Abstract
Background
Pulmonary complications (PPCs) following mandibular fractures are serious post—surgery problems. This study analyzed risk factors of PPCs following mandibular fractures using the National Inpatient Sample (NIS) database, aiming to help clinicians specify surgical protocols and postoperative care for patients.
Method
A retrospective cohort study was conducted to examine patient demographics, hospital characteristics and preoperative comorbidities for identifying risk factors associated with postoperative pulmonary complications (PPCs). The analysis utilized data from the National Inpatient Sample (NIS) database containing patients undergoing mandibular surgery between 2010 and 2019. The cohort was stratified into two groups: those with PPCs and non-PPC cases. Statistical associations were evaluated through univariate and multivariate logistic regression analyses. A threshold of P ≤ 0.001 was set for statistical significance.
Results
The study included 41,984 adult patients (33,017 male; 8,967 female; aged ≥ 18 years), with 3,514 cases of postoperative pulmonary complications (PPCs) subclassified as: 1,347 pneumonia, 2,452 acute respiratory failure (ARF), and 212 pulmonary embolism (PE). For patients with PPCs, there was a significant increase in the age by 8 years, length of stay (LOS) by 12 days, the total charge (TOTCHG) by $163,579, and the mortality rate by 8.9%. Following the analysis, the following risk factors and their incidence were identified: number of comorbidities ≥ 3 (OR = 3.72, 40.4%), fluid and electrolyte disorders (OR = 2.66, 46.7%), obesity (OR = 1.38, 5.0%), congestive heart failure (OR = 1.24, 4.4%), coagulopathy (OR = 1.94, 12.4%), peripheral vascular disorders (OR = 1.53, 5.7%), pulmonary circulation disorders (OR = 7 .93, 4.1%), respiratory diseases (OR = 3.93, 5.2%), other neurological disorders (OR = 1.57, 15.2%), and paralysis (OR = 2.43, 5.0%).
Conclusion
In this study, statistical methods were employed to identify the risk factors for pulmonary complications following mandibular fractures, which can aid in the establishment of a sound surgical procedure and postoperative care.
Keywords: Mandibular fracture, Risk factors, Postoperative pulmonary complications (PPCs), Clinical studies
Background
Mandibular fracture is one of the common traumatic injuries in oral and maxillofacial surgery, and its global incidence has shown a steady annual increase. Surgical intervention, serving as the primary treatment approach for mandibular fractures, has been proven effective in facilitating fracture healing; however, postoperative complications are frequently reported [1, 2].
Pulmonary complications (PPCs), as one of the complications, may exacerbate the physical burden on patients, resulting in prolonged hospital stays, increased mortality rates, and heightened demands on familial and healthcare resources [3, 4]. Famurewa et al. have suggested that improper treatment may adversely affect patients'pulmonary function, thereby contributing to the development of PPCs [5]. However, limited clinical research has systematically analyzed risk factors associated with PPCs following mandibular fractures. Identifying risk factors associated with PPCs enables clinicians to conduct preoperative assessments, develop individualized treatment plans, and implement targeted postoperative care, thereby optimizing patient outcomes and resource utilization. [1, 2, 4, 6]
This study aimed to investigate the risk factors associated with PPCs following mandibular fracture by using the National Inpatient Sample (NIS) database. Furthermore, pneumonia, acute respiratory failure (ARF), and pulmonary embolism (PE) were categorized as serious pulmonary complications (SPCs) [7–9], which pose a significant threat to the stability of patients'vital signs. Consequently, the risk factors associated with these three conditions were also examined in this study.
Material and methodology
Data collection and processing
The study employed a retrospective cohort design to conduct an exploratory analysis of the National Inpatient Sample (NIS) database. The NIS database contains extensive patient hospitalization data, including hospital characteristics (e.g., admission type, bed size, teaching status, location, insurance type, region, total charges, length of stay) and patient demographics (e.g., age, sex, race), covering all regions of the United States. Administered and supported by the Agency for Healthcare Research and Quality (AHRQ), the database has collected patient samples from approximately 1,000 hospitals annually since 1988, covering approximately 97% of U.S. hospitalizations and providing robust support for scientific research, policy development, and other initiatives.
The Nationwide Inpatient Sample (NIS) is a Healthcare Cost and Utilization Project (HCUP) limited datasets, thus Institutional Review Board (IRB) review and ethical approval was not required. NIS database’s use requires neither Institutional Review Board review, an exempt determination, nor users completing the National Institutes of Health human subjects training.
The ICD-10 coding system, the International Classification of Diseases, 10 th Revision, is a globally standardized tool for classifying and coding diseases, injuries, and health problems, developed and maintained by the World Health Organization (WHO). ICD—10 codes are a combination of letters and numbers with a specific structure. The codes are generally divided into different levels, with the first letter representing the major categories of diseases, e.g., A for certain infectious and parasitic diseases, C for tumors, etc.; subsequent numbers further subdivide the disease category. For example, C12.9 represents malignant tumor of the esophagus, unspecified. This coding structure helps to accurately categorize various diseases and facilitates the recording, storage and retrieval of medical information.
