Abstract
Hypertrophic cardiomyopathy (HCM) is a non-rare genetic cardiomyopathy, with an estimated prevalence of 1:500, characterized by an increase in the left ventricular wall thickness in the absence of increased loading conditions. The hypertrophy is mostly asymmetric and predominantly affects the basal septum and anterior wall. Left ventricular outflow tract obstruction, at rest or after provocative tests, is detected in many patients and represents the primary cause of reduced functional capacity, as well as an independent predictor of sudden cardiac death and advanced heart failure. Until ∼1 year ago, symptomatic patients despite maximal therapy with β-blockers or calcium channel blockers plus disopyramide had only basal septal reduction therapy through myectomy or septal alcoholization as additional therapeutic options. Today, a new class of drugs that inhibit cardiac myosin activity is available for patients with obstructive HCM. In light of the new treatment perspectives, the correct clinical-therapeutic classification of affected patients becomes of fundamental importance for the cardiologist. The aim of this position paper is to increase the knowledge of cardiologists in the field of HCM, defining its epidemiological, genetic, and pathological characteristics, identifying the diagnostic criteria and instrumental methods capable of stratifying the risk profile, with the aim of an optimal therapy tailored on the single patient.
Keywords: Hypertrophic cardiomyopathy, Ventricular hypertrophy, Heart failure, Diagnosis, Therapy
Graphical Abstract
Graphical Abstract.
Integrated approach to hypertrophic cardiomyopathy, which includes genetic evaluation, family screening, morphological characterization, evaluation of disease signs and symptoms, electrocardiogram study, multimedia imaging, differential diagnosis with phenocopies, and personalized treatment.
Introduction
Hypertrophic cardiomyopathy (HCM) represents the most frequent hereditary cardiomyopathy with a prevalence in the general population of 1:500. According to the recent guidelines,1-3 it is characterized by the presence of left ventricular (LV) hypertrophy with wall thickness > 15 mm in any myocardial segment, in the absence of increased loading conditions. In first-degree relatives of affected patients, a LV wall thickness > 13 mm is diagnostic. In paediatric patients, the diagnosis of HCM requires a LV wall thickness > 2 SD from the predicted value.
From a pathophysiological point of view, HCM is characterized by hypertrophy and disarray of cardiomyocytes, interstitial replacement fibrosis, and hypertrophy of the smooth muscle cells of the intramyocardial vessels. Diagnosis requires careful clinical evaluation, imaging methods, and genetic analysis, since pathogenic variants in sarcomeric genes are found in ∼40% of affected patients. Moreover, in the diagnostic workup of HCM, the exclusion of phenocopies is mandatory, such as Fabry disease (FD) and cardiac amyloidosis (CA). Developments in imaging techniques, in particular echocardiography and cardiac magnetic resonance imaging (MRI), have broadened the diagnostic capacity in the field of HCM, making the recognition of LV outflow obstruction (LVOTO) easier as well as the tissue characterization of the disease. Together with the improvement in the disease knowledge, a new therapeutic approach has been developed with cardiac myosin inhibitors, acting at the sarcomeric level to reduce the hypercontractility and improve symptoms by reducing the LVOTO.
The aim of this document is to provide the clinical cardiologist with a detailed knowledge of the epidemiological, genetic, diagnostic, and prognostic aspects of HCM, outlining the appropriate diagnostic–therapeutic patient pathway (Graphical Abstract).
Epidemiology of hypertrophic cardiomyopathy
Considered a rare disease for decades, HCM is currently the most common form of cardiomyopathy with an estimated prevalence of 0.2–0.5% in adults and 1.2/1 000 000 in the paediatric population. The increase in the epidemiological impact of HCM is mainly due to improved diagnostic tools: the advancement of imaging techniques, including echocardiography and cardiac MRI, has certainly contributed to the increase in the number of HCM diagnoses and the extension of family screening.4 The extensive use of genetic analysis suggested by the latest European Society of Cardiology (ESC) guidelines has favoured the formulation of early diagnoses and allowed healthy mutation carriers to be followed up over time, contributing to increasing the epidemiological impact of cardiomyopathy.2,3 In recent decades, the prevalence of HCM has increased markedly from 1:5000 (based on data from tertiary referral centres with an influx of highly selected populations) to 1:500, a result obtained from community screening applied in the CARDIA study with subsequent confirmation from international epidemiological studies.5 Estimating all carriers of sarcomere mutations, including those who do not express a phenotype of HCM, leads to a prevalence of 1:200, even though hospital and insurance data arrive at a figure of 1 affected person per 3200 inhabitants.6 The gap between screening data and the real world reflects the low proportion of HCM patients who come to hospital for major clinical events. In fact, the natural history of HCM is generally characterized by slow progression and a low rate of events, even though the clinical and psychological impact associated with cardiomyopathy is still considerable, especially due to the young age of the affected patients, family involvement, repercussions on lifestyle, and physical activity, which justify the need to also consider the psychological aspects and socio-economic consequences of this form of cardiomyopathy.7 The clinical presentation of HCM may occur in the early years of life often in the context of syndromic pictures with complex genetic substrates and poor prognosis or in young adults around the fourth decade with the typical genetic substrate of a sarcomeric disease. The Share Registry has documented how the severity of disease is inversely correlated with age at diagnosis.8 Young age at diagnosis (20–29 years) was associated with a higher incidence of both progression to heart failure and major arrhythmic events, although most events occurred in the third or fourth decade. The mortality rate was four-fold higher in the presence of severe LV hypertrophy (LVH), a large fibrotic component at cardiac magnetic resonance (CMR), detected as late gadolinium enhancement (LGE ≥ 15% of myocardium), left atrial dilatation, a history of sudden cardiac death (SCD), syncope, and the occurrence of episodes of non-sustained ventricular tachycardia.
Genetic testing and family screening in hypertrophic cardiomyopathy
Approximately 30–40% of HCM cases have a genetic aetiology, with a predominantly autosomal dominant (AD) inheritance, characterized by incomplete penetrance and variable expressivity. In adult patients, the majority of causative variants are identified within a core of eight sarcomeric genes, such as MYH7, MYBPC3 (in ∼40% of cases), and TNNT2, TNNI3, TPM1, ACTC1, MYL2, and MYL3. Recently, other loci have been identified as ‘minor genes’ (i.e. ACTN2, CSRP3, TNNC1, FHOD3, FLNC, and PLN) in ∼5% of cases. Genetic testing should include, in addition to sarcomeric genes, also genes essential for the differential diagnosis (phenocopies) and genes responsible for rare syndromic disorders (Tables 1 and 2). Identifying the phenocopies and syndromes is indeed essential both for the availability of targeted therapies (as in the case of FD or transthyretin amyloidosis) and for an individualized clinical management (such as arrhythmia prevention in the case of PRKAG2 glycogenosis).
Table 1.
List of ‘definitive’ sarcomeric genes recommended for inclusion in hypertrophic cardiomyopathy panels
| Gene | Inheritance | Diagnostic yield |
|---|---|---|
| ACTC1 | AD | 0.1–1% |
| MYBPC3 | AD | 15–20% |
| MYH7 | AD | 10–15% |
| MYL2 | AD | <0.1% |
| MYL3 | AD | <0.1% |
| TNNI3 | AD | 1–2% |
| TNNT2 | AD | 1–2% |
| TPM1 | AD | 0.1–1% |
| ACTN2 | AD | <0.1% |
| CSRP3 | AD | <0.1% |
| TNNC1 | AD | <0.1% |
| FHOD3 | AD | <0.1% |
Table 2.
List of genes recommended for inclusion in hypertrophic cardiomyopathy panels for differential diagnosis
| Gene | Inheritance | Diagnostic yield | Disease |
|---|---|---|---|
| GLA | XL | 0.1–0.5% | Fabry disease |
| FHL1 | XL | <0.1% | FHL1-related muscular dystrophy |
| FLNC | AD | <0.1% | FLNC-related filaminopathy |
| LAMP2 | XL | <0.1% | Danon disease |
| PLN | AD | <0.1% | PLN-related cardiomyopathy |
| PRKAG2 | AD | <0.1% | PRKAG2-related cardiomyopathy |
| PTPN11 | AD | <0.1% | Noonan syndrome |
| RAF1 | AD | <0.1% | Noonan syndrome |
| RIT1 | AD | <0.1% | Noonan syndrome |
| TTR | AD | <0.1% | Transthyretin amyloidosis |
The probability of identifying a causative genetic variant is higher in younger patients with a positive family history, while it is lower in older sporadic patients.
Genetic testing is recommended in patients who fulfil the diagnostic criteria for HCM (Figure 1).9-12 The clinical diagnosis must be as accurate as possible to direct the genetic test and facilitate its interpretation. For this reason, it is important that the diagnosis be made by experienced cardiologists. Genetic testing must be performed in the affected proband, and, if a causative genetic variant is identified, the search of this specific variant can be offered to other family members. This cascade testing would indeed identify individuals who are at risk of developing the disease. Close cardiological surveillance over time is then indicated for these individuals. Conversely, if a family member has not inherited the causative variant, the risk of manifesting HCM is negligible and cardiological follow-up should not be continued. Pre-symptomatic genetic testing in the case of an unaffected minor can be performed, but with caution and only balancing the benefits and disadvantages.
Figure 1.
Clinical-genetic course of hypertrophic cardiomyopathy. HCM, hypertrophic cardiomyopathy.
Genetic testing in the HCM patients offers the following opportunities: the identification of the genetic cause, distinguishing phenocopies, the cascade study of the family, the access to genetic counselling, and the access to emerging therapies; e.g. for the MYBPC3 and LAMP2 genes, there are active gene therapy trials (https://clinicaltrials.gov).
Due to technical and knowledge limitations, it is not possible to identify the genetic cause of HCM in ∼60% of the patients with a definitive clinical diagnosis. Genetic testing is currently not useful for risk stratification or prognostic purposes, except for rare cases.
Genetic testing must be performed as part of a pre-test and post-test genetic counselling conducted by appropriately trained health professionals.
Starting from a blood sample, the patient’s genomic DNA is extracted and a panel of genes strongly or moderately associated with HCM is sequenced (Tables 1 and 2).2,9,10 The interpretation process is very complex and requires the interaction between the laboratory personnel and the referring cardiologist. The identified variants are classified into five classes according to current guidelines: pathogenic (Class 5), probably pathogenic (Class 4), variants of uncertain or unknown significance (VUS, Class 3), probably benign (Class 2), and benign (Class 1).11
Genetic testing can therefore have three possible outcomes: (1) Identification of one or more variants that can be interpreted as causative (pathogenic or probably pathogenic variant). In this scenario, the genetic cause is identified and cascade testing in family members is indicated. (2) Identification of one or more variants that are not clearly classifiable (VUS), for which cascade testing in family members is not indicated; VUS must be reassessed over time. (3) The genetic cause of the disease is not identified. For Results (2) and (3), a genetic cause of HCM cannot be ruled out; for this reason, the patient’s family members will still need to be followed up by regular clinical follow-up. In other words, the genetic test is informative in Case 1 whereas in Cases 2 and 3 it is not informative.
Genetic testing is essential for diagnosing HCM, managing at-risk family members, and making therapeutic choices. However, genetic testing requires a strict application of appropriateness criteria. At the moment, the impact of genetic testing over HCM risk stratification and prognosis is still limited.
Macroscopic and microscopic anatomy
Hypertrophic cardiomyopathy is a pathology characterized by great heterogeneity of phenotypic expression.12 The pathology encompasses different degrees of hypertrophy, from the most severe forms to mild and segmental forms that may also be associated with a normal cardiac mass, to forms of HCM ‘without hypertrophy’. In typical cases, LVH is regional and asymmetric and usually involves the basal portion of the septum and, to a lesser extent, the anterior wall (Figures 2 and 3). However, hypertrophy may involve any portion of the myocardium and occasionally also the papillary muscles and the right ventricle. A small percentage of patients show symmetric concentric hypertrophy, and it is this form of HCM that most often enters into differential diagnosis with physiological hypertrophy in athletes, hypertrophy secondary to pressure overload, and hypertrophy related to other disorders (amyloidosis, Fabry, glycogenosis, etc.). Other variants are the mid-ventricular one, in which hypertrophy is localized at the level of the papillary muscles, and the apical one, described mainly in Japan.
Figure 2.
