Abstract
Background
The prognosis of early unfavorable and advanced stage classic Hodgkin lymphoma (cHL) remains suboptimal with the widely used ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) regimen. Novel agents such as brentuximab vedotin (BV) and anti-PD-1 antibody have demonstrated high efficacy and good tolerance in relapsed/refractory cHL and have also shown promising results in the frontline setting. However, concurrent administration of anti-PD-1 antibody plus AVD in comparison with traditional ABVD regimen alone in untreated classic Hodgkin lymphoma (cHL) has yet to be adequately studied in real-world clinical practice.
Methods
We enrolled eligible adult patients with histologically confirmed cHL who had received initial treatment with the ABVD regimen, or the novel combination regimens of anti-PD1-AVD. The study endpoints included modified progression-free survival (mPFS) and complete response (CR) after 2 cycles of therapy. Propensity score matching (PSM) was performed to balance clinical variables between regimens prior to efficacy comparisons.
Results
Of 172 patients, 137 received the ABVD regimen and 35 received the anti-PD1-AVD regimen. With a median follow-up of 37.7 months, significantly prolonged 3-year modified PFS was reported for anti-PD1-AVD versus ABVD (PSM: 91.0 vs. 61.6%, p = 0.032). Significantly improved CR rate was observed with anti-PD1-AVD versus ABVD (PSM: 86.7 vs. 63.8%, p = 0.049).
Conclusions
In this real-world study, concurrent anti-PD1 antibody with AVD showed significantly prolonged modified PFS and improved CR rate after cycle 2 versus ABVD regimen, supporting the use of novel agents in frontline therapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-025-04041-z.
Keywords: Lymphoma, Immune checkpoint inhibitor, Chemotherapy, Modified progression-free survival, Complete response
Introduction
Classic Hodgkin lymphoma (cHL) is a lymphoid neoplasm characterized by the presence of rare malignant Hodgkin/Reed–Sternberg (HRS) cells within a heterogeneous mixture of non-neoplastic inflammatory and immune cells [1]. cHL is generally considered a highly curable disease, with international standard chemotherapy regimens including ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) or escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone), sometimes followed by consolidative radiotherapy [2–5]. Despite the favorable prognosis for most cHL patients, disease relapse and chemotherapy-related toxicity remain significant challenges. Approximately 10–20% of early stage patients and 20–30% of advanced-stage patients experience relapse or refractory after first-line therapy. Additionally, bleomycin-induced pulmonary toxicity negatively impacts treatment outcomes and patient quality of life. Recent efforts have focused on incorporating novel agents into standard chemotherapy to improve efficacy and reduce toxicity.
Novel biologic agents, including the anti-CD30 antibody–drug conjugate (ADC) and anti-programmed death protein 1 (PD1) antibody, have demonstrated promising efficacy and revolutionized the therapy for cHL. The phase III ECHELON-1 study showed that the addition of BV to AVD chemotherapy (BV-AVD) for the treatment of newly diagnosed advanced-stage cHL improved both modified and traditional progression-free survival (PFS) and overall survival (OS) compared to the standard ABVD regimen [6–9], which established BV-AVD as the standard of care in newly diagnosed advanced-stage cHL [10]. Parallel to the above developments, anti-PD1 antibodies have garnered attention as a promising avenue for treating newly diagnosed cHL [11–14]. However, these single-arm studies include a lead-in part with an anti-PD1 antibody monotherapy followed by chemotherapy with or without anti-PD1 antibody, and they lack direct comparisons with conventional standard cHL treatment. A noteworthy exception is the ongoing randomized phase III SWOG S1826 clinical trial, first presented at the 2023 ASCO meeting, which reported that nivolumab plus AVD showed a slight but significant 1-year PFS advantage over BV plus AVD (94 vs. 86%), accompanied by superior tolerability. Recently, 2-year PFS rate has been updated with 92% with N + AVD, as compared with 83% with BV + AVD [15, 16]. The definitive conclusion of this study was premature due to the short median follow-up time of 2.1 years, warranting further evaluation to ascertain long-term outcomes. Currently, there is a notable absence of randomized studies directly comparing the concurrent administration of anti-PD1 antibody-AVD versus ABVD in untreated cHL. The efficacy and side effects between anti-PD1-AVD and ABVD remain inadequately investigated in frontline treatment of cHL in real-world clinical practice. We conducted a real-world, institution-based retrospective analysis to provide comprehensive data on the efficacy and safety profiles of anti-PD1-AVD versus ABVD in the first-line therapy of early unfavorable and advanced-stage cHL, aiming to provide evidence for clinical practice.
