ABSTRACT
Bacteria often make initial contact with their hosts through the ligand-binding domains of large adhesin proteins. Recent analyses of repeats-in-toxin (RTX) adhesins in Gram-negative bacteria suggest that ligand-binding domains can be identified by the way they emerge from “split” domains within the adhesin. Here, using this criterion and an AlphaFold3 model of a 5047-residue RTX adhesin from Aeromonas hydrophila, we identified three different ligand-binding domains in this fibrillar protein. The crystal structures of the two novel domains were solved to 1.4 and 1.95 Å resolution, respectively, and demonstrate excellent agreement with their modeled structures. The other domain was recognized as a carbohydrate-binding module based on its beta-strand topology and confirmed by its micromolar affinity for fucosylated glycans, including the Lewis B and Y antigens. This lectin-like module, which was recombinantly produced with its companion split domain and nearby extender domain, bound to a wide variety of cells including yeasts, diatoms, erythrocytes, and human endothelial cells. In each case, 50 mM free fucose prevented this binding and may offer some protection from infection. The carbohydrate-binding module with its neighboring domains also caused aggregation of yeast and erythrocytes, which was again blocked by the addition of free fucose. The second putative ligand-binding domain has a beta-roll structure supported by a parallel alpha-helix, and the third is a homolog of a von Willebrand Factor A domain. These two domains bind to a more limited range of cell types, and their ligands have yet to be identified.
IMPORTANCE
Characterizing the ligand-binding domains of fibrillar adhesins is important for understanding how bacteria can colonize host surfaces and how this colonization might be blocked. Here, we show that the opportunistic pathogen, Aeromonas hydrophila, uses a carbohydrate-binding module (CBM) to attach to several different cell types. The CBM is one of three ligand-binding domains at the distal tip of the adhesin. Identifying the glycans bound by the CBM as Lewis B and Y antigens has helped explain the range of cell types that the bacterium will bind and colonize, and it has suggested sugars that might interfere with these processes. Indeed, fucose, which is a constituent of the Lewis B and Y antigens, is effective at 50 mM concentrations in blocking the attachment of the CBM to host cells. This will lead to the design of more effective inhibitors against bacterial infections.
KEYWORDS: adhesins, biofilms, protein structure–function, Aeromonas, receptor–ligand interaction, binding proteins, antimicrobial agents
INTRODUCTION
Aeromonas hydrophila is a Gram-negative, rod-shaped bacterium in the family Aeromonadaceae (1, 2). It is widely distributed in aquatic environments, including fresh, brackish, and waste waters. In these ecosystems, the bacterium is typically in sediments and biofilms (3), or associated with other organisms, including plants and animals (4, 5). In humans, diseases caused by A. hydrophila include gastroenteritis, wound infections, and septicemia (6, 7). One unusual vector for infections has been the use of leeches in medicine (8). A. hydrophila also infects many aquatic animals, causing substantial economic losses in aquaculture (9, 10). Antibiotics and immunization against A. hydrophila are two measures used to combat these infections in fish farms (11). However, many strains of A. hydrophila are developing resistance against antibiotics (12, 13), posing an emerging threat made more urgent by global warming, which increases the range of this and other water-borne pathogens like Vibrio cholerae and V. vulnificus (14, 15).
A. hydrophila produces a range of diffusible toxins to inhibit competitors and derive nutrients from target organisms (16). Of these, aerolysin, a pore-forming toxin, has been particularly well characterized (17–19). However, little is known about how this bacterium begins colonization at sites of infection. Recent work on the Antarctic marine bacterium Marinomonas primoryensis, which binds to sea ice, and on V. cholerae, the causative agent for cholera, has implicated long fimbrial adhesins in initiating surface contact with cells through specific ligand-binding domains at the distal end of the adhesins (20, 21). In both of these bacteria, the adhesins are single polypeptide chains of the repeats-in-toxin (RTX) family that are exported through the Type I secretion system but anchored to the bacterial surface by a retention domain that folds internally and is then too large to pass through the channel of the T1SS (22, 23). The RTX long adhesin protein A (LapA) has been well characterized in the beneficial plant bacterium Pseudomonas fluorescens (24, 25). Additionally, the characterization of two gene products from the same operon, LapD and LapG, explains how P. fluorescens can exit a site of colonization when nutrients like phosphate become growth-limiting (26). LapD is an effector protein that responds to low c-di-GMP levels during nutrient starvation by releasing the periplasmic protease LapG to cleave the LapA retention domain, setting the bacterium free to seek a more nutritious environment to colonize.
At the distal, colonizing end of the RTX adhesin, ligand-binding domains determine to what surfaces the bacteria will attach. In M. primoryensis, structural analysis of its RTX adhesin, referred to as the Mp ice-binding protein (MpIBP), revealed the presence of three ligand-binding domains (27). The largest of the three is the ice-binding domain that can anchor the bacterium to ice (28, 29). Next to it are a PA14-type carbohydrate-binding module (CBM), previously referred to as a sugar-binding domain, with affinity for fucosylated glycans (30), and a peptide-binding domain (PBD) that recognizes and binds a C-terminal tripeptide of optimal sequence -Tyr-Thr-Asp (31). The PA14-type CBM was first characterized in the protective antigen of Bacillus anthracis (32) and is widespread in both prokaryotes and eukaryotes (33). The CBM and PBD ligand-binding domains bind to diatoms like Chaetoceros neogracile to help form a mutually beneficial mixed-species biofilm under ice where photosynthesis is optimal (27). Similar analyses of the V. cholerae flagellar-regulated hemagglutination (FrhA) RTX adhesin responsible for colonizing various organisms and cell types (21) have revealed the presence of two ligand-binding domains. One is a homolog of the PBD from M. primoryensis with 65% sequence identity (34). The other is a different type of CBM with less than 20% sequence identity to MpIBPCBM that can bind blood group glycans but is also capable of lysing erythrocytes (35).
Here, we have examined the large RTX adhesin protein of A. hydrophila (AhLap) by AlphaFold3 to predict the structures of all the domains that make up this 0.5-MDa protein. Not only does this modeling correctly predict the start and end of each domain, it can reliably reveal the presence of ligand-binding domains based on their projection out of companion “split domains” (36). By these criteria, there are three C-terminal ligand-binding domains in AhLap. We expressed each of these as recombinant proteins and solved the crystal structures of two. One is a carbohydrate-binding module with affinity for fucosylated glycans that allows it to bind to a wide range of cells, from yeasts to human endothelial cells and erythrocytes. The other two domains have a much more restricted range of targets. By characterizing these domains and elucidating their binding sites and ligands, antagonists can potentially be developed to block binding. This strategy may aid in inhibiting colonization and infection, particularly with antibiotic-resistant strains.
RESULTS
A 0.5-MDa RTX adhesin in A. hydrophila with over 40 domains
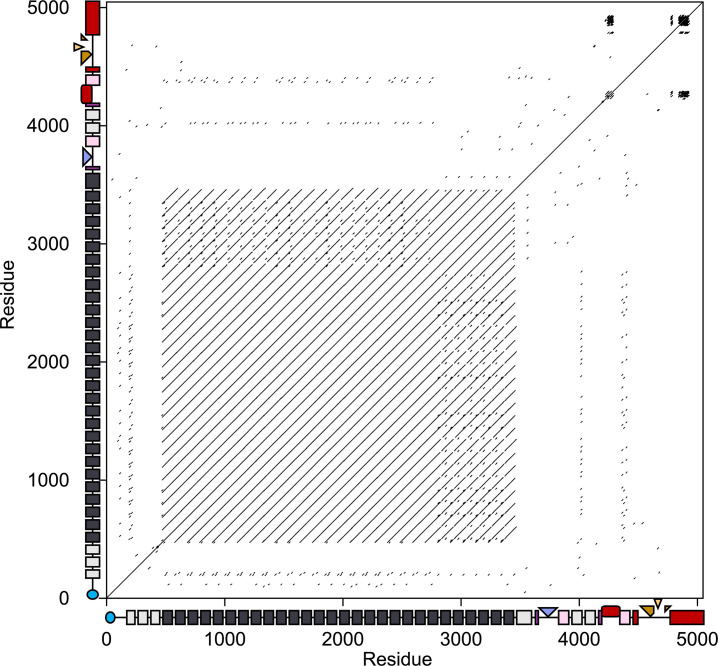
RTX adhesins are typically encoded by the largest or second-largest open reading frame within the genomes of many Gram-negative bacteria. A 15-kb open reading frame in the A. hydrophila genome encoded a 5047-residue protein identified as an RTX adhesin using dot matrix analysis (Fig. 1). The plot showed the classic signature of an RTX adhesin, where residues in the first two-thirds of the sequence (from 482 to 3455) form a long stretch of tandemly repeated immunoglobulin-like (Ig-like) extender domains that present as an array of equally spaced lines parallel to the central diagonal line. In addition, the three tight clusters of shorter repeats forming black patches on the diagonal in the top right-hand corner come from the Ca2+-binding nonapeptide repeats that form the beta-rolls and inspired the name “repeats-in-toxin”. The two clusters near the C terminus correspond to a discontinuous beta-helix that lies immediately before the Type I secretion signal (Fig. 2). The third corresponds to a beta-roll that is one of the three ligand-binding domains.
Fig 1.
Dot matrix analysis of AhLap against itself to detect repetitive regions. The x- and y-axes display the length of AhLap in amino acid residues alongside domain maps of the adhesin. The N-terminal retention domain is in blue, followed by grey and black rectangles representing the extender domains. In the ligand-binding region, Ig-like split domains are in pink, and the projected CBM, RTX domain, and vWFA domain are colored slate blue, dark red, and gold, respectively. The vWFA domain emerges from the C-terminal RTX domain (red). Dashes in the two-dimensional plot record matches with a threshold score of at least 30 using the BLOSUM62 matrix with a window size of 10 residues (37).
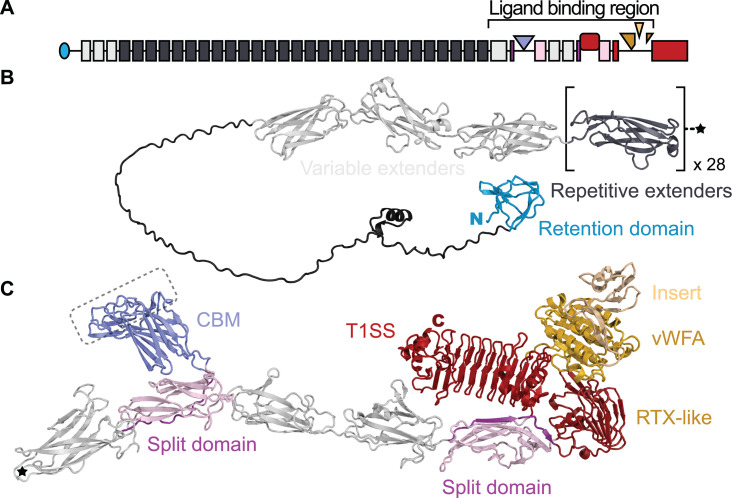
Fig 2.