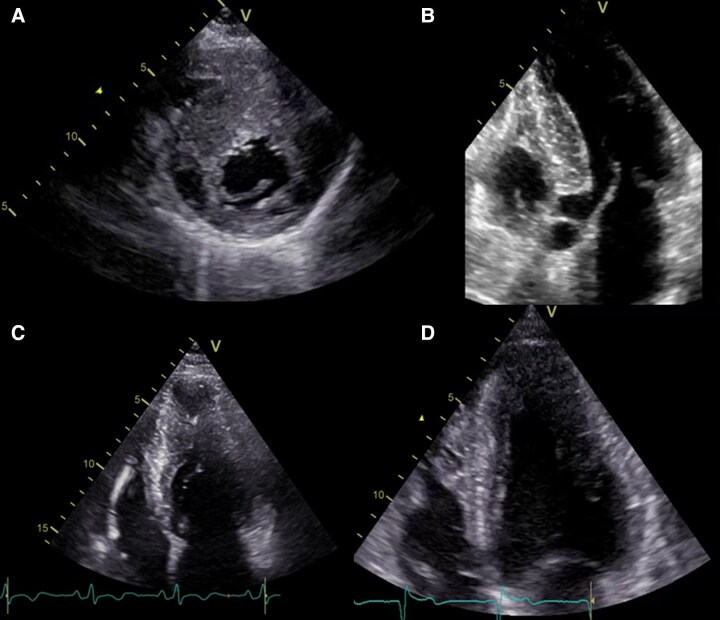
Hypertrophic cardiomyopathy: macroscopic pictures. (A) Asymmetrical septal form (case of sudden death). (B) Anterolateral asymmetric form (case of sudden death). (C) ‘End-stage’ form with reduced ejection fraction (case of death due to heart failure: diffuse intramyocardial fibrosis with thinning of the walls of the left ventricle). (D) ‘End-stage’ form with reduced ejection fraction (case of heart transplantation): diffuse intramyocardial fibrosis prevailing in the septum with thinning (bar = 20 mm in A–D).
Figure 3.
Hypertrophic cardiomyopathy: histological pictures. (A) Hypertrophy of myocytes and ‘disarray’ of individual cardiomyocytes (haematoxylin-eosin). (B) Fascicular ‘disarray’ with interstitial fibrosis (Azan Mallory trichrome). (C) Hypertrophy of the media with narrowing of the intramyocardial arteriole lumen (Azan Mallory trichrome). (D) Patches of replacement fibrosis in the context of cardiomyocyte ‘disarray’ (Azan Mallory trichrome).
In more than 50% of cases, primary morphological alterations of the mitral valve leaflets are present, and in more than 25%, abnormalities of the subvalvular apparatus (chordae tendineae and papillary muscles) are found. The valve leaflets are enlarged and elongated, and the papillary muscles are often hypertrophic, sometimes they are supernumerary, bifid, displaced anteriorly/around the apex, and may have abnormal insertions directly on the valve leaflets. All of these alterations contribute to the genesis of systolic anterior motion (SAM) of the anterior mitral valve leaflet—responsible for LVOTO and functional valve insufficiency.
Deep myocardial crypts have been documented at autopsy of HCM patients from the earliest pathological series. Crypts are visible not only macroscopically but also histologically as large cracks, in the context of the disarray, lined with endocardial tissue and communicating with the ventricular cavities.
Another common phenotypic feature in HCM is the so-called ‘myocardial bridge’ or intramyocardial course of the anterior descending branch of the left coronary artery. A systematic pathology study demonstrated a higher frequency of myocardial bridging and greater depth in HCM than in controls, but depth did not correlate with the presence of septal myocardial scarring or with a particular clinical outcome (death from heart failure or sudden death).13
The ventricular remodelling phase of HCM is characterized by cavity dilatation and extensive myocardial scarring leading to deterioration of contractile function. This progression could be attributed to recurrent myocardial ischaemia.
Systolic obstruction of the mid-ventricular cavity, which results from the complete apposition of hypertrophic walls and papillary muscles, leads to separation of the apical and basal regions of the LV and may be associated with apical ischaemia, scarring, and even aneurysm formation with parietal thinning and dyskinesia/kinesia of the involved segment. Apical aneurysms have been found in 25% of patients with mid-ventricular HCM.
Histopathologically, the most salient features of HCM are hypertrophy and spatial disorganization (disarray) of cardiomyocytes, interstitial and replacement fibrosis, and microcirculation pathology.14 The disarray may be of the muscle bundles with the interposition of collagen bands or of the individual myocytes. It is very variable in extent. Its specificity is not absolute, as it can also be found in hearts with secondary hypertrophy or even in normal hearts and is said to be diagnostic for HCM when it involves at least 5% of the surface area considered by histological examination.
Another important feature of HCM is the presence of fibrosis, i.e. proliferation of fibroblasts with increased deposition of collagen fibres.15 From a morphological point of view, a distinction is made between interstitial fibrosis, which consists of a general increase in pericellular, intercellular, and fascicular connective tissue; perivascular fibrosis; and replacement fibrosis, which manifests itself in the form of microscopic or macroscopic scars also described early in cases of juvenile sudden death. Replacement fibrosis is thought to be the consequence of cardiomyocyte death caused by stress imposed primarily by alterations in sarcomere proteins but also by ischaemia due to various factors, such as reduced capillary density, myocyte disarray, interstitial fibrosis, and increased basal oxygen demand by hypertrophic cardiomyocytes. Furthermore, in patients with severe outflow obstruction and diastolic dysfunction, the pathological increase in intracavitary pressures and wall stress may cause a further increase in extravascular compressive forces at the subendocardial level.
Intramyocardial small vessel disease is demonstrated in autopsy studies in up to 80% of the hearts of HCM patients. The vessel wall is thickened by hypertrophy of the middle tonaca associated with an abundance of disorganized elastic fibres and hyperplasia of the intima, resulting in a reduced vascular lumen.
Signs and symptoms of hypertrophic cardiomyopathy: red flags and diagnostic algorithm
Hypertrophic cardiomyopathy is characterized by a variable clinical presentation and prognosis. In general, as early as 2016, Maron et al.16 highlighted how HCM had become a ‘treatable’ condition thanks to early diagnosis, familial, and genetic screening, the use of cardiac MRI, and population-based studies, which allowed early recognition and treatment of patients at increased risk of arrhythmias, SCD, and heart failure.
A recent study by the Florence group17 found a mortality rate of 1.3%/year, with more than 50% of the causes of death correlated with cardiomyopathy (heart failure, ischaemic stroke, and SCD), with an increase in cases related to time from diagnosis (0.7% during the first decade and 1.8% in the second/third decade), mainly driven by the increase in heart failure/stroke-related events (from 0.6% to 1.3%). This is because HCM has the characteristic of being an evolving pathology with a more or less rapid degeneration of cardiac structures and with consequent clinical manifestations that vary over time.
In most cases, HCM can run asymptomatic or paucisymptomatic. However, certain morpho-functional features specific to the disease can lead to the onset of mild, severe symptoms and even death from heart failure and SCD.
The major determinants of the onset of symptoms in HCM may be:
Degree of myocardial hypertrophy and possible diastolic dysfunction;
Mitral valve alterations, presence of SAM/outflow obstruction on LVOT, and mitral valve insufficiency;
Left atrial dilatation with associated risk of atrial fibrillation/ischemic stroke, mainly due to increased pressures in the left atrium;
Myocardial fibrosis and increased risk of malignant ventricular arrhythmias; and
-
Extensive myocardial fibrosis and possible LV dilatation and dysfunction, i.e. progression to a ‘burn out/end stage phase’. The main symptoms of HCM are as follows:
Dyspnoea on exertion: this is one of the main symptoms of cardiomyopathy; it may depend on hypertrophy per se, diastolic dysfunction, the presence of gradient on LVOTO, mitral insufficiency, or progression to more advanced forms of systolic dysfunction and heart failure; these symptoms may typically be accentuated after meals.
Chest pain: may be present either due to associated coronary artery disease, abnormal course of the coronary arteries, or microvascular involvement.
Palpitations: these are often related to the presence of both supraventricular (particularly atrial fibrillation in patients with left atrial dilatation) and ventricular arrhythmias.
Hypotension/syncope: may be due either to the presence of gradient on LVOTO when presenting predominantly under stress or linked to the presence of arrhythmias.
Reduced exercise tolerance/signs and symptoms of heart failure: may be due to either the presence of gradient on LVOTO or progression to a ‘burn out’ phase with LV systolic dysfunction.
Cardiac arrest/SCD: mainly due to malignant ventricular arrhythmias in high-risk patients.
For each of these symptoms, since they are related to a specific cause, pharmacological, interventional, and surgical therapies have been implemented over the years, which together have led to a marked improvement in the outcome of this pathology.
The diagnosis of HCM requires a careful differential diagnostic process in order to distinguish this condition (the genetic/sarcomeric form) from a series of pathologies that involve an increase in parietal myocardial thicknesses but that underlie a different cause called ‘phenocopies’.
Like sarcomeric HCM, even more phenocopias are pathologies that can evolve over time and progressively present different clinical and instrumental manifestations. This is why the latest European guidelines on cardiomyopathies, published in 2023,2-18 strongly push for the systematic application of a well-defined diagnostic pathway for all patients. Table 3 summarizes the main clinical ‘red flags’ with their clinical suspicion.
Table 3.
Red flags of cardiomyopathies with hypertrophic phenotype
| Clinical variable | Red flags | HCM/possible phenocopy |
|---|---|---|
| Age of onset | Childhood | Mitochondrial disease, Pompe disease |
| Adolescence | Mitochondrial disease, PRKAG2 disease, HCM | |
| Adulthood | Fabry disease, Danon disease, PRKAG2 disease HCM | |
| Old age | Amyloidosis | |
| Inheritance | X-linked | Fabry disease, Danon disease |
| Autosomal D | Amyloidosis, Mitochondrial diseases, PRKAG2 syndrome, RASopathies, HCM | |
| Autosomal R | Friedreich ataxia, Pompe disease | |
| Matrilineare | Mitochondrial disease | |
| Laboratory tests | High transaminases | Danon disease, Pompe disease |
| Reduced GFR | Fabry disease, amyloidosis | |
| CPK rise | Danon disease, mitochondrial disease, PRKAG2 syndrome | |
| High lactic acid | Mitochondrial disease | |
| Systemic manifestations | Muscle weakness | Danon disease, mitochondrial disease, PRKAG2 syndrome |
| SNP involvement | Amyloidosis, Fabry disease | |
| Stroke | Fabry disease, mitochondrial disease | |
| Carpal tunnel syndrome | Amyloidosis (TTR) | |
| Mental retardation | Danon disease, mitochondrial disease | |
| Facial dysmorphism | RASopathies | |
| Walking disorders | Friedreich ataxia | |
| ECG | High voltages | Fabry disease, PRKAG2 syndrome, Danon disease, HCM |
| Low voltages | Amyloidosis | |
| Pre-excitation | PRKAG2 syndrome, Fabry disease, Danon disease, | |
| AV blocks | Amyloidosis, PRKAG2 syndrome, Danon disease | |
| Short PR | Fabry disease, mitochondrial disease | |
| Echocardiography | Massive hypertrophy | PRKAG2 syndrome, Danon disease, HCM |
| Granular sparkling | Amyloidosis | |
| Pericardial effusion | Amyloidosis | |
| Diffuse hypokinesia | Danon disease, PRKAG2 syndrome | |
| Valve thickening | Amyloidosis | |
| Mitral valve alterations | HCM | |
| MRI heart | LGE side postero | Fabry disease |
| Diffuse subendocardial LGE | Amyloidosis | |
| T1 mapping low | Fabry disease | |
| T1 mapping high | Amyloidosis | |
| Apical aneurysm | HCM |
The main phenocopies are as follows:
Secondary hypertrophy: forms due to the presence of a haemodynamic cause such as valvular pathologies (aortic stenosis), intense sports activity (athlete's heart), and uncontrolled arterial hypertension (hypertensive heart disease).
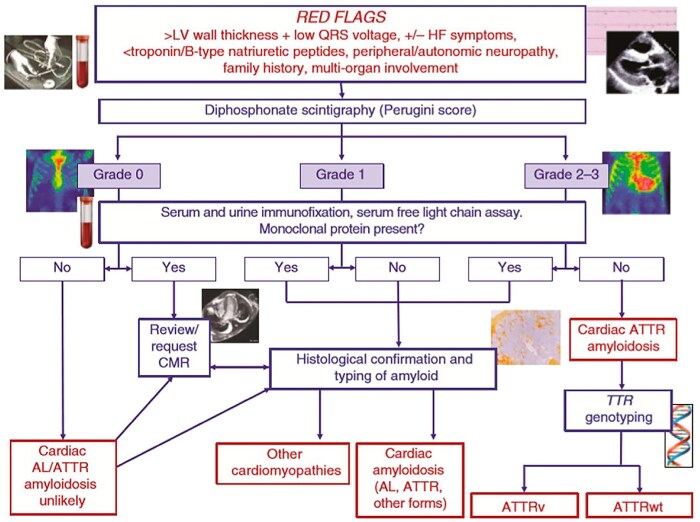
‘Pseudo-hypertrophy’: represented in particular by CA, in its light chain (AL) or transthyretin (TTR) senile or wild-type forms characterized by the presence of ‘amyloid fibrils’ deposited in the intercellular space; inflammatory diseases.
Metabolic diseases: FD, an X-linked genetic disease characterized by a total or partial deficiency of the enzyme α-galactosidase A (α-Gal A) leading to the accumulation of globotriaosylceramide at the cardiac and extracardiac levels; Danon disease, an X-linked clinical syndrome due to the presence of mutations in the Lamp-2 gene; PRKAG2 syndrome, due to an autosomal dominant mutation that leads to a deficiency in the enzyme ‘5′-AMP-activated protein kinase subunit gamma-2’ resulting in hypertrophy, pre-excitation, arrhythmogenicity; Pompe disease, an autosomal recessive genetic disease caused by pathogenic variants in the α-glucosidase acid gene located on chromosome 17q25. 2-q25.3 leading to reduced levels and function of the enzyme.
