressed in the nerve graft but are found circulating in the blood, which makes them potential biomarkers for monitoring the immunosuppression status of an allograft61. Circulating miRNAs have been utilized as a measure of the quality of stored blood; as an addition to noninvasive prenatal diagnostics; and as a means of monitoring treatment efficacy after myocardial infarction or infection57. Thus there is immense potential in unraveling the role of circulating miRNAs in both normal physiology and pathogenic conditions.
miRNAs and pain
The critical role played by miRNAs in the development and pathophysiology of the nervous system is now well established62. In recent years, interest has increased in deciphering the role of miRNAs in modulating pain and analgesia; in defining the importance of miRNAs in pain circuitries; and in the cognitive, emotional, and behavioral components involved in pain63. Animal models have been developed to mimic persistent pain states, facilitating translational research on various types of pain64,65. Although animal models of pain have limitations, it is clear that much can be learned about the mechanisms and maintenance of chronic pain from existing models. In conjunction with human studies utilizing circulating biomolecules that are altered in bodily fluids in pain states, these models have been instrumental in elucidating the role of miRNAs in pain states.
Specific regions of the nervous system express differential levels of miRNAs that are known to regulate neuronal activity and modulate translation in dendrites66. A handful of miRNAs with known function in neuronal activity are also dysregulated in pain, although the mechanisms mediated by interactions between neuronal miRNAs and identified pain targets are not completely elucidated66. Distinct expression patterns of miRNAs in mammalian development as well as tissue-specific expression of miRNAs in disease states suggest that miRNAs play a role in differentiation and maintenance of cell types. In a study investigating the role of miRNAs in peripheral pain pathways, Dicer was conditionally knocked out in neurons that express Nav 1.8 using the Cre-loxP system67. Dicer is the cytoplasmic ribonuclease that is a component of RISC complex and generates small double stranded miRNAs from hairpin loop precursors. Nav 1.8 is a sensory neuron-specific voltage-gated sodium channel that is known to play a critical role in inflammatory pain, cold and noxious mechanosensation. Unlike in postmitotic Purkinje cells where Dicer is necessary for survival68, dorsal root ganglia (DRG) neurons maintained normal function in the absence of this enzyme. Dicer deletion resulted in deficits in inflammatory but not acute pain. Inflammatory agents including carrageenan, formalin, or complete Freund’s adjuvant (CFA) did not alter the pain thresholds in these dicer-null mice. At the cellular level, inflammatory mediators failed to enhance the excitability of Nav 1.8 sensory neurons from null mice. They also observed that nociceptor-specific genes were transcriptionally downregulated, while other genes were either expressed at normal levels or upregulated. Downregulation of nociceptor-associated transcripts including Nav1.8, P2xr3 and CamKII could have reduced functional protein, thus altering the thresholds of activation of sensory neurons. Microarray and deep sequencing revealed that ablation of Dicer in Nav1.8 neurons resulted in a significant reduction or loss of more than 60 miRNAs. Since deletion of Dicer can affect all miRNAs in the Nav1.8 neurons, it was difficult to identify the miRNAs that specifically contributed to pain. However this study showed that miRNAs are crucial for altering pain thresholds after inflammatory stimuli67. Another study using genetic ablation of Dicer showed impaired sciatic nerve regeneration and delayed functional recovery after sciatic nerve crush69.
Measuring changes in miRNA levels in rodent models of pain is another commonly used approach pursued for delineating the molecular mechanisms that underlie pain states. Recent reviews have cataloged the studies linking miRNAs to various pain conditions63,66,70–73. Profiling studies have identified tissue-specific deregulated expression of miRNAs in DRG and spinal cord (SC) after peripheral inflammatory stimulus74,75, nerve injury75–81 and cancer pain82. Additionally, differential regulation of individual miRNAs has been observed in specific regions of the brain in acute and chronic pain models83–85. The few studies that link specific miRNA changes to a pain condition suggest that temporal and spatial regulation of intracellular miRNA expression occurs at specific time points that correspond to early or late phases of pain conditions70,72,86. miRNAs altered in animal models could serve as an efficient strategy to elucidate the pathophysiology of pain, and miRNA changes specific to certain models or pain conditions could provide insights unique to the model being studied.
The natural follow-up from profiling studies is to measure the target mRNA levels in affected tissue after inducing pain. Our miRNA expression profiling study of DRG in spinal nerve ligation (SNL) model of neuropathic pain in rats four weeks after surgery identified significant alterations in expression of 63 miRNAs. We used the same RNA for global transcriptional profiling and observed a general upregulation of mRNAs; however, a strict inverse correlation between the differentially regulated miRNAs that were mostly downregulated, and their predicted mRNA targets was not observed. It is not surprising that a strict inverse correlation between the differentially regulated miRNAs and their predicted mRNA targets was lacking and a number of factors could have contributed to this. A large scale proteomics study performed to investigate the influence of specific miRNAs on protein levels revealed that a number of targets were predominantly regulated by translational repression87. Thus miRNAs could be mediating regulatory effect on mRNAs by suppression of translation and mRNA levels would remain unchanged.
A number of transcription factors were perturbed by SNL in DRG and could exert their influence on gene expression following SNL. In addition, several transcription factors are known to be regulated by miRNAs88,89. Though the classic switch interactions where miRNA induction results in the downregulation of preexisting mRNA targets is well known, other types of interactions such as fine-tuning and neutral mode of action are also possible1. In a “tuning” target-miRNA interaction, the target gene remains functional because the expression is not eliminated by miRNA90. Thus, in its fine-tuning role, a miRNA could adjust protein output that allows for customized expression in a particular cell type, in response to a signaling cue. Neutral miRNA-target mRNA interactions are the one with no particular consequence to the cell because they are neither advantageous nor do they cause any adverse outcome1,90. The complexity of the miRNA regulatory networks, with many miRNAs affecting many target transcripts makes it challenging to extract individual miRNA/target transcript interactions. Prediction of miRNA target sites is an evolving field and any errors in prediction of miRNA target sites would also undermine our ability to observe a correlation between miRNA and mRNA expression. Detailed mechanistic studies of individual genes and the miRNAs targeting them are required to classify the mode of miRNA regulation in pain.
Deep sequencing was used in another study to investigate alterations of miRNA expression following rat sciatic nerve injury in DRG and the proximal stumps of the nerve. They identified 201 and 225 known miRNAs with significant expression variance at five time points in these tissues after injury, in DRG and nerve stump respectively78. Sequencing of miRNAs in primary sensory neurons of L4 and L5 DRG (from two strains of rats that differed in their response to peripheral nerve injury) 3 days after SNL showed only 3 abundant (rno-miR-30d-5p, rno-miR-125b-5p) and one moderately expressed (rno-miR-379-5p) miRNA to be differentially regulated81, suggesting temporal regulation of miRNAs post injury.
Several studies have now successfully established negative correlation between miRNAs and target mRNA levels91–95. Some have successfully provided the proof-of concept for a role of specific miRNAs in influencing behavior using rodent models80,96–98 (Figure 4). miRNA induced attenuation or exacerbation of pain phenotype in rodent models of inflammatory, neuropathic, and cancer pain80,82,96–99 has been demonstrated. miR-103 was shown to simultaneously regulate the three subunits forming the L-type calcium channel (Cav1.2 LTC). Knockdown of miR-103 in naive rats induced hypersensitivity to pain. All three Cav1.2 LTC subunit mRNAs were overexpressed with a concomitant decrease in miR-103 in the dorsal horn after SNL injury. Intrathecal applications of miR-103 repressed Cav1.2-LTC up-regulation and attenuated mechanical hypersensitivity and cold allodynia in SNL rats96.