Methods
Patients
This retrospective study included 172 adult patients (35 in the anti-PD1-AVD group and 137 in the ABVD group) with untreated early unfavorable and advanced (stage III and IV) stage cHL according to National Comprehensive Cancer Network (NCCN) criteria, who initiated their first-line therapy between August 2017 to September 2023 at Sun Yat-Sen University Cancer Center (SYSUCC). NCCN criteria for early unfavorable stage disease included the presence of at least one of the following risk factors: erythrocyte sedimentation rate > 50 mm per hour, presence of B symptoms, involvement of > 3 nodal sites, a mediastinal mass ratio of 1:3 (maximum width of mass/maximum intrathoracic diameter), or a mass > 10 cm in any dimension. Patients with advanced stage were stratified into low-risk (score 0–3) and high-risk (score 4–7) groups. The eligible first-line treatment protocols included the administration of anti-PD1-AVD and ABVD regimens. The decision to administer anti-PD-1-AVD therapy was based on a comprehensive evaluation of clinical indications (particularly bulky disease and advanced-stage presentation), patient-specific characteristics (including age, performance status, and the presence of comorbidities such as neuropathy or pneumonia), and economic factors, as determined by the principal investigator through careful clinical judgment. Response evaluation by PET/CT scanning was based on the 2014 Lugano classification using the 5-point scale [17]. A Deauville score of 1–3 on a PET scan was considered as complete response, whereas a Deauville score of 4 and 5 represented incomplete metabolic response. In instances of equivocal PET2 findings (e.g., Deauville score of 4), the PET-CT scans will undergo independent review by two nuclear medicine specialists to ensure consistent and accurate interpretation. The study was approved by the Committee for Ethical Review of Research involving Human Subjects of Sun Yat-Sen University cancer center (Approval number: B2023-647-01).
Treatment
The ABVD regimen consisted of doxorubicin 25 mg/m2, vindesine 3 mg/m2 (Vinblastine is not available in China), dacarbazine 375 mg/m2, and bleomycin 10U/ m2 or 10 mg/m2 intravenously on days 1 and 15 of each 28-day cycle, for a total of four to six cycles. The anti-PD1-AVD regimen incorporated a PD-1 inhibitor (200 or 240 mg) on day 1 and 15 of each cycle, leveraging the backbone of AVD while omitting bleomycin. Four anti-PD-1 inhibitors were used, including Tislelizumab (62.9%), Sintilimab (22.8%), Toripalimab (11.4%), and Nivolumab (2.8%). Toripalimab and Nivolumab were administered intravenously at a fixed dose of 240 mg every two weeks, while Tislelizumab and Sintilimab were given at 200 mg per dose. Treatment protocols were stratified by disease stage: patients with advanced-stage disease completed six cycles of intravenous therapy, whereas those with early unfavorable-stage disease received four cycles of intravenous therapy combined with 30 Gy involved-site radiotherapy (ISRT), with the alternative option of six cycles of intravenous therapy alone for patients with ISRT contraindications, such as pneumonia, local dermatitis, ulcers, or refusal of radiation therapy. For advanced-stage patients, radiotherapy was selectively administered only to those presenting with residual lesions greater than 2.5 cm following completion of intravenous therapy. Interim response assessment was conducted via PET/CT scans, performed two days prior to the initiation of the third treatment cycle (approximately on Day 26 of the second cycle). Patients with stable disease (SD) or progressive disease (PD) switched to second-line treatment. Other patients with partial remission (PR) during the interim of treatment (after 2 or 4 cycles) continued with the same original regimen or transitioned to second-line treatment at the discretion of treating physicians. The second-line therapy included GVD (gemcitabine, vinorelbine, liposomal doxorubicin) or ICE (ifosfamide, carboplatin, and etoposide) chemotherapy, with or without BV or anti-PD1 antibody, determined by the principal investigator. Two patients in the anti-PD1-AVD group changed to second-line regimens (1 to anti-PD1-GVD and 1 to BV-GVD). Forty patients in ABVD group switched to second-line regimens (among them 55% to anti-PD1 plus chemotherapy, 25% to chemotherapy alone, 12.5% to anti-PD1 monotherapy, 5% to BV plus chemotherapy, 2.5% to anti-PD1 + BV). The initiation and duration of PD-1 maintenance therapy were determined by the principal investigator based on the need to consolidate treatment efficacy in patients with high-risk factors, including multiple extranodal sites involvement or a high International Prognostic Score (IPS). In our retrospective study, PD-1 maintenance therapy was typically administered once monthly for a duration ranging from 2 to 10 months.
End points
The primary endpoints of this retrospective study included modified progression-free survival (modified PFS) and CR rate (CRR) after 2 cycles of the regimen. Modified progression-free survival is defined as the time of diagnosis to disease progression, death from any cause, modified progression (noncomplete remission during primary chemotherapy, with Deauville score of 4 or 5 at interim or end of PET-CT scan, and delivery of subsequent chemotherapy for residual disease) or to last follow-up. A switch to next-line therapy before completing primary therapy due to incomplete response was also considered as an event for this endpoint. The PET2 CRR and ORR were calculated as the proportion of patients who achieve CR, or CR plus PR, respectively, after 2 cycles of first-line therapy evaluated by PET-CT/CT based on the 2014 Lugano criteria. Secondary endpoints included progression-free survival (PFS) and overall survival (OS). Progression-free survival (PFS) is the time from diagnosis to disease progression, death from any cause, or last follow-up. OS is defined as the time from diagnosis to death from any cause or the last follow-up. Adverse events (AEs) were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE, version 5.0).
