Abstract
Objectives
We explored the expression levels and clinical significance of human epidermal growth factor receptor 2 (HER2) in urothelial carcinoma (UC) tissues.
Materials and methods
Patient data were reviewed, and 111 paraffin specimens of UC obtained from the Department of Urology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, from 2020 to 2021 were collected. Immunohistochemistry was used to detect HER2 protein expression in all UC tumor tissues. The χ2 and Fisher exact tests were used to analyze the relationship between HER2 protein expression and clinicopathological data (sex, age, histopathological diagnosis, invasiveness, histopathological grade, maximum tumor diameter, muscle invasion, regional lymph node metastasis, and clinical stage).
Results
In this study, 92 cases (82.88%) showed HER2 protein expression, and there was a statistically significant difference in the distribution of HER2 positivity (immunohistochemistry 2+ and 3+) according to the pathological grades of UC (p = 0.021). Human epidermal growth factor receptor 2 positivity was not associated with sex, age, histopathological diagnosis, invasiveness, maximum tumor diameter, muscle invasion, regional lymph node metastasis, or clinical stage (all p < 0.05).
Conclusions
Human epidermal growth factor receptor 2 protein is highly expressed in UC, and its expression may be closely related to the high pathological grade of UC.
Keywords: Urothelial carcinoma, Human epidermal growth factor receptor 2, Immunohistochemistry
1. Introduction
Urothelial carcinoma (UC) is a common malignant tumor affecting the urinary system. It is the fourth most common cancer among males in the United States, with a higher incidence rate in males compared with females. In 2020, more than 80,000 confirmed cases of UC were reported.[1] Notably, 90% of UC occur in the bladder, whereas 5% to 10% arise in the renal pelvis and ureter.[2] Typically, UC occurs in elderly patients 70 years and older. Risk factors include genetic predisposition (eg, Lynch syndrome), chemical and environmental exposure (eg, cyclophosphamide and aromatic amines), smoking, and male sex.[3] For example, the GLOBOCAN 2020 report indicated 85,000 new cases of bladder cancer in China and 39,000 related deaths.[1] Compared with other primary tumors, UC exhibits unique characteristics, including its heterogeneous nature, and diversity of clinical outcomes even among patients with the same clinical stage and pathological grade, necessitating individualized treatments (doi: 10.1038/s41585-023-00847-7).
The human epidermal growth factor receptor (HER, also known as ERBB) family is a class of transmembrane receptor tyrosine kinases, comprising HER1, HER2, HER3, and HER4, which play an important role in cell development, proliferation, and differentiation.[4] Specifically, research on HER2 is more comprehensive, depicting its close association with the occurrence and development of various malignant tumors. In recent years, anti–HER2-targeted therapy demonstrated great success in breast and gastric cancer treatments. Previous studies have found that 9.4% to 41.2% of UCs exhibit elevated HER2 expression.[5] Other studies demonstrated that HER2 protein overexpression and gene amplification may be closely associated with UC risk classification and poor prognosis.[6] Therefore, clarifying the expression and clinicopathological correlation of HER2 protein in UC is particularly important for the effective clinical application of anti–HER2-targeted therapy.[7–9] This study aimed to detect HER2 protein expression levels in UC tissue using immunohistochemistry (IHC) and analyze its clinicopathological relationship with UC. The investigation into the clinical application of HER2 seeks to provide valuable insights to guide the treatment of UC by targeting HER2.
2. Materials and methods
2.1. Study design and clinical samples
A total of 111 patients with a pathological diagnosis of UC between 2019 and 2021 at the Department of Urology and Pathology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, were enrolled. Eligible patients were at least 18 years old, with a histological or cytological diagnosis of UC, exhibiting at least one measurable lesion, with an Eastern Cooperative Oncology Group performance score of ≤1, and complete clinical data.
Exclusion criteria encompassed missing clinical data, such as the lack of pretreatment computed tomography images and corresponding laboratory test results (routine blood, urine, stool, liver and kidney function, blood biochemistry, and electrocardiogram). Patients with missing or undetectable HER2 expression in pathological sections and those presenting with tumors merging with other anatomical regions were excluded from the study. Informed consent was obtained from all the patients, and the study was approved by the ethics committee of Shandong Provincial Hospital.
Surgical specimens for UC included transurethral resection of bladder tumors, partial cystectomy, radical cystectomy, segmental ureterectomy, and total ureteropelvic resection. Data on patient sex, age, histopathological diagnosis and grade, invasiveness, maximum tumor diameter, muscle invasion, regional lymph node metastasis, and clinical stage were collected.
2.2. Experimental method
Urothelial carcinoma tissue specimens were fixed in 10% neutral formalin, embedded in paraffin, and sectioned at a thickness of 4 μm. Sections were incubated in the primary antibody overnight at 4°C. Subsequently, slides were washed 3 times with 1× phosphate-buffered saline solution for 5 minutes, and then the secondary antibody was added dropwise and incubated at 37°C for 30 minutes. The slides were then washed 3 times in 1× phosphate-buffered saline solution for 5 minutes, and excess water around the tissue on the section was absorbed. Strept avidin-biotin complex solution was added dropwise and incubated at 37°C for 30 minutes. The diaminobenzidine chromogenic solution was added dropwise, and the cells were stained for less than 1 minute. Hematoxylin solution was added dropwise and kept for 2 minutes. The MaxVision method (doi: 10.19746/j.cnki.issn.1009-2137.2021.02.034) was used for immunohistochemical analysis. All sections served as positive controls, whereas normal bladder urothelium, lymphocytes, and blood vessels were used as negative internal controls.
