Abstract
Objectives
Recent studies suggest the existence of an idiopathic pulmonary arterial hypertension (IPAH) phenotype affecting mostly patients with a smoking history, characterised by low diffusion capacity for carbon monoxide (DLCO) without clinically significant emphysema. This study’s objective was to test the hypothesis of a loss of pulmonary capillaries as an underlying mechanism by comparison to other patient groups with and without pulmonary hypertension (PH).
Materials and methods
Between March 2019 and June 2023, patients of four groups were recruited for this observational study: IPAH with preserved (1) and low DLCO (2), combined pulmonary fibrosis and emphysema with PH (3), and emphysema without PH (4). Patients underwent clinical CT and 129Xe MRI including dissolved-phase imaging yielding the ratio of 129Xe in red blood cells and membrane tissues (RBC-M), chemical shift saturation recovery for determining RBC fraction η and diffusion-weighted imaging yielding surface-volume ratio. Kruskal–Wallis tests were used for statistical analysis.
Results
Twenty-nine participants were recruited, of which 22 (age 64 ± 10, 11 male, 5/5/7/5 for the individual groups) could be included in the analysis. RBC-M and η were reduced in IPAH with low versus preserved DLCO and emphysema groups (p ≤ 0.01). CT low-attenuation area percentage was not increased in IPAH with low DLCO compared to any group. 129Xe MRI-derived surface-volume ratio was reduced in IPAH with low versus preserved DLCO (p = 0.04).
Conclusion
Results are consistent with a loss of pulmonary capillaries in patients with IPAH and low DLCO along with destruction of alveolar tissue, likely due to early diffuse emphysema.
Key Points
Question A loss of pulmonary capillaries has been suggested in patients with IPAH and low diffusion capacity without clinically significant emphysema on CT.
Findings 129Xe uptake in red blood cells and lung surface-volume ratio were reduced in IPAH patients with low compared to preserved diffusion capacity.
Clinical relevance This study furthers the understanding of the underlying pathological mechanisms in IPAH with low diffusion capacity, providing evidence that loss of pulmonary capillaries is accompanied by alveolar tissue destruction despite near-normal CT.
Keywords: Pulmonary arterial hypertension, Pulmonary circulation, Pulmonary emphysema, Magnetic resonance imaging, Xenon
Introduction
Idiopathic pulmonary arterial hypertension (IPAH) is a rare disease of the pulmonary vasculature characterised by pre-capillary pulmonary hypertension (PH) and can only be classified in the absence of other causes for PH [1]. Historically, IPAH predominantly affected young women. However, potentially due to changed demographics, IPAH is nowadays frequently diagnosed in elderly patients with cardiopulmonary comorbidities [2]. Among these, a distinct “lung phenotype” has emerged consisting of predominantly male patients with low diffusion capacity of the lung for carbon monoxide (DLCO) and significant smoking history. Despite frequent hypoxaemia, lung function testing and CT show no or minimal signs of parenchymal lung disease [3, 4]. This phenotype is associated with high mortality and distinct from other diseases associated with pulmonary arterial hypertension and low DLCO like pulmonary veno-occlusive disease, pulmonary capillary haemangiomatosis or systemic sclerosis.
Whereas smoking tobacco is not considered a risk factor for pulmonary arterial hypertension, long-standing tobacco exposure is hypothesised to cause direct damage to the alveolar-capillary membrane. Mice exposed to tobacco smoke showed endothelial cell apoptosis and loss of pulmonary capillaries [5]. Histopathology from 24 IPAH patients with a lung phenotype showed vascular pruning with capillary rarefication [6]. Direct damage to the alveolar-capillary membrane resulted in the loss of small pulmonary vessels and the term “vanishing pulmonary capillary syndrome” was proposed for this condition [7].
Smoking effects on the lungs were described in other diagnoses like chronic obstructive pulmonary disease (COPD) and combined pulmonary fibrosis and emphysema (CPFE) [8]. Recently, a vascular phenotype of COPD has been described [1]. There is typically only mild pulmonary arterial pressure elevation in COPD. Yet, a COPD subgroup with poor survival exists, characterised by moderate airflow limitation, severe precapillary PH, relatively low PaCO2, strongly impaired DLCO and progressive right heart failure [9]. CPFE displays a pattern of variable degrees of emphysema and fibrosis on CT. These patients seem to share clinical characteristics with IPAH with a lung phenotype [10].
Previous studies showed that even in patients with no or minimal changes in CT, histopathology may detect emphysematous and fibrotic changes [11]. However, as autopsies are rarely performed, verifying the existence of a vanishing pulmonary capillary syndrome histologically proved difficult.
Hyperpolarised gas MRI may assess lung microstructure and, e.g. emphysematous changes through increases in apparent diffusion coefficient (ADC) [12] noninvasively. Since xenon is taken up in lung tissue and blood and 129Xe experiences strong chemical shifts, 129Xe MRI is sensitive to alveolar-capillary diffusion and microvascular pulmonary blood volume [13]. With the appropriate choice of sequence parameters, 129Xe MRI metrics are specific to the gas uptake region and pulmonary capillaries as opposed to, e.g. dynamic contrast-enhanced MRI. Imaging of the 129Xe dissolved phase and dynamic acquisition of cardiogenic oscillations of 129Xe in red blood cells (RBC) may differentiate cardiopulmonary diseases and may provide information on pre- or postcapillary involvement in PH [14]. Although widespread clinical application is hampered by limited dissemination of necessary equipment, hyperpolarised 129Xe MRI is a useful tool for clinical research and the field of 129Xe MRI continues to grow.
