Abstract
Objective
This study analyzes data from the 2009–2018 National Health and Nutrition Examination Survey (NHANES) to explore the relationship between the Aggregate Index of Systemic Inflammation (AISI), also referred to as the pan-immune-inflammation value (PIV), and Type 2 Diabetes Mellitus (T2DM) among adults in the United States. Furthermore, it evaluates the mediating effect of obesity indicators on this association.
Methods
This study included 9,947 individuals from NHANES and applied appropriate weighting techniques. To examine the relationship between AISI and T2DM, we used various statistical models, including weighted multivariable logistic regression, smooth curve fitting, threshold effect analysis, subgroup analysis, trend tests, mediation analysis, and Shapley additive explanations (SHAP) models.
Results
This research included a total of 9,947 participants, with 3,220 diagnosed with T2DM, while 6,727 remained undiagnosed. Weighted multiple logistic regression with all covariates adjusted indicated that with every one-unit increment in AISI/1000, there was an 88.3% likelihood of T2DM occurrence (OR: 1.883, 95% CI: 1.378–2.571). The stratified analysis identified significant differences in this association based on age, biological sex, level of education, poverty-income ratio (PIR), tobacco consumption status, and body mass index (BMI). Interaction tests revealed a positive association between AISI and T2DM, apart from PIR, BMI, age, education attainment, race, gender, tobacco use status, Estimated Glomerular Filtration Rate(eGFR), platelet count, and high blood pressure, with none of the interaction p-values falling below 0.05. Nevertheless, the occurrence of cardiovascular disease (CVD) among participants may affect the strength of this relationship, where an interaction p-value was less than 0.05. Additionally, smoothing curve fitting revealed a nonlinear relationship between AISI and T2DM, marking a significant change at AISI/1000 of 0.21. Mediation analysis indicated that five obesity-related indicators—LAP, VAI, WHtR, WWI and ABSI — partly mediated the association between AISI/1000 and T2DM.
Conclusion
An increase in AISI is associated with an elevated probability of T2DM, with obesity indicators potentially mediating this relationship. Reducing AISI and managing obesity may help prevent T2DM. However, with the cross-sectional design of this study, causal relationships cannot be established. Future research should utilize longitudinal studies to confirm these findings.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-025-02589-4.
Keywords: Cross-sectional study, NHANES, Aggregate index of systemic inflammation, Type 2 diabetes mellitus, Mediation analysis, Shapley additive explanations (SHAP)
Introduction
Diabetes mellitus (DM) leads to a range of metabolic disturbances. According to predictions from the International Diabetes Federation, the global prevalence of diabetes is expected to rise to 10.0% by 2045, with approximately 90% of cases expected to be Type 2 Diabetes Mellitus (T2DM) [1]. Data from The National Health and Nutrition Examination Survey (2017–2020), the National Health Interview Survey (2018–2019), and estimates from the Centers for Disease Control and Prevention, 37.3 million Americans, or 11.3% of the population, are affected by diabetes [2]. The incidence of T2DM continues to rise annually, making it a major public health concern and a leading cause of heart disease worldwide [3].
Pro-inflammatory diets, insulin resistance, and inflammation are key factors in the pathophysiology of obesity and diabetes [4]. Studies have shown that chronic tissue inflammation is present in insulin-target tissues including adipose tissue, liver and muscle, which has become a hallmark of obesity and type 2 diabetes [5]. Although the precise factors and mechanisms of diabetes are not fully understood, inflammation is considered a major risk factor and plays a critical part in both the pathogenesis and prognosis of the illness [6]. Excessive body fat is also a key risk determinant for the progress of diabetes [7]. An increase in white adipose tissue (WAT) is often associated with inflammation, primarily due to the accumulation of tissue-resident activated (M1) macrophages. Inflammation in WAT can lead to dyslipolysis, promote insulin resistance, increase the release of pro-inflammatory cytokines, and alter adipokine secretion [8]. The accumulation of excess nutrients in adipose tissue stimulates the secretion of inflammation markers, including tumor necrosis factor-alpha and interleukin-6, while simultaneously reducing adiponectin production. This results in a pro-inflammatory state and increased oxidative damage [9].
The Aggregate Index of Systemic Inflammation (AISI), often called the Pan-Immune-Inflammation Value (PIV), serves as an index for evaluating systemic inflammation. It uses a simple and accessible measurement method based on the analysis of complete blood count (CBC) data. AISI is emerging as a biomarker of inflammation and has been studied in conditions such as rheumatoid arthritis [10], diabetic nephropathy [11], and hypertension [12]. Several inflammatory biomarkers are considered predictors of diabetes, including the neutrophil-to-lymphocyte ratio (NLR) [13], the systemic immune-inflammation index (SII) [14], and the red blood cell distribution width-albumin ratio (RAR) [15]. AISI, as a comprehensive inflammatory index, offers an overall assessment of systemic inflammation in the body. Unlike individual inflammatory biomarkers, AISI combines multiple blood cell counts, offering a more thorough evaluation of inflammation. To facilitate targeted treatment in clinical practice, it is crucial to identify reliable and accurate biomarkers for predicting the likelihood of diabetes.
An investigation using data from the National Health and Nutrition Examination Survey (NHANES) conducted from 2009 to 2018, which included 6,412 individuals, found that markers of inflammation including AISI, MLR, NLR, SIRI, SII, as well as dNLR were significantly associated with all-cause mortality, as well as cardiovascular and cardio-cerebrovascular disease (CCD) mortality rates in adults suffering from diabetes in the United States [16]. Nonetheless, the relationship between AISI and T2DM, along with the mediating role of obesity indicators, remains unclear. Therefore, this study aims to examine the correlation between AISI and T2DM utilizing statistics based on the 2009–2018 NHANES database, which is derived from a demographic survey. The research will examine the correlation between AISI and T2DM, along with the mediating role of obesity indicators. This survey provides a comprehensive overview of the health profile of American adults.
Materials and methods
Data source and study sample
Every two years, the National Health and Nutrition Examination Survey, conducted in collaboration with the National Center for Health Statistics, collects health and nutrition-related data from U.S. individuals to assess their well-being [17]. NHANES conducts annual interviews and examinations of approximately 5,000 participants. The survey employs stratified, multistage probability sampling techniques and a cross-sectional design, which were approved by the Research Ethics Review Board of the National Center for Health Statistics, ensuring the generation of a sufficiently large and representative sample. For detailed information on the design of the continuous NHANES surveys, refer to the official NHANES website. Written informed consent was obtained from all participants in the investigation, and additional approval from the Institutional Review Board is not required for secondary analyses. Data were collected from five distinct periods of NHANES between 2009 and 2018, comprising a total of 49,693 participants. Our study focuses on U.S. adults, with similar studies defining U.S. adults as individuals aged 20 and older [18–20]. The detailed methods for extracting laboratory measurements are provided in Supplementary Text 1.