Statistical analysis
Baseline characteristics were compared between groups using the Kruskal–Wallis H test, χ2 tests, or Fisher exact test. All statistical comparisons adhered to a two-sided approach, with statistical significance set at a p value threshold of < 0.05. Modified PFS, PFS, and OS were summarized using the Kaplan–Meier method.
Given the notable disparity in the count of cases between the two groups, propensity score matching (PSM) methods were implemented to match patients to ensure balance in baseline data. The propensity score was calculated incorporating several factors, including age (dichotomized at 45), sex, histology, presence of B symptoms, stage (II, III, and IV), and Eastern Cooperative Oncology Group (ECOG) performance status. For PSM, patients in the anti-PD1-AVD group were matched with patients in the ABVD group using a 1:3 ratio with replacement, nearest-neighbor matching, and caliper of 0.2 times the standard deviation of the propensity score’s logit [18, 19]. Patients in the anti-PD1-AVD group who could be matched with one or two patients in the ABVD group due to propensity score falling beyond the boundaries were retained along with the matched ABVD patients [18]. Owing to this, PSM inevitably reduced sample size [20]. PSM aimed for a standardized difference of < 0.2 for adequate balance. After adjusting for baseline disparities through PSM, response rates were evaluated using conditional and weighted logistic models, and survival endpoints were analyzed with a Cox proportional hazards model [21, 22]. The above analyses were conducted using SPSS, version 27.0, and R, version 4.3.2.
Results
Patients and disposition
Between August 2017 to September 2023, 490 patients with classic Hodgkin lymphoma were screened for eligibility, of which 172 patients met our cohort criteria and were included in the analysis (Fig. 1). In this study cohort, 35 (20.3%) patients received the anti-PD1-AVD regimen for the frontline therapy, and 137 (65.9%) received the ABVD. 39.0% of patients had stage II unfavorable disease, and 61.0% of patients had advanced-stage disease. Regarding treatment response, at the end of 2 cycles, the proportion of patients achieving complete response (PET2-) was 85.7% in the anti-PD1-AVD group, compared to 63.5% in the ABVD group. Throughout the entire treatment period, 94.3% of patients in the anti-PD1-AVD group achieved CR (best of complete response), compared with 86.1% in the ABVD group. After PSM, 30 patients in anti-PD1-AVD group were matched with 69 patients in ABVD group. Table 1 summarizes the baseline features in anti-PD1-AVD vs. ABVD before matching and after PSM matching. Clinical and pathological characteristics were well balanced across all pairwise comparisons (all p > 0.05).
Fig. 1.
Flow diagram of the case selection process and treatment
Table 1.
Baseline characteristics of two cohorts at the initiation of primary therapy
| Before matching | After PSM matching | ||||||
|---|---|---|---|---|---|---|---|
| Group | ABVD | Anti-PD1-AVD | p | ABVD | Anti-PD1-AVD | p | |
| N | 137 | 35 | 69 | 30 | |||
| Age | < 45 | 75.2 | 65.7 | 0.287 | 78.3 | 70.0 | 0.531 |
| ≥ 45 | 24.8 | 34.3 | 21.7 | 30.0 | |||
| Sex | female | 46.0 | 48.6 | 0.850 | 58.0 | 56.7 | 1.000 |
| male | 54.0 | 51.4 | 42.0 | 43.3 | |||
| Histology | Nodular sclerosis | 66.4 | 77.1 | 0.184 | 79.7 | 83.3 | 0.928 |
| Mixed cellularity | 22.6 | 8.6 | 10.0 | 11.6 | |||
| Lymphocyte rich | 2.9 | 8.6 | 2.9 | 0.0 | |||
| Lymphocyte depletion | 1.5 | 0.0 | 0.0 | 0.0 | |||
| without subtyping | 6.6 | 5.7 | 5.8 | 7.4 | |||
| stage | II | 42.3 | 26.5 | 0.192 | 30.4 | 26.7 | 0.912 |
| III | 21.9 | 25.7 | 17.4 | 20.0 | |||
| IV | 35.7 | 48.6 | 52.2 | 53.3 | |||