Study samples
Inclusion criteria: In this study, the risk factors for postoperative pulmonary complications (PPCs) following mandibular fractures were analyzed using the NIS database. The International Classification of Diseases, 9 th Revision, Clinical Modification (ICD-9-CM) and the International Classification of Diseases, 10 th Revision, Clinical Modification (ICD-10-CM) were used to encode samples, thereby ensuring consistency in sample collection and recording and resulting in a high-quality database. In this study, patients who underwent mandibular fracture surgery from 2010 to 2019 were selected as clinical subjects based on ICD-9-CM and ICD-10-CM codes. The patient samples were divided into two groups: those with PPCs and those without PPCs. Referring to a previous study [10], the patients with PPCs were further divided into three groups: pneumonia (ICD-9-CM: 480.0–486; ICD-10-CM: J12.0–J17.0), ARF (ICD-9-CM: 518.81; ICD-10-CM: J96.00, J96.90), and PE (ICD-9-CM: 415.11, 415.12, 415.13, 415.19; ICD-10-CM: I26.02, I26.09, J96.92, J96.93, J96.94, J96.99). Demographic information, including sex, age, and race, was analyzed based on evaluation indicators such as patient cost, insurance, postoperative complications, and comorbidity. Additionally, ICD-9-CM and ICD-10-CM codes were used to query patients'treatments and surgical complications prior to discharge.
All types of mandibular fractures were included in the study group. These included single and multiple fractures, categorized according to the number of fracture sites; displaced and non-displaced fractures, determined by radiographic findings; and open and closed fractures, defined by the integrity of the overlying soft tissue. Furthermore, fractures involving only the mandible, as well as those with concomitant fractures of other facial bones, were also included (ICD-9-CM: 802.20–802.39; ICD-10-CM: S0260-S0269).
Exclusion criteria: Certain data that could potentially confound the study were excluded, including (1) patients under the age of 18 years and (2) cases with missing data. If a patient's race was missing, the race for those samples was standardized to category 6 (other race).
Data analysis
In this study, the Wilcoxon rank sum test was employed for continuous data, and the Chi-square test was utilized for categorical data to assess whether there were significant differences between the PPCs and no PPCs groups. All statistical analyses were performed using SPSS v26, a statistical software package. Furthermore, multivariate logistic regression analysis was conducted to identify the risk factors associated with PPCs. The odds ratio (OR) and 95% confidence interval (95% CI) were calculated to represent the risk levels of the factors. Consistent with prior clinical studies that have utilized a substantial number of NIS samples, a P value of less than or equal to 0.001 was established as the threshold for statistical significance [5, 11].
Table 1 presents the variables utilized in the regression analysis, which encompass patient demographics, hospital characteristics, and comorbidities. Patient demographics reflect inherent patient characteristics, such as age, sex, and race. Hospital characteristics describe attributes of the healthcare facilities selected by patients, including hospital type, bed size, teaching status, location, and insurance type. Comorbidities refer to preexisting health conditions present in patients prior to surgery.
Table 1.
Variables used in binary logistic regression analysis
| Variables categories | Specific variables |
|---|---|
| Patient demographics | Age (≤ 64 years and ≥ 65 years), gender (male and female), race (White, Black, Hispanic, Asian or Pacific Islander, Native American and Other) |
| Hospital characteristics | Type of admission (non-elective, elective), bed size of hospital (small, medium, large), teaching status of hospital (nonteaching, teaching), location of hospital (rural, urban), type of insurance (Medicare, Medicaid, private insurance, self-pay, no charge, other), location of the hospital (northeast, Midwest or north central, south, west) |
| Comorbidities | AIDS, alcohol abuse, deficiency anemia, rheumatoid diseases, chronic blood loss anemia, congestive heart failure, chronic pulmonary disease, coagulopathy, depression, diabetes (uncomplicated), diabetes (with chronic complications), drug abuse, hypertension, hypothyroidism, liver disease, lymphoma, fluid and electrolyte disorders, metastatic cancer, neurological disorders, obesity, paralysis, peripheral vascular disorders, psychoses, pulmonary circulation disorders, renal failure, solid tumor without metastasis, peptic ulcer disease, valvular disease and weight loss |
AIDS Acquired immunodeficiency syndrome, elective admissions refer to patients admitted for pre—arranged, non—emergency medical services. These patients did not present with acute jaw fractures
Results
Incidence of PPCs in mandibular fracture patients
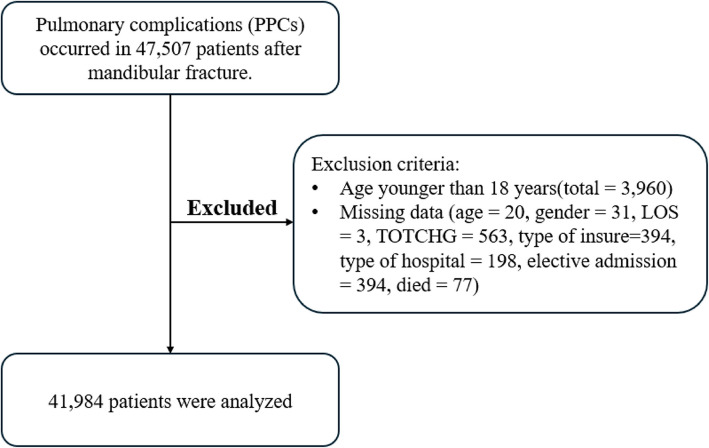
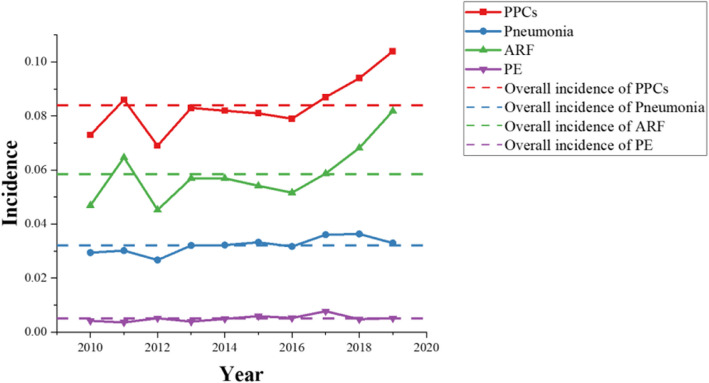
From the NIS database, a total of 47,507 participants who underwent mandibular fracture surgery between 2010 and 2019 were identified, leaving 41,984 patients for clinical analysis were included in this analysis (Fig. 1). As shown in Fig. 2, among these patients, 3,514 had PPCs, accounting for 8.4% of the total, while 1,347 (3.2%) had pneumonia, 2,452 (5.9%) had ARF, and 212 (0.5%) had PE. Figure 2 also illustrates the incidence of pneumonia, ARF, and PE from 2010 to 2019, respectively.