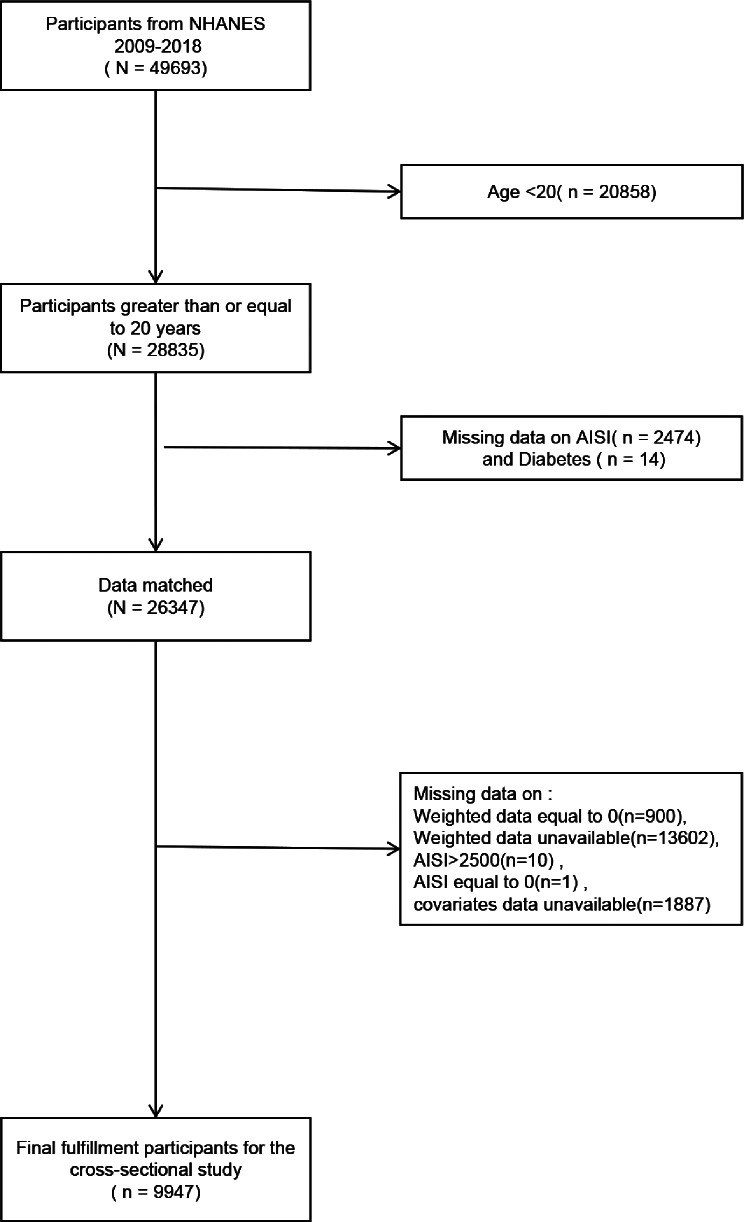
This study excluded U.S. individuals who met one or more of the following criteria: (1) aged under 20 years, (2) absence of data for AISI or diabetes, (3) missing or zero weight data, (4) AISI > 2500, (5) AISI = 0, (6) missing data for PIR, BMI, smoking status, hypertension, eGFR, or education level. After screening, 9,947 participants were included, with the individual admission process outlined in Fig. 1.
Fig. 1.
Flowchart of the sample selection from the 2009–2018 National Health and Nutrition Examination Survey (NHANES)
Exposure variable
In this study, complete blood count (CBC) tests were used to obtain data for calculating inflammatory biomarkers. Participants were required to fast for 9 h, and their fasting status was confirmed by a phlebotomist at the Mobile Examination Center (MEC). During MEC visits, venous blood samples were collected. An automated hematology analyzer was used to process the blood specimens and measure the neutrophil count (NC), monocyte count (MC), platelet count (PC), and lymphocyte count (LC). AISI, a composite biomarker, is calculated as (neutrophils × monocytes × platelets) / lymphocytes [16]. AISI was divided into quartiles based on its P25, P50, and P75 values, with the lowest quartile designated as the control group.
Outcome variable
Individuals were considered diabetic if they self-reported a physician diagnosis had a fasting plasma glucose (FPG) ≥ 7.0 mmol/L, either glycated hemoglobin (HbA1c) ≥ 6.5% or were using diabetic medications [21].
Mediating variables
This study identified five significant mediating variables: Lipid Accumulation Product (LAP), Visceral Adiposity Index (VAI), Waist-to-Height Ratio (WHtR), Weight-Adjusted Waist Index (WWI), and A Body Shape Index (ABSI), all of which are lipid-related obesity indicators.
The researchers collected participants’ basic body measurements and common blood lipid data, including waist circumference (WC), height, BMI, triglycerides (TG), and high-density lipoprotein cholesterol (HDL). The formulas for calculating LAP, VAI, WHtR, and WWI are as follows [22]: (1) Male: LAP = [WC (cm) − 65] × TG (mmol/L); Female: LAP = [WC (cm) − 58] × TG (mmol/L); (2) Male: VAI = WC / [39.68 + (1.88 × BMI)] × (TG / 1.03) × (1.31 / HDL); Female: VAI = WC / [36.58 + (1.89 × BMI)] × (TG / 0.81) × (1.52 / HDL); (3) WHtR = WC (cm) / height (cm); (4) WWI = WC (cm) / (weight (kg))^0.5. The method for ABSI [23] is: ABSI = WC / (BMI^2/3 × height^½). All mediating variables were continuous and included in the regression models to evaluate their moderating effect on the relationship between AISI and T2DM risk.
Covariates
Drawing upon established or observed biological links, and citing related literature, we included variables that may impact the association between AISI and type 2 diabetes [10, 24, 25]. For instance, sex (male, female), ethnicity (non-Hispanic white, other), age, academic attainment (less than high school, high school or equivalent, or college and above), PIR (< 1.0, 1.1–3.0, > 3.0), BMI (< 25, 25–29.9, ≥ 30 kg/m²), and smoking behavior. The CKD-EPI creatinine formula was used to evaluate eGFR [26]. Tobacco consumption history was calculated using the subsequent assessments: “Over the course of your life, have you smoked a total of 100 or more cigarettes?” and “How long have you been smoke-free? “Never smokers were defined as participants who reported smoking fewer than 100 cigarettes during their life. Participants who had smoked more than 100 cigarettes and were still using nicotine were classified as current smokers, whereas others who had exceeded this amount except had ceased were defined as former smokers [27]. We grouped age into three distinct categories: 20 to 39 years, 40 to 59 years, and 60 to 79 years [28]. Platelet (Plt) count was obtained from laboratory data. Hypertension and cardiovascular disease are significant risk determinants in relation to diabetes. Consequently, this analysis took these conditions into account. Hypertension was defined based on the following criteria: systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, or if the contributor was taking hypertension drugs. SBP and DBP were calculated in terms of the mean values from multiple measurements [29]. Self-reported physician diagnoses of cardiovascular disease were used in this study, gathered through personal conversations employing a uniform medical condition survey. Respondents were asked, " Has a physician or healthcare provider ever informed you that you have had acute myocardial infarction, coronary artery disease, angina pectoris, heart failure, or stroke?” Individuals who gave a reply"yes” to any one of these conditions were regarded as having CVD [30].
Statistical methodology
In this study, following the NHANES analysis guidelines, AISI data were sourced from the Mobile Examination Center (MEC). We appropriately applied the sample weights (WTSAF2YR) in our data analysis to ensure the representativeness of the data. Specifically, weighted methods were used to analyze population characteristics, univariate regression, multivariable regression, subgroup analyses, and interaction tests. In contrast, smooth curve fitting, threshold effect analysis, and mediation analysis were conducted without incorporating sample weights. The formula for calculating the sample weights was Weight = 1/5 × WTSAF2YR. Since the AISI data were sourced from the Mobile Examination Center, this step ensured that the data accurately represented the broader population. In describing the study population, we presented continuous variables as weighted means with standard deviations, while categorical variables were reported as weighted percentages. To assess differences between the AISI and T2DM cohorts, weighted t-tests were applied to continuous variables, and weighted chi-square tests were used for categorical variables.
