ABSTRACT
Elderly patients with locally advanced head and neck squamous cell carcinoma (LA-HNSCC) are often excluded from clinical trials. However, in an aging population with competing comorbidities, many are ineligible for standard of care cisplatin-based chemoradiotherapy and represent an unmet need for which effective treatments are urgently needed. NBTXR3, a first-in-class intratumoral radioenhancer made of hafnium oxide nanoparticles, enhances the tumor-killing effect of radiotherapy. In this paper, we describe the study design of NANORAY-312, a global, open-label, randomized (1:1) phase III pivotal study comparing NBTXR3 + radiotherapy ± cetuximab (Investigator’s choice) versus radiotherapy ± cetuximab (Investigator’s choice) in 500 eligible patients, aged ≥60 years, with platinum-ineligible LA-HNSCC. The primary endpoint is progression-free survival, with a key secondary endpoint being overall survival.
Clinical trial registration
https://clinicaltrials.gov identifier is NCT04892173; EudraCT Number: 2021-002163-22.
KEYWORDS: NBTXR3, radiotherapy, cetuximab, H&N, LA-HNSCC, cisplatin-ineligible, radioenhancer, hafnium oxide nanoparticles
Plain Language Summary
Locally advanced head and neck cancer is a type of cancer that starts in the tissues of the head or neck region and has spread to nearby areas. Older patients with this cancer often have other health problems or may be frail, which can make it harder for them to tolerate the usual treatment of cisplatin (an anti-cancer drug) combined with radiation. This can make it difficult for some older patients to receive the standard treatment, so there is a need for other treatment options.
NBTXR3 is a new type of treatment that helps improve how radiation therapy works. It is a special kind of nanoparticle that is injected into cancer cells. When radiation is given, these nanoparticles help the radiation kill more cancer cells and slow down cancer growth. Research suggests NBTXR3 also helps the immune system recognize and attack cancer cells more effectively and that NBTXR3 can make cancer treatment more effective, with better results for patients.
NANORAY-312 is a study looking at how NBTXR3 works when used with radiation (and some patients may also receive cetuximab, a drug that helps the immune system fight cancer) in treating older patients with locally advanced head and neck cancer. The study is testing whether this treatment can be a good alternative for those who cannot receive cisplatin. The study is ongoing, and its main goal is to see whether patients who receive NBTXR3 will live longer than those who will not receive the treatment.
1. Introduction
1.1. Background and rationale
Globally, head and neck cancers are the seventh most common cancer [1]. Approximately 90% of head and neck cancers are squamous cell carcinomas (HNSCC), with around 350,000 deaths worldwide per year occurring due to HNSCC [1–3]. Key risk factors include tobacco and alcohol use, human papillomavirus (HPV) infection, and in some regions, the use of areca nut in betel quid chewing [2,4,5].
Around 60% of patients with HNSCC are diagnosed with locally advanced HNSCC (LA-HNSCC) [1]. Establishing locoregional control of HNSCC is crucial to prevent both the progression of the disease and to protect the organs in this anatomically sensitive region that play a vital role in functions such as speaking, swallowing, breathing, and cranial nerve function [6]. Because of these vital functions, locoregional progression is often the primary cause of morbidity and mortality in patients with HNSCC, even for those patients with distant metastases [6].
The European Head and Neck Society (EHNS), the European Society for Medical Oncology (ESMO), the European Society for Radiotherapy & Oncology (ESTRO) (EHNS-ESMO-ESTRO), and the National Comprehensive Cancer Network (NCCN) guidelines recommend a multimodality approach for treating LA-HNSCC. This approach may include surgery, radiotherapy (RT), chemotherapy, or targeted therapy, depending on the patient’s health and tumor characteristics [1–3]. RT is crucial in the treatment of LA-HNSCC [2,3], but one of its limitations is its potential to damage healthy tissues adjacent to the tumor, which is especially concerning when tumors are close to vital anatomical structures [2,3,6–8]. Chemoradiotherapy is a standard treatment for LA-HNSCC and can improve tumor control and overall survival (OS) [1–3].
Cisplatin-based chemoradiotherapy is recommended as the first-line treatment for LA-HNSCC [2,3]. Studies with high-dose cisplatin-based chemoradiotherapy have demonstrated improved patient outcomes compared to RT alone (Table 1) [2,3,9–11]. In the SENIOR study, an international cohort study of patients ≥65 years with HNSCC receiving definitive chemoradiation, those who received cumulative cisplatin doses ≥200 mg/m2 had significantly higher OS (p = 0.02), increased progression-free survival (PFS) (p = 0.003), and lower locoregional failures (p = 0.004) compared to those receiving <200 mg/m2 [12]. However, most patients who received cisplatin or a cisplatin-containing regimen did not reach the prognostically critical threshold of 200 mg/m2, the median cumulative cisplatin dose was 180 mg/m2 [12]. The SENIOR study authors suggested that single-agent cisplatin be considered as the standard for older patients with good performance status (PS) who are cisplatin-eligible, while better options are needed for frail or cisplatin-ineligible patients [12]. The use of cisplatin-based chemoradiotherapy may be restricted in certain patients [1,3,13,14], especially those with absolute contraindications (e.g., renal disfunction, hearing loss, etc.), or relative contraindications (e.g., PS, advanced age, comorbidities, etc.) [1,13,15,16]. The factors limiting cisplatin eligibility, particularly older patients, are shown in Table 2 [1,13,14,16,17]. Elderly patients represent a large and growing portion of the LA-HNSCC patient population, currently about one-third of LA-HNSCC patients are ≥70 years old, and this is projected to increase to around 64% over the next 20 years [1,16]. It is estimated that by 2030, 70% of all cancers will be diagnosed in patients ≥65 years [18]. Treating older patients with LA-HNSCC presents challenges as diagnosis may be delayed due to atypical symptoms or comorbidities, and participation in clinical trials may be limited due to an upper age restriction or other eligibility criteria that may exclude a large portion of older patients from clinical trials [16,19–22]. Patients ≥65 years generally have worse outcomes than younger patients, which is in part due to suboptimal treatment [1,13,16,18]. Furthermore, cisplatin-ineligible patients with LA-HNSCC usually have more comorbidities and are likely to have a shorter OS compared with the cisplatin-eligible population [1,16]. Including these patients in clinical trials is crucial to generate evidence-based data that can enhance their care and outcomes [1,19].
Table 1.