Mitochondrial diseases (e.g. MELAS syndrome) that lead to hypertrophy in 20–40% of patients and involve organs with high energy metabolism (brain, kidney, and skeletal muscle), although with extreme variability, the onset is more frequent in paediatric age.
Rare diseases/syndromes (e.g. Kearns–Sayre syndrome, Friedreich's ataxia, and RASopathies), rare syndromes with significant extracardiac involvement.
Rare diseases/paediatric diseases: e.g. RASopathies.
Electrocardiographic red flags in the differential diagnosis
The critical reading of the electrocardiogram (ECG) is a crucial element in the evaluation of patients with HCM. A pathological ECG may be the only manifestation of the disease at an early stage. Furthermore, although the presence of LVH, considered as a wall thickness ≥ 15 mm, is the diagnostic marker of HCM, it is not disease specific. In this context, reading the ECG with a ‘red flags approach’ is extremely useful for the differential diagnosis between sarcomeric HCM and its phenocopies (storage diseases and infiltrative diseases)19 (Figure 4). Fabry disease, characterized by glycosphingolipid accumulation that increases the speed of the conduction system, should be suspected in patients with LVH and a short PR interval. Other frequent signs are the presence of the right bundle branch block, R wave in aVL ≥ 1.1 mV, and ST depression in the lower leads. The finding of Q waves with positive T waves in the same leads (QT vector discordance) and giant T waves (0.10 mm) in the anterolateral leads supports the diagnosis of HCM. Short PR interval and/or ventricular pre-excitation can also be commonly found in other storage diseases (glycogenosis, Danon disease, PRKAG2 mutations, and Pompe disease) as well as in mitochondrial diseases (MELAS and MERFF). In the long term, storage and mitochondrial diseases may progress to atrioventricular conduction delay and result in advanced AV block. The latter is also a common complication of infiltrative disorders such as CA.
Figure 4.
Electrocardiographic red flags in the differential diagnosis of hypertrophic cardiomyopathy. AVB, atrioventricular block; RBBB, right bundle branch block; LVH, left ventricular hypertrophy; ST lat depr, lateral ST depression; Pre-exc ventr, ventricular pre-excitation; Neg Lat T, T negative in lateral leads; QT VT, QT vector discordance.
Hypertrophic cardiomyopathy and storage diseases are invariably accompanied by high amplitude QRS complexes with LVH voltage criteria, ST depression, and T-wave inversion in lateral leads,20-22 the latter being typical of HCM with predominantly apical localization. In CA, on the other hand, the expansion of the myocardial interstitium secondary to extracellular infiltration can cause low QRS voltages and, above all, a relative reduction in QRS voltage with respect to the degree of ventricular hypertrophy, unlike in HCM. In the latter, the reduction in QRS voltage occurs only in ‘end-stage’ forms of the disease when hypertrophy gives way to extensive fibrosis. Pseudonecrosis Q waves (i.e. in the presence of uninjured coronary arteries), common in HCM, are also frequent in CA, secondary to amyloid deposition, are mainly anterior in location, and coexist with low voltages.
Basic and advanced echocardiography
Due to its accessibility and its ability to provide detailed information on cardiac morphology and function, basic and advanced echocardiography plays a central role in both the first diagnosis and follow-up of HCM patients and in the screening of first-degree relatives at risk.
The first step is LV telediastolic thickness measurement with two-dimensional parasternal long-axis and short-axis images. To avoid overestimation, it is essential to exclude false tendons, ventricular trabeculae, and structures such as the moderator band or supraventricular ridge. The diagnosis of HCM is made in the presence of a myocardial thickness ≥ 15 mm in any segment, not explained by overload conditions. This cut-off may be reduced in first-degree relatives of diagnosed patients in whom a cut-off value ≥ 13 mm is considered diagnostic for HCM.23
Closely related to the quantification of ventricular hypertrophy is the analysis of its distribution, which will most often be asymmetrical, with a prevalent involvement of the basal anterior interventricular septum (IVS) determining an anterior septal thickness to posterior wall ratio >1.3 in normotensive patients and >1.5 in hypertensive patients.24
The correlation between hypertrophy distribution and septal morphology allows five different HCM geometries to be identified (Figure 5):
Figure 5.
Different hypertrophic cardiomyopathy geometries at two-dimensional echocardiography: (A) Reverse septal curvature. (B) Sigmoid septum. (C) Mid-ventricular. (D) Apical.
Reverse septal curvature characterized by hypertrophy of the IVS with convexity of the middle tract predominant towards the LV cavity, which often takes on a crescent shape.
Sigmoid septum with a generally oval LV cavity, IVS concave towards the cavity, and a prominent basal bulge.
Mid-ventricular with predominant mid-ventricular hypertrophy.
Apical with predominantly apical segment hypertrophy.
Neutral septum characterized by a generally linear IVS with no predominant convexity or concavity towards the LV cavity.
This classification is predictive of positivity in genetic testing, with a lower rate in patients with sigmoid septum and a higher rate in patients with reverse curvature.
Evaluation of hypertrophy must be complemented by careful characterization of abnormalities of the mitral valve and papillary muscles. A common finding in patients with HCM is elongation of both mitral leaflets resulting in a point of coaptation at the level of the leaflet body rather than at the ends with a consequent extension of the residual part of the anterior leaflet towards the outflow tract in systole. This phenomenon may be associated with abnormalities of the papillary muscles such as dislocation of the base of the anterolateral papillary muscle, bifid papillary muscles, abnormal insertions of the papillary muscle directly on the anterior flap, hypertrophy of the papillary muscles, hypermobile accessory papillary muscles, and, less commonly, the presence of accessory cords. These alterations will represent the anatomical substrate of mitral valve SAM and dynamic obstruction of the LV outflow tract and will also be responsible for the establishment of a systolic coaptation gap, leading to mitral insufficiency of variable degree with typically posterolateral jet with meso-telesystolic expression.25
Left ventricular ejection fraction (LVEF) assessed by biplane Simpson's method is generally normal or hypernormal in most HCM patients. Left ventricular dysfunction, defined as an LVEF < 50%, is observed in 4–9% of reported cases and is associated with high rates of all-cause mortality, need for cardiac transplantation or implantation of LV assist devices.26 The evaluation of systolic velocities of the septal and lateral loops with pulsed tissue Doppler can also be correlated with the ejection fraction data: a reduction in systolic velocities (Sa < 13 cm/s) may correlate with the presence of subclinical disease prior to the onset of frank hypertrophy, and a value < 4 cm/s indicates severe impairment of systolic function with independent prognostic value for hospital death and decompensation.27
The assessment of diastolic function in HCM patients is more complex.
In fact, due to the regionality of the disease, E′ annular velocities may be reduced secondary to myofibrillar disarray, and the possible concomitant presence of mitral insufficiency may further influence their estimation.
Conventional indices that correlate with adverse outcomes and/or increased filling pressures include the E/e′ ratio taking into account potential limitations stated, a tricuspid regurgitant jet velocity > 2.8 m/s, and an increase in left atrium size. Reporting these data in the ultrasound report is also crucial for prognostic purposes: an anteroposterior diameter > 48 mm is an independent predictor of cardiovascular mortality and heart failure. Similar results are observed for a biplane left atrial volume of more than 34 mL/m2.28
With regard to transmitral flow pattern assessment, evidence of a restrictive pattern (E/A ≥ 2; deceleration time ≤ 150 ms) is associated with a poor prognosis regardless of the degree of FE impairment.29
In addition to these commonly used diastolic assessment indices, it is also possible to associate a further parameter represented by the duration of pulmonary vein A-wave inversion (Ar) vs. mitral A-wave duration (Adur) indicative of high filling pressures when >30 ms. In contrast to the previous parameters, a significant difference between Ar and Adur will maintain diagnostic validity for high filling pressures even in the presence of haemodynamically significant mitral insufficiency.
Advanced echocardiography tools allow detailed analysis of cardiac function in HCM patients, improving diagnostic and predictive accuracy compared with traditional approaches, and are listed below.
Left cavity strain
Global longitudinal strain of the LV is a sensitive measure to detect regional and global changes in LV function, even in the presence of normal ejection fraction. In patients with HCM, strain abnormalities occur predominantly in areas of hypertrophy, such as in the septum in cases of classic septal hypertrophy or in the apex if there is an apical variant. Global longitudinal strain is an important predictor of event-free survival in patients with HCM and normal FE, emphasizing its prognostic value.
Left atrial strain is an emerging parameter, as in other diseases, for estimating the risk of recurrence of decompensation or atrial fibrillation, frequent events in the advanced stages of the disease.
Three-dimensional echocardiography
This method makes it possible to estimate the volumes and ejection fraction of the LV, as well as the LV mass. It must be emphasized that it is sometimes difficult to identify the blood–muscle interface due to prominent trabeculae or hypertrophic areas with particular disarray. There are automatic calculation systems that include a different edge identification setting, closer to that of the cardiac MRI, which may be useful in these patients. Three-dimensional echocardiography also allows a more accurate visualization of the SAM and the geometry of the LV outflow tract, e.g. assessing the area of the LVOT after myectomy.
Ultrasound with contrast medium
Its use makes it possible above all to provide accurate visualization of apical areas, often sites of hypertrophy, aneurysms, or thrombi, to identify myocardial crypts and to improve myocardial target localization for therapeutic procedures, such as during septal ablation.
Multiparametric assessment of left ventricular outflow tract obstruction
Left ventricular outflow obstruction is an important pathophysiological mechanism in HCM and a major cause of symptoms such as dyspnoea, chest pain, pre-syncope, and syncope.30 The presence of LVOTO is also associated with poor prognosis and an increased risk of SCD.31 The presence of LVOTO differentiates obstructive HCM from the non-obstructive form and radically alters the therapeutic approach and prognostic stratification of the patient.2,3 Approximately 30% of patients present with LVOTO at rest, while in a further 30% LVOTO may be dislodged by provocative manoeuvres or exercise.32
Echocardiography is the pivotal examination for the evaluation of LVOTO, as it allows the site of obstruction at rest and during exercise to be quantified and localized. Indeed, in patients who do not present LVOTO in basal conditions, provocative manoeuvres can be used or an echocardiogram during exercise can be performed, whereas the use of dobutamine is contraindicated.2,3 Re-evaluation during exercise is recommended in all patients who do not present a gradient at rest but are highly symptomatic (Figure 6). If the exercise echocardiogram also proves negative for LVOTO, in some of these patients, it may be useful to repeat the examination after a carbohydrate-rich meal, as demonstrated in a recent study.33 Echocardiography allows the mechanisms of LVOTO to be defined by measuring the extent of LVH and the presence of abnormalities of the mitral valvular and subvalvular apparatus such as elongation of the valve leaflets, the presence of thickened secondary chordae, and abnormalities of the papillary muscles that contribute in varying degrees to determining mitral valve SAM and thus LVOTO.34 Associated with SAM, mitral regurgitation with posteriorly and laterally directed jets is typically present.
Figure 6.
Evaluation and treatment of left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Exercise echocardiography may be considered on an individual basis in presence of symptoms referable to latent obstruction and when the presence of a left ventricular outflow tract gradient is relevant to lifestyle advice and drug therapy decisions. *Valsalva manoeuvre, squatting, change from clinostatism to orthostatism. **Consider repeat examination after meal. HCM, hypertrophic cardiomyopathy.
Colour Doppler and pulsed Doppler allow detection of the presence and location of LVOTO, while continuous Doppler allows the calculation of the extent of LVOTO. The Doppler signal typically has a meso-telesystolic (sabre-like) peak and may be preceded by an initial acceleration followed by rapid deceleration due to rapid contact of the anterior mitral leaflet with the IVS (lobster claw appearance). The best projections for measuring the gradient are apical five-chamber and apical three-chamber. It is important to have a good alignment with the outflow tract and avoid the possible signal from mitral regurgitation, whose jet has a rounder appearance, higher velocity, and holosystolic duration. If it is not possible to assess LVOTO flow without overlapping mitral regurgitation, it is possible to estimate the magnitude of LVOTO using the formula LVOTO = [(4V2 + PA) − PAS], where V is the mitral regurgitation velocity (V), PA the estimated atrial pressure, and PAS the measured systemic arterial pressure. The presence and magnitude of the gradient should also be assessed by provocative manoeuvres such as the Valsalva manoeuvre, switching from clinostatism to orthostatism or squatting (Figure 6). The presence of LVOTO in the absence of SAM should raise the suspicion of subaortic membrane, usually associated with a Doppler profile similar to aortic stenosis and aortic valve abnormalities (regurgitation).
The echocardiogram is also crucial in defining the indication for septal reduction procedures (septal alcoholization or surgical myectomy)2,3 and in planning these procedures. Prior to and during cardiac myectomy surgery, the transoesophageal echocardiogram is crucial in defining the thickness and longitudinal extent of the myectomy, the presence of any additional surgical targets such as abnormalities of the mitral valvular and subvalvular apparatus, papillary muscle changes, and the presence of muscle bands or subaortic membranes. It should be remembered that both morphological and functional valvular assessments can be altered by loading conditions (vasodilatation from anaesthetics and positive pressure ventilation with reduced venous return). In the post-operative period, the transoesophageal echocardiogram makes it possible to verify the effectiveness of the intervention and to rule out possible complications (aortic insufficiency, interventricular defect, and coronary septal fistulas).
