Abstract
Human T-cell lymphotropic virus type 1 (HTLV-1) infection is associated with a diverse range of lymphoproliferative and neurodegenerative diseases, yet pathogenic mechanisms induced by the virus remain obscure. This complex retrovirus contains typical structural and enzymatic genes but also unique regulatory and accessory genes in four open reading frames (ORFs) of the pX region of the viral genome (pX ORFs I to IV). The regulatory proteins encoded by pX ORFs III and IV, Tax and Rex, respectively, have been extensively characterized. In contrast the contribution of the four accessory proteins p12I, p27I, p13II, and p30II, encoded by pX ORFs I and II, to viral replication and pathogenesis remained unclear. Proviral clones that are mutated in either pX ORF I or II, while fully competent in cell culture, are severely limited in their replicative capacity in a rabbit model. Emerging evidence indicates that the HTLV-1 accessory proteins are critical for establishment of viral infectivity, enhance T-lymphocyte activation, and potentially alter gene transcription and mitochondrial function. HTLV-1 pX ORF I expression is critical to the viral infectivity in resting primary lymphocytes, suggesting a role for p12I in lymphocyte activation. The endoplasmic reticulum and cis-Golgi localizing p12I, encoded from pX ORF I, activates NFAT, a key T-cell transcription factor, through calcium-mediated signaling pathways and may lower the threshold of lymphocyte activation via the JAK/STAT pathway. In contrast p30II localizes to the nucleus and represses viral promoter activity, but may regulate cellular gene expression through p300/CBP or related coactivators of transcription. p13II targets mitochondrial proteins, where it alters the organelle morphology and may influence energy metabolism. Collectively, studies of the molecular functions of the HTLV-1 accessory proteins provide insight into strategies used by retroviruses that are associated with lymphoproliferative diseases.
INTRODUCTION
Human T-cell lymphotropic virus type 1 (HTLV-1) infection currently persists in 10 to 20 million people worldwide but is a particular problem in regions of endemicity in the Caribbean, Japan, Africa, and South America and among at-risk groups in the United States (10, 39, 85, 94). The virus is the etiologic agent of adult T-cell lymphoma or leukemia, an aggressive malignancy of CD4+ T lymphocytes, and initiates the neurodegenerative disease tropical spastic paraparesis-HTLV-1-associated myelopathy (15, 55, 118). The virus infection is also associated with a variety of other immune-mediated disorders, most likely through its ability to induce T-lymphocyte activation (10, 56, 88). HTLV-1 is a highly cell-associated virus, and transmission occurs through routes that promote lymphocyte transfer (44, 119). While the epidemiology and diseases associated with HTLV-1 are well characterized, the molecular mechanisms used by the virus to establish persistent infection and subsequently promote lymphocyte proliferation while evading elimination by the host immune response remain poorly defined.
The genome of HTLV-1 encodes the common structural and enzymatic proteins typical of all retroviruses (i.e., Gag, Pol, and Env). In addition, as a complex retrovirus, HTLV-1 uses alternative splicing and internal initiation codons to produce several regulatory and accessory proteins encoded by four open reading frames (ORFs) predominantly located in the pX region (pX ORF I to IV) of the viral genome between env and the 3′ long terminal repeat (42). A doubly spliced, 2.1-kb mRNA containing elements of the 5′ long terminal repeat, pol, and the pX region encodes the regulatory proteins Tax (pX ORF IV) and Rex (pX ORF III) (49, 68, 77, 95). Through interaction with cellular transcription factors, Tax potently activates transcription not only from the viral promoter (Tax-responsive element) but also from the enhancer elements of many cellular genes involved in host cell proliferation (47, 97, 104, 105, 128). Rex is responsible for nuclear export of unspliced or singly spliced viral RNA (52). For a more detailed discussion on Tax and Rex function the reader is referred to a number of recent reviews (59, 77, 95, 105).
HTLV-1 ACCESSORY PROTEINS EXPRESSED FROM pX GENE REGION
In addition to Tax and Rex, pX ORFs I and II produce alternatively spliced forms of mRNA, which encode four accessory proteins, p12I, p27I, p13II, and p30II (Table 1) (13, 24, 45, 71). These alternative mRNA and protein products were initially believed to be dispensable for viral replication (37, 107). However, over the past several years a number of reports have been published demonstrating that the HTLV-1 accessory proteins are critical for viral infectivity and maintenance of high viral loads, host cell activation, and regulation of gene transcription (3, 12, 25, 29, 60, 75, 96, 129, 130). These studies illustrate the critical function of these HTLV-1 accessory proteins during viral replication, and as a result these proteins should more appropriately be considered essential proteins that regulate virus-host cell interactions during the natural infection.
TABLE 1.
Summary of HTLV-1 accessory proteins
| Proteina | pX ORF | Subcellular distribution | In vitro functional activity | In vivo effects of proviral clone gene mutation |
|---|---|---|---|---|
| p12I | I | ER and cis-Golgi | Calcium-mediated NFAT activation; decreases IL-2 requirement for T-cell activation | Abolished infectivity in rabbit model; reduced infectivity in nondividing primary human T cells |
| p13II | II | Mitochondria and nucleus | Mitochondrial swelling and disruption of Δψ | Reduced viral load in rabbit modelb |
| p30II | II | Nucleus | Transcriptional regulator; binds p300/CBP | Reduced viral load in rabbit modelb |
In addition to the bicistronic mRNA encoding Tax and Rex, HTLV-1 expresses at least five other species of pX mRNA, including those encoding the less well characterized p21Rex (Fig. 1) (13, 24, 71). mRNA species specific for four accessory proteins—p12I, p27I, p13II, and p30II—can be detected in cells transfected with HTLV-1 molecular clones, HTLV-1 immortalized or transformed cell lines, and freshly isolated leukocytes from virus-infected subjects with or without disease (13, 19, 24, 71). Similar to the circumstances of Tax and Rex, there exists limited detailed knowledge about the specific quantities and timing of expression for these four accessory proteins during the course of the natural infection. Indirect evidence of their expression in vivo has been revealed from studies of the immune response to the viral infection. Serum antibodies (20, 35) as well as cytotoxic T cells (103) directed against recombinant proteins or peptides representing these proteins have been detected in diseased patients, as well as from asymptomatic carriers. Thus, proteins encoded by pX ORF I and II are produced during the course of the viral infection at levels sufficient to elicit both specific antibody and cell-mediated immune responses in infected subjects.
FIG. 1.
Diagram of HTLV-1 genome and alternatively spliced mRNA and protein species from pX ORF. Lines below the proviral genome (top) represent spliced RNA, and boxes below each line represent protein products. The ORF and protein size in kilodaltons are represented to the right of each predicted protein.
Interestingly, analogous gene regions encoding the accessory proteins, especially the pX ORF I-encoded p12I, are highly conserved in the closely related virus HTLV-2 and the nonhuman primate counterpart of HTLV-1, simian T-cell lymphotropic virus type 1 (23, 110, 115). Further illustration of the conserved nature of these gene regions comes from studies of another member of the deltaretroviruses, bovine leukemia virus (BLV). This virus, like HTLV-1, contains an X region between the env sequences and the 3′ long terminal repeat. Two proteins are expressed from this region of BLV: the protein R3, which shares a common nuclear localization signal (NLS) with the Rex protein of HTLV-1, and G4, an arginine-rich protein that may exist as two isoforms following protease processing (62, 76, 122). Similar to HTLV-1, deletion of homologous sequences from BLV infectious molecular clones encoding these accessory proteins, R4 and G3, results in decreased viral loads in the experimental sheep model (62, 123). Collectively these studies illustrate that these retroviruses, which are all associated with lymphoproliferative disorders, during the course of their evolution have retained conserved gene regions that apparently serve analogous functional roles.
ROLE OF pX ORF I P12I IN VIRAL REPLICATION AND T-CELL ACTIVATION
Alternative splicing events that combine the second exon of Tax with additional downstream sequences produce pX ORF I mRNA. This message potentially encodes two accessory proteins, the 152-amino-acid p27I and the 99-amino-acid p12I. Translation of the latter is initiated at an internal methionine codon in the p27I ORF. Alternatively, p12I can be translated from a singly spliced message produced by direct splicing of nucleotide 119 to the splice acceptor at position 6383 (24, 71). Interestingly, transfection of expression plasmids containing HA1-tagged versions of either the full-length p27I cDNA or the cDNA for the singly spliced p12I yield only the smaller p12I (71). In contrast, using in vitro transcription-translation systems, Ciminale et al. (24) produced p27I from the doubly spliced mRNA. Thus, it is possible that p12I is preferentially produced from the p27I mRNA and that removal of the internal p12I AUG start codon could yield detectable levels of p27I. Pique et al. (103) demonstrated production of cytotoxic T cells in HTLV-1-infected subjects that were reactive against peptides representing all putative pX accessory proteins, including p27I. These results suggest that p27I, along with p12I, p13II, and p30II, is produced during the course of the natural infection in vivo.