Fig. 1.
Flow of data exclusion
Fig. 2.
PPC and SPCs from 2010 to 2019
The bacteria that cause postoperative infection
Blood samples from patients are incubated in petri dishes to monitor bacterial growth. It can be seen from Fig. 3 that the infection of postoperative complications can be caused by a variety of cells, among which Staphylococcus accounts for the highest proportion (35.63%), while Anaerobes accounts for the lowest proportion (5.75%). In addition, the bacteria that cause infection include Enterococcus (8.05%), Streptococcus (14.94%), Pseudomonas (11.49%), E.coli (12.64%), and Candida (11.49%).
Fig. 3.
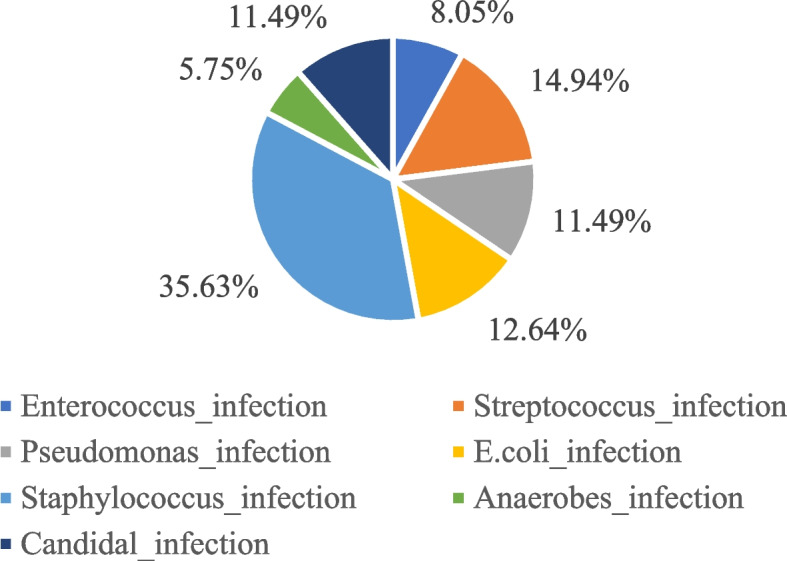
Percentage of bacteria that cause postoperative infection
Patient demographics between two groups
As shown in Table 2, out of the total 41,984 patients, about 3514 patients suffered from pulmonary complications and their median age was 43. Whereas, about 38,470 patients had no pulmonary complications and their median age was about 35. There was a difference of 8 years in the age between the two groups and it is evident that age was a factor influencing the difference between the two groups (P < 0.001). Furthermore, the age distribution also showed a significant difference between the two groups, with a 5.7% higher rate of PPCs in patients over 65 years of age (16.5% vs 10.8%, P < 0.001). In terms of racial distribution, the proportion of patients with PPCs was 6.9% higher among white patients and 6.3% lower among black patients than among patients with no PPCs. However, the results from both groups showed no statistical significance for sex.
Table 2.