Figure 4. miRNA administration in rodent models of pain.

Specific miRNAs that are altered in animal model of neuropathic, inflammatory and cancer pain have been identified as potential therapeutic targets and are summarized in the table. Schematic indicates therapeutic delivery strategies that have been successful at reversing the pain phenotype including viral vectors (adeno-associated virus (AAV), herpes simplex virus (HSV), or lentivirus (LV)), anti-miRNA oligonucleotides (AMO), and stabilized locked nucleic acid (LNA) mimics. Figure modified from http://www.doyourownpestcontrol.com/rodent_cdc.jpg
The let-7 family of miRNAs has been implicated in pain processing, specifically in opioid tolerance owing to its upregulation after morphine treatment and consequential downregulation of mu opioid receptors100,101. A recent study demonstrated that miR-7a dysfunction underlies maintenance of neuropathic pain through regulation of DRG neuronal excitability80. Decrease of miR-7a in injured DRG was associated with neuropathic pain. Adeno-associated viral vector was used for local induction of miR-7a in DRG and this alleviated neuropathic pain in SNL model rats. Naïve rats developed both mechanical allodynia and thermal hyperalgesia when miR-7 expression was blocked. miR-7a was ineffective in reversing inflammatory pain and these results imply a role for miR-7a in neuropathic pain. The beta2 subunit of voltage-gated sodium channels is crucial for the cell surface expression and it is targeted by miR-7a. Downregulation of beta subunit protein by miR-7a suppressed hyperexcitability of nociceptive DRG neurons demonstrating that miR-7a downregulation is causally involved in maintenance of neuropathic pain through regulation of neuronal excitability80.
While earlier studies revealed the role of miR-21 in tumorigenesis102,103, it was upregulated in spinal cord in response to spinal cord injury104–106. Although astrocytes are not the only cells to express miR-21 in the injured spinal cord, GFAP+ astrocytes adjacent to the lesion area expressed high levels of miR-21107. Astrocytes becomes reactive following spinal cord injury and initial hypertrophy (increase in volume due to the enlargement of its component) followed by hyperplasia (increase in number) around the injury site contributing to progression of the glial scar. Upregulation of the inhibitory extracellular matrix components is known to contribute to glial scar and it is a significant barrier for axonal regeneration. However, glial scar serves to repair the blood–brain barrier and limit infiltration of inflammatory cells and cellular degeneration108. The role of miR- 21 in astrogliosis was investigated using transgenic mice that overexpress either miR-21 or a miRNA sponge (see Text Box 1) designed to inhibit miR-21 function in astrocytes. Overexpression of miR-21 attenuated the beneficial hypertrophic response to injury and miR-21 sponge augmented the hypertrophic phenotype107. Inhibition of miR-21 decreased scar formation without interfering with blood–brain barrier repair. These results suggest miR-21 can be a potential target for manipulating gliosis and enhancing functional outcome after spinal cord injury62. Peripherally, Sakai and Suzuki109 characterized miR-21 in DRG neurons after SNL injury. They found increased expression of miR-21 in the injured DRG and were able to reverse the pain phenotype associated with this injury by administering an inhibitor to decrease miR-21.
Inflammatory pain models have specific miRNA signatures and responses to miRNA therapy. For example, miR-124 is enriched in central nervous system (CNS) tissues and has low expression in microglia and peripheral macrophages110–112. In a rodent model of inflammatory pain induced by formalin, animals had measurably less endogenous miR-124 in spinal cord and delivery of miR-124 mimics (see Text Box 1) attenuated hyperalgesia98. In another study, delivery of miR-124 mimics reversed persistent hyperalgesia induced by carrageenan and prevented development of mechanical allodynia in SNI model97. Delivery of miR-124 analogues in multiple inflammatory pain models consistently reduced thermal hyperalgesia suggesting that increasing miR-124 can be a therapeutic strategy for the treatment of pain.
A miRNA microarray profiling study identified downregulation of miR-219 in spinal cord from CFA model. miR-219 negatively regulates NMDA receptors and calcium/calmodulin-dependent protein kinase II γ (CaMKII γ), two target genes that are known to be important in central sensitization113. CFA induced inflammatory pain reduced miR-219 expression and increased the expression of CaMKII γ. Overexpression of miR-219 prevented and reversed CFA-induced thermal hyperalgesia, mechanical allodynia and spinal neuronal sensitization. Knockdown of miR-219 by miR-219-sponge produced thermal hyperalgesia and mechanical allodynia and increased Fos protein expression in naive mice suggesting that miR-219 contributes to the modulation of chronic pain and the increase in CaMKIIγ expression mediates miR-219 downregulation-induced pain behavior. This study is also novel for highlighting how two different epigenetic mechanisms, DNA methylation and non-coding RNAs can collectively contribute to pain state. Methylation of cytosine bases found in cytosine-guanine dinucleotides are predominantly present in enriched regions of the DNA referred to as CpG islands. DNA methylation is an important epigenetic modification and this study demonstrated that chronic inflammatory pain can induce hypermethylation of CpG islands in the miR-219 promoter. Methylation of cytosine is usually associated with stable transcriptional repression when present in promoters. A demethylation agent increased miR-219 and decreased CaMKII expression, resulting in alleviation of pain in CFA model99. An earlier report had linked DNA methylation to miRNA modulation in neuropathic pain. A drastic decrease in the expression of miR-200b and miR-429 along with increase in DNA methyltransferase 3a (Dnmt3a) was observed in nucleus accumbens neurons, 7 days after SNL. Dnmt3a, commonly referred to as de novo methyltransferases, is one of the two enzymes responsible for establishing methylation patterns rather than maintaining the existing methylation patterns. Future studies no doubt will unravel additional convergence of multiple epigenetic mechanisms in mediating pain.
In a first genome-wide miRNA profiling study investigating the development and maintenance of tumor-mediated chronic pain using a mouse model of metastatic bone cancer pain, dysregulation of 57 miRNAs in sensory neurons corresponding to areas affected by tumor was observed in the DRG. Six candidate miRNAs, (three upregulated and three downregulated) were chosen for in vivo functional analysis and intrathecal delivery of miRNA inhibitors or mimics into lumbar DRGs were undertaken to demonstrate the efficacy of miRNA manipulation in vivo. Intrathecal application of inhibitors showed that inhibiting the tumor-induced upregulation of miR-1a-3p or miR-34c-5p, but not of miRNA-544-3p, in sensory neurons, markedly attenuated tumor-mediated hyperalgesia. Reversing the decrease of miR-483-3p, but not of miR-291b-5p, with miRNA mimics attenuated tumor-mediated hyperalgesia but increase in miR-370-3p in DRGs led to exaggerated tumor mediated hyperalgesia. They further investigated the impact of miRNA-1a-3p inhibition on the expression of target genes using a nanostring device which digitally quantifies a wide set of mRNA transcripts, and confirmed that Chloride channel 3 (Clcn3) was a target for miRNA-1a-3p. Though there are several ways by which Clcn3-mediated Cl− flux could affect sensory neuron function, the exact mechanism underlying how decreased Clcn3 levels cause hyperalgesia is unknown. In addition to confirming the inverse correlation in expression and miRNA binding to the 3’UTR, siRNAs designed against Clcn3 mRNA were used to demonstrate that a knockdown of Clcn3 expression in DRG was associated with exaggerated mechanical hypersensitivity. Thus, downregulating expression of Clcn3 in the DRG had the same functional effect as inhibition of miR-1a-3p, again indicating the in vivo significance of miR-1a-3p mediated regulation of Clnc3 as a key modulator of nociceptive hypersensitivity associated with cancer pain82.