| IPS | 0–3 | 44.5 | 48.6 | 0.301 | 79.2 | 63.6 | 0.278 |
| 4–7 | 13.1 | 22.6 | 20.8 | 36.4 | |||
| ECOG | 0 | 65.0 | 62.9 | 0..640 | 72.5 | 60.0 | 0.187 |
| 1 | 34.3 | 34.3 | 27.5 | 36.7 | |||
| 2 | 2 | 2.9 | 0.0 | 3.3 | |||
| B symptoms | yes | 40.9 | 45.7 | 0.702 | 30.4 | 43.3 | 0.312 |
| Extranodal involvement | yes | 44.5 | 48.6 | 0.706 | 56.5 | 53.3 | 0.942 |
| mass | > 7 cm | 22.6 | 17.6 | 0.645 | 25.0 | 22.2 | |
| > 10 cm | 10.2 | 0.0 | 0.074 | 10.3 | 0.0 | ||
| Involvement of nodal areas | ≥ 3 | 73.7 | 77.1 | 0.829 | 75.4 | 83.3 | 0.539 |
Data presented as percentage (%) unless otherwise indicated. PSM, propensity score matching
Efficacy
Response rate
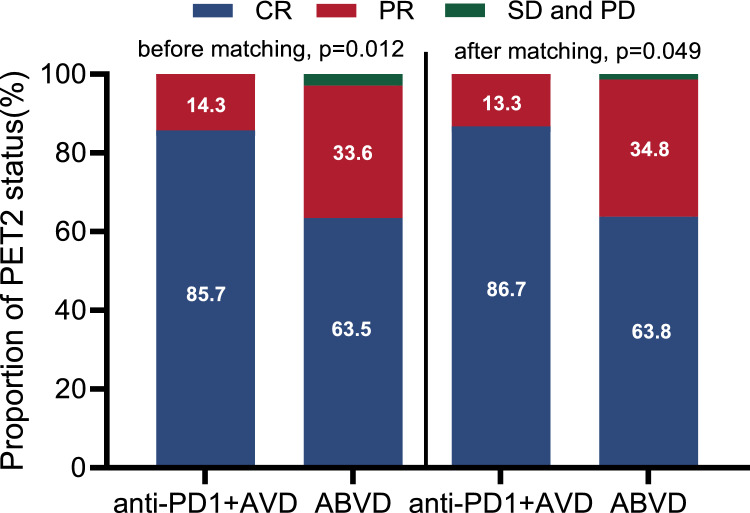
CRR and PRR before and after matching are shown in Fig. 2. Patients from the anti-PD1-AVD group had a superior CRR compared to the ABVD after 2 cycles of the regimen. CRR at PET2 was 85.7 vs. 63.5% (p = 0.012) between the anti-PD1-AVD and ABVD groups before PSM matching. The difference remained consistent after PSM matching (86.7 vs. 63.8%, p = 0.049). ORR at PET2 was also evaluated and failed to exhibit statistically significant variations among distinct treatment cohorts (Table 2).
Fig. 2.
Complete response rate (CRR) and partial response rate (PRR) after two cycles of two regimens. CRR and PRR in anti-PD1-AVD versus ABVD group at PET2 before matching (p = 0.012) and after PSM matching (p = 0.049). CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; PSM: propensity score matching; PET2, end of cycle two positron emission tomography
Table 2.
Response rates and survival outcomes in different cohorts
| Before matching | After PSM matching | |||||
|---|---|---|---|---|---|---|
| anti-PD1-AVD vs ABVD | anti-PD1-AVD | ABVD | p | anti-PD1-AVD | ABVD | p |
| Response rate | ||||||
| CRR, (95% CI) | 85.7(69.7–95%.2) | 63.5(54.9–71.6) | 0.012* | 86.7(69.3–96.2) | 63.8(51.3–75.0) | 0.049* |
| ORR, (95% CI) | 100.0(90.0–100.0) | 97.1(92.7–99.2) | 0.995 | 100.0(88.4–100.0) | 98.5(92.2–99.9) | 0.999 |
| Survival | ||||||
| 1-year mPFS, (95%CI) | 94.3(86.9–100.0) | 75.8(68.7–83.7) | 96.7(90.5–100.0) | 76.0(66.0–87.5) | ||
| 2-year mPFS, (95%CI) | 89.6(78.7–100.0) | 65.6(57.5–75.0) | 91.0(79.4–100.0) | 69.2(58.0–82.6) | ||
| 3-year mPFS, (95%CI) | 89.6(78.7–100.0) | 59.2(50.4–69.6) | 0.018* | 91.0(69.5–100.0) | 61.6(49.3–76.8) | 0.032* |
| 1-year PFS, (95%CI) | 100.0(100.0–100.0) | 83.8(77.5–90.6) | 100.0(100.0–100.0) | 85.2(76.7–94.7) | ||
| 2-year PFS, (95%CI) | 90.4(78.6–100.0) | 73.4(65.5–82.2) | 88.5(74.8–100.0) | 73.8(62.6–87.1) | ||
| 3-year PFS, (95%CI) | 90.4(78.6–100.0) | 66.7(57.9–76.9) | 0.037* | 88.5(74.8–100.0) | 65.9(53.4–81.2) | 0.072 |
| 3-year OS, (95%CI) | 91.8(60.4–81.6) | 93.5(89.3–98.0) | 0.983 | 90.5(78.8–100.0) | 96.4 (91.5–100.0) | 0.292 |
| 5-year OS, (95%CI) | – | 90.6 (85.0–96.7) | – | 96.4 (91.5–100.0) | ||
Survival
The median follow-up time was 37.7 months (IQR 19.2–51.7 months). Before matching, patients in anti-PD1-AVD group had a longer modified progression-free survival (3-year mPFS 89.6 vs 59.2%, HR: 0.24, p = 0.018, Fig. 3A) and progression-free survival (3-year PFS 90.4% vs 66.7%, HR: 0.20, p = 0.037, Fig. 3B) in comparison with the ABVD group. After applying PSM as an adjustment method for baseline variables, the 3-year mPFS rate in the anti-PD1-AVD and ABVD groups was 91.0% and 61.6% (HR: 0.20, p = 0.032, Fig. 3C), respectively. However, PFS difference did not remained statistically significant after PSM analysis. The 3-year PFS rate was 88.5% in the anti-PD1-AVD group and 65.9% in the ABVD group (HR: 0.26, p = 0.072, Fig. 3D). There was no significant discrepancy in OS across two groups (Figure S1). Detailed response and survival information in two cohorts are presented in Table 2.