We hypothesise that patients with IPAH and low DLCO differ from other patient groups by a loss of pulmonary capillaries, as judged by the ratio of 129Xe in RBCs and membrane tissues (M). The purpose of this study was to test this hypothesis and to evaluate alveolar microstructure by comparing results from 129Xe MRI in IPAH with low DLCO with those from IPAH with preserved DLCO, as well as with those from PH patients with CPFE and with those from patients with emphysema without echocardiographic signs of PH.
Materials and methods
Ethical approval for this observational, prospective study was granted by the institutional review board of Hannover Medical School. MRI was performed at Hannover Medical School between 03/2019 and 06/2023. Participants gave written informed consent.
Participants
Four patient groups were consecutively recruited from the outpatient clinic of the Department of Respiratory Medicine and Infectious Diseases of Hannover Medical School:
Patients with IPAH and preserved DLCO (≥ 45% of predicted, based on findings in [4]) and forced expiratory volume in 1 s (FEV1) of ≥ 60% predicted;
Patients with IPAH and low DLCO (< 45% predicted) and FEV1 ≥ 60% predicted without clinically significant parenchymal lung disease on CT;
Patients with CPFE on chest CT and PH;
Patients with emphysema without PH diagnosis.
Lung function testing, blood gas analyses, right-heart catheterisation (groups 1–3), as well as echocardiography (group 4) were part of clinical assessments. Right heart catheterisation was done at the time of PH diagnosis (except for patients with emphysema in whom echocardiography did not show signs of PH). PH was defined as mean pulmonary arterial pressure > 20 mmHg [1]. Clinical chest CT was available within 12 months. Exclusion criteria were pregnancy and MRI contraindications.
129Xe application
Locally produced hyperpolarised 129Xe (isotopically enriched 129Xe, polarisation 20–30%, Model 9810, Polarean) was authorised as a medical device for research applications. Doses were topped up with nitrogen to 1 L and dispensed into Tedlar bags (Jensen Inert Products). Subjects were instructed to exhale forcefully and inhale the dose in one breath. For the first two doses, in participants with vital capacity > 3 L, a second Tedlar bag containing air was connected to achieve a volume of 1/3 vital capacity [15]. For the last dose, participants were coached to further inhale room air after 129Xe to reach full lung inflation which may be beneficial for the repeatability of chemical shift saturation recovery (CSSR) measurements [16]. MRI was performed in breathhold. Vital signs were monitored by a physician during 129Xe applications.
Imaging
129Xe MRI was performed at 1.5 T/17.6 MHz (Avanto, Siemens) using a linearly-polarised birdcage coil with a 16-channel receive array (Rapid Biomedical) and self-developed pulse sequences. Common sequence parameters are summarised in Table 1.
Table 1.
Sequence parameters
| Sequence | TR/ms | TE/ms | Flip angle/° | Reconstructed FOV/mm | Reconstructed matrix |
|---|---|---|---|---|---|
| Ventilation imaging | 3.2 | 1.6 | ~10 | 384 × 384 × 384 | 96 × 96 × 96 |
| Dissolved-phase imaging | 18 | Echo train 1: 0.61/2.23/3.85 Echo train 2: 1.42/3.04 | 21 (dissolved)/0.4 (gas) | 320 × 320 × 320 | 48 × 48 × 48 |
| Fixed-TR dynamic spectroscopy | 36 | 1.2 | 60 | n/a | n/a |
| CSSR | Variable | 0.7 | ~27 (excitation using maximum RF power) | n/a | n/a |
| Diffusion-weighted imaging | 57 | 41.6 | 7.4 | 400 × 400 × 240 | 64 × 64 × 6 |
The sequence parameters for dissolved-phase imaging are comparable to those previously established for multi-echo techniques and to 129Xe clinical trial consortium recommendations for multi-site trials [15, 28]. Compared to the recommendations, ventilation imaging uses a radial trajectory facilitating acceleration. Diffusion-weighted imaging uses shorter diffusion times to facilitate the estimation of surface-volume ratio. The impact of frequency-selective excitation in fixed-TR dynamic spectroscopy on dissolved-phase ratios is assessed in the supplemental material
The first dose was used for ventilation imaging using a 3D radial balanced steady-state free precession sequence and transmitter calibration. A second dose (600–800 mL 129Xe, dose equivalent to ~130 mL) was used for fixed-TR dynamic dissolved-phase spectroscopy and dissolved-phase imaging. A third dose (500–600 mL 129Xe) was used for diffusion-weighted imaging and CSSR with a combined imaging time of 11 s [17].
Dissolved-phase imaging [17] was performed using five echoes in the dissolved phase. Ratio maps M-gas, RBC-gas, and RBC-M were formed and whole-lung averages were calculated within an automatically generated mask based on gas-phase SNR. Whole-lung RBC-M was defined as the primary dependent variable.
Fixed-TR dynamic spectroscopy was not spatially selective and employed excitation at 222.5 ppm using a 2.2 ms frequency-selective radiofrequency pulse systematically increasing RBC signals. Sequence parameters included 512 points, a bandwidth of 16.7 kHz, and 160 measurements. Two Lorentzians including a phase term were fitted to real parts of spectra after zeroth order phasing corresponding to M and RBC. The median RBC-M chemical shift difference was determined. The function
| 1 |
was fitted to the ratio of magnitudes to quantify the average RBC-M ratio (m) and oscillation amplitude (a). Nmeas denotes the number of measurements (discarding the first 10%), n is the excitation number, r is proportional to heart rate, φ a phase factor and d accounts for signal drifts.
