Abstract
Background
Renal cell carcinoma (RCC) has traditionally been considered to be radioresistant. Response rates are believed to be improved by a high dose of stereotactic body radiotherapy (SBRT). A retrospective analysis was conducted of patients treated with SBRT for metastatic disease from RCC.
Material and methods
We reviewed records from 20 patients who underwent SBRT for a total of 30 RCC metastases from 2015 to 2020. Patients were included who had a confirmed primary RCC and radiographic evidence of metastasis, either synchronous or metachronous. The most common SBRT fractionation was 30 Gy in 3 fractions.
Results
Median age was 60 years (range, 40–77 years) and 60% were male. After a median follow-up of 18 months (range, 3–36 months), overall survival was estimated to be 85% and 70%, at 1 and 2 years, respectively, and local control at 2 years was 83.33%. Only 5 patients had documented progression of disease, all of whom received biologically effective dose inferior to 100 Gy, and no patients treated with a higher biologically effective dose had disease, which progressed. The most common acute toxicity was grade 1 fatigue (20%). No grade 3 or higher acute toxicity occurred.
Conclusions
Treatment with SBRT in patients with RCC metastases yielded a high local control rate, promising survival rate, and low toxicity.
Keywords: Renal cell carcinoma, Metastases, Stereotactic body radiotherapy, Radioresistance
1. Introduction
Renal cancer represents 3% of all cancers and is the third most common urological malignancy after prostate and bladder cancer. More than 70% of patients have tumors with clear cell histology.[1] One in 3 patients has metastatic disease at diagnosis, and 30% of patients with localized carcinoma will develop metastases during follow-up. Lung, bone, liver, and brain are the most frequent sites of metastases.[2] Systemic treatment currently represents the standard of care for metastatic renal cell carcinoma (mRCC). Recent advances in the treatment of mRCC, from the introduction of antiangiogenic tyrosine kinase inhibitors (TKIs) to the recent approval of immune checkpoint inhibitors, have resulted in improved overall survival (OS).[3] Among all mRCC patients, a subgroup with oligometastatic disease, those who have a limited number of metastases, has been identified with an improved prognosis and a prolonged disease course.[4] Oligorecurrent disease is likely included in the spectrum of the oligometastatic disease described by Hellman and Weichselbaum.[5] For these patients, aggressive management with local therapies such as surgical excision and stereotactic body radiotherapy (SBRT) to active sites of disease may be of particular benefit.
Renal cell carcinoma has often been considered radioresistant; consequently, radiation has been mostly reserved for patients with metastatic disease. Endothelial cell apoptosis determines the radiosensitivity of several malignant tumors, including RCC. Conventional fractionated radiation involves daily fractions of 1.8–3.0 Gy, causing programmed cell death or P53-mediated apoptosis, which probably does not result in an adequate endothelial apoptotic response for tumor death in RCC. By contrast, higher radiotherapy fractions that efficiently destroy tumor microvasculature would be expected to have better results in tumors that are highly dependent on angiogenesis such as RCC.[6] Moreover, in the last 2 decades, several studies have shown the benefit of radiation in oligometastatic disease for numerous tumor types, especially SBRT, which allows accurate delivery of high doses of radiation to target lesions while minimizing the dose to surrounding organs at risk. Recently, a range of retrospective studies and one prospective study have investigated SBRT in the management of metastasis from renal cancer.[7–14]
This report aims to evaluate SBRT in the treatment of metastases from renal cancer, including its effects on local disease control and OS, and to determine SBRT-related toxicities.
2. Materials and methods
Patients were retrospectively identified by searching an institutional database of patients treated with radiation for RCC at the Le Littoral clinic of oncology between December 2015 and September 2020. Patients included in the study had a minimum of 3-month follow-up. In total, 20 patients with oligometastatic RCC, either synchronous (8 patients) or metachronous (12 patients), were included, for a total of 30 lesions treated with SBRT. All patients displayed radiographic evidence of distant metastasis, as assessed by computed tomography (CT), bone scan, and brain magnetic resonance imaging (MRI) if indicated. Histologic confirmation of primary RCC was mandatory for inclusion, which was obtained by nephrectomy (17 patients) or needle biopsy (3 patients). Stereotactic body radiotherapy was chosen for patients with oligometastatic disease after considering the feasibility of SBRT and consensus by a multidisciplinary committee. Patients also received a variety of targeted agents after SBRT at the discretion of the treating medical oncologist, beginning 2 to 4 weeks after the last course of SBRT. Characteristics of patients included in the study are shown in Table 1.
Table 1.