Biochemical Features of a Signaling Molecule
Amino acid sequence analysis of p12I reveals a highly hydrophobic protein with 32% of its amino acids being leucine and 17% being proline (71). Hydropathy and immunogenicity plots alike demonstrate a minimal number of soluble regions and suggest two transmembrane domains extending from amino acid 12 to 30 and amino acid 48 to 67 (71, 117). These putative transmembrane domains roughly overlap with two predicted leucine zipper motifs, which form alpha-helices. These structural features could contribute to membrane localization or homo-oligomerization of the protein (Fig. 2). Using HA1-and AU1-tagged versions of p12I transiently overexpressed in HeLa-Tat cells, Trovato et al. (117) demonstrated by immunoprecipitation and immunoblot analysis that the protein indeed forms at least dimers, if not oligomers. However, the presence of several helix-breaking proline residues within the predicted leucine zippers warrants caution about whether functional leucine zippers or simply hydrophobic protein domains contribute to the oligomerization of the protein. Furthermore, p12I contains at least three predicted SH3-binding motifs (42, 117) (Fig. 2). Similar motifs in cellular signaling proteins are typically proline rich with a minimal core of PXXP and are often preceded by an arginine residue at +2 (18, 73). Interestingly, the sequences encoding the first and third PXXP motifs of p12I (amino acids 8 to 11 and 70 to 74) are highly conserved among viral strains, suggesting a role for these domains in the function of p12I (42). In addition, a dileucine motif (DXXXLL) is found at amino acid positions 26 to 31 (Fig. 2). As shown for HIV Nef, dileucine motifs are commonly involved in directing protein trafficking through endosomal compartments by mediating association of the protein with adapter protein 1 (AP-1) to AP-3 (30, 106). However, no functional role has yet been established for the proposed motif in HTLV-1 p12I. A ubiquitylation motif surrounds the lysine at position 88 of HTLV-1 p12I. While the functional significance of this motif remains unclear, arginine substitution at this position, which is commonly found among natural HTLV-1 strains, significantly enhances the half-life of the protein (117).
FIG. 2.
Diagram of p12I (top), p30II (middle), and p13II (bottom) with predicted functional motifs in shaded boxes. Abbreviations: TM, transmembrane region; LZip, leucine zipper motif; DxxxLL, dileucine motif; PxxP, SH3 binding motif; K/R, lysine-to-arginine variant at position 88 (arginine at this position increases stability of protein). Boxes below p30II (middle) indicate regions sharing sequence homology with the DNA binding domain and homeodomain of Oct-1. Numbers below bars are amino acid position numbers.
Cytoplasmic Expression and Cellular Protein Interactions
When transiently expressed in fibroblasts HTLV-1 p12I localizes to the endoplasmic reticulum (ER) and cis-Golgi compartments (38, 61, 70, 72). Intriguingly, we recently demonstrated that p12I associates with two ER-resident calcium-binding proteins, calreticulin and calnexin (38). Calreticulin, a highly conserved and ubiquitous protein, serves as one of the major calcium-binding proteins in the ER, participates in calcium signaling, and has been linked to activation of the transcription factor nuclear factor of activated T cells (NFAT) (74, 90). Furthermore, calreticulin functions as a regulator of neoangiogenesis, and the N terminus of the protein, designated vasostatin, is used as a therapeutic angiogenesis inhibitor (102). It would be advantageous for a virus to target such a conserved protein in order to dysregulate calcium signaling pathways and activate NFAT in infected T lymphocytes.
Overall HTLV-p12I shares sequence homology with bovine papillomavirus (BPV) E5 and Epstein-Barr virus LMP-1 (43). The region of highest homology starts after the first and extends into the second transmembrane domain of p12I. Interestingly, p12I functionally cooperates with BPV E5 in transformation of mouse C127 fibroblasts and, like E5, binds to the 16-kDa subunit of the vacuolar H+ ATPase (16K) (43). Although this association appears to be required for the E5-mediated transformation of epithelial cells, no clear correlation was found between p12I-16K interaction and cooperative transformation with BPV E5, leaving the functional significance of the p12I-16K interaction to be determined. Attempts to further map the motif in p12I responsible for the association with 16K did not clearly identify a specific domain in the viral protein. Although the region between amino acids 36 and 48 of p12I is necessary for the interaction, it alone is not sufficient for binding (72). Interestingly, Nef, a key accessory protein of simian immunodeficiency virus and human immunodeficiency virus (HIV), binds the catalytic subunit NBP-1 of the ATPase (78). NBP-1 association of Nef mediated by the Nef C-terminal flexible loop is critical for Nef-dependent internalization of CD4 and viral infectivity (81).
Several reports have suggested an involvement of HTLV-1 p12I in the modulation of T-cell-specific signal transduction pathways. Using transient transfections in HeLa-Tat cells, Mulloy et al. (93), using transient-coexpression assays, reported that HTLV-1 p12I interacts with the immature forms of the interleukin-2 receptor β (IL-2Rβ) and γ chains, resulting in reduced surface expression of the receptor chains. The IL-2R binding region of p12I mapped to amino acids 37 to 47, which lie directly in front of the C-terminal proposed transmembrane domain of the protein. The p12I-binding site on the IL-2R chain overlaps with the binding site for JAK kinases 1 and 3 and the adapter protein Shc. Although p12I does not influence JAK3 kinase activity directly, Nicot et al. (96) recently demonstrated a modest increase in STAT5 activity in 293T cells transfected with p12I and all components of the IL-2R signaling complex and in primary human lymphocytes transduced with a p12I-expressing lentiviral vector. As a consequence, p12I-expressing cells displayed a decreased requirement for IL-2 to induce proliferation during suboptimal stimulation with anti-CD3 and anti-CD28 antibodies (96). Conversely, peripheral blood-derived lymphocyte cell lines immortalized by transfection with HTLV-1 infectious molecular clones with selected elimination of pX ORF I have intact IL-2R signaling pathways (28). Thus, following immortalization p12I does not appear to be necessary for the activation of the IL-2R-associated Janus kinases, JAK1 and JAK3, or their downstream effectors STAT3 and STAT5. Taken together, these findings indicate that p12I may induce STAT activity to confer a growth advantage to infected cells during the early stages of infection that precede the immortalized T-cell state. It remains to be elucidated by which JAK3-independent pathway p12I induces STAT5 activation.
When coexpressed in HeLa-Tat cells, p12I binds immature forms of the major histocompatibility complex class I (MHC-I) and directs its degradation in the proteasome (60, 61). In this system p12I localizes to the ER and decreases the surface expression of transfected MHC-I in HeLa-Tat cells and endogenous MHC-I in Jurkat cells transduced with a p12I encoding lentiviral vector. These data suggest that p12I-mediated down regulation of MHC-I surface expression might aid the virus in escaping immune surveillance. In contrast, T-lymphocytes immortalized with the wild type and p12I-mutant clones ACH and ACH.p12 expressed equal levels of MHC-I and -II, indicating that if p12I modulates MHC-I surface expression it is likely to occur only during the early stages of infection (28). In a similar manner, the accessory proteins p10I and p11V of HTLV-2 also associate with MHC-I, but these do not bind to either 16K or IL-2Rβ or -γ (60). These results are intriguing, because HIV type 1 (HIV-1) Nef binds to and down regulates the cell surface expression of MHC-I and is believed to contribute to immune evasion by HIV-1 (100). Despite this evidence down regulation of MHC-I of virus-infected cells also does not appear to explain the early loss of infectivity of a molecular clone of HTLV-1 that lacks ORF I expression, as virus infection is blocked as early as 1 week postinoculation, prior to the time one would expect an active immune response (29). It remains to be shown whether HTLV-1 p12I down regulates MHC-I expression on infected peripheral blood mononuclear cells (PBMC) in vivo and actively contributes to viral spread or persistence. To address this question studies of early virus replication immediately after inoculation of virus-infected cells in appropriate animal models will be required.
Regulation of Viral Infectivity by HTLV-1 p12I
Initial studies reported that deletion of pX ORF I from HTLV-1 infectious molecular clones had no adverse effects on the virus' ability to infect and transform primary lymphocytes in vitro (37, 107). In contrast, our research group demonstrated that selective elimination of pX ORF I from the molecular clone ACH resulted in dramatically reduced viral infectivity in vivo (29). Rabbits inoculated with ACH.p12, which is mutated and does not express pX ORF I mRNA, failed to establish persistent infection as indicated by reduced anti-HTLV-1 antibody responses, failure to demonstrate viral p19 antigen production in PBMC cultures, and only transient detection of provirus by PCR (29). The most striking difference between these in vitro and in vivo studies is that standard in vitro coculture techniques used to transmit virus to naïve PBMC utilize target cells stimulated by IL-2 and mitogen. However, in vivo the majority of circulating and tissue-associated lymphocytes are nondividing. To test whether p12I is critical for optimal viral infectivity in nonactivated primary cells in vitro, we designed coculture assays that would allow transmission of the virus to resting primary lymphocytes (3). These assays were based on the coculture of a variety of HTLV-1 producing cells with naïve (nondividing) PBMC in the absence of exogenous stimuli to more accurately reflect the virus-cell interactions during the natural infection. Under these conditions, we demonstrated a dramatic reduction in the viral infectivity of the p12I mutant ACH.p12 in primary lymphocytes. Furthermore, upon addition of mitogen to the coculture, the mutant's ability to infect primary cells was restored (3). These data provided the first evidence that HTLV-1 p12I is required for optimal viral infectivity in nondividing primary lymphocytes and suggested a role of p12I in T-lymphocyte activation. Analogously, studies of HIV-1 Nef indicate that the accessory protein is dispensable for transmission of the virus to activated target cells in vitro but is required for viral infectivity in nondividing lymphocytes (22, 31, 79, 99, 101, 112, 127).