Utility of miRNAs in reversing hyperalgesia associated with diabetic neuropathy and muscle inflammation has been explored using viral vectors expressing miRNA mimics. Increase in voltage gated sodium channel isoforms Nav1.7 and Nav1.3 protein in the DRG of rats with streptozotocin-induced diabetes was reversed using a nonreplicating herpes simplex virus-based vector expressing a miRNA against Nav alpha subunits114. Artificial miRNAs to downregulate acid-sensing ion channel 3 (ASIC3) expression was delivered into the muscle using a herpes simplex viral vector and this resulted in reduction of both muscle and paw mechanical hyperalgesia in mouse model of carrageenan-induced muscle inflammation115.
While a link between miRNA therapeutics and pain has been established in animal models, miRNA alterations in human pain conditions are being explored for their utility as biomarkers as well as therapeutics. Profiling studies in cancer have yielded many specific alterations in miRNAs that are being developed as biomarkers and explored as potential therapeutics, but translating these types of studies to neurological conditions proves more difficult because of the difficulty in obtaining healthy and diseased tissue from each individual116. Screening, diagnosis, and prognosis of various human diseases through miRNA profiling has capitalized on the stability of miRNAs in all bodily fluids and their aberrant expression in diseases, often correlate with disease severity or progression117,118. Altered miRNA expression profiles have been reported for immune disorders such as rheumatoid arthritis119 and systemic lupus erythematosus120 as well as for pain conditions such as irritable bowel syndrome121, chronic bladder syndrome122 and endometriosis123. Cerebrospinal fluid from fibromyalgia patients showed differential expression of nine miRNAs and miR-145-5p correlated positively with pain ratings based on the fibromyalgia impact questionnaire124 (Figure 5).
Figure 5. Circulating miRNAs altered in painful disorders.

Schematic showing altered miRNAs in bodily fluids of patients with painful conditions124,125,142–147. Altered miRNAs are potential biomarkers for disease progression and diagnosis as well as therapeutic targets for disease intervention. Alterations in circulating miRNAs were useful for stratifying patients with CRPS, a multifactorial neuropathic condition with complex pathophysiology. Figure modified from http://en.wikipedia.org/wiki/File:Circulatory_System_en.svg
Analysis of miRNA signature in whole blood from patients with complex regional pain syndrome (CRPS)125, a severe and multifactorial neuropathic pain condition characterized by dysregulated immune responses, demonstrated the utility of circulating miRNAs in patient stratification. Clustering of patients based on miRNA signature revealed additional miRNAs and inflammatory markers that were not significant when the whole population were analyzed together. These stratifications and identification of subset specific biomarkers are especially beneficial for disorders such as CRPS where the patients present with a variety of clinical symptoms. This study was also the first to show that circulating miRNAs can serve as potential biomarkers in pain125.
Pathological pain is correlated with cognitive and mood disorders126. In our efforts to obtain additional insights into comorbid conditions commonly associated with CRPS, we performed correlation analyses of all miRNAs detected in whole blood with clinical parameters. Strong correlations were observed between miRNAs and comorbidities such as migraine, high blood pressure, cholesterol, thyroid disease, and use of narcotics and antiepileptic medications125. For example hsa-miR-339-5p was correlated with narcotics use in CRPS patients. A recent study showed that miR-339 can downregulate mu-opioid receptor at the post-transcriptional level in response to opioid treatment127. Opioid analgesics fentanyl and morphine act mainly on mu-opioid receptors. Fentanyl is currently the most widely used synthetic opioid in clinical practice and it is up to 100 times more potent than morphine. miR-339-3p was upregulated in the hippocampus of mice chronically treated with morphine and fentanyl. miR-339-3p inhibited the production of mu-opioid receptor protein by destabilizing the mRNA127. This shows that miRNAs associated with clinical parameters may provide insights on mechanisms by linking it to observations in animal models and vice versa.
To investigate the functional consequences of circulating miRNAs in pain we recently characterized exosomes derived from mouse RAW 264.7 macrophage cell lines and demonstrated that macrophages primed with the inflammatory stimulus lipopolysaccharide (LPS) release exosomes containing a distinct miRNA signature. Next generation sequencing of exosomal RNA showed the reads that mapped to miRNAs were more abundant after LPS stimulation. Exosomes from LPS-stimulated cells showed a dose dependent NF-κB activation compared with exosomes from control cells, confirming that exosomes were functional in recipient cells. To determine the effect of exosomes in mediating pain hypersensitivity, exosomes were directly injected into CFA-treated paws. Although exosomes derived from both unstimulated and LPS-stimulated cells reduced CFA-induced thermal hyperalgesia, those from stimulated cells also attenuated paw swelling and showed an analgesic response at earlier time points after delivery128. The absence of pain and swelling in saline-treated paws after exosome injection demonstrates that exosomal delivery does not induce a hypersensitivity response. This combined with the fact that exosomes can be loaded with nucleic acids or drugs suggests a potential role for exosomes in pain therapy. Exosomes may provide an avenue to regulate inflammatory response and reduce chronic inflammatory pain by modulating dysregulated signaling pathways. Exosomes derived from a patient and reintroduced after loading with the molecule of interest should be better tolerated by the immune system129–131. Further investigations of exosomal biology and other microvesicle-mediated transport will no doubt provide insights on how regulation of cellular processes can be mediated via circulation. Although little is known about the mechanisms of recognition and uptake of circulating miRNAs, it was recently demonstrated that hematopoietic cells, which include the cells of the immune system, release mRNA and miRNAs that are taken up by the neurons132. Using transgenic mice expressing Cre recombinase under the hematopoietic-specific promoter in a Cre reporter background, Purkinje neurons expressed the reporter. Having ruled out cell fusion, they identified Cre-recombinase mRNA, but not the protein in the extracellular vesicles. Induction of peripheral inflammation increased the number of Cre-recombined neurons demonstrating that mRNA can be transferred from immune cells in blood to neurons in central nervous system via extracellular vesicles. Cre-recombined Purkinje neurons contained three miRNAs not found in non-recombined neurons and the same three miRNAs were also present in exosomes isolated from blood of mice 24 h after induction of peritonitis132. Thus exosomes provide yet another mode of regulation of the nervous system mediated by the immune system.