Patient characteristics (n = 20).
| Variables | Values |
|---|---|
| Age, yr | 60.4 (range, 40–77) |
| Sex, n (%) | |
| Male | 12 (60) |
| Female | 8 (40) |
| KPS, median (range) | 90 (70–100) |
| Histology, n (%) | |
| Clear cell | 15 (75) |
| Chromophobe | 3 (15) |
| Papillary | 1 (5) |
| Tubulo-papillary | 1 (5) |
| Systemic therapy | |
| First-line | 12 (60%) |
| Sunitinib | 11 |
| Pembrolizumab-axitinib | 1 |
| Second-line | 7 (35%) |
| Pazopanib | 5 |
| Axitinib | 1 |
| Nivolumab | 1 |
| Third-line | 1 (5%) |
| Nivolumab | 1 |
KPS = Karnofsky performance status.
This study was conducted in accordance with relevant ethical standards including the Helsinki Declaration.
2.1. Treatment
Before treatment, all patients were placed in a custom-made immobilization device and underwent contrast-enhanced CT imaging (2- to 3-mm slices) for radiation planning purposes. Diagnostic images (MRI, bone scan, etc) were anatomically correlated to the CT scans to assist with gross tumor volume delineation. Gross tumor volume was equal to the clinical target volume. Planning target volume was generated by adding 2 to 5 mm to gross tumor volume to account for setup reproducibility and lesion location at the physician’s discretion. Prescribed doses varied from 28 to 60 Gy in 3 to 5 fractions. Because of the lack of an available standard for the dose of radiation therapy, the prescription was at the discretion of the physician and based on the size and location of the tumor and nearby organs at risk (Table 2).
Table 2.
Metastasis location (n = 30) and fractionation regimen.
| Location | No. lesions (%) | Total radiation dose / fractions × dose per fraction | BED |
|---|---|---|---|
| Bone | 18 (60%) | ||
| 8 | 27 Gy / 3 × 9 Gy | 51.3 Gy | |
| 10 | 30 Gy / 3 × 10 Gy | 60 Gy | |
| Brain | 8 (27%) | 24 Gy / 3 × 8 Gy | 43.2 Gy |
| Lung | 3 (10%) | 60 Gy / 4 × 15 Gy | 150 Gy |
| Choroid | 1 (3%) | 28 Gy / 5 × 5.6 Gy | 43.68 Gy |
BED = biologically equivalent dose.
Treatment was delivered by Truebeam Novalis Tx (Varian Medical Systems). Before each treatment, image guidance cone-beam CT scans were acquired, and appropriate adjustments were made to correlate bony anatomy. Most target lesions could be visualized on the cone-beam CT scan to confirm that they were encompassed by the planning target volume (Fig. 1).
Figure 1.

Dose distribution, with the prescription isodose (90%), at the periphery of the planning target volume, on axial, coronal, and sagittal views.
During treatment, all patients underwent weekly surveillance. Acute toxicity was scored using the Common Terminology Criteria for Adverse Events v3.0 scoring system.
2.2. Statistics
The primary purpose of this study was to demonstrate the efficacy of SBRT for metastasis from RCC, which is generally considered radioresistant. End points were OS, local control (LC), and SBRT-related acute toxicity.
Local response was assessed clinically and radiologically according to RECIST criteria during follow-up. Local progression was defined as either clinical or radiological progression of the treated metastasis. Survival and time to LC were calculated from the date of the start of SBRT treatment. Data were analyzed using the Kaplan-Meier method to estimate OS (Figs. 2, 3).
Figure 2.
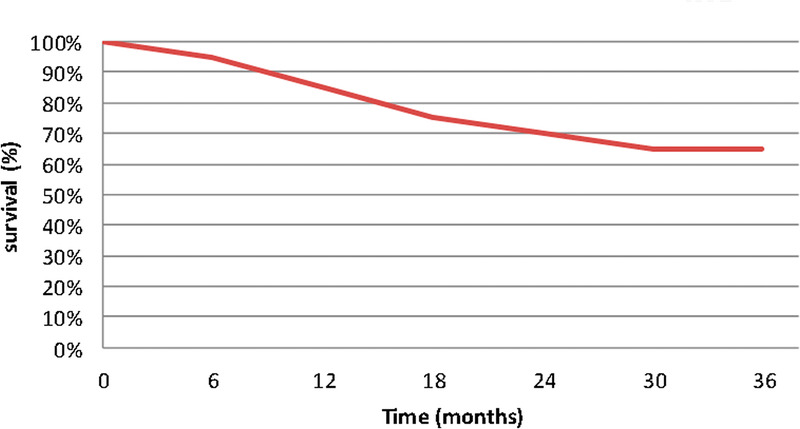
Kaplan-Meier curve, OS after SBRT. OS = overall survival; SBRT = stereotactic body radiation therapy.