To explore the association between AISI and T2DM, multiple logistic regression with weighting was made. This analysis included the following models: In Model 1, no adjustments were carried out. In Model 2, adjustments were applied for race, gender and age. In Model 3, adjustments were performed on age, gender, ethnicity, educational attainment, PIR, BMI, tobacco use status, glomerular filtration rate, platelet count, hypertension, and CVD. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to examine the association between AISI and T2DM. In regression analysis, p-values were calculated using the Wald test.
The weighted multivariable regression subgroup analysis was conducted using Model 3 and was further employed to evaluate additional factors influencing the association between AISI and T2DM. We performed a weighted multivariable regression subgroup analysis after adjusting for all covariates to examine the interactions between variables such as education level, PIR, race, gender, age, smoking status, body mass index, hypertension and cardiovascular disease. The results were visualized using a forest plot. Interaction p-values were used to evaluate the consistency of effects across subgroups: non-significant p-values indicated consistent results for the entire population, while significant p-values suggested that specific subgroups might exhibit different outcomes. In the subgroup analysis, p-values for continuous variables were calculated using the weighted t-test, while for categorical variables, p-values were computed using the weighted chi-square test. For trend tests, p-values were computed using the chi-square test.
In the model with all covariates adjusted, we analyzed the associations between AISI and T2DM using smooth curve fitting (SCF) and generalized additive models (GAM). If a nonlinear association was detected, recursive algorithms were used to determine significant inflection points, and the threshold effect of AISI on the prevalence of T2DM was assessed. Furthermore, stratified analysis based on CVD was conducted to evaluate whether the association between AISI and T2DM was affected by the CVD (yes/no) subgroup. This comprehensive statistical approach provides a detailed understanding of the correlation between AISI and T2DM within the study participants.
This study also performed multiple mediation analyses on the fully adjusted model to evaluate the indirect effects of indicators such as LAP, VAI, WHtR, WWI, and ABSI as mediators in the relationship between AISI and T2DM risk. Mediation effects were quantified by calculating direct effects, indirect effects, and total effects. All estimated intermediary effects are represented in Fig. 5 and Table S1. The mediating effect was quantified as the percentage of mediation. In this study, mediation analysis employed the Bootstrap resampling method, evaluating the significance of the mediation effect by calculating the confidence intervals and p-values for the indirect effects.
Fig. 5.
Analysis of the mediation by LAP (A), VAI (B), WHtR (C), WWI (D), ABSI (E) of the associations of AISI/1000 with diabetes. Adjusted variables: race, age, sex, income to poverty ratio, education level, smoke, BMI, high blood pressure, eGFR, CVD, platelet count, neutrophil count, monocyte count
In this study, for missing covariate values, we initially applied listwise deletion. To further assess the robustness of the results, we performed multiple imputation and conducted sensitivity analyses using the imputed data. Additionally, several sensitivity analyses were conducted to validate the conclusions of the study. Data analyses were implemented using R software (version 4.2.0, http://www.R-project.org, The R Foundation) and empowerStats (version 4.2, http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA). A p-value of 0.05 was used as the threshold for statistical significance.
Results
Participants characteristics
Table 1 presents the demographic characteristics of respondents stratified by T2DM status. This study analyzed the population using weighted data. The results showed that the T2DM group consisted of 56,125,718 individuals, while the non-T2DM group included 135,999,189 individuals. In the T2DM group, 46.59% were male, and 53.41% were female. Additionally, 40.04% were non-Hispanic White, while 59.96% were from other racial backgrounds. The mean AISI value in the T2DM group was 295.66 ± 5.19. In the non-T2DM group, 48.61% were male, 51.39% were female, 42.47% were non-Hispanic White, and 57.53% were from other racial backgrounds. The mean AISI value for the non-T2DM group was 273.86 ± 3.13. In contrast with the non-T2DM cluster, individuals in the T2DM cluster exhibited an increased age, education attainment, BMI, nicotine use status, and high blood pressure prevalence, with all differences being statistically significant (P < 0.05). Significant statistical differences were also found between the two groups in platelet count, eGFR, PIR, and the prevalence of cardiovascular disease (P < 0.05).
Table 1.
Baseline characteristics of all participants by type 2 diabetes mellitus
| Characteristics | Non-Diabetes | Diabetes | p-Value |
|---|---|---|---|
| (n = 6727, N = 135999189) | (n = 3220, N = 56125718) | ||
| AISI | 273.863 ± 3.132 | 295.661 ± 5.195 | 0.001 |
| Platelet count (1000 cells/uL) | 237.537 ± 0.876 | 228.671 ± 1.369 | < 0.001 |
| eGFR (mL/min/1.73 m2) | 108.928 ± 0.447 | 101.812 ± 0.607 | < 0.001 |
| Gender (%) | 0.131 | ||
| Male | 48.613 | 46.594 | |
| Female | 51.387 | 53.406 | |
| Age group(%) | < 0.001 | ||
| 20–39 | 40.479 | 26.110 | |
| 40–59 | 36.787 | 37.283 | |
| 60–79 | 22.734 | 36.606 | |
| Race(%) | 0.079 | ||
| Non-Hispanic White | 42.466 | 40.040 | |
| Other | 57.534 | 59.960 | |
| Educational attainment(%) | < 0.001 | ||
| Less than high school | 24.622 | 17.733 | |
| High school or equivalent | 21.120 | 21.692 | |
| College or above | 54.258 | 60.575 | |
| Family income-to-poverty ratio(%) | 0.001 | ||
| ≤ 1.0 | 19.404 | 23.015 | |
| 1.1–3.0 | 41.868 | 42.437 | |
| > 3.0 | 38.728 | 34.548 | |
| BMI(%) | < 0.001 | ||
| < 25 | 30.614 | 27.113 | |
| 25-29.9 | 33.566 | 29.666 | |
| ≥ 30 | 35.821 | 43.221 | |
| Smoking status(%) | < 0.001 | ||
| Never smoker | 52.815 | 58.931 | |
| Former smoker | 28.829 | 17.913 | |
| Current smoker | 18.356 | 23.156 | |
| Hypertension(%) | < 0.001 | ||
| No | 87.051 | 80.915 | |
| Yes | 12.949 | 19.085 | |
| CVD(%) | < 0.001 | ||
| No | 85.638 | 95.969 | |
| Yes | 14.362 | 4.031 |
BMI, body mass index; CVD, cardiovascular disease
Association between AISI and T2DM
Due to the small effect size, we scaled the AISI value by a factor of 1000 and examined the relationship between AISI/1000 and T2DM. The results of the univariate logistic regression analysis are shown in Table 2. In the univariate analysis, we selected variables with p < 0.1, and based on previous studies [31–33] and their clinical significance, we ultimately chose income-to-poverty ratio, education level, race, age, sex, smoking, eGFR, high blood pressure, CVD, BMI and platelet count as covariates for subsequent multivariable regression analysis. The outcomes of the regression analysis with multiple variables among AISI/1000 and T2DM is exposed in Table 3. In Model 1, covariates are not accounted for. Model 2 adjusts for racial background, gender, and age, while all covariates are included in Model 3. AISI/1000 showed a positive correlation with T2DM. Following complete adjustment for all covariates, the positive relationship still remained (OR: 1.883, 95% CI: 1.378–2.571, P < 0.001). With each unit increase in AISI/1000, the likelihood of having T2DM elevated by 88.3%. To further investigate the relationship between AISI/1000 and T2DM, we grouped AISI/1000 based on quartile groups. With Quartile 1 as the baseline group, we performed a fully adjusted regression analysis for AISI/1000 quartiles. A significantly higher OR was observed for Quartile 4 in comparison with Quartile 1 (OR: 1.563, 95% CI: 1.287–1.897, P < 0.001). The possibility of developing T2DM in the top quartile of AISI/1000 was more than twice as high as in the lowest percentile. This suggests that an increase in AISI/1000 may be associated with an elevated risk of developing type 2 diabetes.