In CSSR spectroscopy, two 2.4 ms rectangular radiofrequency pulses saturated the dissolved-phase magnetisation. Dissolved-phase signal-buildup/gas uptake at delay times of 3–600 ms was probed using a 1.2 ms Gaussian pulse without localisation and free-induction decays sampled with bandwidth 16.7 kHz. 129Xe in the three compartments was quantified after zeroth- and first-order phasing by numerical integration of real parts. CSSR data were analysed by fitting a generalised model of 129Xe septal uptake [18] yielding membrane permeability κ defined as the ratio of diffusion coefficient and membrane thickness, describing delayed uptake to RBCs, RBC fraction η derived from relative RBC signal intensity at the time of saturation of alveolar septa with 129Xe, as well as capillary transit time τ derived from RBC signal increase at high delay times.
From diffusion-weighted 129Xe MRI, whole-lung time-dependent ADCs were calculated and the whole-lung ratio of alveolar surface to gas volume quantified from data with 920 µs, 1140 µs, 1400 µs, 1660 µs, and 1960 µs diffusion time with 11, 9, 8, 7, and 7 repetitions of the diffusion-sensitising gradients at a constant b-value of 3 s/cm2. A second-order approximation of ADC time dependence was assumed [19].
Chest CT was evaluated by a radiologist (J.V.C., 21 years of experience). Lungs were segmented and low-attenuation area percentage (voxels with attenuation ≤ −950 HU) was quantified automatically using Aview (version 1.1.42.53, Coreline Soft).
Statistical analysis
Statistical analysis was performed by AK using subroutines in R (version 4.1.2). Kruskal–Wallis tests were performed to assess differences between groups. Post-hoc analysis was performed using Dunn tests including Holm p-value adjustment (kwManyOneDunnTest, package PMCMRplus, version 1.9.10) to test pairwise differences between IPAH with low DLCO and all other groups. Correlations were assessed using Pearson’s correlation. The significance level was 0.05 two-sided. Results are presented as median (interquartile range).
Results
Participants
Out of the 29 initially recruited participants 7 were excluded resulting in 22 participants for analysis: 5 participants with IPAH and preserved DLCO, 5 participants with IPAH and low DLCO, 7 PH participants with CPFE and 5 participants with pulmonary emphysema without PH. Results for exclusion were notable emphysema or parenchymal lung disease on chest CT in patients of the IPAH low DLCO group (four cases) or suspected pulmonary veno-occlusive disease based on their clinical phenotype (two cases) or histological results (one case). Data from diffusion-weighted imaging and CSSR could not be evaluated in one CPFE patient due to inadequate SNR.
Table 2 summarises participant demographics and clinical characteristics. The median interval between right heart catheterisation and MRI was 1.1 years. Participants with IPAH and preserved DLCO were comparatively young (median 55 years), the majority never smoked. FEV1 and DLCO were preserved with near-normal PaO2 and low PaCO2. Right-heart catheterisation at PH diagnosis showed severe pre-capillary PH. Patients received double (four cases) or triple (one case) combination therapy for PH. Four patients received macitentan, three received a PDE5 inhibitor, two riociguat, one selexipag, and one imatinib.
Table 2.
Participant demographics and clinical characteristics
| All patients | IPAH preserved DLCO | IPAH low DLCO (lung phenotype) | CPFE | Emphysema | |
|---|---|---|---|---|---|
| Number | 22 | 5 | 5 | 7 | 5 |
| Age at study, years | 65 (57–71) | 55 (43–59) | 65 (61–72) | 71 (63–79) | 66 (62–70) |
| Sex, male, % | 50 | 60 | 40 | 71 | 20 |
| DLCO, % pred. | 33 (28–40) | 72 (66–82) | 29 (27–31) | 33 (24–38) | 29 (19–36) |
| FEV1, % pred. | 73 (63–94) | 94 (87–94) | 74 (71–92) | 81 (66–94) | 39 (23–47) |
| FVC, % pred. | 94 (79–104) | 89 (88–110) | 98 (93–102) | 80 (79–103) | 71 (70–102) |
| TLC, % pred. | 100 (84–107) | 89 (83–102) | 100 (93–105) | 86 (67–103) | 113 (106–127) |
| RV, % pred. | 106 (85–137) | 86 (85–98) | 121 (88–131) | 96 (61–132) | 173 (156–213) |
| Body mass index, kg/m2 | 26 (24–29) | 25 (21–28) | 27 (26–34) | 27 (25–29) | 22 (21–26) |
| World Health Organization functional class | |||||