2.3. Interpretation of results
Immunohistochemical results were independently read and interpreted by 2 experienced senior pathologists using a double-blind method. When there was a difference in the readings, a third senior pathologist made a second judgment to reach a consensus. The HER2 protein scoring standard is based on the 2021 edition of the Clinical Pathological Expert Consensus on HER2 testing in UC in China and is outlined as follows: (0: no staining or <10% of invasive cancer cells with incomplete and weakly stained membranes; 1+: ≥10% of invasive cancer cells with incomplete and weakly stained membranes; 2+: ≥10% of invasive cancer cells with weak-moderate full membrane staining or <10% of invasive cancer cells with strong staining of the intact cell membrane; 3+: ≥10% of invasive cancer cells with strong staining of the intact cell membrane).[10]
Ki-67 was assessed by counting more than 500 tumor cells at high magnification and counting the percentage of Ki-67 staining in tumor cell nuclei, regardless of staining intensity. All other markers were analyzed according to the Remmele and Stegner[4] immune response score.
2.4. Statistical methods
Statistical analyses were conducted using the SPSS software (SPSS 26.0; IBM Corp, Armonk, NY). The χ2 test was used to analyze the relationship between HER2 and UC myometrial invasion, clinical stage, maximum tumor diameter, UC histopathological grade, age, and sex. The adjusted χ2 test was used to analyze the relationship between HER2 and regional lymph node metastasis and Ki-67 proliferation index in UC. The relationship between HER2 and regional lymph node metastasis and Ki-67 proliferation index in bladder cancer was analyzed using Fisher exact test. p < 0.05 was considered statistically significant.
3. Results
3.1. Baseline of clinical patients
A total of 111 patients were included in this study, including 78 males and 33 females, with a median age of 66 (40–93) years. The primary tumor sites were distributed as follows: 78 patients (70.27%) had tumors in the bladder, 17 patients (15.32%) in the ureter, and 16 patients (14.41%) in the renal pelvis. Among patients with the primary tumor located in the bladder (78 cases), 44 cases (56.41%) exhibited a tumor diameter ≥2 cm, whereas 34 cases (43.59%) had a tumor diameter <2 cm. Among the cases with the primary tumor in the ureter (17 cases), 10 cases (58.82%) had a tumor diameter ≥2 cm, and 7 cases (41.18%) had a tumor diameter <2 cm. Among cases with primary tumors in the renal pelvis (16 cases), 12 cases (75.00%) presented with a tumor diameter ≥2 cm, and 4 cases (25.00%) presented with a tumor diameter <2 cm. Based on the 2016 edition of the World Health Organization urology pathology and genetics diagnostic criteria (doi: 10.1016/j.eururo.2016.02.028), 82 cases were high grade (primary bladder: 52 cases, primary ureter: 16 cases, primary renal pelvis: 14 cases), and 29 cases were low grade (primary bladder: 26 cases, primary ureter: 1 case, primary renal pelvis: 2 cases). As for the invasiveness, the study included 84 cases of invasive UC (primary bladder: 54 cases, primary ureter: 16 cases, primary renal pelvis: 14 cases) and 27 cases of noninvasive UC (primary bladder: 24 cases, primary ureter: 1 case, primary renal pelvis: 2 cases). The histopathological diagnosis of each specimen was reviewed by 2 pathologists and attending physicians. Among patients with primary tumor located in the bladder, 49 patients underwent transurethral resection of bladder tumor, 20 patients underwent radical cystectomy, 7 patients underwent partial cystectomy, and 2 patients did not undergo surgical treatment or bladder drug instillation and received only programmed cell death protein 1 inhibitor immunotherapy. Among patients with primary tumors located in the ureter, 11 patients underwent radical nephroureterectomy, and 6 patients underwent segmental ureterectomy. As for patients with primary tumors located in the renal pelvis, 14 patients underwent radical nephroureterectomy and partial cystectomy; 2 patients did not undergo surgery and received programmed cell death protein 1 inhibitor immunotherapy. (Detailed clinicopathological data of patients with UC are shown in Table 1 and in Fig. 1A–D.)
Table 1.
Clinicopathological features of the analyzed cohort.
| Bladder cancer | Ureteral cancer | Renal pelvis cancer | |
|---|---|---|---|
| Total | 78 | 17 | 16 |
| Age, yr | |||
| Range | 40–93 | 58–85 | 57–84 |
| Median age | 66 | 72 | 72.5 |
| Gender | |||
| Male | 65 | 10 | 3 |
| Female | 13 | 7 | 13 |
| Invasiveness | |||
| Invasive | 54 | 16 | 14 |
| Noninvasive | 24 | 1 | 2 |
| Grading WHO 2016 | |||
| High-grade | 52 | 16 | 14 |
| Low-grade | 26 | 1 | 2 |
| Tumor size, cm | |||
| ≥2 | 44 | 7 | 12 |
| <2 | 34 | 10 | 4 |
| Muscle invasion | |||
| MIUC | 21 | 13 | 12 |
| NMIUC | 57 | 4 | 4 |
| pN | |||
| N1 | 2 | 3 | 2 |
| N0 | 76 | 14 | 14 |
| Stage | |||
| I | 57 | 4 | 4 |
| II | 8 | 5 | 2 |
| III | 9 | 4 | 6 |
| IV | 4 | 4 | 4 |
MIUC = muscle-invasive urothelial cancer; NMIUC = non–muscle-invasive urothelial cancer; pN = primary lymph node stage; WHO = World Health Organization.