Selection of prospective randomized clinical trials with either high-dose cisplatin-based radiotherapy or cetuximab-based radiotherapy in patients with LA-HNSCC.
| Author (Study) | Study phase | Study arms | No. of pts | Primary endpoint | LRC rate | mPFS | mOS | ≥Grade 3 toxicities |
|---|---|---|---|---|---|---|---|---|
| RT vs high dose cisplatin + RT: | ||||||||
| Adelstein et al. [9] (Intergroup study) |
Ph III* | Standard RT† vs Standard RT + cisplatin (100 mg/m2)‡ vs Daily RT + 5-FU (1000 mg/m2) + cisplatin (75 mg/m2)∫ |
295 | Survival | – | – | 3-yr projected OS: 23% vs 37% (p = 0.014) vs 27% (p=NS) |
52% vs 89% (p <0.0001) vs 77% (p <0.001) |
| High dose cisplatin + RT: | ||||||||
| Noronha et al. [11] (CTRI/2012/10/003062) |
Ph III | Q3W cisplatin (100 mg/m2) vs QW cisplatin (30 mg/m2)** |
150 150 |
LRC | 2-yr LRC rate: 73.1% vs 58.5% (p = 0.014) |
28.6 mo. vs 17.7 mo. (p = 0.21) |
NR vs 39.5 mo. (p = 0.48) |
Acute: 84.6% vs 71.6% (p = 0.006) |
| RT vs RT + cetuximab: | ||||||||
| Bonner et al. [24,25] (NCT00004227) |
Ph III | High-dose RT vs High-dose RT + weekly cetuximab†† |
213 211 |
Duration of LRC | 14.9 mo. vs 24.2 mo. (p = 0.005) |
12.4 mo. vs 17.1 mo. (p = 0.006) |
29.3 mo. vs 49.0 mo. (p = 0.018) |
Similar in the 2 groups‡‡ |
| RT + cetuximab + CT vs RT + cetuximab or RT + cisplatin: | ||||||||
| Ang et al. [26] (RTOG 0522) |
Ph III∫∫ | RT + cetuximab†† + cisplatin (100 mg/m2) vs RT + cisplatin (100 mg/m2) |
444 447 |
PFS failure*** | 3-yr LRF: 25.9% vs 19.9% (p = 0.97) |
3-yr PFS: 58.9% vs 61.2% (p = 0.76) |
3-yr OS: 75.8% vs 72.9% (p = 0.32) |
Acute: 89% vs 87% (p = 0.61) |
| Tao et al. [27] (GORTEC 2007–01) |
Ph III | RT + cetuximab†† + CT††† vs RT + cetuximab†† |
204 201 |
PFS | 3-yr LRF: 21.6% vs 38.8% (p<0.001) |
3-yr PFS: 52.3% vs 40.5% (p = 0.015) mPFS: 37.9 mo. vs 22.4 mo. |
3-yr OS: 60.8% vs 54.9% (p = 0.11) mOS: 53.4 mo. vs 44.5 mo. |
91% vs 91% |
*Study prematurely stopped as it did not meet the accrual goal of 362 patients. †Standard RT: single daily fractionated RT at 2.0 Gy/d (70 Gy in total). ‡Cisplatin (concurrent bolus injection) on days 1, 22, and 43. ∫Split course of single daily fractionated RT (30 Gy in first cycle and 30–40 Gy in third cycle) + three cycles of concurrent 5-FU (continuous infusion over 4d) + bolus cisplatin (Q4W x 3). **93% of patients received CRT in the adjuvant setting. ††Weekly cetuximab: initial dose of 400 mg/m2 followed by 250 mg/m2 for the duration of RT. ‡‡Except for acneiform rash and infusion-related events which were higher with the cetuximab regimen. ∫∫Stage III or IV HNSCC. ***Defined as LRF/progression, distant metastasis, or death. †††Concurrent CT: 3 cycles of carboplatin (70 mg/m2/d on days 1 to 4) + 5-FU (600 mg/m2/d on days 1 to 4) with continuous infusions.
CRT, chemoradiotherapy; CT, chemotherapy; d, day; 5-FU, fluorouracil; HNSCC, head and neck squamous cell carcinoma; LA, locally advanced; LRC, locoregional control; LRF; locoregional failure; m, median; mo., months; No., number; NR, not reached; NS, not significant; OS, overall survival; PFS, progression-free survival; Ph, phase; pts, patients; RT, radiotherapy; QW, once-a-week; Q3W, once-every-3-weeks; Q4W, once-every-4-weeks; vs, versus.
Table 2.
Potential cisplatin-ineligibility factors for patients with LA-HNSCC.
| Factors may include [1,13,14,16,17]: | |
|---|---|
| Absolute contraindications: | |
| (i) | Renal dysfunction, such as creatinine clearance < 50–60 mL/min (cisplatin is known to be nephrotoxic it may exacerbate existing renal impairment or compromised kidney function which could lead to severe complications) |
| (ii) | Hearing loss or neurotoxicity, such as grade ≥ 2 tinnitus or preexisting hearing loss or grade ≥ 2 neuropathy (cisplatin can cause ototoxicity and neurotoxicity, and cisplatin-based therapy should be avoided in these patients to prevent further deterioration) |
| (iii) | Allergic reactions or hypersensitivity to cisplatin or platinum-based therapies |
| (iv) | Pregnant or lactating patients |
| (v) | Patients with HIV/AIDs and a CD4 count <200 μL |
| Relative contraindications: | |
| vi) | Advanced age, such as ≥70 years (older patients may have more comorbidities, or increasing hearing loss, with the potential for an increased risk of complications from cisplatin treatment) |
| (vii) | A poor PS, such as an ECOG PS score ≥ 2 (cisplatin-based treatment can be taxing, patients with a compromised PS may not be able to withstand treatment and may be at higher risk for non-adherence and/or adverse events) |
| (viii) | Poor nutritional status (cisplatin may cause acute gastrointestinal symptoms such as nausea, vomiting and diarrhea, and it may further exacerbate the poor nutritional status)
|
| (ix) | Comorbidities (e.g., severe bone marrow, cardiovascular, respiratory, metabolic, hepatic dysfunction), which may include one or more of the following assessments:
|
ACE, Adult Comorbidity Evaluation; AIDs, acquired immunodeficiency syndrome; BMI, body mass index; CARG, Cancer and Aging Research Group; CIRS-G, Cumulative Illness Rating Scale for Geriatrics; ECOG, Eastern Cooperative Oncology Group; G-8, Geriatric 8; HIV, human immunodeficiency virus; HNCIG, Head and Neck Cancer Intergroup; HNSCC, head and neck squamous cell carcinoma; LA, locally advanced; mCCI, Modified Charlson Comorbidity Index; PS, performance status.
For cisplatin-ineligible patients with LA-HNSCC, cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, and docetaxel are approved alternative therapies [1,3,13,23]. The Bonner et al. trial demonstrated that adding cetuximab to RT significantly improved locoregional control (p = 0.005), PFS (p = 0.006), and OS (p = 0.018) compared to RT alone in patients with LA-HNSCC (Table 1) [1,3,24,25]. The RTOG 0522 study evaluated RT + cetuximab + cisplatin versus RT + cisplatin, and the GORTEC 2007–01 study evaluated RT + cetuximab + carboplatin + fluorouracil versus RT + cetuximab; however, the addition of cisplatin or a platinum containing regimen to RT + cetuximab did not significantly improve OS (p = 0.32 and p = 0.11, respectively) (Table 1) [3,26–29]. In the recent NRG-HN004 trial comparing RT + cetuximab with RT + durvalumab in cisplatin-ineligible patients with locoregionally advanced HNSCC (most ≥70 years), the 2-year PFS was 63.7% (95% CI 51.3–76.1) versus 50.6% (95% CI 41.5–59.8), respectively (p = 0.89), and the authors concluded that RT + durvalumab did not improve outcomes over RT + cetuximab, highlighting the need for alternative radiosensitizing strategies [30].