Due to its excellent spatial resolution and the absence of limitations related to the acoustic window, cardiac magnetic resonance is important for accurately assessing the distribution of hypertrophy, the presence of any myocardial crypts or areas of thinning (to be avoided during the surgical approach in order to reduce the risk of interventricular defect), and papillary muscle abnormalities with any abnormal attachments on the mitral leaflets or accessory muscle bands that may contribute to LVOTO. When not contraindicated, cardiac MRI should always be performed for diagnostic completion, especially as part of pre-operative planning.
Cardiac computed axial tomography has excellent spatial resolution with the possibility of tomographic reconstruction and accurate morphological assessment. The examination can be useful in cases of complex, biventricular obstruction, to assess anatomical relationships with the coronary arteries, the presence of muscle bridges, or any significant coronary artery disease. The use of ionizing radiation and iodinated contrast medium, together with necessary technology that is not yet widely available, limit its routine use.
Role of cardiac magnetic resonance
CMR is currently considered an indispensable imaging method in the study of cardiomyopathies, including those with a hypertrophic phenotype. It allows precise definition of the extent and location of hypertrophy, calculation of ventricular myocardial mass, and fine tissue characterizations of documented utility in both diagnostic and prognostic definitions. The repeatability of CMR during follow-up of patients with ventricular hypertrophy is favoured by the non-use of ionizing radiation.
On morphological analysis, images obtained with steady-state free precession imaging cine sequences are characterized by an excellent signal contrast between the ventricular cavity and the myocardial wall, making it possible to define the ‘pattern’ of hypertrophy and its extent with extreme precision and also to identify additional morphological alterations potentially associated with HCM, such as myocardial crypts, mitral flaps of greater than normal length, and apical aneurysms.35 In addition, in patients with HCM, CMR may demonstrate abnormalities in papillary muscles in terms of thickness, number, and position. Abnormally displaced papillary muscles, such as those with an ‘apicalized’ implant, may contribute over time to the determinism of ‘apical’ forms. In this regard, the presence of initial apical hypertrophy (with a thickness cut-off > 5.6 mm/m2), associated with systolic obliteration of the ventricular cavity in the apex, and the presence in the apical segments of LGE are markers considered sufficient to define apical forms of HCM.36 However, as emphasized in the ESC guidelines for cardiomyopathies, the greatest usefulness of CMR lies in the non-invasive tissue assessment applicable not only in the differential diagnosis with other phenocopies of hypertrophy, such as CA and/or Anderson–Fabry disease, but also for defining markers of poor prognosis either as evolution in the ‘dilated phase’ of HCM or expression of malignant ventricular arrhythmias.4
Tissue analysis by CMR is obtained by means of contrast agent-free techniques such as native T1 mapping of myocardial segments: values that are low compared with normal ranges may indicate myocardial accumulation of sphingolipids, directing the diagnostic suspicion towards FD; conversely, values that are diffusely and markedly elevated compared with normal are typically observed in cases of hypertrophy by CA. Other pre-contrast sequences, such as T2 STIR and T2 mapping, are instead capable of demonstrating the presence of areas of myocardial oedema, indicative of phases of active tissue damage in subjects with HCM potentially at risk of unfavourable clinical evolution.36
Once contrast medium has been administered, LGE images can be obtained to study the presence and distribution patterns of areas of pathological accumulation of contrast medium in the myocardium: in sarcomeric forms, areas of intramyocardial LGE are predominantly distributed in myocardial segments with greater hypertrophy; in FD, it is not uncommon to observe LGE involving the inferolateral wall of the LV; in patients with CA, LGE images are characterized by a lack of ‘myocardial signal cancellation’ with a distribution that generally follows a base > apex gradient.
Comparison studies between cardiac MRI and histological specimens have defined how in HCM, areas of LGE represent tissue characterized by fibrosis more or less associated with coronary microcirculation disease. The presence of myocardial LGE is an extremely common finding in sarcomeric HCM, described in 60–85% of cases; it follows that a purely qualitative assessment of the presence of LGE may be useful for diagnostic purposes, but not for a prognostic definition.34 The quantitative analysis of the extent of myocardial LGE, although not easy to standardize methodologically, has identified a threshold of LGE ≥ 15% of the LV mass in identifying patients at increased risk of malignant ventricular arrhythmias (Figure 7) and ‘dilatative’ evolution.34 This parameter was included in the ‘flow chart’ for risk stratification, used in the decision for possible implantation of a defibrillator in primary prevention in patients with intermediate–low clinical risk (ESC score < 6%).2,3 In this scenario, the calculation of myocardial ECV (extracellular volume) from pre- and post-contrast T1 mapping techniques showed no advantage over LGE but could be used effectively in follow-up studies. Serious studies of cardiac CMR have shown that myocardial fibrosis expressed as LGE is not a static but a dynamic phenomenon over time and patients with a higher rate of fibrosis progression have a poorer prognosis.37 Finally, in addition to the amount of myocardial scarring, its characteristics in terms of inhomogeneity between healthy/fibrotic tissue show a correlation with arrhythmic risk in subjects with HCM.37
Figure 7.
Short-axis cardiac magnetic resonance imaging images of left ventricle in patient with history of malignant ventricular arrhythmias and sarcomeric hypertrophic cardiomyopathy. Left T2-weighted image with hyperintense areas of septal tissue oedema. In the centre same post-contrast image (LGE) with areas of myocardial fibrosis in the septal location that follow those of the T2-weighted image. Right quantification of areas of LGE (using 6 SD method) with extent of myocardial fibrosis estimated to be ∼15% of left ventricular mass. LGE, late gadolinium enhancement.
Arrhythmia management and prevention of sudden cardiac death
Hypertrophic cardiomyopathy can expose patients to an increased risk of SCD. However, this risk, once thought to be considerably increased, is now lower. The overall annual mortality related to this condition is relatively low, ranging between 0.5% and 1–2%. The specific annual risk of SCD is around 0.8% but may vary significantly depending on factors such as age, clinical profile, and the presence of specific risk markers.38 In young patients, fatal events tend to occur suddenly, in the absence of prodromes or apparent causes, whereas in older individuals, the disease manifests itself more frequently through complications such as heart failure or stroke.
A relevant finding is the presence of non-sustained ventricular tachycardias, which can be detected by Holter ECG in about a quarter of HCM patients. Although the correlation between duration, frequency of these arrhythmias, and prognosis is not completely elucidated, their observation during exercise testing is considered an increased risk indicator for sudden death events. For this reason, the practice of strenuous physical activity in patients with HCM remains controversial. Some studies, however, have suggested that physical activity may be safe in patients with a positive genotype but without evidence of significant hypertrophy, leading to a small increase in LV volume in the absence of ventricular arrhythmias or impaired diastolic function.39
Several tools have been developed to estimate the risk of sudden death in patients with HCM, including the model proposed by the ESC and the combined model by the American Heart Association (AHA) and the American College of Cardiology (ACC).40 These models differ in methodology, parameters considered, and clinical applicability (Table 4).
Table 4.
Comparison of the European Society of Cardiology/American Heart Association risk scores for sudden death
| Features | ESC HCM risk-SCD score | AHA HCM-SCD score |
|---|---|---|
| Age of the patient | ≥16 years | ≥16 years |
| Ventricular wall thickness | Including | Including |
| Size of the left atrium | Including | Including |
| Maximum left ventricular outflow tract gradient | Including | Including |
| Family history of sudden death | Including | Including |
| Non-sustained ventricular tachycardia | Including | Including |
| Unexplained syncope | Including | Including |
| Left ventricular ejection fraction (EF ≤ 50%) | No | Including |
| Apical aneurysm | No | Including |
| Extended LGE (>15%) | No | Including |
| Risk calculation | At 5 years: low (<4%), intermediate (4–6%), high (>6%), less adaptable to individual situations, possible underestimation of risk | At 5 years, with ICD recommendation, greater clinical discretion, possible overestimation of risk |
The ESC HCM Risk-SCD score was introduced in 2014 and is based on a quantitative model that considers seven main clinical factors: patient age, maximum LV wall thickness, LV outflow tract gradient, left atrium size, family history of sudden death, episodes of unexplained syncope, and presence of non-sustained ventricular tachycardia.
This score estimates the percentage risk of sudden death at 5 years, stratifying patients into three categories: low (<4%), intermediate (4–6%), and high risk (≥6%). Despite its usefulness, it has significant limitations, especially in paediatric patients, elite athletes, and subjects with metabolic or infiltrative diseases, where clinical applicability is more complex. In low- or intermediate-risk patients, additional factors such as LV systolic dysfunction, the presence of apical aneurysms, extensive areas of fibrosis detected by LGE at CMR, and multiple genetic mutations can be used in shared decision-making, although solid data on their independent predictive role are still lacking.
The AHA/ACC approach is more qualitative and is based on the integration of structural and functional factors, such as those considered by the ESC score, and also includes additional elements, specifically LVEF < 50%, presence of apical aneurysms, and extensive areas of fibrosis documented by LGE at CMR.41
With this model, implantation of an automatic defibrillator is considered appropriate for adult HCM patients with one or more major risk factors. However, even this model has limitations in its application to patients under 16 years of age, elite athletes, and individuals with infiltrative or metabolic diseases. Furthermore, it is not applicable to those who have already undergone invasive reduction of LV outflow tract obstruction, such as surgical myectomy or septal alcoholization.
For paediatric patients (1–16 years), a specific model, known as HCM Risk-Kids,2,42 has been developed that excludes factors such as age and family history of sudden death, which are considered less predictive in this age group. Despite progress, the prognostic impact of fibrosis detected by CMR in paediatric subjects still remains uncertain, further limiting the possibility of accurate risk stratification.
To date, there are no randomized trials that have demonstrated the efficacy of drugs in preventing sudden death in patients with hypertrophic heart disease. Pharmacological therapies such as β-blockers, disopyramide, and amiodarone are useful for symptom control and management of LV outflow tract obstruction but have not shown significant benefit in reducing the risk of sudden death. Similarly, invasive interventions such as septal thickness reduction have shown limited impact in preventing sudden death.
A still poorly understood aspect concerns the effect of the selective myosin inhibitor, mavacamten, on the risk of SCD. Although this drug has shown promising results in improving exercise tolerance and reducing outflow tract obstruction, its impact on sudden death remains uncertain.
In cases of recurrent ventricular arrhythmias or cardiac arrest, implantable cardioverter defibrillator (ICD) is the recommended therapy.2,3 Appropriate ICD interventions often occur more than 10 years after implantation, emphasizing the importance of accurate risk stratification (Figure 8). In ICD wearers, the most common documented arrhythmia is sustained monomorphic ventricular tachycardia, which can be successfully treated by antitachycardia therapy. In cases where drugs are ineffective or contraindicated, transcatheter ablation may be considered.
Figure 8.
Arrhythmia management and prevention of sudden death. ESC, European Society of Cardiology; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter defibrillator; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction.
Interventional and surgical treatment
The therapeutic management of obstructive HCM has been refined through individualized approaches based on the severity of the disease, the response to drug therapy, and the clinical condition of the patients. When medical therapy fails to control symptoms or reduce the LVOTO gradient, interventional treatments are indicated, such as alcohol septal ablation (ASA) and surgical myectomy.
Alcohol septal ablation is a minimally invasive procedure performed via transcatheter.42 It consists of the injection of absolute alcohol through a catheter inserted into the septal artery with the aim of inducing a controlled myocardial infarction in the hypertrophic portion of the IVS. This causes a thinning of the septum, reducing the LVOTO gradient and improving blood flow. The main indications are as follows:
Refractory symptoms: dyspnoea, chest pain, and syncope persisting despite optimal drug therapy.
High surgical risk: patients with significant comorbidities who cannot undergo open heart surgery.
Favourable anatomy: need for an accessible and adequate septal artery to ensure success of the procedure.
Clinical studies show that ASA improves symptoms in 70–80% of patients, with a significant reduction in LVOTO gradient and improvement in the New York Heart Association (NYHA) functional class. However, the procedure may have complications such as the following43:
Complete atrioventricular block (5–10%): may require implantation of a permanent pacemaker.
Adverse remodelling: in rare cases, the LV may dilate over time.
Extensive infarction: if the injected alcohol affects unwanted areas.
Patient selection and operator experience are crucial to minimize risks and improve outcomes.
In patients with severe symptoms or in the presence of a persistent gradient > 50 mmHg despite drug therapy, surgery becomes the preferred treatment option. The standard procedure is transaortic septal myectomy.44
Transaortic septal myectomy is a surgical procedure that consists of removing a portion of the IVS to relieve obstruction of the LVOTO.
Myectomy is indicated by the ESC and AHA/ACC guidelines as the procedure of first choice. The main indication (Class I/B) concerns patients with LVOT > 50 mmHg and functional class (NYHA/Ross) III/IV despite optimized medical therapy.