Figure 1.
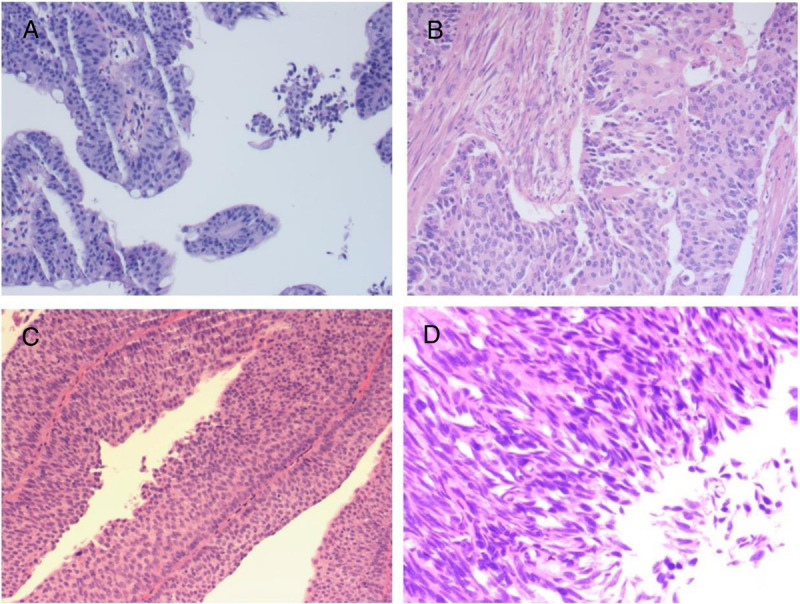
Histomorphological spectrum of UC. (A) Low-grade invasive urothelial bladder carcinoma; (B) high-grade invasive urothelial bladder carcinoma; (C) low-grade noninvasive urothelial bladder carcinoma; (D) high-grade noninvasive urothelial bladder carcinoma, original magnification ×200. UC = urothelial carcinoma.
3.2. Expression of HER2 in UC
Among the 111 UC tissues, 92 (82.88%) showed HER2 protein expression, of which 26 (23.42%) had HER2 expression 1+, 61 (54.95%) had HER2 expression 2+, and 5 (4.50%) had HER2 expression 3+. Among the 78 primary bladder tumor tissues, 70 (89.74%) had HER2 protein expression, of which 20 (25.64%) had HER2 expression 1+, 45 (57.69%) had HER2 expression 2+, and 5 (6.41%) had HER2 expression 3+. Among the 17 primary ureteral tumor tissues, 8 (47.06%) had HER2 protein expression, of which 3 (17.65%) had HER2 expression 1+, and 5 (29.41%) had HER2 expression 2+. As for the 16 primary renal pelvis tumor tissues, 14 (87.50%) had HER2 protein expression, including 3 (18.75%) with HER2 expression 1+ and 11 (68.75%) with HER2 expression 2+ (Figs. 2–4, respectively).
Figure 2.
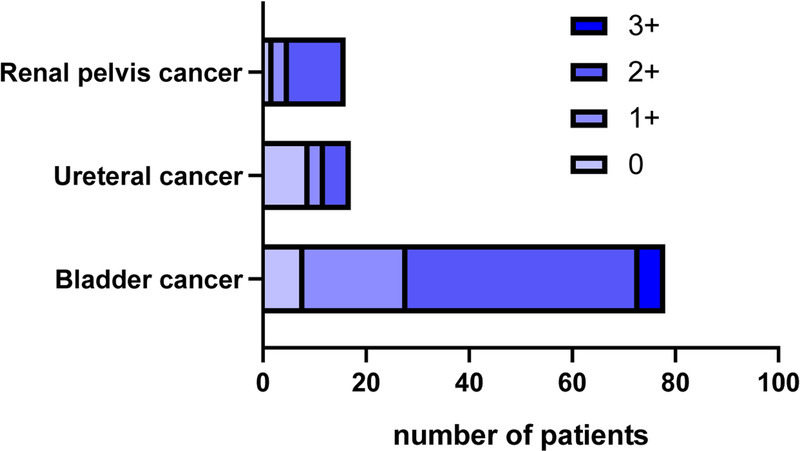
HER2 expression levels in UC from different sources. HER2 = human epidermal growth factor receptor 2; UC = urothelial carcinoma.
Figure 4.

HER2 in UC by IHC. Example of HER2-positive urothelial bladder carcinoma. (A) HER2 IHC scored 0+; (B) HER2 IHC scored 1+; (C) HER2 IHC scored 2+; (D) HER2 IHC scored 3+, original magnification ×200. HER2= human epidermal growth factor receptor 2; UC = urothelial carcinoma; IHC = immunohistochemistry.