Table 2.
Univariate logistic regression analyses
| Variables | OR (95%CI) | P-value |
|---|---|---|
| Gender | ||
| Male | Reference | |
| Female | 1.084 (0.976, 1.204) | 0.135 |
| Age | ||
| 20–39 | Reference | |
| 40–59 | 1.571 (1.367, 1.806) | <0.001 |
| 60–79 | 2.496 (2.135, 2.919) | <0.001 |
| Race | ||
| Non-Hispanic White | Reference | |
| Other | 1.105 (0.989, 1.236) | 0.083 |
| Educational attainment | ||
| Less than high school | Reference | |
| High school or equivalent | 1.426 (1.166, 1.744) | 0.001 |
| College or above | 1.550 (1.333, 1.802) | <0.001 |
| Family income-to-poverty ratio | ||
| ≤ 1.0 | Reference | |
| 1.1–3.0 | 0.855 (0.734, 0.995) | 0.046 |
| > 3.0 | 0.752 (0.655, 0.864) | <0.001 |
| BMI | ||
| < 25 | Reference | |
| 25-29.9 | 0.998 (0.865, 1.151) | 0.977 |
| ≥ 30 | 1.362 (1.180, 1.573) | <0.001 |
| Smoking status | ||
| Never smoker | Reference | |
| Former smoker | 0.557 (0.479, 0.647) | <0.001 |
| Current smoker | 1.131 (0.967, 1.321) | 0.127 |
| Hypertension | ||
| No | Reference | |
| Yes | 1.586 (1.359, 1.851) | <0.001 |
| CVD | ||
| No | Reference | |
| Yes | 0.250 (0.191, 0.328) | <0.001 |
| Serum creatinine(mg/dL) | 1.449 (1.202, 1.745) | <0.001 |
| eGFR (mL/min/1.73 m2) | 0.991 (0.989, 0.993) | <0.001 |
| Platelet count (1000 cells/uL) | 0.997 (0.997, 0.998) | <0.001 |
OR odds ratio, CI confidence interval
Table 3.
Weighted ORs (95% CIs) of the association between AISI and type 2 diabetes mellitus
| Variables | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| OR(95%CI), P-value | OR(95%CI), P-value | OR(95%CI), P-value | |
| AISI/1000 | 1.581 (1.221, 2.048), < 0.001 | 1.419 (1.101, 1.828), 0.009 | 1.883 (1.378, 2.571), < 0.001 |
| AISI/1000 quartiles | |||
| Quartiles 1 | Reference | Reference | Reference |
| Quartiles 2 | 1.148 (0.970, 1.358), 0.113 | 1.145 (0.961, 1.363), 0.134 | 1.232 (1.028, 1.477), < 0.001 |
| Quartiles 3 | 1.251 (1.071, 1.459), 0.006 | 1.232 (1.056, 1.438), 0.010 | 1.378 (1.157, 1.641), < 0.001 |
| Quartiles 4 | 1.397 (1.175, 1.660), < 0.001 | 1.309 (1.103, 1.554), 0.003 | 1.563 (1.287, 1.897), < 0.001 |
| p for trend | < 0.001 | 0.001 | < 0.001 |
Model 1, no covariates were adjusted. Model 2, race, age, and sex were adjusted. Model 3, race, age, sex, income to poverty ratio, education level, smoke, BMI, high blood pressure, eGFR, CVD, platelet count were adjusted. 95%CI, 95% confidence interval; OR, odds ratio; AISI, aggregate index of systemic inflammation. p < 0.05 was considered statistically significant
Interaction test and subgroup analysis
To explore the robustness of the association between AISI/1000 and Type 2 Diabetes Mellitus across different population subgroups, interaction tests and subgroup analyses were conducted using fully adjusted models, as presented in Fig. 2. The outcomes revealed that the association between elevated AISI and T2DM risk was not consistently significant across all subgroups. The association between AISI and the probability of T2DM reached statistical significance (p < 0.05) in specific cohort subgroups. These included males, individuals aged 60–79 years, participants with educational levels either below high school or at college level and above, those with a poverty-income ratio exceeding 1.1, individuals with a BMI of 25 or higher and former smokers. In contrast, among the female subgroup, those aged 20–59 years, individuals with a high school education or equivalent, a PIR < 1.0, BMI < 25, non-smokers, and current smokers, the association between elevated AISI and T2DM risk was not statistically significant (p > 0.05). Notably, no meaningful interactions were discovered in subgroups of sex, age, race, attainment of education, PIR, BMI, nicotine exposure, or hypertension, suggesting that these variables did not affect the positive association between AISI and T2DM (all interaction p-values > 0.05). However, a significant interaction was observed in the subgroup based on cardiovascular disease status (P for interaction = 0.039). In the CVD cohort, each one-unit increase in AISI/1000 was associated with a 2.283-fold higher risk of progression to T2DM. Conversely, in participants without CVD, a similar increase in AISI/1000 was linked to a 0.589-fold rise in T2DM risk. This suggests that considering the moderating role of CVD in the impact of risk factors may be an important consideration when developing personalized intervention strategies.
Fig. 2.
Forest plot for the correlation among AISI/1000 and T2DM
Nonlinear relationships between AISI and T2DM
Smooth curve fitting in Figs. 3 and 4 is used to depict the nonlinear association between AISI/1000 and T2DM. Adjustments for race, age, gender, educational attainment, PIR, BMI, tobacco use status, glomerular filtration rate, platelet count, hypertension, and cardiovascular disease were made in the analysis. The likelihood ratio test indicated a statistically significant difference between the linear regression model and the segmented linear relationship analysis model(p < 0.05). The inflection points in the AISI/1000-T2DM relationship are shown in Table 4, occurring at an AISI/1000 value of 0.21. Stratified analysis based on CVD status revealed that in the CVD group, an inverted U-shaped curve was observed, with the inflection point occurring at an AISI/1000 value of 0.674, as shown in Table 4 and Fig. 4.
Fig. 3.

Smoothed curve fitting: Dose-response relationship between AISI/1000 and T2DM
Fig. 4.

Association between AISI/1000 and T2DM stratified by CVD
Table 4.