Figure 3.

Kaplan-Meier curve, local progression-free survival after SBRT. SBRT = stereotactic body radiation therapy.
2.3. Follow-up
Patients returned for follow-up 1 month after the end of their treatment and then every 3 months thereafter, during which a comprehensive history was obtained and physical examination was performed. Appropriate imaging studies (CT, MRI, or bone scan) were performed 3 months after the completion of treatment and then every 3–6 months.
3. Results
The median follow-up of the study cohort was 18 months (range, 3–36 months). In total, 20 patients were included, with a total of 30 lesions treated with SBRT. The median age was 60 years (range, 40–77 years) and 60% of patients were male. All patients have a Karnofsky index of 70% or higher (Table 1). Clear cell carcinoma was the most frequent histology type. Total nephrectomy was performed in 5 of 8 patients with metachronous disease, and 8 patients underwent surgical excision of lesions other than those treated with SBRT (3 for soft tissue, 2 for brain, 2 for bone, and 1 for thyroid metastasis). The median number of metastases per patient was 1.7 (range, 1–3) with a median size of 2.3 cm (range, 1.4–3.5 cm).
The most common fractionation was 30 Gy given in 3 fractions (10 Gy per fraction). For all fractionation regimens used, the biologically effective dose (BED) ranged from 43.2 to 150 Gy, with a mean BED of 61.5 ± 17.7 Gy (Table 2). Among 30 metastases treated with SBRT, only 5 had documented disease progression. These included 3 vertebral metastases treated with 3 × 10 Gy (BED = 60 Gy) and 2 brain metastases treated with 3 × 8 Gy fractionation (BED = 43.2 Gy). Overall, LC was excellent, with an LC of 83.33% at 2 years. Overall survival was estimated at 85% and 70% for all patients, at 1 and 2 years, respectively.
Treatment was well tolerated, as shown in Table 3. The most common acute toxicity was grade 1 fatigue (20%). One patient treated for lumbar vertebral metastasis experienced grade 2 diarrhea. There was no documented grade 3 or higher acute toxicity.
Table 3.
Toxicity according to CTCAE v3.0 scoring system (n = 20).
| Charateristics | Grade I | Grade II | Grade III |
|---|---|---|---|
| Fatigue | 20% | 0% | 0% |
| Diarrhea | 10% | 5% | 0% |
| Nausea | 5% | 0% | 0% |
| Cough | 5% | 0% | 0% |
| Radiodermatitis | 5% | 0% | 0% |
CTCAE = Common Terminology Criteria for Adverse Events.
4. Discussion
In recent decades, SBRT has been widely used for the treatment of distant metastasis, especially in oligometastatic disease. Stereotactic body radiotherapy offers some advantages, such as a less invasive ablative option of cure, while at the same time obtaining very good LC and a low rate of toxicity.[15] The present study demonstrates the efficacy and safety of SBRT in the treatment of RCC metastatic disease. We found a LC rate of 83.3% at 2 years and OS of 85% and 70% at 1 and 2 years, respectively. Our results demonstrate that RCC is not necessarily radioresistant but sensitive to higher doses per fraction. These findings corroborate previous literature on the efficacy of SBRT for mRCC. Wersäll et al.[7] reported a large series of 162 treated tumors in 58 patients with either extracranial metastatic or inoperable renal tumors, with an excellent LC of 90% with mild side effects. Patients with 1–3 metastases, local recurrence after nephrectomy, or inoperable primary tumors benefited most. Only 3 progressions were reported in that study, all of which had a large retroperitoneal volume and a fraction dose, which did not exceed 8 Gy and a total dose less than 40 Gy.[7] Regarding cranial metastases, Kano et al.[8] demonstrated in their retrospective study that stereotactic radiosurgery achieved LC in 92% of 531 cranial metastases. Overall survival was better for patients with good performance status and those with few metastases.[8] A systemic review of cranial and extracranial RCC metastases showed that SBRT achieved LC at 1 year of 89%–92%, with a median OS ranging from 6.7 to 25.6 months, and grade 3–4 toxicity was observed in 0%–6% of cases.[16] Svedman et al.[9] conducted a prospective phase II study evaluating the safety and local efficacy of SBRT in metastatic or inoperable primary renal cancer in 30 patients with a total of 82 lesions. Local control was reported at 98%, although 19% of patients had less than 6 months of follow-up. Only 2 cases of progression were identified, and adverse effects were grade 1–2 in 90% of cases.[9]
Compared with conventional fractionated external beam radiation therapy (C-FEBT), SBRT achieves better outcomes in terms of radiological LC and prolonged duration of symptomatic control. Amini and colleagues[10] compared SBRT with C-FEBT in the treatment of bone metastases among 46 mRCC patients. In their retrospective study, the symptom control rate at 2 years was better in the SBRT group than the C-FEBT group (74.2% vs. 35.7%), with equivalent low toxicity. A recent retrospective study by Altoos et al.[11] evaluated the radiographic response rate in 53 thoracic, abdominal, skin, and soft tissue RCC metastases to SBRT (25–50 Gy delivered in 1–10 fractions) compared with conventional fractionated radiotherapy (20–55 Gy). After a median follow-up of 16 months, LC at 24 months was significantly greater and more durable in patients receiving SBRT as compared with conventional fractionated RT (93% vs. 35%, p < 0.001). Predictors for local response included a BED ≥100 and fraction size ≥9 Gy.[11] Stereotactic body radiotherapy seems to provide better LC than C-FEBT.
