ABSTRACT
Objectives
To analyze short‐term weight gain among obese children after intracapsular tonsillectomy.
Methods
A retrospective cohort included all children with a body mass index (BMI) > 95th percentile obtaining intracapsular tonsillectomy between 2021 and 2023 at a tertiary children's hospital. Measurements at least 30 days and at least 90 days postoperatively were recorded. BMI was expressed as a percentage of the 95th percentile (%BMIp95) and grouped by initial %BMIp95 ≤ 120 (class I obesity) or %BMIp95 > 120 (class II and III obesity).
Results
There were 68 children that underwent intracapsular tonsillectomy at a mean age of 7.2 years (SD: 3.8) with obstructive breathing indications for 91% (N = 62). Mean %BMIp95 at surgery was 121 (95% confidence interval [CI]: 117–125). Class I obesity was noted for N = 39 (57%) and class II/III obesity was noted for N = 29 children (43%). At a mean of 6.9 months (SD: 5.3), the change in %BMIp95 was 1.77 (95% CI: −1.03 to 4.57) for class I and 3.27 (95% CI: 0.55 to 6.00) for class II/III obese children (p = 0.45). Measurements at least 90 days after surgery (mean: 12.8 months (SD: 5.2) showed no differences in %BMIp95 change between children with class I (1.17; 95% CI: −3.63 to 5.96) and class II/III obesity (3.00; 95% CI: −2.25 to 8.25) (p = 0.60).
Conclusion
Weight gain after intracapsular tonsillectomy was similar between children with low and high obesity class. Consistent growth trajectories continued beyond 3 months, suggesting intracapsular tonsillectomy may be an appropriate technique to address obstructive breathing in obese children.
Level of Evidence
III.
Keywords: intracapsular tonsillectomy, pediatric obesity, pediatric tonsillectomy, post‐tonsillectomy weight gain, tonsillectomy outcomes
Weight gain after intracapsular tonsillectomy was similar between children with low and high obesity class. Consistent growth trajectories continued beyond 3 months, suggesting intracapsular tonsillectomy may be an appropriate technique to address obstructive breathing in obese children.
1. Introduction
There has been a body of literature dedicated to exploring the relationship between pediatric tonsillectomy and changes in postoperative weight [1, 2, 3, 4, 5]. Potential increases in weight after surgery raise concerns for residual obstructive sleep apnea, which is more frequent in overweight or obese children [6, 7]. However, any correlation between weight gain and tonsillectomy remains uncertain. While some studies have found increases in younger children [2], children who are obese [3], or have Down syndrome [4], other research fails to sustain this relationship [5].
The intracapsular technique to tonsillectomy is continuing to gain acceptance [8]. This approach is designed to improve postoperative pain, reduce bleeding, and enhance quicker recovery by avoiding tonsillar capsule violation [9, 10]. As our specialty evolves in its appreciation and understanding of intracapsular tonsillectomy, it is valuable to recognize whether the postoperative weight gain dilemma applies to this technique. To date, no study has explored the relationship between intracapsular tonsillectomy and postoperative weight changes in children.
The primary objective of this study is to examine the weight gain trajectories of obese children who underwent intracapsular tonsillectomy. Specifically, we were interested in exploring whether patients could maintain consistent weight in the months after tonsillectomy regardless of obesity class. We hypothesized that intracapsular tonsillectomy would result in similar weight distributions following surgery and that obesity class would not cause varied impacts on postoperative weight.
2. Materials and Methods
A retrospective cohort study included all children (< 18 years) who underwent intracapsular tonsillectomy at Children's Medical Center Dallas between January 2021 and December 2023. The institution prospectively enrolls patients in a database at the time of tonsillectomy to monitor perioperative outcomes. Review of this data was approved by the University of Texas Southwestern Medical Center Institutional Review Board (STU‐2023‐0260).
Only children that had a body mass index (BMI) greater than the 95th percentile for age and sex at the time of surgery were reviewed. Anthropomorphic data was documented in the electronic medical record at the time of surgery and reviewed for inclusion candidacy. All children needed at least one additional weight measurement greater than 30 days postoperatively to be included. Additional weights beyond three months were recorded if available.
The primary endpoint was the BMI percentage of the 95th percentile (%BMIp95), which is a metric to improve precision when analyzing longitudinal changes in obesity [11, 12]. Patients were grouped into Class I obesity if the %BMIp95 was ≤ 120 or Class II/III obesity if the %BMIp95 > 120. Absolute %BMIp95 was then compared between these groups as well as a variety of patient outcomes.
