Technological innovation: from high-resolution imaging to targeted neuromodulation
The quest for high spatiotemporal resolution in brain imaging has driven remarkable advances. For instance, multimodal approaches integrating electroencephalography (EEG) with functional magnetic resonance imaging (fMRI) or near-infrared spectroscopy (NIRS) now enable simultaneous mapping of hemodynamic and electrophysiological correlates of cognition (Gao et al., 2022). Meanwhile, innovations in network neuroscience, such as whole-brain connectivity analyses (Figure 1), have unlocked personalized targeting strategies for non-invasive neuromodulation (Cash and Zalesky, 2024).
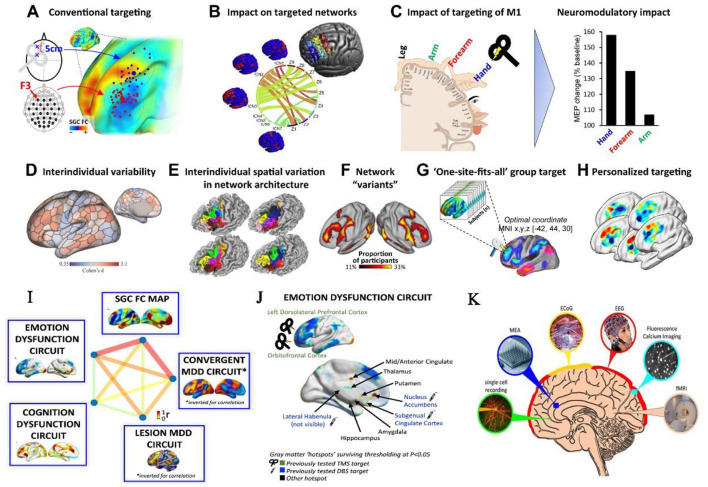
Figure 1.
Representative brain imaging and stimulation methods for personalized interventions targeting cognitive and affective functions. (A–H) Rationale for personalized targeting based on brain connectivity. (I, J) Cognitive and affective circuits that can be targeted by intervention. Copyright © 2024 Elsevier B.V. (K) Commonly used techniques for recording brain activity. Copyright © The University of Queensland.
In the realm of neuromodulation, non-invasive techniques like transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) continue to dominate due to their safety and reversibility. A systematic review in this Research Topic, Effects of transcranial direct current stimulation on modulating executive functions in healthy populations, highlights tDCS-mediated enhancements in executive functions (e.g., working memory and inhibitory control) through prefrontal cortex modulation (You et al.). However, effect variability tied to stimulation parameters and interindividual differences underscores the need for precision protocols. Similarly, a pooled analysis of transcutaneous auricular vagus nerve stimulation (taVNS) side effects of non-invasive taVNS reaffirms its safety profile while advocating for rigorous dose optimization and longitudinal monitoring (Giraudier et al.).
Clinical translation: bridging mechanisms and interventions
The synergy between imaging and stimulation holds transformative potential for cognitive disorders (Figure 1). A pivotal study in this Research Topic, Effect of continuous theta burst stimulation on the glymphatic system and cognition in cerebral small vessel disease, demonstrates how continuous theta burst stimulation (cTBS) modulates glymphatic clearance and default-mode network connectivity, improving information processing speed and executive function in patients (Dai et al.). Such findings and neuroimaging biomarkers (e.g., functional connectivity strength) offer objective metrics for evaluating therapeutic efficacy.
However, clinical translation demands careful navigation of neuroplasticity and ethical risks. A study in this Research Topic, The safety and efficacy of applying a high-current temporal interference electrical stimulation in humans, addressed this Research Topic, evaluating the safety and efficiency of a proposed high-current temporal interference electrical stimulation (Wang et al.). Personalized neuromodulation protocols must balance innovation with ethical responsibility to mitigate unintended consequences. Moreover, the application of imaging biomarkers for early diagnosis (e.g., functional connectivity anomalies in Alzheimer's disease) requires standardized, reproducible methodologies across multicenter datasets.