| I, n (%) | 2 (12) | 2 (40) | 0 (0) | 0 (0) | / |
| II, n (%) | 4 (24) | 2 (40) | 1 (20) | 1 (14) | / |
| III, n (%) | 10 (59) | 1 (20) | 3 (60) | 6 (86) | / |
| IV, n (%) | 1 (6) | 0 (0) | 1 (20) | 0 (0) | / |
| 6-min walk distance, m | 346 (251–387) | 558 (484–546) | 252 (177–335) | 353 (250–373) | / |
| N-terminal pro-B-type natriuretic peptide, ng/L | 279 (69–1576) | 60 (40–141) | 306 (132–2675) | 1212 (643–2786) | / |
| Haemoglobin, g/dL | 15.5 (14.3–16.9) | 15.4 (14.4–17.0) | 15.8 (13.6–16.7) | 15.2 (14.5–16.5) | 15.6 (14.9–17.4) |
| Arterial partial pressure of oxygen, mmHg | 61 (54–72) | 77 (65–79) | 57 (54–62) | 58 (53–62) | 60 (55–68) |
| Partial pressure of carbon dioxide, mmHg | 36 (34–39) | 34 (33–39) | 35 (32–40) | 34 (33–36) | 39 (35–42) |
| Oxygen saturation, % | 92 (90–95) | 96 (95–97) | 91 (88–93) | 91 (88–92) | 92 (92–95) |
| Oxygen supplementation, n (%) | 13 (59) | 0 (0) | 4 (80) | 5 (71) | 4 (80) |
| Smoking status, n (%) | |||||
| Active | 3 (14) | 0 (0) | 1 (20) | 2 (29) | 0 (0) |
| Former | 15 (68) | 2 (40) | 3 (60) | 5 (71) | 5 (100) |
| Never | 4 (18) | 3 (60) | 1 (20) | 0 (0) | 0 (0) |
| Pack years | 42 (5–50) | 0 (0–3) | 30 (23–44) | 50 (45–65) | 40 (13–49) |
| Haemodynamics | |||||
| Right atrial pressure, mmHg | 10 (7–12) | 8 (5–9) | 10 (7–12) | 12 (8–13) | / |
| Mean pulmonary arterial pressure, mmHg | 47 (40–54) | 53 (42–59) | 44 (43–48) | 47 (36–54) | / |
| Pulmonary arterial wedge pressure, mmHg | 11 (8–14) | 9 (8–11) | 14 (10–15) | 11 (6–15) | / |
| Cardiac index, L/min/m2 | 2.2 (2.0–2.4) | 1.8 (1.6–1.9) | 2.3 (2.2–2.6) | 2.2 (2.0–2.4) | / |
| Pulmonary vascular resistance, Wood units | 8 (6–13) | 14 (8–16) | 7 (7–8) | 9 (5–11) | / |
| Mixed venous oxygen saturation, % | 63 (57–67) | 67 (56–68) | 60 (58–66) | 54 (42–66) | / |
Values describing a distribution are shown as median (interquartile range)
CPFE combined pulmonary fibrosis and emphysema, DLCO diffusion capacity of the lung for carbon monoxide, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, IPAH idiopathic pulmonary arterial hypertension, RV residual volume, TLC total lung capacity
Patients with IPAH and low DLCO were comparatively older (median 65 years) and had a substantial smoking history (median 30 pack-years). FEV1 was mildly reduced, DLCO was severely reduced, and these participants were more hypoxaemic. These participants also had severe PH comparable to participants with IPAH and preserved DLCO. All patients were treated with a PDE5 inhibitor, two also received macitentan.
Patients with CPFE and PH were similar in age to patients with IPAH and low DLCO and predominantly male. Patients with CPFE and PH also had a substantial smoking history (median 50 pack-years). DLCO was similar to patients with IPAH and low DLCO. All patients were treated with a PDE5 inhibitor, one additionally with macitentan and selexipag.
Patients with emphysema without PH diagnosis, similar in age to patients with IPAH and low DLCO, had markedly reduced FEV1 and presented with comparable, very low DLCO (median 29% pred.). Echocardiography showed no signs of PH in these patients.
Imaging
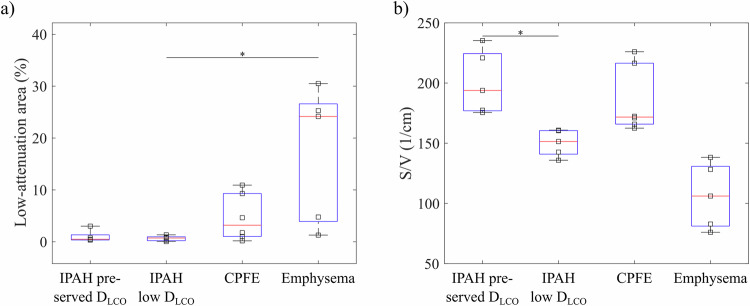
Representative CT images for participants of all groups are depicted in Figs. 1a–4a, respectively. Low-attenuation area percentages are shown in Fig. 5a. Values were significantly different between groups (p = 0.02) but not increased in the IPAH with low DLCO group compared to other groups.
Fig. 3.
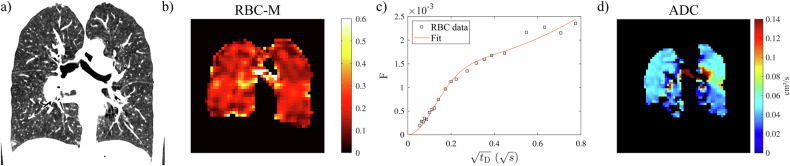
Representative participant with CPFE (61-year-old male, DLCO 39% pred., 70 pack-years): (a) CT (low-attenuation area percentage 10.9%), (b) ratio of 129Xe in RBC and M (whole-lung value 0.117) from 129Xe dissolved-phase MRI, (c) uptake of 129Xe to RBCs normalised by a gas signal as a function of delay time tD in CSSR (η = 0.16, τ = 0.82 s) and (d) ADC at 3 ms diffusion time from diffusion-weighted imaging. The surface/volume ratio from diffusion-weighted 129Xe MRI for this participant is 216 cm–1. ADC, apparent diffusion coefficient; CPFE, combined pulmonary fibrosis and emphysema; CSSR, chemical shift saturation recovery; DLCO, diffusion capacity of the lung for carbon monoxide; M, membrane tissues; RBC, red blood cell, tD, delay time
Fig. 1.