The intracapsular tonsillectomy technique utilized in this study was consistent for all cases. Coblation technology was used to remove essentially all tonsillar tissue to the level of the capsule. Adenoidectomy was also performed with coblation. A single fellowship‐trained pediatric otolaryngologist performed all surgeries regardless of the indication for tonsillectomy.
The following patient characteristics were included: age at surgery (years), sex (male/female), race (Asian, Black or African American, White, other, unknown/refused), ethnicity (Hispanic/Non‐Hispanic), surgical indication (obstructive/sleep apnea, infectious, other/unknown), payor category (government, commercial, other/unknown), and median household income based on zip code in United States dollars (USD). Patient race and ethnicity were self‐identified by caregivers.
Several comorbidities were recorded based on International Classification of Diseases, 10th Revision‐ Clinical Modification (ICD‐10‐CM) terminology present during the surgical admission. This included: Trisomy 21, obesity, obstructive sleep apnea, congenital defects, complex chronic diagnosis, or complicated patient (includes a diagnosis of reactive airway, craniofacial diagnosis, neuromuscular disorder, prematurity, or congenital cardiac disease). Postoperative outcomes within 30 days of surgery included: length of index admission (days), return to the hospital for bleeding (yes/no), return to the hospital for vomiting (yes/no), return to the hospital for pain (yes/no), surgical complication (yes/no), emergency department (ED) readmission (yes/no), inpatient (IP) readmission (yes/no), or operating room (OR) control of bleeding (yes/no). Nursing line calls within 30 days of surgery were recorded including the primary reason for the call as documented by the triage nurse. The number of calls related to a postoperative problem was specifically noted.
All statistics were performed with Stata (StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC.). Continuous data are presented as means with standard deviations (SD) or 95% confidence intervals. Categorical data are presented as counts with percentages. Univariable analysis utilized student's t‐testing for continuous variables and Fisher's exact test for categorical variables. A paired t‐test was utilized for comparing mean %BMIp95 between different time points. Analysis also included a mixed effect model to determine the relationship between time and weight when factoring in the variability of different trajectories among individual patients. Statistical significance was set at p < 0.05. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed [13].
3. Results
A total of 68 patients met the inclusion criteria for the study. The demographic characteristics of the analyzed cohort are summarized in Table 1. There were 39 children noted to have Class I obesity, and 29 children had class II or III obesity at the time of tonsillectomy. Intracapsular tonsillectomy was performed at an age of 7.7 years (SD: 3.8) and was often indicated for sleep‐disordered breathing or obstructive sleep apnea (91%, N = 62). Class II/III obesity patients were noted to be more typically male compared to the cohort who were Class I (p = 0.02). Other demographic characteristics, including medical comorbidities, race, age, household income, and payor category, were similar between the two cohorts.
TABLE 1.
Characteristics of intracapsular tonsillectomies by obesity class.
| Characteristic | Class I obesity (%BMIp95 ≤ 120) | Class II and III (%BMIp95 > 120) | P value |
|---|---|---|---|
| Total, No. (%) | 39 (57) | 29 (43) | — |
| Age, mean (SD), y | 7.3 (3.9) | 8.3 (3.6) | 0.30 |
| Males, No. (%) | 18 (46) | 22 (76) | 0.02 |
| Race, No. (%) | |||
| White | 25 (64) | 22 (76) | 0.46 |
| Black or African American | 9 (23) | 6 (21) | |
| Other | 5 (13) | 1 (3.5) | |
| Hispanic, No. (%) | 20 (51) | 18 (62) | 0.69 |
| Indication, No. (%) | |||
| Obstructive/sleep apnea | 35 (90) | 27 (93) | 0.05 |
| Infectious | 0 | 2 (6.9) | |
| Other/unknown | 4 (10) | 0 | |
| Payor category, No. (%) | |||
| Government | 33 (85) | 24 (83) | 0.99 |
| Commercial | 6 (15) | 5 (17) | |
| Household income, median USD (SD) | $41,130 ($14,072) | $43,383 ($17,335) | 0.56 |
| Complicated patient flag, No. (%) | 10 (26) | 7 (24) | 0.99 |
| Trisomy 21, No. (%) | 1 (2.6) | 1 (3.5) | 0.99 |
| OSA, No. (%) | 19 (49) | 17 (59) | 0.47 |
| Congenital defect, No. (%) | 2 (5.1) | 1 (3.5) | 0.99 |
| Complex chronic diagnosis, No. (%) | 5 (13) | 3 (10) | 0.99 |
Postoperative complications were similar between the class I obesity and class II/III cohorts (Table 2). No patients in the study required operative interventions for bleeding. Postoperative calls to the nursing line with questions/concerns were not significantly different between the two groups (p = 0.56). Patients in both cohorts had a similar length of stay (p = 0.33) and readmission rates to the ED (p = 0.57) or an inpatient unit (p = 0.99).
