ABSTRACT
Background
The increased consumption of antifungal agents increases the emergence of resistant fungal strains among immunocompromised patients. The present study aims to determine the antifungal consumption and resistance pattern among adult and pediatric cancer patients with systemic Candida infections.
Method
A descriptive, retrospective study was conducted by reviewing admitted patients' electronic medical records from 2019 to 2023. Consumption was estimated using Days of Therapy (DOT) metrics. Culture reports of Candida‐positive isolates were collected from the hospital microbiology laboratory to determine the resistance pattern of Candida species.
Results
Consumption of antifungals showed a declining trend, with highest consumption of oral voriconazole (mean DOT/1000 pt. days 18653.49) and oral fluconazole (mean DOT/1000 pt. days 15258.14). Candida albicans was the most isolated pathogen 768 (53.60%) and among all Candida species, major resistance was found in C. tropicalis 58 (4.05%). All Candida species except C. parapsilosis and C. guilliermondii exhibited more resistance to fluconazole.
Conclusion
The findings indicated that the number of resistant isolates is clinically important 133 (9.2%) in the immunocompromised population, which underscores the necessity of conducting culture sensitivity analyses of antifungals. This approach is crucial for early detection and mitigation of antifungal resistance and optimizing therapy.
Keywords: antifungal resistance, antifungal stewardship, consumption, culture sensitivity, surveillance
Consumption of antifungals showed a declining trend, with the highest consumption of oral voriconazole (mean DOT/1000 pt. days 18653.49) and oral fluconazole (mean DOT/1000 pt. days 15258.14). Candida albicans was the most isolated pathogen 768 (53.60%) and among all Candida species, major resistance was found in Candida tropicalis 58 (4.05%). All Candida species except Candida parapsillosis and Candida guilliermondii exhibited more resistance to fluconazole.
1. Introduction
Cancer patients are vulnerable to a wide range of infections, with heightened susceptibility to bacterial and fungal pathogens due to the adverse effect of cytotoxic chemotherapy on host defense mechanisms [1]. Systemic fungal infections continue to pose a significant threat, resulting in elevated rates of mortality and morbidity in cancer patients. Chemotherapy or transplant procedures are often delayed in such patients, which may affect their overall survival [2]. The prevalence of cancer has been increasing globally over the past few decades; thus, the population at risk of systemic fungal infections is also growing, leading to an escalation in the prescription of antifungal agents for both treatment and prevention [3]. The predominant sources of invasive infections include Candida, Aspergillus, and Cryptococcus species [4]. Systemic fungal infections are of global concern due to the lack of standardized diagnostic instruments and the rise of drug‐resistant species. An estimated billion individuals worldwide are thought to be affected by pathogenic fungi, resulting in around 1.5 million documented deaths annually [5].
Additionally, the global health threat posed by the emergence of multidrug‐resistant C. auris is also noteworthy, and some European hospitals report up to a 30% prevalence of azole‐resistant Aspergillus, accompanied by a mortality rate exceeding 90% [6]. In Pakistan, studies have reported resistance among Candida species, with significant variability observed in their resistance profiles to antifungal agents. A study involving critically ill patients found that C. auris was completely resistant to fluconazole (100%), with 28.6% resistance to voriconazole and 7.9% resistance to amphotericin B [7]. Another study reported a higher resistance to voriconazole (29.6%) in C. auris , emphasizing the increasing challenge of antifungal resistance in the region [8]. In patients with invasive C. glabrata infections, approximately 18.4% of the isolates were found resistant to fluconazole [9]. Therefore, monitoring antifungal drug consumption is crucial for effective regulation and resistance prevention.
In high‐risk patients, using antifungals as empirical therapy substantially impacts consumption, as evident from the upsurge in prescription rates of these drugs in intensive care units (ICUs) and hematology‐oncology units [10]. The increased consumption affects the distribution of fungal species and diminishes the susceptibility of target pathogens. Pakistan, with its diverse climate, population, and healthcare infrastructure, presents a unique set of challenges in combating fungal infections. To track trends and mitigate antifungal resistance, it is essential to implement consumption monitoring and antifungal stewardship approaches at both local and national levels [11].
The common metrics for assessing antimicrobial consumption are Days of Therapy (DOT) and Defined Daily Dose (DDD). World health organization (WHO) Collaborating Center for Drug Statistics recommends DDD as a statistical measure for drug consumption, whereas CDC recommends DOT methodology for estimating drug consumption [12]. DOT refers to administering a single medication within a day, irrespective of the number of doses. In contrast, DDD represents the presumed average daily maintenance dose for adults using a drug for its primary purpose, but its relevance diminishes in pediatric patients due to weight‐based prescriptions [13]. The aim of the present study was to determine the trend of antifungal drug usage and resistance pattern among adult and pediatric cancer patients between 2019 and 2023 in Pakistan.
2. Material and Methods
2.1. Study Setting
This study was conducted at Shaukat Khanum memorial cancer hospital and research centre (SKMCH&RC) which is a specialized cancer care hospital equipped with 195 beds and providing a comprehensive array of healthcare services.
2.2. Study Design
A descriptive, retrospective study was conducted by reviewing electronic medical records (EMR) of hospitalized patients in all hospital departments who were prescribed antifungals between 2019 and 2023.
2.3. Inclusion and Exclusion Criteria
All male and female cancer patients, irrespective of age, who were admitted to the hospital and prescribed antifungals either empirically or as definitive treatment for systemic fungal infections were included in the study. Data on both oral and intravenous formulations of antifungals were recorded. Outpatient data were excluded as exact estimates of days of therapy (DOT) cannot be calculated. Duplicate entries were also excluded.
2.4. Antifungal Prescription
Antifungal therapy is categorized as empiric, prophylactic, pre‐emptive, or targeted, depending on factors such as radiological findings, culture results, fungal microscopy, and biomarkers [14].
Empiric: Administered to neutropenic patients with persistent fever that does not respond to 4–7 days of broad‐spectrum antibiotics, or to those with recurring fever and no symptoms of invasive fungal infection (IFI), in the absence of positive fungal test results. For non‐neutropenic patients, it is started in critically ill individuals who have risk factors for fungal infection and no other identifiable cause for fever.
Prophylactic: Given to patients at high risk of developing IFI, even if they show no signs or symptoms of infection and have negative fungal test results.
Pre‐emptive: Focuses on early treatment of suspected IFI, utilizing clinical, radiological, or laboratory indicators to assess the likelihood of the infection.