miRNAs have been reported to act as agonists of Toll-like receptors (TLRs) and activate downstream signaling pathway in target cells133. Additionally, miRNAs have been shown to act extracellularly by directly interacting with cell-surface receptors; miR-21, miR-29a and let-7b act as agonists of Toll-like receptors (TLR) 7 and 8, influencing downstream signaling pathway in target cells133. Another recent study showed that secreted miRNAs may represent a new class of pain mediators; miRNA let-7b induced rapid inward currents and action potentials in DRG neurons coexpressing TLR7 and TRPA1 receptors. miRNA-induced neuronal activation required miRNAs with a GUUGUGU motif indicating that GU-rich core is critical. DRG neurons from mice lacking either TLR7 or TRPA1 did not respond to let-7b. In earlier studies describing actions of let-7b at TLR7, myeloid differentiation factor 88 (Myd88), an adapter protein associated with TLR7 was critical for the canonical signaling of most TLRs including TLR7. In Myd88-deficient DRG neurons, let-7b-induced inward currents were largely intact, although the amplitude of currents was slightly reduced compared to that of wild type neurons. TLR7 and TRPA1 co-localized with let-7b, both on the cell surface and inside cells and let-7 activated TLR7 and TRPA1 in HEK293 cells. Expression of TRPA1 alone was not effective confirming that TLR7 is essential for mediating the let-7b-induced currents in HEK293 cells. Interestingly, let-7b was more potent than the synthetic TLR7 agonists loxoribine and imiquimod in inducing inward currents in DRG neurons. Single-channel recordings in both DRG neurons and HEK293 cells confirmed that let-7b could bind TLR7 on the extracellular surface to induce single-channel activities of TRPA1. Spontaneous pain was induced upon intraplantar injection of let-7b, and an inhibitor of let-7b reduced formalin-induced TRAPA1 currents and spontaneous pain134. A single intraplantar injection of let-7b produced mechanical allodynia that was dependent on TLR7, Myd88 and TrpA1 activation in a dose-dependent manner that was abolished in Tlr7 knockout mice and reduced in Trpa1 and Myd88 knockout mice. Pretreatment with an inhibitor of let-7b reduced formalin-induced inflammatory pain in wild type animals. These studies demonstrated that extracellular let-7b is required and sufficient for inducing inflammatory pain via the activation of TLR7 and TRPA1. Additionally, let-7b is highly enriched in DRG tissues and endogenous let-7b can be released from DRG neurons in an activity-dependent manner134. These studies indicate an unconventional role for miRNAs that could potentially act in an autocrine fashion on nociceptors, or mediate novel signaling mechanisms.
Studies using rodent models of pain highlighting the effectiveness of synthetic miRNAs in reducing hyperalgesia have been beneficial in providing the proof-of principle for miRNA-based therapy. Different animal models have also been used to determine tissue specific expression changes under various acute and chronic pain conditions. The difficulty in obtaining human tissue samples of interest has led to the analysis of circulating miRNA in patients with chronic pain disorders. For miRNAs and their mRNA targets conserved across species, observations in patients can be validated in rodents. Though miRNA therapeutics for pain is still unrealized, other diseases have benefited and are forging ahead with clinical trials. Miraversen has been proven to be an effective therapeutic for chronic hepatitis C135. This is not surprising because liver is relatively easy to be targeted for therapy and when administered in the absence of a carrier, miRNA oligos are taken up by the liver and kidney and rapidly excreted in urine136. miRNAs found in plasma and blood samples have emerged as potential biomarkers for pain. Although pain is the common denominator in a variety of disorders, the underlying neurobiological mechanisms differ between pain conditions and circulating miRNAs may provide insight into unique miRNA signatures that could be exploited for diagnostic uses as well as therapeutics. Identifying several miRNAs as biomarkers, rather than relying on one specific molecule or parameter, may increase the chances of successful treatment in an extremely heterogeneous group of patients suffering from pain. Exploring the basis for the altered expression of such biomarkers, their relationship to disease progression and utility in predicting disease progression and clinical outcome will undoubtedly be beneficial in unravelling the significance of miRNAs in the context of pain.
Challenges associated with miRNA research
Lack of overt phenotype in loss-of-function studies has been an impediment in the miRNA field. Though the initial identification of miRNAs in C. elegans was accomplished using classic genetic screens, the vast majority of deletion and inhibition studies lacked significant phenotypes. The targets of lin-4 and let-7 miRNAs were identified based on discrete developmental timing events, but identifying mRNA targets of miRNAs that are ubiquitously expressed in adult tissues is more challenging. The task of determining valid miRNA: mRNA interactions requires multiple bioinformatics prediction algorithms, individual validation of binding, and translational suppression in conjunction with forward genetic techniques to uncover new potential targets.5 With miRNAs acting as a rheostat, the influence of miRNAs under a normal or homeostatic state is often described as modest. It has been proposed that under stress or disease states, the functions of miRNAs are more pronounced3.
The majority of the 25 miRNA knockout mice that have been examined thus far lack severe defects in embryonic development3. This is not surprising considering that miRNAs are finetuners of gene regulation. With multiple target binding sites within a gene, and the presence of several miRNA family members with the same seed sequence, compensatory mechanisms can easily override any functional perturbations resulting from loss or inhibition of an individual miRNA. It has been proposed that investigating miRNA signaling under conditions of cellular stress or injury perturbing cellular homeostasis may be required for unraveling the role of miRNAs. Several potential mechanisms by which miRNAs mediate stress signaling have been proposed. An increase or decrease in expression of an miRNA can act as a critical intermediate in modulating signaling pathways. A miRNA can serve as a negative or positive feedback loop to amplify or dampen a signal. miRNAs can also have a buffering role by targeting both positive and negative regulators in a signal transduction pathway, thus enabling a cell or tissue to return to a homeostatic state3. An example for negative regulation has been demonstrated for miR-146a. This miRNA is induced in THP-1 monocytic cell lines in response to LPS and proinflammatory cytokines. Promoter analysis of miR-146a showed that its induction in response to LPS, TNFα, and IL-1β was dependent on NF-KB. One of the branches of TLR4 signaling cascade is initiated when adaptor protein MyD88 is recruited to the receptor. MyD88 serves as a bridge between TLR4 and IL-1 receptor-associated kinase 1 (IRAK1) that then recruits TNF receptor-associated factor 6 (TRAF6) into the complex. miR-146a can target the UTRs of TRAF6 and IRAK1 genes. Thus miR-146a which is activated by NF-KB, negatively feeds back on this signaling cascade by targeting TRAF6 and IRAK1, the two upstream activators of the pathway137. NF-kB pathway also has been shown to employ a miRNA-mediated positive feedback circuit. Lin28B-dependent downregulation of let-7 causes upregulation of IL-6 which is a target of let-7, and this result in further stimulation of NF-kB138. Applying these emerging principles of miRNA regulation of stress signaling pathways to our understanding of the roles of miRNAs in disease states including pain, will be beneficial in elucidating their role.
Another challenging aspect of miRNA research is the absence of high-throughput biological approaches to identify miRNA targets. Elucidation of functional consequences of aberrant miRNA expression in a disease state is dependent on understanding the target genes being regulated in a physiologically relevant context. To describe the mechanistic basis of miRNA function in the context of a single downstream target is an oversimplification of the mode of action of miRNAs4. Thus, a combination of systems biology approaches with functional studies of individual target genes will help capture global perturbations affecting signaling cascades.
Though significant progress has been made, substantial challenges remain in the delivery and design of effective synthetic RNAs. Success of antimiR or antagomiR therapy will depend on achieving meaningful down regulation of the targeted miRNA. Anti-miRs accumulate predominantly in liver and kidneys. Hence, it is not surprising that the first miRNA-based therapeutic to reach the clinic is against a liver-specific miRNA139. As with any good pharmacological modulator, cell permeability, stability, and rate of excretion will be crucial for a successful therapeutic outcome30.
Conclusions
It is thought that each miRNA can bind and repress multiple mRNAs, which ultimately affects protein levels that can be crucial in regulating multiple physiological pathways. Induction of extensive miRNA expression changes in chronic pain conditions indicates their involvement in mediating underlying mechanisms of chronic pain, ranging from neuronal hyperexcitability and neuroinflammation to possibly altered higher brain function86. miRNAs represent an emerging new tool for patient management, with a wide range of potential clinical applications, including diagnosis, prognosis, and prediction of treatment efficiency. Larger patient cohorts will be needed to reach definitive conclusions regarding the prognostic power of extracellular miRNAs in pain. miRNAs also have potential therapeutic applications, but more effort is needed before miRNAs are translated from research tools to clinical use. Investigating the role of circulating miRNAs and exosome-mediated information transfer and employing systems biology approaches will undoubtedly advance our understanding of miRNA biology under conditions of homeostasis and disease in the context of pain.