TABLE 2.
30‐day postoperative events for tonsillectomies by obesity class.
| Postoperative event | Class I obesity (%BMIp95 ≤ 120) | Class II and III (%BMIp95 > 120) | P value |
|---|---|---|---|
| Length of stay, mean d (SD) | 1.5 (1.9) | 2.8 (7.5) | 0.33 |
| Return for bleeding | 0 | 0 | — |
| Return for vomiting | 0 | 0 | — |
| Return for pain | 0 | 1 (3.5) | 0.43 |
| Surgical complication flag | 0 | 0 | — |
| ED readmits | 1 (2.6) | 2 (6.9) | 0.57 |
| IP readmits | 2 (5.1) | 2 (6.9) | 0.99 |
| OR control bleeding | 0 | 0 | — |
| Any nursing call | 7 (18) | 7 (24) | 0.56 |
| Postop problem calls | 5 (13) | 6 (21) | 0.51 |
Note: Values represent number (%) unless otherwise specified.
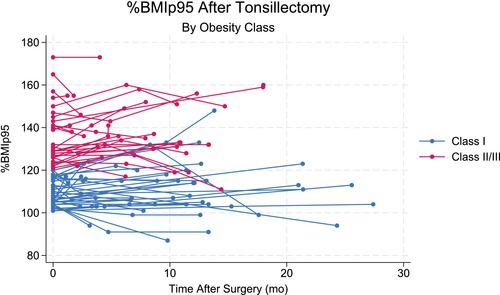
The changes in %BMIp95 postoperatively are summarized in Table 3. All 68 had at least one height and weight measurement greater than 30 days postoperatively. Further, 40 had a second measurement greater than 90 days postoperatively. A scatterplot of this data is shown in Figure 1. For the children with class I obesity, initial %BMIp95 was a mean of 109.1 (SD: 6.2), (median: 107, IQR: 104–116) and the second %BMIp95 was 110.9 (SD: 10.9) (median: 113, IQR: 103–117) (p = 0.21). The third %BMIp95 was a mean of 111.2 (SD: 13.7), (median: 112, IQR: 103.5–119) which was no different than the second %BMIp95 (p = 0.92) or the first %BMIp95 (p = 0.62).
TABLE 3.
BMI data by obesity class.
| Postoperative event | Class I obesity (%BMIp95 ≤ 120) | Class II and III (%BMIp95 > 120) | P value |
|---|---|---|---|
| Baseline BMI | 109.1 (6.2) | 136.6 (13.5) | < 0.001 |
| Second BMI | 110.9 (10.9) | 139.8 (12.6) | < 0.001 |
| Months after T&A Second BMI obtained | 7.8 (6.0) | 5.7 (3.9) | 0.11 |
| Difference between first and second BMI | 1.8 (8.6) | 3.3 (7.7) | 0.45 |
| Third BMI | 111.2 (13.7) | 135.3 (14.8) | < 0.001 |
| Months after T&A third BMI obtained | 14.1 (5.8) | 10.9 (3.5) | 0.05 |
| Difference between first and third BMI | 1.2 (11.4) | 3.0 (9.9) | 0.60 |
FIGURE 1.

Scatterplot of %BMIp95 for children with class I and class II/III obesity by months after tonsillectomy.
For the children with class II/III obesity, initial %BMIp95 was a mean of 136.6 (SD: 13.5), (median: 131, IQR: 126–143) and the second %BMIp95 was 139.8 (SD: 12.6) (median: 136, IQR: 132–146). These differences were statistically different (p = 0.02) but not clinically different. The third %BMIp95 was a mean of 135.3 (SD: 14.8), (median: 133, IQR: 123.5–149.5) which was no different than the second %BMIp95 (p = 0.27) or the first %BMIp95 (p = 0.24).