We have recently reported that p12I expression in Jurkat cells results in an approximately 20-fold activation of NFAT-dependent gene expression, while AP-1- or NF-κB-mediated transcription remained unchanged (5). HTLV-1 p12I specifically induced NFAT-mediated transcription in synergy with the Ras/mitogen-activated protein kinase (MAPK) pathway. Inhibition of calcium-dependent signals by cyclosporine, BAPTA-AM and a dominant negative mutant of NFAT2 abolished the p12I-mediated activation of NFAT-dependent transcription. In contrast, inhibition of more proximal signaling, such as that through phospholipase C-gamma, did not affect p12I-induced NFAT activity (5). Importantly, p12I functionally substituted for thapsigargin, which selectively depletes intracellular calcium stores. Thus, HTLV-1 p12I in a calcium-dependent manner appears to activate NFAT-mediated transcription in lymphoid cells. These recent studies collectively implicate a novel mechanism by which this HTLV-1 accessory protein may dysregulate common T-cell activation pathways critical for the virus to establish persistent infection.
Subcellular localization studies indicated that p12I colocalizes with the ER-resident, calcium-binding proteins calreticulin and calnexin (38). Most strikingly, expression of p12I results in increased cytosolic calcium, indicating that HTLV-1 p12I induces release of calcium from the ER to activate NFAT (W. Ding, J. Virol., in press). Thus, the viral protein appears to act in the ER to activate calcium-mediated signaling, which would be an obvious advantage for the virus by activating T cells during the early stages of HTLV-1 infection. Cellular stimuli that would normally induce only partial activation of T cells (e.g., through AP-1) could through the influence of p12I become fully activated due to enhanced NFAT activity. These stimuli could be triggered by cytokines or chemokines released from infected neighboring cells or by direct contact between viral envelope proteins and certain cell surface receptors on newly targeted lymphocytes prior to viral entry (9, 114). Calreticulin and calnexin each have been demonstrated to modulate calcium storage and control protein folding, including several viral glycoproteins, in the ER (74, 91). Within the ER, p12I may serve to modulate calcium-mediated signals involved in cell activation. Alternatively, these proteins may serve as molecular chaperones to regulate the folding of p12I. Further studies will be required to determine the possible role of p12I in calcium storage and release from the ER. Interestingly, Johnson et al. (60, 61) have reported that p12I binds to the heavy chain of MHC-I and prevents its association with β2-microglobulin, impairing the traffic of the protein complex. Calreticulin also acts as a chaperone in the assembly and expression of MHC-I molecules in activated human T lymphocytes (8). One potential mechanism to explain the ability of p12I to interfere with MHC-I complex transport is by binding and retaining calreticulin-MHC-I complexes in the ER or cis-Golgi.
Together our data support the tenet that HTLV-1 p12I causes an increase in calcium release from the ER to activate NFAT. Interestingly, the cellular protein CAML (Ca2+-modulating cyclophilin ligand) induces calcium release from the ER in a fashion proposed for HTLV-1 p12I (120). Like HTLV-1 p12I, CAML contains two putative transmembrane domains, colocalizes with calreticulin in the ER, and leads to NFAT activation (53, 54). Thus, the accessory protein p12I of HTLV-1 appears to mimic the function of a host cell protein to increase cytosolic calcium and thus facilitate pathological T-cell activation and eventually viral infection and replication.
Role in T-Cell Activation
Calcium-dependent activation of NFAT facilitates productive infection of primary lymphocytes by HIV (69). Primary CD4+ T cells stably expressing NFAT2 became highly susceptible to HIV infection, while cells transduced with empty vector did not. Susceptibility of these cells to HIV infection could be restored by phytohemagglutinin treatment, which is most likely due to the phytohemagglutinin-induced upregulation of NFAT activity. These data concur with our findings that addition of mitogens can rescue the infectivity of a p12I mutant viral clone in resting PBMC (3), most likely by overriding the requirement for p12I-induced activation of NFAT. It will therefore be critical to examine the effect of cyclosporine in HTLV-1 replication in primary lymphocytes and compare the drug's capacity to affect replication of wild type and p12I mutant clones. Interestingly, cyclosporine reduces the infectivity of HIV and is strongly dependent on the presence of a functional nef gene (1). Manninen et al. have delineated the induction of NFAT activity in Nef-expressing Jurkat cells (83, 84). Similarly to HTLV-1 p12I, this induction required calcium signaling and the synergistic action of the Ras/MAPK pathway. In sharp contrast to HTLV-1 p12I, however, Nef-mediated NFAT activation is dependent on plasma membrane localization of the viral protein, as well as association with PAK2, suggesting an involvement of the PAK2/Vav/CASK pathway. This indicates that HIV Nef induces NFAT activity via a molecular mechanism that is clearly distinct from that used by HTLV-1 p12I. Nevertheless, despite the apparent differences in the mechanisms of calcium regulation between HTLV-1 p12I and HIV Nef, the two accessory proteins apparently play a critical role in enhancing viral infectivity in primary lymphocytes by upregulation of NFAT.
pX ORF II P30II: MODULATOR OF TRANSCRIPTION
The accessory proteins encoded by pX ORF II of HTLV-1 are produced from two alternatively spliced mRNAs. The larger of the two proteins, p30II, is encoded by a doubly spliced message including the first and second exon of Tax spliced to the splice acceptor site at position 6478 (13, 24). An internal methionine codon in p30II can be used to produce a smaller protein, p13II, which contains the C-terminal 87 amino acids of p30II. Alternatively, p13I can be produced from a singly spliced message by splicing of the first Tax exon directly to the splice acceptor at position 6875 (13, 24, 71). Initial studies suggested that ORF II was dispensable for Tax, Rex, or Env expression, as well as viral replication and immortalization of primary lymphocytes in vitro (37, 107, 108). In addition, Chou et al. (21) described the isolation of a viral clone from leukemic cells that contained a premature stop codon in pX ORF II. They concluded that pX ORF II was not necessary for the outgrowth of leukemic clones in vivo but could not rule out a function for pX ORF II during early infection. To specifically test the functional role of pX ORF II in viral replication in vivo, we inoculated rabbits with lethally irradiated cell lines expressing the wild-type molecular clone of HTLV-1 (ACH) and a clone containing selected mutations in pX ORF II (ACH.p30/13) (12). While all ACH-inoculated rabbits became infected as early as 2 weeks postinoculation, ACH.p30/13-inoculated animals failed to become infected or maintained low proviral copy numbers in their blood leukocytes. These animals also had weak and transient ex vivo p19 antigen production from their PBMC cultures and anti-HTLV-1 antibody titers that declined towards the end of the study. Most strikingly, using quantitative competitive PCR, we demonstrated a dramatically reduced (up to 100-fold) viral load for the ACH.p30/13-infected animals (4, 12). Taken together, these data suggested that pX ORF II is indeed necessary for maintenance of high viral loads in vivo.
Several lines of evidence indicate that p30II acts as a transcription factor. Importantly, the protein localizes to the nucleus, specifically the nucleolus of cells transiently transfected with a p30II expression vector (71, 130). Amino acids 71 to 98 of p30II are able to functionally substitute for the NLS of Rex (32). Furthermore, p30II contains serine/threonine-rich regions that share distant homology to the activation domain of cellular transcription factors, such as Oct-1/2, Pit-1, and POU-1 (24) (Fig. 2). Functionally p30II behaves like a transcription factor and differentially modulates CREB-responsive (CRE) promoters in transient-transfection reporter gene assays (130). Preliminary mutational analysis implicated a central core region within p30II (amino acids 62 to 132) that may mediate the transcriptional enhancement observed. Interestingly, while repressing CRE-mediated transcription, at low concentrations p30II activated viral Tax-responsive-element-dependent transcription independently of Tax expression (130). Recent molecular analyses of transcriptional regulation by p30II showed that the viral protein colocalizes with p300 in cell nuclei and regulates gene expression by binding to the KIX domain of CBP/p300 (129). Furthermore, p30II was able to disrupt CREB-Tax-CBP/p300 complexes bound to the viral 21-bp repeats. Taken together, these data suggest that p30II acts as a repressor of transcription by sequestering CBP/p300 from the pool of available transcription factors. Therefore, at higher concentrations p30II may serve to promote viral persistence by reducing viral gene expression and thus reducing immune recognition of infected cells. It will be important for future studies to define relevant p30II target genes and perhaps yet-unidentified direct p30II-responsive DNA elements. These may include promoters of genes critical for T-cell function, such as the IL-2 promoter, which contains Oct-1-responsive elements (87). In addition, further structure-function analyses will help define the roles of five lysine residues within the transactivation domain of p30II. Intriguingly, these five residues are all preceded by at least one serine residue (SK motif) and thus present potential acetylation sites for CBP/p300. As CBP/p300-mediated acetylation has become a common theme for regulation of protein function (16), it will be interesting to test whether the intrinsic histone acetyltransferase activity of CBP/p300 can in fact function to acetylate and potentially regulate HTLV-1 p30II. While p30II acetylation remains to be evaluated, p30II may function to directly inhibit acetylation of histone H3 and H4, as well as lysine 320 (K320) of the cell cycle regulator p53 through the p300/CBP-associated factor (R. Harrod and G. Franchini, unpublished observation).