Two-piecewise logistic regression model analysis results of the threshold relationship between AISI and type 2 diabetes mellitus
| Model | OR (95%CI) | P-value |
|---|---|---|
| Inflection point (K) | 0.210 | |
| AISI/1000<0.210 | 6.347 (2.291, 17.587) | < 0.001 |
| AISI/1000>0.210 | 1.667 (1.294, 2.148) | < 0.001 |
| Log-likelihood ratio | 0.019 | |
| Stratified by CVD | ||
| Yes | ||
| Inflection point (K) | 0.674 | |
| AISI/1000<0.674 | 15.773 (4.388, 56.695) | < 0.001 |
| AISI/1000>0.674 | 0.694 (0.088, 5.473) | 0.729 |
| Log-likelihood ratio | 0.022 | |
| No | ||
| Inflection point (K) | 0.149 | |
| AISI/1000<0.149 | 14.893 (2.219, 99.982) | 0.005 |
| AISI/1000>0.149 | 1.650 (1.291, 2.109) | < 0.001 |
| Log-likelihood ratio | 0.028 | |
Mediation effect analysis with involving obesity indicators as a mediating variable
Mediation analysis was carried out to assess the latent intervening role of obesity indicators in the relationship between AISI/1000 and the probability of being diagnosed with T2DM, as detailed in Table S1. The lipid accumulation product (LAP), visceral adiposity index (VAI), waist-to-height ratio (WHtR), weight-adjusted waist index (WWI), shape index (ABSI) mediated 3.496%, 2.210%, 1.610%, 1.265%, and 1.329% of the association between AISI/1000 and T2DM, respectively (Figs. 5A, B, C, D, E). However, as shown in Table S1, neither waist circumference (WC) nor body roundness index (BRI) demonstrated any mediating effect in the relationship between AISI/1000 and T2DM. Table S2 and Figure S1 present the results of the mediation analysis performed across different populations. When stratified by gender, the mediating effect of LAP accounted for 4.955% of the relationship between AISI/1000 and T2DM in the male population, and 3.392% in the female population. Although LAP, VAI, WHtR, WWI, and ABSI demonstrated significant effects in the overall sample, no significant mediating effects were found in specific subgroups. For example, in the male group, VAI, WHtR, WWI, and ABSI; in the female group, VAI, WHtR, and WWI; in the 20–39 age group, LAP, VAI, WHtR, and WWI; in the 40–59 age group, VAI and ABSI; and in the 60–79 age group, LAP, VAI, WHtR, WWI, and ABSI did not show significant mediation effects. After adjusting for all covariates, Table S3 shows the association between AISI/1000 and obesity indicators. Specifically, the correlations between AISI/1000 and LAP, VAI, WHtR, WWI, and ABSI were (β = 12.994, 95% CI: 5.686, 20.302), (β = 0.432, 95% CI: 0.112, 0.753), (β = 0.035, 95% CI: 0.025, 0.044), (β = 0.409, 95% CI: 0.325, 0.494), and (β = 0.740, 95% CI: 0.609, 0.871), respectively. These results suggest that obesity indicators can be considered potential mediators in the relationship between AISI/1000 and T2DM.
Sensitivity analysis
This study also performed sensitivity analysis to validate the reliability and accuracy of the previous results. After conducting multiple imputation for all missing covariates, main results derived from the imputed data were consistent with the earlier findings (Table S4 and Figure S2).
SHAP model
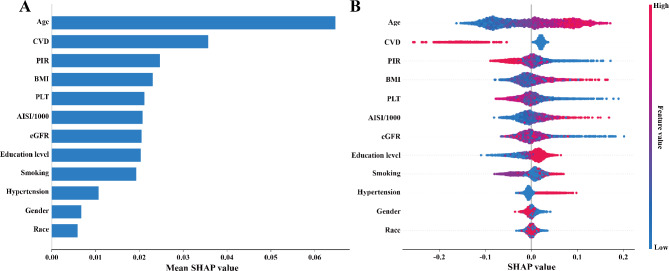
Additionally, this study employed the SHAP (Shapley Additive Explanations) method, based on a random forest model, to assess the contribution and importance of each variable (age, race, sex, PIR, educational attainment, smoking, BMI, high blood pressure, eGFR, CVD, and platelet count) in predicting T2DM. The SHAP model revealed that the top five variables most influential in predicting T2DM were age, CVD, PIR, BMI, and platelet count (Fig. 6A). Among these, AISI/1000 exhibited a positive correlation with T2DM (Fig. 6B).
Fig. 6.
Variable importance for random forest (A) and SHAP dependence plots (B)
Discussion
This study built on a cross-sectional approach utilized data from 9,947 adult individuals in the United States, sourced from NHANES database. The study’s findings reveal an association between AISI and T2DM. A positive connection was found between AISI and the probability of T2DM. This association remained robust after accounting for several confounders, such as baseline demographic characteristics and laboratory parameters. This correlation was consistent in subgroup analyses with the same adjustment strategy. Notably, the relationship between AISI and the likelihood of being diagnosed with type 2 diabetes mellitus was unrelated to potential confounding causes, encompassing race, sex, attainment for education, PIR, age, BMI, smoking condition, estimated renal filtration rate (eGFR), platelet count, and hypertension status. However, the presence of cardiovascular disease influenced the strength of this association. Individuals with CVD were more likely to develop T2DM at the same AISI levels compared to those without CVD. It is important to note that many CVD patients have unhealthy dietary habits, which can significantly contribute to the onset and progression of T2DM. Moreover, smooth curve fitting revealed a nonlinear relationship between AISI/1000 and Type 2 Diabetes Mellitus, with a turning point at an AISI/1000 value of 0.21. When stratified by cardiovascular disease, an inverted U-shaped link was observed during the CVD population, with a turning point of 0.674. To further investigate the potential associations among AISI, obesity indicators, and T2DM, a mediation analysis was conducted. The results showed that LAP, VAI, WHtR, WWI, and ABSI were all identified as partial mediators of the association between AISI/1000 and T2DM. These observations suggest that AISI may influence T2DM risk through the regulation of obesity.
This study provides valuable insights for clinical practice. By monitoring inflammatory markers such as AISI, healthcare providers may identify high-risk patients for diabetes early and intervene promptly, potentially slowing disease progression. Additionally, the findings suggest that controlling systemic inflammation and managing obesity may play a key role in diabetes prevention, offering scientific evidence to support the development of more targeted intervention strategies.
This study highlights the nonlinear relationship between AISI and T2DM. AISI, as a novel inflammatory biomarker, has been relatively underexplored in previous research. However, a large nationwide cross-sectional study in the United States, involving 6,412 U.S. adults, revealed a notable association between AISI and mortality from any cause among diabetic adults [16]. This suggests that AISI may serve as a marker for predicting long-term outcomes in diabetic populations. Additionally, a two-year prospective study found that AISI, along with other inflammatory indices derived from complete blood count (CBCII), is a reliable predictor of metabolic syndrome (MetS) progression in patients with T2DM [34]. AMP-activated protein kinase (AMPK) signaling plays a critical role in maintaining cellular homeostasis. An animal experiment demonstrated that the combined treatment of metformin and atorvastatin may enhance AMPK signaling through synergistic activation, exerting both preventive and therapeutic effects on diabetic cardiomyopathy (DCM). These effects are mediated through mechanisms such as antioxidation, anti-inflammation, and anti-apoptosis [35]. Therefore, our findings corroborate previous evidence that elevated AISI levels contribute to an increased likelihood of being diagnosed with T2DM.