Figure 2 represents a box plot showing the changes in weight between the class I and Class II/III obesity patients. At a mean of 6.9 months (SD: 5.3), the change in %BMIp95 was 1.77 (95% CI: −1.03 to 4.57) for class I and 3.27 (95% CI: 0.55 to 6.00) for class II/III obese children (p = 0.45). For 40 children, latest measurements were taken at least 3 months after surgery. There were no long‐term differences in mean change in %BMIp95 between children with class I obesity (1.17; 95% CI: −3.63 to 5.96) and class II/III obesity (3.00; 95% CI: −2.25 to 8.25) (p = 0.60).
FIGURE 2.

Box plot of %BMIp95 for children with class I and class II/III obesity at surgery, at least 30 days after surgery, and at least 3 months postoperatively.
Finally, a mixed effects linear regression model was created to determine the impact time had on patient %BMIp95. Among class I obese children, the model was not significant (β: 0.09, p = 0.41, 95% CI: −0.12 to −0.30) but there was a significant random effect by individual patient (p < 0.001). Similarly, the model was not significant for class II/III obese children (β: 0.28, p = 0.07, 95% CI: −0.02 to −0.59) but with a significant random effect by individual patient (p < 0.001).
4. Discussion
In this retrospective cohort study, preoperative %BMIp95 was similar to postoperative %BMIp95 after intracapsular tonsillectomy regardless of obesity class. Even when %BMIp95 was obtained at least 3 months after surgery, a consistent distribution of %BMIp95 was identified. These findings suggest that intracapsular tonsillectomy does not result in significant changes in postoperative weight metrics among obese children. This is consistent with previous investigations of %BMIp95 after traditional tonsillectomy procedures [5] and adds to a growing understanding of outcomes after intracapsular tonsillectomy. Clinicians can utilize this information when counseling families ahead of intracapsular tonsillectomy when selected in obese children.
The association between tonsillectomy and weight gain has been extensively examined in the literature [5, 6, 7, 8, 9, 14, 15, 16, 17]. For perspective, sleep‐disordered breathing and obstructive sleep apnea have been strongly linked to growth failure in children [18]. The weight gain after tonsillectomy seen in this subset of patients was historically seen as advantageous in managing patients with growth failure. However, as childhood obesity rates have risen over the past few decades, there has been increased interest in assessing whether tonsillectomy is an independent risk factor for weight gain in pediatric patients.
The reason for associated weight gain after pediatric tonsillectomy is uncertain. Children with OSA have been shown to expend more calories while sleeping due to their obstructed breathing, a factor which improves following adenotonsillectomy [19]. The resultant decrease in energy expenditure at the same level of caloric intake may contribute to weight gain. For children who undergo tonsillectomy to manage recurrent tonsillitis, reduced frequency in severe throat infections could result in overeating and subsequent weight gain [14]. Alternatively, other studies have pointed to hormonal dysregulation postoperatively with derangements in insulin‐like growth factor‐I (IGF‐1) and IGF‐binding protein 3 (IGFB‐3) [20]. For intracapsular tonsillectomy, where a small portion of tonsillar tissue remains, future studies might consider exploring biochemical markers that may differ from children who have had total tonsillectomy.
Katz et al. presented the first randomized controlled trial investigating weight gain after tonsillectomy as part of the Childhood Adenotonsillectomy Trial (CHAT). Their study determined that BMI z‐scores were significantly increased following T&A. In their cohort, 51% of overweight patients were noted to become obese after T&A, corresponding with prior systemic reviews suggesting that overweight children may be at higher risk [15]. However, the conclusions of this study have been questioned in recent years. Kirkham et al. reanalyzed the CHAT data focusing exclusively on undesirable weight gain and concluded that the weight gain was related to catch‐up growth in underweight children [17]. Jensen et al. also reanalyzed the CHAT data using %BMIp95 instead of z‐scores since z‐scores can become compressed as they reach the upper limits of the BMI curve. In their analysis, there was no significant increase in weight after T&A [5]. More recently, the Pediatric Adenotonsillectomy Trial for Snoring (PATS) again performed a randomized controlled trial, failing to find a significant increase in BMIp95% [17]. These recent analyses utilizing %BMIp95 call into question whether tonsillectomy is truly an independent factor for weight gain.