Fig. 3.
Kaplan–Meier curves of modified progression free survival (mPFS) and progression free survival (PFS) in two cohorts before matching after PSM matching. A mPFS between anti-PD1-AVD and ABVD group before matching. B PFS between anti-PD1-AVD and ABVD group before matching. C mPFS between anti-PD1-AVD and ABVD group after PSM matching. D PFS between anti-PD1-AVD and ABVD group after PSM matching
Safety
The safety profiles are summarized in Table 3. Although the difference in hematologic AEs between the two groups did not reach statistical significance, the ABVD regimen showed a numerically higher incidence of leukopenia and neutropenia compared to the anti-PD1-AVD regimen. Notably, there were higher rates of immune-related endocrine diseases in the anti-PD1-AVD group, including hypothyroidism (20.0%), hyperthyroidism (10.0%), and adrenal insufficiency (6.7%). It is worth noting that in the ABVD group, one patient who developed a second tumor (EBV + DLBCL) after being cured of Hodgkin lymphoma. Toxicity profiles of different anti-PD1antibodies plus AVD are shown in Table S1.
Table 3.
Treatment-related and immune-related adverse events in anti-PD-1-AVD and ABVD groups after PSM matching
| Regimen | ABVD | Anti-PD-1-AVD | p | ||
|---|---|---|---|---|---|
| n | 69 | 30 | |||
| % | Any grade | Grade3-4 | Any grade | Grade3-4 | |
| Leukopenia | 60.9 | 11.6 | 36.7 | 10.0 | 0.075 |
| Neutropenia | 53.6 | 33.3 | 33.4 | 16.7 | 0.145 |
| Thrombocytopenia | 1.5 | 0.0 | 0.0 | 0.0 | 0.697 |
| Anemia | 13 | 1.4 | 11.1 | 0.0 | 1.000 |
| Vomiting | 11.8 | 0 | 3.3 | 0.0 | 0.268 |
| Fever | 10.1 | 2.9 | 13.3 | 0.0 | 0.418 |
| Hypothyroidism | 0.0 | 0.0 | 20.0 | 3.3 | < 0.001 |
| Hyperthyroidism | 0.0 | 0.0 | 10.0 | 0.0 | 0.026 |
| Adrenal Insufficiency | 0.0 | 0.0 | 6.7 | 0.0 | 0.090 |
| Rash | 7.2 | 1.4 | 13.3 | 3.3 | 0.701 |
| Neuropathy | 0.0 | 0.0 | 13.3 | 0.0 | 0.007 |
| ALT or AST increased | 5.8 | 5.9 | 10 | 3.3 | 0.424 |
| Second tumor | 1.4 | 0.0 | 1.000 | ||
Data presented as percentage (%) unless otherwise indicated
Discussion
Therapeutic approaches for classic Hodgkin lymphoma have evolved significantly in recent years, propelled by innovative biological agents, notably the CD30-targeting antibody–drug conjugate brentuximab vedotin and anti-PD1 antibodies. The BV-AVD has challenged the ABVD regimen as the new standard frontline therapy for advanced cHL based on PFS and OS benefits. Although anti-PD1 antibodies have shown promising efficacy in the frontline setting of cHL, the absence of direct comparative data with standard-of-care chemotherapy precludes a comprehensive assessment of their performance. In this study, we reported for the first time a significantly improved CR rate after cycle 2, modified PFS with concurrent administration of anti-PD1 antibody with AVD compared to a matched cohort of patients who received ABVD in untreated early unfavorable and advanced-stage cHL.