Targeted: Involves treatment of confirmed fungal infections based on positive culture or other diagnostic results.
Microbiological confirmation is achieved through blood cultures, tissue biopsies, or non‐invasive diagnostic methods. Imaging techniques such as computed tomography (CT) or magnetic resonance imaging (MRI) are employed to assess the extent of infection and identify potential sites of fungal involvement.
2.5. Data Collection
Antifungal usage data for the specified years were retrieved from the hospital's EMR after getting approval from Institutional review board and ethical committee (IRB) of hospital. The consumption data comprises antifungals dispensed between 2019 and 2023, which was presented in an Excel spreadsheet consisting of antifungal details (both brand and generic names), dosage forms, doses (mg), patient diagnosis, admission time, and drug dispense days. Additionally, distinct data pertaining to patient admission records for 5 years (2019–2023) were obtained from the system with assistance from the hospital's Information Technology (IT) department. All the data were anonymized during extraction in order to maintain patient confidentiality.
2.5.1. Calculation of Antifungal Consumption Through the DOT Method
To determine antifungal usage through the DOT method, the administration of a single agent on a particular day, irrespective of the number of doses or dosage strength, was considered as each DOT. For instance, a patient receiving two antifungal drugs would be recorded as 2 DOTs (1 for each drug administered). The total DOTs were standardized to 100 patient admissions and 1000 patient‐days to present cumulative usage. Patient Admissions and patient days are both key metrics used in healthcare to measure the volume of patient care provided in a healthcare facility. Patient admission refers to the number of individual patients who are admitted and patient days refer to the total number of days that patients spend in hospital during a specific period.
2.5.2. Culture Sensitivity Testing
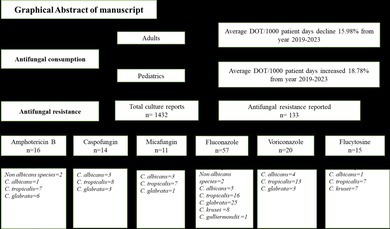
Culture sensitivity reports were also extracted from the Hospital information management system (HIMS) of admitted patients between 2020 and 2023, as culture sensitivity testing was not carried out before 2020 due to a lack of resources. Only Candida positive reports were included because culture sensitivity testing for Aspergillus was unavailable. A total of 1432 culture sensitivity reports were identified in which susceptibility of fungal pathogens was tested against six antifungals, namely amphotericin B, caspofungin, micafungin, voriconazole, fluconazole, and flucytosine. Samples were collected from blood and other sterile body sites. The study primarily included patients with systemic fungal infections and incorporated nasal and ear swabs due to their clinical significance in immunocompromised individuals and potential systemic dissemination. The inclusion of these non‐invasive samples proved helpful for antifungal stewardship in high‐risk populations. The susceptibility of Candida isolates to antifungal agents was assessed using the broth microdilution method in accordance with CLSI (Clinical and Laboratory Standard Institute) document M60‐Ed2 [15]. Test results are presented as sensitive, intermediate sensitive, or resistant. Quality control was established utilizing C. albicans (ATCC 90028) and C. parapsilosis (ATCC 22019) strains to ensure the reliability and reproducibility of the testing results.
2.6. Data Analysis
Data were entered and analyzed through microsoft excel and spss version 21. Descriptive statistics were applied to determine means of yearly drug consumption among adult and pediatric patients along with frequency and percentages of positive isolates from specimens. Moreover, the Student t‐test was applied to determine the trend of drug consumption among adult and pediatric populations. The Chi‐square test was used to determine the association of antifungal resistance with fungal species and categorical variables. A P value < 0.05 is considered to be statistically significant.
3. Results
3.1. Basic Demographics and Clinical Characteristics of Patients
Patient demographic data is shown in Table 1. The total number of admissions were highest in the year 2023 (19259) and the year 2021(19130) as compared to the other 3 years. The average length of hospital stay from the year 2019 to the year 2023 was (4.15, 4.2, 4.16, 4.17, 4.16, respectively). The majority of patients were diagnosed with malignant neoplasm: 256 (41.2%); 185 (41.2%); 188 (42.1%); 240 (47.4%) in the last 5 years, respectively. Patient mortality was highest in the year 2021 (41.3%) as compared to the year 2019 (40.9%) as shown in Table 1.
TABLE 1.
Patients' basic demographics and clinical characteristics of patients.
| Parameters | Year 2019 | Year 2020 | Year 2021 | Year 2022 | Year 2023 |
|---|---|---|---|---|---|
| Total no. of admissions | 18,623 | 15,492 | 19,130 | 18,679 | 19,259 |
| Adults (> 18 years) | 13,858 | 12,453 | 41,226 | 13,770 | 14,213 |
| Pediatrics (≤ 18 years) | 4765 | 3639 | 4904 | 4909 | 5046 |
| Average length of stay (days) | 4.15 | 4.2 | 4.16 | 4.17 | 4.16 |
| Patient days (Adults) | 57510.7 | 52302.6 | 171500.16 | 57420.9 | 59126.08 |
| Patient days (Pediatrics) | 19774.75 | 15283.8 | 20400.64 | 20470.53 | 20991.36 |
| Gender | |||||
| Male | 388 (62.5%) | 279 (62.3%) | 254 (56.8%) | 305 (60.3%) | 289 (59.2%) |
| Female | 232 (37.4%) | 169 (37.7%) | 193 (43.2%) | 201 (39.7%) | 199 (41.4%) |
| Age group | |||||
| Adult (> 18 years) | 410 (66.1%) | 303 (67.6%) | 280 (62.6%) | 289 (57.1%) | 286 (58.6%) |
| Pediatrics (≤ 18 years) | 210 (33.8%) | 145 (32.3) | 167 (37.3%) | 217 (42.8%) | 202 (41.3%) |
| Type of cancer | |||||
| Malignant neoplasm | 256 (41.2%) | 185 (41.2%) | 188 (42.1%) | 240 (47.4%) | 211 (43.2%) |
| Lymphoma | 184 (29.7%) | 130 (29.0%) | 128 (28.6%) | 139 (27.4%) | 160 (32.7%) |
| Leukemia | 160 (25.8%) | 117 (26.1%) | 111 (24.8%) | 111 (21.9%) | 97 (19.8%) |
| Multiple myeloma | 4 (0.6%) | 3 (0.7%) | 0 (0.0%) | 5 (0.9%) | 3 (0.6%) |
| Carcinoma | 0 (0.0) | 2 (0.4%) | 2 (0.44%) | 3 (0.6%) | 2 (0.4%) |
| Sarcoma | 0 (0.0%) | 0 (0.0%) | 2 (0.44%) | 1 (0.1%) | 3 (0.6%) |
| Carcinoid | 0 (0.0%) | 1 (0.2%) | 1 (0.2%) | 1 (0.19%) | 1 (0.2%) |
| Melanoma | 0 (0.0%) | 0 (0.0%) | 2 (0.4%) | 0 (0.0%) | 0 (0.0%) |
| Other | 16 (2.5%) | 16 (3.5%) | 13 (2.9%) | 6 (1.1%) | 11 (2.3%) |
| Mortality | 254 (40.9%) | 177 (39.5%) | 185 (41.3%) | 176 (34.7%) | 147 (30.1%) |
3.2. Pattern and Trend of Total Antifungals Consumption
Total antifungal consumption (TAC) from 2019 to 2023 in days of therapy (DOT/100 patient admission) is shown in Figure 1. Mean antifungal consumption was higher in 2019 (161.77 DOT/100 patient admission) and decreased by 8.0% in year 2023 (148.80 DOT/100 patient admission). Among all antifungals, the mean highest DOT/100 patient admission was PO voriconazole (370.48).