The pathophysiology of T2DM is characterized by chronic inflammatory responses, which are strongly associated with the complications of the disease. For example, compared to healthy individuals, diabetic patients exhibit significant transcriptional changes in classical monocytes. Additionally, it has been shown to compromise the functional responses of CD+ T cells and natural killer (NK) cells, potentially impairing immune responses and leading to altered differentiation pathways for these immune cells [36]. Macrophage accumulation in the kidneys of diabetic patients is closely linked to kidney damage [37]. Similarly, the infiltration of M1 macrophages significantly impairs wound healing in diabetic patients [38]. As a vital component of the innate immune response, Toll-like receptor 4 (TLR4) plays a key role, believed to contribute to diabetic cardiomyopathy (DCM) by initiating pro-inflammatory cascades triggered by hyperglycemia. Elevated TLR4 levels have also been noted in the cardiac tissues of mice and rats with diabetes [39].
T2DM is a widespread endocrine disorder, characterized by several risk factors, including poor glycemic control, dyslipidemia, visceral adiposity, hypertension and insulin resistance, all of which contribute to the heightened cardiovascular risk observed in T2DM patients. These factors are strongly associated with persistent mild inflammation, which plays a crucial role in the emergence and development of CVD in these individuals [40]. In clinical practice, blood cell counts are easily measurable, providing rapid and cost-effective results. A study conducted in Spain with 1,035 children aged 8 to 13 years found that overweight and obese children, particularly boys, who had elevated leukocyte levels and an increased neutrophil-to-lymphocyte ratio, exhibited abnormal indicators of insulin resistance [41]. These findings may provide valuable insights into predicting the likelihood of T2DM and could inform future medical and preventive strategies. A systematic review and meta-analysis revealed that septic critical illness is associated with NLR. Additionally, potential clinical utility has been demonstrated in conditions such as pneumonia, pertussis, urinary tract infections, diabetic foot infections, and Crimean-Congo hemorrhagic fever [42]. Based on the inference that combining two blood cell count-derived results may lack specificity for predicting type 2 diabetes, we propose that AISI, which is calculated using lymphocyte cell count, monocyte count, neutrophil count, and platelet count, could offer greater reliability. A prospective case-control study showed that compared to healthy pregnant women, those with gestational diabetes exhibited heightened platelet reactivity, which persisted for up to two months postpartum [43]. Chronic inflammation is characterized by a pro-thrombotic state, driven by the reciprocal activation of neutrophils and platelets. For those individuals with T2DM, elevated secretion of neutrophil-derived S100A8/A9 has been shown to further increase platelet counts, potentially contributing to a pro-thrombotic state [44]. This suggests that neutrophils contribute to the progression of T2DM. NF-κB signaling is a crucial transcriptional mechanism initiated by macrophage activation and serves as a pivotal regulator connecting inflammation to insulin resistance. Mechanistically, the CREB/ATF bZIP transcription factor (CREBZF) in macrophages prolongs the range of nuclear p65 activity through the competitive inhibition of IκBα binding to p65 which enhances NF-κB vitality and reduces insulin sensitivity [45]. In individuals suffering from type 1 diabetes mellitus, monocytes excessively secrete IL-1β and IL-6, resulting in chronic inflammation. The heightened activity of these monocytes is linked to increased susceptibility to type 1 diabetes and its progression [46]. A multicenter intervention study demonstrated that during insulin-induced hypoglycemic reactions, both in individuals regardless of type 2 diabetes status, the absolute number of circulating lymphocytes and monocytes significantly increased, with levels remaining increased for up to one week post-hypoglycemia. A meaningful increase in the ratio of CD16 + monocytes was explored, accompanied by a corresponding decrease in CD14+ monocytes. This trend remained consistent for up to one week in the non-diabetic population. Furthermore, under hypoglycemic conditions, vitro-stimulated monocytes exhibited enhanced secretion of tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β) [47].
Inflammation is an essential physiological defense mechanism; however, chronic or inordinate inflammation can result in various illnesses. Excessive obesity is associated with changes in the function of adipose tissue, adipocyte apoptosis, and persistent mild inflammatory state. Circulating inflammatory markers of low intensity, encompassing high-sensitivity C-reactive protein (hs-CRP) together with interleukin-6 (IL-6), are frequently elevated in individuals suffering from obesity and type 2 diabetes mellitus, reflecting a shared pathophysiological link between these conditions [40]. Neutrophil infiltration is detected in the fat depot of obese respondents with T2DM. Within this tissue, neutrophils secrete neutrophil elastase (NE) and myeloperoxidase (MPO), which are thought to influence the progression of insulin resistance as well as inflammation in adipose tissue, as demonstrated in T2DM mouse models [44]. Most obese individuals exhibit inflamed adipose tissue, resembling chronically damaged tissue, characterized by immune cell infiltration and tissue remodeling. Obesity exerts systemic effects through circulating metabolic and immune mediators, which are closely linked to the inflammation in adipose tissue [48]. Evidence has shown that obesity is closely associated with an increase in neutrophil levels in the bloodstream [49]. This elevation in neutrophils is thought to contribute to the inflammatory processes and metabolic disturbances frequently observed in obese individuals. Neutrophil, platelet, monocyte, and lymphocyte counts are routinely evaluated in clinical blood tests. As a result of their simplicity, cost-effectiveness, and extensive applicability, these indicators are widely used to assess systemic inflammation and immune status. This study focuses on AISI, an inflammatory marker derived from lymphocyte count, monocyte count, neutrophil count, and platelet count. Mediation analysis was employed to investigate the associations among T2DM, obesity-related indicators, and AISI, aiming to elucidate potential mechanistic pathways linking these variables. The analysis revealed that LAP, VAI, WHtR, WWI, and ABSI partially mediate the relationship between AISI and T2DM. These results suggest that improving obesity may play a role in alleviating inflammation in patients with T2DM.
Our study infers a nonlinear relationship between AISI and T2DM, providing valuable insights for the early identification of diabetic patients. Mediation analysis further suggests that obesity may play a mediating role between inflammation and T2DM, suggesting that controlling obesity could play a key role in the prevention of T2DM. However, when we conducted mediation analyses in different subgroups, we found that these mediation effects were not significant in certain populations. This discrepancy may suggest that the mediation effect observed in the overall sample could be influenced by factors not accounted for in the subgroup analyses. It may also reflect heterogeneity between subgroups, resulting in variations in the presence and strength of the mediation effects across groups. Further research is needed to investigate the mediating mechanisms in diverse populations to better understand how these variables function across various groups.
This study, based on the NHANES database, examined the relationship between AISI and type 2 diabetes (T2DM) in a sample of U.S. adults from 2009 to 2018. While previous research has indicated an association between AISI and T2DM mortality, no study has thoroughly analyzed the direct relationship between AISI and T2DM. This study not only explored this correlation but also investigated the mediating effect of obesity indicators in this relationship. Furthermore, the use of the Shapley Additive Explanations (SHAP) model enhanced the interpretability and clinical relevance of the findings. The results may provide valuable insights for clinical practice. By elucidating the relationship between AISI and T2DM, along with associated risk factors such as obesity, this study could help clinicians identify high-risk individuals at an earlier stage and design more targeted intervention strategies. However, the effective application of these findings in clinical settings requires further validation. This study provides a potential theoretical foundation for the future prevention and management of diabetes.
Strength and limitation
The large, representative NHANES database used in this study is a major strength, enabling an in-depth analysis of the AISI-T2DM relationship and ensuring the generalizability of our findings. Furthermore, AISI, as an easily obtainable laboratory marker, could assist clinicians in identifying high-risk T2DM patients early, while also highlighting the potential intermediary influence of obesity indicators in the AISI-T2DM association.