MITOCHONDRIA: TARGET OF pX ORF II P13II
Less is known about the function of the smaller protein, p13II, encoded by pX ORF II. Initial studies demonstrated p13II localization to the nucleus (71), but more-recent reports show mitochondrial localization of the protein (25, 33, 34). This localization is mediated by an atypical mitochondrial targeting sequence (MTS) in the N terminus of p13II (Fig 2). The 10-amino-acid MTS also targets green fluorescent protein to mitochondria when fused to the N terminus of green fluorescent protein (25). Importantly, a fusion protein of the p13II MTS with HIV Rev can localize to mitochondria, indicating that the p13II MTS is, at least in part, able to override the potent NLS of Rev (34). Functionally, expression of p13II alters mitochondrial morphology and disrupts the mitochondrial inner membrane potential, suggesting a role for p13II in induction of apoptosis (25). Intriguingly, proteins that localize to mitochondria have been described for other human viruses including Vpr and Tat of HIV, vMIA of human cytomegalovirus, and BHRF-1 of Epstein-Barr virus (11, 40). The retroviral proteins Vpr and Tat of HIV have been shown to disrupt mitochondrial inner membrane potential, resulting in rapid swelling of mitochondria and release of cytochrome c (58). At this point the biological significance of p13II mitochondrial localization and disruption of membrane potentials remains unclear. Thus far, it has not been demonstrated that p13II indeed induces apoptosis, leaving open the possibility for other mitochondrion-based functions of the viral protein. Such functions could simply include an increased respiratory activity of mitochondria, which is often accompanied by swelling. Thus, p13II may facilitate later stages of HTLV-1 infection such as assembly and release. Furthermore, while screening a cDNA library from an HTLV-1-infected rabbit cell line by Saccharomyces cerevisiae two-hybrid assay, Hou et al. (57) discovered the association of p13II with two novel cellular proteins designated C44 and C254. While C254 appears to be rabbit actin-binding protein 280, C44 shares homology with archeal adenylate kinases, the eukaryotic homologues of which localize to mitochondria. Interestingly, the human homologue of C44 is expressed in the Jurkat T-cell line and proliferating, but not resting, PBMC (57). The implications of this finding remain unclear but allow speculation about a potential role of p13II in cellular activation. Furthermore, Mahana et al. (80) reported an increase in Vav phosphorylation in rabbit cells transfected with an HTLV-1 molecular clone that contains two mutations in pX ORF II, resulting in expression of truncated p13II and p30II. Vav is a hematopoietically restricted guanine nucleotide exchange factor for the Rac/Rho family of GTPases and is necessary for T-cell activation (92). These findings suggest that p13II may play a role in controlling the activation state of Vav, which may relate to viral infectivity and leukemogenesis.
ACCESSORY GENE PRODUCTS OF RELATED DELTARETROVIRUSES: PARALLEL ROLES IN INFECTIVITY AND PATHOGENESIS
Examination of the genome sequence of HTLV-2, simian T-cell lymphotropic virus, and BLV reveals genes and conserved organizational structure similar to those of HTLV-1 (17, 51, 89, 109, 111, 113, 115, 121). While recent molecular and epidemiological studies have expanded knowledge of the number of strains of each of these viruses, HTLV-1, HTLV-2, and BLV belong to a class of complex pathogenic retroviruses all associated with lymphoproliferative diseases (7, 67, 118, 122). Each of these viruses encodes conserved regulatory and accessory genes from pX region ORFs in the 3′ portion of the viral genome. The availability of infectious molecular clones of HTLV-2 and BLV has provided important findings that substantiate the role of homologous gene products in the pathogenesis of these other members of the deltaretroviruses.
HTLV-2 shares 60% amino acid identity with HTLV-1, and infection by this highly related virus is associated, albeit less frequently than HTLV-1, with leukemia and neurologic disease (116). Because each of these viruses shares genome structures and in vitro biological properties, HTLV-2 remains an important model for the dissection of HTLV pathogenesis. Cockerell et al. (27) reported the first successful infection of rabbits with a molecular clone of HTLV-2. Like work in the HTLV-1 system, it was first reported that deletion of genes between env and the last exon of tax/rex of this HTLV-2 molecular clone had no effect on infectivity of the virus in cell culture systems (50). Subsequently, it was reported by this same research group that this clone had reduced infectivity in the rabbit model system, further verifying the importance of this gene region in infectivity (26). The analogous protein in HTLV-2, compared to HTLV-1 p12I, appears to be p10I, also encoded by pX ORF I. Johnson et al. (60) reported the common property of pX ORF I gene products of HTLV-1 and HTLV-2 to bind MHC-I molecules and perhaps down regulate this important surface protein on infected cells. Thus, like HTLV-1, proteins encoded in the pX region of HTLV-2 are likely to be essential for viral replication during the natural infection. Further studies will be required to determine the role of these accessory genes in the disease syndromes associated with HTLV-2 infections.
BLV infection of sheep offers a reliable model of disease associated with deltaretrovirus infections. Similarly to initial reports of HTLV-1 deletion mutants, BLV molecular clones that disrupted the expression of pX ORF genes, encoding the G4 and R3 accessory proteins, failed to influence virus replication in cell culture systems but reduced the ability of the virus to replicate in sheep (6, 123, 125). The BLV G4 protein shares structural features and cellular distribution patterns with HTLV-1 p13II, while BLV R3 appears to be functionally related more closely to HTLV-1 p12I (L. Willems, personal communication). BLV wild type and mutant proviruses that contained deletions in the G4 or R3 genes infected B lymphocytes and permitted the infected cell to resist apoptotic signals (36). To test the functional properties of the viral proteins, Kerkhofs et al. (62) tested the oncogenic potential of R3 and G4, by determining their ability to transform primary rat embryo fibroblasts. In this system, G4 (analogous to HTLV-1 p13II), but not R3 (analogous to HTLV-1 p12I), cooperated with the Ha-ras oncogene to induce tumors in nude mice. A yeast two-hybrid system, as well as confocal microscopy, was used by Lefebvre et al. (76) to demonstrate that G4 interacts with farnesyl pyrophosphate (FPP) synthetase, an enzyme in the mevalonate/squalene pathway that is critical for synthesis of FPP, a substrate required for prenylation of Ras. Analogously, HTLV-1 p13II was also found to specifically interact with FPP synthetase and to colocalize with G4 in mitochondria. Whether these observations explain the function of G4 is yet to be determined, but this report illustrates new directions for research in the role of these accessory proteins in signal transduction pathways, leading to cell transformation and potential therapeutic approaches to eliminate virus replication. Interestingly, infectious molecular clones of BLV with mutations in gene regions encoding G4 and R3 were limited in their ability to maintain proviral loads in infected sheep (62). More importantly, while wild-type BLV typically produces lymphosarcomas in the majority of infected sheep during the course of the infection, none out of 13 sheep infected with viruses with mutations in G4 or in R3 and G4 developed disease (62). Whether this diminished pathogenic ability is specifically related to these gene products or a generalized attenuation of replication capacity by the virus has not been resolved. Despite this the BLV model provides an important system to test the potential role of the regulatory and accessory genes in the pathogenesis of the deltaretroviruses.
CONCLUSIONS AND FUTURE DIRECTIONS
Much of the work on HTLV-1-mediated T-cell activation and transformation has focused on the role of the transcriptional activator Tax, which potently activates numerous cellular genes involved in host cell proliferation (59). The oncogenic potential of Tax has been demonstrated in animal models, as well as in vitro transformation assays (9, 14, 41, 98). Therefore, Tax apparently may be responsible for many of the required events necessary for HTLV-1-mediated lymphocyte immortalization. However, it is uncertain whether Tax aids the virus in establishing persistent infection within a cell, a prerequisite for basal transcription of Tax itself. Emerging evidence indicates that while potentially dispensable for viral replication under activation conditions in vitro, expression of the accessory proteins encoded by pX ORFs I and II is critical for efficient HTLV-1 infection in vivo.
Based on recent findings from our own laboratory and others, we propose a molecular function for the pX ORF I-encoded p12I in HTLV-1-induced T-cell activation (Fig. 3). This calcium-dependent mechanism is independent on and most likely precedes Tax expression during a natural infection. Through this mechanism, p12I could enhance viral transmission to nondividing lymphocytes, most likely by activating target cells during the very early stages of infection through induction of NFAT-dependent gene expression.
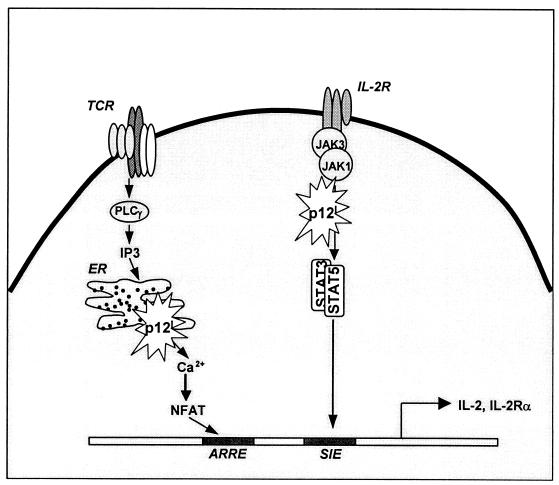
FIG. 3.
At left is shown a model of p12I function during T-cell receptor-mediated activation through calcium-mediated enhancement of NFAT responsive promoters. Abbreviations: ARRE, NFAT/AP-1, AP-1 responsive element-nuclear component; PLCγ, phospholipase Cγ. At right, the schematic shows that in the presence of p12I, IL-2-mediated signaling through STAT 5 (STAT responsive element [SIE]) may lower the threshold to trigger T-cell activation through the IL-2R.