Representative participant with IPAH and preserved DLCO (67-year-old female, DLCO 82% pred., no smoking history): (a) CT (low-attenuation area percentage 3.0%), (b) ratio of 129Xe in RBC and M (whole-lung value 0.144) from 129Xe dissolved-phase MRI, (c) uptake of 129Xe to RBCs normalised by a gas signal as a function of delay time tD in CSSR (η = 0.28, τ = 0.91 s), and (d) ADC at 3 ms diffusion time from diffusion-weighted imaging. The surface/volume ratio from diffusion-weighted 129Xe MRI for this participant is 221 cm−1. ADC, apparent diffusion coefficient; CSSR, chemical shift saturation recovery; DLCO, diffusion capacity of the lung for carbon monoxide; IPAH, idiopathic pulmonary arterial hypertension; M, membrane tissues; RBC, red blood cell; tD, delay time
Fig. 4.
Representative participant with emphysema (63-year-old female, DLCO % pred., 16 pack-years): (a) CT (low-attenuation area percentage 24.2%), (b) ratio of 129Xe in RBC and M (whole-lung value 0.190) from 129Xe dissolved-phase MRI, (c) uptake of 129Xe to RBCs normalised by a gas signal as a function of delay time tD in CSSR (η = 0.22, τ = 0.49 s), and (d) ADC at 3 ms diffusion time from diffusion-weighted imaging. The surface/volume ratio from diffusion-weighted 129Xe MRI for this participant is 138 cm–1. ADC, apparent diffusion coefficient; CSSR, chemical shift saturation recovery; DLCO, diffusion capacity of the lung for carbon monoxide; M, membrane tissues; RBC, red blood cell; tD, delay time
Fig. 5.
a Low attenuation area percentage in the different study groups. b Lung S/V ratio from diffusion-weighted imaging in all four study groups. Significant differences between the group IPAH and low DLCO with all other groups from post-hoc analysis are marked by asterisks. CPFE, combined pulmonary fibrosis and emphysema; DLCO, diffusion capacity of the lung for carbon monoxide; IPAH, idiopathic pulmonary arterial hypertension; S/V, surface/volume
Lung surface-volume ratio from time-dependent diffusion measurements was significantly different between groups (p < 0.001), see Fig. 5b. In contrast to low-attenuation area percentage, the surface-volume ratio was significantly reduced in participants of the IPAH with low DLCO group compared to the IPAH with preserved DLCO group (p = 0.04). The median surface-volume ratio was lowest in the emphysema group.
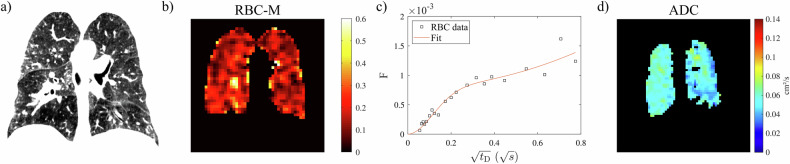
Representative ratio maps of 129Xe in RBC and M from dissolved-phase imaging are shown in Figs. 1b–4b. The RBC-M ratio was diffusely low throughout the lung parenchyma in the IPAH with low DLCO group (Fig. 2b). Whole-lung averages were significantly different between groups (p = 0.001), Fig. 6a. In post-hoc analysis, RBC-M was significantly reduced in the IPAH with low DLCO group compared to both the IPAH with preserved DLCO group (p = 0.006) and the emphysema group (p = 0.006). No significant difference was observed in comparison to the CPFE group (p = 0.45).
Fig. 2.
Representative participant within the group IPAH low DLCO (64-year-old female, DLCO 32% pred., 30 pack-years): (a) CT (low-attenuation area percentage 1.3%), (b) RBC-M ratio maps from 129Xe dissolved-phase MRI (whole-lung value 0.054), (c) CSSR uptake to RBC (RBC fraction η = 0.10, capillary transit time τ = 0.44 s), and (d) ADC at 3 ms diffusion time. RBC-M is diffusely low corresponding to low RBC uptake in CSSR at intermediate delay whereas ADC is mostly increased throughout the lung parenchyma. The surface/volume ratio from diffusion-weighted 129Xe MRI for this participant is 151 cm–1. ADC, apparent diffusion coefficient; CSSR, chemical shift saturation recovery; DLCO, diffusion capacity of the lung for carbon monoxide; IPAH, idiopathic pulmonary arterial hypertension; M, membrane tissues; RBC, red blood cell; tD, delay time
Fig. 6.
a RBC-M, (b) M-gas, and (c) RBC-gas ratio from dissolved-phase imaging, as well as (d) membrane permeability κ, (e) RBC fraction η, as well as (f) capillary transit time τ from CSSR spectroscopy. Significant differences between the group IPAH and low DLCO with all other groups from post-hoc analysis are marked by asterisks. CPFE, combined pulmonary fibrosis and emphysema; DLCO, diffusion capacity of the lung for carbon monoxide; IPAH, idiopathic pulmonary arterial hypertension; M, membrane tissues; RBC, red blood cell
The RBC fraction η from CSSR was significantly different between the groups (p = 0.001), Fig. 6e. η was significantly reduced in the IPAH with low DLCO group compared to the IPAH with preserved DLCO group (p = 0.004) and to the emphysema group (p = 0.004). No difference was observed with respect to the CPFE group (p = 0.33). The parameters κ and τ were not significantly different between the four groups (p = 0.36 and 0.05). Interestingly, capillary transit time τ tended to be lower in the IPAH with low DLCO (0.24 s (0.20–0.50 s)) than in the IPAH with preserved DLCO group (0.91 s (0.66–1.12 s)), Fig. 6f.