NBTXR3, a first-in-class intratumoral radioenhancer, was designed to enhance the tumor-killing effects of RT [8,31–34]. Comprised of functionalized hafnium oxide nanoparticles, NBTXR3 is injected intratumorally under local or general anesthesia and remains inert until exposed to ionizing radiation [8,31–33,35,36]. When activated by RT, these nanoparticles absorb X-rays and release a large amount of high-energy electrons, causing an increased production of reactive oxygen species (ROS) (i.e., oxidative stress) that enhance cytotoxicity [8,33,35]. NBTXR3 intensifies the energy deposited inside tumor cells during RT, enhancing tumor cell death compared to RT alone, without additional harm to adjacent non-injected healthy tissues [8,31–37]. Efficacy and safety were demonstrated in a phase II/III clinical trial (Act.In.Sarc/NCT02379845) involving patients with locally advanced soft tissue sarcoma (LA-STS), the results showed a significant increase in the rate of pathological complete response in the NBTXR3 followed by RT group compared with the RT alone group (16% versus 8%, p = 0.044) [33,37].
In a phase I dose escalation/expansion study (NCT01946867) in cisplatin-ineligible elderly patients with LA-HNSCC, the feasibility of the NBTXR3 injection followed by RT was shown with no unexpected toxicities, and promising initial antitumor activity was observed with a prolonged median PFS (11.4 months) and median OS (18.1 months) in the all treated population [32,33,38]. Furthermore, the patient population in this study had several negative prognostic factors, including the majority of patients having a high burden of comorbidities as 67% of patients had an Age-adjusted modified Charlson Comorbidity Index (ACCI) ≥4 [38], which has been shown to correlate with a worse survival for patients with head and neck cancer [32,38,39]. Taken together, these promising efficacy results observed in patients with LA-HNSCC with a poor prognosis warrant further evaluations in larger trials.
Other nanoparticles, including gold, silver, and gadolinium, have been evaluated in preclinical studies using irradiated head and neck cancer cell models, demonstrating enhanced tumor destruction compared with RT alone [40]. However, NBTXR3 is currently the only nanoparticle being investigated in clinical trials with RT in HNSCC [40]. In addition to the radioenhancing effects of NBTXR3, NBTXR3 potentially possesses immunomodulatory properties [41–43]. In anti-programmed cell death 1 (PD1)-resistant lung cancer murine models, NBTXR3 enhanced the immune responses, and when NBTXR3 + RT was combined with anti-PD1 and other checkpoint inhibitors, such as anti-CTLA4, a synergistic antitumor immunomodulatory effect was observed [41,43]. A phase I clinical trial evaluating NBTXR3 followed by RT and anti-PD-1 in locoregionally recurrent or metastatic HNSCC (NCT03589339) has shown promising early efficacy results [44]. A phase II clinical trial is ongoing evaluating NBTXR3 followed by radiotherapy and pembrolizumab in recurrent or metastatic HNSCC (NCT04862455) [45].
Based on the preclinical and clinical results with NBTXR3, a pivotal phase III study is being conducted comparing NBTXR3 activated by Investigator’s choice of RT alone or RT with cetuximab (NBTXR3/RT ± cetuximab) versus Investigator’s choice of RT alone or RT with cetuximab (RT ± cetuximab) for patients with platinum-ineligible LA-HNSCC (NANORAY-312, NCT04892173) [31]. Radiopaque visualization will also be used in this study to monitor the presence of NBTXR3 inside or outside of the tumor.
1.2. Objectives
The NANORAY-312 study hypothesis is that patients treated with NBTXR3 activated RT ± cetuximab will have longer survival outcomes (i.e., PFS and OS) compared with patients treated with RT ± cetuximab.
The primary objective of NANORAY-312 is to evaluate PFS with a key secondary objective of overall survival. Other secondary objectives include locoregional control, distant control, head and neck cancer specific event-free survival outcomes, safety and tolerability, patient reported outcomes (PROs), and health-related quality of life (HRQoL). Table 3 provides a detailed list of the study objectives and endpoints.
Table 3.
NANORAY-312 study objectives and endpoints of NBTXR3/RT ± cetuximab versus RT ± cetuximab.
| Primary objective | Primary endpoint |
|---|---|
| To evaluate survival outcomes (PFS) | PFS: time from randomization to locoregional recurrence, locoregional progression, distant progression, or death from any cause, whichever occurs first |
| Key secondary objective | Key secondary endpoint |
| To evaluate long-term survival (OS) | OS defined as time from randomization to death from any cause |
| Other secondary objective | Other secondary endpoint |
| To evaluate locoregional control | Time to locoregional progression: time from randomization to locoregional progression or death, whichever occurs first |
| To evaluate distant control | Time to distant progression: time from randomization to distant progression or death whichever occurs first |
| To evaluate H&N cancer specific event-free survival outcomes | H&N cancer-specific event-free survival: time from randomization to locoregional recurrence, locoregional progression, distant progression, or H&N cancer-related death, as per RECIST 1.1, whichever occurs first |
| To evaluate long-term H&N cancer-specific survival outcomes | H&N cancer-specific survival: time from randomization to H&N cancer-related death |
| To evaluate safety and tolerability | AEs, safety laboratory parameters, vital signs, ECOG PS, and physical examinations |
| To assess PROs including symptoms, function, and HRQoL | Change from baseline over time in symptoms, function, and HRQoL using the EORTC QLQ-H&N35 and the EQ-5D-5 L instrument |
| To evaluate the tumor response |
|
AEs, adverse events; CR, complete response; DoR, duration of response; ECOG, Eastern Cooperative Oncology Group; EORTC, European Organization for Research and Treatment of Cancer; EQ-5D-5 L, 5-level EuroQol 5-dimension; GY, gray; H&N, head and neck; HRQoL, health-related quality of life; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PRO, patient-reported outcome; PR, partial response; PS, performance status; QLQ-H&N35, Quality of Life Questionnaire – Head and Neck Cancer Module; RECIST, Response Evaluation Criteria In Solid Tumors; RT, radiotherapy.
1.3. Trial design
NANORAY-312 is a global, open-label, randomized, 2-arm, phase III registrational study to investigate the efficacy and safety of NBTXR3/RT ± cetuximab (Arm A) versus RT ± cetuximab (Arm B) in treatment-naïve, platinum-ineligible, elderly patients with LA-HNSCC. Patients are randomized in a 1:1 ratio to either treatment arm. The study is designed to assess the superiority of Arm A over Arm B, and an interim analysis of PFS superiority will be performed.
2. Methods: participants, interventions, and outcomes
2.1. Study setting
This trial is being conducted globally in hospitals and medical centers in over 30 countries, recruiting patients in North America, Europe, and the Asia-Pacific region. Figure 1 shows an overview of the countries involved, and a list of the currently enrolled study sites can be found at https://clinicaltrials.gov/ct2/show/study/NCT04892173.
Figure 1.