Perhaps the most fundamental question about the contribution of p12I to HTLV-1 replication is whether the accessory protein indeed influences events early in the life cycle of the virus, e.g., prior to integration. Alternatively this accessory protein could enhance viral expression after integration. It has been difficult to address this question due to the lack of a reliable single-round infection assay for HTLV-1. If p12I does indeed increase viral infectivity rather than replication, it will be critical to determine whether p12I is present in newly infected cells prior to transcription of viral genes. Interestingly, HIV Nef, which appears to be functionally homologous, enhances infectivity during the early stages of the virus replication cycle (2, 79, 86, 112, 127). In addition, it has been shown that about 10 to 100 copies of HIV Nef protein are contained in HIV particles (2, 82). If p12I influences replication rather than infectivity, this would add another level of complexity, namely, the seemingly overlapping functions of p12I and Tax. Both proteins have been shown to activate pathways involved in T-cell activation. While p12I activates NFAT2, Tax has been reported to induce IL-2 and IL-2Rα expression partly through NFAT1 transcriptional activity (14, 48). This apparent redundancy could be resolved if p12I and Tax expression were temporally regulated during the viral replication cycle. For example, p12I could be expressed before Tax and lower the threshold for full, Tax-mediated cell activation. This is an attractive model, as Tax has been shown to up regulate expression from the serum response element (59). This leads to synthesis of the AP-1 subunits c-Fos and c-Jun and would thus provide the synergistic signal required for p12I-mediated activation of NFAT-dependent gene expression. Therefore, a kinetic analysis of the synthesis of the HTLV-1 regulatory and accessory proteins is necessary.
While less is known about the function of p30II and especially p13II in the viral life cycle, emerging evidence suggests that these proteins may act during later stages of infection to promote viral persistence and potentially aid in virus assembly. It will be interesting to evaluate the effect of single p13II or p30II knockout mutations on the replicative potential of HTLV-1 viral clones in vivo and in vitro. In addition, future studies on the p13II protein will be designed to elucidate whether the mitochondrial swelling observed in the presence of the protein is indicative of p13II-induced apoptosis or increased mitochondrial activity, which may aid during the assembly process of the virus. More detailed structure-function analysis of the p30II protein will help identify the minimal region mediating its transcriptional effects and those involved in regulation of p30II function itself. Findings resulting from such studies will aid in the design of specific p12I, p13II, and p30II functional mutants, which can subsequently be reintroduced into infectious molecular clones. Such detailed mutational analyses will be important in order to test the effect of specific mutations on protein function in the context of the whole virus in vitro or in vivo. In this regard, the BLV system offers the opportunity to test specific mutations of analogous gene regions in a disease model.
In conclusion, emerging evidence indicates that the accessory proteins of HTLV-1, which were once thought to be dispensable for viral replication, are critically involved in viral transmission and propagation and may in fact be multifunctional proteins.
Acknowledgments
Work performed in our laboratory was supported by National Institutes of Health grants RR-14324, CA-92009, and CA-70259 (R30) from the National Cancer Institute awarded through The Ohio State University, Comprehensive Cancer Center. M. Lairmore is supported by an Independent Scientist Career Award from the National Institutes of Health (K02 AI01474). B. Albrecht was sponsored by a Boehringer Ingelheim Predoctoral Fellowship.
We thank past and present members of the laboratory for their invaluable technical contributions and stimulating discussions. We are indebted to Patrick Green and Kathy Boris-Lawrie for continued collaborative support and constructive comments and to Tim Vojt for preparation of illustrations. We thank all investigators who shared unpublished information.
REFERENCES
- 1.Aiken, C. 1998. Mechanistic independence of Nef and cyclophilin A enhancement of human immunodeficiency virus type 1 infectivity. Virology 248:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiken, C., and D. Trono. 1995. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J. Virol. 69:5048-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht, B., N. D. Collins, M. T. Burniston, J. W. Nisbet, L. Ratner, P. L. Green, and M. D. Lairmore. 2000. Human T-lymphotropic virus type 1 open reading frame I p12I is required for efficient viral infectivity in primary lymphocytes. J. Virol. 74:9828-9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albrecht, B., N. D. Collins, G. C. Newbound, L. Ratner, and M. D. Lairmore. 1998. Quantification of human T-cell lymphotropic virus type 1 proviral load by quantitative competitive polymerase chain reaction. J. Virol. Methods 75:123-140. [DOI] [PubMed] [Google Scholar]
- 5.Albrecht, B., C. D. D'Souza, W. Ding, S. Tridandapani, K. M. Coggeshall, and M. D. Lairmore. 2002. Activation of nuclear factor of activated T cells by human T-lymphotropic virus type 1 accessory protein p12I. J. Virol. 76:3493-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexandersen, S., S. Carpenter, J. Christensen, T. Storgaard, B. Viuff, Y. Wannemuchler, J. Belousov, and J. A. Roth. 1993. Identification of alternatively spliced mRNAs encoding potential new regulation proteins in cattle infected with bovine leukemia virus. J. Virol. 67:39-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allan, J. S., M. Leland, S. Broussard, J. Mone, and G. Hubbard. 2001. Simian T-cell lymphotropic viruses (STLVs) and lymphomas in African nonhuman primates. Cancer Investig. 19:383-395. [DOI] [PubMed] [Google Scholar]
- 8.Arosa, F. A., O. de Jesus, G. Porto, A. M. Carmo, and M. de Sousa. 1999. Calreticulin is expressed on the cell surface of activated human peripheral blood T lymphocytes in association with major histocompatibility complex class I molecules. J. Biol. Chem. 274:16917-16922. [DOI] [PubMed] [Google Scholar]
- 9.Ballard, D. W. 2001. Molecular mechanisms in lymphocyte activation and growth. Immunol. Res. 23:157-166. [DOI] [PubMed] [Google Scholar]
- 10.Bangham, C. R. 2000. HTLV-1 infections. J. Clin. Pathol. 53:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber, G. N. 2001. Host defense, viruses and apoptosis. Cell Death Differ. 8:113-126. [DOI] [PubMed]
- 12.Bartoe, J. T., B. Albrecht, N. D. Collins, M. D. Robek, L. Ratner, P. L. Green, and M. D. Lairmore. 2000. Functional role of pX open reading frame II of human T-lymphotropic virus type 1 in maintenance of viral loads in vivo. J. Virol. 74:1094-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berneman, Z. N., R. B. Gartenhaus, M. S. Reitz, W. A. Blattner, A. Manns, B. Hanchard, O. Ikehara, R. C. Gallo, and M. E. Klotman. 1992. Expression of alternatively spliced human T-lymphotropic virus type 1 pX mRNA in infected cell lines and in primary uncultured cells from patients with adult T-cell leukemia/lymphoma and healthy carriers. Proc. Natl. Acad. Sci. USA 89:3005-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bex, F., and R. B. Gaynor. 1998. Regulation of gene expression by HTLV-I Tax protein. Methods 16:83-94. [DOI] [PubMed] [Google Scholar]
- 15.Blattner, W. A. 1999. Human retroviruses: their role in cancer. Proc. Assoc. Am. Physicians 111:563-572. [DOI] [PubMed] [Google Scholar]
- 16.Blobel, G. A. 2000. CREB-binding protein and p300: molecular integrators of hematopoietic transcription. Blood 95:745-755. [PubMed] [Google Scholar]
- 17.Cann, A. J., J. D. Rosenblatt, W. Wachsman, N. P. Shah, and I. S. Chen. 1985. Identification of the gene responsible for human T-cell leukaemia virus transcriptional regulation 5264. Nature 318:571-574. [DOI] [PubMed] [Google Scholar]
- 18.Cantrell, D. 1996. T cell antigen receptor signal transduction pathways. Annu. Rev. Immunol. 14:259-274. [DOI] [PubMed] [Google Scholar]
- 19.Cereseto, A., Z. Berneman, I. Koralnik, J. Vaughn, G. Franchini, and M. E. Klotman. 1997. Differential expression of alternatively spliced pX mRNAs in HTLV-I-infected cell lines. Leukemia 11:866-870. [DOI] [PubMed] [Google Scholar]
- 20.Chen, Y. M. A., S. H. Chen, C. Y. Fu, J. Y. Chen, and M. Osame. 1997. Antibody reactivities to tumor-suppressor protein p53 and HTLV-I Tof Rex and Tax in HTLV-I-infected people with differing clinical status. Int. J. Cancer 71:196-202. [DOI] [PubMed] [Google Scholar]
- 21.Chou, K. S., A. Okayama, N. Tachibana, T. H. Lee, and M. Essex. 1995. Nucleotide sequence analysis of a full-length human T-cell leukemia virus type I from adult T-cell leukemia cells: a prematurely terminated pX open reading frame II. Int. J. Cancer 60:701-706. [DOI] [PubMed] [Google Scholar]
- 22.Chowers, M. Y., M. W. Pandori, C. A. Spina, D. D. Richman, and J. C. Guatelli. 1995. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology 212:451-457. [DOI] [PubMed] [Google Scholar]
- 23.Ciminale, V., D. M. D'Agostino, L. Zotti, and L. Chieco-Bianchi. 1996. Coding potential of the X region of human T-cell leukemia/lymphotropic virus type II. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13(Suppl. 1):S220-S227. [DOI] [PubMed] [Google Scholar]