The efficacy of radiotherapy is highly correlated with the dose applied and fractionation regimen. While RCC tumors seem to be radioresistant using conventionally fractionated radiotherapy, dose escalation has been shown to overcome radioresistance in RCC tumors.[12] Memorial Sloan Kettering Cancer Center reported a 3-year LC of 88% after SBRT with a single high fractional dose (24 Gy) for extracranial RCC metastases, while LC dropped to only 20% when lower doses were used (a single low fraction of <24 Gy or low hypofractionation doses).[13] Biologically effective dose seems to be correlated with better response rates. Many studies have shown that a BED of at least 100 Gy was predictive of a better control rate.[11,12,14] A multicenter retrospective study demonstrated that 1- and 3-year LC rates were 98.1% and 91.9% for pulmonary metastasis from RCC treated by SBRT with a median BED of 117 Gy, with 1- and 3-year OS of 84.3% and 43.8%, respectively. Treatment with BED ≥130 Gy of pulmonary metastases demonstrated a trend toward superior LC.[12] In our study, the BED used was generally inferior to 100 Gy in 90% of cases. The 5 patients who developed local progression received doses with a BED of 60 and 43.2 Gy, whereas no patient receiving higher BED developed recurrence. Nevertheless, despite the low BED used in our study, LC remained satisfactory and was comparable with the literature.
Stereotactic body radiotherapy may also delay the time to introduction of systemic therapy. Zhang et al.[17] showed in their retrospective study that SBRT offered prolonged LC and delayed introduction of systemic therapy by a median of 15.2 months while offering benefits in terms of quality of life. Liu et al.[18] demonstrated in a retrospective study that SBRT can be safely combined with TKI therapy. The median OS in the TKI + SBRT group was significantly longer than that of the TKI group (63.2 vs. 29.8 months; p < 0.001), and only 5.9% of patients experienced SBRT-related grade 3 toxicity. In a recent multicenter prospective study of 17 patients with extracranial RCC metastases who were stable after 4 months on TKI or checkpoint inhibitor therapy and received SBRT to 1 lesion, radiographic response in the target lesion was seen in 13 patients (76%), with complete response in 5 patients (29%), and partial response in 8 (47%), including an abscopal effect in 1 patient. There was no grade 2 or higher toxicity in this study.[19] An abscopal effect was reported by Xie et al.[20] in a patient with mRCC who achieved a systemic complete response only 2.2 months after starting treatment with concurrent SBRT and anti-programmed death-1 antibody, pembrolizumab. In our study, many patients received targeted therapy immediately after SBRT, making it difficult to distinguish the relative impacts on OS of SBRT and systemic therapy and their combination.
Limitations
Our study had several limitations, especially the small number of patients and the absence of a comparison group. These were related to the relative rarity of oligometastatic renal cancer disease and the limited availability and recent introduction of SBRT in our country. Furthermore, the use of systemic therapy in 60% of patients might have had a favorable impact on OS in this population with an initial favorable prognosis, thus making it difficult to evaluate the effect of SBRT on OS. Despite this, our data corroborate previous literature findings suggesting that SBRT may provide good LC for metastasis from RCC.
5. Conclusions
Based on our results and emerging data from other investigators, SBRT seems to be an effective treatment option for metastases from RCC. Stereotactic body radiotherapy was associated with a high local tumor control and a low risk of toxicity. Higher BED seems to be associated with better results. Further prospective, randomized trials are required to compare stereotactic radiotherapy to other options, to establish the optimal dose and fractionation regimen, and to identify the patient groups that may benefit the most from this intervention.
Acknowledgments
None.