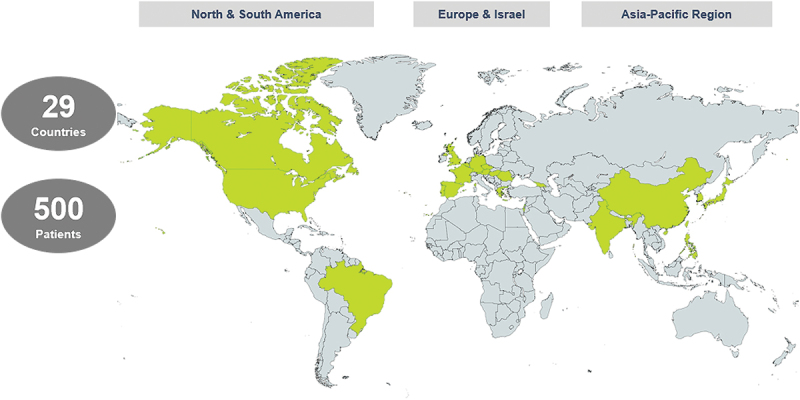
NANORAY-312 planned global participation.
2.2. Eligibility criteria
Eligible patients are ≥60 years, biopsy-confirmed SCC of the oral cavity, oropharynx, hypopharynx, or supraglottic larynx, with tumor categories of T3–4 any N or T2 if ≥N2 (as per the American Joint Committee on Cancer [AJCC] tumor-node-metastasis [TNM] staging classification of malignant tumors, 8th Edition [AJCC TNM v8]). For patients with oropharyngeal cancer, HPV status must be known. Patients must have one primary tumor amenable for intratumoral injection, must be ineligible for platinum-based chemotherapy (Table 4), and amenable to definitive treatment with RT, with an Eastern Cooperative Oncology Group (ECOG) PS ≤ 2 and adequate organ and bone marrow function. Involved lymph nodes that are amenable for intranodal injection should be ≥1 cm in the longest dimension and have <180-degree encasement of the carotid artery on magnetic resonance imaging (MRI) or computed tomography (CT) scan. Further details on inclusion and exclusion criteria are provided in Table 4.
Table 4.
Inclusion and exclusion criteria for NANORAY-312.
| Inclusion criteria |
Exclusion criteria |
||
| 1 | Signed ICF | 1 | HNSCC categories T1, T2 N0, T2 N1, or M1 according to the 8th Edition of the AJCC Cancer Staging Manual |
| 2 | Men and women aged ≥60 years | 2 | Has received prior antineoplastic systemic therapy or intervention (including pharmacological – both marketed and investigational, RT, or surgery) for the treatment of HNSCC |
| 3 | Biopsy confirmed SCC of the oral cavity, oropharynx, hypopharynx, or supraglottic larynx (archived biopsies are allowed); if no biopsies are available, a new biopsy must be obtained to provide confirmation of SCC | 3 | Patients with known severe Grade 3 or 4 hypersensitivity reactions to cetuximab and patients with known prior or ongoing interstitial lung disease to be excluded as a candidate for cetuximab treatment as per the Investigator’s choice before randomization† |
| 4 | For participants with oropharyngeal cancer, HPV status must be known | 4 | Known history of HIV. Chronically ongoing active hepatitis B or C infection as defined in AASLD/EASL guidelines |
| 5 | Tumor categories T3–T4 any N or T2, if ≥N2 according to the 8th Edition of the AJCC Cancer Staging Manual | 5 | Locoregionally recurrent HNSCC that has been previously treated with surgery, chemotherapy, and/or RT are not eligible for the study |
| 6 | Has one primary tumor lesion that is amenable for IT injection, as determined by the Investigator | 6 | Ulceration or other characteristics (e.g., bleeding diathesis) that may, in the opinion of the Investigator, increase the risk of severe tumor bleeding |
| 7 | Ineligible to receive platinum based chemotherapy for the treatment of LA-HNSCC as defined by having at least one of the following:
|
7 | SCC originating in the nasopharynx, paranasal sinus, salivary gland, or thyroid gland; or non-squamous histology (e.g., melanoma or neuroendocrine carcinoma), or SCC of unknown primary origin |
| 8 | Must be able to tolerate RT with curative intent as determined by the study Investigator. | 8 | Prior or concurrent malignancy (including a second synchronous HNSCC) whose natural history or treatment has the potential to interfere with the safety or efficacy assessment of the investigational regimen |
| 9 | Amenable to definitive treatment with RT. For patients with an oral cavity cancer, the decision for definitive treatment with RT requires consultation with the H&N surgeon and the site’s MDTB | 9 | Clinically significant cardiac arrhythmias (e.g., ventricular tachycardia, ventricular fibrillation, torsades de pointes, second- or third-degree atrioventricular heart block without a permanent pacemaker in place) |
| 10 | ECOG PS ≤ 2 | 10 | Class IV congestive heart failure as defined by the NYHA functional classification system <6 months prior to screening |
| 11 | Adequate organ and bone marrow function at screening* | 11 | A pregnant or nursing woman, or women of childbearing potential and men who are sexually active and not willing/able to use medically acceptable forms of contraception starting from the time that the ICF is signed through 150 days (18 months for South Korean subjects) after the last cetuximab dose/RT fraction. A woman who is ≥1 year postmenopausal or who is surgically sterile is not considered to be of childbearing potential (pregnancy test is not required) |
| 12 | Life expectancy ≥6 months | 12 | Any condition for that, in the opinion of the Investigator, participation would not be in the best interest of the individual (e.g., compromises the patient’s well-being) or that could prevent, limit, or confound the protocol/CIP-specified assessments, including patients under legal protection |
| 13 | Patients participating in another clinical study, except for a non-interventional trial/registry, at the time of signing the ICF | ||
| 14 | Ongoing or active bacterial or fungal infection (includes infection requiring treatment with antimicrobial therapy for which participants will be required to complete 2 weeks before randomization), symptomatic viral infection, any other clinically significant infection, or use of immune suppressive agents | ||
†These patients can still be eligible for the study, only if RT alone or NBTXR3 + RT alone is chosen and documented by the Investigator before randomization. *Adequate organ and bone marrow function at screening, define as: (i) hemoglobin >9.0 g/dL; (ii) platelet count > 100,000 cells/mm3; (iii) leukocytes > 3000 cells/mm3; (iv) absolute neutrophil count > 1500 cells/mm3; (v) ALT ≤ 3 × ULN; (vi) AST ≤ 3 × ULN; (vi) total bilirubin ≤1.5 mg/dL (in patients with Gilbert’s syndrome, if total bilirubin is > 1.5 × ULN, measure direct and indirect bilirubin and if direct bilirubin is ≤ 1.5 × ULN, the patient may be eligible); (vii) total serum magnesium within normal ranges if the patients is a candidate for cetuximab treatment as per the Investigator’s choice prior to randomization; and (vii) estimated creatinine clearance ≥30 mL/min (calculated by Cockcroft and Gault).
AASLD, American Association for the Study of Liver Diseases; AJCC, American Joint Committee on Cancer; EASL, European Association for the Study of the Liver; ECOG, Eastern Cooperative Oncology Group; H&N, head and neck; HNSCC, head and neck squamous cell carcinoma; HIV, human immunodeficiency virus; HPV, human papilloma virus; ICF, informed consent form; IT, intratumoral; LA, locally advanced; LN, lymph node; MDTB, multidisciplinary tumor board; NYHA, New York Heart Association; PS, performance status; RT, radiotherapy; SCC, squamous cell carcinoma; ULN, upper limit of normal.