- 24.Ciminale, V., G. N. Pavlakis, D. Derse, C. P. Cunningham, and B. K. Felber. 1992. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV type I. J. Virol. 66:1737-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciminale, V., L. Zotti, D. M. D'Agostino, T. Ferro, L. Casareto, G. Franchini, P. Bernardi, and L. Chieco-Bianchi. 1999. Mitochondrial targeting of the p13(II) protein coded by the x-II ORF of human T-cell leukemia/lymphotropic virus type I (HTLV-I). Oncogene 18:4505-4514. [DOI] [PubMed] [Google Scholar]
- 26.Cockerell, G. L., J. Rovnak, P. L. Green, and I. S. Y. Chen. 1996. A deletion in the proximal untranslated pX region of human T-cell leukemia virus type II decreases viral replication but not infectivity in vivo. Blood 87:1030-1035. [PubMed] [Google Scholar]
- 27.Cockerell, G. L., M. G. Weiser, J. Rovnak, B. Wicks-Beard, B. Roberts, A. Post, I. S. Chen, and M. D. Lairmore. 1991. Infectious transmission of human T-cell lymphotropic virus type II in rabbits. Blood 78:1532-1537. [PubMed] [Google Scholar]
- 28.Collins, N. D., C. D'Souza, B. Albrecht, M. D. Robek, L. Ratner, W. Ding, P. L. Green, and M. D. Lairmore. 1999. Proliferation response to interleukin-2 and Jak/Stat activation of T cells immortalized by human T-cell lymphotropic virus type 1 is independent of open reading frame I expression. J. Virol. 73:9642-9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins, N. D., G. C. Newbound, B. Albrecht, J. L. Beard, L. Ratner, and M. D. Lairmore. 1998. Selective ablation of human T-cell lymphotropic virus type 1 p12(I) reduces viral infectivity in vivo. Blood 91:4701-4707. [PubMed] [Google Scholar]
- 30.Craig, H. M., M. W. Pandori, and J. C. Guatelli. 1998. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc. Natl. Acad. Sci. USA 95:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cullen, B. R. 1994. The role of Nef in the replication cycle of the human and simian immunodeficiency viruses. Virology 205:1-6. [DOI] [PubMed] [Google Scholar]
- 32.D'Agostino, D. M., V. Ciminale, L. Zotti, A. Rosato, and L. Chieco-Bianchi. 1997. The human T-cell lymphotropic virus type 1 Tof protein contains a bipartite nuclear localization signal that is able to functionally replace the amino-terminal domain of Rex. J. Virol. 71:75-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Agostino, D. M., L. Zotti, T. Ferro, I. Cavallori, M. Silic-Benussi, L. Chieco-Bianchi, and V. Ciminale. 2001. Expression and functional properties of proteins encoded in the x-II ORF of HTLV-I. Virus Res. 78:35-43. [DOI] [PubMed] [Google Scholar]
- 34.D'Agostino, D. M., L. Zotti, T. Ferro, G. Franchini, L. Chieco-Bianchi, and V. Ciminale. 2000. The p13II protein of HTLV type 1: comparison with mitochondrial proteins coded by other human viruses. AIDS Res. Hum. Retrovir. 16:1765-1770. [DOI] [PubMed] [Google Scholar]
- 35.Dekaban, G. A., A. A. Peters, J. C. Mulloy, J. M. Johnson, R. Trovato, E. Rivadeneira, and G. Franchini. 2000. The HTLV-I orfI protein is recognized by serum antibodies from naturally infected humans and experimentally infected rabbits. Virology 274:86-93. [DOI] [PubMed] [Google Scholar]
- 36.Dequiedt, F., E. Hanon, P. Kerkhofs, P. P. Pastoret, D. Portetelle, A. Burny, R. Kettmann, and L. Willems. 1997. Both wild-type and strongly attenuated bovine leukemia viruses protect peripheral blood mononuclear cells from apoptosis. J. Virol. 71:630-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derse, D., J. Mikovits, and F. Ruscetti. 1997. X-I and X-II open reading frames of HTLV-I are not required for virus replication or for immortalization of primary T-cells in vitro. Virology 237:123-128. [DOI] [PubMed] [Google Scholar]
- 38.Ding, W., B. Albrecht, R. Luo, W. Zhang, J. R. Stanley, G. C. Newbound, and M. D. Lairmore. 2001. Endoplasmic reticulum and cis-Golgi localization of human T-lymphotropic virus type 1 p12I: association with calreticulin and calnexin. J. Virol. 75:7672-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edlich, R. F., J. A. Arnette, and F. M. Williams. 2000. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I). J. Emerg. Med. 18:109-119. [DOI] [PubMed] [Google Scholar]
- 40.Everett, H., and G. McFadden. 2001. Viruses and apoptosis: meddling with mitochondria. Virology 288:1-7. [DOI] [PubMed] [Google Scholar]
- 41.Ferreira, O. C., Jr., V. Planelles, and J. D. Rosenblatt. 1997. Human T-cell leukemia viruses: epidemiology, biology, and pathogenesis. Blood Rev. 11:91-104. [DOI] [PubMed] [Google Scholar]
- 42.Franchini, G. 1995. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood 86:3619-3639. [PubMed] [Google Scholar]
- 43.Franchini, G., J. C. Mulloy, I. J. Koralnik, A. Lo Monico, J. J. Sparkowski, T. Andresson, D. J. Goldstein, and R. Schlegel. 1993. The human T-cell leukemia/lymphotropic virus type I p12I protein cooperates with the E5 oncoprotein of bovine papillomavirus in cell transformation and binds the 16-kilodalton subunit of the vacuolar H+ ATPase. J. Virol. 67:7701-7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furnia, A., R. N. Lal, E. Maloney, S. Wiktor, E. Pate, D. Rudolph, D. Waters, W. Blattner, and A. Manns. 1999. Estimating the time of HTLV-I infection following mother-to-child transmission in a breast-feeding population in Jamaica. J. Med. Virol. 59:541-546. [DOI] [PubMed] [Google Scholar]
- 45.Furukawa, K., K. Furukawa, and H. Shiku. 1991. Alternatively spliced mRNA of the pX region of human T lymphotropic virus type I proviral genome. FEBS Lett. 295:141-145. [DOI] [PubMed] [Google Scholar]
- 46.Reference deleted.
- 47.Giebler, H. A., J. E. Loring, K. Vanorden, M. A. Colgin, J. E. Garrus, K. W. Escudero, A. Brauweiler, and J. K. Nyborg. 1997. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol. Cell. Biol. 17:5156-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Good, L. F., S. B. Maggirwar, and S. C. Sun. 1996. Activation of the IL-2 gene promoter by HTLV-I Tax involves induction of NF-AT complexes bound to the CD28-responsive element. EMBO J. 15:3744-3750. [PMC free article] [PubMed] [Google Scholar]
- 49.Green, P. L., and I. S. Y. Chen. 1994. Molecular features of the human T-cell leukemia virus. Mechanisms of transformation and leukemogenicity, p. 277-311. In J. A. Levy (ed.), The Retroviridae. Plenum Press, New York, N.Y.
- 50.Green, P. L., T. M. Ross, I. S. Y. Chen, and S. Pettiford. 1995. Human T-cell leukemia virus type II nucleotide sequences between env and the last exon of tax/rex are not required for viral replication or cellular transformation. J. Virol. 69:387-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greene, W. C., W. J. Leonard, Y. Wano, P. B. Svetlek, N. J. Peffer, J. G. Sodroski, C. A. Rosen, W. C. Goh, and W. A. Haseltine. 1986. Trans-activation gene of HTLV-II induces IL-2 receptor and IL-2 cellular gene expression. Science 232:877-880. [DOI] [PubMed] [Google Scholar]
- 52.Heger, P., O. Rosorius, J. Hauber, and R. H. Stauber. 1999. Titration of cellular export factors, but not heteromultimerization, is the molecular mechanism of trans-dominant HTLV-1 Rex mutants. Oncogene 18:4080-4090. [DOI] [PubMed] [Google Scholar]
- 53.Holloway, M. P., and R. J. Bram. 1996. A hydrophobic domain of Ca2+-modulating cyclophilin ligand modulates calcium influx signaling in T lymphocytes. J. Biol. Chem. 271:8549-8552. [DOI] [PubMed] [Google Scholar]
- 54.Holloway, M. P., and R. J. Bram. 1998. Co-localization of calcium-modulating cyclophilin ligand with intracellular calcium pools. J. Biol. Chem. 273:16346-16350. [DOI] [PubMed] [Google Scholar]
- 55.Hollsberg, P. 1997. Pathogenesis of chronic progressive myelopathy associated with human T-cell lymphotropic virus type I. Acta Neurol. Scand. 95:86-93. [DOI] [PubMed] [Google Scholar]
- 56.Hollsberg, P. 1999. Mechanisms of T-cell activation by human T-cell lymphotropic virus type I. Microbiol. Mol. Biol. Rev. 63:308-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hou, X., S. Foley, M. Cueto, and M. A. Robinson. 2000. The human T-cell leukemia virus type I (HTLV-I) X region encoded protein p13(II) interacts with cellular proteins. Virology 277:127-135. [DOI] [PubMed] [Google Scholar]
- 58.Jacotot, E., L. Ravagnan, M. Loeffler, K. F. Ferri, H. L. Vieira, N. Zamzami, P. Costantini, S. Druillennec, J. Hoebeke, J. P. Briand, T. Irinopoulou, E. Daugas, S. A. Susin, D. Cointe, Z. H. Xie, J. C. Reed, B. P. Roques, and G. Kroemer. 2000. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med. 191:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeang, K. T. 2001. Functional activities of the human T-cell leukemia virus type I Tax oncoprotein: cellular signaling through NF-kappa B. Cytokine Growth Factor Rev. 12:207-217. [DOI] [PubMed]
- 60.Johnson, J. M., J. C. Mulloy, V. Ciminale, J. Fullen, C. Nicot, and G. Franchini. 2000. The MHC class I heavy chain is a common target of the small proteins encoded by the 3′ end of HTLV type 1 and HTLV type 2. AIDS Res. Hum. Retrovir. 16:1777-1781. [DOI] [PubMed] [Google Scholar]
- 61.Johnson, J. M., C. Nicot, J. Fullen, V. Ciminale, L. Casareto, J. C. Mulloy, S. Jacobson, and G. Franchini. 2001. Free major histocompatibility complex class I heavy chain is preferentially targeted for degradation by human T-cell leukemia/lymphotropic virus type 1 p12I protein. J. Virol. 75:6086-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kerkhofs, P., H. Heremans, A. Burny, R. Kettmann, and L. Willems. 1998. In vitro and in vivo oncogenic potential of bovine leukemia virus G4 protein. J. Virol. 72:2554-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reference deleted.