Figure 3.

HER2 expression under different clinical and pathological characteristics. HER2 = human epidermal growth factor receptor 2; MIUC = muscle-invasive urothelial cancer; NMIUC = non–muscle-invasive urothelial cancer; UC = urothelial carcinoma.
3.3. HER2 positivity (IHC 2+ and 3+) in invasive and noninvasive UCs
The HER2 IHC protein–positive rate (IHC 2+ and 3+) in the 84 invasive UCs was 62% (52/84). The positivity rate of HER2 IHC protein expression was 70% (38/54) in UCs originating from the bladder, 29% (5/16) in UCs originating from the ureter, and 64% (9/14) in UCs originating from the renal pelvis. There was no significant difference in the HER2-positive rate between invasive and noninvasive UCs (p = 0.355). Among UCs originating from the bladder, there was no significant difference in the HER2-positive rate between invasive UC of the bladder and noninvasive UC of the bladder (p = 0.083).
3.4. HER2 positivity (IHC 2+ and 3+) in high- and low-grade UC
The HER2-positive rate (IHC 2+ and 3+) in high-grade UCs was 66% (54/82). The positivity rate of HER2 protein expression was 75% (39/52) in UC originating from the bladder, 31% (5/16) in UC originating from the ureter, and 71% (10/14) in UC originating from the renal pelvis. There was a statistically significant difference in the HER2-positive rate between high- and low-grade UCs (p = 0.021). Among UCs originating from the bladder, there was a statistically significant difference in the HER2-positive rate between high- and low-grade UCs of the bladder (p = 0.005).
3.5. Association of HER2 positivity with clinicopathological features of UC
Among the 111 cases of UC investigated, a statistically significant difference was depicted between HER2 positivity and high-grade versus low-grade UCs (p = 0.021). No significant associations were found with muscle invasion (p = 0.890), invasiveness (p = 0.355), tumor size (cutoff value of 2 cm, p = 0.624), clinical stage (I + II, III + IV, p = 0.944), gender (p = 0.267), age (cutoff value of 55 years, p = 0.850), and regional lymph node metastasis (p = 0.598). Among 78 cases of bladder cancer tissue, the difference between HER2 positivity and high- and low-grade was statistically significant (p = 0.005), and it was not associated with muscle invasion (p = 0.177), invasiveness(p = 0.083), tumor size (cutoff value of 2 cm, p = 0.922), clinical stage (I + II, III + IV, p = 0.261), gender (p = 0.073), age (cutoff value of 55 years, p = 0.549), and regional lymph node metastasis (p = 0.534, 2-sided). There was a statistically significant difference between the primary site of HER2 positivity and UCs (p = 0.022, Tables 2, 3)
Table 2.
Relationship between HER2 positivity and some clinicopathological features of UC.
| Characteristics | HER2 | χ 2 | p |
|---|---|---|---|
| Muscle invasion | 0.019 | 0.890 | |
| MIUC | 27/46 (58.7%) | ||
| NMIUC | 39/65 (60.0%) | ||
| Invasiveness | 0.857 | 0.355 | |
| Invasive UC | 52/84 (62.0%) | ||
| Noninvasive UC | 14/27 (51.9%) | ||
| Stage | 0.005 | 0.944 | |
| I + II | 48/81 (59.3%) | ||
| III + IV | 18/30 (60.0%) | ||
| Tumor size, cm | 0.240 | 0.624 | |
| ≥2 | 38/66 (57.6%) | ||
| <2 | 28/45 (62.2%) | ||
| Gender | 1.230 | 0.267 | |
| Male | 49/78 (62.8%) | ||
| Female | 17/33 (51.5%) | ||
| Age, yr | 0.036 | 0.850 | |
| ≤55 | 8/14 (57.1%) | ||
| >55 | 58/97 (59.8%) | ||
| Grading WHO 2016 | 5.324 | 0.021 | |
| High-grade | 54/82 (65.9%) | ||
| Low-grade | 12/29 (41.4%) | ||
| pN | 0.277 | 0.598 | |
| N1 | 3/7 (42.9%) | ||
| N0 | 63/104 (60.6%) | ||
| Primary tumor | 7.638 | 0.022 | |
| Bladder | 50/78 (64.1%) | ||
| Ureter | 5/17 (29.4%) | ||
| Renal pelvis | 11/16 (68.8%) |
HER2 = human epidermal growth factor receptor 2; MIUC = muscle-invasive urothelial cancer; NMIUC = non–muscle-invasive urothelial cancer; pN = primary lymph node stage; UC = urothelial carcinoma; WHO = World Health Organization.
Table 3.