FIGURE 1.
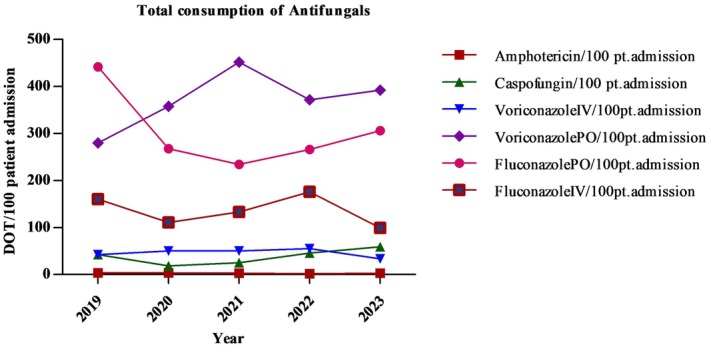
Year‐wise antifungal consumption (DOT/100 patient admission).
3.3. Mean Antifungal Consumption in Adult and Pediatric Cancer Patients
Average antifungal consumption and percentage change in consumption of individual antifungals from 2019 to 2023 is shown in Table 2. Mean antifungal (AF) consumption among adult patients decreased by 15.98% between 2019 (9.57 DOT/1000 patient days) and 2023 (8.04 DOT/1000 patient days). However, mean AF consumption among pediatric patients increased by 18.78% between 2019 (18.74 DOT/1000 patient days) and 2023 (22.26 DOT/1000 patient days). In the year 2021, there was a significant difference in AF consumption in terms of DOT/1000 patient days among adult and pediatric patients (p = 0.03). Fluconazole PO was the most prescribed antifungal among adults in 2019 (24.97 DOT/1000 patient days) and Amphotericin IV was most prescribed among pediatrics (45.56 DOT/1000 patient days). In 2020, fluconazole PO was most prescribed among adults (16.16 DOT/1000 patient days), while in pediatrics, amphotericin IV was most prescribed (51.69 DOT/1000 patient days). In 2021 and 2022, voriconazole PO (6.67 DOT/1000 patient days) and (13.32 DOT/1000 patient days) among adults and amphotericin IV in pediatrics were most prescribed (38.72 DOT/1000 patient days) and (42.26 DOT/1000 patient days) respectively. In 2023, fluconazole PO in adults (15.27DOT/1000 patient days) and amphotericin IV in pediatric patients was prescribed frequently (54.40 DOT/1000 patient days), as shown in Table 2.
TABLE 2.
Average consumption of antifungals between 2019 and 2023 in days of therapy per 1000 patient days (DOT/1000 patient days).
| DOT/1000 patient days | Age group | Year | Percentage change in consumption (2019–2023) | ||||
|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2022 | 2023 | |||
| Amphotericin IV | Adults | 8.61 | 9.71 | 2.86 | 4.55 | 7.90 | −8.258% |
| Caspofungin IV | Adults | 1.46 | 1.19 | 0.40 | 2.12 | 2.60 | 78.0% |
| Voriconazole IV | Adults | 1.84 | 3.40 | 0.75 | 2.54 | 1.69 | −8.15% |
| Voriconazole PO | Adults | 10.61 | 16.02 | 6.67 | 13.32 | 15.24 | 43.63% |
| Fluconazole PO | Adults | 24.97 | 16.16 | 3.48 | 11.48 | 15.27 | −38.56% |
| Fluconazole IV | Adults | 9.91 | 7.59 | 2.31 | 10.92 | 5.56 | −43.89% |
| Average drug used (mean) | 9.57 | 9.01 | 2.74 | 7.49 | 8.04 | −15.98% | |
| Amphotericin IV | Pediatrics | 45.56 | 51.69 | 38.72 | 42.26 | 54.40 | 19.4% |
| Caspofungin IV | Pediatrics | 5.06 | 0.92 | 0.98 | 4.10 | 5.53 | 9.28% |
| Voriconazole IV | Pediatrics | 3.24 | 4.12 | 2.99 | 5.67 | 2.43 | −25% |
| Voriconazole PO | Pediatrics | 29.43 | 46.19 | 38.53 | 37.66 | 44.78 | 52.16% |
| Fluconazole PO | Pediatrics | 23.41 | 22.05 | 18.38 | 24.57 | 21.25 | −9.22% |
| Fluconazole IV | Pediatrics | 5.76 | 4.65 | 7.79 | 6.84 | 5.14 | −10.76% |
| Average drug used (mean) | 18.74 | 21.60 | 17.90 | 20.18 | 22.26 | 18.78% | |
| p | p = 0.26 | p = 0.22 | p** = 0.03 | p = 0.08 | p = 0.16 | ||
Note: The numbers in bold are peak values during 5 years. Student's t‐test is used to calculate p‐value. p < 0.05 is considered to be significant. “−” Indicates percentage decrease in DOT/1000 patient days from 2019 to 2023.
Abbreviations: IV = intravenous; PO = per oral. * p < 0.1; ** p < 0.05; ***p < 0.01.