Obese patients are at risk for undesirable weight gain after T& A. Lewis et al. performed a retrospective case–control series that showed that the weight gain experienced by pediatric patients undergoing T&A is often higher in patients who are obese [3]. Kashiwazaki et al. showed that the rate of growth %BMIp95 following T&A was higher among Obesity Class III patients than Class I or II [21]. Together, these studies raised concern that the most obese children are at the highest risk for postoperative weight gain. The current study performed a similar analysis to Kashiwazaki et al., comparing the growth rates for Obesity Class I and Class II/III following tonsillectomy. Notably, with the intracapsular technique utilized in this cohort, the same trend in obesity was not identified. It is not possible with the current study design to conclusively state that intracapsular tonsillectomy definitively avoids the weight gain seen in obese children, but it raises a question that can be investigated in the future.
There are several limitations to this study. The patients who underwent intracapsular tonsillectomy in this study did so mainly due to provider surgical preference. In the future, a prospective randomized trial comparing the weight gain trends between standard tonsillectomy and intracapsular tonsillectomy would better clarify the effect that surgical technique has on weight gain. Furthermore, children in this study were followed for a mean of 6.9 months. Future studies could include a cohort with a longer follow‐up period to determine the long‐term trajectory of weight gain following intracapsular tonsillectomy.
5. Conclusion
Weight gain after intracapsular tonsillectomy was no different between children with low and high obesity class. These similar growth trajectories continued even beyond 3 months, which is consistent with prior studies on standard tonsillectomy. Intracapsular tonsillectomy may be an appropriate technique to address obstructive breathing in obese children, and continued diligence to postoperative weight changes in children after tonsillectomy will be valuable.
Conflicts of Interest
Stephen Chorney is an educational consultant for Smith & Nephew.
Acknowledgments
The authors have nothing to report.
Funding: The authors received no specific funding for this work.
Presentation Statement: This manuscript was presented at the AAO‐HNSF 2024 Annual Meeting & OTO EXPO, September 28, 2024, in Miami, Florida.
References
- 1. Buono P., Maines E., Azzolini N., et al., “Short‐Term Weight Gain After Tonsillectomy Does Not Lead to Overweight: A Systematic Review,” Nutrients 16, no. 2 (2024): 324: 1–25, 10.3390/nu16020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith D. F., Vikani A. R., Benke J. R., Boss E. F., and Ishman S. L., “Weight Gain After Adenotonsillectomy Is More Common in Young Children,” Otolaryngology and Head and Neck Surgery 148, no. 3 (2013): 488–493. [DOI] [PubMed] [Google Scholar]
- 3. Lewis T. L., Johnson R. F., Choi J., and Mitchell R. B., “Weight Gain After Adenotonsillectomy: A Case Control Study,” Otolaryngology and Head and Neck Surgery 152, no. 4 (2015): 734–739, 10.1177/0194599815568957. [DOI] [PubMed] [Google Scholar]
- 4. Ruiz A. G., Gao D., Ingram D. G., Hickey F., Haemer M. A., and Friedman N. R., “Does Tonsillectomy Increase Obesity Risk in Children With Down Syndrome?,” Journal of Pediatrics 211 (2019): 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jensen A. M., Herrmann B. W., Mitchell R. B., and Friedman N. R., “Growth After Adenotonsillectomy for Obstructive Sleep Apnea: Revisited,” Laryngoscope 132, no. 6 (2022): 1289–1294, 10.1002/lary.29863 PMID: 34551129. [DOI] [PubMed] [Google Scholar]
- 6. Mitchell R. B. and Kelly J., “Outcome of Adenotonsillectomy for Obstructive Sleep Apnea in Obese and Normal‐Weight Children,” Otolaryngology and Head and Neck Surgery 137 (2007): 43–48. [DOI] [PubMed] [Google Scholar]