Table 1. Comparison of miRNA and siRNA.
Small interfering RNAs (siRNAs) repress target mRNAs by binding with 100% complementarity to target mRNAs, forming a double stranded (dsRNA) motif that is degraded by the RNA-induced silencing complex (RISC complex). An association of non-coding RNA, mRNA, and proteins including Ago2, make up the RISC complex and guide the process of RNA interference (RNAi)140. Both miRNAs and siRNAs are bound by Ago2, a small RNA scaffold that has endonucleolytic activity, but the smaller region of complementarity between miRNAs and their target mRNA ensures that not all miRNA:mRNA interactions leads to mRNA degradation141. These two types of non-coding RNAs are similar in size and function, but have important distinctions in the mechanisms employed to regulate endogenous mRNA translation.
| miRNA | siRNA |
|---|---|
| ~21-bp RNA | Similar length |
| Derived from longer ~70-bp imperfectly base-paired hairpin | Derived from longer, perfectly complementary double-stranded RNA |
| Precursor of endogenous origin | Precursors of endogenous or exogenous origin |
| Nuclear processing by Drosha and secondary processing in the cytoplasm by Dicer | Processed in the cytoplasm by Dicer |
| Translational repression or mRNA degradation | Degradation of mRNA by RNAi |
| Heterosilencing; i.e., target remote loci | Autosilencing; i.e., target the same locus |
| miRNA can regulate several target RNAs | Gene (family) specific silencing |
| Highly conserved among species | Rarely conserved |
| Only common feature of the targets are the short sequences complementary to 6–7 bases seed sequence of the miRNA | High complementarity to target mRNA (often 100%) |
| Clinical use | |
| Drug targets or drug agents themselves | Valuable laboratory tool |
| Expression levels (potential diagnostic or biomarker tools) | Several siRNAs in clinical trials as therapeutic agents |
Acknowledgments
We acknowledge grant support from the Rita Allen Foundation and National Institutes of Health (1R21NS082991-01) to S.A.
References
- 1.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esteller M Non-coding RNAs in human disease. Nature reviews Genetics 2011;12:861–74. [DOI] [PubMed] [Google Scholar]
- 3.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell 2012;148:1172–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson EN. MicroRNAs as Therapeutic Targets and Biomarkers of Cardiovascular Disease. Science Translational Medicine 2014;6:239ps3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 2009;19:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambros V The functions of animal microRNAs. Nature 2004;431:350–5. [DOI] [PubMed] [Google Scholar]
- 7.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993;75:843–54. [DOI] [PubMed] [Google Scholar]
- 8.Olsen PH, Ambros V. The lin-4 Regulatory RNA Controls Developmental Timing in Caenorhabditis elegans by Blocking LIN-14 Protein Synthesis after the Initiation of Translation. Developmental Biology 1999;216:671–80. [DOI] [PubMed] [Google Scholar]
- 9.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Muller P, Spring J, Srinivasan A, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 2000;408:86–9. [DOI] [PubMed] [Google Scholar]
- 10.Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature 2004;432:235–40. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001;409:363–6. [DOI] [PubMed] [Google Scholar]
- 12.Gregory R, Chendrimada T, Shiekhattar R. MicroRNA Biogenesis: Isolation and Characterization of the Microprocessor Complex. In: Ying S-Y, ed. MicroRNA Protocols: Humana Press; 2006:33–47. [DOI] [PubMed] [Google Scholar]
- 13.Djuranovic S, Nahvi A, Green R. A parsimonious model for gene regulation by miRNAs. Science 2011;331:550–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baskerville S, Bartel DP. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA 2005;11:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y, Lu Y, Zhang Q, Liu J-J, Li T-J, Yang J-R, Zeng C, Zhuang S-M. MicroRNA-26a/b and their host genes cooperate to inhibit the G1/S transition by activating the pRb protein. Nucleic acids research 2012;40:4615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olena AF, Patton JG. Genomic organization of microRNAs. J Cell Physiol 2009;222:540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proceedings of the National Academy of Sciences of the United States of America 2004;101:2999–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffiths-Jones S The microRNA Registry. Nucleic acids research 2004;32:D109–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic acids research 2006;34:D140–D4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic acids research 2011;39:D152–D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh WJ, Lin FM, Huang HD, Wang H. Investigating microRNA-Target Interaction-Supported Tissues in Human Cancer Tissues Based on miRNA and Target Gene Expression Profiling. PloS one 2014;9:e95697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu B, Li J, Cairns MJ. Identifying miRNAs, targets and functions. Briefings in Bioinformatics 2014;15:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Q, Wang Y, Hao Y, Juan L, Teng M, Zhang X, Li M, Wang G, Liu Y. miR2Disease: a manually curated database for microRNA deregulation in human disease. Nucleic acids research 2009;37:D98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nature reviews Genetics 2008;9:102–14. [DOI] [PubMed] [Google Scholar]
- 25.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nature reviews Genetics 2010;11:597–610. [DOI] [PubMed] [Google Scholar]
- 26.Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS biology 2005;3:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farh KK-H, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, Burge CB, Bartel DP. The Widespread Impact of Mammalian MicroRNAs on mRNA Repression and Evolution. Science 2005;310:1817–21. [DOI] [PubMed] [Google Scholar]
- 28.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Molecular cell 2007;27:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majoros WH, Ohler U. Spatial preferences of microRNA targets in 3’ untranslated regions. BMC genomics 2007;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Rooij E The Art of MicroRNA Research. Circulation research 2011;108:219–34. [DOI] [PubMed] [Google Scholar]
- 31.Friedman RC, Farh KK-H, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome research 2009;19:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu S-D, Lin F-M, Wu W-Y, Liang C, Huang W-C, Chan W-L, Tsai W-T, Chen G-Z, Lee C-J, Chiu C-M, Chien C-H, Wu M-C, Huang C-Y, Tsou A-P, Huang H-D. miRTarBase: a database curates experimentally validated microRNA–target interactions. Nucleic acids research 2011;39:D163–D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature 2009;460:479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, Rothballer A, Ascano M, Jr., Jungkamp A-C, Munschauer M, Ulrich A, Wardle GS, Dewell S, Zavolan M, Tuschl T. Transcriptome-wide Identification of RNA-Binding Protein and MicroRNA Target Sites by PAR-CLIP. Cell;141:129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lennox KA, Owczarzy R, Thomas DM, Walder JA, Behlke MA. Improved Performance of Anti-miRNA Oligonucleotides Using a Novel Non-Nucleotide Modifier. Molecular therapy Nucleic acids 2013;2:e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Meth 2007;4:721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tay FC, Lim JK, Zhu H, Hin LC, Wang S. Using artificial microRNA sponges to achieve microRNA loss-of-function in cancer cells. Advanced drug delivery reviews. [DOI] [PubMed] [Google Scholar]