3.4. Demographic Data of Culture‐Positive Patients
Among 1432 culture reports, 677 (47.3%) were of female and 755(52.7%) were of male, 238 (16.6%) were of pediatrics and 1194 (83.4%) were adults. The majority of patients, 1153(80.5%), were from Punjab province, and most of the positive samples, 571(39.9%), were from the inpatient department (IPD). C. albicans was most frequently isolated and contributed to 768 (53.6%) of total isolates, followed by C. tropicalis 527(36.8%), and least was C. guilliermondii 10(0.7%), as shown in Table 3.
TABLE 3.
Demographic characteristics of culture‐positive patients.
| Basic demographics | Frequency, n = 1432 | Percentage (%) |
|---|---|---|
| Gender | ||
| Male | 755 | 52.7 |
| Female | 677 | 47.3 |
| Age group | ||
| Adult | 1194 | 83.4 |
| Pediatric | 238 | 16.6 |
| State | ||
| Punjab | 1153 | 80.5 |
| Khyber Pakhtunkhwa | 156 | 10.9 |
| Fata | 64 | 4.5 |
| Federal capital | 8 | 0.6 |
| Gilgit Baltistan | 7 | 0.5 |
| Sindh | 5 | 0.3 |
| Department | ||
| IPD | 571 | 39.9 |
| EAR | 343 | 24.0 |
| ICU | 323 | 22.6 |
| OPD | 195 | 13.6 |
| Fungal isolates | ||
| Candida albicans | 768 | 53.6 |
| Candida tropicalis | 527 | 36.8 |
| Candida glabrata | 50 | 3.5 |
| Candida parapsilosis | 35 | 2.4 |
| Non albicans Candida species a | 30 | 2.1 |
| Candida krusei | 12 | 0.8 |
| Candida guilliermondii | 10 | 0.7 |
Abbreviations: EAR = emergency and acute response department; ICU = intensive care unit; IPD = in patient department; OPD = outpatient department.
Species type not classified.
3.5. Percentage Prevalence of Cultures Positive Specimen for Fungal Isolates
Among 1432 fungal isolate positive samples, 878 (61.3%) were urine, 271 (18.9%) blood (central), 81 (5.7%) wound swabs, 41 (2.9%) nasal swabs, 37 (2.6%) tissue samples, and 31 (2.2%) blood for peripheral smear samples, while 93 (6.5%) samples were collected from other sterile body sites and fluids such as pleural fluid, tracheal aspirate, pus, central venous catheter tip, central intravenous catheter tip, and ear swab. The year‐wise frequency (percentage) of positive samples is shown in Table 4.
TABLE 4.
Prevalence of positive samples for fungal pathogens.
| Type of sample/specimen | Year | p | ||||
|---|---|---|---|---|---|---|
| 2020 (n = 225) | 2021 (n = 325) | 2022 (n = 449) | 2023 (n = 433) | Total (n = 1432) | ||
| Urine | 145 (64.4%) | 206 (63.4%) | 272 (60.6%) | 255 (58.9%) | 878 (61.3%) | 0.005* |
| Blood (central) | 32 (14.2%) | 55 (16.9%) | 88 (19.6%) | 96 (22.2%) | 271 (18.9%) | |
| Wound swab | 13 (5.8%) | 28 (8.6%) | 24 (5.3%) | 16 (3.7%) | 81 (5.7%) | |
| Nasal swab | 8 (3.6%) | 12 (3.7%) | 11 (2.4%) | 10 (2.3%) | 41 (2.9%) | |
| Tissue | 1 (0.4%) | 3 (0.9%) | 22 (4.9%) | 11 (2.5%) | 37 (2.6%) | |
| Blood (peripheral) | 6 (2.7%) | 5 (1.5%) | 8 (1.8%) | 12 (2.8%) | 31 (2.2%) | |
p < 0.001 is highly significant.
3.6. Sensitivity and Resistance Pattern of Isolated Fungal Pathogens
Antifungal susceptibility analysis revealed that the highest resistance was observed in C. tropicalis, which was resistant to fluconazole in 16 (3.0%) of cases, 13 (2.5%) to voriconazole, 8 (1.5%) to caspofungin, and equally resistant 7 (1.3%) to amphotericin B, micafungin, and flucytosine. C. glabrata was resistant to fluconazole in 25 (50.0%) of cases, 6 (12.0%) to amphotericin B, 3(6.0%) to voriconazole and caspofungin, 1(2.0%) to micafungin. C. albicans was resistant to fluconazole in 5(0.7%) of cases, 4(0.5%) to voriconazole, 3(0.4%) to caspofungin and micafungin, and 1(0.1%) to amphotericin B and flucytosine. In the case of non‐C. albicans species, resistance was observed to amphotericin B in 2 (6.7%) and fluconazole in 2(6.7%) cultures. Only 1 (10.0%) C. guilliermondii resistance to fluconazole was observed. No resistance was observed in C. parapsilosis . Results showed that Candida species were mainly resistant to fluconazole, as shown in Table 5.
TABLE 5.
Sensitivity and Resistance pattern of isolated fungal pathogens.