- 7. Johnson R. F., Hansen A., Narayanan A., Yogesh A., Shah G. B., and Mitchell R. B., “Weight Gain Velocity as a Predictor of Severe Obstructive Sleep Apnea Among Obese Adolescents,” Laryngoscope 130, no. 5 (2020): 1339–1342. [DOI] [PubMed] [Google Scholar]
- 8. Huoh K. C., Haidar Y. M., and Dunn B. S., “Current Status and Future Trends: Pediatric Intracapsular Tonsillectomy in the United States,” Laryngoscope 131 (2021): S1–S9. [DOI] [PubMed] [Google Scholar]
- 9. Windfuhr J. P. and Werner J. A., “Tonsillotomy: It's Time to Clarify the Facts,” European Archives of Oto‐Rhino‐Laryngology 270 (2013): 2985–2996. [DOI] [PubMed] [Google Scholar]
- 10. Windfuhr J. P., Savva K., Dahm J. D., and Werner J. A., “Tonsillotomy: Facts and Fiction,” European Archives of Oto‐Rhino‐Laryngology 272 (2015): 949–969. [DOI] [PubMed] [Google Scholar]
- 11. Freedman D. S., Butte N. F., Taveras E. M., et al., “BMI z‐Scores Are a Poor Indicator of Adiposity Among 2‐To 19‐Year‐Olds With Very High BMIs, NHANES 1999–2000 to 2013–2014,” Obesity 25, no. 4 (2017): 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freedman D. S., Woo J. G., and Daniels S. R., “Longitudinal Changes in Various BMI Metrics and Adiposity in 3‐ to 7‐Year‐Olds,” Pediatrics 150, no. 6 (2022): e2022058302, 10.1542/peds.2022-058302. [DOI] [PubMed] [Google Scholar]
- 13. von Elm E., Altman D. G., Egger M., et al., “The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies,” PLoS Medicine 4, no. 10 (2007): e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeyakumar A., Fettman N., Armbrecht E. S., and Mitchell R., “A Systematic Review of Adenotonsillectomy as a Risk Factor for Childhood Obesity,” Otolaryngology and Head and Neck Surgery 144, no. 2 (2011): 154–158, 10.1177/0194599810392328 PMID: 21634056. [DOI] [PubMed] [Google Scholar]
- 15. Katz E. S., Moore R. H., Rosen C. L., et al., “Growth After Adenotonsillectomy for Obstructive Sleep Apnea: An RCT,” Pediatrics 134, no. 2 (2014): 282–289, 10.1542/peds.2014-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kevat A., Bernard A., Harris M. A., et al., “Impact of Adenotonsillectomy on Growth Trajectories in Preschool Children With Mild‐Moderate Obstructive Sleep Apnea,” Journal of Clinical Sleep Medicine 19, no. 1 (2023): 55–62, 10.5664/jcsm.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kirkham E. M., Ishman S., Baldassari C. M., et al., “Weight Gain After Adenotonsillectomy in Children With Mild Obstructive Sleep‐Disordered Breathing: Exploratory Analysis of the PATS Randomized Clinical Trial,” JAMA Otolaryngology. Head & Neck Surgery 150, no. 10 (2024): 859–867, 10.1001/jamaoto.2024.2554 PMID: 39172463; PMCID: PMC11342222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bonuck K., Parikh S., and Bassila M., “Growth Failure and Sleep Disordered Breathing: A Review of the Literature,” International Journal of Pediatric Otorhinolaryngology 70, no. 5 (2006): 769–778, 10.1016/j.ijporl.2005.11.012 PMID: 16460816. [DOI] [PubMed] [Google Scholar]
- 19. Marcus C. L., Carroll J. L., Koerner C. B., Hamer A., Lutz J., and Loughlin G. M., “Determinants of Growth in Children With the Obstructive Sleep Apnea Syndrome,” Journal of Pediatrics 125, no. 4 (1994): 556–562, 10.1016/s0022-3476(94)70007-9 PMID: 7931873. [DOI] [PubMed] [Google Scholar]
- 20. Nieminen P., Löppönen T., Tolonen U., Lanning P., Knip M., and Löppönen H., “Growth and Biochemical Markers of Growth in Children With Snoring and Obstructive Sleep Apnea,” Pediatrics 109, no. 4 (2002): e55, 10.1542/peds.109.4.e55 PMID: 11927728. [DOI] [PubMed] [Google Scholar]
- 21. Kashiwazaki R., Jensen A. M., Haemer M., and Friedman N. R., “The Effects of Adenotonsillectomy for Obstructive Sleep Apnea on Growth Trajectory in Children With Obesity,” Otolaryngology and Head and Neck Surgery 170, no. 1 (2024): 277–283, 10.1002/ohn.512 PMID: 37668178. [DOI] [PubMed] [Google Scholar]