- 38.Prosser HM, Koike-Yusa H, Cooper JD, Law FC, Bradley A. A resource of vectors and ES cells for targeted deletion of microRNAs in mice. Nature biotechnology 2011;29:840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park Chong Y, Jeker Lukas T, Carver-Moore K, Oh A, Liu Huey J, Cameron R, Richards H, Li Z, Adler D, Yoshinaga Y, Martinez M, Nefadov M, Abbas Abul K, Weiss A, Lanier Lewis L, de Jong Pieter J, Bluestone Jeffrey A, Srivastava D, McManus Michael T. A Resource for the Conditional Ablation of microRNAs in the Mouse. Cell reports;1:385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proceedings of the National Academy of Sciences of the United States of America 2008;105:10513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Zen K, Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell research 2008;18:997–1006. [DOI] [PubMed] [Google Scholar]
- 42.Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H, Melamed N, Bentwich Z, Hod M, Goren Y, Chajut A. Serum microRNAs are promising novel biomarkers. PloS one 2008;3:e3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grasedieck S, Sorrentino A, Langer C, Buske C, D√∂hner H, Mertens D, Kuchenbauer F. Circulating microRNAs in hematological diseases: principles, challenges, and perspectives. Blood 2013;121:4977–84. [DOI] [PubMed] [Google Scholar]
- 44.Boon RA, Vickers KC. Intercellular transport of microRNAs. Arterioscler Thromb Vasc Biol 2013;33:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic acids research 2011;39:7223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Current Opinion in Cell Biology 2014;29:116–25. [DOI] [PubMed] [Google Scholar]
- 47.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014;14:195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoorvogel W Functional transfer of microRNA by exosomes. Blood 2012;119:646–8. [DOI] [PubMed] [Google Scholar]
- 49.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009;9:581–93. [DOI] [PubMed] [Google Scholar]
- 50.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654–9. [DOI] [PubMed] [Google Scholar]
- 51.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A, Buzas EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 2011;68:2667–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borràs FE, Breakefield X, Budnik V, Buzas E, Camussi G, Clayton A, Cocucci E, Falcon-Perez JM, Gabrielsson S, Gho YS, Gupta D, Harsha HC, Hendrix A, Hill AF, Inal JM, Jenster G, Krämer-Albers E-M, Lim SK, Llorente A, Lötvall J, Marcilla A, Mincheva-Nilsson L, Nazarenko I, Nieuwland R, Nolte-’t Hoen ENM, Pandey A, Patel T, Piper MG, Pluchino S, Prasad TSK, Rajendran L, Raposo G, Record M, Reid GE, Sánchez-Madrid F, Schiffelers RM, Siljander P, Stensballe A, Stoorvogel W, Taylor D, Thery C, Valadi H, van Balkom BWM, Vázquez J, Vidal M, Wauben MHM, Yáñez-Mó M, Zoeller M, Mathivanan S. Vesiclepedia: A Compendium for Extracellular Vesicles with Continuous Community Annotation. PLoS biology 2012;10:e1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic acids research 2012;40:D1241–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villarroya-Beltri C, Baixauli F, Gutierrez-Vazquez C, Sanchez-Madrid F, Mittelbrunn M. Sorting it out: Regulation of exosome loading. Seminars in cancer biology 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vickers KC, Remaley AT. Lipid-based carriers of microRNAs and intercellular communication. Current opinion in lipidology 2012;23:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem 2010;56:1733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiland M, Gao X-H, Zhou L, Mi Q-S. Small RNAs have a large impact: Circulating microRNAs as biomarkers for human diseases. RNA biology 2012;9:850–9. [DOI] [PubMed] [Google Scholar]
- 58.Ajit SK. Circulating microRNAs as biomarkers, therapeutic targets, and signaling molecules. Sensors (Basel) 2012;12:3359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cogswell JP, Ward J, Taylor IA, Waters M, Shi Y, Cannon B, Kelnar K, Kemppainen J, Brown D, Chen C, Prinjha RK, Richardson JC, Saunders AM, Roses AD, Richards CA. Identification of miRNA Changes in Alzheimer’s Disease Brain and CSF Yields Putative Biomarkers and Insights into Disease Pathways. Journal of Alzheimer’s Disease 2008;14:27–41. [DOI] [PubMed] [Google Scholar]
- 60.Machida A, Ohkubo T, Yokota T. Circulating MicroRNAs in the Cerebrospinal Fluid of Patients with Brain Diseases. In: Kosaka N, ed. Circulating MicroRNAs: Humana Press; 2013:203–9. [DOI] [PubMed] [Google Scholar]
- 61.Rau C-S, Jeng J, Jeng S-F, Lu T-H, Chen Y-C, Liliang P-C, Wu C-J, Lin C-J, Hsieh C-H. Entrapment neuropathy results in different microRNA expression patterns from denervation injury in rats. BMC Musculoskeletal Disorders 2010;11:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhalala OG, Srikanth M, Kessler JA. The emerging roles of microRNAs in CNS injuries. Nat Rev Neurol 2013;9:328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kress M, Hüttenhofer A, Landry M, Kuner R, Favereaux A, Greenberg DS, Bednarik J, Heppenstall P, Kronenberg F, Malcangio M, Rittner H, Üçeyler N, Trajanoski Z, Mouritzen P, Birklein F, Sommer C, Soreq H. microRNAs in nociceptive circuits as predictors of future clinical applications. Frontiers in Molecular Neuroscience 2013;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain 2010;151:12–7. [DOI] [PubMed] [Google Scholar]
- 65.Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An overview of animal models of pain: disease models and outcome measures. The journal of pain : official journal of the American Pain Society 2013;14:1255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elramah S, Landry M, Favereaux A. MicroRNAs regulate neuronal plasticity and are involved in pain mechanisms. Front Cell Neurosci 2014;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao J, Lee MC, Momin A, Cendan CM, Shepherd ST, Baker MD, Asante C, Bee L, Bethry A, Perkins JR, Nassar MA, Abrahamsen B, Dickenson A, Cobb BS, Merkenschlager M, Wood JN. Small RNAs control sodium channel expression, nociceptor excitability, and pain thresholds. J Neurosci 2010;30:10860–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schaefer A, O’Carroll D, Tan CL, Hillman D, Sugimori M, Llinas R, Greengard P. Cerebellar neurodegeneration in the absence of microRNAs. The Journal of experimental medicine 2007;204:1553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu D, Raafat A, Pak E, Clemens S, Murashov AK. Dicer-microRNA pathway is critical for peripheral nerve regeneration and functional recovery in vivo and regenerative axonogenesis in vitro. Experimental neurology 2012;233:555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kynast KL, Russe OQ, Geisslinger G, Niederberger E. Novel findings in pain processing pathways: implications for miRNAs as future therapeutic targets. Expert Rev Neurother 2013;13:515–25. [DOI] [PubMed] [Google Scholar]
- 71.Sakai A, Suzuki H. Emerging roles of microRNAs in chronic pain. Neurochemistry International. [DOI] [PubMed] [Google Scholar]
- 72.Bali KK, Kuner R. Noncoding RNAs: key molecules in understanding and treating pain. Trends Mol Med 2014;20:437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan P-H, Pao Y-Y, Cheng J-K, Hung K-C, Liu C-C. MicroRNA-based therapy in pain medicine: Current progress and future prospects. Acta Anaesthesiologica Taiwanica 2013;51:171–6. [DOI] [PubMed] [Google Scholar]