Patient characteristics and outcomes following mandibular fractures
| Characteristics | No PPCs | PPCs | P |
|---|---|---|---|
| Total (n = count) | 38,470 | 3,514 | |
| Total incidence (%) | 8.4 | ||
| Age (median, years) | 35.0 (25.0, 51.0) | 43.0 (28.0, 57.0) | < 0.001 |
| Age group (%) | |||
| 18–44 | 65.0 | 52.8 | < 0.001 |
| 45–64 | 24.2 | 30.7 | |
| 65–74 | 4.7 | 7.7 | |
| ≥ 75 | 6.1 | 8.8 | |
| Sex (%) | |||
| Male | 78.6 | 79.1 | 0.466 |
| Female | 21.4 | 20.9 | |
| Race (%) | |||
| White | 50.7 | 57.6 | < 0.001 |
| Black | 24.6 | 18.3 | |
| Asian or Pacific Islander | 1.6 | 1.3 | |
| Native American | 1.1 | 1.0 | |
| Other | 9.5 | 10.8 | |
| Number of Comorbidity (%) | |||
| 0 | 38.5 | 12.9 | < 0.001 |
| 1 | 26.6 | 23.7 | |
| 2 | 17.3 | 23.1 | |
| ≥ 3 | 17.6 | 40.4 | |
| LOS (median, d) | 3(2–5) | 15(7–24) | < 0.001 |
| TOTCHG (median, $) | 48,336 (27,347–91,379) | 211,915 (105,311–392,487) | < 0.001 |
| Type of insure (%) | |||
| Medicare | 13.5 | 18.4 | < 0.001 |
| Medicaid | 26.8 | 25.4 | |
| Private insurance | 28.5 | 33.0 | |
| Self-pay | 19.5 | 12.5 | |
| No charge | 1.9 | 1.1 | |
| Other | 9.7 | 9.6 | |
| Bed size of hospital (%) | |||
| Small | 6.70 | 5.70 | 0.003 |
| Medium | 21.1 | 19.4 | |
| Large | 72.2 | 74.9 | |
| Elective admission (%) | 8.2 | 3.8 | < 0.001 |
| Type of hospital (teaching %) | 84.0 | 85.0 | 0.108 |
| Location of hospital (urban, %) | 97.6 | 98.1 | 0.057 |
| Region of hospital (%) | |||
| Northeast | 19.8 | 12.7 | < 0.001 |
| Midwest or North Central | 18.5 | 20.3 | |
| South | 39.3 | 45.6 | |
| West | 22.3 | 21.3 | |
| Died (%) | 1.7 | 10.6 | < 0.001 |
LOS Length of stay, TOTCHE Total charge
Hospital characteristics between two groups
Among the hospitals chosen by patients, there was a significant difference between PPCs and non-PPC patients across hospital regions (P < 0.001). There was also a significant difference between PPCs and non-PPC patients between the types of insurance utilized by the patients (P < 0.001). Furthermore, patients with PPCs had a 4.4% lower elective admission rate than patients without PPCs (3.8% vs 8.2%). However, there was no significant difference in hospital location between the two groups. In addition, there was no statistically significant difference in bed size of hospital and type of hospital (Table 2).
Adverse effects of PPCs following mandibular fracture
As shown in Table 2, following mandibular fracture surgery, compared with patients without PPCs, the median LOS and TOTCHG for patients with PPCs were 15 days and $211,915, respectively, which are 5 times and 4.4 times higher than those of the former group, and the mortality rate also increased by 624%. Furthermore, patients with PPCs had a 2.3% higher proportion of patients with three or more comorbidities compared to patients without PPCs.
Risk and protective factors associated with PPCs following mandibular fracture
As shown in Table 3, through variable regression analysis, it was found that multiple comorbidities (n ≥ 3, OR = 3.72, 95%CI = 2.90–4.76) were risk factors. Furthermore, four protective factors were identified in this study: female (OR = 0.73, 95%CI = 0.66–0.80), self-pay (OR = 0.81, 95%CI = 0.79–0.91), no charge (OR = 0.74, 95%CI = 0.51–1.07), and elective admission (OR = 0.47, 95%CI = 0.39–0.56).
Table 3.
Risk factors associated with PPCs following mandibular fractures
| Variable | Multivariate logistic regression | ||
|---|---|---|---|
| OR | 95% CI | P | |
| Age ≥ 65 years old | 1.10 | 0.95–1.28 | 0.220 |
| Female | 0.73 | 0.66–0.80 | < 0.001 |
| Race | |||
| White | Ref | − | − |
| Black | 0.77 | 0.69–0.85 | < 0.001 |
| Hispanic | 0.96 | 0.85–1.09 | 0.520 |
| Asian or Pacific Islander | 0.68 | 0.49–0.94 | 0.020 |
| Native American | 0.78 | 0.54–1.13 | 0.190 |
| Other | 1.12 | 0.98–1.27 | 0.090 |
| Number of Comorbidity | |||
| 0 | Ref | − | − |
| 1 | 2.38 | 2.08–2.71 | < 0.001 |
| 2 | 2.94 | 2.48–3.49 | < 0.001 |
| ≥ 3 | 3.72 | 2.90–4.76 | < 0.001 |
| Type of insurance | |||
| Medicare | Ref | − | − |
| Medicaid | 1.16 | 0.99–1.35 | 0.060 |
| Private insurance | 1.27 | 1.10–1.47 | < 0.001 |
| Self-pay | 0.81 | 0.68–0.96 | 0.010 |
| No charge | 0.74 | 0.51–1.07 | 0.110 |
| Other | 1.19 | 1.00–1.42 | 0.050 |
| Bed size of hospital | |||
| Small | Ref | − | − |
| Medium | 1.06 | 0.89–1.26 | 0.550 |
| Large | 1.21 | 1.03–1.41 | 0.020 |
| Elective admission | 0.47 | 0.39–0.56 | < 0.001 |
| Teaching hospital | 1.07 | 0.96–1.20 | 0.210 |
| Urban hospital | 1.23 | 0.92–1.63 | 0.160 |
| Region of hospital | |||
| Northeast | Ref | − | − |
| Midwest or North Central | 1.43 | 1.25–1.63 | < 0.001 |
| South | 1.72 | 1.53–1.93 | < 0.001 |
| West | 1.38 | 1.21–1.57 | < 0.001 |
OR Odds ratio, CI Confidence interval
Preoperative comorbidities associated with PPCs following mandibular fracture.
In conjunction with the aforementioned analysis, preoperative comorbidities are identified as a significant factor contributing to the poor prognosis of patients.