In patients with LVOTO > 50 mmHg and syncope, the recommendation class drops to IIa/C. In experienced centres, intervention may also be considered for NYHA/Ross functional Class II who have (over gradient > 50 mmHg) moderate–severe functional mitral insufficiency from SAM, atrial fibrillation, or moderate–severe left atrial dilatation. In patients with symptomatic atrial fibrillation, ablation of the atrial fibrillation and/or surgical closure of the auricle (IIb/C) may also be considered.45
Operative mortality is low (<1%) in experienced centres. Operative patients have a longer survival and a significantly reduced risk of SCD compared with those treated with ASA. Some potential complications must be considered, however, and include the following:
-
Intraoperative complications
Intraoperative bleeding: due to resection of the IVS.
Mitral valve injury: may occur during surgery if the anterior flap is damaged.
Conduction system injury: can cause complete atrioventricular block, requiring implantation of a permanent pacemaker.
-
Postoperative cardiac complications
Acute cardiac failure: in cases of inadequate septal resection or excessive resection.
Cardiac tamponade: due to postoperative bleeding.
Postoperative arrhythmias: atrial fibrillation or ventricular tachycardias may develop in the short term.
-
Long-term complications
Residual mitral regurgitation: if the surgery does not adequately correct the SAM.
Restenosis of the efflux tract: due to recurrent hypertrophy or excessive scarring.
Need for reintervention: in cases of failure or recurrence of LVOTO gradient.
If LVOTO is aggravated by mitral valve dysfunction, it is possible to combine myectomy with valve correction (Class IIa/C). This may include mitral valve plastic or, in rare cases, valve replacement with a mechanical or biological prosthesis.46,47 A comparison between myectomy and ASA is shown in Table 5.
Table 5.
Comparison of septal myectomy and alcoholic septal ablation
| Feature | Myectomy septoplasty | ASA |
|---|---|---|
| Type of procedure | Open-heart surgery | Cardiac catheterization |
| Invasiveness | Invasive | Minimally invasive |
| Long-term effectiveness | Lasting | Good, but risk of VS dilatation |
| Operative mortality | <1% in expert centres | 1–3% |
| Major complications | Bleeding, restenosis | AV blockage, septal infarction |
| Patient selection | Low surgical risk; severe IM from SAM | Elderly patients or patients with comorbidities |
The management of obstructive HCM requires a multidisciplinary approach that takes into account the severity of symptoms, the gradient in LVOT, and the anatomical characteristics of the patient. The ESC 2023 and AHA/ACC 2024 guidelines emphasize the importance of treating patients in specialized centres. The choice between ASA and myectomy must be carefully evaluated to ensure the best possible clinical outcome.
Conventional medical therapy
Conventional medical therapy for HCM has long been the only therapy available for this complex disease that is considered an orphan of specific medical therapy. Nevertheless, there are numerous studies demonstrating the efficacy of conventional therapy in the clinical management of patients with obstructive and non-obstructive HCM symptomatic for angina, dyspnoea, and heart palpitation.
β-blockers
Non-vasodilator β-blockers (e.g. nadolol, metoprolol, and bisoprolol) are the most commonly used drugs in patients with HCM. In symptomatic patients with outflow gradient, both European2 and American1 guidelines agree in recommending non-vasodilator β-blockers as first-line drugs (Figure 9).
Figure 9.
Comparison of flow chart of treatment in patients with hypertrophic obstructive cardiomyopathy. Adapted from European Society of Cardiology guidelines 2023 (2) and American Heart Association/American College of Cardiology 2024 (1). ACC, American College of Cardiology; AHA, American Heart Association; ESC, European Society of Cardiology; LVOTO, left ventricular outflow tract obstruction.
Currently, nadolol (40–160 mg/day), bisoprolol (5–10 mg/day), and metoprolol (100–200 mg/day) are the most widely used β-blockers.48
A recent small randomized trial demonstrated in the group of patients treated with metoprolol (vs. placebo) a significant reduction in the intraventricular gradient at rest and during exercise, an improvement in symptoms and quality of life.49
In many centres with expertise in the management of cardiomyopathies, nadolol is the β-blocker of first choice for patients with intraventricular gradient, as it is well tolerated, effective in reducing the gradient, has an excellent antiarrhythmic profile, and requires a single administration per day.
A reasonable approach is to start with a quarter of a full dose of the β-blocker of choice (e.g. nadolol 20 mg once daily, metoprolol 25 mg twice daily, or bisoprolol 2.5 mg once daily) and increase the same amount every 1–2 weeks up to the maximum tolerated dose (usually 80 mg for nadolol, 100 mg twice daily for metoprolol, and 10 mg for bisoprolol). The titration of the active substance will depend on the symptoms (e.g. asthenia) and the response in terms of heart rate and blood pressure.
Patients with HCM may also present with symptoms such as angina and dyspnoea associated with microvascular ischaemia, diastolic dysfunction, and elevated filling pressures. β-blockers, through their chronotropic-negative (reduction in heart rate) and inotropic-negative (reduction in cardiac muscle contraction force) effects, may improve symptoms and are therefore recommended in symptomatic patients regardless of the intraventricular gradient. The use of β-blockers in patients with a restrictive diastolic profile, i.e. with a fixed stroke volume and a cardiac output that depends mainly on heart rate, must be very cautious as iatrogenic chronotropic incompetence may be poorly tolerated by the patient.
Calcium channel blockers
In patients with obstructive HCM who are still symptomatic despite β-blocker therapy or who are intolerant or have contraindications to such drugs, international guidelines recommend the use of non-dihydropyridine calcium channel antagonists such as verapamil and diltiazem.2 These drugs exert their effects by inhibiting the L-type calcium channel in cardiomyocytes and vascular smooth muscle, thereby promoting dilation of peripheral vessels as well as sustaining negative inotropic and chronotropic effects. Negative inotropy is achieved through the reduction of calcium entry through L-type channels in ventricular working cardiomyocytes, while negative chronotropy is mediated by the reduced rate of spontaneous activation of the sinus node. Diltiazem or verapamil also reduces atrioventricular conduction and prolongs atrioventricular delay (negative dromotropic effect), through lengthening the refractory period in the atrioventricular node. Calcium channel blockers must be used with caution in patients with a tendency to hypotension or with known atrioventricular blockade. Side effects of calcium channel blockers include headache, dizziness, nausea, constipation, oedema, and flushing. The combination of non-dihydropyridine calcium channel antagonists with β-blockers is always dangerous and contraindicated due to the high risk of developing severe conduction blocks.
In a direct comparison of verapamil and diltiazem, no differences in the efficacy of the two drugs were observed. Diltiazem was associated, however, with a lower incidence of side effects.50
The recommended starting dose is 40 mg three times a day for verapamil (maximum dose 480 mg/day) and 60 mg three times a day for diltiazem (maximum dose 360 mg/day).51 Close monitoring of the effect of these drugs is necessary in patients with severe outflow obstruction (≥100 mmHg) or high pulmonary pressures as they may cause pulmonary oedema.
Disopyramide
Disopyramide is an antiarrhythmic sodium channel blocker with a potent negative inotropic effect that interferes with sarcomere proteins, thus being effective in reducing the LV outflow gradient and improving symptoms in a fair proportion of patients. Its biochemical mechanisms have recently been investigated in a series of myocardial tissue samples from patients undergoing myectomy, demonstrating a potent antiarrhythmic protective effect on ventricular arrhythmias in particular by suppressing post-potentials.52 This aspect gives disopyramide an important safety and efficacy profile. Nevertheless, disopyramide is underused by clinicians as recent real-world studies have shown.53 Also controversial are the long-term results on the improvement of functional capacity and on its efficacy in reducing the gradient at the ventricular outflow. Currently, disopyramide is a second-line drug in the treatment of patients with obstructive HCM when β-blockers or calcium channel blockers have proved ineffective.2,3
Other molecules
Discussed and with still little evidence is the indication for sartan therapy in patients with HCM. Valsartan was found to be effective in patients with sarcomeric non-obstructive forms with mild phenotypic expression in slowing down reverse remodelling as well as in improving quality of life, but not in improving functional capacity.54,55
The possible effect of sacubitril/valsartan was investigated in a recent Phase 2 trial involving 115 patients with HCM: a 16-week treatment with sacubitril/valsartan was well tolerated but had no effect on exercise capacity, cardiac structure or function.56 In contrast, different evidence has been published so far on the role of sodium-glucose cotransporter-2 (SGLT2) inhibitors in patients with obstructive HCM: a recent real-world dataset from electronic medical records showed that the use of SGLT2 inhibitors in patients with obstructive HCM is safe and associated with better survival, fewer hospitalizations, and fewer cardiovascular symptoms.57
New therapeutic perspectives: cardiac myosin inhibitors
Cardiac myosin inhibitors are the first treatment specifically developed for HCM and constitute an innovative and effective therapeutic option for adult patients with symptomatic obstructive HCM, although studies in non-obstructive and paediatric patients are ongoing. They are allosteric inhibitors developed to counteract an over-activation of cardiac myosin that is central to the pathogenesis of HCM, due to dysregulation and reduced representation of the ‘super-relaxed’ state of myosin itself. In the hearts of HCM patients, genetic mutations tend to increase the number of active myosins compared with normal hearts. In a cascade of downstream maladaptive mechanisms, this phenomenon leads to the development of hypertrophy, hyperdynamic contraction, increased energy cost of force generation, and fibrosis, i.e. the overt HCM phenotype. Understanding of these pathophysiological processes has led to the development of small molecules capable of binding to the catalytic domain of β-myosin, thereby favouring an ‘off’ conformation and reducing the number of myosin molecules functionally accessible for interaction with actin.58 Available drugs include mavacamten (approved for the treatment of symptomatic obstructive HCM in 2022 by the US Food and Drug Administration and in 2023 by the European Medicines Agency) and aficamten (pending approval for the same indication). Mavacamten59 binds to myosin and reduces the rate of inorganic phosphate release, stabilizing a low-energy myosin conformation. Aficamten has a similar mechanism of action, although it has a shorter half-life and stabilizes a myosin off-state that is likely different from mavacamten. Randomized Phase 3 clinical trials with myosin inhibitors in patients with obstructive HCM have consistently shown a marked reduction in outflow gradients, improved exercise capacity and quality of life, reduced circulating levels of N-terminal pro Brain Natriuretic Peptide (NT-proBNP) and high-sensitivity troponin, and beneficial effects on diastolic function.
The Phase 3 EXPLORER-HCM Study randomized 251 patients with symptomatic obstructive HCM to mavacamten or placebo, in addition to their previous medical therapy with beta-blockers or calcium antagonists.60 The primary endpoint was a composite of functional capacity assessed by cardiopulmonary exercise testing and symptomatic burden expressed as NYHA class. After a treatment period of 30 weeks, patients were considered responder if they achieved an increase in peak exercise oxygen consumption (pVO2) ≥ 1.5 mL/kg/min (compared with initial assessment) with an improvement of ≥1 NYHA class or an increase in pVO2 ≥ 3.0 mL/kg/min without worsening of NYHA class. This endpoint was achieved by 37% of patients assigned to mavacamten, compared with 17% of placebo patients (P = 0.0005). A hierarchical evaluation of the predefined secondary endpoints (all with P > 0.001) showed a consistent and significant benefit of mavacamten in terms of increase in pVO2 compared with baseline, NYHA functional class (improvement of at least one NYHA class in 65% vs. 31% in the placebo arm), reduction in post-exercise gradient (almost 50 mmHg, compared with no change in the placebo group), and quality of life. Specifically, at the end of the treatment period, the Kansas City Cardiomyopathy Questionnaire (KCCQ) had improved by 13.6 points in the mavacamten arm vs. 4.2 in the placebo. Overall, 27% of treated patients had a complete therapeutic response (defined as abolition of the exercise gradient associated with NYHA Class I at the final assessment, thus equivalent to an optimal surgical outcome) compared with 1% of those treated with placebo.
The recent Phase 3 SEQUOIA-HCM trial randomized 282 patients to aficamten and placebo. The primary endpoint was the change from Day 0 to Week 24 in pVO2 assessed by cardiopulmonary testing.61 The pre-specified secondary endpoints (hierarchically tested) included change in KCCQ-CSS, improvement in NYHA functional class, change in pressure gradient after Valsalva manoeuvre, occurrence of a gradient < 30 mmHg after Valsalva manoeuvre, and duration of candidacy for surgical myectomy or septal alcoholization, all assessed at Week 24. At the last evaluation, the mean increase in pVO2 was 1.8 mL/kg/min [95% confidence interval (CI), 1.2–2.3) in the aficamten group vs. 0.0 mL/kg/min (95% CI, −0.5 to 0.5) in the placebo group (P < 0.001). All secondary endpoints showed a significant advantage of aficamten over placebo.