Statement of ethics
This study has been reviewed and granted exemption approval by the Ethics Committee of our institution University Hospital Ibn Roch. All participants provided consent for their participation and publication of this study. This study was conducted in accordance with relevant ethical standards including the Helsinki Declaration.
Funding source
None.
Author contributions
All authors of this manuscript have directly participated in planning, execution, and/or analysis of this study.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
How to cite this article: Rida H, Zaine H, Jouhadi H, Benider A, Samlali H, Samlali R. Stereotactic body irradiation for metastasis from renal carcinoma: A retrospective study. Curr Urol 2025;19(3):187–191. doi: 10.1097/CU9.0000000000000191
Contributor Information
Hind Zaine, Email: Dr.h.zaine@gmail.com.
Hassan Jouhadi, Email: Hjouhadi@gmail.com.
Hamza Samlali, Email: hamzasamlali1986@gmail.com.
Redouane Samlali, Email: samlali.r@menara.ma.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
References
- 1.Lipworth L Morgans AK Edwards TL, et al. Renal cell cancer histological subtype distribution differs by race and sex. BJU Int 2016;117(2):260–265. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med 1996;335(12):865–875. [DOI] [PubMed] [Google Scholar]
- 3.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 2017;376(4):354–366. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17(8):2530–2540. [DOI] [PubMed] [Google Scholar]
- 5.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol 2011;8(6):378–382. [DOI] [PubMed] [Google Scholar]
- 6.De Meerleer G Khoo V Escudier B, et al. Radiotherapy for renal-cell carcinoma. Lancet Oncol 2014;15(4):e170–e177. [DOI] [PubMed] [Google Scholar]
- 7.Wersäll PJ Blomgren H Lax I, et al. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol 2005;77(1):88–95. [DOI] [PubMed] [Google Scholar]
- 8.Kano H, Iyer A, Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Outcome predictors of gamma knife radiosurgery for renal cell carcinoma metastases. Neurosurgery 2011;69(6):1232–1239. [DOI] [PubMed] [Google Scholar]
- 9.Svedman C Karlsson K Rutkowska E, et al. Stereotactic body radiotherapy of primary and metastatic renal lesions for patients with only one functioning kidney. Acta Oncol 2008;47(8):1578–1583. [DOI] [PubMed] [Google Scholar]
- 10.Amini A Altoos B Bourlon MT, et al. Local control rates of metastatic renal cell carcinoma (RCC) to the bone using stereotactic body radiation therapy: Is RCC truly radioresistant? Pract Radiat Oncol 2015;5(6):e589–e596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altoos B Amini A Yacoub M, et al. Local control rates of metastatic renal cell carcinoma (RCC) to thoracic, abdominal, and soft tissue lesions using stereotactic body radiotherapy (SBRT). Radiat Oncol 2015;10:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoerner-Rieber J Duma M Blanck O, et al. Stereotactic body radiotherapy (SBRT) for pulmonary metastases from renal cell carcinoma—A multicenter analysis of the German working group “Stereotactic Radiotherapy”. J Thorac Dis 2017;9(11):4512–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zelefsky MJ Greco C Motzer R, et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys 2012;82(5):1744–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stinauer MA Kavanagh BD Schefter TE, et al. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: Impact of single fraction equivalent dose on local control. Radiat Oncol 2011;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agolli L. Stereotactic body radiation therapy could improve disease control in oligometastatic patients with renal cell carcinoma: Do we need more evidence? Ann Transl Med 2019;7(suppl 3):S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kothari G, Foroudi F, Gill S, Corcoran NM, Siva S. Outcomes of stereotactic radiotherapy for cranial and extracranial metastatic renal cell carcinoma: A systematic review. Acta Oncol 2015;54(2):148–157. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y Schoenhals J Christie A, et al. Stereotactic ablative radiation therapy (SAbR) used to defer systemic therapy in oligometastatic renal cell cancer. Int J Radiat Oncol Biol Phys 2019;105(2):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y Zhang Z Han H, et al. Survival after combining stereotactic body radiation therapy and tyrosine kinase inhibitors in patients with metastatic renal cell carcinoma. Front Oncol 2021;11:607595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dengina N, Mitin T, Gamayunov S, Safina S, Kreinina Y, Tsimafeyeu I. Stereotactic body radiation therapy in combination with systemic therapy for metastatic renal cell carcinoma: A prospective multicentre study. ESMO Open 2019;4(5):e000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie G, Gu D, Zhang L, Chen S, Wu D. A rapid and systemic complete response to stereotactic body radiation therapy and pembrolizumab in a patient with metastatic renal cell carcinoma. Cancer Biol Ther 2017;18(8):547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]