- 74.Bai G, Ambalavanar R, Wei D, Dessem D. Downregulation of selective microRNAs in trigeminal ganglion neurons following inflammatory muscle pain. Mol Pain 2007;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kusuda R, Cadetti F, Ravanelli MI, Sousa TA, Zanon S, De Lucca FL, Lucas G. Differential expression of microRNAs in mouse pain models. Mol Pain 2011;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aldrich BT, Frakes EP, Kasuya J, Hammond DL, Kitamoto T. Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience 2009;164:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strickland IT, Richards L, Holmes FE, Wynick D, Uney JB, Wong L-F. Axotomy-induced miR-21 promotes axon growth in adult dorsal root ganglion neurons. PloS one 2011;6:e23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu B, Zhou S, Wang Y, Ding G, Ding F, Gu X. Profile of MicroRNAs following Rat Sciatic Nerve Injury by Deep Sequencing: Implication for Mechanisms of Nerve Regeneration. PloS one 2011;6:e24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.von Schack D, Agostino MJ, Murray BS, Li Y, Reddy PS, Chen J, Choe SE, Strassle BW, Li C, Bates B, Zhang L, Hu H, Kotnis S, Bingham B, Liu W, Whiteside GT, Samad TA, Kennedy JD, Ajit SK. Dynamic changes in the microRNA expression profile reveal multiple regulatory mechanisms in the spinal nerve ligation model of neuropathic pain. PloS one 2011;6:e17670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakai A, Saitow F, Miyake N, Miyake K, Shimada T, Suzuki H. miR-7a alleviates the maintenance of neuropathic pain through regulation of neuronal excitability. Brain 2013;136:2738–50. [DOI] [PubMed] [Google Scholar]
- 81.Bali K, Hackenberg M, Lubin A, Kuner R, Devor M. Sources of individual variability: miRNAs that predispose to neuropathic pain identified using genome-wide sequencing. Molecular Pain 2014;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bali KK, Selvaraj D, Satagopam VP, Lu J, Schneider R, Kuner R. Genome-wide identification and functional analyses of microRNA signatures associated with cancer pain. EMBO molecular medicine 2013;5:1740–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poh KW, Yeo JF, Ong WY. MicroRNA changes in the mouse prefrontal cortex after inflammatory pain. Eur J Pain 2011;15:801 e1–12. [DOI] [PubMed] [Google Scholar]
- 84.Imai S, Saeki M, Yanase M, Horiuchi H, Abe M, Narita M, Kuzumaki N, Suzuki T, Narita M. Change in MicroRNAs Associated with Neuronal Adaptive Responses in the Nucleus Accumbens under Neuropathic Pain. The Journal of Neuroscience 2011;31:15294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arai M, Genda Y, Ishikawa M, Shunsuke T, Okabe T, Sakamoto A. The miRNA and mRNA Changes in Rat Hippocampi after Chronic Constriction Injury. Pain Medicine 2013;14:720–9. [DOI] [PubMed] [Google Scholar]
- 86.Sakai A, Suzuki H. Emerging roles of microRNAs in chronic pain. Neurochemistry international 2014. [DOI] [PubMed] [Google Scholar]
- 87.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature 2008;455:58–63. [DOI] [PubMed] [Google Scholar]
- 88.Shalgi R, Lieber D, Oren M, Pilpel Y. Global and Local Architecture of the Mammalian microRNA–Transcription Factor Regulatory Network. PLoS Comput Biol 2007;3:e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hobert O Gene regulation by transcription factors and microRNAs. Science 2008;319:1785–6. [DOI] [PubMed] [Google Scholar]
- 90.Flynt AS, Lai EC. Biological principles of microRNA-mediated regulation: shared themes amid diversity. Nature reviews Genetics 2008;9:831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen H-P, Zhou W, Kang L-M, Yan H, Zhang L, Xu B-H, Cai W-H. Intrathecal miR-96 Inhibits Nav1.3 Expression and Alleviates Neuropathic Pain in Rat Following Chronic Construction Injury. Neurochemical research 2014;39:76–83. [DOI] [PubMed] [Google Scholar]
- 92.Sengupta JN, Pochiraju S, Kannampalli P, Bruckert M, Addya S, Yadav P, Miranda A, Shaker R, Banerjee B. MicroRNA-mediated GABAAα-1 receptor subunit down-regulation in adult spinal cord following neonatal cystitis-induced chronic visceral pain in rats. PAIN® 2013;154:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Im YB, Jee MK, Choi JI, Cho HT, Kwon OH, Kang SK. Molecular targeting of NOX4 for neuropathic pain after traumatic injury of the spinal cord. Cell Death Dis 2012;3:e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun Y, Li XQ, Sahbaie P, Shi XY, Li WW, Liang DY, Clark JD. miR-203 regulates nociceptive sensitization after incision by controlling phospholipase A2 activating protein expression. Anesthesiology 2012;117:626–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ni J, Gao Y, Gong S, Guo S, Hisamitsu T, Jiang X. Regulation of mu-opioid type 1 receptors by microRNA134 in dorsal root ganglion neurons following peripheral inflammation. Eur J Pain 2013;17:313–23. [DOI] [PubMed] [Google Scholar]
- 96.Favereaux A, Thoumine O, Bouali-Benazzouz R, Roques V, Papon MA, Salam SA, Drutel G, Leger C, Calas A, Nagy F, Landry M. Bidirectional integrative regulation of Cav1.2 calcium channel by microRNA miR-103: role in pain. EMBO J 2011;30:3830–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Willemen H, Huo X-J, Mao-Ying Q-L, Zijlstra J, Heijnen C, Kavelaars A. MicroRNA-124 as a novel treatment for persistent hyperalgesia. Journal of neuroinflammation 2012;9:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kynast KL, Russe OQ, Möser CV, Geisslinger G, Niederberger E. Modulation of CNS-specific microRNA-124a alters the inflammatory response in the formalin test in mice. Pain 2012. [DOI] [PubMed] [Google Scholar]
- 99.Pan Z, Zhu L-J, Li Y-Q, Hao L-Y, Yin C, Yang J-X, Guo Y, Zhang S, Hua L, Xue Z-Y, Zhang H, Cao J-L. Epigenetic Modification of Spinal miR-219 Expression Regulates Chronic Inflammation Pain by Targeting CaMKIIγ. The Journal of Neuroscience 2014;34:9476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He Y, Yang C, Kirkmire CM, Wang ZJ. Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J Neurosci 2010;30:10251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.HE Y, WANG ZJ. Let-7 microRNAs and opioid tolerance. Frontiers in Genetics 2012;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene 2007;26:2799–803. [DOI] [PubMed] [Google Scholar]
- 103.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 2005;65:6029–33. [DOI] [PubMed] [Google Scholar]
- 104.Liu N-K, Wang X-F, Lu Q-B, Xu X-M. Altered microRNA expression following traumatic spinal cord injury. Experimental neurology 2009;219:424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yunta M, Nieto-Díaz M, Esteban FJ, Caballero-López M, Navarro-Ruíz R, Reigada D, Pita-Thomas DW, Águila Ád, Muñoz-Galdeano T, Maza RM. MicroRNA Dysregulation in the Spinal Cord following Traumatic Injury. PloS one 2012;7:e34534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nieto-Díaz M, Esteban FJ, Reigada D, Muñoz-Galdeano T, Yunta M, Caballero-López M, Navarro-Ruiz R, del Águila Á, Maza RM. MicroRNA dysregulation in Spinal Cord Injury: causes, consequences and therapeutics. Frontiers in Cellular Neuroscience 2014;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, Kessler JA. microRNA-21 regulates astrocytic response following spinal cord injury. J Neurosci 2012;32:17935–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci 2004;5:146–56. [DOI] [PubMed] [Google Scholar]
- 109.Sakai A, Suzuki H. Nerve injury-induced upregulation of miR-21 in the primary sensory neurons contributes to neuropathic pain in rats. Biochemical and biophysical research communications 2013;435:176–81. [DOI] [PubMed] [Google Scholar]
- 110.Veremeyko T, Siddiqui S, Sotnikov I, Yung A, Ponomarev ED. IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation. PloS one 2013;8:e81774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mishima T, Mizuguchi Y, Kawahigashi Y, Takizawa T. RT-PCR-based analysis of microRNA (miR-1 and -124) expression in mouse CNS. Brain Res 2007;1131:37–43. [DOI] [PubMed] [Google Scholar]