Patients from Phase 3 studies with both drugs were subsequently enrolled in open-label extension studies, which also demonstrated a reduction in LV mass and left atrium size in the mid- to long-term, as well as confirmation of the efficacy demonstrated during the blinded trials. Both mavacamten and aficamten showed a favourable safety and tolerability profile, which seem to be confirmed for mavacamten by the first reports on real-world use. The most feared adverse effect is a reduction of LVEF below 50%, which was observed in 5–10% of patients with obstructive HCM who received mavacamten in clinical trials (and slightly less in aficamten patients) but which generally did not lead to decompensation episodes or hospitalization and was reversible upon discontinuation with subsequent reduction of the drug, in the case of mavacamten, or a simple dose reduction with aficamten. However, the use of the drug requires close monitoring of LVEF in the long term, as indicated by a risk mitigation strategy (REMS) imposed by the Food and Drug Administration Agency in the USA and similar programmes in Europe and elsewhere. Based on these findings, the current ESC guidelines2 place mavacamten in Class IIa for the treatment of symptomatic obstructive HCM, while the very recent AHA/ACC guidelines1 speak more broadly of cardiac myosin inhibitors, in Class I for the same indication. In both cases, HCMs are indicated where first-line therapy with beta-blockers or calcium channel blockers has not produced a favourable result; however, while the ESC guidelines2 propose HCMs as an intermediate option before surgery or septal alcoholization, the AHA/ACC guidelines1 place HCMs on the same level, as an alternative to invasive therapies (Figure 9). Future developments point to an expansion of indications for HCM in HCM patients. Two large Phase 3 studies are currently underway to establish the potential benefit of mavacamten and aficamten in symptomatic patients with non-obstructive HCM (ODISSEY-HCM and ACACIA-HCM, respectively) and two smaller studies in paediatric patients with symptomatic obstructive HCM (SCOUT-HCM with mavacamten and CEDAR-HCM, respectively).
Phenocopies of hypertrophic cardiomyopathy: differential diagnosis and treatment of Fabry disease
Fabry disease is a rare X-linked lysosomal disease caused by pathogenic mutations in the GLA gene (Xq21.3-q22) that encodes for the enzyme α-Gal A.62 Deficient or absent α-Gal A activity results in the accumulation of glycosphingolipids, predominantly globotriaosylceramide (Gb3) and its derivative globotriaosylsphingosine (lyso-Gb3) in lysosomes of different organs and tissues including vascular endothelium, kidney, heart, peripheral, and central nervous system. Estimated prevalence of the classical form of AFD is 1:30 000–40 000 adult males. However, neonatal screening programmes have reported a high prevalence of pathogenic variants ranging from 1:1 250 to 1:7800.
Fabry disease exists in a classical form and a late-onset non-classical form. Patients with a classic phenotype have severely reduced or no α-Gal A enzyme activity and manifest the disease from early childhood with neuropathic pain, cornea verticillata, gastrointestinal symptoms, hypohidrosis, hearing loss, and angiokeratomas. Long-term manifestations of the disease include renal failure, HCM, cardiac arrhythmias, and stroke. Late-onset phenotype is less severe and has residual α-Gal A enzyme activity; it usually manifests in the third to fourth decade and predominantly involves the kidney and/or heart. Cardiac involvement is characterized by the development of progressive LVH with risk of heart failure, arrhythmias, and sudden death.
Fabry disease accounts for 0.5–1% of cases of cardiomyopathy with a hypertrophic phenotype and the differential diagnosis from the classic sarcomeric form sometimes represents a challenge for the cardiologist especially when extracardiac manifestations are absent or not readily recognized.
In the heart, the accumulation of glycosphingolipids occurs in all cell types: cardiomyocytes, endocardium, intramyocardial vessels, valves, and conduction tissue leading clinically to the appearance of cardiomyopathy with a hypertrophic phenotype, heart failure, angina, valve abnormalities, and arrhythmias (both hypokinetic and hyperkinetic).63
Fabry disease should be suspected in cases of the following:
Cardiomyopathy with hypertrophic phenotype with the absence of patrilineal transmission.
Familiarity and/or history of renal failure and juvenile stroke.
Extracardiac red flags such as angiokeratomas, cornea verticillata, neuropathic pain, gastrointestinal disturbances, hypo/anhydrosis, cold/hot intolerance, hearing loss, and basilar artery dolichoectasia.
Cardiac red flags such as short PR, chronotropic incompetence, LVH with ‘binary sign’, papillary muscle hypertrophy, thickening of the interatrial septum and valve leaflets, late enhancement in the inferoposterolateral site and low T1 mapping values at cardiac MRI, and increased cardiac markers (ultrasensitive troponin and NT-proBNP).
In cases of diagnostic suspicion, the diagnosis is simple (Figure 10) and is based on the α-Gal A enzyme activity assay on ‘dried blood spots’, a test in which small amounts of blood are dried on filter paper. In males with a classical phenotype, undetectable α-Gal A activity or activity below 3% of the expected value is diagnostic. Although the α-Gal A activity assay is diagnostic in the male, genetic testing is essential to confirm the disease and the possible genotype–phenotype correlation. In the female, on the other hand, residual enzyme activity may be normal as a consequence of the skewed or ‘non-random’ X-chromosome inactivation, so genetic testing is necessary for a definitive diagnosis.64 If a proband is identified, family genetic screening must be initiated. Clinical monitoring is essential to assess disease progression and requires a multidisciplinary approach.
Figure 10.
Pathogenesis of cardiac damage, main clinical manifestations, extracardiac and cardiac red flags and diagnostic pathway in Fabry disease cardiomyopathy.
A diagnosis of FD is essential, since for ∼20 years, a specific therapy is available, that aimed to prevent or slow the progression of the organ damage. The approved treatments for FD include enzyme replacement therapy (ERT) and chaperone therapy65 (Table 6).
Table 6.
Currently approved therapies for treatment of Fabry disease
| Enzyme replacement therapy | |
| Agalsidase alpha | 0.2 mg/kg/ev every other week |
| Agalsidase beta | 1 mg/kg/ev every other week |
| Pegunigalsidase alpha | 1 mg/kg/ev every other week |
| Chaperone therapy | |
| Migalastat | 123 mg per os every other day |
Enzyme replacement therapy is administered intravenously every fortnight and has been approved since 2001 in two enzyme formulations; both contain recombinant human α-Gal and are produced in different cell lines with different genetic engineering techniques and different dosages:
Agalsidase alpha, produced in human fibroblast cells (0.2 mg/kg/ev every fortnight).
Agalsidase beta, produced in ovarian cells of Chinese hamsters (1 mg/kg/ev every fortnight).
Long-term follow-up studies have shown that ERT is well tolerated and reduces cardiac and renal disease progression; it stabilizes intracellular Gb3 deposits and cardiomyocyte size; however, complete removal is difficult even after long-term therapy.66 Moreover, the presence of an immune-mediated inflammatory process may limit its efficacy.67
Recently, a new ERT has been approved: pegunigalsidase alpha, a recombinant human α-Gal A chemically produced in plant cells (tobacco) modified with polyethylene glycol, with the theoretical potential for reduced immunogenicity. Trials have shown a non-inferiority of pegunigalsidase alpha to the ERT currently in use.68
Oral therapy based on migalastat, a chaperone molecule administered at a dose of 123 mg every other day in patients with susceptible GLA variants, is also possible.
Finally, new therapeutic strategies based on gene and mRNA therapy are under development.
Phenocopies of hypertrophic cardiomyopathy: differential diagnosis and treatment of cardiac amyloidosis
Cardiac amyloidosis is linked to the deposition of amyloid fibres in the myocardial extracellular space: the alteration of the substrate leads to progressive dysfunction of ventricular diastole and systole, valvular disease, atrial cardiomyopathy, conduction and rhythm disturbances, and clinical decompensation.69-72 The vast majority of CA cases result from misfolded light-chain proteins produced by abnormal clonal proliferation of plasma cells [light-chain amyloidosis (AL-CA) and transthyretin (ATTR-CA)]: cardiac involvement is the main cause of morbidity and mortality. Transthyretin (TTR) is a tetramer produced by the liver and the disease occurs when it dissociates into oligomers and monomers that subsequently misfold and generate amyloid fibres; ATTR-CA may be secondary to an inherited autosomal dominant point mutation in the TTR gene (ATTRv) or to an acquired process associated with ageing, (wild-type, ATTR-CAwt); ATTRv has an earlier presentation, with a mixed clinical phenotype consisting of cardiomyopathy and peripheral sensorimotor and autonomic neuropathy. Median survival without treatment is 3–5 years vs. 6 months in advanced AL-CA. Cardiac amyloidosis is clinically manifested not only at cardiovascular level but also at systemic level, with the appearance of certain red flags even years before cardiac involvement, such as sensorimotor and dysautonomic polyneuropathy, carpal tunnel syndrome, atraumatic rupture of the biceps brachii tendon, stenosis of the lumbar vertebral canal, and particularly in the case of AL-CA, macroglossia, periorbital purpura, renal failure, and proteinuria.
The diagnostic suspicion of AC must be supported by laboratory and imaging findings according to an established diagnostic flow chart (Figure 11). Primarily, wall thickening of the LV, the absence of significant dilatation, and biatrial dilatation are typical features of CA. A reduction in the amplitude of QRS complexes (especially in AL-CA) or a discrepancy between QRS voltages and LV thickness defines the pseudohypertrophy characteristic of CA. Plasma elevation of troponin and B-type natriuretic peptides is an element of suspicion in at-risk categories (patients with monoclonal gammopathy or carriers of transthyretin gene mutations). Once suspicion has been raised on the basis of these red flags, the flow chart suggests further diagnostic investigations with a scintigraphy with diphosphonate tracers. The absence of myocardial uptake and a monoclonal component on serum and urinary immunofixation makes the diagnosis of AL-CA or ATTR-CA unlikely. In such cases, a cardiac MRI, with suggestive pre- and post-contrast findings, and also an amyloidogenic protein typing with endomyocardial biopsy are useful. An intense myocardial uptake (Perugini 2 and 3) in the absence of demonstration of a monoclonal component allows a final diagnosis of ATTR-CA, without the need for histological confirmation. The search for mutations in the transthyretin gene then allows amyloidosis to be classified as ATTRv or ATTRwt. Histological examination and typing of amyloid on extracardiac cardiac tissue are also necessary in the presence of intense uptake (Perugini 2 and 3) and evidence of a monoclonal component or with mild uptake (Perugini 1).
Figure 11.
Diagnostic–therapeutic course of cardiac amyloidosis. AL, light chain; ATTR, amyloidosis transthyretin; ATTRwt, wild-type amyloidosis transthyretin; CMR, cardiac magnetic resonance.
After establishing a diagnosis of AL-CA, the goal of clinical management is to suppress further production of amyloidogenic light chains. Organ amyloidosis results in decreased tolerance to therapeutic regimes derived from multiple myeloma treatment. High-dose melphalan (HDM) and autologous stem cell transplantation (SCT) represented an initial option for adapting patients with non-advanced disease.73 With this approach, ∼40% of patients achieve a response, which may last >12 years. The majority of patients (70–80%) not suitable for HDM/SCT are instead treated with daratumumab-based regimens. This monoclonal antibody therapy, which targets CD38 on plasma cells and is typically combined with bortezomib, cyclophosphamide, and dexamethasone (Dara-VCD) on the basis of the encouraging results of the ANDROMEDA trial, is becoming the first therapy of choice.74
Advances in therapeutic strategies have also revolutionized the management of ATTR amyloidosis.69-72 Treatments available in clinical practice include TTR stabilizers such as tafamidis and acoramidis, which prevent dissociation of TTR tetramers into misfolded monomers, and gene silencing therapies such as patisiran, inotersen, and vutrisiran, which suppress TTR synthesis. Other approaches undergoing Phase 2 and 3 studies include therapies targeting amyloid deposits through monoclonal antibodies (coramitug, ALXN2220, ongoing Phase 2 and 3 studies) and CRISPR-Cas9 gene editing with nexiguran ziclumeran. Pharmacological and device-based therapy of heart failure using adrenergic, renin–angiotensin–aldosterone system, and SGLT2 inhibitors is complementary, given the biopsy evidence of fibrosis and humoural activation of the two neuronal systems.1,2 Furthermore, economic and logistical barriers further complicate access to advanced therapies, particularly in resource-limited settings. As therapies continue to evolve, the integration of early diagnosis, combination treatments, and patient-centred care will be critical to transform the management of this complex disease.
Conclusions
Over the past 50 years, the evolution of imaging techniques and the introduction of new drugs have revolutionized the clinical and therapeutic management of patients with HCM. In particular, the introduction of cardiac myosin inhibitors is going to change the therapeutic approach of obstructive HCM.
The new therapeutic landscape requires a renewed and thorough understanding of the disease, from clinical, diagnostic, and prognostic perspectives. Identifying affected patients today requires a careful assessment of the presence or absence of outflow obstruction, even under dynamic conditions, and an in-depth diagnostic investigation aimed at excluding the most common phenocopies that can benefit from targeted treatment.75
The figure of the cardiologist expert in cardiomyopathies is increasingly emerging and who can define the risk profile in the single patient, which may vary from paucisymptomatic with normal life expectancy to severe conditions associated with a high risk of arrhythmia and heart failure. The ‘cardiomyopathy expert’ cardiologist should also deal with family members, who may be carriers of the same genetic variant or manifest the same disease. The clinical diagnosis must guide the genetic test and facilitate its interpretation, in collaboration with the geneticist, who is an integral part of the multidisciplinary ‘cardiomyopathy’ team.