Univariate analysis showed that patients with the following comorbidities were more likely to develop PPCs: alcohol abuse (19.8%), deficiency anemia (7.1%), congestive heart failure (4.4%), chronic pulmonary disease (10.4%), coagulopathy (12.4%), depression (7.4%), diabetes, uncomplicated (5.8%), diabetes with chronic complications (3.3%), drug abuse (11.1%), hypertension (25.9%), hypothyroidism (3.1%), liver disease (4.0%), fluid and electrolyte disorders (46.7%), other neurological disorders (15.2%), obesity (5.0%), paralysis (5.0%), peripheral vascular disorders (5.7%), psychoses (6.1%), pulmonary circulation disorders (4.1%), renal failure (3.7%) and weight loss (20.2%). The results of the multivariate logistic regression analysis identified the following risk factors for PPCs: congestive heart failure (OR = 1.24, 95%CI = 1.00–1.54), coagulopathy (OR = 1.94, 95%CI = 1.68–2.23), fluid and electrolyte disorders (OR = 2.66, 95%CI = 2.40–2.94), other neurological disorders (OR = 1.57, 95%CI = 1.38–1.77), obesity (OR = 1.38, 95%CI = 1.15–1.65), paralysis (OR = 2.43, 95%CI = 1.97–3.01), peripheral vascular disorders (OR = 1.53, 95%CI = 1.27–1.85), pulmonary circulation disorders (OR = 7.93, 95%CI = 6.00–10.48) and weight loss (OR = 2.99, 95%CI = 2.66–3.37) (Table 4).
Table 4.
Relationship between PPCs and preoperative comorbidities
| Comorbidities | Univariate analysis | Multivariate logistic regression | ||||
|---|---|---|---|---|---|---|
| No PPCs | PPCs | P | OR | 95% CI | P | |
| Preoperative comorbidities | ||||||
| Acquired immune deficiency syndrome | 286 (0.7%) | 26 (0.7%) | 0.981 | 0.82 | 0.53–1.26 | 0.360 |
| Alcohol abuse | 7,027 (18.7%) | 697 (19.8%) | 0.110 | 0.71 | 0.63–0.79 | < 0.001 |
| Deficiency anemia | 1,224 (3.2%) | 250 (7.1%) | < 0.001 | 1.1 | 0.94–1.3 | 0.240 |
| Rheumatoid arthritis/collagen vascular diseases | 216 (0.6%) | 25 (0.7%) | 0.260 | 0.75 | 0.48–1.18 | 0.210 |
| Chronic blood loss anemia | 161 (0.4%) | 32 (0.9%) | < 0.001 | 1.16 | 0.77–1.76 | 0.470 |
| Congestive heart failure | 690 (1.8%) | 154 (4.4%) | < 0.001 | 1.24 | 1.00–1.54 | 0.050 |
| Chronic pulmonary disease | 3,409 (8.9%) | 366 (10.4%) | 0.002 | 0.88 | 0.77–1.01 | 0.060 |
| Coagulopathy | 1,109 (2.9%) | 435 (12.4%) | < 0.001 | 1.94 | 1.68–2.23 | < 0.001 |
| Depression | 2,660 (6.9%) | 259 (7.4%) | 0.309 | 0.72 | 0.62–0.84 | < 0.001 |
| Diabetes, uncomplicated | 1,904 (4.9%) | 203 (5.8%) | 0.031 | 0.82 | 0.69–0.97 | 0.020 |
| Diabetes with chronic complications | 687(1.8%) | 117 (3.3%) | < 0.001 | 0.98 | 0.78–1.23 | 0.860 |
| Drug abuse | 4,813 (12.5%) | 391 (11.1%) | 0.017 | 0.74 | 0.65–0.85 | < 0.001 |
| Hypertension | 8,041 (20.9%) | 910 (25.9%) | < 0.001 | 0.72 | 0.64–0.8 | < 0.001 |
| Hypothyroidism | 1,095 (2.8%) | 109 (3.1%) | 0.385 | 0.67 | 0.54–0.84 | < 0.001 |
| Liver disease | 1,169 (3.0%) | 140 (4.0%) | 0.002 | 0.75 | 0.61–0.92 | 0.010 |
| Lymphoma | 47 (0.1%) | 7 (0.2%) | 0.223 | 0.87 | 0.37–2.06 | 0.750 |
| Fluid and electrolyte disorders | 5,187 (13.5%) | 1,642 (46.7%) | < 0.001 | 2.66 | 2.40–2.94 | < 0.001 |
| Metastatic cancer | 90 (0.2%) | 23 (0.7%) | < 0.001 | 1.07 | 0.62–1.82 | 0.820 |
| Other neurological disorders | 2,091 (5.4%) | 533 (15.2%) | < 0.001 | 1.57 | 1.38–1.77 | < 0.001 |
| Obesity | 1,094 (2.8%) | 196 (5.0%) | < 0.001 | 1.38 | 1.15–1.65 | < 0.001 |
| Paralysis | 389 (1.0%) | 176 (5.0%) | < 0.001 | 2.43 | 1.97–3.01 | < 0.001 |
| Peripheral vascular disorders | 717(1.9%) | 201 (5.7%) | < 0.001 | 1.53 | 1.27–1.85 | < 0.001 |
| Psychoses | 2,426 (6.3%) | 215 (6.1%) | 0.661 | 0.75 | 0.64–0.88 | < 0.001 |
| Pulmonary circulation disorders | 129 (0.3%) | 145 (4.1%) | < 0.001 | 7.93 | 6.00–10.48 | < 0.001 |
| Renal failure | 773 (2.0%) | 130 (3.7%) | < 0.001 | 0.83 | 0.66–1.04 | 0.110 |
| Solid tumor without metastasis | 192 (0.5%) | 33 (0.9%) | < 0.001 | 0.81 | 0.53–1.23 | 0.320 |
| Peptic ulcer disease excluding bleeding | 55 (0.1%) | 8 (0.2%) | 0.214 | 1.03 | 0.46–2.29 | 0.940 |
| Valvular disease | 447 (1.2%) | 61 (1.7%) | 0.003 | 0.66 | 0.48–0.90 | 0.010 |
| Weight loss | 1,423 (3.7%) | 710 (20.2%) | < 0.001 | 2.99 | 2.66–3.37 | < 0.001 |
OR Odds ratio, CI Confidence interval
Postoperative complications associated with PPCs following mandibular fractures