The pivotal finding of our study underscores the superiority of concurrent anti-PD1 antibodies with AVD over the conventional ABVD regimen in terms of modified PFS and CR rate at PET2, both before and after compensating with the PSM method. This provides compelling evidence for the clinical adoption of anti-PD1-AVD in the frontline treatment of cHL. Anti-PD1 antibodies such as nivolumab, pembrolizumab, sintilimab and tislelizumab have been evaluated in relapsed/refractory cHL with overall response rates of 69, 72, 80 and 87% and CR rates of 16, 28, 34 and 63%, respectively [23–26]. Notably, sintilimab and tislelizumab are PD-1 inhibitors developed and evaluated in China [26, 27]. Several phase II studies have recently investigated the concomitant or sequential anti-PD1 antibody with chemotherapy for treating newly diagnosed cHL, yielding encouraging efficacy and good tolerability [11–14, 28]. The lack of direct comparative data with conventional standard chemotherapy has hindered the approval of anti-PD1 antibodies for frontline treatment of cHL. Our study makes a significant contribution by demonstrating that anti-PD1 antibody plus AVD is superior to conventional ABVD regimen, filling this critical knowledge gap. Monotherapy with tislelizumab in frontline treatment of early stage cHL has also been reported with a CR rate of 47.8% after 2 cycles [29]. The survival analysis favored anti-PD1-AVD over the ABVD regimen for frontline treatment of cHL. Both modified and traditional PFS were improved in patients treated with anti-PD1-AVD compared to ABVD. After two years of treatment, the PFS curve shows a plateau at 88.5% with PSM in the anti-PD1-AVD arm, indicating a high possibility of cure. The 2-year PFS rate in our study is inferior compared to the result obtained in a previous phase II study evaluating concurrent pembrolizumab with chemotherapy for untreated cHL, which showed a 2-year PFS rate of 97% among the 29 response-evaluable patients [13]. Notably, the single-arm study included early favorable disease (20%).
Recent evidence suggests that PFS serves as a valid surrogate for overall survival (OS) in Hodgkin lymphoma [30]. Given the generally favorable prognosis of Hodgkin lymphoma, evaluating OS after first-line treatment necessitates trials with extended follow-up durations and impractically large patient cohorts to achieve adequate statistical power, owing to the low frequency of OS events. Considering the limitations of our sample size and the impact of salvage therapy, we did not designate OS as a primary endpoint in this retrospective study. Nevertheless, we reported 3-year OS rates for both treatment groups (the median follow-up time was 37.7 months in our study), and as shown in Table 2, no significant difference was observed between the anti-PD1-AVD and ABVD groups. In contrast, there was a significant improvement in 3-year modified PFS.
A concurrent anti-PD1 antibody with chemotherapy induced a rapid and profound response in our study. The CR rate after cycle 2 with anti-PD1-AVD was 86.7% versus 63.8% with PSM. Similar observations align with previous findings, where anti-PD1 antibodies, given concurrently rather than sequentially with chemotherapy, induced faster and more robust responses. In the randomized phase II NIVAHL trial, the interim CR rate was 87% with 2 × N-AVD versus 51% with 4 × nivolumab for early unfavorable cHL [28, 31, 32]. These observations were also supported by the NU16H08 study, which showed concurrent pembrolizumab with AVD yielded a CR rate of 66% after 2 cycles, outperforming 37% with 3 cycles of pembrolizumab alone [13, 14]. The rapid response suggests that concurrent anti-PD1 antibody with chemotherapy is a highly feasible option for untreated cHL. This approach is especially beneficial for patients with bulky or advanced-stage disease, as it may provide rapid tumor shrinkage and relief of disease-related symptoms. Additionally, this concurrent approach avoids the possibility of early immune-related adverse events during anti-PD1 antibody monotherapy that could delay or disrupt the administration of subsequent therapy and potentially allow disease progression. Notably, the ABVD arm exhibited a relatively high PET2 positivity rate of 36.5% (50/137), aligning with the 31% PET2 positivity rate reported in a retrospective study of ABVD-treated patients from European cancer centers [33]. This elevated PET2 positivity rate may be attributed to the presence of mediastinal bulky disease (observed in 9 patients) and extranodal involvement (observed in 24 patients).
The treatment-related adverse events (AEs) observed in the anti-PD1-AVD group of our retrospective study align with the known safety profiles of Nivolumab-AVD and Pembrolizumab-AVD therapies [13, 28, 31]. Compared to these regimens, anti-PD1-AVD demonstrated relatively lower hematologic toxicity but a higher incidence of thyroid dysfunction, potentially due to differences in anti-PD1 antibody types and dosing frequencies. We also explored toxicity variations among different anti-PD1 antibodies combined with AVD. However, the limited sample size precludes definitive conclusions. Notably, no tumor flare reactions were reported in this study. Our study demonstrated that anti-PD1-AVD was better tolerated than ABVD, with less neutropenia. This suggests that anti-PD1-AVD could be applicable for elderly or frail patients. Regarding the concern of anti-PD-1 antibody cytotoxicity with chemotherapy, most studies evaluating anti-PD1 antibodies in frontline treatment of cHL have been conducted with a sequential strategy [11, 14]. Even in the study of concurrent pembrolizumab with chemotherapy for untreated cHL, pembrolizumab was given every 3 weeks, which may cause inconvenience as it required four additional visits throughout 6 cycles [13]. Our study showed that administering the anti-PD1 antibody every 2 weeks was feasible with the chemotherapy.