AlphaFold3 structure of AhLap in ribbon format in relation to the adhesin domain map. (A) Domain map of AhLap as presented in Fig. 1. (B) Structure of the retention domain (blue) and extender (light and dark grey) region of the adhesin. The square brackets around the dark grey extender domain indicate that there are 28 highly similar versions of this fold. The star denotes the end of the repetitive extender region. (C) Structure of the remainder of the AhLap adhesin from the star to the C-terminal end. The domains here in the ligand-binding region are colored as described in the Fig. 1 legend. The putative ligand-binding site on the CBM is indicated by the dashed line boundary. Note that the insert in the vWFA domain is colored yellow to distinguish it from the main section (gold). The N and C termini of these two adhesin halves are marked N in blue and C in red, respectively.
When this adhesin was modeled by AlphaFold3, a total of 42 discrete domains could be distinguished (Fig. 2). The N-terminal retention domain (blue) had the same Ca2+-independent fold seen in the ice-binding adhesin of M. primoryensis (27), in LapA of P. fluorescens (22), and in the FrhA adhesin of V. cholerae (34). The first three extender domains (light grey in Fig. 1, 2A and B) are distinct from the following more highly repetitive extender domains (dark grey in Fig. 1, 2A and B), which is why their repeat signature of lines parallel to the diagonal is muted. A similar observation was made with the ice-binding adhesin of M. primoryensis, where the first three domains were varied in their sequences, while subsequent Ig-like extender domains were identical (27).
The ligand-binding region of AhLap has three putative ligand-binding domains
Immediately after the tandem extender domains is the more varied ligand-binding region (Fig. 2C). Following the last, slightly larger extender domain (leftmost light grey domain in Fig. 2C) is an Ig-like split domain, with the N-terminal portion of the domain in purple, and the portion that is C-terminal to the ligand-binding domain in pink. These split domains serve to project a ligand-binding domain away from the axis of the adhesin, with its ligand-binding site farthest from the junction with the split domain (34). Here, this first ligand-binding domain (colored slate blue in Fig. 2A and C) shares beta-strand topology and low sequence identity with other carbohydrate-binding modules found in bacterial adhesins (Fig. 3A through C) (30, 38). This unit is followed by two Ig-like extender domains (light grey) that lead into a second split domain (purple and pink). Budding out from this second split domain is a 17-kDa putative ligand-binding domain (dark red), which AlphaFold3 suggests is an RTX-type beta-roll containing nonapeptide Ca2+-binding sequences. After the fold of the second split domain is completed, the polypeptide chain leads into the C-terminal RTX beta-roll domain (red). This solenoid structure also serves as a split domain from which a von Willebrand Factor A (vWFA) domain emerges (bronze). Finally, the von Willebrand Factor A domain itself serves as a split domain to bud off an insertion sequence (peach) before completing its fold and returning to the beta-roll domain that is followed immediately by the Type I secretion signal, containing short alpha-helices, that marks the C-terminal end of the adhesin.
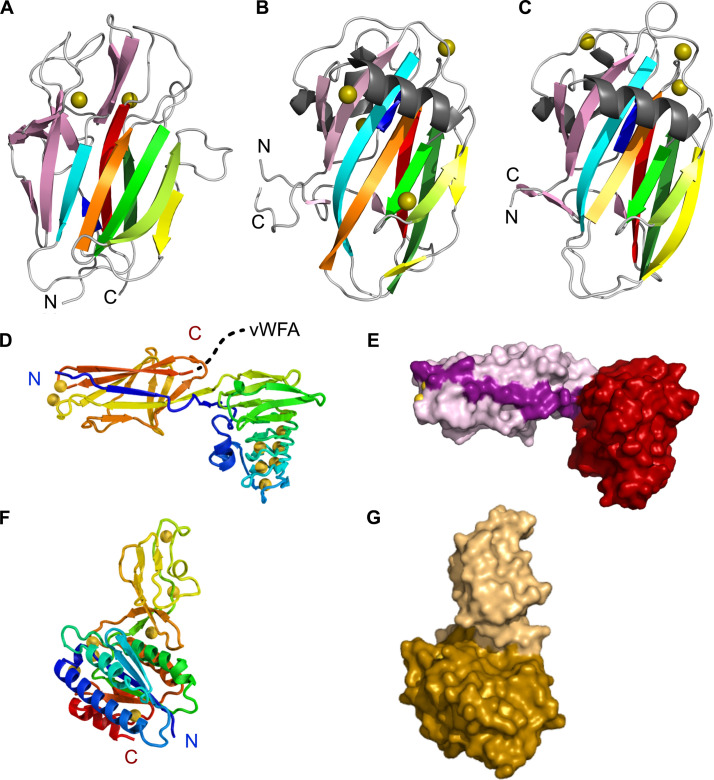
Fig 3.
Structures of the three AhLap ligand-binding domains. (A) The AlphaFold3 model of AhLapCBM is shown as a nine-stranded beta-sandwich fold made up of a four-stranded antiparallel beta-sheet on one side and a five-stranded antiparallel beta-sheet on the other side. (B) Crystal structure of the CBM from MpIBP (PDB: 5J6Y) showing the similarity of its beta-strand topology (30). (C) Crystal structure of the CBM from MnLap (PDB: 6M8M), again showing the similarity of its beta-strand topology (38). (D) Crystal structure of the RTX-like two-domain construct (from residue 4168 to 4498) in rainbow ribbon format that traces the path of the polypeptide chain through the two domains. Numerous Ca2+ ions (gold spheres) are present, especially in the RTX solenoid domain. The dotted line from the C terminus leads into the vWFA domain. (E) Space-filling presentation of the RTX-like two-domain construct. The split domain strand that leads into the RTX domain is colored a darker pink than the rest of the split domain. (F) Crystal structure of one vWFA molecule from the crystal asymmetric unit. The rainbow ribbon tracing shows how the insert sequence buds out of the Rossman fold before the latter is complete. (G) Space-filling mode of the vWFA structure, with the main section corresponding to the Rossman fold colored gold and the insert sequence that is missing from some other vWFA domains colored beige.
Structures of the ligand-binding domains
Carbohydrate-binding module
To study the carbohydrate-binding module (CBM) of AhLap, a 49,630-Da, three-domain construct (CBM3) that included this module, along with its split domain and the preceding extender domain (Asn3455 to Gly3940), was produced in Escherichia coli from a synthetic gene as a His-tagged recombinant protein (colored light grey, pink, and slate blue in Fig. 2C and corresponding to the three N-terminal domains). CBM3 was purified by nickel affinity chromatography, size-exclusion chromatography, followed by another round of nickel affinity chromatography, and stored in aliquots at −80°C at a concentration of 23 mg/mL.
During size exclusion chromatography on a Superdex column, AhLapCBM3 eluted as a monomer with an apparent molecular weight of ~50 kDa (Fig. S1A). To check for binding interaction with the dextran-based column matrix, the chromatography was repeated with 10 mM glucose in the column buffer, and the elution profile (red line) gave the same apparent molecular weight from which we concluded that CBM3 is a monomer in solution.
When the modeled structure of the putative AhLapCBM (Fig. 3A) is compared to the crystal structures of the PA14 CBMs of M. primoryensis IBP (30) and Marinobacter nauticus (formerly Marinobacter hydrocarbonoclasticus) (38), their beta-strand topologies are identical. This is a key observation given that AhLapCBM and MpCBMs share only 20% sequence identity, and AhCBM and MnCBMs have 23% sequence identity. In contrast, the MpCBM and MnCBMs share 40% sequence identity. At the outer edge of the CBMs is a set of peptide loops that connect the strands of the core beta-sandwich fold. This region in AhLapCBM is the presumed ligand-binding site (circled in Fig. 2C) based on a comparison with the MpCBM and MnCBMs, where co-crystal structures show the presence of a bound Ca2+ ion helping coordinate their sugar ligands (30, 38).
RTX-like ligand-binding domain
Immediately C-terminal to the split domain from which the CBM buds are two regular extender domains (grey in Fig. 2C). After these, another split domain begins (colored pink) that leads into a second putative ligand-binding domain (colored dark red) before returning to finish the split domain (pink). These two domains were expressed together, purified, and crystallized (Fig. 3D and E). Their combined structure (PDB ID: 9CSE) was solved by collecting data from Ho3+-soaked crystals and then using this solution to determine a 1.95 Å-resolution structure from a native data set (Table S1). In the rainbow-colored ribbon presentation (Fig. 3D), the polypeptide chain continues on from the first beta-strand of the split domain (colored blue) to form the ligand-binding domain (colored blue to green). The first section of the beta-roll domain is a short alpha-helix that runs parallel with, and leads into, a short beta-solenoid domain composed of tandem RTX nonapeptide repeats with a consensus sequence X-G-G-X-G-(N/D)-D-X-(L/I/F)-X that together bind nine Ca2+ ions on the inside of the solenoid. After four coils of the beta-roll, the domain ends in a short beta-sandwich motif before the polypeptide chain returns to complete the split domain (colored yellow to red). There is no obvious ligand-binding site on this domain, but based on observations of other split + ligand-binding domain combinations (34), its binding site is likely to be the most solvent-exposed surface. In the space-filling presentation (Fig. 3E), the split domain is colored pink with the strand leading into the beta-roll domain (red) shown in a darker pink.
vWFA domain with an insertion sequence
Immediately following the second Ig-like split domain lies the main RTX beta-roll that ends in the C-terminal Type I secretion signal. But, after just two turns of this beta-roll domain, the polypeptide chain diverges to fold into a vWFA domain (Fig. 2C). This 28.3-kDa domain was produced as a His-tagged recombinant protein and purified for crystallography. Its crystal structure (PDB ID: 9DAS) was solved for the Ca2+-bound protein on our home X-ray source (39). Subsequently, the structure resolution was improved to 1.4 Å with a data set collected at the APS synchrotron (Table S2). The rainbow-colored ribbon presentation (Fig. 3F) shows how the N and C termini (blue and red, respectively) lie adjacent to each other, which is a typical feature of ligand-binding domains because they bud out of their companion split domains. The vWFA domain has a classic Rossman fold of which the first half (in red and orange) is laid down before a 9-kDa insertion sequence (yellow and light green) emerges around the mid-point between residues 4628 and 4704 in the full-length adhesin. This insertion sequence folds into a short alpha-helix and an antiparallel beta-sheet with at least one Ca2+ ion present that coordinates OD1 and OD2 of Asp4647 and carbonyl of Arg4641. The polypeptide chain returns to complete the C-terminal portion (residues 4709–4773) of the Rossman fold colored green and blue. It is not known if the insert, colored peach in the space-filling presentation (Fig. 3G), modifies the binding specificity of the vWFA domain (colored gold) or if it is a ligand-binding domain on its own. In the latter case, the vWFA domain would be its split domain. In the crystal structure, there are two molecules of the vWFA domain in the asymmetric unit, with two salt bridges formed between molecule B Arg4641 NE1 and NE2 and molecule A Asp4635 CD1, respectively, as well as seven hydrogen-bond contacts on the surface between the insertion regions of molecules A and B. However, when the PDBePISA program (40) was used to evaluate the macromolecular interface between vWFA domains, the complexation significance score was zero on a scale of 0–1, suggesting that the vWFA domain only makes protein–protein contacts in the crystal. Indeed, the apparent molecular weight of the vWFA domain during size-exclusion chromatography is consistent with it being a monomer in solution (Fig. S1B).