Nevertheless, there are several limitations to this study. The cross-sectional, retrospective nature of this finding restricts our ability to infer causal relationships between AISI and T2DM, highlighting the need for further research. Although potential confounders were accounted for in the data analysis, unmeasured or residual confounding factors may still exist. Additionally, the diagnosis of CVD was based on questionnaires filled out by participants, which may introduce recall bias and affect diagnostic accuracy. Future research should utilize prospective studies to assess causal links and apply objective diagnostic measures to minimize bias in disease diagnosis. However, the generalizability of our findings may also be limited due to the homogeneity of the study population. The current study primarily focused on adult individuals in the United States, necessitating further external validation in diverse racial and ethnic populations to determine whether the observed associations are consistent across different groups. Future research should incorporate more racially and ethnically diverse cohorts to better understand the link between AISI and type 2 diabetes mellitus among a broader cohort, ensuring the findings are applicable to a wider range of individuals.
Conclusions
Our findings indicate that an increase in AISI/1000 is associated with a higher prevalence of T2DM. Additionally, several obesity indicators, including LAP, VAI, WHtR, WWI and ABSI, may serve as potential mediators in this association. While these results highlight the importance of managing inflammation and obesity, the cross-sectional design limits causal interpretation. Future research should employ longitudinal studies to further investigate these associations and more effectively address the burden of T2DM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We extend our sincere gratitude to the participants and staff of NHANES, as well as to all individuals who contributed to this study.
Abbreviations
- AISI
The aggregate index of systemic inflammation
- T2DM
Type 2 diabetes mellitus
- NHANES
The National Health and Nutrition Examination Survey
- PIV
The pan-immune-inflammation value
- PIR
Poverty-income ratio
- BMI
Body mass index
- eGFR
Estimated glomerular filtration rate
- CVD
Cardiovascular disease
- WAT
White adipose tissue
- CBC
The complete blood count
- NLR
The neutrophil-to-lymphocyte ratio
- SII
The systemic immune-inflammation index
- RAR
The red blood cell distribution width-albumin ratio
- MEC
The Mobile Examination Center
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- LAP
The lipid accumulation product
- VAI
Visceral adiposity index
- WHtR
Waist-to-height ratio
- WWI
Weight-adjusted waist index
- ABSI
The A Body Shape Index
- WC
Waist circumference
- BRI
Body roundness index
- TG
Triglycerides
- HDL
High-density lipoprotein cholesterol
- OR
Odds ratios
- CI
Confidence range
- SCF
Smooth curve fitting
- GAM
Generalized additive models
Author contributions
YS and ZH designed the study. LC, HC and YS collected the data. PZ analyzed the data. YS and HC drafted the manuscript. CW and JL revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
No financial support was received for the research.
Data availability
The datasets used in this study are publicly available and can be accessed at the following link: https://www.cdc.gov/nchs/nhanes/.
Declarations
Ethics approval and consent to participate
The data utilized in this study, which were neither generated nor analyzed by the authors, can be accessed through the NHANES database.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Qi MH, Zhang HY, Hou YY, Nguepi Tsopmejio IS, Liu W, Chang WG, Chen C, Wang Z, Li W. Ginseng-derived GABAFG ameliorates type 2 diabetes mellitus by modulating autophagy-lysosome pathway and gut microbiota. J Adv Res. 2025. [DOI] [PubMed]
- 2.Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Lernmark Å, Metzger BE, Nathan DM, Kirkman MS. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2023;46:e151–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouyang X, Peng L, Huang Z, Wang T, Wang J, Wu H, Zhong J, Wu B, Wu L, Li Y, et al. Effects of adipose tissues on the relationship between type 2 diabetes mellitus and reduced heart rate variability: mediation analysis. Cardiovasc Diabetol. 2024;23:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hariharan R, Odjidja EN, Scott D, Shivappa N, Hébert JR, Hodge A, de Courten B. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes Rev. 2022;23:e13349. [DOI] [PubMed] [Google Scholar]
- 5.Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo J, Qin X, Zhang X, Zhang Y, Yuan F, Shi W, Liu B, Wei Y. Prognostic implications of systemic immune-inflammation index in myocardial infarction patients with and without diabetes: insights from the NOAFCAMI-SH registry. Cardiovasc Diabetol. 2024;23:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henning RJ. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018;14:491–509. [DOI] [PubMed] [Google Scholar]
- 8.Qi L, Groeger M, Sharma A, Goswami I, Chen E, Zhong F, Ram A, Healy K, Hsiao EC, Willenbring H, Stahl A. Adipocyte inflammation is the primary driver of hepatic insulin resistance in a human iPSC-based microphysiological system. Nat Commun. 2024;15:7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13:851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin X, Zou J, Yang J. The association between the aggregate index of systemic inflammation and risk of rheumatoid arthritis: retrospective analysis of NHANES 1999–2018. Front Med (Lausanne). 2024;11:1446160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alirezaei A, Toudeshki KK, Taherian M, Pashapour H, Rahmani F, Norouzi N, Fazeli SA. Comparison of complete blood count parameters in different severity of proteinuria among patients with type 2 diabetes mellitus. J Res Med Sci. 2024;29:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiu J, Lin X, Chen Q, Yu P, Lu J, Yang Y, Chen W, Bao K, Wang J, Zhu J, et al. The aggregate index of systemic inflammation (AISI): a novel predictor for hypertension. Front Cardiovasc Med. 2023;10:1163900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen HL, Wu C, Cao L, Wang R, Zhang TY, He Z. The association between the neutrophil-to-lymphocyte ratio and type 2 diabetes mellitus: a cross-sectional study. BMC Endocr Disord. 2024;24:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aljuraiban GS, Alharbi FJ, Aljohi AO, Almeshari AZ, Alotaibi MN, AlShammari SS, Al-Musharaf S, Aldhwayan MM, Abudawood M. Systemic Immune-Inflammation index in relation to diabetes markers in Saudi adults: A retrospective study. Med (Kaunas). 2024;60. [DOI] [PMC free article] [PubMed]
- 15.Liu J, Wang X, Gao TY, Zhang Q, Zhang SN, Xu YY, Yao WQ, Yang ZH, Yan HJ. Red blood cell distribution width to albumin ratio associates with prevalence and long-term diabetes mellitus prognosis: an overview of NHANES 1999–2020 data. Front Endocrinol (Lausanne). 2024;15:1362077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Fan X, Xu Y, Wang K, Xu T, Han T, Hu C, Li R, Lin X, Jin L. Association between inflammatory biomarkers and mortality in individuals with type 2 diabetes: NHANES 2005–2018. Diabetes Res Clin Pract. 2024;209:111575. [DOI] [PubMed] [Google Scholar]