2.3. Interventions
Eligible patients are treated with the Investigator’s choice of RT alone or RT in combination with cetuximab. Following the Investigator’s choice, patients are randomized 1:1 on Day 1 to either Arm A or Arm B of the trial. Patients randomized to Arm A receive NBTXR3 activated by RT ± cetuximab, while patients randomized to Arm B receive RT ± cetuximab.
NBTXR3 is administered under local or general anesthetic as an intratumoral/intranodal injection at the dose level of 33% of the gross tumor volume (GTV). During the screening period, within 4 weeks prior to randomization, NBTXR3 injection volume is calculated as a percentage of the computed GTV contour of the lesion(s) by MRI. After the injection, the patient is monitored clinically.
If the Investigator’s choice of treatment includes cetuximab, patients receive a loading dose of cetuximab of 400 mg/m2 (over 120 min) of body surface area at 1 week before the start of RT, with subsequent weekly infusion of cetuximab of 250 mg/m2 (over 60 min) for the duration of RT (7 weeks).
All patients in both arms of the study receive 70 Gy of RT (intensity-modulated radiation therapy [IMRT]) in 35 fractions of 2 Gy per fraction over approximately 7 weeks.
For cetuximab and RT, any dose interruptions/reductions (including missed doses) and the reason for the interruption/reduction will be recorded in the eCRF. Cetuximab may be resumed according to the most current prescribing information. RT may be withheld due to adverse events (AEs). RT may resume after the toxicity has resolved to Grade 1 or baseline level(s) (of Version 5 of the National Cancer Institute-Common Toxicity Criteria for Adverse Events [NCI-CTCAE]) and the patient has an ECOG PS ≤ 2. A retrospective radiation therapy quality assessment (RTQA) will be performed to monitor compliance with RT planning with an independent qualified IMRT-QA reviewer.
NBTXR3 is a single injection therapy, typically the primary tumor and lymph nodes can be injected during the same procedure, however intratumoral and intranodal injections may be performed as two different procedures. RT and/or cetuximab will be discontinued in case of intolerable safety concern, noncompliance, disease progression, need of further therapy for HNSCC (including surgery other than post-RT neck dissection), or if the Investigator believes that it is in the best interest of the patient. Patients also have the right to withdraw from the trial at any time for any reason, without any reprisal.
2.4. Outcomes
The primary endpoint of PFS is defined as time from randomization to locoregional recurrence, locoregional progression, distant progression, or death from any cause, whichever occurs first. PFS primary analysis will be based on central imaging assessments. OS, the key secondary endpoint, is defined as time from randomization to death from any cause. Other endpoints are shown in Table 3.
2.5. Participant timeline and evaluations
The first patient was randomized to NANORAY-312 in January 2022. Enrollment is currently ongoing.
Patients have a screening period of up to 28 days, a treatment period with an end-of-treatment (EOT) visit 4 weeks after the last RT fraction. Patients have a follow-up assessment post-RT completion, then every 12 weeks for 2 years, and then every 24 weeks thereafter until death, lost to follow-up, withdrawal of consent, or end of study, whichever occurs first.
Safety is assessed throughout the study, from the signing of the informed consent form (ICF), through the treatment and follow-up period. The treatment period begins after randomization and continues until the EOT visit. Patients in Arm A undergo a CT scan or CT-Simulation after NBTXR3 administration, which is reviewed by the Investigator for the presence or absence of NBTXR3 (radiopaque visualization) prior to the start of RT. All patients will have functional imaging fluorodeoxyglucose positron emission tomography (FDG PET)/CT at 12 weeks post-RT completion (the first follow-up visit), with optional 16-week PET/CT, to evaluate the response to treatment and to identify which patients will have a nodal resection. Patients with FDG-positive disease will undergo post-radiotherapy neck dissection within 20 weeks post-RT, as determined by the Investigator. Evaluation of treatment response by imaging will be performed based on Response Evaluation Criteria in Solid Tumors Version 1.1 (RECIST 1.1). An Independent Central Review (ICR) will assess images used for tumor measurement assessment at screening, during treatment, and follow-up. Patients who have disease progression/recurrence and/or receive further therapy for HNSCC will be followed for survival information. Hematology and biochemistry laboratory assessments will be performed. PROs and HRQoL assessments are based on the EORTC Quality of Life Questionnaire-Head and Neck Cancer Module (QLQ-H&N35) and the EuroQoL 5-dimension 5-level (EQ-5D-5 L) instrument and will be completed by the patients during the screening period, at the EOT visit, and at every follow-up visit.
2.6. Sample size and statistical methods
A total of 500 patients (250 per treatment arm) will be randomized in this trial.
For analysis of primary and key secondary endpoints, a stratified log-rank test will be used to compare PFS and OS in the NBTXR3 treatment arm (Arm A) versus control (Arm B). The Kaplan–Meier method will be used to estimate the median PFS and median OS. When proportional hazards can be assumed, a stratified Cox proportional hazards model with Efron’s method of tie handling will be used to assess the magnitude of the treatment effect, i.e., the hazard ratio (HR). PFS primary analysis will be based on central imaging assessments.
The population for the primary and key secondary endpoints is the Intent-to-Treat (ITT) population (all randomized patients regardless of the treatment they received). Safety analyses will be performed on the All Treated population (all patients who receive at least one dose of study treatment [i.e., at least one NBTXR3 injection, even if incomplete, and/or ≥1 fraction of RT, and/or ≥1 dose of cetuximab, even if incomplete]). Subgroup analyses will be performed for both PFS and OS.
Statistical methods and censoring rules for time-to-event endpoints are described in the Statistical Analysis Plan (SAP). Missing safety data will generally not be imputed.
3. Methods: assignment of interventions
3.1. Sequence generation and method of assigning treatment
After the screening assessments are performed and the Investigator has selected treatment (RT alone or RT + cetuximab), the patient will be randomized 1:1 to either Arm A or Arm B via a centralized interactive voice/web response system (IXRS).
3.2. Masking
This is an open-label study. Personnel involved with study analysis will be blinded to aggregated data of tumor assessments by treatment arm made by the ICR and by the Investigators until database lock and identification of protocol/Clinical Investigational Plan (CIP) deviations have occurred. There will be an unblinded independent biometrics team conducting the interim statistical analyses for PFS, providing details to the Independent Data Monitoring Committee (IDMC).
4. Methods: data collection and management
4.1. Data collection
An eCRF, which is password protected, is used to store and transmit patient information. All study source documents are maintained by the investigator and made available for inspection by authorized persons for study-related monitoring, audits, Institutional Review Board (IRB)/Independent Ethics Committee (IEC) review, and regulatory inspections. Records will be retained in accordance with the current International Council for Harmonization (ICH) Guidelines on Good Clinical Practice (GCP).
5. Ethics
The study protocol/CIP/amendment(s), written ICF, any ICF updates, subject recruitment procedures, and any written information provided to patients is approved by each participating site’s IRB/IEC.