Crystal structures of the ligand-binding domains closely match AlphaFold3 predictions
When the crystal structures of the two putative ligand-binding domains (red and gold ribbon) are overlaid on their AlphaFold3 models (green ribbon) to compare their folds (Fig. S2A and B), the agreement between individual domains is extremely close, with RMSD values of below 0.5 Å (Table S4). Deviations are slightly higher in the loops and at the termini.
AhLapCBM3 binds fucosylated glycans
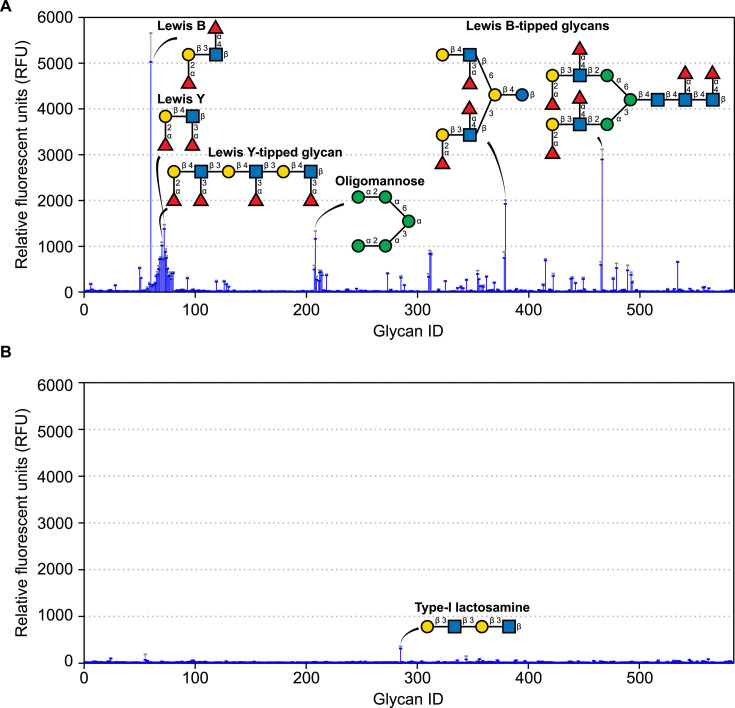
One of the challenges of studying the ligand-binding domains of bacterial adhesins lies in determining what molecules they bind. Putative CBMs can be assayed using glycan arrays. To prepare for this analysis, the AhLapCBM3 construct shown in Fig. 3A was labeled at its N-terminal end by fusion to green fluorescent protein (GFP). This CBM3 bound avidly to a small set of glycans on the array (Fig. 4A). Strong binding was observed to the Lewis B and Lewis Y antigens as well as to more complex glycans that were tipped with these antigens. These glycans all share terminal fucose residues. Binding was also seen to a branched oligomannose.
Fig 4.
Glycan array analysis. (A) Analysis using GFP-tagged AhLapCBM3 to probe 580+ mammalian glycans. Binding is shown in blue as relative fluorescence units with the standard deviation from four replicates indicated by error bars above the peaks. Six of the main glycans bound are shown in schematic representation where the sugar moieties are fucose (red triangles), galactose (yellow circles), glucose (blue squares), and mannose (green circles). (B) Analysis using GFP-tagged AhLapRTX domain showing the glycan structure of an interactor that is marginally above background levels.
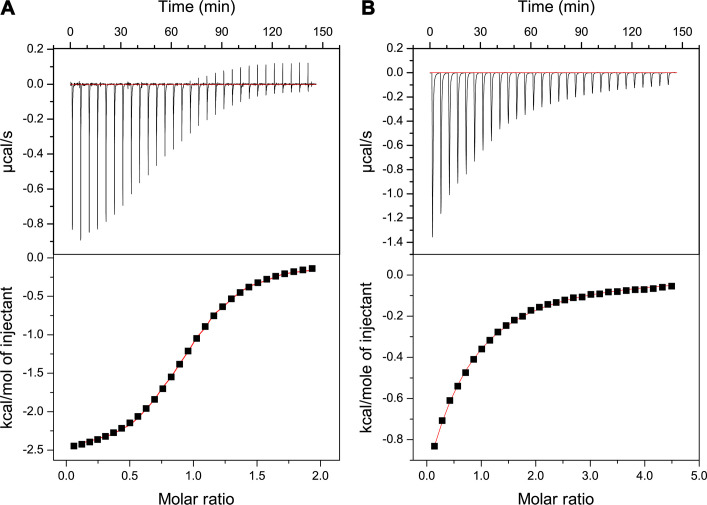
Isothermal titration calorimetry was used to measure the stoichiometry and strength of binding of the Lewis B antigen to AhLapCBM3. When the ligand concentration was fixed at 3.5 mM and the protein concentration was varied between 170 and 467 µM, the stoichiometry of binding was 1:1, and the average KD value of five measurements was 20.8 µM (Fig. 5A). The stoichiometry and KD value are in line with values obtained for the CBMs of V. cholerae, Aeromonas veronii, and M. primoryensis when tested with fucose as the ligand (30, 35). However, when free fucose was assessed as the ligand for AhLapCBM3, its KD value of ~386 µM ± 15.2 µM showed much weaker binding for this simple sugar (Fig. 5B). This suggests that other elements of the Lewis B antigen besides the fucosyl moiety contribute to the glycan binding.
Fig 5.
Isothermal titration calorimetry. (A) Analysis of AhLapCBM3 binding Lewis B antigen. Top panel shows the thermogram for the interaction between Lewis B antigen and AhLapCBM3 plotted as µcal/s per event. The lower panel shows the fitted curve of these data from which the stoichiometry and KD were calculated. (B) Analysis of AhLapCBM3 binding free fucose. Top panel shows the thermogram for the interaction between l-fucose and AhLapCBM3 plotted as µcal/s per event. The lower panel shows the fitted curve of these data from which the KD was calculated.
The putative RTX-like ligand-binding domain does not bind mammalian glycans
The binding partner for the RTX-like domain (AhLapRTX) is not known. To determine if this domain is another type of CBM, it was also tagged with GFP at its N terminus and sent for glycan array analysis. It bound to only one glycan slightly above background levels (Fig. 4B). This was galactose-tipped Type-I lactosamine. Therefore, we do not consider it to be a glycan-binding domain. vWFA domains in animals function in binding cells and extracellular matrix proteins like collagen (41, 42). For this reason, the AhLapvWFA domain linked to GFP was not sent for glycan binding analysis.
AhLapCBM3 has affinity for a variety of human cells
Having identified fucosylated glycans as a binding target for the AhLapCBM3, we looked at this protein’s affinity for different cell types and biofilms. CBMs from RTX adhesins of V. cholerae and A. veronii also bound to fucosylated glycans that included some blood group antigens (35). When these CBMs were fluorescently labeled and incubated with human erythrocytes, they bound to these cells but also lysed them in a concentration-dependent manner. When the GFP-tagged AhLapCBM3 prepared for glycan array analysis was incubated with human erythrocytes, it bound to the cells and aggregated them together into huge, tight, fluorescent clusters (Fig. 6A). However, it did not lyse them. When this experiment was repeated in the presence of 50 mM l-fucose, there was no binding of the CBM3 to erythrcytes and no aggregation of the cells (Fig. 6B), which is consistent with the binding interaction being mediated through a fucosyl moiety on the erythrocyte glycans. When the other two putative ligand-binding domains were GFP-tagged and mixed with human erythrocytes, they showed no affinity and no ability to cause aggregation of these cells (Fig. 6C and D).
Fig 6.
Binding of AhLapCBM3 to human erythrocytes, with and without fucose present. (A) Erythrocytes incubated with GFP-tagged AhLapCBM3 construct that includes a split domain and one extender domain (Fig. 3A through C). The left-hand panel is viewed in brightfield and the right-hand panel is viewed to see the green fluorescence of GFP. (B) Erythrocytes incubated with the same GFP-tagged AhLapCBM3 construct in the presence of 50 mM fucose. (C) Erythrocytes incubated with the GFP-tagged AhLapvWFA construct. (D) Erythrocytes incubated with the GFP-tagged AhLapRTX construct. The white scale bar indicates 10 µm.