Relationship between HER2 positivity and some clinicopathological features of bladder cancer.
| Characteristics | HER2 | χ 2 | p |
|---|---|---|---|
| Muscle invasion | 1.825 | 0.177 | |
| MIBC | 16/21 (76.2%) | ||
| NMIBC | 34/57 (59.6%) | ||
| Invasiveness | 2.996 | 0.083 | |
| Invasive BC | 38/54 (70.4%) | ||
| Noninvasive BC | 12/24 (50.0%) | ||
| Stage | 1.264 | 0.261 | |
| I + II | 40/65 (61.5%) | ||
| III + IV | 10/11 (90.9%) | ||
| Tumor size, cm | 0.010 | 0.922 | |
| ≥2 | 28/44 (63.6%) | ||
| <2 | 22/34 (64.7%) | ||
| Gender | 3.220 | 0.073 | |
| Male | 45/65 (69.2%) | ||
| Female | 5/13 (38.5%) | ||
| Age, yr | 0.359 | 0.549 | |
| ≤55 | 8/14 (57.1%) | ||
| >55 | 42/64 (65.6%) | ||
| Grading WHO 2016 | 8.051 | 0.005 | |
| High-grade | 39/52 (75.0%) | ||
| Low-grade | 11/26 (42.3%) | ||
| pN | 0.534 (2-sided) | ||
| N1 | 2/2 (100.0%) | ||
| N0 | 48/76 (63.2%) |
BC = bladder cancer; HER2 = human epidermal growth factor receptor 2; MIBC = muscle-invasive bladder cancer; NMIBC = non–muscle-invasive bladder cancer; pN = primary lymph node stage; WHO = World Health Organization.
4. Discussion
4.1. Differential expression of HER2 in UC
There were significant differences in the positivity rates of HER2 in different studies. Bellmunt et al.[11] found amplification of the HER2 gene in 20% of Hispanic patients with bladder UC and 4% of Greek patients with bladder UC. Jiang et al.[5] reported that 0% to 59% of patients with bladder UCs exhibit HER2 gene amplification, and 0% to 89% present with HER2 protein overexpression. In Chinese patients with bladder UC, or even in patients from different provinces, the expression of HER2 is likely to differ from that in foreign populations. The study population was distributed across Shandong Province, China. The HER2 protein was expressed in 92 cases (82.88%) of UC, and the HER2-positive rate (IHC 2+ and 3+) was 59% (66/111). Among UCs originating from the bladder, 70 cases (89.74%) expressed HER2 protein, and the HER2-positive rate (IHC 2+ and 3+) was 64% (50/78). Human epidermal growth factor receptor 2 is a potential therapeutic target for UC, and its expression is important for treatment. Currently, HER2-targeting antibody-drug conjugates have achieved good curative effects in some clinical trials. However, the relationship between HER2 expression levels, treatment effects, and disease prognosis requires further exploration. In this study, HER2 protein was highly expressed in both UC and bladder cancer. In UC, the rate of HER2 overexpression was significantly different from that reported previously. We believe that this difference stems from the heterogeneity and diversity of UCs, immobilization methods, antibody brands, and research and evaluation methods.
This study found that the positivity rates were 64.1% (50/78) in the bladder, 29.4% (5/17) in the ureter, and 68.8% (11/16) in the renal pelvis. Urothelial carcinomas show differences in HER2 expression at different primary sites, with UCs originating from the renal pelvis and bladder exhibiting higher HER2 expression rates than those originating from the ureters. In this study, HER2 expression was the highest in UCs, whereas the positivity rate of HER2 in 3+ UCs was significantly lower than that in 1+ and 2+ UCs. In addition, HER2-negative lesions were frequently found in specific areas of high-grade UC specimens, suggesting substantial heterogeneity in UC, especially in high-grade and poorly differentiated tumors. The differences in HER2 overexpression may be related to tumor heterogeneity. Previous studies have highlighted a higher heterogeneity within invasive UC tumors (35%) with HER2 3+ compared with invasive breast cancer (5%) with HER2 3+.[12,13] This heterogeneity posed challenges for immunohistochemical analysis, surpassing the complexity observed in breast cancer. The pattern of the number of HER2-positive tumors more than advanced tumors in breast cancer does not apply to UCs. Once UC invades the muscle, the morphological diversity, molecular heterogeneity, and various tumorigenic pathways further complicate targeted therapeutic approaches.
The frequency of HER2 protein overexpression is the result of multiple factors, including ERBB2 mutation or amplification and HER2 overexpression in different histological subtypes of UC, which may lead to differences in tumor biology and prognosis. Genome profiling and IHC revealed high HER2 amplification, mutation, and overexpression in UC.[14] However, gene amplification does not perfectly correlate with the overexpression of cell surface receptors, and genomic analysis may underestimate the extent of protein overexpression.[15] Immunohistochemical evaluation of a large clinical sequencing cohort of multiple tumor types revealed that bladder cancer exhibited the highest HER2 overexpression relative to all other tissues, including breast and gastric cancers.[16] Kiss et al.[17] collected clinicopathological information from 127 muscle-invasive bladders and found that gene amplification was not the only driver of HER2 overexpression in bladder cancer, with ERBB2 amplification detected in some cases without HER2 overexpression, and HER2 overexpression detected without ERBB2 amplification in other cases. This suggests that HER2 overexpression in bladder cancer is regulated by mechanisms other than gene amplification.