Results from fixed-TR dynamic spectroscopy of the 129Xe dissolved phase are summarised in supporting Fig. 1. Average RBC-M was different between groups (p = 0.002) and reduced in IPAH with low DLCO compared to IPAH with preserved DLCO (p = 0.02) and the emphysema group (p = 0.03). Oscillation amplitudes were significantly different between groups (p = 0.02) but post-hoc analysis showed no significant differences. Both IPAH groups tended to have low oscillation amplitudes. The RBC-M chemical shift difference was significantly different between groups (p = 0.002) and reduced in the IPAH with low DLCO group compared to the IPAH with preserved DLCO group (p = 0.005).
Ventilation defect percentage was significantly different between groups (p = 0.02) and highest in the emphysema group, but not different between IPAH with low DLCO compared to IPAH with preserved DLCO (p = 0.67).
Correlations
Cardiac index from right-heart catheterisation and capillary transit time τ were inversely correlated in the first three groups (R = −0.64, p = 0.008). There was a moderate correlation between CT-derived low-attenuation area and surface-volume ratio from 129Xe MRI (R = −0.45, p = 0.04). RBC-M chemical shift difference was correlated with oxygen partial pressure from blood gas analysis (R = 0.55, p = 0.006). RBC-M from fixed-TR dynamic spectroscopy was strongly correlated with whole-lung averages of imaging (R = 0.98, p < 0.001), supporting Fig. 2, and with η (R = 0.81, p < 0.001), but not with κ (R = −0.17, p = 0.45). Surface-volume ratio from diffusion-weighted imaging was strongly correlated with whole-lung M-Gas from dissolved-phase imaging (R = 0.82, p < 0.001) but not RBC-M (R = −0.18, p = 0.44).
Discussion
This study was performed to non-invasively detect a potential loss of pulmonary capillaries in patients with IPAH and low DLCO, as well as to evaluate lung microstructure. We found a significantly reduced RBC-M ratio, as well as RBC fraction η in the IPAH with a low DLCO group compared to both the preserved DLCO group and emphysema groups. At the same time, the lung surface-volume ratio derived from 129Xe ADCs in lung airspaces was significantly decreased in IPAH with low DLCO compared to IPAH with preserved DLCO although CT did not show signs of clinically significant macroscopic emphysema in IPAH with low DLCO.
These findings support the hypothesis of a loss of pulmonary capillaries in participants of the IPAH with low DLCO group, as seen by the significant reduction in RBC-M and the RBC fraction η. Furthermore, results from 129Xe MRI also suggest early emphysematous changes in IPAH with low DLCO as seen by the reduced lung surface-volume ratio despite negligible low-attenuation area on CT. This indicates that alveolar tissue destruction as part of early diffuse emphysema is likely contributing to DLCO reduction in IPAH with low DLCO. Four of five participants with IPAH and low DLCO were former or active smokers. One participant never smoked but was a metalworker with occupational exposure to toxic fumes for several decades.
Our findings suggest that the IPAH with low DLCO group mainly reflects the vascular phenotype of COPD, corresponding to World Health Organization PH group 3, with predominantly pulmonary capillary loss or dysfunction with relatively mild FEV1 reduction and diffuse alveolar tissue destruction as part of early emphysema leading to severe alveolar membrane dysfunction with severely reduced DLCO and resulting hypoxia. Measured pathophysiological changes are summarised in Table 3 with reference values from healthy subjects [17].
Table 3.
Summary of pathophysiological changes
| Quantity | IPAH preserved DLCO | IPAH low DLCO (lung phenotype) | CPFE | Emphysema |
|---|---|---|---|---|
| Airspace enlargement | ||||
| LAA (CT) | 0 | 0 | + | ++ |
| S/V (129Xe MRI) | 0 | − | (−) | −− |
| Capillary rarefication | ||||
| RBC fraction η | 0 | − | − | 0 |
| RBC-TP | 0 | − | − | 0 |
| Capillary blood flow | ||||
| Capillary transit time τ | + | − | 0 | 0 |
| RBC oscillation amplitude | − | − | 0 | 0 |
| Alveolar membrane function | ||||
| Membrane permeability κ | − | − | − | − |
| RBC chemical shift (oxygenation) | 0 | −− | −− | − |
| Ventilation heterogeneity | ||||
| Ventilation defect percentage | + | + | + | ++ |
| FEV1 as % of predicted value | 0 | − | − | −− |
Reference values for parameters from CSSR measurements in a group of 12 healthy volunteers reported in [17] (7 male/5 female, age 49 years ± 14 years) are κ: 0.051 cm/s ± 0.023 cm/s, η: 0.247 ± 0.041, τ: 0.67 s ± 0.18 s. Similarly, for fixed-TR dynamic spectroscopy, reference values are average RBC-M: 1.04 ± 0.17, RBC oscillation amplitude: 3.5% ± 0.5%, RBC chemical shift 18.7 ppm ± 0.4 ppm
++ strongly increased, + increased, 0 equal, − reduced, −− strongly reduced, CPFE combined pulmonary fibrosis and emphysema, DLCO diffusion capacity of the lung for carbon monoxide, FEV1 forced expiratory volume in 1 s, GP gas phase, IPAH idiopathic pulmonary arterial hypertension, LAA low-attenuation area, M membrane tissues, RBC red blood cell, S/V surface/volume
The different findings from CT and MRI may suggest an increased sensitivity of 129Xe diffusion-weighted MRI for early emphysema detection. Recent work showed the potential of 129Xe diffusion-weighted imaging to detect airspace enlargement in normal-appearing areas of the fibrotic lung [20]. 129Xe diffusion-weighted imaging and assessment of RBC signal may add value to patient phenotyping.