Challenges and future directions
Despite progress being achieved in this field, there are some critical gaps that warrant researchers' attention.
1. Multimodal data integration. How can we unify structural, functional, and metabolic imaging data to build cross-scale models of cognition? Machine learning and radiomics approaches may offer solutions (Hua et al., 2025). The coupling and interaction among different modalities of data might also carry interesting process information (Zeng et al., 2024).
2. Dynamic brain imaging in naturalistic contexts and wearable intervention devices. Capturing neural dynamics during real-world cognitive tasks remains technically challenging, necessitating advances in portable devices and real-time analytics. Meanwhile, wearable intervention devices can enable broader utility (Qi et al., 2025).
3. Validating causal mechanisms. Combining neuromodulation with pre-post imaging (e.g., TMS-EEG or tDCS-fMRI) can strengthen causal inferences (Bergmann and Hartwigsen, 2021). For example, studies pairing TMS with event-related potentials (ERPs) in this Research Topic reveal neuroplasticity markers in traumatic brain injury recovery.
Conclusion
The four studies in this Research Topic exemplify the diversity and utility of modern brain imaging and stimulation tools, spanning methodological refinement, clinical validation, and safety assessment. Moving forward, research must prioritize standardization (aligned with FAIR principles), interdisciplinary collaboration (e.g., engineering and cognitive science), and ethical frameworks to ensure responsible innovation. By addressing these challenges, we move closer to the ultimate goal of decoding cognition to enable precision interventions, paving the way for individualized therapies in neuropsychiatric disorders.
Funding Statement
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Hospital-level scientific research fund of Yunfu People's Hospital (A20231006), the Start-Up Fund for Introduced Talents and Scientific Research at Beijing Normal University (28709-312200502501), Overseas Expert Project of Guangdong Province (30802-110690303), National major project of brain science and brain-like research (2021ZD0204300), National major scientific research instrument development project (61827811), University of Macau (MYRG2022-00054-FHS, MYRG-GRG2023-00038-FHS-UMDF, and MYRG-GRG2024-00259-FHS), and the Macao Science and Technology Development Fund (FDCT 0014/2024/RIB1).
Author contributions
MX: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YHe: Writing – original draft. YHu: Writing – original draft. ZY: Conceptualization, Funding acquisition, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Bergmann T. O., Hartwigsen G. (2021). Inferring causality from noninvasive brain stimulation in cognitive neuroscience. J. Cogn. Neurosci. 33, 195–225. 10.1162/jocn_a_01591 [DOI] [PubMed] [Google Scholar]
- Cash R. F. H., Zalesky A. (2024). Personalized and circuit-based transcranial magnetic stimulation: evidence, controversies, and opportunities. Biol. Psychiatry 95, 510–522. 10.1016/j.biopsych.2023.11.013 [DOI] [PubMed] [Google Scholar]
- Gao F., Wang R. E., Armada-Da-Silva P., Wang M. Y., Lu H., Leong C., et al. (2022). How the brain encodes morphological constraints during Chinese word reading: an EEG-fNIRS study. Cortex 154, 184–196. 10.1016/j.cortex.2022.05.016 [DOI] [PubMed] [Google Scholar]
- Hua L., Huang C. P., Zeng X. L., Gao F., Yuan Z. (2025). Individualized brain radiomics-based network tracks distinct subtypes and abnormal patterns in prodromal Parkinson's disease. Neuroimage 306:121012. 10.1016/j.neuroimage.2025.121012 [DOI] [PubMed] [Google Scholar]
- Qi Z., Liu H., Jin F., Wang Y., Lu X., Liu L., et al. (2025). A wearable repetitive transcranial magnetic stimulation device. Nat. Commun. 16:2731. 10.1038/s41467-025-58095-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X. L., Hua L., Ma G. L., Zhao Z. Y., Yuan Z. (2024). Dysregulated neurofluid coupling as a new noninvasive biomarker for primary progressive aphasia. Neuroimage 303:120924. 10.1016/j.neuroimage.2024.120924 [DOI] [PubMed] [Google Scholar]