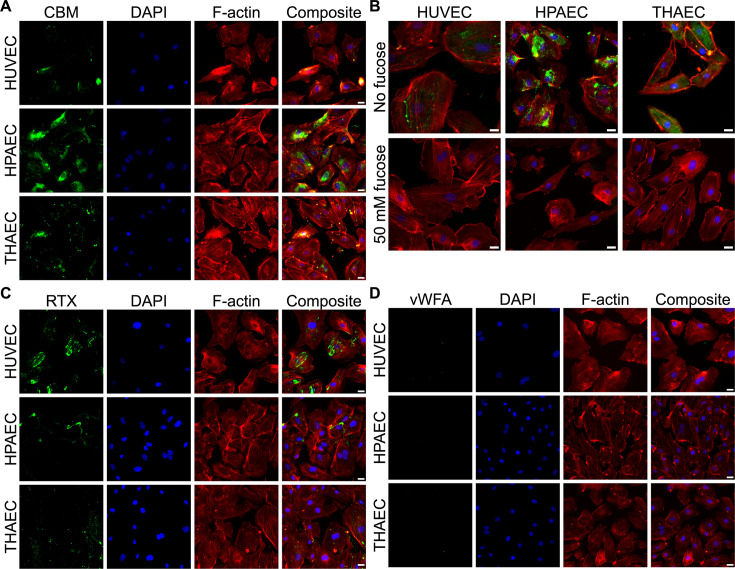
To determine if the CBM3 can bind to other human cell types, three different types of endothelial cells, HUVEC (human umbilical vein endothelial cells), HPAEC (human pulmonary artery endothelial cells), and THAEC (telomerase-immortalized human aorta endothelial cells) were probed with GFP-labeled AhLapCBM3. Extensive patches of fluorescence labeling were seen on the surface of all three cell types (Fig. 7A). For reference, the same cells were stained with DAPI to show the nuclei and phalloidin to visualize the actin cytoskeleton, with a superimposition of all three images shown to the right of each set. A similar superimposition of the three images for the three endothelial cell types was made in the absence (top) and presence of 50 mM fucose (bottom) (Fig. 7B). Fucose completely blocked all binding of the GFP-labeled AhLapCBM3 to all three endothelial cell types.
Fig 7.
AhLapCBM3 binding to endothelial cells in the absence and presence of fucose. (A) The three images in the first column show fluorescence from the incubation of GFP-tagged AhLapCBM3 with human umbilical vein endothelial cells (HUVECs), human pulmonary artery endothelial cells (HPAECs), and telomerase-immortalized human aorta endothelial cells (THAECs). Images in the second column show the same cells stained with DAPI to visualize the nuclei. Images in the third column show the same cells stained with phalloidin to visualize the actin cytoskeleton. Images in the fourth column show composite images of the three staining patterns superimposed. (B) Composite staining images of the three endothelial cell types were repeated in the absence (top row) and presence (bottom row) of 50 mM fucose. The white scale bar indicates 10 µm. (C) The three images in the first column show fluorescence from the incubation of GFP-tagged AhLapRTX with HUVECs, HPAECs, and THAECs. Images in the second column show the same cells stained with DAPI to visualize the nuclei. Images in the third column show the same cells stained with phalloidin to visualize the actin cytoskeleton. Images in the fourth column show composite images of the three staining patterns superimposed. (D) The 12 panels shown here are equivalent to those in (A) and (C), except that the cells in the first column were probed with GFP-tagged AhLapvWFA.
AhLapRTX, but not AhLapvWFA, has affinity for endothelial cells
When GFP-tagged AhLapRTX was incubated with the same array of endothelial cells, it also bound to all three of them (Fig. 7C). It is not currently known what component of the endothelial cells is recognized and bound by this domain. When GFP-tagged AhLapvWFA was incubated with the same endothelial cells, none of them were bound by this protein (Fig. 7D).
Binding of AhLap ligand-binding domains to yeasts
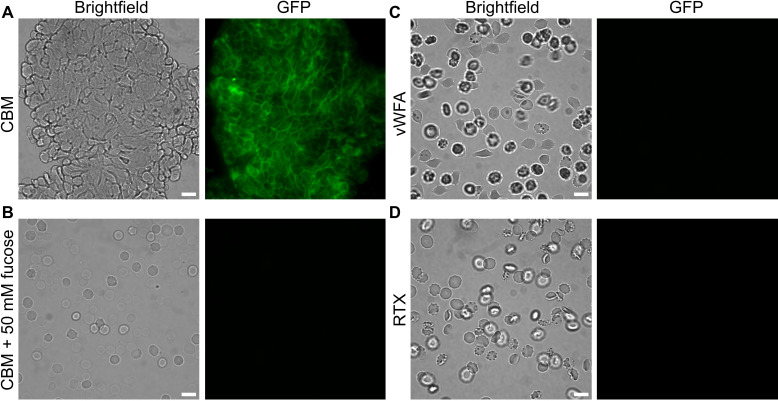
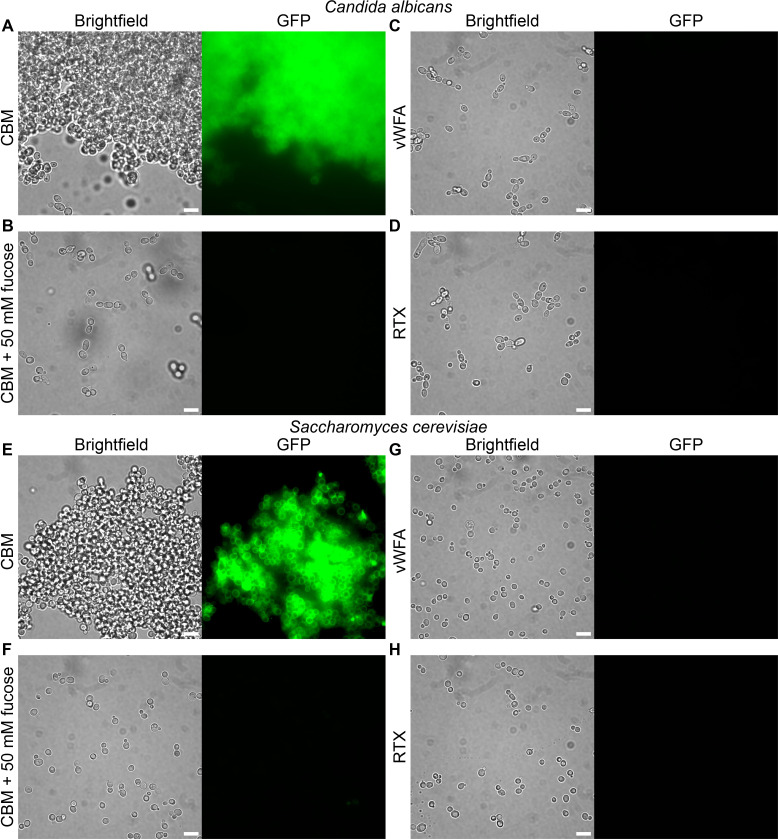
When two yeast species, Candida albicans and Saccharomyces cerevisiae, were incubated with GFP-labeled AhLapCBM3, both yeasts were intensively labeled (Fig. 8A and E). The addition of this three-domain construct induced aggregation of the yeasts into large cell clusters, very much like the aggregation of erythrocytes in Fig. 6A. This was despite the S. cerevisiae being a non-flocculating strain. To test for the presence of functional amyloids, the cell aggregates were stained with the amyloid-specific dye thioflavin T. Imaging by microscopy showed no increase in fluorescence by ThT (data not shown), suggesting an absence of amyloids. This contrasts with typical aggregation by C. albicans and S. cerevisiae, in which cell-to-cell contacts are mediated, in part, by functional amyloid formation (43). This, in turn, supports that the observed aggregation is mediated by AhLapCBM3 rather than the yeasts’ native adhesins. Again, preincubation with 50 mM fucose blocked all fluorescent staining and aggregation, with the yeast dispersed as single cells (Fig. 8B and F). In contrast, the GFP-tagged AhLapRTX and AhLapvWFA domains showed no binding to either yeast species and no tendency to aggregate the yeast (Fig. 8C, D, G, H).
Fig 8.
AhLapCBM3 binding to yeasts in the presence and absence of fucose. (A) The two panels show Candida albicans incubated with GFP-labeled AhLapCBM3 and visualized in brightfield (left) and fluorescence (right). (B) The two panels repeat the conditions in (A) but with 50 mM fucose present. Panels (E) and (G) are repeats of (A) and (B) with Saccharomyces cerevisiae instead of C. albicans. (C) The two panels show C. albicans incubated with GFP-labeled AhLapvWFA and visualized in brightfield (left) and fluorescence (right). (D) The two panels show C. albicans incubated with GFP-labeled AhLapRTX and visualized in brightfield (left) and fluorescence (right). Panels (G) and (H) are repeats of (C) and (D) with S. cerevisiae instead of C. albicans. The white scale bar indicates 10 µm.
Binding of AhLapCBM3 to diatoms and biofilms
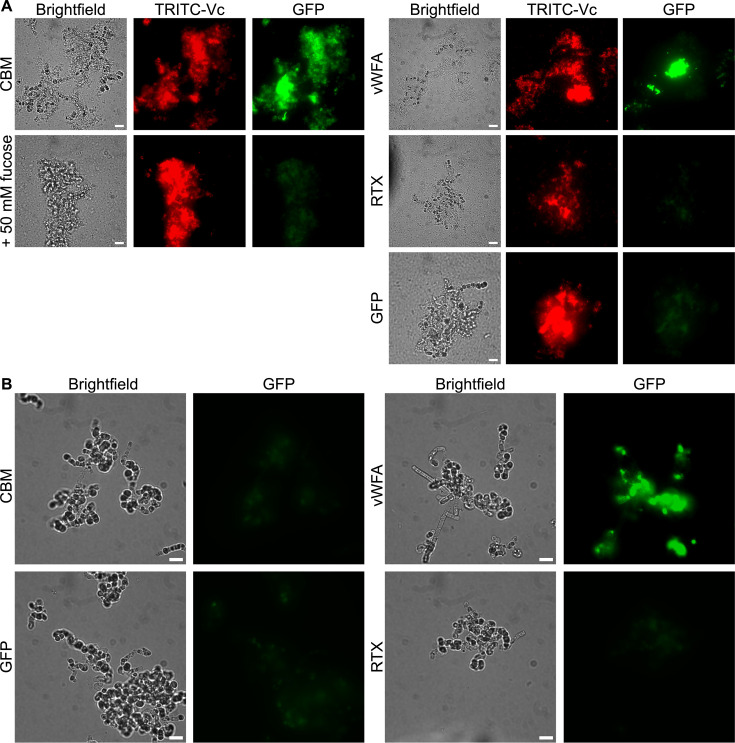
Marine bacteria, like V. cholerae, use their adhesins to colonize a wide variety of organisms and microorganisms in their aqueous habitat (44). When V. cholerae encountered the diatom Extubocellulus spinifer, the bacteria bound to them and formed biofilms (Fig. 9A). The brightfield image shows several diatoms enmeshed in bacteria and the biofilm the bacteria produce in this situation. The bacteria were stained red with TRITC. These mixed species biofilms provided an opportunity to test AhLapCBM3 for binding to all three components: diatoms, bacteria, and biofilm matrix. In the absence of V. cholerae, and hence the absence of biofilm, GFP-tagged AhLapCBM3 failed to bind to the diatoms (Fig. 9B). However, after the addition of V. cholerae, AhLapCBM3 bound to the zones of biofilm produced by the red fluorescently labeled bacteria around the diatoms (Fig. 9A). Fluorescence was particularly intense in some areas of the mixed species biofilm. There was no obvious binding to the free bacteria.