The HER2 detection standard used in this study was based on the 2021 edition of the Clinical Pathological Expert Consensus on HER2 testing in UC in China. Before October 2021, the Chinese UC HER2 detection standard was based on the evaluation criteria used for breast cancer. Simultaneously, a clinical trial (RC-48) evaluating an antibody-drug conjugate targeting HER2 in the treatment of advanced UC revealed that the response rates were not significantly different between fluorescence in situ hybridization (FISH) + and FISH-IHC 2+ tumors.[18] This may indicate that the degree of HER2 overexpression does not exert the same impact on the treatment efficiency in UC, as observed in breast cancer. Therefore, in the cases with HER2 2+, additional evidence is required to substantiate the potential impact of further improvement in FISH staining to guide the treatment of UC, similar to considerations in breast cancer.
4.2. HER2-positive rate of UC correlates with invasiveness and clinical stage
There were no statistically significant differences in HER2 positivity, invasiveness, or muscle invasiveness in this study. Notably, the correlation between HER2 positivity and invasiveness was greater in UCs originating in the bladder. Moreover, no significant difference was depicted in the distribution of HER2 positivity among different clinical stages of UC. Human epidermal growth factor receptor 2 positivity was associated with the invasive characteristics of UC cells; however, there was no significant difference in muscle invasion or clinical stage distribution, suggesting that other factors may play a role in the progression of UC. Eltze et al.[19] found that HER2 amplification was not related to the primary tumor stage or lymph node status in 153 bladder cancer tumor tissues. Human epidermal growth factor receptor 2 overexpression is thought to be an early event in urothelial tumorigenesis and rarely occurs in subsequent tumor development. Therefore, there may be a lack of correlation between tumor stage and the depth of myometrial invasion.[20]
4.3. The correlation between HER2-positive rate and pathological grade in UC
High-grade UCs had a higher HER2-positive rate in this study, which is consistent with the results of several previous studies.[5,21] It should be noted that high-grade tumors have both histological types with better prognoses and histological types with poor prognoses. Zhao et al.[22] found that HER2 expression was significantly associated with tumor grade (high- vs. low-grade). In 2 different cohort studies, El-Moneim et al.[23] reported that HER2 overexpression was statistically associated with high-grade tumors but did not find any association with the clinical stage. Similarly, in a study including 138 patients with bladder cancer, Krüger et al.[24] found that HER2 overexpression was more common in the high-grade cancer group than in the low-grade cancer group.
4.4. Association of HER2 positivity with prognosis in UC
In recent years, several studies have found that the expression of HER2 may be associated with the prognosis of breast and gastric cancers. The HER2-overexpressing subtype accounts for 20% to 30% of breast cancers, and HER2 receptor overexpression is caused by mechanisms such as HER2 gene amplification. Patients with HER2-overexpressing breast cancer have a poor prognosis.[25] Monoclonal antibodies and small-molecule inhibitors targeting HER2 have been widely used for the clinical treatment of HER2-positive metastatic breast cancer. The binding of monoclonal antibodies to HER2 extracellular receptors, which block HER2 signaling, can significantly improve the prognosis of patients with HER2-positive breast cancer.[26] In gastric cancer, the study showed that the positivity rate of HER2 is different across various gastric cancer types and locations, and the expression of HER2 in tumors of the same patient is different, similar to that of UC. According to the ToGA test, the overall positive rate of HER2 expression in gastric cancer was 22.1%, of which intestinal-type gastric cancer and gastroesophageal junction cancer had the highest positive rates (31.8% and 32.2%, respectively), and diffuse and distal tumors had lower positive rates (6.1% and 21.4%, respectively).[27] Similar to UC, gastric exhibits marked heterogeneity.[28] With further understanding of gastric cancer, it was found that HER2-positive gastric cancer has a worse prognosis, and HER2 heterogeneity is a poor prognostic factor.[29]
In recent years, the relationship between the prognosis of patients with UC and HER2 expression has been controversial. Indeed, previous reports suggest that HER2 status, alone or in combination with HER3 or epidermal growth factor receptor in non–muscle-invasive and muscle-invasive cancers, is negatively correlated with prognosis.[30,31] However, data on this association are not conclusive. In a retrospective single-institution study, patients with HER2 gene amplification had shorter survival than patients with HER2-negative UC.[32] Bolenz et al.[31] studied 198 patients who underwent lymphadenectomy for radical bladder cancer and found that HER2 positivity provided independent prognostic information for recurrence and death in patients with bladder cancer. Schneider et al.[33] found that HER2 amplification as a prognostic factor was significantly associated with increased cancer-specific mortality; however, this association was not found in a less-studied case series.[34] Based on these results, it has been suggested that HER2 status may be a tool for preoperative risk stratification in patients with bladder cancer. However, the prognostic value of HER2 positivity in bladder cancer remains controversial. Some studies suggest that HER2 cannot be used as a basis for judging prognosis, whereas others suggest that HER2 positivity is associated with poor cancer prognosis,[35] and few believe that it is associated with better prognosis.[36] These findings suggest that further differentiation of HER2-positive patients is needed to screen for patients who would benefit from HER2-targeted therapy.