Owing to the complex structure of mandibular fractures and their functional significance, improper management may result in a variety of complications, thereby seriously affecting the prognosis and quality of life of patients. Univariate analysis indicated that patients were likely to develop multiple complications after surgery: urinary tract infection (8.2%), respiratory disease (5.2%), genitourinary disease (18.9%), postoperative delirium (5.2%), urinary retention (3.6%), wound rupture/non healing (2.5%). Multivariate analysis revealed a significant association between PPCs and various postoperative complications: hemorrhage/seroma/hematoma (OR = 1.7, 95%CI = 1.08–2.68), urinary tract infection (OR = 1.27, 95%CI = 1.07–1.50), respiratory disease (OR = 3.93, 95%CI = 3.26–4.73), genitourinary disease (OR = 4.07, 95%CI = 3.63–4.57), gastrointestinal complication (OR = 1.81, 95%CI = 0.75–4.35), postoperative delirium (OR = 2.85, 95%CI = 2.38–3.41), postoperative shock (OR = 2.09, 95%CI = 0.32–13.89), urinary retention (OR = 1.92, 95%CI = 1.57–2.36), wound rupture/non healing (OR = 2.85, 95%CI = 2.18–3.71) (Table 5).
Table 5.
Relationship between PPCs and postoperative complications
| Complications | Univariate analysis | Multivariate logistic regression | ||||
|---|---|---|---|---|---|---|
| No PPCs | PPCs | P | OR | 95% CI | P | |
| Jawbone inflammation | 549 (1.4%) | 20 (0.6%) | < 0.001 | 0.35 | 0.22–0.55 | < 0.001 |
| Nerve injury | 234 (0.6%) | 31 (0.9%) | 0.050 | 1.35 | 0.91–2.00 | 0.130 |
| Hemorrhage/seroma/hematoma | 129 (0.3%) | 30 (0.9%) | < 0.001 | 1.70 | 1.08–2.68 | 0.020 |
| Urinary tract infection | 879 (2.3%) | 289 (8.2%) | < 0.001 | 1.27 | 1.07–1.50 | 0.010 |
| Respiratory disease | 428 (1.1%) | 182 (5.2%) | < 0.001 | 3.93 | 3.26–4.73 | < 0.001 |
| Genitourinary disease | 1,719 (4.5%) | 663 (18.9%) | < 0.001 | 4.07 | 3.63–4.57 | < 0.001 |
| Gastrointestinal complication | 27 (0.1%) | 7 (0.2%) | 0.010 | 1.81 | 0.75–4.35 | 0.190 |
| Wound infection | 206 (0.5%) | 46 (1.3%) | < 0.001 | 0.92 | 0.50–1.67 | 0.780 |
| Postoperative delirium | 570 (1.5%) | 183 (5.2%) | < 0.001 | 2.85 | 2.38–3.41 | < 0.001 |
| Postoperative shock | 4 (0.01%) | 2 (0.1%) | 0.027 | 2.09 | 0.32–13.89 | 0.440 |
| Urinary retention | 567 (1.5%) | 128 (3.6%) | < 0.001 | 1.92 | 1.57–2.36 | < 0.001 |
| Wound rupture/non healing | 270 (0.7%) | 87 (2.5%) | < 0.001 | 2.85 | 2.18–3.71 | < 0.001 |
OR Odds ratio, CI Confidence interval
Risk factors associated with SPCs following mandibular fractures
SPCs, including pneumonia, ARF, and PE, seriously affect the physical and mental health of patients. After screening, the common risk factors for SPCs are presented in Table 6 for analysis and study, including preoperative comorbidities (coagulopathy, fluid and electrolyte disorders, other neurological disorders, pulmonary circulation disorders and weight loss) and postoperative complications (hemorrhage/seroma/hematoma, urinary tract infection, respiratory disease, genitourinary disease and gastrointestinal complication).
Table 6.
Common risk factors for SPCs (pneumonia, ARF, and PE) following mandibular fractures
Discussion
Data from the NIS database (2010–2019) were analyzed to identify risk factors associated with PPCs following mandibular fracture.
Demographic analysis revealed an 8-year age difference between patients with PPCs and those without complications. Age has been established as a key predictor of PPC risk, serving as a critical component in postoperative assessment protocols [12, 13]. Age-related physiological decline in respiratory function, including diminished respiratory control and muscle strength, has been directly linked to increased PPC susceptibility [14, 15].
Fluid and electrolyte disorders and obesity were identified as risk factors predisposing patients to PPCs. These factors demonstrated significant associations with pneumonia, ARF and PE. Orea-Tejeda et al. [16] identified fluid distribution abnormalities that increase pulmonary capillary permeability, ultimately causing edema, pleural effusion, and measurable declines in pulmonary function. Mafort et al. [17] demonstrated that obesity-induced adipose accumulation drives cytokine-mediated systemic complications that directly impair respiratory mechanics.