The study has several potential limitations that warrant careful consideration. First, the non-randomized design and restricted sample size, despite our efforts to mitigate bias through PSM for baseline characteristics, may limit the generalizability of the findings. While our study represents one of the largest real-world cohorts comparing anti-PD1-AVD with ABVD in newly diagnosed cHL patients, the sample size remains relatively modest, particularly in the anti-PD1-AVD subgroup (n = 35). This limitation is shared by other studies in this field, such as the real-world study by Wen et al. [34], which included only 7 patients on anti-PD1-AVD, as well as other pivotal trials with relatively small sample sizes, such as those involving 30, 40 and 51 patients [11, 13, 14, 35]. Second, the anti-PD1-AVD group included four different anti-PD1 antibodies due to variations in drug availability, cost, and physician experience. Unfortunately, the limited case numbers precluded subset analyses to evaluate efficacy and safety across different anti-PD1 antibodies, patients aged ≥ 60, or high-risk IPS subgroups. Third, incomplete medical records, particularly regarding adverse events such as infertility, may have hindered a comprehensive toxicity analysis. These limitations collectively reduced the statistical power and precision of our analyses, necessitating cautious interpretation of the results. Nevertheless, the significant differences in 3-year mPFS and CR rates between the two regimens, along with the alignment of our findings with emerging evidence from other studies [13–15], suggest a strong efficacy signal for anti-PD1-AVD. We fully agree that future studies with larger, randomized cohorts and standardized treatment protocols are needed to validate these findings and further refine the role of immune checkpoint inhibitors in the frontline treatment of cHL.
In conclusion, our matched cohort retrospective study demonstrated the favorable efficacy and acceptable safety profile of anti-PD1-AVD compared to the ABVD regimen. This suggests that anti-PD1-AVD could be a viable choice as frontline therapy for patients with newly untreated early unfavorable and advanced cHL. More prospective studies with long follow-up time are needed to validate our findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are deeply grateful to Sun Yat-sen University Cancer Center for establishing a prestigious platform that offers a rich trove of classic Hodgkin’s lymphoma cases for our retrospective study.
Author contributions
Conceptualization and design were done by Panpan Liu, Zhiming Li, and Yu Wang; methodology was done by Panpan Liu and Mengqiu Wu; acquisition of data was done by all authors; data analysis was done by Mengqiu Wu, Peng Sun, and Baitian Zhao; Writing—original draft was done by Mengqiu Wu, Panpan Liu, and Peng Sun; Study supervision was done by Panpan Liu, Zhiming Li, Yu Wang, Zhongjun Xia, He Huang, Huiqiang Huang, and Qingqing Cai. All authors reviewed the manuscript and approved submitting.
Funding
This work was funded in part by Guangdong Basic and Applied Basic Research Foundation (2023A1515010123) and from the Guangzhou Municipal Science and Technology Program key project (2024B03J1249).
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This retrospective study was approved by the Committee for Ethical Review of Research involving Human Subjects of Sun Yat-Sen University (Approval number: B2023-647–01). Written informed consent was waived in light of the retrospective nature and need to collect and report data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mengqiu Wu, Peng Sun, and Baitian Zhao have contributed equally to this work.
Contributor Information
Yu Wang, Email: wangyu@sysucc.org.cn.
Zhiming Li, Email: lizhm@sysucc.org.cn.
Panpan Liu, Email: liupp@sysucc.org.cn.
References
- 1.Brice P, de Kerviler E, Friedberg JW (2021) Classical Hodgkin lymphoma. Lancet (London, England) 398(10310):1518–1527 [DOI] [PubMed] [Google Scholar]
- 2.Johnson P, Federico M, Kirkwood A, Fosså A, Berkahn L, Carella A et al (2016) Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med 374(25):2419–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carde P, Karrasch M, Fortpied C, Brice P, Khaled H, Casasnovas O et al (2016) Eight cycles of ABVD versus four cycles of BEACOPPescalated plus four cycles of BEACOPPbaseline in stage III to IV, international prognostic score ≥ 3, high-risk Hodgkin lymphoma: first results of the phase III EORTC 20012 intergroup trial. J Clin Oncol: Off J Am Soc Clin Oncol 34(17):2028–2036 [DOI] [PubMed] [Google Scholar]
- 4.Engert A (2016) ABVD or BEACOPP for advanced Hodgkin lymphoma. J Clin Oncol: Off J Am Soc Clin Oncol 34(11):1167–1169 [DOI] [PubMed] [Google Scholar]
- 5.Gillessen S, Plütschow A, Fuchs M, Markova J, Greil R, Topp MS et al (2021) Intensified treatment of patients with early stage, unfavourable Hodgkin lymphoma: long-term follow-up of a randomised, international phase 3 trial of the German Hodgkin Study Group (GHSG HD14). Lancet Haematol 8(4):e278–e288 [DOI] [PubMed] [Google Scholar]
- 6.Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A et al (2018) Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med 378(4):331–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straus DJ, Długosz-Danecka M, Alekseev S, Illés Á, Picardi M, Lech-Maranda E et al (2020) Brentuximab vedotin with chemotherapy for stage III/IV classical Hodgkin lymphoma: 3-year update of the ECHELON-1 study. Blood 135(10):735–742 [DOI] [PubMed] [Google Scholar]
- 8.Straus DJ, Długosz-Danecka M, Connors JM, Alekseev S, Illés Á, Picardi M et al (2021) Brentuximab vedotin with chemotherapy for stage III or IV classical Hodgkin lymphoma (ECHELON-1): 5-year update of an international, open-label, randomised, phase 3 trial. Lancet Haematol 8(6):e410–e421 [DOI] [PubMed] [Google Scholar]
- 9.Ansell SM, Radford J, Connors JM, Długosz-Danecka M, Kim WS, Gallamini A et al (2022) Overall survival with brentuximab vedotin in stage III or IV Hodgkin’s lymphoma. N Engl J Med 387(4):310–320 [DOI] [PubMed] [Google Scholar]
- 10.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Hodgkin Lymphoma. Version 3.2024.