NANORAY-312 is being conducted in accordance with the protocol/CIP, the Note for Guidance on ICH GCP Harmonized Tripartite Guideline E6 (R1)/Integrated Addendum E6 (R2); International Standard Operations 14155, United States Food and Drug Administration Code of Federal Regulations (Title 21 Parts 50, 56, 312), requirements for the conduct of clinical studies as provided in the European Union Directive 2001/20/EC; the general guidelines indicated in the Declaration of Helsinki; and all applicable regulatory requirements.
Patients participating in this study must provide written informed consent. Patients are informed that their participation is voluntary, and consent can be withdrawn at any point.
6. Conclusion
Cisplatin-ineligible elderly patients with LA-HNSCC have limited therapeutic options, underscoring the high unmet need for effective treatments. The NANORAY-312 approach of combining the radioenhancer NBTXR3 with RT ± cetuximab holds promise for improving treatment outcomes in this patient population, and results from this study are eagerly awaited. Furthermore, elderly patients usually have limited access to clinical trials, but NANORAY-312 uniquely caters to this demographic, and information from this study will offer valuable insights to advance our understanding of treating cisplatin-ineligible elderly patients with LA-HNSCC.
Acknowledgments
We thank all patients and their families, all investigators and site staff at participating centers, and the data managers.
This research was presented at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, June 3–7, 2022, Chicago, IL, USA (Abstract TPS6110); and at the 2022 American Society for Radiation Oncology (ASTRO) Annual Meeting, October 23–26, 2022, San Antonio, TX, USA (Abstract e313).
Funding Statement
This study is sponsored by Nanobiotix SA (Paris, France). Some employees of the funding organization contributed to the preparation of this manuscript and are included as co-authors.
Article highlights
Background and rationale
Head and neck (H&N) cancers are the seventh most common cancer globally.
Approximately 90% of H&N cancers are H&N squamous cell carcinomas (HNSCC).
About 60% of HNSCCs are diagnosed as locally advanced (LA-HNSCC).
Older patients make up a large portion of the LA-HNSCC population but are largely underrepresented in clinical trials.
Current guidelines for LA-HNSCC recommend multimodality treatment.
Cisplatin-based chemoradiotherapy is a preferred choice of treatment, but not all patients are eligible or can tolerate it.
Cetuximab, a monoclonal antibody, is an alternative treatment for cisplatin-ineligible patients.
However, the treatment of cisplatin-ineligible elderly patients with LA-HNSCC remains challenging, and with the growing aging population and projected increase in elderly patients with LA-HNSCC in the coming years, access to clinical trials and to effective treatments are urgently needed.
NBTXR3
NBTXR3 is a first-in-class radioenhancer made up of hafnium oxide nanoparticles.
NBTXR3 is injected into the tumor and activated by ionizing radiation.
NBTXR3 enhances the tumor-killing effect of radiotherapy without additional harm to surrounding non-injected healthy tissue.
Preclinical and clinical trials in several tumor types have demonstrated that NBTXR3 boosts tumor cell death with radiotherapy.
Promising efficacy results were observed in a phase I study with NBTXR3 + radiotherapy in chemotherapy-ineligible elderly patients with LA-HNSCC, and the treatment was well tolerated.
NANORAY-312
NANORAY-312 is a global, open-label, randomized (1:1), two-arm, pivotal phase III study that is comparing NBTXR3 + radiotherapy ± cetuximab (Investigator’s choice) with radiotherapy ± cetuximab (Investigator’s choice) in platinum-ineligible elderly patients with LA-HNSCC.
The target for enrollment is 500 patients (250 patients per study arm).
Eligible patients are aged ≥60 years, biopsy-confirmed T3–4 any N or T2, if ≥N2 (8th edition of the American Joint Committee on Cancer [AJCC] tumor-node-metastasis [TNM] classification) squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, or supraglottic larynx, with one primary tumor amenable for intratumoral injection.
Human papillomavirus status must be known for patients with oropharyngeal cancer.
Involved lymph nodes that are amenable for intranodal injection should be ≥1 cm in the longest dimension and have <180-degree encasement of the carotid artery on magnetic resonance imaging or computed tomography scan.
The primary endpoint is progression-free survival.
The key secondary endpoint is overall survival.
Other secondary endpoints include ORR, safety and tolerability, and quality of life.
The study started in January 2022 and enrollment is ongoing.
Author contributions
The authors are responsible for the content of this manuscript, and the views and opinions described in the publication reflect those of the authors.
Christophe Le Tourneau, Caroline Hoffmann, Eteri Natelauri, Amanda Psyrri, and Sue S. Yom: Conceptualization, Investigation. Sandra Nuyts, Trevor G. Hackman, Maria Lesnik, France Nguyen: Investigation. All Authors: Writing – Original draft preparation, Writing – Reviewing and Editing.
Disclosure statement
Christophe Le Tourneau has received honoraria from Bristol-Myers Squibb; Celgene; GlaxoSmithKline; Merck Serono; MSD; Nanobiotix; Novartis; Rakuten Medical; Roche; and has received consulting fees from or has been an advisory board member of Amgen; AstraZeneca; AstraZeneca; Bristol-Myers Squibb; GlaxoSmithKline; Merck Serono; MSD; Nanobiotix; Roche; and has received payment for travel/accommodations/expenses from AstraZeneca; Bristol-Myers Squibb; and MSD. Zoltán Takácsi-Nagy has received honoraria from Nanobiotix. Sandra Nuyts has received honoraria from Merck Serono; MSD. Sébastien Thureau has no relationships to disclose. Feng Liu has no relationships to disclose. Caroline Hoffmann has received honoraria from Nanobiotix, has received research funding from Merck KGaA, is currently employed at and has stock from Owkin. Trevor Hackman no conflicts or relationships to disclose. Maria Lesnik hasn’t received any honoraria nor fundings and has no relationship to disclose. Anaïs Debard, Laetitia Finzi, Omar I Vivar, and Leonard A Farber are employed by Nanobiotix. Kiran Devisetty is employed by Johnson & Johnson. France Nguyen has no relationships to disclose. Xavier Liem has no relationships to disclose. Eteri Natelauri has no relationships to disclose. Amanda Psyrri has no relationships to disclose. Martin Burian has no relationships to disclose. Jinming Yu has no relationships to disclose. Sue S. Yom has received research grants from Bristol Myers Squibb and EMD Serono and royalties from Springer and UpToDate. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing assistance was provided by Bingham Mayne and Smith and funded by Nanobiotix SA.
Ethical disclosure
The study protocol/CIP/amendment(s), written ICF, any ICF updates, subject recruitment procedures, and any written information provided to patients is approved by each participating site’s IRB/IEC
NANORAY-312 is being conducted in accordance with the protocol/CIP, the Note for Guidance on ICH GCP Harmonized Tripartite Guideline E6 (R1)/Integrated Addendum E6 (R2); International Standard Operations 14,155, United States Food and Drug Administration Code of Federal Regulations (Title 21 Parts 50, 56, 312), requirements for the conduct of clinical studies as provided in the European Union Directive 2001/20/EC; the general guidelines indicated in the Declaration of Helsinki; and all applicable regulatory requirements.