In results, risk factors pertain to circulatory diseases, including congestive heart failure, coagulopathy, peripheral vascular diseases, and pulmonary circulation disorders. Ramalho et al. [18] revealed that reduced respiratory volumes elevate cardiovascular risk regardless of pulmonary status, while heart failure patients show heightened thrombosis susceptibility due to coagulopathies [19]. The heart failure-pulmonary circulation axis involves compromised cardiac output leading to vascular remodeling, pulmonary blood pooling, and subsequent edema development. These pathophysiological changes directly impair gas exchange, clinically manifesting as dyspnea [20]. Similarly, pulmonary circulation disorders demonstrated the strongest PPC association (OR = 7.93), particularly for pulmonary embolism (n = 102, 48.1%). Despite its utility in mandibular reconstruction, vascularized bone techniques may exacerbate PPC risks in patients with preexisting peripheral vascular disease, emphasizing the imperative for meticulous intraoperative vascular management [21, 22].
Respiratory diseases is a risk factor associated with PPCs, demonstrating strong correlations with pneumonia, acute respiratory failure (ARF), and pulmonary embolism (PE). Viral pathogens induce epithelial destruction and thrombotic cascades, potentially progressing to acute respiratory distress syndrome (ARDS) or pulmonary fibrosis [23]. Bacterial superinfections exacerbate pulmonary inflammation and accelerate functional decline [24]. Fernandez-Bustamante et al. [25] demonstrated that perioperative anesthetic agents exacerbate obstructive apnea through dual mechanisms: parapharyngeal hypotonia and blunted hypercapnic responsiveness. Research by De Carvalho Sampaio [26] et al. confirmed that maxillomandibular fixation induces transient respiratory impairment, with full recovery post-removal. Maxillomandibular fixation remains viable for patients with preserved preoperative nasal airflow. Contraindications include pre-existing pulmonary compromise or oncologic histories, where fixation risks catastrophic respiratory sequelae.
Albaiceta et al. [27] demonstrated bidirectional lung-brain communication mediated by neurological pathways, establishing the nervous system as critical to pulmonary complication mechanisms. Neurological disorders emerged as independent PPC risk factors. Traumatic brain injury induces neurogenic pulmonary edema, acute respiratory distress syndrome (ARDS), and pneumonia. Conversely, acute/chronic pulmonary conditions may precipitate neurological dysfunction [28, 29]. Paralysis independently predicts PPC development. This paralysis-associated risk, validated across clinical studies, now informs perioperative risk stratification [30].
This study has notable strengths. It's the first clinical research on PPCs and related risk factors in mandibular fracture. The large—scale national sample from the US improves the generalizability of the findings. Moreover, the study uses statistical methodology with strict inclusion and exclusion criteria. However, the study has limitations. First, the use of maxillomandibular fixation, a known risk factor for respiratory complications in maxillofacial trauma treatment, wasn't analyzed. Second, lacking data on the time from injury to mandibular fracture surgery limits accurate risk assessment and preventive strategy development, as this time interval is crucial for postoperative complications.
The clinical importance of this study lies in its ability to help clinicians identify high—risk patients before mandibular fracture surgery. By identifying these risk factors, targeted preventive measures can be implemented, this can effectively reduce the incidence of PPCs and improve patient outcomes.
Conclusion
This study identified risk factors associated with PPCs following mandibular fractures by using the NIS database from 2010 to 2019. The following risk factors were identified: number of comorbidities ≥ 3, fluid and electrolyte disorders, obesity, congestive heart failure, coagulopathy, peripheral vascular disorders, pulmonary circulation disorders, respiratory disease, other neurological disorders, and paralysis. Clinicians can specify reasonable surgical protocols and postoperative care based on these risk factors.
Acknowledgements
No applicable.
Abbreviations
- PPCs
Postoperative pulmonary complications
- SPCs
Severe pulmonary complications
- AIDS
Acquired immunodeficiency syndrome
- ARF
Acute respiratory failure
- PE
Pulmonary embolism
- LOS
Length of stay
- OR
Odds rate
- 95% CI
95% confidence interval
Authors’ contributions
Lina Yu conceptualized the study. Shuwei Liao collected the data. Guanxiong Zhu and Liting zeng analyzed and interpreted the data. Yang Yu and Zeyu Zhang drafted the manuscript. Hongru Zhang and Jingyuan Wang critically revised the manuscript. All the authors approved the final version for submission and publication.
Funding
This research received no external funding.
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This retrospective cohort study was deemed exempt because it used publicly available data (National Inpatient Sample, NIS) and not contain any human participants or animals. As is the case with other studies utilizing the NIS, this study did not require ethical review, as the data are anonymous and cannot be linked to any individuals.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuwei Liao, Guanxiong Zhu and Liting Zeng contributed equally to this work.