- 64.Reference deleted.
- 65.Reference deleted.
- 66.Reference deleted.
- 67.Khorana, A. A., J. D. Rosenblatt, and F. M. Young. 2001. Immunopathogenesis of HIV and HTLV-1 infection: mechanisms for lymphomagenesis. Cancer Treat. Res. 104:19-74. [DOI] [PubMed] [Google Scholar]
- 68.King, J. A., J. M. Bridger, M. Lochelt, P. Lichter, T. F. Schulz, V. Schirrmacher, and K. Khazaie. 1998. Nucleocytoplasmic transport of HTLV-1 RNA is regulated by two independent LTR encoded nuclear retention elements. Oncogene 16:3309-3316. [DOI] [PubMed] [Google Scholar]
- 69.Kinoshita, S., B. K. Chen, H. Kaneshima, and G. P. Nolan. 1998. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell 95:595-604. [DOI] [PubMed] [Google Scholar]
- 70.Koralnik, I. J., J. Fullen, and G. Franchini. 1993. The p12, p13, and p30 proteins encoded by human T-cell leukemia/lymphotropic virus type-1 open reading frames I and II are localized in three different cellular compartments. J. Virol. 67:2360-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koralnik, I. J., A. Gessain, M. E. Klotman, A. Lo Monico, Z. N. Berneman, and G. Franchini. 1992. Protein isoforms encoded by the pX region of human T-cell leukemia/lymphotropic virus type 1. Proc. Natl. Acad. Sci. USA 89:8813-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koralnik, I. J., J. C. Mulloy, T. Andresson, J. Fullen, and G. Franchini. 1995. Mapping of the intermolecular association of the human T cell leukaemia/lymphotropic virus type I p12(I) and the vacuolar H+-ATPase 16 kDa subunit protein. J. Gen. Virol. 76:1909-1916. [DOI] [PubMed] [Google Scholar]
- 73.Koretzky, G. A., and N. J. Boerth. 1999. The role of adapter proteins in T cell activation. Cell. Mol. Life Sci. 56:1048-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krause, K. H., and M. Michalak. 1997. Calreticulin. Cell 88:439-443. [DOI] [PubMed] [Google Scholar]
- 75.Lairmore, M. D., B. Albrecht, C. D'Souza, J. W. Nisbet, W. Ding, J. T. Bartoe, P. L. Green, and W. Zhang. 2000. In vitro and in vivo functional analysis of human T cell lymphotropic virus type 1 pX open reading frames I and II. AIDS Res. Hum. Retrovir. 16:1757-1764. [DOI] [PubMed] [Google Scholar]
- 76.Lefebvre, L., A. Vanderplasschen, V. Ciminale, H. Heremans, O. Dangoisse, J. C. Jauniaux, J. F. Toussaint, V. Zelnik, A. Burny, R. Kettmann, and L. Willems. 2002. Oncoviral bovine leukemia virus G4 and human T-cell leukemia virus type 1 p13II accessory proteins interact with farnesyl pyrophosphate synthetase. J. Virol. 76:1400-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li, X. H., and R. B. Gaynor. 2000. Mechanisms of NF-κB activation by the HTLV type 1 tax protein. AIDS Res. Hum. Retrovir. 16:1583-1590. [DOI] [PubMed] [Google Scholar]
- 78.Lu, X., H. Yu, S. H. Liu, F. M. Brodsky, and B. M. Peterlin. 1998. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity 8:647-656. [DOI] [PubMed] [Google Scholar]
- 79.Luo, T. C., J. L. Foster, and J. V. Garcia. 1997. Molecular determinants of Nef function. J. Biomed. Sci. 4:132-138. [DOI] [PubMed] [Google Scholar]
- 80.Mahana, W., T. M. Zhao, R. Teller, M. A. Robinson, and T. J. Kindt. 1998. Genes in the pX region of human T cell leukemia virus I influence Vav phosphorylation in T cells. Proc. Natl. Acad. Sci. USA 95:1782-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mandic, R., O. T. Fackler, M. Geyer, T. Linnemann, Y. H. Zheng, and B. M. Peterlin. 2001. Negative factor from SIV binds to the catalytic subunit of the V-ATPase to internalize CD4 and to increase viral infectivity. Mol. Biol. Cell 12:463-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mangasarian, A., and D. Trono. 1997. The multifaceted role of HIV Nef. Res. Virol. 148:30-33. [DOI] [PubMed] [Google Scholar]
- 83.Manninen, A., R. G. Herma, and K. Saksela. 2000. Synergistic activation of NFAT by HIV-1 nef and the Ras/MAPK pathway. J. Biol. Chem. 275:16513-16517. [DOI] [PubMed] [Google Scholar]
- 84.Manninen, A., P. Huotari, M. Hiipakka, G. H. Renkema, and K. Saksela. 2001. Activation of NFAT-dependent gene expression by Nef: conservation among divergent Nef alleles, dependence on SH3 binding and membrane association, and cooperation with protein kinase C-θ. J. Virol. 75:3034-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Manns, A., M. Hisada, and L. Lagrenade. 1999. Human T-lymphotropic virus type I infection. Lancet 353:1951-1958. [DOI] [PubMed] [Google Scholar]
- 86.Marsh, J. W. 1999. The numerous effector functions of Nef. Arch. Biochem. Biophys. 365:192-198. [DOI] [PubMed] [Google Scholar]
- 87.Matthias, P. 1998. Lymphoid-specific transcription mediated by the conserved octamer site: who is doing what? Semin. Immunol. 10:155-163. [DOI] [PubMed] [Google Scholar]
- 88.Mccallum, R. M., D. D. Patel, J. O. Moore, and B. F. Haynes. 1997. Arthritis syndromes associated with human T cell lymphotropic virus type I infection. Med. Clin. N. Am. 81:261-276. [DOI] [PubMed] [Google Scholar]
- 89.Meertens, L., J. Rigoulet, P. Mauclere, M. Van Beveren, G. M. Chen, O. Diop, G. Dubreuil, M. C. Georges-Goubot, J. L. Berthier, J. Lewis, and A. Gessain. 2001. Molecular and phylogenetic analyses of 16 novel simian T cell leukemia virus type 1 from Africa: close relationship of STLV-1 from Allenopithecus nigroviridis to HTLV-1 subtype B strains. Virology 287:275-285. [DOI] [PubMed] [Google Scholar]
- 90.Mesaeli, N., K. Nakamura, E. Zvaritch, P. Dickie, E. Dziak, K. H. Krause, M. Opas, D. H. MacLennan, and M. Michalak. 1999. Calreticulin is essential for cardiac development. J. Cell Biol. 144:857-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Michalak, M., E. F. Corbett, N. Mesaeli, K. Nakamura, and M. Opas. 1999. Calreticulin: one protein, one gene, many functions. Biochem. J. 344:281-292. [PMC free article] [PubMed] [Google Scholar]
- 92.Moores, S. L., L. M. Selfors, J. Fredericks, T. Breit, K. Fujikawa, F. W. Alt, J. S. Brugge, and W. Swat. 2000. Vav family proteins couple to diverse cell surface receptors. Mol. Cell. Biol. 20:6364-6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mulloy, J. C., R. W. Crowley, J. Fullen, W. J. Leonard, and G. Franchini. 1996. The human T-cell leukemia/lymphotropic virus type 1 p12I protein binds the interleukin-2 receptor β and γc chains and affects their expression on the cell surface. J. Virol. 70:3599-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murphy, E. L., J. P. Figueroa, W. N. Gibbs, A. Brathwaite, M. Holding-Cobham, D. Waters, B. Cranston, B. Hanchard, and W. A. Blattner. 1989. Sexual transmission of human T-lymphotropic virus type I (HTLV-I). Ann. Intern. Med. 111:555-560. [DOI] [PubMed] [Google Scholar]
- 95.Neuveut, C., and K. T. Jeang. 2000. HTLV-I Tax and cell cycle progression. Prog. Cell Cycle Res. 4:157-162. [DOI] [PubMed]
- 96.Nicot, C., J. C. Mulloy, M. G. Ferrari, J. M. Johnson, K. Fu, R. Fukumoto, R. Trovato, J. Fullen, W. J. Leonard, and G. Franchini. 2001. HTLV-1 p12(I) protein enhances STAT5 activation and decreases the interleukin-2 requirement for proliferation of primary human peripheral blood mononuclear cells. Blood 98:823-829. [DOI] [PubMed] [Google Scholar]
- 97.Nyborg, J. K., M. H. Matthews, W. T. Golde, and W. S. Dynan. 1995. The identification of cellular proteins that interact with the HTLV-I LTR, p. 131-140. In Human retroviruses. Alan R. Liss, Inc., New York, N.Y.