Results from fixed-TR dynamic spectroscopy of the dissolved phase are consistent with those from dissolved-phase imaging despite the systematic difference in RBC-M owing to frequency-selective excitation and an offset in the scatter plot (supporting Fig. 2), possibly due to T2* differences or non-Lorentzian line-shapes. The fact that RBC-M strongly correlates with η but not κ suggests that RBC-M in these patients is relatively unaffected by membrane permeability and mostly reflective of capillary density within alveolar tissue. The relative RBC signal from dissolved-phase imaging has been suggested to be reflective of capillary volume in analogy to the Roughton–Forster model [21].
Post-hoc analysis of RBC oscillation amplitudes showed no significant differences likely due to limited sample size. There was a trend towards reduced oscillation amplitudes in precapillary PAH compared to patients with emphysema without PH. The reduced RBC-M chemical shift difference in patients with IPAH and low DLCO is likely due to reduced oxygenation [22]. Furthermore, we observed a tendency for higher capillary flow in the IPAH with low DLCO group compared to the IPAH group with preserved DLCO. This is consistent with the hypothesis of capillary loss leading to increased capillary flow in remaining capillaries, shortening the oxygenation time. However, the influence of cardiac index on capillary transit time should be considered given that data from right-heart catheterisation showed a higher cardiac index in the IPAH with a low DLCO group on average. Interestingly, both capillary transit time and the product of RBC fraction η and surface-volume ratio were lower in IPAH with low DLCO compared to emphysema patients without PH, possibly reflecting a larger loss of capillary surface area.
Limitations
Measures of 129Xe gas uptake, particularly RBC-M, depending on age and sex in healthy subjects with increased values in males and younger age [23]. In addition, dissolved-phase ratios depend on haemoglobin [24] and could potentially also depend on oxygenation [25]. Future work should concentrate on the further establishment of reference values, as well as on the exact influence of confounding variables. Correlations between invasive haemodynamics and MRI findings must be interpreted with caution as these measurements were not obtained simultaneously and PAH treatments were initiated in between, potentially affecting pulmonary blood flow.
The parameters from the generalised CSSR model in [18] have not been validated by comparison to gold standard measurements. This study showed a correlation of capillary transit time with cardiac index. Results for κ may be affected by noise and uncertainties in transmitter calibration.
The inability of some participants to hold their breath long enough may have influenced the results. In particular, imprecision and bias may arise from the dependence of dissolved-phase ratios on lung inflation [26]. This was partly addressed by administering gas volumes normalised to spirometry. Previous studies suggest an influence of lung size which possibly cannot be addressed by standardised breathing manoeuvres [27].
The generalizability of the results of this single-centre study to other centres with different patient cohorts and imaging methods is an open question. Multi-centre studies with larger sample sizes are needed but are hampered by limited dissemination of 129Xe MRI.
Conclusion
Our results are consistent with a loss of pulmonary capillaries in patients with IPAH and low DLCO in conjunction with the destruction of alveolar membranes, likely due to early diffuse emphysema.
Supplementary information
Acknowledgements
This work was funded by the German Center for Lung Research (DZL).
Abbreviations
- ADC
Apparent diffusion coefficient
- COPD
Chronic obstructive pulmonary disease
- CPFE
Combined pulmonary fibrosis and emphysema
- CSSR
Chemical shift saturation recovery
- DLCO
Diffusion capacity of the lung for carbon monoxide
- FEV1
Forced expiratory volume in 1 s
- IPAH
Idiopathic pulmonary arterial hypertension
- M
Membrane tissues
- PH
Pulmonary hypertension
- RBC
Red blood cell
Funding
This study has received funding from the German Center for Lung Research (DZL). Open Access funding enabled and organised by Projekt DEAL.
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Jens Vogel-Claussen.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
None.
Methodology
Prospective
Cross-sectional study
Performed at one institution
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Agilo Luitger Kern and Da-Hee Park contributed equally to this work.
Karen M. Olsson and Jens Vogel-Claussen jointly supervised to this work.
Supplementary information
The online version contains supplementary material available at 10.1007/s00330-024-11209-1.