References
- 1.Hsieh TY, et al. Risk Factors Associated With Complications After Treatment of Mandibular fractures. JAMA Facial Plast Surg. 2019;21(3):213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odom EB, Snyder-Warwick AK. Mandibular fracture Complications and Infection: The Influence of Demographics and Modifiable Factors. Plast Reconstr Surg. 2016;138(2):282e–9e. [DOI] [PubMed] [Google Scholar]
- 3.Kirmeier E, et al. Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): a multicentre, prospective observational study. Lancet Respir Med. 2019;7(2):129–40. [DOI] [PubMed] [Google Scholar]
- 4.Loeffelbein DJ, et al. Perioperative risk factors for postoperative pulmonary complications after major oral and maxillofacial surgery with microvascular reconstruction: A retrospective analysis of 648 cases. J Craniomaxillofac Surg. 2016;44(8):952–7. [DOI] [PubMed] [Google Scholar]
- 5.Famurewa BA, et al. Effects of maxillomandibular fixation and rigid internal fixation on pulmonary function in patients with mandibular fractures. Int J Oral Maxillofac Surg. 2020;49(9):1193–8. [DOI] [PubMed] [Google Scholar]
- 6.Stacey DH, et al. Management of mandibular fractures. Plast Reconstr Surg. 2006;117(3):48e–60e. [DOI] [PubMed] [Google Scholar]
- 7.Morris K, et al. Identification of risk factors for postoperative pulmonary complications in general surgery patients in a low-middle income country. PLoS ONE. 2022;17(10): e0274749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Summers C, Todd RS, Vercruysse GA, Moore FA. Acute Respiratory Failure. Perioper Med. 2022:576–86. 10.1016/B978-0-323-56724-4.00039-3. Epub 2021 Mar 5. PMCID: PMC7946379.
- 9.Rivera-Lebron B, et al. Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium. Clin Appl Thromb Hemost. 2019;25:1076029619853037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haines KL, Agarwal S. Postoperative Pulmonary Complications-A Multifactorial Outcome. JAMA Surg. 2017;152(2):166–7. [DOI] [PubMed] [Google Scholar]
- 11.Stulberg JJ, Haut ER. Practical Guide to Surgical Data Sets: Healthcare Cost and Utilization Project National Inpatient Sample (NIS). JAMA Surg. 2018;153(6):586–7. [DOI] [PubMed] [Google Scholar]
- 12.Kannari L, et al. Non-Surgical Site-Related Complications in Mandibular Fracture Surgery - A Problem of Elderly Patients? J Oral Maxillofac Surg. 2024;82(1):47–55. [DOI] [PubMed] [Google Scholar]
- 13.Xue Q, et al. Developing Machine Learning Algorithms to Predict Pulmonary Complications After Emergency Gastrointestinal Surgery. Front Med (Lausanne). 2021;8: 655686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen D, et al. Frailty is an independent risk factor for postoperative pulmonary complications in elderly patients undergoing video-assisted thoracoscopic pulmonary resections. Aging Clin Exp Res. 2022;34(4):819–26. [DOI] [PubMed] [Google Scholar]
- 15.Vaz Fragoso CA, Gill TM. Respiratory impairment and the aging lung: a novel paradigm for assessing pulmonary function. J Gerontol A Biol Sci Med Sci. 2012;67(3):264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orea-Tejeda A, et al. The impact of hydration status and fluid distribution on pulmonary function in COPD patients. Sci Rep. 2022;12(1):1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mafort TT, et al. Obesity: systemic and pulmonary complications, biochemical abnormalities, and impairment of lung function. Multidiscip Respir Med. 2016;11:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramalho SHR, Shah AM. Lung function and cardiovascular disease: A link. Trends Cardiovasc Med. 2021;31(2):93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farid-Zahran M, Méndez-Bailón M, Pedrajas JM, Alonso-Beato R, Galeano-Valle F, Sendín Martín V, Marco-Martínez J, Demelo-Rodríguez P. Prognostic Significance of Heart Failure in Acute Pulmonary Embolism: A Comprehensive Assessment of 30-Day Outcomes. J Clin Med. 2024;13(5):1284. 10.3390/jcm13051284. PMID: 38592126; PMCID: PMC10931925. [DOI] [PMC free article] [PubMed]
- 20.Chen J, Aronowitz P. Congestive Heart Failure. Med Clin North Am. 2022;106(3):447–58. [DOI] [PubMed] [Google Scholar]
- 21.Shao S, et al. Jaw reconstruction with vascularized fibular flap: The 11-year experience among 104 patients. World J Surg Oncol. 2020;18(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babu MR, et al. Deep vein thrombosis: A rare complication in oral and maxillofacial surgery: A review of two cases. Contemp Clin Dent. 2013;4(2):236–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi Y, et al. Acute and post-acute respiratory complications of SARS-CoV-2 infection: population-based cohort study in South Korea and Japan. Nat Commun. 2024;15(1):4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SJ, et al. Respiratory pathogen and clinical features of hospitalized patients in acute exacerbation of chronic obstructive pulmonary disease after COVID 19 pandemic. Sci Rep. 2024;14(1):10462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Bustamante A, et al. Postoperative Pulmonary Complications, Early Mortality, and Hospital Stay Following Noncardiothoracic Surgery: A Multicenter Study by the Perioperative Research Network Investigators. JAMA Surg. 2017;152(2):157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Carvalho Sampaio TR, et al. Does Maxillomandibular Fixation Affect Respiratory Function? A Systematic Review J Craniofac Surg. 2022;33(8):2455–9. [DOI] [PubMed] [Google Scholar]
- 27.Albaiceta GM, et al. The central nervous system during lung injury and mechanical ventilation: a narrative review. Br J Anaesth. 2021;127(4):648–59. [DOI] [PubMed] [Google Scholar]
- 28.Chacon-Aponte AA, et al. Brain-lung interaction: a vicious cycle in traumatic brain injury. Acute Crit Care. 2022;37(1):35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park H, Lee CH. The Impact of Pulmonary Disorders on Neurological Health (Lung-Brain Axis). Immune Netw. 2024;24(3): e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aragón-Benedí C, et al. Model for predicting early and late-onset postoperative pulmonary complications in perioperative patients receiving neuromuscular blockade: a secondary analysis. Sci Rep. 2023;13(1):5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.