- 17.Mo T, Wei M, Fu J. Dietary inflammatory index and type 2 diabetes in US women: a cross-sectional analysis of the National health and nutrition examination survey, 2007–2018. Front Nutr. 2024;11:1455521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu D, Sun J, Wang P, Xu Q, Ma C. Overall sleep quality is associated with advanced stages in patients with Cardiovascular-Kidney-Metabolic syndrome. J Am Heart Assoc. 2025;14:e038674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei Y, Tao S, Yang Y, Xie F, Xie W. Association between prognostic nutritional index and all-cause mortality and cardiovascular disease mortality in American adults with non-alcoholic fatty liver disease. Front Nutr. 2025;12:1526801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo W, Xue H, Li Q, Wen Z, Zhou Z, Dong Y, He M, Li Y, Li F, Tong Y. Association between visceral fat metabolism score and cataract risk in US adults: National health and nutrition examination survey 1999 to 2008. Am J Ophthalmol. 2025;274:184–195. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Shao X, Xu W, Xue B, Zhong S, Yang Q. The relationship between weight-adjusted-waist index and diabetic kidney disease in patients with type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2024;15:1345411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun X, Song J, Yan R, Diao J, Liu Y, Zhu Z, Lu W. The association between lipid-related obesity indicators and severe headache or migraine: a nationwide cross sectional study from NHANES 1999 to 2004. Lipids Health Dis. 2025;24:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Kang J, Liu W, Shen Y. Association between a body shape index and colorectal cancer in US population: a cross-sectional study based on NHANES. Front Nutr. 2025;12:1535655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan MY, Weng L, Yang ZH, Zhu SX, Wu S, Su JH. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio with type 2 diabetes mellitus: recent findings from NHANES 2007–2018. Lipids Health Dis. 2024;23:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou H, Li T, Li J, Zhuang X, Yang J. The association between visceral adiposity index and risk of type 2 diabetes mellitus. Sci Rep. 2024;14:16634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. [DOI] [PMC free article] [PubMed]
- 27.Qiu Z, Chen X, Geng T, Wan Z, Lu Q, Li L, Zhu K, Zhang X, Liu Y, Lin X, et al. Associations of serum carotenoids with risk of cardiovascular mortality among individuals with type 2 diabetes: results from NHANES. Diabetes Care. 2022;45:1453–61. [DOI] [PubMed] [Google Scholar]
- 28.Wu C, Ren C, Song Y, Gao H, Pang X, Zhang L. Gender-specific effects of oxidative balance score on the prevalence of diabetes in the US population from NHANES. Front Endocrinol (Lausanne). 2023;14:1148417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Y, Liu X, Zhu X, Zhao B, Hu B, Sheng X, Chen L, Yu M, Yang T, Zhao J. Increasing trend of diabetes combined with hypertension or hypercholesterolemia: NHANES data analysis 1999–2012. Sci Rep. 2016;6:36093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao S, Zhang Q, Yang HY, Tong JY, Yang RQ. The association between triglyceride glucose-body mass index and all-cause and cardiovascular mortality in diabetes patients: a retrospective study from NHANES database. Sci Rep. 2024;14:13884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Yang C, An J, Fan Y, Dong X. Evaluating sleep’s role in type 2 diabetes mellitus: evidence from NHANES. Brain Behav Immun Health. 2025;44:100953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Z, Gong C, Wang B. The relationship between dietary index for gut microbiota and diabetes. Sci Rep. 2025;15:6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J, Xu W, Wu S, Song D. Vitamin B6 status, type 2 diabetes mellitus, and periodontitis: evidence from the NHANES database 2009–2010. BMC Oral Health. 2025;25:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fajkić A, Jahić R, Begić E, Dervišević A, Kurtović A, Lepara O. Complete blood count inflammation derived indexes as predictors of metabolic syndrome in type 2 diabetes mellitus. Technol Health Care. 2024;32:2321–30. [DOI] [PubMed] [Google Scholar]
- 35.Jia W, Bai T, Zeng J, Niu Z, Fan D, Xu X, Luo M, Wang P, Zou Q, Dai X. Combined administration of Metformin and Atorvastatin attenuates diabetic cardiomyopathy by inhibiting inflammation, apoptosis, and oxidative stress in type 2 diabetic mice. Front Cell Dev Biol. 2021;9:634900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee H, Joo JY, Song JM, Kim HJ, Kim YH, Park HR. Immunological link between periodontitis and type 2 diabetes Deciphered by single-cell RNA analysis. Clin Transl Med. 2023;13:e1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Li L, Zhang Z, Chen P, Shu H, Yang C, Chu Y, Liu J. Ferroptosis: an important player in the inflammatory response in diabetic nephropathy. Front Immunol. 2023;14:1294317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang L, Zhang D, Li W, Lin H, Ding C, Liu Q, Wang L, Li Z, Mei L, Chen H, et al. Biofilm microenvironment triggered self-enhancing photodynamic Immunomodulatory microneedle for diabetic wound therapy. Nat Commun. 2023;14:7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo W, Lin K, Hua J, Han J, Zhang Q, Chen L, Khan ZA, Wu G, Wang Y, Liang G. Schisandrin B attenuates diabetic cardiomyopathy by targeting MyD88 and inhibiting MyD88-Dependent inflammation. Adv Sci (Weinh). 2022;9:e2202590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gram-Kampmann EM, Olesen TB, Hansen CD, Hugger MB, Jensen JM, Handberg A, Beck-Nielsen H, Krag A, Olsen MH, Højlund K. A six-month low-carbohydrate diet high in fat does not adversely affect endothelial function or markers of low-grade inflammation in patients with type 2 diabetes: an open-label randomized controlled trial. Cardiovasc Diabetol. 2023;22:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodríguez-Rodríguez E, Salas-González MD, Ortega RM, López-Sobaler AM. Leukocytes and Neutrophil-Lymphocyte ratio as indicators of insulin resistance in overweight/obese School-Children. Front Nutr. 2021;8:811081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russell CD, Parajuli A, Gale HJ, Bulteel NS, Schuetz P, de Jager CPC, Loonen AJM, Merekoulias GI, Baillie JK. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: A systematic review and meta-analysis. J Infect. 2019;78:339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guglielmini G, Falcinelli E, Piselli E, Mezzasoma AM, Tondi F, Alfonsi L, De Luca C, Fino V, Favilli A, Parrettini S, et al. Gestational diabetes mellitus is associated with in vivo platelet activation and platelet hyperreactivity. Am J Obstet Gynecol. 2025;232:120.e121-120.e114. [DOI] [PubMed]
- 44.Herrero-Cervera A, Soehnlein O, Kenne E. Neutrophils in chronic inflammatory diseases. Cell Mol Immunol. 2022;19:177–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Su W, Liu Z, Hu Z, Shen J, Zheng Z, Ding D, Huang W, Li W, Cai G, et al. Macrophage CREBZF orchestrates inflammatory response to potentiate insulin resistance and type 2 diabetes. Adv Sci (Weinh). 2024;11:e2306685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pant T, Lin CW, Bedrat A, Jia S, Roethle MF, Truchan NA, Ciecko AE, Chen YG, Hessner MJ. Monocytes in type 1 diabetes families exhibit high cytolytic activity and subset abundances that correlate with clinical progression. Sci Adv. 2024;10:eadn2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verhulst CEM, van Heck JIP, Fabricius TW, Stienstra R, Teerenstra S, McCrimmon RJ, Tack CJ, Pedersen-Bjergaard U, de Galan BE. Sustained Proinflammatory effects of hypoglycemia in people with type 2 diabetes and in people without diabetes. Diabetes. 2022;71:2716–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34:4270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott HA, Ng SH, McLoughlin RF, Valkenborghs SR, Nair P, Brown AC, Carroll OR, Horvat JC, Wood LG. Effect of obesity on airway and systemic inflammation in adults with asthma: a systematic review and meta-analysis. Thorax. 2023;78:957–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in this study are publicly available and can be accessed at the following link: https://www.cdc.gov/nchs/nhanes/.