Fig 9.
AhLap ligand-binding domain studies with diatoms, with and without V. cholerae present to generate biofilms. (A) The six panels on the left show binding of GFP-labeled AhLapCBM3 to a co-culture biofilm formed by TRITC-labeled V. cholerae on E. spinifer. The left-hand column of panels shows the brightfield view of the co-culture. The middle column shows the red TRITC fluorescence of V. cholerae colonizing E. spinifer. The right-hand column shows the green GFP fluorescence of bound AhLapCBM3. The top row of cells were incubated in the absence of fucose, and the bottom row of cells in the presence of 50 mM fucose. The nine panels on the right have the same horizontal sequence of brightfield, followed by TRITC staining to visualize V. cholerae, and GFP analysis. In the top sequence, the cells were incubated with GFP-labeled AhLapvWFA. In the middle sequence, the cells were labeled with AhLapRTX, and in the bottom sequence, the cells were mixed with free GFP to check for nonspecific labeling. (B) Here, diatoms in the absence of V. cholerae were incubated with the three GFP-tagged ligand-binding domains as indicated and with the GFP control. Brightfield and fluorescence images are compared side by side. The white scale bar indicates 10 µm.
When GFP-labeled AhLapCBM3 was added to the mix of bacteria and diatoms, its green fluorescence coincided with the locations of the red V. cholerae. Also, the most intense patches of green matched closely to those regions of high bacterial density. When these binding experiments were repeated in the presence of 50 mM fucose, biofilms were still formed, but the binding by GFP-tagged AhLapCBM3 was almost eliminated. These results are consistent with AhLapCBM3 binding to polysaccharides released by V. cholerae during biofilm formation.
AhLapvWFA binds to diatoms and biofilms
The widespread presence of vWFA domains in adhesins from diverse bacterial species, including the plant bacterium Pseudomonas putida (45), suggests that proteins other than those of the mammalian extracellular matrix can be their targets. For this reason, we tested GFP-labeled AhLapvWFA for binding to all the same cells tested with the other two ligand-binding domains. There was no binding of the vWFA domain to the endothelial cells (Fig. 7D), to human erythrocytes (Fig. 6C), or to the two yeasts tested (Fig. 8C and G), but unlike the CBM3 and RTX domains, it did bind directly to the diatom E. spinifer, both in the absence and presence of biofilm-producing V. cholerae (Fig. 9A and B).
DISCUSSION
As recently reported by Sherik et al. (35) and Graham et al. (36), AlphaFold is adept at folding the long chain of domains that make up RTX adhesins and at recognizing the split domains out of which ligand-binding domains protrude. In AhLap, there are a total of 42 domains of which three can be classified as ligand-binding. Expression constructs for these three domains were originally designed and synthesized prior to the advent of AlphaFold by relying on sequence alignments, homology modeling, and guesswork. AlphaFold3 is far superior at predicting the beginning and end of each domain but fortunately, these three constructs were not lacking any portions of domains needed for folding. In addition, the crystal structures for AhLapRTX and AhLapvWFA ligand-binding domains are in excellent agreement with the AlphaFold3 models (Fig. S2A and B).
The three ligand-binding domains of AhLap are markedly different from each other in their structures and range of targets that they adhere to. AhLapCBM3, which has micromolar affinity for fucosylated glycans like the Lewis B and Y antigens, was the most promiscuous binder. It bound to human erythrocytes, a range of endothelial cells, and to yeasts, but not directly to the diatom E. spinifer. However, when V. cholerae was present and had a chance to form biofilm on the diatoms, AhLapCBM3 was able to bind to this surface. The V. cholerae strain used to generate the biofilms has had both of its toxin genes eliminated so they posed no known health risks to the experimenters.
The other two ligand-binding domains in AhLap give this one RTX adhesin additional versatility in binding different targets. Thus, the vWFA domain was able to directly bind to the E. spinifer diatoms, which AhLapCBM3 could not do. This additional interaction leads to biofilm formation, which helps the bacteria’s colonization of its aqueous habitats, protects it from predation by amoebas, and provides a way to evade host defenses during ingestion or breaching of the skin. There were no signs of AhLapvWFA binding to yeasts, human erythrocytes, or endothelial cells. The RTX-like domain is positioned in AhLap as a ligand-binding module emerging from a split domain. It did not bind to any of the microorganisms tested, nor to the erythrocytes, but it did bind in patches to all three human endothelial cell types.
AhLapRTX appears to be a novel ligand-binding domain, and there are as yet no indications of what macromolecules it might adhere to. In a recent survey of 11 RTX adhesins, there is one structural homolog present in an adhesin from V. vulnificus (34). These RTX ligand-binding domains are separate from the larger C-terminal RTX domain adjacent to the Type I secretion signal that has been suggested to initiate the folding of the adhesin during secretion into a Ca2+-rich environment (46). However, there is a report of an extended, segmented C-terminal RTX domain from an RTX toxin being used to bind fibronectin, which shows the potential of this fold to be involved in adhesion (47).
Once ligands are recognized for the different AhLap adhesive domains, it should be possible to develop antagonists to block binding. For example, millimolar concentrations of free fucose were effective in blocking the binding of AhLapCBM3 to all of its targets (erythrocytes, endothelial cells, yeasts, and biofilms). The same strategy was previously used to block the CBMs of V. cholerae and A. veronii from binding and lysing human erythrocytes (35), and the CBM of M. primoryensis from binding the diatom Chaetoceros neogracile (30). In the situation where two or more ligand-binding domains are linked together, as they are in the adhesins attached to their bacterial host, it might be necessary to simultaneously provide antagonists to each of the ligand-binding domains to release their collective grip. An example mentioned previously, the MpIBP adhesin, contains a PBD lying adjacent to the CBM which binds C-terminal tripeptides with the optimal sequence -Tyr-Thr-Asp (31). To release this adhesin section from C. neogracile, it has been necessary to simultaneously provide both fucose and the peptide antagonist (data not shown). V. cholerae and M. primoryensis are both examples where there is a single dominant adhesin at work in the strains examined. Other bacteria, like P. fluorescens, can simultaneously express two adhesins (48). In this situation, blocking bacterial binding will require a thorough analysis of all adhesins at play that might extend the niches and hosts colonized by the bacteria. In a situation where ligands cannot be found for a particular ligand-binding domain, it should be possible to still block adhesion by raising polyclonal antibodies to the domain. This was the case for the ice-binding domain of the M. primoryensis ice adhesin, where an antibody to this domain completely blocked the binding of the bacteria to ice (29).
This structure–function study on AhLap, which is one type of fibrillar adhesin from the pathogen Aeromonas hydrophila, illustrates the complexity of the interactions the bacteria might display in different stages of its life cycle and with the different hosts and surfaces that make up its niche. Bioinformatic strategies outlined here for recognizing RTX adhesins can quickly zero in on these structures in other Gram-negative bacteria, and the recognition of split domains by AI-driven folding programs can reveal the number and types of ligand-binding domains present (36). Identifying their ligands is not simple but offers the possibility of developing low molecular weight antagonists that can block or reduce colonization. However, colonization and infection by the bacteria could also be blocked by immunization through using the ligand-binding domains as antigens. These approaches offer a tailored tactic to combat specific bacterial pathogens that is unlikely to result in resistance and can complement the use of antibiotics as the latter wane in their effectiveness.
MATERIALS AND METHODS
Bioinformatics and protein modeling
The RTX adhesin AhLap sequence (UniProtKB ID: A0KNW4) was identified by database searches as described for RTX adhesins in general (36). AhLap was compared to itself using EMBOSS Dotmatcher (https://www.bioinformatics.nl/cgi-bin/emboss/dotmatcher) to generate a dot matrix analysis to locate repetitive sequences such as the BIg extender domains and the nonapeptide repeats that make up the C-terminal RTX beta-solenoid domain close to the C terminus.
The structure of the adhesin was predicted by AlphaFold3 (49). This allowed the AhLap domain map to be compared alongside the dot matrix analysis. The model for the entire adhesin was rendered in PyMOL V2.5.2 (Schrödinger Inc.) and examined to reveal split domains that indicate associated ligand-binding domains.
Cloning and expression of AhLap ligand-binding domains
DNA sequences encoding the carbohydrate-binding construct (AhLapCBM3) (residues 3455NDAP-----AAEG3940), RTX-beta-roll domain (RTX) (residues 4168AVAD---- VLPG4498), and von Willebrand factor A domain (vWFA) (4497PGQN----NNLP4776) were synthesized by GeneArt (Life Technologies) to allow codon optimization for expression in E. coli. The DNA constructs were ligated into the pET28a expression vector, which provides an N-terminal hexahistidine tag. Positive clones were confirmed by DNA sequencing and transformed into E. coli BL21 (DE3) cells for protein production.
Single colonies of the clones were each picked into 25 mL cultures of LB broth +0.1 mg/mL kanamycin and grown overnight at 37 °C. Overnight cultures were used to inoculate 1 L cultures, which were then grown at 37 °C until an OD600 of 1.0 was reached. Isopropyl β-d-1-thiogalactopyranoside was added to a final concentration of 1 mM to induce protein expression of AhLapCBM3 and AhLapRTX, and 0.4 mM to induce expression of AhLapvWFA, respectively, at 20 °C. The cultures were grown overnight.