Contributor Information
Cristina Chimenti, UOC Malattie Apparato Cardiovascolare, AOU ‘Policlinico Umberto I’, Dipartimento di Scienze Cliniche, Internistiche, Anestesiologiche e Cardiovascolari, Sapienza Università di Roma, Viale del Policlinico, 155, Roma 00161, Italy.
Attilio Iacovoni, UOC Cardiologia 1, ASST Papa Giovanni XXIII, Bergamo, Italy.
Andrea Montalto, UOC Cardiochirurgia, Azienda Ospedaliera Sant’Anna e San Sebastiano, Caserta, Italy.
Michele Emdin, Cardiologia e Medicina Cardiovascolare, Fondazione Toscana Gabriele Monasterio (FTGM)—Stabilimento di Pisa, Italy.
Iacopo Olivotto, SOC Cardiologia Pediatrica e della Transizione, AOU Meyer—IRCCS, Firenze, Italy.
Cristina Basso, UOC Patologia Cardiovascolare, Azienda Ospedaliera- Dipartimento di Scienze Cardio-toraco-vascolari e di Sanità Pubblica, Università degli Studi, Padova, Italy.
Benedetta Carla De Chiara, SC Cardiochirurgia e del Trapianto di Cuore, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy.
Irene Bottillo, UOC Laboratorio di Genetica Medica, Dipartimento di Medicina Sperimentale, A.O. San Camillo Forlanini, Roma, Italy; Sapienza Università, Roma, Italy.
Claudio Mario Ciampi, UOC Cardiologia, Ospedale Garibaldi-Nesima, Azienda di Rilievo Nazionale e Alta Specializzazione ‘Garibaldi’, Catania, Italy.
Santo Dellegrottaglie, Laboratorio RM Cardiovascolare—Unità Imaging Cardiovascolare Avanzato, Ospedale Medico-Chirurgico Accreditato Villa dei Fiori, Acerra, NA, Italy.
Massimo Di Marco, UOC Cardiologia-UTIC, Ospedale Santo Spirito, Pescara, Italy.
Piero Gentile, Cardiologia 2—Insufficienza Cardiaca e Trapianto, Dipartimento Cardiotoracovascolare ‘A. De Gasperis’, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy; Scuola di Medicina e Chirurgia, Università Bicocca, Milano, Italy.
Francesca Girolami, SOC Cardiologia Pediatrica e della Transizione, AOU Meyer—IRCCS, Firenze, Italy.
Paola Grammatico, UOC Laboratorio di Genetica Medica, Dipartimento di Medicina Sperimentale, A.O. San Camillo Forlanini, Roma, Italy; Sapienza Università, Roma, Italy.
Maria Iascone, Laboratorio di Genetica Medica, ASST Papa Giovanni XXIII, Bergamo, Italy.
Eluisa La Franca, Dipartimento di Cardiologia, Istituto Mediterraneo per i Trapianti e Terapie ad Alta Specializzazione (ISMETT)-IRCCS, UPMC, Palermo, Italy.
Carla Lofiego, SOD Cardiologia-UTIC, Dipartimento di Scienze Cardiovascolari, AOU delle Marche, Ancona, Italy.
Andrea Matteucci, UOC Cardiologia Clinica e Riabilitativa, Presidio Ospedaliero San Filippo Neri—ASL Roma 1, Roma, Italy.
Daniele Pasqualucci, Cardiologia con UTIC, Azienda Ospedaliera ‘G. Brotzu’, Cagliari, Italy.
Samuele Pentiricci, Cardiochirurgia, ASST Papa Giovanni XXIII, Bergamo, Italy.
Enrica Perugini, UOC Cardiologia, Ospedale Maggiore, Bologna, Italy.
Maurizio Pieroni, Cardiomiopatie, AOU Careggi—Università degli Studi, Firenze, Italy.
Giovanni Quarta, UOC Cardiologia 1, ASST Papa Giovanni XXIII, Bergamo, Italy.
Federica Re, UOC Cardiologia, Dipartimento di Scienze Cardio-Toraco-Vascolare, Azienda Ospedaliera San Camillo Forlanini, Roma, Italy.
Laura Scelsi, SC Cardiologia, Dipartimento Cardio-Toraco-Vascolare, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Giancarlo Todiere, Cardiologia e Medicina Cardiovascolare, Fondazione Toscana Gabriele Monasterio (FTGM)—Stabilimento di Pisa, Italy.
Maria Alfarano, UOC Malattie Apparato Cardiovascolare, AOU ‘Policlinico Umberto I’, Dipartimento di Scienze Cliniche, Internistiche, Anestesiologiche e Cardiovascolari, Sapienza Università di Roma, Viale del Policlinico, 155, Roma 00161, Italy.
Monica De Gaspari, UOC Patologia Cardiovascolare, Azienda Ospedaliera- Dipartimento di Scienze Cardio-toraco-vascolari e di Sanità Pubblica, Università degli Studi, Padova, Italy.
Claudio Bilato, U.O.C. Cardiologia, Ospedali dell’Ovest Vicentino, Azienda ULSS 8 Berica, Vicenza, Italy.
Marco Corda, Cardiologia con UTIC, Azienda Ospedaliera ‘G. Brotzu’, Cagliari, Italy.
Leonardo De Luca, SC Cardiologia, Dipartimento Cardio-Toraco-Vascolare, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Giovanna Geraci, U.O.C. Cardiologia, Presidio Ospedaliero Sant’Antonio Abate, ASP Trapani, Erice, Italy.
Massimo Milli, Cardiologia Firenze 1 (Ospedali S. Maria Nuova e Nuovo San Giovanni di Dio), Azienda USL Toscana Centro, Firenze, Italy.
Alessandro Navazio, S.O.C. Cardiologia Ospedaliera, Presidio Ospedaliero Arcispedale Santa Maria Nuova, Azienda USL di Reggio Emilia—IRCCS, ReggioEmilia, Italy.
Vittorio Pascale, UTIC-Emodinamica e Cardiologia Interventistica, Ospedale Civile Pugliese, Catanzaro, Italy.
Carmine Riccio, U.O.S.D. Follow-up del Paziente Post-Acuto, Dipartimento Cardio-Vascolare, AORN Sant’Anna e San Sebastiano, Caserta, Italy.
Pietro Scicchitano, U.O. Cardiologia-UTIC, Ospedale ‘F. Perinei’, Altamura BA, Italy.
Emanuele Tizzani, Dipartimento di Cardiologia, Ospedale degli Infermi, Rivoli, TO, Italy.
Michele Massimo Gulizia, UOC Cardiologia, Ospedale Garibaldi-Nesima, Azienda di Rilievo Nazionale e Alta Specializzazione ‘Garibaldi’, Catania, Italy.
Federico Nardi, Dipartimento di Cardiologia, Ospedale Santo Spirito, Casale Monferrato AL, Italy.
Domenico Gabrielli, UOC Cardiologia, Dipartimento di Scienze Cardio-Toraco-Vascolare, Azienda Ospedaliera San Camillo Forlanini, Roma, Italy; Fondazione per il Tuo cuore—Heart Care Foundation, Firenze, Italy.
Furio Colivicchi, UOC Cardiologia Clinica e Riabilitativa, Presidio Ospedaliero San Filippo Neri—ASL Roma 1, Roma, Italy.
Massimo Grimaldi, U.O.C. Cardiologia-UTIC, Ospedale Miulli, Acquaviva delle Fonti, BA, Italy.
Fabrizio Oliva, Fondazione per il Tuo cuore—Heart Care Foundation, Firenze, Italy; U.O.C. Cardiologia-UTIC, Ospedale Miulli, Acquaviva delle Fonti, BA, Italy; Cardiologia 1-Emodinamica, Dipartimento Cardiotoracovascolare ‘A. De Gasperis’, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy; Associazione Nazionale Medici Cardiologi Ospedalieri—ANMCO, Firenze, Italy.
Funding
This paper was published as part of a supplement financially supported by Centro Servizi ANMCO SrL - Società Benefit.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Disclaimer
This Position Paper was originally published in the Italian language as ‘Position Paper ANMCO: Cardiomiopatia ipertrofica: dalla diagnosi al trattamento', in the official journal of the Italian Federation of Cardiology (IFC) ‘Giornale Italiano di Cardiologia’, published by Il Pensiero Scientifico Editore. This paper was translated by a representative of the Italian Association of Hospital Cardiologists (ANMCO) and reprinted with permission of IFC and Il Pensiero Scientifico Editore.
References
- 1. Ommen SR, Ho CY, Asif IM, Balaji S, Burke MA, Day SM et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024;149:e1239–e1311. [DOI] [PubMed] [Google Scholar]
- 2. Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C et al. 2023 ESC guidelines for the management of cardiomyopathies. Eur Heart J 2023;44:3503–3626. [DOI] [PubMed] [Google Scholar]
- 3. Bertero E, Canepa M, Pieroni M, Olivotto I. Update sulla gestione del paziente con cardiomiopatia ipertrofica alla luce delle nuove linee guida nord-americane. G Ital Cardiol (Rome) 2025;26:185–194. [DOI] [PubMed] [Google Scholar]
- 4. Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 2015;65:1249–1254. [DOI] [PubMed] [Google Scholar]
- 5. Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA study. Coronary artery risk development in (young) adults. Circulation 1995;92:785–789. [DOI] [PubMed] [Google Scholar]
- 6. Maron MS, Hellawell JL, Lucove JC, Farzaneh-Far R, Olivotto I. Occurrence of clinically diagnosed hypertrophic cardiomyopathy in the United States. Am J Cardiol 2016;117:1651–1654. [DOI] [PubMed] [Google Scholar]
- 7. Del Franco A, Menale S, Chiti C, Biagioni G, Tomberli A, Zampieri M et al. The evolving paradigm and current perception of hypertrophic cardiomyopathy: implications for management. Prog Cardiovasc Dis 2023;80:8–13. [DOI] [PubMed] [Google Scholar]
- 8. Ho CY, Day SM, Ashley EA, Michels M, Pereira AC, Jacoby D et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 2018;138:1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilde AAM, Semsarian C, Márquez MF, Sepehri Shamloo A, Ackerman MJ, Ashley EA et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus statement on the state of genetic testing for cardiac diseases. Heart Rhythm 2022;19:e1–e60. [DOI] [PubMed] [Google Scholar]
- 10. Hayesmoore JB, Bhuiyan ZA, Coviello DA, du Sart D, Edwards M, Iascone M et al. EMQN: recommendations for genetic testing in inherited cardiomyopathies and arrhythmias. Eur J Hum Genet 2023;31:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Basso C, Michaud K, d'Amati G, Banner J, Lucena J, Cunningham K et al. Cardiac hypertrophy at autopsy. Virchows Arch 2021;479:79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Basso C, Thiene G, Mackey-Bojack S, Frigo AC, Corrado D, Maron BJ. Myocardial bridging, a frequent component of the hypertrophic cardiomyopathy phenotype, lacks systematic association with sudden cardiac death. Eur Heart J 2009;30:1627–1634. [DOI] [PubMed] [Google Scholar]
- 14. Maron BJ, Roberts WC. Quantitative analysis of cardiac muscle cell disorganization in the ventricular septum of patients with hypertrophic cardiomyopathy. Circulation 1979;59:689–706. [DOI] [PubMed] [Google Scholar]
- 15. Basso C, Thiene G, Corrado D, Buja G, Melacini P, Nava A. Hypertrophic cardiomyopathy and sudden death in the young: pathologic evidence of myocardial ischemia. Hum Pathol 2000;31:988–998. [DOI] [PubMed] [Google Scholar]
- 16. Maron BJ, Rowin EJ, Casey SA, Maron MS. How hypertrophic cardiomyopathy became a contemporary treatable genetic disease with low mortality: shaped by 50 years of clinical research and practice. JAMA Cardiol 2016;1:98–10. [DOI] [PubMed] [Google Scholar]
- 17. Maurizi N, Olivotto I, Maron MS, Bonacchi G, Antiochos P, Tomberli B et al. Lifetime clinical course of hypertrophic cardiomyopathy: outcome of the historical florence cohort over 5 decades. JACC Adv 2023;2:100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rapezzi C, Arbustini E, Caforio AL, Charron P, Gimeno-Blanes J, Heliö T et al. Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC working group on myocardial and pericardial diseases. Eur Heart J 2013;34:1448–1445. [DOI] [PubMed] [Google Scholar]
- 19. Aguiar Rosa S, Thomas B, Pieroni M, Maurizi N, Zampieri M, Cappelli F et al. Role of cardiovascular magnetic resonance in the clinical evaluation of left ventricular hypertrophy: a 360° panorama. Int J Cardiovasc Imaging 2023;39:793–809. [DOI] [PubMed] [Google Scholar]
- 20. Aimo A, Milandri A, Barison A, Pezzato A, Morfino P, Vergaro G et al. Electrocardiographic abnormalities in patients with cardiomyopathies. Heart Fail Rev 2024;29:151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vitale G, Ditaranto R, Graziani F, Tanini I, Camporeale A, Lillo R et al. Standard ECG for differential diagnosis between Anderson–Fabry disease and hypertrophic cardiomyopathy. Heart 2022;108:54–60. [DOI] [PubMed] [Google Scholar]
- 22. Finocchiaro G, Sheikh N, Biagini E, Papadakis M, Maurizi N, Sinagra G et al. The electrocardiogram in the diagnosis and management of patients with hypertrophic cardiomyopathy. Heart Rhythm 2020;17:142–151. [DOI] [PubMed] [Google Scholar]
- 23. Rapezzi C, Lorenzini M, Longhi S, Milandri A, Gagliardi C, Bartolomei I et al. Cardiac amyloidosis: the great pretender. Heart Fail Rev 2015;20:117–124. [DOI] [PubMed] [Google Scholar]