In recent years, antibody-drug conjugate drugs targeting HER2 have shown therapeutic effects in HER2-positive UC. However, variations in HER2 expression in UC across studies can be attributed to differences in interpretation standards and experimental techniques. Whereas previous studies have mostly referred to the HER2 detection standards for breast cancer, this study utilized standard staining techniques and was based on the 2021 China HER2 detection for UC clinical pathology expert consensus. The purpose of this study was to clarify the relationship between HER2 expression and clinical pathology in patients with UC, offering guidance for HER2-targeted therapy. However, this study had certain limitations. This was a single-center clinical pathological study including 111 patients. The relatively small sample size may introduce errors, necessitating a larger population analysis in the future. Moreover, this study lacks information on patient prognosis and its association with HER2 expression. These limitations provide directions for future research.
5. Conclusions
Human epidermal growth factor receptor 2 is highly expressed in UC, and HER2 expression may be closely related to the high pathological grade of UC. Elevated HER2 expression in patients with UC is a potential target for tumor therapy.
Acknowledgments
None.
Statement of ethics
Informed consent was obtained from all the patients, and the study was approved by the ethics committee of Shandong Provincial Hospital (SWYX: NO.2021-492). All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding statement
This work was supported by Integrated Research on Intelligent Precision Diagnosis and Treatment of High-Risk Tumors of the Urinary System (grant 6020119027) and Shandong Provincial Natural Science Foundation (ZR2023LZL005, ZR2021QH313).
Author contributions
All authors listed gave a substantive contribution to this study and to this original article.
YC: Conceptualization, writing—original draft preparation;
DZ: Conceptualization, formal analysis and investigation;
ZW: Formal analysis and investigation;
KZ: Statistical analysis;
AG: Methodology;
JC: Methodology;
CW: Formal analysis and investigation;
SD: Writing—review and editing, conceptualization.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Footnotes
How to cite this article: Chang Y, Zhao D, Wang Z, Zhu K, Guo A, Cao J, Wu C, Ding S. Expression of HER2 in urothelial carcinoma and its significance. Curr Urol 2025;19(3):201–207. doi: 10.1097/CU9.0000000000000249
Contributor Information
Yao Chang, Email: cy92330@163.com.
Delong Zhao, Email: doctorzhaodelong@163.com.
Zicheng Wang, Email: wzc2020127@163.com.
Kejia Zhu, Email: zhukejia@sdfmu.edu.cn.
Andong Guo, Email: guoandong0123@163.com.
Jishuang Cao, Email: 1506269132@qq.com.
Chenrui Wu, Email: 419480870@qq.com.
Conflicts of interest statement
No conflict of interest has been declared by the authors.
References
- 1.Sung H Ferlay J Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez Pena MDC Chaux A Eich ML, et al. Immunohistochemical assessment of basal and luminal markers in non–muscle invasive urothelial carcinoma of bladder. Virchows Arch 2019;475(3):349–356. [DOI] [PubMed] [Google Scholar]
- 3.D'Angelo A Chapman R Sirico M, et al. An update on antibody-drug conjugates in urothelial carcinoma: State of the art strategies and what comes next. Cancer Chemother Pharmacol 2022;90(3):191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue [in German]. Pathologe 1987;8(3):138–140. [PubMed] [Google Scholar]
- 5.Jiang Q, Xie MX, Zhang XC. Complete response to trastuzumab and chemotherapy in recurrent urothelial bladder carcinoma with HER2 gene amplification: A case report. World J Clin Cases 2020;8(3):594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afatinib is active in platinum-refractory ERBB-mutant urothelial carcinoma. Cancer Discov 2016;6(6):OF13. [DOI] [PubMed] [Google Scholar]
- 7.Matsubara H Yamada Y Naruse K, et al. Potential for HER-2/neu molecular targeted therapy for invasive bladder carcinoma: Comparative study of immunohistochemistry and fluorescent in situ hybridization. Oncol Rep 2008;19(1):57–63. [PubMed] [Google Scholar]
- 8.Gunia S, Koch S, Hakenberg OW, May M, Kakies C, Erbersdobler A. Different HER2 protein expression profiles aid in the histologic differential diagnosis between urothelial carcinoma in situ (CIS) and non-CIS conditions (dysplasia and reactive atypia) of the urinary bladder mucosa. Am J Clin Pathol 2011;136(6):881–888. [DOI] [PubMed] [Google Scholar]
- 9.Latif Z Watters AD Dunn I, et al. HER2/neu overexpression in the development of muscle-invasive transitional cell carcinoma of the bladder. Br J Cancer 2003;89(7):1305–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumor Pathology Committee of Chinese Anti-Cancer Association; Expert Committee on Urothelial Carcinoma of Chinese Society of Clinical Oncology . Clinical pathological expert consensus on HER-2 testing in urothelial carcinoma in China [in Chinese]. Zhonghua Zhong Liu Za Zhi 2021;43(10):1001–1006. [DOI] [PubMed] [Google Scholar]