References
- 1.Humbert M, Kovacs G, Hoeper MM et al (2022) 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 43:3618–3731. 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 2.Hoeper MM, Pausch C, Grünig E et al (2020) Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Hear Lung Transplant 39:1435–1444. 10.1016/j.healun.2020.09.011 [DOI] [PubMed] [Google Scholar]
- 3.Olsson KM, Fuge J, Meyer K, Welte T, Hoeper MM (2017) More on idiopathic pulmonary arterial hypertension with a low diffusing capacity. Eur Respir J 50:1700354. 10.1183/13993003.00354-2017 [DOI] [PubMed] [Google Scholar]
- 4.Trip P, Nossent EJ, de Man FS et al (2013) Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: patient characteristics and treatment responses. Eur Respir J 42:1575–1585. 10.1183/09031936.00184412 [DOI] [PubMed] [Google Scholar]
- 5.Seimetz M, Parajuli N, Pichl A et al (2011) Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell 147:293–305. 10.1016/j.cell.2011.08.035 [DOI] [PubMed] [Google Scholar]
- 6.Nossent EJ, Smits J, Seegers C et al (2022) A new clinical phenotype with non-plexiform vasculopathy and distinctive microvessel remodeling among idiopathic pulmonary arterial hypertension patients. In: D96 EMBARCADERO: MACHINES AND DEEP PHENOTYPING OF THE PULMONARY CIRCULATION. American Thoracic Society, pp A5302–A5302. 10.1164/ajrccm-conference.2022.205.1_MeetingAbstracts.A5302
- 7.Hoeper MM, Vonk-Noordegraaf A (2017) Is there a vanishing pulmonary capillary syndrome? Lancet Respir Med 5:676–678. 10.1016/S2213-2600(17)30291-6 [DOI] [PubMed] [Google Scholar]
- 8.Christenson SA, Smith BM, Bafadhel M, Putcha N (2022) Chronic obstructive pulmonary disease. Lancet 399:2227–2242. 10.1016/S0140-6736(22)00470-6 [DOI] [PubMed] [Google Scholar]
- 9.Kovacs G, Agusti A, Barberà JA et al (2018) Pulmonary vascular involvement in chronic obstructive pulmonary disease. Is there a pulmonary vascular phenotype? Am J Respir Crit Care Med 198:1000–1011. 10.1164/rccm.201801-0095PP [DOI] [PubMed] [Google Scholar]
- 10.Hoeper MM, Dwivedi K, Pausch C et al (2022) Phenotyping of idiopathic pulmonary arterial hypertension: a registry analysis. Lancet Respir Med 10:937–948. 10.1016/S2213-2600(22)00097-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Kersh K, Perez RL, Smith JS, Fraig M (2013) Smoking-related interstitial fibrosis (SRIF) and pulmonary hypertension. Case Rep 2013:bcr2013008970. 10.1136/bcr-2013-008970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tafti S, Garrison WJ, Mugler JP et al (2020) Emphysema index based on hyperpolarised 3 He or 129 Xe diffusion MRI: performance and comparison with quantitative CT and pulmonary function tests. Radiology 297:201–210. 10.1148/radiol.2020192804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern AL, Vogel-Claussen J (2018) Hyperpolarized gas MRI in pulmonology. Br J Radiol 91:20170647. 10.1259/bjr.20170647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Bier EA, Swaminathan A et al (2019) Diverse cardiopulmonary diseases are associated with distinct xenon magnetic resonance imaging signatures. Eur Respir J 54:1900831. 10.1183/13993003.00831-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qing K, Ruppert K, Jiang Y et al (2014) Regional mapping of gas uptake by blood and tissue in the human lung using hyperpolarized xenon-129 MRI. J Magn Reson Imaging 39:346–359. 10.1002/jmri.24181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart NJ, Horn FC, Norquay G et al (2017) Reproducibility of quantitative indices of lung function and microstructure from 129Xe chemical shift saturation recovery (CSSR) MR spectroscopy. Magn Reson Med 77:2107–2113. 10.1002/mrm.26310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kern AL, Pink I, Bonifacius A et al (2024) Alveolar membrane and capillary function in COVID-19 convalescents: insights from chest MRI. Eur Radiol. 10.1007/s00330-024-10669-9 [DOI] [PMC free article] [PubMed]
- 18.Kern A, Olsson K, Kaireit T et al (2020) Assessment of interstitial membrane permeability using a generalized model of 129Xe septal uptake in the lung. In: Proceedings of the International Society for Magnetic Resonance in Medicine. ISMRM, pp 2371
- 19.Kern AL, Gutberlet M, Moher Alsady T et al (2020) Investigating short-time diffusion of hyperpolarized 129 Xe in lung air spaces and tissue: a feasibility study in chronic obstructive pulmonary disease patients. Magn Reson Med 84:2133–2146. 10.1002/mrm.28264 [DOI] [PubMed] [Google Scholar]
- 20.Chan H-F, Weatherley ND, Biancardi AM et al (2023) Voxel-wise comparison of Co-registered quantitative CT and hyperpolarised gas diffusion-weighted MRI measurements in IPF. Diagnostics 13:3497. 10.3390/diagnostics13233497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Rankine L, Bier EA et al (2021) Using hyperpolarized 129 Xe gas-exchange MRI to model the regional airspace, membrane, and capillary contributions to diffusing capacity. J Appl Physiol (1985) 130:1398–1409. 10.1152/japplphysiol.00702.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolber J, Cherubini A, Leach MO, Bifone A (2000) Hyperpolarized129Xe NMR as a probe for blood oxygenation. Magn Reson Med 43:491–496 [DOI] [PubMed] [Google Scholar]
- 23.Plummer JW, Willmering MM, Cleveland ZI, Towe C, Woods JC, Walkup LL (2023) Childhood to adulthood: accounting for age dependence in healthy-reference distributions in 129Xe gas-exchange MRI. Magn Reson Med 89:1117–1133. 10.1002/mrm.29501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bechtel A, Lu J, Mummy D et al (2023) Establishing a hemoglobin adjustment for 129Xe gas exchange MRI and MRS. Magn Reson Med 90:1555–1568. 10.1002/mrm.29712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladefoged J, Andersen AM (1967) Solubility of Xenon-133 at 37 °C in water, saline, olive oil, liquid paraffin, solutions of albumin, and blood. Phys Med Biol 12:307. 10.1088/0031-9155/12/3/307 [Google Scholar]
- 26.Garrison WJ, Qing K, He M et al (2023) Lung volume dependence and repeatability of hyperpolarized 129Xe MRI gas uptake metrics in healthy volunteers and participants with COPD. Radiol Cardiothorac Imaging 5:e220096. 10.1148/ryct.220096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robert HB, Robert AW, Kirk G, Drummond MB, Mitzner W (2015) Lung density changes with growth and inflation. Chest 148:995–1002. 10.1378/chest.15-0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niedbalski PJ, Hall CS, Castro M et al (2021) Protocols for multi‐site trials using hyperpolarized 129Xe MRI for imaging of ventilation, alveolar‐airspace size, and gas exchange: a position paper from the 129Xe MRI clinical trials consortium. Magn Reson Med 86:2966–2986. 10.1002/mrm.28985 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.