| Drug | Fungal pathogens | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non albicans species (not classified) (n = 30) | Candida albicans (n = 768) | Candida tropicalis (n = 527) | Candida glabrata (n = 50) | Candida krusei (n = 12) | Candida parapsilosis (n = 35) | Candida gulliermondii (n = 10) | Total (n = 1432) | |||
| Amphotericin B | Sensitive | 28 (93.3%) | 767 (99.9%) | 520 (98.7%) | 44 (88.0%) | 9 (75%) | 35 (100%) | 10 (100%) | 1413 (98.7%) | < 0.001*** |
| Resistant | 2 (6.7%) | 1 (0.1%) | 7 (1.3%) | 6 (12.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 16 (1.1%) | ||
| Intermediate sensitivity | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (25.0%) | 0 (0.0%) | 0 (0.0%) | 3 (0.2%) | ||
| Caspofungin | Sensitive | 24 (80.0%) | 765 (99.6%) | 519 (37.0%) | 43 (3.1%) | 8 (0.6%) | 35 (2.5%) | 10 (0.7%) | 1404 (98.0%) | < 0.001*** |
| Resistant | 0 (0.0%) | 3 (0.4%) | 8 (1.5%) | 3 (6.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 14 (1.0%) | ||
| Intermediate sensitivity | 6 (20.0%) | 0 (0.0%) | 0 (0.0%) | 4 (8.0%) | 4 (33.3%) | 0 (0.0%) | 0 (0.0%) | 14 (1.0%) | ||
| Micafungin | Sensitive | 30 (100.0%) | 765 (99.6%) | 520 (98.7%) | 49 (98.0%) | 12 (100.0%) | 35 (100.0%) | 10 (100.0%) | 1421 (99.2%) | NS |
| Resistant | 0 (0.0%) | 3 (0.4%) | 7 (1.3%) | 1 (2.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 11 (0.8%) | ||
| Fluconazole | Sensitive | 28 (93.3%) | 763 (99.3) | 511 (97.0%) | 25 (50.0%) | 4 (33.3%) | 35 (100.0%) | 9 (90.0%) | 1375 (96.0%) | < 0.001*** |
| Resistant | 2 (6.7%) | 5 (0.7%) | 16 (3.0%) | 25 (50.0%) | 8 (66.7%) | 0 (0.0%) | 1 (10.0%) | 57 (4.0%) | ||
| Voriconazole | Sensitive | 30 (100.0%) | 764 (99.5%) | 514 (97.5%) | 47 (94.0%) | 12 (100.0%) | 35 (100.0%) | 10 (100.0%) | 1412 (98.6%) | < 0.01** |
| Resistant | 0 (0.0%) | 4 (0.5%) | 13 (2.5%) | 3 (6.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 20 (1.4%) | ||
| Flucytosine | Sensitive | 30 (100.0%) | 767 (99.9%) | 520 (98.7%) | 50 (100.0%) | 5 (41.7%) | 35 (100.0%) | 10 (0.7%) | 1417 (99.0%) | < 0.001*** |
| Resistant | 0 (0.0%) | 1 (0.1%) | 7 (1.3%) | 0 (0.0%) | 7 (58.3%) | 0 (0.0%) | 0 (0.0%) | 15 (1.0%) | ||
p < 0.01.
p < 0.001.
3.7. Sensitivity and Resistance Pattern of Antifungals Among Adult and Pediatric Cancer Patients
Among cultures taken from adult patients, 13 (1.1%) isolates showed resistance to amphotericin B as compared to 3 (1.3%) isolates from pediatric patients; 1 (0.4%) isolate was resistant to caspofungin among pediatric patients as compared to 13 (1.1%) isolates in adults; 14 (5.9%) isolates were resistant to fluconazole in pediatrics as compared to 43 (3.6%) isolates in adults; 4 (1.7%) isolates were resistant to voriconazole in pediatrics as compared to 16 (1.3%) isolates in adults. No resistance was observed in isolates from pediatric patients to micafungin and flucytosine, as shown in Table 6.
TABLE 6.
Antifungal sensitivity and resistance among adult and pediatric patients.
| Drug | Culture sensitivity | Age group | p | |
|---|---|---|---|---|
| Adult | Pediatric | |||
| Amphotericin B | Sensitive | 1178 (98.7%) | 235 (98.7%) | 0.85 |
| Resistant | 13 (1.1%) | 3 (1.3%) | ||
| Intermediate sensitivity | 3 (0.2%) | 0 (0.0%) | ||
| Caspofungin | Sensitive | 1168 (97.8%) | 236 (99.2%) | 0.51 |
| Resistant | 13 (1.1%) | 1 (0.4%) | ||
| Intermediate sensitivity | 13 (1.1%) | 1 (0.4%) | ||
| Micafungin | Sensitive | 1183 (99.1%) | 238 (100%) | 0.22 |
| Resistant | 11 (0.9%) | 0 (0.0%) | ||
| Fluconazole | Sensitive | 1151 (96.4%) | 224 (94.1%) | 0.10 |
| Resistant | 43 (3.6%) | 14 (5.9%) | ||
| Voriconazole | Sensitive | 1178 (98.7%) | 234 (98.3%) | 0.76 |
| Resistant | 16 (1.3%) | 4 (1.7%) | ||
| Flucytosine | Sensitive | 1179 (98.7%) | 238 (100%) | 0.15 |
| Resistant | 15 (1.3%) | 0 (0.0%) | ||
4. Discussion
This study investigated trends in antifungal consumption and antifungal resistance patterns in the last 5 years among cancer patients with systemic fungal infections. Mycoses Study Group Education and Research Consortium recommended that antifungal stewardship initiatives are crucial components of comprehensive stewardship programs within facilities that utilize antifungal drugs [16].
This study showed a 16.8% decrease in patient admissions from the Year 2019 to 2020. However, it increased to 3.4% in 2023 as compared to 2019. In 2020, the decrease in cancer patient admissions may have been influenced by various factors, including disruptions in healthcare services due to the COVID‐19 pandemic, as the peak of COVID‐19 cases in Pakistan occurred in mid‐2020 along with fear of contracting the virus leading to avoidance of healthcare facilities, and changes in healthcare‐seeking behavior [17]. Literature reported that since March 2020, there have been no additions of new cancer patients for treatment, and a two‐thirds reduction occurs in patients receiving chemotherapy, and the number of patients receiving radiation treatment reduced to half [18]. Conversely, the increase in cancer patient admissions by 3.4% in 2023 compared to 2019 suggests a recovery in healthcare services after the pandemic.
Findings of the demographic spread of fungal infections indicated a higher prevalence among adults compared to pediatric populations over the course of 5 years. Additionally, there was a notable predominance of male cancer patients in comparison to female cancer patients. These findings are consistent with the study that reported a 1.4–3.5 times higher rate of systemic fungal infections diagnosis among males than females due to variations in the regulation of sex hormones and immune responses within the host [19].
This study's results showed that fungal infection prevalence was higher in patients diagnosed with lymphomas and leukemia's. Kumar et al. reported the prevalence of systemic fungal infections to be 22%–97% in pediatric acute leukemia patients with febrile neutropenia [20]. Patients undergoing chemotherapy or hematopoietic stem cell transplantation (HSCT) often result in immune suppression, which makes them more vulnerable to fungal infections [21]. Moreover, chemotherapy can lead to neutropenia and disrupt the mucosal barrier, thus increasing the risk of fungal infections. Patients with lymphomas and leukemias often require prolonged hospitalization for treatment, exposing them to healthcare‐associated fungal infections [22].