Purification of AhLap ligand-binding domains
Induced cells were pelleted in a JS4.2 rotor (Beckman Coulter) at 4,600 × g for 30 min at 4 °C. The cell pellets were resuspended in 50 mL of 50 mM Tris-HCl (pH 9.0), 500 mM NaCl, 2 mM CaCl2, and 0.1 mM phenylmethylsulfonyl fluoride (lysis buffer). Cells were lysed by sonication, and the cell debris was removed through centrifugation in a JA25.5 rotor (Beckman Coulter) at 50,750 × g for 40 min. The supernatant was loaded onto a Ni-NTA affinity chromatographic column (Qiagen) in 50 mM Tris-HCl (pH 9.0), 500 mM NaCl, and 2 mM CaCl2. After washing the column in the same buffer supplemented with 5 mM imidazole, the bound protein was eluted with this buffer containing 400 mM imidazole. Fractions enriched in target proteins were subjected to size-exclusion chromatography on a Superdex 200 Increase 10/300 column (GE Healthcare). For AhLapCBM3, the peak fractions were re-loaded onto a Ni-NTA column for further purification, and the eluted peak was dialyzed against 20 mM Tris-HCl (pH 9.0) overnight at 4 °C. The peak fractions of AhLapRTX from size-exclusion chromatography were dialyzed in 50 mM Tris-HCl (pH 9.0) (buffer A) overnight and subjected to Q-Sepharose ion-exchange chromatography on a High Performance HiLoad 16/10 column (GE Healthcare) to elute proteins with a linear gradient of buffer B containing 50 mM Tris-HCl (pH 9.0) and 1 M NaCl. The purified AhLapRTX was dialyzed against 50 mM NaCl and 20 mM Tris-HCl (pH 7.6), overnight. All three recombinant proteins were checked for purity by SDS-PAGE with Coomassie blue staining before they were concentrated, aliquoted, flash-frozen, and stored at −80 °C until further use.
Protein crystallization
Initial crystallization trials with AhLapRTX and AhLapvWFA were done by sitting-drop vapor-diffusion and microbatch-under-oil methods with various crystallization screen kits (Qiagen) at 295 K. Only conditions that were compatible with millimolar Ca2+ concentrations were used. Thin multi-plate crystals of AhLapRTX (5 mg/mL) grew in precipitant condition of 20% (w/v) PEG 8000, 0.1 M MES (pH 6.0), and 0.2 M calcium acetate. Single crystals of AhLapRTX were obtained by adding 10% v/v of Tacsimat (pH 7.0) and 0.02 M HEPES-NaOH (pH 6.8) from the Silver Bullets library of additives (Hampton Research) to the above crystallization condition. The crystals of AhLap-vWFA (10 mg/mL) grew in 20% PEG 3350 (w/v) and 0.2 M CaCl2. All diffraction-size crystals were flash-frozen in liquid nitrogen with a cryoprotectant that included crystallization solution plus 30% ethylene glycol or 30% glycerol or 40% PEG 400, respectively, for the two different protein crystals. Since there were no homologous structures in the RCSB PDB that corresponded to the amino acid sequence of AhLapRTX, native crystals were soaked in 0.7 mM HoCl3 in its cryoprotectant for heavy atom derivatization for phasing. Crystals were soaked in the solution for approximately 1–10 min before flash freezing in liquid nitrogen.
Structure determination
Single-wavelength calcium anomalous X-ray diffraction data for AhLapvWFA crystals were collected at 100 K on the home source with a wavelength of 2.29 Å using a Rigaku MicroMax-007 HF rotating-anode X-ray generator equipped with a chromium target and an R-AXIS IV++ image-plate detector. Native data for AhLapvWFA crystals were collected at a wavelength of 1.03318 Å on the 23ID-B beam line at the Argonne National Laboratory (Advanced Photon Source, Lemont, USA). For HoCl3-soaked derivatized crystals of AhLapRTX, single-wavelength anomalous diffraction data were collected at a wavelength of 1.5348 Å, and native crystal data sets were collected at a wavelength of 0.9775 Å at 100 K on the CLSID08-1 beamline at the Canadian Light Source (Saskatoon, Canada). All diffraction image datasets from the two proteins were indexed and integrated with XDS (50) and then scaled with AIMLESS in CCP4 (51). AutoSol from the PHENIX suite (52) was used to identify Ca-atom and Ho-atom anomalous substructure sites as well as to calculate phases. After getting phasing solutions, the AutoBuild (53, 54) was then applied to build and refine the initial models. To increase the resolution of the AhLapvWFA structure, the Molecular Replacement method with PHASER in the CCP4 suite (55) was performed with synchrotron data, and a low-resolution structure solved on the home source was used as a model template. Subsequent structure building for the two proteins was done by iterative manual model building using COOT (56) with refinement using PHENIX (57) and REFMAC5 (58). Figures were generated in PyMOL V2.5.2 (Schrödinger Inc.). Crystallographic data collection and refinement statistics are summarized in Tables S1 and S2.
Addition of fluorescent tags to ligand-binding domains and to bacteria
All three ligand-binding domains, AhLapCBM3, AhLapRTX, and AhLapvWFA, were fluorescently tagged by inserting the GFP gene sequence in frame at the 5ʹ-end of their gene sequences. The GFP-tagged proteins were produced and purified as described above for the non-tagged proteins.
V. cholerae classical strain, with both toxin genes deleted, was grown overnight at 37 °C in 5 mL of LB Miller media. Bacteria were pelleted by centrifugation at 10,000 × g for 5 min and resuspended in 10 mL of seawater/F2 media with 10 µL of TRITC from a 10 mg/mL stock (Invitrogen) added for 15 min. Bacteria were pelleted again, and the supernatant was removed and discarded. The cells were resuspended in seawater/F2 media (5 mL) and washed one time by another cycle of pelleting and resuspension as described above.
Glycan array analysis
Mammalian glycan array version 5.4 was screened with AhLapCBM3 and AhLapRTX by the Consortium for Functional Glycomics (Harvard Medical School). This printed glycan array consisted of 585 mammalian glycans that each appeared six times. The array was incubated with GFP-tagged AhLapCBM3 and AhLapRTX at two different concentrations, 5 and 50 µg/mL. The green fluorescence of the fusion protein was used to measure the relative fluorescence units (RFU) of the bound protein. The highest and lowest values from each set of six replicates were discarded to remove outliers, and an average value of the remaining four replicates was used in the comparisons.
Isothermal titration calorimetry
Isothermal titration calorimetry (ITC) measurements for AhLapCBM3 binding Lewis B antigen were done using a MicroCal iTC200 calorimeter (Malvern) set at 30 °C. AhLapCBM3 was dialyzed overnight against 50 mM Tris-HCl (pH 9.0), 200 mM NaCl, and 2 mM CaCl2 and then diluted to different concentrations from 170 to 467 µM in the same buffer. The final thermogram was produced using 370 µM. Lewis B antigen (Biosynth) solution at a concentration of 3.55 mM in the same buffer was added as 5 µL aliquots into the AhLapCBM3 solution (350 µL) at 5 min intervals from a computer-controlled syringe rotating at 400 rpm. After a total of 30 injections, the data were analyzed by Origin V5.0 (Malvern).
ITC measurements for AhLapCBM3 binding free fucose were done using a MicroCal VP-ITC calorimeter (Malvern) set at 30 °C. AhLapCBM3 was dialyzed overnight against 50 mM Tris-HCl (pH 9.0), 200 mM NaCl, and 2 mM CaCl2 and then diluted to different concentrations in the same buffer. The final thermogram was produced using 323 µM. Free fucose (Fisher Scientific, Cat # F006510G) solution at a concentration of 6.5 mM in the same buffer was added as 10 µL aliquots into the AhLapCBM3 solution (1.5 mL) at 5 min intervals from a computer-controlled syringe rotating at 400 rpm. After a total of 30 injections, the data were analyzed by Origin V7.0 (Malvern).
Erythrocyte and endothelial cell binding experiments
Type-O blood was obtained from ZenBio (NC, USA). Erythrocytes were prepared by pelleting cells at 1,000 × g in Krebs–Henseleit solution (KRT) buffer (120 mM NaCl, 5 mM KCl, 1 mM MgSO4, 3 mM CaCl2, 10 mM Tris-HCl, pH 7.4). Erythrocytes were washed an additional three times in KRT buffer and resuspended to 10% (v/v) in KRT buffer. Erythrocyte binding experiments were done using GFP-tagged AhLapCBM3 at a concentration of 9.5 µM. The labeled protein was incubated with 1.4% (v/v) erythrocytes in KRT buffer for 30 min. Erythrocytes were then pelleted by centrifugation for 3 min at 4,500 × g, and the supernatant was discarded. This procedure was repeated three times to wash out unbound GFP-tagged AhLapCBM3 before the erythrocyte pellet was finally resuspended in 20 µL of buffer and examined on slides by fluorescence microscopy. Parallel experiments were performed to test if l-fucose could release GFP-tagged AhLapCBM3 that was already bound to the erythrocytes. Here GFP-tagged AhLapCBM3 was incubated with erythrocytes and 50 mM of l-fucose. The remainder of the experiment followed the same procedure as described above. Images were obtained using an Olympus IX83 inverted fluorescence microscope equipped with an Andor Zyla 4.2 Plus camera.
Red fluorescent protein-expressing HUVECs were obtained from Angio-Proteomie, Boston, MA. THAECs and HPAECs were a generous gift from Dr. Mark Ormiston, Queen’s University. Cells were cultured in Endothelial Growth Medium (EGM-2, Lonza, Switzerland) and were first grown to 80% confluence and then transferred to a 24-well plate with 12 mm circular glass cover at 2 × 104 cells/mL. The cells were maintained in a humidified incubator at 37 °C and 5% CO2. Experiments were conducted after 20 h of growth. Cells were incubated with 0.1 mg/mL of protein for 30 min before washing three times with phosphate-buffered saline (PBS) followed by a 5-min incubation with 2% paraformaldehyde. Fixed cells were washed three times with PBS before staining F-actin for 45 min with TRITC-labelled phalloidin. Cells were washed three more times with PBS then with water before mounting on slides with coverslips and 5 µL of Fluoromount-G with DAPI (Invitrogen). Imaging was performed on an Olympus IX83 inverted fluorescence microscope or a Leica Mica confocal microscope. ImageJ (https://imagej.net/ij) was used to create composite images of the individual channels.
Yeast binding experiments
Fungal strains used in this study are listed in Table S4. Growth and manipulation of C. albicans and S. cerevisiae followed basic procedures for budding yeast cultures. All strains were maintained on YPD plates containing 1% yeast extract (BD-Bacto), 2% peptone (BD-Bacto), and 2% glucose (BioShop). Experimental cultures were inoculated the night before in 10 mL of YPD culture and grown overnight at 30 °C. The overnight culture was used to inoculate fresh YPD for growth to the mid-logarithmic phase the next morning. Prior to performing the experiment, cultures were diluted to 1.0 × 105 cells/mL. Yeast cells in a 1 mL aliquot of the culture were pelleted and resuspended in 1 mL of Tris-buffered saline (TBS; 50 mM Tris-HCl, 150 mM NaCl, pH 7.6) containing AhLapCBM, AhLapvWFA, or AhLapRTX in the presence or absence of 50 mM fucose. The reactions were incubated overnight at room temperature and visualized by microscopy. Following visualization, thioflavin T (Thermo Scientific Chemicals, Cat# 211760050) suspended in TBS was added to each reaction to a final concentration of 100 nM. The samples were incubated for 30 min at 30 °C and visualized by microscopy.