- 98.Ozden, S., L. Coscoy, and D. Gonzalez-Dunia. 1996. HTLV-I transgenic models: an overview. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13(Suppl 1):S154-S161. [DOI] [PubMed] [Google Scholar]
- 99.Petit, C., F. Buseyne, C. Boccaccio, J. P. Abastado, J. M. Heard, and O. Schwartz. 2001. Nef is required for efficient HIV-1 replication in cocultures of dendritic cells and lymphocytes. Virology 286:225-236. [DOI] [PubMed] [Google Scholar]
- 100.Piguet, V., O. Schwartz, S. Legall, and D. Trono. 1999. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol. Rev. 168:51-63. [DOI] [PubMed] [Google Scholar]
- 101.Piguet, V., and D. Trono. 1999. The Nef protein of primate lentiviruses. Rev. Med. Virol. 9:111-120. [DOI] [PubMed] [Google Scholar]
- 102.Pike, S. E., L. Yao, J. Setsuda, K. D. Jones, B. Cherney, E. Appella, K. Sakaguchi, H. Nakhasi, C. D. Atreya, J. Teruya-Feldstein, P. Wirth, G. Gupta, and G. Tosato. 1999. Calreticulin and calreticulin fragments are endothelial cell inhibitors that suppress tumor growth. Blood 94:2461-2468. [PubMed] [Google Scholar]
- 103.Pique, C., A. Uretavidal, A. Gessain, B. Chancerel, O. Gout, R. Tamouza, F. Agis, and M. C. Dokhelar. 2000. Evidence for the chronic in vivo production of human T cell leukemia virus type I Rof and Tof proteins from cytotoxic T lymphocytes directed against viral peptides. J. Exp. Med. 191:567-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pise-Masison, C. A., R. Mahieux, M. Radonovich, H. Jiang, and J. N. Brady. 2001. Human T-lymphotropic virus type I Tax protein utilizes distinct pathways for p53 inhibition that are cell type-dependent. J. Biol. Chem. 276:200-205. [DOI] [PubMed] [Google Scholar]
- 105.Pise-Masison, C. A., R. Mahieux, M. Radonovich, H. Jiang, J. Duvall, C. Guillerm, and J. N. Brady. 2000. Insights into the molecular mechanism of p53 inhibition by HTLV type 1 Tax. AIDS Res. Hum. Retrovir. 16:1669-1675. [DOI] [PubMed] [Google Scholar]
- 106.Riggs, N. L., H. M. Craig, M. W. Pandori, and J. C. Guatelli. 1999. The dileucine-based sorting motif in HIV-1 Nef is not required for down-regulation of class I MHC. Virology 258:203-207. [DOI] [PubMed] [Google Scholar]
- 107.Robek, M. D., F. H. Wong, and L. Ratner. 1998. Human T-cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J. Virol. 72:4458-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roithmann, S., C. Pique, A. Le Cesne, L. Delamarre, D. Pham, T. Tursz, and M. C. Dokhelar. 1994. The open reading frame I (ORF I)/ORF II part of the human T-cell leukemia virus type 1 X region is dispensable for p40tax, p27rex, or envelope expression. J. Virol. 68:3448-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosen, C. A., J. G. Sodroski, L. Willems, R. Kettmann, K. Campbell, R. Zaya, A. Burny, and W. A. Haseltine. 1986. The 3′ region of bovine leukemia virus genome encodes a trans-activator protein. EMBO J. 5:2585-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saksena, N. K., A. Srinivasan, Y. C. Ge, S. H. Xiang, A. Azad, W. Bolton, V. Herve, S. Reddy, O. Diop, M. Miranda-Saksena, W. D. Rawlinson, A. M. Vandamme, and F. Barre-Sinoussi. 1997. Simian T cell leukemia virus type I from naturally infected feral monkeys from Central and West Africa encodes a 91-amino acid p12 (ORF-I) protein as opposed to a 99-amino acid protein encoded by HTLV type I from humans. AIDS Res. Hum. Retrovir. 13:425-432. [DOI] [PubMed] [Google Scholar]
- 111.Salemi, M., J. Desmyter, and A. M. Vandamme. 2000. Tempo and mode of human and simian T-lymphotropic virus (HTLV/STLV) evolution revealed by analyses of full-genome sequences. Mol. Biol. Evol. 17:374-386. [DOI] [PubMed] [Google Scholar]
- 112.Schrager, J. A., and J. W. Marsh. 1999. HIV-1 Nef increases T cell activation in a stimulus-dependent manner. Proc. Natl. Acad. Sci. USA 96:8167-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seiki, M., T. Watanabe, A. Komuro, I. Miyoshi, M. Hayami, and M. Yoshida. 1984. Characterization of simian retrovirus genome related to human T-cell leukemia virus type I. Princess Takamatsu Symp. 15:241-249. [PubMed]
- 114.Serfling, E., F. Berberich-Siebelt, S. Chuvpilo, E. Jankevics, S. Klein-Hessling, T. Twardzik, and A. Avots. 2000. The role of NF-AT transcription factors in T cell activation and differentiation. Biochim. Biophys. Acta 1498:1-18. [DOI] [PubMed] [Google Scholar]
- 115.Shaw, G. M., M. A. Gonda, G. H. Flickinger, B. H. Hahn, R. C. Gallo, and F. Wongstaal. 1984. Genomes of evolutionarily divergent members of the human T-cell leukemia virus family (HTLV-I and HTLV-II) are highly conserved, especially in pX. Proc. Natl. Acad. Sci. USA 81:4544-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Silva, E. A., K. Otsuki, A. C. Leite, A. H. Alamy, D. Sa-Carvalho, and A. C. Vicente. 2002. HTLV-II infection associated with a chronic neurodegenerative disease: clinical and molecular analysis. J. Med. Virol. 66:253-257. [DOI] [PubMed] [Google Scholar]
- 117.Trovato, R., J. C. Mulloy, J. M. Johnson, S. Takemoto, M. P. Deoliveira, and G. Franchini. 1999. A lysine-to-arginine change found in natural alleles of the human T-cell lymphotropic leukemia virus type 1 p12 protein greatly influences its stability. J. Virol. 73:6460-6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Uchiyama, T. 1997. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu. Rev. Immunol. 15:15-37. [DOI] [PubMed] [Google Scholar]
- 119.Ureta-Vidal, A., C. Angelin-Duclos, P. Tortevoye, E. Murphy, J. F. Lepere, R. P. Buigues, N. Jolly, M. Joubert, G. Carles, J. F. Pouliquen, G. de The, J. P. Moreau, and A. Gessain. 1999. Mother-to-child transmission of human T-cell-leukemia/lymphoma virus type I: implication of high antiviral antibody titer and high proviral load in carrier mothers. Int. J. Cancer 82:832-836. [DOI] [PubMed] [Google Scholar]
- 120.von Bulow, G. U., H. Russell, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, and R. J. Bram. 2000. Molecular cloning and functional characterization of murine transmembrane activator and CAML interactor (TACI) with chromosomal localization in human and mouse. Mamm. Genome 11:628-632. [DOI] [PubMed] [Google Scholar]
- 121.Watanabe, T., M. Seiki, H. Tsujimoto, I. Miyoshi, M. Hayami, and M. Yoshida. 1985. Sequence homology of the simian retrovirus genome with human T-cell leukemia virus type I. Virology 144:59-65. [DOI] [PubMed] [Google Scholar]
- 122.Willems, L., A. Burny, D. Collete, O. Dangoisse, F. Dequiedt, J. S. Gatot, P. Kerkhofs, L. Lefebvre, C. Merezak, T. Peremans, D. Portetelle, J. C. Twizere, and R. Kettmann. 2000. Genetic determinants of bovine leukemia virus pathogenesis. AIDS Res. Hum. Retrovir. 16:1787-1795. [DOI] [PubMed] [Google Scholar]
- 123.Willems, L., P. Kerkhofs, F. Dequiedt, D. Portetelle, M. Mammerickx, A. Burny, and R. Kettmann. 1994. Attenuation of bovine leukemia virus by deletion of R3 and G4 open reading frames. Proc. Natl. Acad. Sci. USA 91:11532-11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reference deleted.
- 125.Willems, L., R. Kettmann, F. Dequiedt, D. Portetelle, V. Voneche, I. Cornil, P. Kerkhofs, A. Burny, and M. Mammerickx. 1993. In vivo infection of sheep by bovine leukemia virus mutants. J. Virol. 67:4078-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu, D., K. Takahashi, N. Liu, A. Koguchi, M. Makara, J. Sasaki, M. Goryo, and K. Okada. 1999. Distribution of T-lymphocyte subpopulation in blood and spleen of normal cattle and cattle with enzootic bovine leukosis. J. Comp. Pathol. 120:117-127. [DOI] [PubMed] [Google Scholar]
- 127.Wu, Y., and J. W. Marsh. 2001. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science 293:1503-1506. [DOI] [PubMed] [Google Scholar]
- 128.Yin, M. J., and R. B. Gaynor. 1996. Complex formation between CREB and Tax enhances the binding affinity of CREB for the human T-cell leukemia virus type 1 21-base-pair repeats. Mol. Cell. Biol. 16:3156-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang, W., J. W. Nisbet, B. Albrecht, W. Ding, F. Kashanchi, J. T. Bartoe, and M. D. Lairmore. 2001. Human T-lymphotropic virus type 1 p30II regulates gene transcription by binding CREB binding protein/p300. J. Virol. 75:9885-9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang, W., J. W. Nisbet, J. T. Bartoe, W. Ding, and M. D. Lairmore. 2000. Human T-lymphotropic virus type 1 p30II functions as a transcription factor and differentially modulates CREB-responsive promoters. J. Virol. 74:11270-11277. [DOI] [PMC free article] [PubMed] [Google Scholar]