Results showed that overall consumption of antifungals was higher in 2019 and declined by 16.76% and 16.81% using both measures (DOT/100 patient admission and DOT/1000 patient days) in 2020. The observed decline in antifungal consumption can be attributed to a reduction in the number of cancer patient admissions during the COVID‐19 pandemic. This decrease in admissions likely led to fewer cases requiring antifungal treatment, thereby contributing to the overall reduction in antifungal usage during the study period. However, in Year 2021, a 7.54% (145.98 DOT/100 patient admission), 5.58% (152.75 DOT/100 patient admission) in Year 2022, and 8.02% (148.80 DOT/100 patient admission) in Year 2023 decline in antifungal consumption occurred. With improved access to diagnostic tools, particularly culture‐based sensitivity testing, clinicians may have been able to more accurately identify fungal pathogens and determine their susceptibility to specific antifungals. This enhanced diagnostic capability likely allowed for more targeted treatment regimens leading to a decline in overall antifungal consumption. Geomaere et al. also reported a decline in average antifungal consumption at the national level in Belgium hospitals [11]. Our results differed from a study that reported increased antifungal consumption over time in a tertiary care hospital in Brazil [23]. Another study reported higher consumption in terms of DOT/1000 patient days (348.12 ± 85.41) in the hemato‐oncology department [24].
In this study utilization of amphotericin B, caspofungin, PO fluconazole and IV fluconazole decrease over time, but IV voriconazole and PO voriconazole consumption increases. Overall, the consumption of voriconazole and fluconazole was higher in our study population. Fluconazole is more effective against C. albicans [25], and administering fluconazole prophylactically can help prevent fungal infections among patients undergoing cytotoxic cancer therapy [26]. This is reflected in our study as the prevalence of C. albicans isolates is highest, leading to increased fluconazole consumption. These findings were partially similar to a study conducted in a tertiary care hospital in Brazil where high consumption of voriconazole and amphotericin was observed [23]. The increased consumption of voriconazole is due to its empirical and prophylactic use for invasive fungal infections and in patients with febrile neutropenia in hemato‐oncology setting [27].
A study conducted in a Lebanese hospital evaluating antifungal consumption and Candida species distribution showed higher consumption of azoles and the highest consumption in hemato‐oncology (days of therapy/1000 patient days = 348.12 ± 85.41). Our results were similar to a study conducted in Belgium where overall fluconazole consumption was highest compared to other antifungals [11]. Another study reported the highest fluconazole consumption in acute care hospitals in Spain [28]. A major factor associated with reduced amphotericin B and caspofungin consumption is cost. Moreover, amphotericin B can cause nephrotoxicity and serious infusion‐related reactions, requiring careful monitoring during treatment [29].
Among positive cultures in our study, the most isolated were C. albicans (53.6%) and C. tropicalis (36.8%), and the least isolated was C. guilliermondii (0.7%). Results of a previous study conducted in a similar setting in 2017 showed the highest prevalence of C. tropicalis followed by C. albicans among positive isolates in patients with hematological malignancies (41.1%; 28.6%) and solid tumors (36.9%;28.2%) [25]. Ecological niche reflects a higher prevalence of C. tropicalis in countries with warm, tropical, and subtropical climates such as Pakistan [30]. Similarly, a tertiary care hospital Laboratory of Pakistan study showed that C. tropicalis followed by C. parapsilosis were most isolated from burn wound specimens [31]. Our results are consistent with a previous study conducted in a tertiary care hospital in Brazil, which showed the most isolated microorganisms were C. albicans (36.5%) and least C. guilliermondii (1.7%) [23]. In the majority of patients, isolates were recovered from urine (61.3%), similar to a previous study conducted in Lebanon tertiary care hospital (48%), and the most isolated was C. albicans (76%) [24].
In 58 culture reports, C. tropicalis showed resistance to antifungals with greater resistance 16 (3.0%) to fluconazole, and in 38 culture reports, C. glabrata showed resistance to antifungals with more resistance 25 (50%) to fluconazole. C. albicans showed resistance in 17 culture reports with the highest 5(0.7%) resistance to fluconazole, and C. krusei showed resistance to fluconazole 8(66.7%) however, C. parapsilosis showed 100% sensitivity to all utilized antifungals in the hospital and C. guilliermondii showed resistance in only 1 (10%) of the culture reports. Our findings differed from a previous study conducted in a bone marrow unit in Pakistan that describes 100% sensitivity of C. albicans to amphotericin B and 90% to fluconazole, while 92% sensitivity of C. tropicalis to amphotericin B and 88% to fluconazole [32]. Results of another study conducted in a tertiary care hospital in Peshawar showed that 62% of Candida isolates were resistant to fluconazole and 10.2% were resistant to voriconazole [33]. However, in our study, Candida isolates were 57 (4.0%) resistant to fluconazole, 20 (1.4%) to voriconazole, and 16 (1.1%) to amphotericin B.
Fluconazole, introduced in the early 1990s, is widely favored for treating systemic Candida infections due to its affordability and low toxicity. However, its prolonged and widespread use has been associated with the development of resistance. Literature reported a concerning rise in fluconazole resistance from approximately 32% before the COVID‐19 pandemic to 48% during the pandemic [34]. Fluconazole resistance is particularly notable in C. albicans , which was initially highly susceptible but has shown increasing rates of resistance over time. Non‐albicans C. species, such as C. glabrata and C. krusei , pose an even greater challenge [35]. The causative agents and their levels of resistance changed over time. The observed variations in antifungal sensitivity and resistance patterns over time highlight the need for AFST [36].
Regularly monitoring fungal pathogens within a specific geographical area and their susceptibility to commonly used antifungal treatments is essential for antifungal stewardship [37]. It is crucial for public health to consistently and periodically monitor the local prevalence of pathogens and their susceptibility to commonly prescribed medications. This monitoring helps promote the judicious use of traditional antimicrobials [38].
5. Conclusion
Consumption of antifungals from 2019 to 2023 showed a declining trend, with the highest consumption of PO voriconazole and PO fluconazole. C. albicans was the most isolated pathogen among all Candida species, and greater resistance was found in C. tropicalis. All Candida species except C. parapsilosis and C. guilliermondii exhibited more resistance to fluconazole. The number of resistant isolates is clinically important, underscoring the necessity of conducting culture sensitivity analyses of antifungals when managing fungal infections in the immunocompromised population. Cancer patients are overprescribed antifungals because of the high risk of systemic fungal infections. Judicious use and regular monitoring of antifungal consumption are mandatory to reduce the occurrence of fungal resistance. This approach is crucial for early detection and mitigation of antifungal resistance. Without such measures, antifungal resistance could escalate similarly to antibacterial resistance.
5.1. Strength and Limitation
DOT methodology is used in this study to measure drug consumption patterns in pediatric and adult patients, which is a strength of our study as DDD methodology is not applied in pediatrics and other medicines that require adjustment based on body weight. Another strength of this study is that antifungal sensitivity and resistance patterns are evaluated as studies focusing on antifungal antibiograms are lacking in Pakistan [39].