Diatom culturing
The diatom Extubocellulus spinifer was cultured in Gulf of Maine sea water (Bigelow National Center for Algae and Microbiota, East Boothbay, USA) supplemented with Guillard’s (F/2) marine water enrichment solution (Sigma-Aldrich, Cat # G0154) as previously described (34). Diatom cultures were grown at 20 °C shaking at 70 µE/m2⋅s and split 1:1 weekly.
Biofilm formation
V. cholerae classical strain with both cholera toxin genes deleted (a generous gift from Dr. Karl Klose, University of Texas at San Antonio) was cultured in 5 mL LB broth overnight at 37 °C with shaking (34). The culture was pelleted at 4000 × g and the medium was replaced with 1 mL Gulf of Maine sea water supplemented with Guillard’s (F/2) marine water enrichment solution. Diatom cultures (25 mL) were also pelleted at 4,000 × g and resuspended in 1 mL of seawater medium. The diatom suspension (100 µL) and the V. cholerae suspension (100 µL) were mixed and incubated at 20 °C for 2 days. At this time, the top 100 µL of the mixed culture was removed, and 50 µL of 1 mg/mL fluorescently tagged protein was added and incubated for 18 h at 20 °C in the dark. Cultures were pelleted at 1,500 × g for 2 min and rinsed by resuspension in 500 µL of TBS before being pelleted again. This washing procedure was repeated one more time to remove any unbound fluorescently tagged protein. The final pellet was resuspended in 50 µL of TBS and imaged on an Olympus IX83 inverted fluorescence microscope using the 100× oil objective.
ACKNOWLEDGMENTS
The authors would like to thank both Kim Munro in the Protein Function Discovery (PFD) facility and Matthew S. Fishman at Queen’s University for assisting with ITC analyses. The authors also acknowledge glycan array analysis by the Protein-Glycan Interaction Resource of the CFG and the National Center for Functional Glycomics (NCFG) at Beth Israel Deaconess Medical Center, Harvard Medical School (supporting grant R24 GM137763). We are grateful to Karl Klose (University of Texas San Antonio, USA) for Vibrio cholerae strains, to Mark Ormiston (Queen’s University, ON) for supplying telomerase immortalized human aortic endothelial cells and human pulmonary aortic endothelial cells, and to Jocelyn Lee for editorial advice and help with this manuscript.
This work was supported by CIHR Foundation Grant FRN 148422 and NSERC Discovery Grant RGPIN-2022-03845 to P.L.D., who held the Canada Research Chair in Protein Engineering; by CIHR Project Grant PJT-169149 and NSERC Discovery Grant RGPIN-2019-05924 to J.S.A.; and by Canada Foundation for Innovation Award 31967 and a FEAS Excellence in Research Award from Queen’s University to C.E. Part of the research described in this paper was performed using beamline CMCF-08ID at the Canadian Light Source, a national research facility of the University of Saskatchewan, which is supported by the Canada Foundation for Innovation (CFI), the Natural Sciences and Engineering Research Council (NSERC), the National Research Council (NRC), the Canadian Institutes of Health Research (CIHR), the Government of Saskatchewan, and the University of Saskatchewan. Part of this research used resources of the Advanced Photon Source; a U.S. Department of Energy (DOE) Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Contributor Information
Peter L. Davies, Email: daviesp@queensu.ca.
Matthew R. Chapman, University of Michigan-Ann Arbor, Ann Arbor, Michigan, USA
Han Remaut, VIB/Vrije Universiteit Brussel, Brussels, Belgium.
DATA AVAILABILITY
Crystallographic data for the AhLapRTX (PDB ID: 9CSE) and AhLapvWFA (PDB ID: 9DAS) domains have been deposited in the Protein Data Bank. Additional data are available upon request.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.03158-24.
Size-exclusion chromatography of AhLapCBM3.
AlphaFold3 models of AhLap ligand-binding domains overlaid on corresponding crystal structures.
Tables S1 to S4.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Fernández-Bravo A, Figueras MJ. 2020. An update on the genus Aeromonas: taxonomy, epidemiology, and pathogenicity. Microorganisms 8:129. doi: 10.3390/microorganisms8010129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Janda JM, Abbott SL. 2010. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev 23:35–73. doi: 10.1128/CMR.00039-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cai W, Willmon E, Burgos FA, Ray CL, Hanson T, Arias CR. 2019. Biofilm and sediment are major reservoirs of virulent Aeromonas hydrophila (vAh) in catfish production ponds. J Aquat Anim Health 31:112–120. doi: 10.1002/aah.10056 [DOI] [PubMed] [Google Scholar]
- 4. Elhariry HM. 2011. Biofilm formation by Aeromonas hydrophila on green-leafy vegetables: cabbage and lettuce. Foodborne Pathog Dis 8:125–131. doi: 10.1089/fpd.2010.0642 [DOI] [PubMed] [Google Scholar]
- 5. Zepeda-Velazquez AP, Gómez-De-Anda F-R, Aguilar-Mendoza LF, Castrejón-Jiménez NS, Hernández-González JC, Varela-Guerrero JA, de-la-Rosa-Arana J-L, Vega-Sánchez V, Reyes-Rodríguez NE. 2023. Bullfrogs (Lithobates catesbeianus) as a potential source of foodborne disease. J Food Prot 86:100067. doi: 10.1016/j.jfp.2023.100067 [DOI] [PubMed] [Google Scholar]
- 6. Azzopardi EA, Azzopardi SM, Boyce DE, Dickson WA. 2011. Emerging gram-negative infections in burn wounds. J Burn Care Res 32:570–576. doi: 10.1097/BCR.0b013e31822ac7e6 [DOI] [PubMed] [Google Scholar]
- 7. Abdella B, Abozahra NA, Shokrak NM, Mohamed RA, El-Helow ER. 2023. Whole spectrum of Aeromonas hydrophila virulence determinants and the identification of novel SNPs using comparative pathogenomics. Sci Rep 13:7712. doi: 10.1038/s41598-023-34887-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sartor C, Limouzin-Perotti F, Legré R, Casanova D, Bongrand M-C, Sambuc R, Drancourt M. 2002. Nosocomial Infections with Aeromonas hydrophila from Leeches. Clin Infect Dis 35:E1–5. doi: 10.1086/340711 [DOI] [PubMed] [Google Scholar]
- 9. Griffin MJ, Goodwin AE, Merry GE, Liles MR, Williams MA, Ware C, Waldbieser GC. 2013. Rapid quantitative detection of Aeromonas hydrophila strains associated with disease outbreaks in catfish aquaculture. J Vet Diagn Invest 25:473–481. doi: 10.1177/1040638713494210 [DOI] [PubMed] [Google Scholar]
- 10. Maldonado-Miranda JJ, Castillo-Pérez LJ, Ponce-Hernández A, Carranza-Álvarez C. 2022. Chapter 19 - Summary of economic losses due to bacterial pathogens in aquaculture industry, p 399–417. In Dar GH, Bhat RA, Qadri H, Al-Ghamdy KM, Hakeem KR (ed), Bacterial Fish Diseases. Academic Press. [Google Scholar]
- 11. Mzula A, Wambura PN, Mdegela RH, Shirima GM. 2019. Current state of modern biotechnological-based Aeromonas hydrophila vaccines for aquaculture: a systematic review. Biomed Res Int 2019:3768948. doi: 10.1155/2019/3768948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drk S, Puljko A, Dželalija M, Udiković-Kolić N. 2023. Characterization of third generation cephalosporin- and carbapenem-resistant Aeromonas isolates from municipal and hospital wastewater. Antibiotics (Basel) 12:513. doi: 10.3390/antibiotics12030513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kosikowska U, Stec J, Andrzejczuk S, Mendrycka M, Pietras-Ożga D, Stępień-Pyśniak D. 2022. Plasmid-mediated fluoroquinolone resistance genes in quinolone-susceptible Aeromonas spp. phenotypes isolated from recreational surface freshwater reservoir. Front Cell Infect Microbiol 12:885360. doi: 10.3389/fcimb.2022.885360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vezzulli L, Pezzati E, Brettar I, Höfle M, Pruzzo C. 2015. Effects of global warming on Vibrio ecology. Microbiol Spectr 3. doi: 10.1128/microbiolspec.VE-0004-2014 [DOI] [PubMed] [Google Scholar]
- 15. Chowdhury FR, Nur Z, Hassan N, von Seidlein L, Dunachie S. 2017. Pandemics, pathogenicity and changing molecular epidemiology of cholera in the era of global warming. Ann Clin Microbiol Antimicrob 16:10. doi: 10.1186/s12941-017-0185-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Majeed S, De Silva LADS, Kumarage PM, Heo G-J. 2023. Occurrence of potential virulence determinants in Aeromonas spp. isolated from different aquatic environments. J Appl Microbiol 134:lxad031. doi: 10.1093/jambio/lxad031 [DOI] [PubMed] [Google Scholar]
- 17. Fivaz M, Abrami L, Tsitrin Y, van der Goot FG. 2001. Aerolysin from Aeromonas hydrophila and related toxins. Curr Top Microbiol Immunol 257:35–52. doi: 10.1007/978-3-642-56508-3_3 [DOI] [PubMed] [Google Scholar]
- 18. Abrami L, Fivaz M, van der Goot FG. 2000. Surface dynamics of aerolysin on the plasma membrane of living cells. Int J Med Microbiol 290:363–367. doi: 10.1016/S1438-4221(00)80042-9 [DOI] [PubMed] [Google Scholar]
- 19. Buckley JT. 1992. Crossing three membranes. Channel formation by aerolysin. FEBS Lett 307:30–33. doi: 10.1016/0014-5793(92)80896-o [DOI] [PubMed] [Google Scholar]
- 20. Guo S, Garnham CP, Whitney JC, Graham LA, Davies PL. 2012. Re-evaluation of a bacterial antifreeze protein as an adhesin with ice-binding activity. PLoS ONE 7:e48805. doi: 10.1371/journal.pone.0048805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Syed KA, Beyhan S, Correa N, Queen J, Liu J, Peng F, Satchell KJF, Yildiz F, Klose KE. 2009. The Vibrio cholerae flagellar regulatory hierarchy controls expression of virulence factors. J Bacteriol 191:6555–6570. doi: 10.1128/JB.00949-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith TJ, Font ME, Kelly CM, Sondermann H, O’Toole GA. 2018. An N-terminal retention module anchors the giant adhesin LapA of Pseudomonas fluorescens at the cell surface: a novel subfamily of type I secretion systems. J Bacteriol 200:e00734-17. doi: 10.1128/JB.00734-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo S, Langelaan DN, Phippen SW, Smith SP, Voets IK, Davies PL. 2018. Conserved structural features anchor biofilm-associated RTX-adhesins to the outer membrane of bacteria. FEBS J 285:1812–1826. doi: 10.1111/febs.14441 [DOI] [PubMed] [Google Scholar]