- 24. Nagueh SF, Phelan D, Abraham T, Armour A, Desai MY, Dragulescu A et al. Recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: an update from the American Society of Echocardiography, in collaboration with the American Society of Nuclear Cardiology, the Society for Cardiovascular Magnetic Resonance, and the Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2022;35:533–569. [DOI] [PubMed] [Google Scholar]
- 25. Mitchell CC, Frye C, Jankowski M, Symanski J, Lester SJ, Woo A et al. A practical approach to echocardiographic imaging in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr 2023;36:913–932. [DOI] [PubMed] [Google Scholar]
- 26. Marstrand P, Han L, Day SM, Olivotto I, Ashley EA, Michels M et al. Hypertrophic cardiomyopathy with left ventricular systolic dysfunction: insights from the SHaRe registry. Circulation 2020;141:1371–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bayrak F, Kahveci G, Mutlu B, Sonmez K, Degertekin M. Tissue Doppler imaging to predict clinical course of patients with hypertrophic cardiomyopathy. Eur J Echocardiogr 2008;9:278–283.doi: 10.1080/00015385.2017.1291155 [DOI] [PubMed] [Google Scholar]
- 28. Yang H, Woo A, Monakier D, Jamorski M, Fedwick K, Wigle ED et al. Enlarged left atrial volume in hypertrophic cardiomyopathy: a marker for disease severity. J Am Soc Echocardiogr 2005;18:1074–1082. [DOI] [PubMed] [Google Scholar]
- 29. Biagini E, Spirito P, Rocchi G, Ferlito M, Rosmini S, Lai F et al. Prognostic implications of the Doppler restrictive filling pattern in hypertrophic cardiomyopathy. Am J Cardiol 2009;104:1727–1731. [DOI] [PubMed] [Google Scholar]
- 30. Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med 2003;348:295–303. [DOI] [PubMed] [Google Scholar]
- 31. Massera D, Long C, Xia Y, James L, Adlestein E, Alvarez IC et al. Unmasking obstruction in hypertrophic cardiomyopathy with postprandial resting and treadmill stress echocardiography. J Am Soc Echocardiogr 2024;37:971–980. [DOI] [PubMed] [Google Scholar]
- 32. Sherrid MV. On the cause of systolic anterior motion in obstructive hypertrophic cardiomyopathy. J Am Soc Echocardiogr 2024;37:782–786. [DOI] [PubMed] [Google Scholar]
- 33. Sivalokanathan S. The role of cardiovascular magnetic resonance imaging in the evaluation of hypertrophic cardiomyopathy. Diagnostics 2022;12:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hughes RK, Shiwani H, Rosmini S, Augusto JB, Burke L, Jiang Y et al. Improved diagnostic criteria for apical hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 2024;17:501–512. [DOI] [PubMed] [Google Scholar]
- 35. Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014;130:484–495. [DOI] [PubMed] [Google Scholar]
- 36. Todiere G, Aquaro GD, Piaggi P, Formisano F, Barison A, Masci PG et al. Progression of myocardial fibrosis assessed with cardiac magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 2012;60:922–929. [DOI] [PubMed] [Google Scholar]
- 37. Aquaro GD, Grigoratos C, Bracco A, Proclemer A, Todiere G, Martini N et al. Late gadolinium enhancement-dispersion mapping: a new magnetic resonance imaging technique to assess prognosis in patients with hypertrophic cardiomyopathy and low-intermediate 5-year risk of sudden death. Circ Cardiovasc Imaging 2020;13:e010489. [DOI] [PubMed] [Google Scholar]
- 38. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. [DOI] [PubMed] [Google Scholar]
- 39. Dejgaard LA, Haland TF, Lie OH, Ribe M, Bjune T, Leren IS et al. Vigorous exercise in patients with hypertrophic cardiomyopathy. Int J Cardiol 2018;250:157–163. [DOI] [PubMed] [Google Scholar]
- 40. Maron BJ, Desai MY, Nishimura RA, Spirito P, Rakowski H, Towbin JA et al. Management of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2022;79:390–414. [DOI] [PubMed] [Google Scholar]
- 41. Weng Z, Yao J, Chan RH, He J, Yang X, Zhou Y et al. Prognostic value of LGE-CMR in HCM: a meta-analysis. JACC Cardiovasc Imaging 2016;9:1392–1402. [DOI] [PubMed] [Google Scholar]
- 42. Polovina M, Tschöpe C, Rosano G, Metra M, Crea F, Mullens W et al. Incidence, risk assessment and prevention of sudden cardiac death in cardiomyopathies. Eur J Heart Fail 2023;25:2144–2163. [DOI] [PubMed] [Google Scholar]
- 43. Maurizi N, Antiochos P, Owens A, Lakdwala N, Saberi S, Russell MW et al. Long-term outcomes after septal reduction therapies in obstructive hypertrophic cardiomyopathy: insights from the SHARE registry. Circulation 2024;150:1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Panaich SS, Badheka AO, Chothani A, Mehta K, Patel NJ, Deshmukh A et al. Results of ventricular septal myectomy and hypertrophic cardiomyopathy (from nationwide inpatient sample [1998–2010]). Am J Cardiol 2014;114:1390–1395. [DOI] [PubMed] [Google Scholar]
- 45. Yacoub MH, Afifi A, Saad H, Aguib H, ElGuindy A. Current state of the art and future of myectomy. Ann Cardiothorac Surg 2017;6:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cui H, Schaff HV, Nishimura RA, Geske JB, Dearani JA, Lahr BD et al. Conduction abnormalities and long-term mortality following septal myectomy in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2019;74:645–655. [DOI] [PubMed] [Google Scholar]
- 47. Hong JH, Nguyen A, Schaff HV. Management of the mitral valve in patients with obstructive hypertrophic cardiomyopathy. Indian J Thorac Cardiovasc Surg 2020;36:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ammirati E, Contri R, Coppini R, Cecchi F, Frigerio M, Olivotto I. Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives. Eur J Heart Fail 2016;18:1106–1118. [DOI] [PubMed] [Google Scholar]
- 49. Dybro AM, Rasmussen TB, Nielsen RR, Andersen MJ, Jensen MK, Poulsen SH. Randomized trial of metoprolol in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2021;78:2505–2517. [DOI] [PubMed] [Google Scholar]
- 50. Frishman WH. Calcium channel blockers: differences between subclasses. Am J Cardiovasc Drugs 2007;7:17–23. [DOI] [PubMed] [Google Scholar]
- 51. Authors/Task Force members; Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 52. Coppini R, Ferrantini C, Pioner JM, Santini L, Wang ZJ, Palandri C et al. Electrophysiological and contractile effects of disopyramide in patients with obstructive hypertrophic cardiomyopathy: a translational study. JACC Basic Transl Sci 2019;4:795–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bertero E, Chiti C, Schiavo MA, Tini G, Costa P, Todiere G et al. Real-world candidacy to mavacamten in a contemporary hypertrophic obstructive cardiomyopathy population. Eur J Heart Fail 2024;26:59–64. [DOI] [PubMed] [Google Scholar]
- 54. Ho CY, Day SM, Axelsson A, Russell MW, Zahka K, Lever HM et al. Valsartan in early-stage hypertrophic cardiomyopathy: a randomized Phase 2 trial. Nat Med 2021;27:1818–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ireland CG, Burstein DS, Day SM, Axelsson Raja A, Russell MW, Zahka KG et al. Quality of life and exercise capacity in early stage and subclinical hypertrophic cardiomyopathy: a secondary analysis of the VANISH trial. Circ Heart Fail 2024;17:e011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Velicki L, Popovic D, Okwose NC, Preveden A, Tesic M, Tafelmeier M et al. Sacubitril/valsartan for the treatment of non-obstructive hypertrophic cardiomyopathy: an open label randomized controlled trial (SILICOFCM). Eur J Heart Fail 2024;26:1361–1368. [DOI] [PubMed] [Google Scholar]
- 57. Aglan A, Fath AR, Eldaly AS, Anderson AS, Phillips JS, Maron BJ et al. Impact of sodium-glucose cotransporter 2 inhibitors on mortality in hypertrophic cardiomyopathy. JACC Adv 2024;3:100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lehman SJ, Crocini C, Leinwand LA. Targeting the sarcomere in inherited cardiomyopathies. Nat Rev Cardiol 2022;19:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Braunwald E, Saberi S, Abraham TP, Elliott PM, Olivotto I. Mavacamten: a first-in-class myosin inhibitor for obstructive hypertrophic cardiomyopathy. Eur Heart J 2023;44:4622–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Olivotto I, Oreziak A, Barriales-Villa R, Abraham TP, Masri A, Garcia-Pavia P et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet 2020;396:759–769. [DOI] [PubMed] [Google Scholar]
- 61. Maron MS, Masri A, Nassif ME, Barriales-Villa R, Arad M, Cardim N et al. Aficamten for symptomatic obstructive hypertrophic cardiomyopathy. N Engl J Med 2024;390:1849–1861. [DOI] [PubMed] [Google Scholar]
- 62. Desnick RJ, Ioannou YA, Eng CM. α Galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS (eds.), The Metabolic and Molecular Bases of Inherited Disease. New York, NY: McGraw-Hill; 2001. p3733–3774. [Google Scholar]
- 63. Pieroni M, Namdar M, Olivotto I, Desnick RJ. Anderson–Fabry disease management: role of the cardiologist. Eur Heart J 2024;45:1395–1409. [DOI] [PubMed] [Google Scholar]
- 64. Chimenti C, Pieroni M, Morgante E, Antuzzi D, Russo A, Russo MA et al. Prevalence of Fabry disease in female patients with late-onset hypertrophic cardiomyopathy. Circulation 2004;110:1047–1053. [DOI] [PubMed] [Google Scholar]
- 65. Linhart A, Germain DP, Olivotto I, Akhtar MM, Anastasakis A, Hughes D et al. An expert consensus document on the management of cardiovascular manifestations of Fabry disease. Eur J Heart Fail 2020;22:1076–1096. [DOI] [PubMed] [Google Scholar]
- 66. Frustaci A, Verardo R, Galea N, Alfarano M, Magnocavallo M, Marchitelli L et al. Long-term clinical-pathologic results of enzyme replacement therapy in prehypertrophic fabry disease cardiomyopathy. J Am Heart Assoc 2024;13:e032734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Frustaci A, Verardo R, Grande C, Chimenti C, Galea N, Piselli P et al. Immune-mediated myocarditis in Fabry disease cardiomyopathy. J Am Heart Assoc 2018;7:e009052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Germain DP, Linhart A. Pegunigalsidase alfa: a novel, pegylated recombinant alpha-galactosidase enzyme for the treatment of Fabry disease. Front Genet 2024;15:1395287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Emdin M, Vergaro G, Aimo A, Fontana M (eds.), Cardiac Amyloidosis: Diagnosis and Treatment. Heidelberg Germany: Springer-Verlag GmbH; 2024. p332. [Google Scholar]
- 70. Porcari A, Chimenti C, Merlo M, Musca F, Vergaro G, Iacoviello M et al. Percorsi diagnostico-terapeutici assistenziali per i pazienti con amiloidosi cardiaca—documento di consenso SIC/ANMCO. A cura della Rete Italiana dell’Amiloidosi Cardiaca (RIAC) [diagnostic–therapeutic care pathways for patients with cardiac amyloidosis—SIC/ANMCO Consensus document. Edited by the Italian Cardiac Amyloidosis Network (RIAC)]. G Ital Cardiol (Rome) 2024;25:900–920. [DOI] [PubMed] [Google Scholar]
- 71. Fontana M, Ioannou A, Cuddy S, Dorbala S, Masri A, Moon JC et al. The last decade in cardiac amyloidosis: advances in understanding pathophysiology, diagnosis and quantification, prognosis, treatment strategies, and monitoring response. JACC Cardiovasc Imaging 2025:S1936-878X(24)00458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vergaro G, Aimo A, Barison A, Genovesi D, Buda G, Passino C et al. Keys to early diagnosis of cardiac amyloidosis: red flags from clinical, laboratory and imaging findings. Eur J Prev Cardiol 2020;27:1806–1815. [DOI] [PubMed] [Google Scholar]
- 73. Sanchorawala V. Systemic light chain amyloidosis. N Engl J Med 2024;390:2295–2307. [DOI] [PubMed] [Google Scholar]
- 74. Kastritis E, Palladini G, Minnema MC, Wechalekar AD, Jaccard A, Lee HC et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med 2021;385:46–58. [DOI] [PubMed] [Google Scholar]
- 75. Chimenti C, Iacovoni A, Montalto A et al. Cardiomiopatia ipertrofica dalla diagnosi al trattamento. G Ital Cardiol 2025. In press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.