This study has few limitations. Data on susceptibility testing of only Candida species are observed because susceptibility testing of Aspergillus species is not carried out in the hospital. Culture sensitivity records of the Year 2019 were not available because, in the Year 2019, AFST was not started. The consumption of micafungin and flucytosine cannot be estimated as they are not prescribed in this setting. In addition, this study was conducted in a single center, which limits the generalizability of results, and regional focus may limit its applicability to populations outside the studied area, as local epidemiological and resistance patterns may differ.
5.2. Future Direction
Efforts, including raising awareness about the prudent use of antifungals, crafting a comprehensive national action plan for monitoring and managing resistance, and accurately estimating antifungal usage, should be made on both international and national fronts to mitigate this burden.
Author Contributions
Conceptualization: M.A., Z.S., and H.E. Data curation: Z.A., and M.R.K.N. Formal analysis: M.R.K.N., M.A., M.A., K.A. Investigation: Z.A. Writing original draft: Z.A. Review and editing: Z.S., M.R.K.N., M.A., and H.E. All authors have critically reviewed the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics Statement
The study was approved by the Institutional review board and ethical committee of the Shaukat Khanum memorial cancer hospital and research center (EX‐05‐09‐23‐06).
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
Authors would like to acknowledge Shaukat Khanum memorial cancer hospital and research center for allowing the conduct of research.
Data Availability Statement
Data available on request from the authors.
References
- 1. Teoh F. and Pavelka N., “How Chemotherapy Increases the Risk of Systemic Candidiasis in Cancer Patients: Current Paradigm and Future Directions,” Pathogens 5, no. 1 (2016): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruhnke M., Cornely O. A., Schmidt‐Hieber M., et al., “Treatment of Invasive Fungal Diseases in Cancer Patients—Revised 2019 Recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO),” Mycoses 63, no. 7 (2020): 653–682. [DOI] [PubMed] [Google Scholar]
- 3. Gross B. N., Steib‐Bauert M., Kern W. V., et al., “Hospital Use of Systemic Antifungal Drugs: A Multi‐Center Surveillance Update From Germany,” Infection 43 (2015): 423–429. [DOI] [PubMed] [Google Scholar]
- 4. Friedman D. Z. and Schwartz I. S., “Emerging Fungal Infections: New Patients, New Patterns, and New Pathogens,” Journal of Fungi 5, no. 3 (2019): 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown G. D., Denning D. W., Gow N. A., Levitz S. M., Netea M. G., and White T. C., “Hidden Killers: Human Fungal Infections,” Science Translational Medicine 4, no. 165 (2012): 165rv13. [DOI] [PubMed] [Google Scholar]
- 6. van Paassen J., Russcher A., In 't Veld‐van Wingerden A. W., Verweij P. E., and Kuijper E. J., “Emerging Aspergillosis by Azole‐Resistant Aspergillus fumigatus at an Intensive Care Unit in The Netherlands, 2010 to 2013,” Eurosurveillance 21, no. 30 (2016): 30300. [DOI] [PubMed] [Google Scholar]
- 7. Sayeed M. A., Farooqi J., Jabeen K., Awan S., and Mahmood S. F., “Clinical Spectrum and Factors Impacting Outcome of Candida Auris: A Single Center Study From Pakistan,” BMC Infectious Diseases 19 (2019): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sayeed M. A., Farooqi J., Jabeen K., and Mahmood S. F., “Comparison of Risk Factors and Outcomes of Candida Auris Candidemia With Non‐Candida Auris Candidemia: A Retrospective Study From Pakistan,” Medical Mycology 58, no. 6 (2019): 721–729. [DOI] [PubMed] [Google Scholar]
- 9. Memon S., Farooqi J., Zafar U., Naqvi S. F., Zafar A., and Jabeen K., “Antifungal Susceptibility Profile of Invasive Candida Glabrata Isolates (2009–2020) From a Tertiary Care Hospital Laboratory in Pakistan,” Journal of Medical Microbiology 70, no. 12 (2021): 1459. [DOI] [PubMed] [Google Scholar]
- 10. Kanj S. S., Omrani A. S., Al‐Abdely H. M., et al., “Survival Outcome of Empirical Antifungal Therapy and the Value of Early Initiation: A Review of the Last Decade,” Journal of Fungi 8, no. 11 (2022): 1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goemaere B., Lagrou K., Spriet I., et al., “Systemic Antifungal Drug Use in Belgium—One of the Biggest Antifungal Consumers in Europe,” Mycoses 62, no. 6 (2019): 542–550. [DOI] [PubMed] [Google Scholar]
- 12. Pollack L. A. and Srinivasan A., “Core Elements of Hospital Antibiotic Stewardship Programs From the Centers for Disease Control and Prevention,” Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 59 Suppl 3 (2014): S97–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zombori L., Paulus S., Shah M. A., McGarrity O., and Hatcher J., “Antibiotic Spectrum Index as an Antimicrobial Stewardship Tool in Paediatric Intensive Care Settings,” International Journal of Antimicrobial Agents 61, no. 2 (2023): 106710. [DOI] [PubMed] [Google Scholar]
- 14. de Souza M. C. P., Dos Santos A. G., and Reis A. M. M., “Drug Utilization Study of Systemic Antifungal Agents in a Brazilian Tertiary Care Hospital,” International Journal of Clinical Pharmacy 38 (2016): 1398–1406. [DOI] [PubMed] [Google Scholar]
- 15. CLSI , Performance Standards for Antifungal Susceptibility Testing of Filamentous Fungi, 2nd ed. (Malvern, PA, USA, 2020). [Google Scholar]