- 24. Hinsa SM, Espinosa-Urgel M, Ramos JL, O’Toole GA. 2003. Transition from reversible to irreversible attachment during biofilm formation by Pseudomonas fluorescens WCS365 requires an ABC transporter and a large secreted protein. Mol Microbiol 49:905–918. doi: 10.1046/j.1365-2958.2003.03615.x [DOI] [PubMed] [Google Scholar]
- 25. Boyd CD, Smith TJ, El-Kirat-Chatel S, Newell PD, Dufrêne YF, O’Toole GA. 2014. Structural features of the Pseudomonas fluorescens biofilm adhesin LapA required for LapG-dependent cleavage, biofilm formation, and cell surface localization. J Bacteriol 196:2775–2788. doi: 10.1128/JB.01629-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collins AJ, Smith TJ, Sondermann H, O’Toole GA. 2020. From input to output: the Lap/c-di-GMP biofilm regulatory circuit. Annu Rev Microbiol 74:607–631. doi: 10.1146/annurev-micro-011520-094214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo S, Stevens CA, Vance TDR, Olijve LLC, Graham LA, Campbell RL, Yazdi SR, Escobedo C, Bar-Dolev M, Yashunsky V, Braslavsky I, Langelaan DN, Smith SP, Allingham JS, Voets IK, Davies PL. 2017. Structure of a 1.5-MDa adhesin that binds its Antarctic bacterium to diatoms and ice. Sci Adv 3:e1701440. doi: 10.1126/sciadv.1701440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garnham CP, Campbell RL, Davies PL. 2011. Anchored clathrate waters bind antifreeze proteins to ice. Proc Natl Acad Sci U S A 108:7363–7367. doi: 10.1073/pnas.1100429108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bar Dolev M, Bernheim R, Guo S, Davies PL, Braslavsky I. 2016. Putting life on ice: bacteria that bind to frozen water. J R Soc Interface 13:20160210. doi: 10.1098/rsif.2016.0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guo S, Vance TDR, Zahiri H, Eves R, Stevens C, Hehemann JH, Vidal-Melgosa S, Davies PL. 2021. Structural basis of ligand selectivity by a bacterial adhesin lectin involved in multispecies biofilm formation. MBio 12:e00130-21. doi: 10.1128/mBio.00130-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo S, Zahiri H, Stevens C, Spaanderman DC, Milroy LG, Ottmann C, Brunsveld L, Voets IK, Davies PL. 2021. Molecular basis for inhibition of adhesin-mediated bacterial-host interactions through a peptide-binding domain. Cell Rep 37:110002. doi: 10.1016/j.celrep.2021.110002 [DOI] [PubMed] [Google Scholar]
- 32. Petosa C, Collier RJ, Klimpel KR, Leppla SH, Liddington RC. 1997. Crystal structure of the anthrax toxin protective antigen. Nature New Biol 385:833–838. doi: 10.1038/385833a0 [DOI] [PubMed] [Google Scholar]
- 33. Rigden DJ, Mello LV, Galperin MY. 2004. The PA14 domain, a conserved all-beta domain in bacterial toxins, enzymes, adhesins and signaling molecules. Trends Biochem Sci 29:335–339. doi: 10.1016/j.tibs.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 34. Lloyd CJ, Guo S, Kinrade B, Zahiri H, Eves R, Ali SK, Yildiz F, Voets IK, Davies PL, Klose KE. 2023. A peptide-binding domain shared with an Antarctic bacterium facilitates Vibrio cholerae human cell binding and intestinal colonization. Proc Natl Acad Sci USA 120:e2308238120. doi: 10.1073/pnas.2308238120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sherik M, Eves R, Guo S, Lloyd CJ, Klose KE, Davies PL. 2024. Sugar-binding and split domain combinations in repeats-in-toxin adhesins from Vibrio cholerae and Aeromonas veronii mediate cell-surface recognition and hemolytic activities. MBio. doi: 10.1128/mbio.02291-23:e0229123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Graham LA, Hansen T, Yang Y, Sherik M, Ye Q, Soares BP, Kinrade B, Guo S, Davies PL. 2024. Adhesin domains responsible for binding bacteria to surfaces they colonize project outwards from companion split domains. Proteins 92:933–945. doi: 10.1002/prot.26689 [DOI] [PubMed] [Google Scholar]
- 37. Madeira F, Pearce M, Tivey ARN, Basutkar P, Lee J, Edbali O, Madhusoodanan N, Kolesnikov A, Lopez R. 2022. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res 50:W276–W279. doi: 10.1093/nar/gkac240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vance TDR, Guo S, Assaie-Ardakany S, Conroy B, Davies PL. 2019. Structure and functional analysis of a bacterial adhesin sugar-binding domain. PLoS One 14:e0220045. doi: 10.1371/journal.pone.0220045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo S, Campbell R, Davies PL, Allingham JS. 2019. Phasing with calcium at home. Acta Crystallogr F Struct Biol Commun 75:377–384. doi: 10.1107/S2053230X19004151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krissinel E, Henrick K. 2007. Inference of macromolecular assemblies from crystalline state. J Mol Biol 372:774–797. doi: 10.1016/j.jmb.2007.05.022 [DOI] [PubMed] [Google Scholar]
- 41. Whittaker CA, Hynes RO. 2002. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol Biol Cell 13:3369–3387. doi: 10.1091/mbc.e02-05-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deckmyn H, Vanhoorelbeke K. 2006. When collagen meets VWF. Blood 108:3628–3628. doi: 10.1182/blood-2006-09-046540 [DOI] [Google Scholar]
- 43. Ramsook CB, Tan C, Garcia MC, Fung R, Soybelman G, Henry R, Litewka A, O’Meally S, Otoo HN, Khalaf RA, Dranginis AM, Gaur NK, Klotz SA, Rauceo JM, Jue CK, Lipke PN. 2010. Yeast cell adhesion molecules have functional amyloid-forming sequences. Eukaryot Cell 9:393–404. doi: 10.1128/EC.00068-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Conner JG, Teschler JK, Jones CJ, Yildiz FH. 2016. Staying alive: Vibrio cholerae's cycle of environmental survival, transmission, and dissemination. Microbiol Spectr 4. doi: 10.1128/microbiolspec.VMBF-0015-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guo S, Vance TDR, Stevens CA, Voets IK, Davies PL. 2019. RTX adhesins are key bacterial surface megaproteins in the formation of biofilms. Trends Microbiol 27:453–467. doi: 10.1016/j.tim.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 46. Bumba L, Masin J, Macek P, Wald T, Motlova L, Bibova I, Klimova N, Bednarova L, Veverka V, Kachala M, Svergun DI, Barinka C, Sebo P. 2016. Calcium-driven folding of RTX domain β-rolls ratchets translocation of RTX proteins through type I secretion ducts. Mol Cell 62:47–62. doi: 10.1016/j.molcel.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 47. Goldsmith JA, DiVenere AM, Maynard JA, McLellan JS. 2021. Structural basis for antibody binding to adenylate cyclase toxin reveals RTX linkers as neutralization-sensitive epitopes. PLoS Pathog 17:e1009920. doi: 10.1371/journal.ppat.1009920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Collins AJ, Pastora AB, Smith TJ, O’Toole GA. 2020. MapA, a second large RTX adhesin conserved across the pseudomonads, contributes to biofilm formation by Pseudomonas fluorescens. J Bacteriol 202:e00277-20. doi: 10.1128/JB.00277-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abramson J, Adler J, Dunger J, Evans R, Green T, Pritzel A, Ronneberger O, Willmore L, Ballard AJ, Bambrick J, et al. 2024. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature New Biol 630:493–500. doi: 10.1038/s41586-024-07487-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kabsch W. 2010. XDS. Acta Crystallogr D Biol Crystallogr 66:125–132. doi: 10.1107/S0907444909047337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Evans P. 2006. Scaling and assessment of data quality. Acta Crystallogr D Biol Crystallogr 62:72–82. doi: 10.1107/S0907444905036693 [DOI] [PubMed] [Google Scholar]
- 52. Terwilliger TC, Adams PD, Read RJ, McCoy AJ, Moriarty NW, Grosse-Kunstleve RW, Afonine PV, Zwart PH, Hung LW. 2009. Decision-making in structure solution using Bayesian estimates of map quality: the PHENIX AutoSol wizard. Acta Crystallogr D Biol Crystallogr 65:582–601. doi: 10.1107/S0907444909012098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Terwilliger TC, Grosse-Kunstleve RW, Afonine PV, Moriarty NW, Zwart PH, Hung LW, Read RJ, Adams PD. 2008. Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard. Acta Crystallogr D Biol Crystallogr 64:61–69. doi: 10.1107/S090744490705024X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66:213–221. doi: 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. 2007. Phaser crystallographic software. J Appl Crystallogr 40:658–674. doi: 10.1107/S0021889807021206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60:2126–2132. doi: 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 57. Afonine PV, Grosse-Kunstleve RW, Chen VB, Headd JJ, Moriarty NW, Richardson JS, Richardson DC, Urzhumtsev A, Zwart PH, Adams PD. 2010. Phenix.model_vs_data: a high-level tool for the calculation of crystallographic model and data statistics. J Appl Crystallogr 43:669–676. doi: 10.1107/S0021889810015608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Murshudov GN, Skubák P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin AA. 2011. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr 67:355–367. doi: 10.1107/S0907444911001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Size-exclusion chromatography of AhLapCBM3.
AlphaFold3 models of AhLap ligand-binding domains overlaid on corresponding crystal structures.
Tables S1 to S4.
Data Availability Statement
Crystallographic data for the AhLapRTX (PDB ID: 9CSE) and AhLapvWFA (PDB ID: 9DAS) domains have been deposited in the Protein Data Bank. Additional data are available upon request.