Patients participating in this study must provide written informed consent. Patients are informed that their participation is voluntary, and consent can be withdrawn at any point.
Data sharing statement
Nanobiotix is committed to sharing the data underlying this study once the study has been completed and published. Data will be shared on request from qualified scientific and medical researchers, as necessary for legitimate research. Interested researchers can request access to anonymized patient-level data and supporting documents from clinical studies to do further research that could help to advance medical science or improve patient care. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel which will ensure thorough scrutiny on the application of the global data protection regulation. Nanobiotix is not involved in the decisions made by the independent review panel. Nanobiotix takes all necessary measures to ensure that patient privacy is safeguarded. Contact: dataprivacy@nanobiotix.com.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Haddad RI, Harrington K, Tahara M, et al. Managing cisplatin-ineligible patients with resected, high-risk, locally advanced squamous cell carcinoma of the head and neck: Is there a standard of care? Cancer Treat Rev. 2023. Sep;119:102585. doi: 10.1016/j.ctrv.2023.102585 [DOI] [PubMed] [Google Scholar]; •• A review discussing cisplatin-ineligibility factors in patients with LA-HNSCC and the management of patients with resected high-risk disease.
- 2.Machiels JP, René Leemans C, Golusinski W, et al. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS–ESMO–ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(11):1462–1475. doi: 10.1016/j.annonc.2020.07.011 [DOI] [PubMed] [Google Scholar]
- 3.NCCN . National comprehensive cancer Network© (NCCN©). NCCN clinical practice guidelines in oncology (NCCN Guidelines©). Head and neck cancers. Version 2. 2025. [2025 Apr]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- 4.Joshi P, Dutta S, Chaturvedi P, et al. Head and neck cancers in developing countries. Rambam Maimonides Med J. 2014. Apr;5(2):e0009. doi: 10.5041/RMMJ.10143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson DE, Burtness B, Leemans CR, et al. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020. Nov 26;6(1):92. doi: 10.1038/s41572-020-00224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zumsteg ZS, Luu M, Yoshida EJ, et al. Combined high-intensity local treatment and systemic therapy in metastatic head and neck squamous cell carcinoma: an analysis of the national cancer data base. Cancer. 2017. Dec 1;123(23):4583–4593. doi: 10.1002/cncr.30933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pottier A, Borghi E, Levy L.. New use of metals as nanosized radioenhancers. Anticancer Res. 2014. Jan;34(1):443–453. [PubMed] [Google Scholar]; • Describes the mechanism of action of NBTXR3.
- 8.Maggiorella L, Barouch G, Devaux C, et al. Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol. 2012. Sep;8(9):1167–1181. doi: 10.2217/fon.12.96 [DOI] [PubMed] [Google Scholar]; • Describes the ninefold radiation dose enhancement effect of NBTXR3.
- 9.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003. Jan 1;21(1):92–98. doi: 10.1200/JCO.2003.01.008 [DOI] [PubMed] [Google Scholar]
- 10.Szturz P, Wouters K, Kiyota N, et al. Low-dose vs. High-dose Cisplatin: lessons learned from 59 chemoradiotherapy trials in head and neck cancer [review]. Front Oncol. 2019. Feb 21;9:86. doi: 10.3389/fonc.2019.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noronha V, Joshi A, Patil VM, et al. Once-a-week versus once-every-3-weeks cisplatin chemoradiation for locally advanced head and neck cancer: a phase III randomized noninferiority trial. J Clin Oncol. 2018. Apr 10;36(11):1064–1072. doi: 10.1200/JCO.2017.74.9457 [DOI] [PubMed] [Google Scholar]
- 12.Rühle A, Weymann M, Behrens M, et al. A multicenter evaluation of different chemotherapy regimens in older adults with head and neck squamous cell carcinoma undergoing definitive chemoradiation. Int J Radiat Oncol Biol Phys. 2024. Apr 1;118(5):1282–1293. doi: 10.1016/j.ijrobp.2023.10.025 [DOI] [PubMed] [Google Scholar]
- 13.Porceddu SV, Scotté F, Aapro M, et al. Treating patients with locally advanced squamous cell carcinoma of the head and neck unsuitable to receive Cisplatin-based therapy. Front Oncol. 2019;9:1522. doi: 10.3389/fonc.2019.01522 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A review discussing cisplatin risk factors, identification of cisplatin-ineligible patients with LA-HNSCC and alternative treatment options for these patients.
- 14.Kim SS, Liu HC, Mell LK. Treatment considerations for patients with locoregionally advanced head and neck cancer with a contraindication to Cisplatin. Curr Treat Opt Oncol. 2023. Mar;24(3):147–161. doi: 10.1007/s11864-023-01051-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bøje CR, Dalton SO, Primdahl H, et al. Evaluation of comorbidity in 9388 head and neck cancer patients: a national cohort study from the DAHANCA database. Radiother Oncol. 2014. Jan;110(1):91–97. doi: 10.1016/j.radonc.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 16.Dickstein DR, Lehrer EJ, Hsieh K, et al. Management of older adults with locally advanced head and neck cancer. Cancers (Basel). 2022. Jun 5;14(11):2809. doi: 10.3390/cancers14112809 [DOI] [PMC free article] [PubMed] [Google Scholar]; • A review providing an overview of treatment considerations, and current and future treatment options for older patients with LA-HNSCC.
- 17.Abdulla M, Belal AA, Sakr A, et al. Eligibility criteria to cisplatin in head and neck squamous cell carcinoma: Egyptian expert opinion. Health Sci Rep. 2023. Jan;6(1):e1037. doi: 10.1002/hsr2.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadambi S, Loh KP, Dunne R, et al. Older adults with cancer and their caregivers — current landscape and future directions for clinical care. Nat Rev Clin Oncol. 2020. Dec;17(12):742–755. doi: 10.1038/s41571-020-0421-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maggiore R, Zumsteg ZS, BrintzenhofeSzoc K, et al. The older adult with locoregionally advanced head and neck squamous cell carcinoma: knowledge gaps and future direction in assessment and treatment. Int J Radiat Oncol Biol phys. 2017. Jul 15;98(4):868–883. doi: 10.1016/j.ijrobp.2017.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fasano M, D’Onofrio I, Belfiore MP, et al. Head and neck squamous cell carcinoma in elderly patients: role of radiotherapy and chemotherapy. Cancers (Basel). 2022. Jan 18;14(3):472. doi: 10.3390/cancers14030472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okuda H, Shibata H, Watanabe T, et al. Nonsurgical treatment strategies for elderly head and neck cancer patients: an emerging subject worldwide. Cancers (Basel). 2022. Nov 19;14(22):5689. doi: 10.3390/cancers14225689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haehl E, Rühle A, David H, et al. Radiotherapy for geriatric head-and-neck cancer patients: what is the value of standard treatment in the elderly? Radiat Oncol. 2020. Feb 04;15(1):31. doi: 10.1186/s13014-020-1481-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patil VM, Noronha V, Menon N, et al. Results of phase III randomized trial for use of docetaxel as a radiosensitizer in patients with head and neck cancer, unsuitable for Cisplatin-based Chemoradiation. J Clin Oncol. 2023. May 1;41(13):2350–2361. doi: 10.1200/JCO.22.00980 [DOI] [PubMed] [Google Scholar]
- 24.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010. Jan;11(1):21–28. doi: 10.1016/S1470-2045(09)70311-0 [DOI] [PubMed] [Google Scholar]; • Randomized phase III study demonstrating RT + cetuximab significantly improves OS at 5-years vs RT alone in patients with LA-HNSCC.