- 16. Johnson M. D., Lewis R. E., Dodds Ashley E. S., et al., “Core Recommendations for Antifungal Stewardship: A Statement of the Mycoses Study Group Education and Research Consortium,” Journal of Infectious Diseases 222 (2020): S175–S198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saeed U., Sherdil K., Ashraf U., et al., “Identification of Potential Lockdown Areas During COVID‐19 Transmission in Punjab, Pakistan,” Public Health 190 (2021): 42–51, 10.1016/j.puhe.2020.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yusuf A., “Cancer Care in the Time of COVID‐19—A Perspective From Pakistan,” Ecancermedicalscience 14 (2020): 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rayens E., Rayens M. K., and Norris K. A., “Demographic and Socioeconomic Factors Associated With Fungal Infection Risk, United States, 2019,” Emerging Infectious Diseases 28, no. 10 (2022): 1955–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar J., Singh A., Seth R., Xess I., Jana M., and Kabra S. K., “Prevalence and Predictors of Invasive Fungal Infections in Children With Persistent Febrile Neutropenia Treated for Acute Leukemia–A Prospective Study,” Indian Journal of Pediatrics 85 (2018): 1090–1095. [DOI] [PubMed] [Google Scholar]
- 21. Otto W. R. and Green A. M., “Fungal Infections in Children With Haematologic Malignancies and Stem Cell Transplant Recipients,” British Journal of Haematology 189, no. 4 (2020): 607–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jordan A., James A. E., Gold J. A., et al., “Investigation of a Prolonged and Large Outbreak of Healthcare‐Associated Mucormycosis Cases in an Acute Care Hospital—Arkansas, June 2019–May 2021,” Open Forum Infectious Diseases 9, no. 10 (2022): ofac510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marins T. A., Marra A. R., Edmond M. B., et al., “Evaluation of Candida Bloodstream Infection and Antifungal Utilization in a Tertiary Care Hospital,” BMC Infectious Diseases 18, no. 1 (2018): 187, 10.1186/s12879-018-3094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Awad L., Tamim H., Abdallah D., et al., “Correlation Between Antifungal Consumption and the Distribution of Candida Species in Different Hospital Departments of a Lebanese Medical Centre,” BMC Infectious Diseases 18 (2018): 589, 10.1186/s12879-018-3512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bilal H. M., Ayaz S., and Iqbal N., “Predictors of Candidemia Infections and Its Associated Risk of Mortality Among Adult and Pediatric Cancer Patients: A Retrospective Study in Lahore, Punjab, Pakistan,” Archives of Cancer Science and Therapy 2 (2018): 1–7. [Google Scholar]
- 26. Ramírez‐Carmona W., Fernandes G. L. P., Díaz‐Fabregat B., et al., “Effectiveness of Fluconazole as Antifungal Prophylaxis in Cancer Patients Undergoing Chemotherapy, Radiotherapy, or Immunotherapy: Systematic Review and Meta‐Analysis,” APMIS 131, no. 11 (2023): 668–684, 10.1111/apm.13324. [DOI] [PubMed] [Google Scholar]
- 27. Mishra P., Agrawal N., Bhurani D., and Agarwal N. B., “Invasive Fungal Infections in Patients With Acute Myeloid Leukemia Undergoing Intensive Chemotherapy,” Indian Journal of Hematology and Blood Transfusion 36 (2020): 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fondevilla E., Grau S., Mojal S., Palomar M., Matas L., and Gudiol F., “Consumption of Systemic Antifungal Agents Among Acute Care Hospitals in Catalonia (Spain), 2008–2013,” Expert Review of Anti‐Infective Therapy 14, no. 1 (2016): 137–144. [DOI] [PubMed] [Google Scholar]
- 29. Tragiannidis A., Gkampeta A., Vousvouki M., Vasileiou E., and Groll A. H., “Antifungal Agents and the Kidney: Pharmacokinetics, Clinical Nephrotoxicity, and Interactions,” Expert Opinion on Drug Safety 20, no. 9 (2021): 1061–1074. [DOI] [PubMed] [Google Scholar]
- 30. Raza A., Zafar W., Mahboob A., Nizammudin S., Rashid N., and Sultan F., “Clinical Features and Outcomes of Candidaemia in Cancer Patients: Results From Pakistan,” JPMA. Journal of the Pakistan Medical Association 66 (2016): 584–589. [PubMed] [Google Scholar]
- 31. Jabeen K., Khan M., Umar S., Shaheen N., and Farooqi J., “Spectrum of Fungal Pathogens in Burn Wound Specimens: Data From a Tertiary Care Hospital Laboratory in Pakistan,” Journal of Burn Care & Research 42, no. 2 (2021): 241–244. [DOI] [PubMed] [Google Scholar]
- 32. Hussain W., Bashir S., Mirza I. A., et al., “Frequency and Antifungal Susceptibility Pattern of Candida Species Causing Candidemia in Bone Marrow Transplant Unit,” Pharmaceutical and Biosciences Journal 9 (2021): 45–49. [Google Scholar]
- 33. Khan M., Ahmed J., Gul A., Ikram A., and Lalani F. K., “Antifungal Susceptibility Testing of Vulvovaginal Candida Species Among Women Attending Antenatal Clinic in Tertiary Care Hospitals of Peshawar,” Infection and Drug Resistance 11 (2018): 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Routsi C., Meletiadis J., Charitidou E., et al., “Epidemiology of Candidemia and Fluconazole Resistance in an ICU Before and During the COVID‐19 Pandemic Era,” Antibiotics 11, no. 6 (2022): 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee Y., Puumala E., Robbins N., and Cowen L. E., “Antifungal Drug Resistance: Molecular Mechanisms in Candida Albicans and Beyond,” Chemical Reviews 121, no. 6 (2021): 3390–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Díaz‐García J., Mesquida A., Sánchez‐Carrillo C., et al., “Monitoring the Epidemiology and Antifungal Resistance of Yeasts Causing Fungemia in a Tertiary Care Hospital in Madrid, Spain: Any Relevant Changes in the Last 13 Years?,” Antimicrobial Agents and Chemotherapy 65, no. 4 (2021): e01827–20, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gupta A. K., Venkataraman M., Renaud H. J., Summerbell R., Shear N. H., and Piguet V., “The Increasing Problem of Treatment‐Resistant Fungal Infections: A Call for Antifungal Stewardship Programs,” International Journal of Dermatology 60, no. 12 (2021): e474–e479. [DOI] [PubMed] [Google Scholar]
- 38. Majumder M. M. I., Mahadi A. R., Ahmed T., Ahmed M., Uddin M. N., and Alam M. Z., “Antibiotic Resistance Pattern of Microorganisms Causing Urinary Tract Infection: A 10‐Year Comparative Analysis in a Tertiary Care Hospital of Bangladesh,” Antimicrobial Resistance & Infection Control 11, no. 1 (2022): 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saleem Z., Godman B., Azhar F., et al., “Progress on the National Action Plan of Pakistan on Antimicrobial Resistance (AMR): A Narrative Review and the Implications,” Expert Review of Anti‐Infective Therapy 20, no. 1 (2022): 71–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