- 11.Bellmunt J Werner L Bamias A, et al. HER2 as a target in invasive urothelial carcinoma. Cancer Med 2015;4(6):844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chivukula M, Bhargava R, Brufsky A, Surti U, Dabbs DJ. Clinical importance of HER2 immunohistologic heterogeneous expression in core-needle biopsies vs resection specimens for equivocal (immunohistochemical score 2+) cases. Mod Pathol 2008;21(4):363–368. [DOI] [PubMed] [Google Scholar]
- 13.Cottu PH Asselah J Lae M, et al. Intratumoral heterogeneity of HER2/neu expression and its consequences for the management of advanced breast cancer. Ann Oncol 2008;19(3):596–597. [DOI] [PubMed] [Google Scholar]
- 14.Yan M Schwaederle M Arguello D, et al. HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev 2015;34(1):157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer 2004;5(1):63–69. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Chen X, Lin T. Emerging strategies for the improvement of chemotherapy in bladder cancer: Current knowledge and future perspectives. J Adv Res 2022;39:187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiss B Wyatt AW Douglas J, et al. Her2 alterations in muscle-invasive bladder cancer: Patient selection beyond protein expression for targeted therapy. Sci Rep 2017;7:42713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong J Shen L Wang W, et al. Safety, pharmacokinetics and efficacy of RC48-ADC in a phase I study in patients with HER2-overexpression advanced solid cancer. J Clin Oncol 2018;36:e16059. [Google Scholar]
- 19.Eltze E, Wülfing C, Von Struensee D, Piechota H, Buerger H, Hertle L. Cox-2 and Her2/neu co-expression in invasive bladder cancer. Int J Oncol 2005;26(6):1525–1531. [PubMed] [Google Scholar]
- 20.Goodman AL, Osunkoya AO. Human epidermal growth factor receptor 2 expression in micropapillary urothelial carcinoma of the bladder: An analysis of 27 cases. Hum Pathol 2016;57:160–164. [DOI] [PubMed] [Google Scholar]
- 21.Bellmunt J Valderrama BP Puente J, et al. Recent therapeutic advances in urothelial carcinoma: A paradigm shift in disease management. Crit Rev Oncol Hematol 2022;174:103683. [DOI] [PubMed] [Google Scholar]
- 22.Zhao J Xu W Zhang Z, et al. Prognostic role of HER2 expression in bladder cancer: A systematic review and meta-analysis. Int Urol Nephrol 2015;47(1):87–94. [DOI] [PubMed] [Google Scholar]
- 23.El-Moneim H Tawfik H El-Sherbiny Y, et al. Analysis of Her2/neu overexpression and amplification in urothelial carcinoma of the bladder associated with Cox-2 overexpression [J]. 2011;7:8–24. [Google Scholar]
- 24.Krüger S Weitsch G Büttner H, et al. HER2 overexpression in muscle-invasive urothelial carcinoma of the bladder: Prognostic implications. Int J Cancer 2002;102(5):514–518. [DOI] [PubMed] [Google Scholar]
- 25.Mitri Z, Constantine T, O'Regan R. The HER2 receptor in breast cancer: Pathophysiology, clinical use, and new advances in therapy. Chemother Res Pract 2012;2012:743193–743197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pernas S, Tolaney SM. HER2-positive breast cancer: New therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol 2019;11:1758835919833519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bang YJ van Cutsem E Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010;376(9742):687–697. [DOI] [PubMed] [Google Scholar]
- 28.Van Cutsem E Bang YJ Feng-Yi F, et al. HER2 screening data from ToGA: Targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015;18(3):476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaito A Kuwata T Tokunaga M, et al. HER2 heterogeneity is a poor prognosticator for HER2-positive gastric cancer. World J Clin Cases 2019;7(15):1964–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cimpean AM, Tarlui V, Cumpănaş AA, Bolintineanu S, Cumpănaş A, Raica M. Critical overview of HER2 assessement in bladder cancer: What is missing for a better therapeutic approach? Anticancer Res 2017;37(9):4935–4942. [DOI] [PubMed] [Google Scholar]
- 31.Bolenz C Shariat SF Karakiewicz PI, et al. Human epidermal growth factor receptor 2 expression status provides independent prognostic information in patients with urothelial carcinoma of the urinary bladder. BJU Int 2010;106(8):1216–1222. [DOI] [PubMed] [Google Scholar]
- 32.Rizzo A Mollica V Giunchi F, et al. Impact of HER2 assessment by CISH in urothelial carcinoma: A retrospective single-center experience. Pathol Res Pract 2021;220:153410. [DOI] [PubMed] [Google Scholar]
- 33.Schneider SA Sukov WR Frank I, et al. Outcome of patients with micropapillary urothelial carcinoma following radical cystectomy: ERBB2 (HER2) amplification identifies patients with poor outcome. Mod Pathol 2014;27(5):758–764. [DOI] [PubMed] [Google Scholar]
- 34.Ching CB Amin MB Tubbs RR, et al. HER2 gene amplification occurs frequently in the micropapillary variant of urothelial carcinoma: Analysis by dual-color in situ hybridization. Mod Pathol 2011;24(8):1111–1119. [DOI] [PubMed] [Google Scholar]
- 35.Erben P Wezel F Wirtz R, et al. Role of the human ErbB family receptors in urothelial carcinoma of the bladder: mRNA expression status and prognostic relevance [in German]. Aktuelle Urol 2017;48(4):356–362. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson P, Sjödahl G, Chebil G, Liedberg F, Höglund M. HER2 and EGFR amplification and expression in urothelial carcinoma occurs in distinct biological and molecular contexts. Oncotarget 2017;8(30):48905–48914. [DOI] [PMC free article] [PubMed] [Google Scholar]