- 25.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006. Feb 9;354(6):567–578. doi: 10.1056/NEJMoa053422 [DOI] [PubMed] [Google Scholar]
- 26.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014. Sep 20;32(27):2940–2950. doi: 10.1200/JCO.2013.53.5633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao Y, Auperin A, Sire C, et al. Improved outcome by adding concurrent chemotherapy to Cetuximab and radiotherapy for locally advanced head and neck carcinomas: results of the GORTEC 2007–01 phase III randomized trial. J Clin Oncol. 2018. Jun 7;36(31):3084–3090. doi: 10.1200/JCO.2017.76.2518 [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Yang C, Gan Y, et al. Radiotherapy plus cetuximab or cisplatin in head and neck squamous cell carcinoma: an updated systematic review and meta-analysis of randomized controlled trials. Eur Arch Otorhinolaryngol. 2023. Jan 01;280(1):11–22. doi: 10.1007/s00405-022-07572-8 [DOI] [PubMed] [Google Scholar]
- 29.Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393(10166):40–50. doi: 10.1016/S0140-6736(18)32779-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mell LK, Torres-Saavedra PA, Wong SJ, et al. Radiotherapy with cetuximab or durvalumab for locoregionally advanced head and neck cancer in patients with a contraindication to cisplatin (NRG-HN004): an open-label, multicentre, parallel-group, randomised, phase 2/3 trial. Lancet Oncol. 2024. Dec;25(12):1576–1588. doi: 10.1016/S1470-2045(24)00507-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Tourneau C, Hoffmann C, Takacsi-Nagy Z, et al. NANORAY-312: a phase III pivotal study of NBTXR3 activated by investigator’s choice of radiotherapy alone or radiotherapy in combination with cetuximab for platinum-based chemotherapy-ineligible elderly patients with locally advanced head and neck squamous cell carcinoma. J Clin Oncol. 2022;40(16_suppl):TPS6110–TPS6110. [Google Scholar]; •• Abstract of protocol of NANORAY-312 with NBTXR3 + RT ± cetuximab.
- 32.Le Tourneau C, Calugaru V, Takacsi-Nagy Z, et al. Phase I study of novel radioenhancer NBTXR3 activated by radiotherapy in cisplatin-ineligible locally advanced HNSCC patients. Int J Radiat Oncol Biol Phys. 2021. Nov 01;111(3):e392. doi: 10.1016/j.ijrobp.2021.07.1141 [DOI] [Google Scholar]; • Phase I study with NBTXR3 activated by RT in elderly patients with LA-HNSCC showing promising antitumor efficacy.
- 33.Hoffmann C, Calugaru V, Borcoman E, et al. Phase I dose-escalation study of NBTXR3 activated by intensity-modulated radiation therapy in elderly patients with locally advanced squamous cell carcinoma of the oral cavity or oropharynx. Eur J Cancer. 2021. Mar;146:135–144. doi: 10.1016/j.ejca.2021.01.007 [DOI] [PubMed] [Google Scholar]; •• Phase I study with NBTXR3 activated by RT in elderly patients with LA-HNSCC demonstrated a good safety profile with promising antitumor efficacy.
- 34.Marill J, Anesary NM, Zhang P, et al. Hafnium oxide nanoparticles: toward an in vitropredictive biological effect? Radiat Oncol. 2014. Jun 30;9(1):150. doi: 10.1186/1748-717X-9-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liem X, de Baère T, Vivar OI, et al. International guidelines for intratumoral and intranodal injection of NBTXR3 nanoparticles in head and neck cancers. Head Neck. 2024. Jun;46(6):1253–1262. doi: 10.1002/hed.27739 [DOI] [PubMed] [Google Scholar]
- 36.Ginat DT, Juloori A, Vivar OI, et al. Imaging features of intratumoral injection of NBTXR3 for head and neck squamous cell carcinoma lymph node metastases. Diagnostics (Basel). 2022. Sep 5;12(9):2156. doi: 10.3390/diagnostics12092156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonvalot S, Rutkowski PL, Thariat J, et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.sarc): a multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019. Aug;20(8):1148–1159. doi: 10.1016/S1470-2045(19)30326-2 [DOI] [PubMed] [Google Scholar]; • Randomized phase II/III study demonstrating NBTXR3 activated by RT enhanced the efficacy of RT with double the amount of pathological complete responses versus RT alone in patients with LA-STS.
- 38.Le Tourneau C, Takacsi-Nagy Z, Liem X, et al. 887P antitumor activity of the radioenhancer NBTXR3 on injected lesions to estimate overall survival: exploratory analyses from a phase I in cisplatin-ineligible locally advanced HNSCC patients. Ann Oncol. 2023;34:S568–S569. doi: 10.1016/j.annonc.2023.09.2032 [DOI] [Google Scholar]
- 39.Charlson ME, Charlson RE, Peterson JC, et al. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008. Dec;61(12):1234–1240. doi: 10.1016/j.jclinepi.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 40.Vlastou E, Kougioumtzopoulou A, Platoni K, et al. The emerging role of nanoparticles combined with either radiotherapy or hyperthermia in head and neck cancer: a current review. Cancers (Basel). 2025;17(5):899. doi: 10.3390/cancers17050899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Da Silva J, Bienassis C, Schmitt P, et al. Radiotherapy-activated NBTXR3 nanoparticles promote ferroptosis through induction of lysosomal membrane permeabilization. J Exp Clin Cancer Res. 2024. Jan 03;43(1):11. doi: 10.1186/s13046-023-02938-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darmon A, Zhang P, Marill J, et al. Radiotherapy-activated NBTXR3 nanoparticles modulate cancer cell immunogenicity and TCR repertoire. Cancer Cell Int. 2022. Jun 03;22(1):208. doi: 10.1186/s12935-022-02615-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Y, Paris S, Bertolet G, et al. NBTXR3 improves the efficacy of immunoradiotherapy combining nonfucosylated anti-CTLA4 in an anti-PD1 resistant lung cancer model. Front Immunol. 2022;13:1022011. doi: 10.3389/fimmu.2022.1022011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen C, Frakes JM, Hackman TG, et al. Early signs of efficacy in patients with anti-PD-1 naïve and anti-PD-1 resistant HNSCC treated with NBTXR3/SBRT in combination with nivolumab or pembrolizumab in the phase I trial study 1100. J Clin Oncol. 2024;42(16_suppl):6035–6035. doi: 10.1200/JCO.2024.42.16_suppl.6035 [DOI] [Google Scholar]
- 45.Khadela A, Shah Y, Mistry P, et al. Immunomodulatory therapy in head and neck squamous cell carcinoma: recent advances and clinical prospects. Technol Cancer Res Treat. 2023. Jan;22:15330338221150559. doi: 10.1177/15330338221150559 [DOI] [PMC free article] [PubMed] [Google Scholar]