- 112.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nature medicine 2011;17:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kocerha J, Faghihi MA, Lopez-Toledano MA, Huang J, Ramsey AJ, Caron MG, Sales N, Willoughby D, Elmen J, Hansen HF, Orum H, Kauppinen S, Kenny PJ, Wahlestedt C. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proceedings of the National Academy of Sciences of the United States of America 2009;106:3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chattopadhyay M, Zhou Z, Hao S, Mata M, Fink D. Reduction of voltage gated sodium channel protein in DRG by vector mediated miRNA reduces pain in rats with painful diabetic neuropathy. Molecular Pain 2012;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Walder RY, Gautam M, Wilson SP, Benson CJ, Sluka KA. Selective targeting of ASIC3 using artificial miRNAs inhibits primary and secondary hyperalgesia after muscle inflammation. Pain 2011;152:2348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hébert SS, De Strooper B. Alterations of the microRNA network cause neurodegenerative disease. Trends in Neurosciences;32:199–206. [DOI] [PubMed] [Google Scholar]
- 117.Siddeek B, Inoubli L, Lakhdari N, Rachel PB, Fussell KC, Schneider S, Mauduit C, Benahmed M. MicroRNAs as potential biomarkers in diseases and toxicology. Mutation research Genetic toxicology and environmental mutagenesis 2014;764–765:46–57. [DOI] [PubMed] [Google Scholar]
- 118.De Guire V, Robitaille R, Tetreault N, Guerin R, Menard C, Bambace N, Sapieha P. Circulating miRNAs as sensitive and specific biomarkers for the diagnosis and monitoring of human diseases: promises and challenges. Clin Biochem 2013;46:846–60. [DOI] [PubMed] [Google Scholar]
- 119.Ammari M, Jorgensen C, Apparailly F. Impact of microRNAs on the understanding and treatment of rheumatoid arthritis. Current opinion in rheumatology 2013;25:225–33. [DOI] [PubMed] [Google Scholar]
- 120.Shen N, Liang D, Tang Y, de Vries N, Tak PP. MicroRNAs--novel regulators of systemic lupus erythematosus pathogenesis. Nature reviews Rheumatology 2012;8:701–9. [DOI] [PubMed] [Google Scholar]
- 121.Fourie NH, Peace RM, Abey SK, Sherwin LB, Rahim-Williams B, Smyser PA, Wiley JW, Henderson WA. Elevated circulating miR-150 and miR-342-3p in patients with irritable bowel syndrome. Experimental and molecular pathology 2014;96:422–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gheinani A, Burkhard F, Monastyrskaya K. Deciphering microRNA code in pain and inflammation: lessons from bladder pain syndrome. Cell Mol Life Sci 2013;70:3773–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ohlsson Teague EMC, Print CG, Hull ML. The role of microRNAs in endometriosis and associated reproductive conditions. Human Reproduction Update 2010;16:142–65. [DOI] [PubMed] [Google Scholar]
- 124.Bjersing JL, Lundborg C, Bokarewa MI, Mannerkorpi K. Profile of Cerebrospinal microRNAs in Fibromyalgia. PloS one 2013;8:e78762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Orlova IA, Alexander GM, Qureshi RA, Sacan A, Graziano A, Barrett JE, Schwartzman RJ, Ajit SK. MicroRNA modulation in complex regional pain syndrome. J Transl Med 2011;9:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain 2003;106:127–33. [DOI] [PubMed] [Google Scholar]
- 127.Wu Q, Hwang CK, Zheng H, Wagley Y, Lin H-Y, Kim DK, Law P-Y, Loh HH, Wei L-N. MicroRNA 339 down-regulates μ-opioid receptor at the post-transcriptional level in response to opioid treatment. The FASEB Journal 2013;27:522–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McDonald MK, Tian Y, Qureshi RA, Gormley M, Ertel A, Gao R, Aradillas Lopez E, Alexander GM, Sacan A, Fortina P, Ajit SK. Functional significance of macrophage-derived exosomes in inflammation and pain. PAIN®. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 2005;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nature medicine 1998;4:594–600. [DOI] [PubMed] [Google Scholar]
- 131.Viaud S, Ploix S, Lapierre V, Thery C, Commere PH, Tramalloni D, Gorrichon K, Virault-Rocroy P, Tursz T, Lantz O, Zitvogel L, Chaput N. Updated technology to produce highly immunogenic dendritic cell-derived exosomes of clinical grade: a critical role of interferon-gamma. J Immunother 2011;34:65–75. [DOI] [PubMed] [Google Scholar]
- 132.Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Turco DD, Starmann J, Macas J, Karpova D, Devraj K, Depboylu C, Landfried B, Arnold B, Plate KH, Hoglinger G, Sultmann H, Altevogt P, Momma S. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS biology 2014;12:e1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fabbri M, Paone A, Calore F, Galli R, Croce CM. A new role for microRNAs, as ligands of Toll-like receptors. RNA biology 2013;10:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Park CK, Xu ZZ, Berta T, Han Q, Chen G, Liu XJ, Ji RR. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 2014;82:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Janssen HLA, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV Infection by Targeting MicroRNA. New England Journal of Medicine 2013;368:1685–94. [DOI] [PubMed] [Google Scholar]
- 136.Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov 2014;13:622–38. [DOI] [PubMed] [Google Scholar]
- 137.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proceedings of the National Academy of Sciences of the United States of America 2006;103:12481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 2009;139:693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lindow M, Kauppinen S. Discovering the first microRNA-targeted drug. The Journal of cell biology 2012;199:407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sen GL, Blau HM. A brief history of RNAi: the silence of the genes. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2006;20:1293–9. [DOI] [PubMed] [Google Scholar]
- 141.Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009;136:642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Murata K, Yoshitomi H, Tanida S, Ishikawa M, Nishitani K, Ito H, Nakamura T. Plasma and synovial fluid microRNAs as potential biomarkers of rheumatoid arthritis and osteoarthritis. Arthritis research & therapy 2010;12:R86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Murata K, Furu M, Yoshitomi H, Ishikawa M, Shibuya H, Hashimoto M, Imura Y, Fujii T, Ito H, Mimori T, Matsuda S. Comprehensive microRNA analysis identifies miR-24 and miR-125a-5p as plasma biomarkers for rheumatoid arthritis. PloS one 2013;8:e69118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Adachi T, Nakanishi M, Otsuka Y, Nishimura K, Hirokawa G, Goto Y, Nonogi H, Iwai N. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin Chem 2010;56:1183–5. [DOI] [PubMed] [Google Scholar]
- 145.Widera C, Gupta SK, Lorenzen JM, Bang C, Bauersachs J, Bethmann K, Kempf T, Wollert KC, Thum T. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. Journal of molecular and cellular cardiology 2011;51:872–5. [DOI] [PubMed] [Google Scholar]
- 146.Fourie NH, Peace RM, Abey SK, Sherwin LB, Rahim-Williams B, Smyser PA, Wiley JW, Henderson WA. Elevated circulating miR-150 and miR-342-3p in patients with irritable bowel syndrome. Experimental and molecular pathology 2014;96:422–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang WT, Zhao YN, Han BW, Hong SJ, Chen YQ. Circulating microRNAs identified in a genome-wide serum microRNA expression analysis as noninvasive biomarkers for endometriosis. The Journal of clinical endocrinology and metabolism 2013;98:281–9. [DOI] [PubMed] [Google Scholar]