- 11.Ramchandren R, Domingo-Domènech E, Rueda A, Trněný M, Feldman TA, Lee HJ et al (2019) Nivolumab for newly diagnosed advanced-stage classic Hodgkin lymphoma: safety and efficacy in the phase II checkmate 205 study. J Clin Oncol: Off J Am Soc Clin Oncol 37(23):1997–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen PB, Lu X, Chen Q, O’Shea K, Chmiel JS, Slonim LB et al (2023) Sequential pembrolizumab and AVD are highly effective at any PD-L1 expression level in untreated Hodgkin lymphoma. Blood Adv 7(12):2670–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch RC, Ujjani CS, Poh C, Warren EH, Smith SD, Shadman M et al (2023) Concurrent pembrolizumab with AVD for untreated classic Hodgkin lymphoma. Blood 141(21):2576–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen PB, Savas H, Evens AM, Advani RH, Palmer B, Pro B et al (2021) Pembrolizumab followed by AVD in untreated early unfavorable and advanced-stage classical Hodgkin lymphoma. Blood 137(10):1318–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrera AF, LeBlanc ML, Castellino SM, Li H, Rutherford SC, Evens AM, et al. (2023) SWOG S1826, a randomized study of nivolumab(N)-AVD versus brentuximab vedotin(BV)-AVD in advanced stage (AS) classic Hodgkin lymphoma (HL). Journal of Clinical Oncology. 41(17_suppl):LBA4-LBA.
- 16.Herrera AF, LeBlanc M, Castellino SM, Li H, Rutherford SC, Evens AM et al (2024) Nivolumab+AVD in advanced-stage classic Hodgkin’s lymphoma. N Engl J Med 391(15):1379–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol: Off J Am Soc Clin Oncol 32(27):3059–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zlotta AR, Ballas LK, Niemierko A, Lajkosz K, Kuk C, Miranda G et al (2023) Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. Lancet Oncol 24(6):669–681 [DOI] [PubMed] [Google Scholar]
- 19.Gerritsen JKW, Zwarthoed RH, Kilgallon JL, Nawabi NL, Jessurun CAC, Versyck G et al (2022) Effect of awake craniotomy in glioblastoma in eloquent areas (GLIOMAP): a propensity score-matched analysis of an international, multicentre, cohort study. Lancet Oncol 23(6):802–817 [DOI] [PubMed] [Google Scholar]
- 20.Stuart EA (2010) Matching methods for causal inference: a review and a look forward. Stat Sci: Rev J Inst Math Stat 25(1):1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iacoboni G, Navarro V, Martín-López A, Rejeski K, Kwon M, Jalowiec KA et al (2024) Recent bendamustine treatment before apheresis has a negative impact on outcomes in patients with large B-cell lymphoma receiving chimeric antigen receptor T-cell therapy. J Clin Oncol: Off J Am Soc Clin Oncol 42(2):205–217 [DOI] [PubMed] [Google Scholar]
- 22.Hong Z, Xu J, Chen Z, Xu H, Huang Z, Weng K et al (2023) Additional neoadjuvant immunotherapy does not increase the risk of anastomotic leakage after esophagectomy for esophageal squamous cell carcinoma: a multicenter retrospective cohort study. Int J Surg (London, England) 109(8):2168–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL et al (2018) Nivolumab for relapsed/refractory classic hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II checkmate 205 trial. J Clin Oncol: Off J Am Soc Clin Oncol 36(14):1428–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen R, Zinzani PL, Lee HJ, Armand P, Johnson NA, Brice P et al (2019) Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood 134(14):1144–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y, Su H, Song Y, Jiang W, Sun X, Qian W et al (2019) Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. Lancet Haematol 6(1):e12–e19 [DOI] [PubMed] [Google Scholar]
- 26.Song Y, Gao Q, Zhang H, Fan L, Zhou J, Zou D et al (2020) Treatment of relapsed or refractory classical Hodgkin lymphoma with the anti-PD-1, tislelizumab: results of a phase 2, single-arm, multicenter study. Leukemia 34(2):533–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Y, Gao Q, Zhang H, Fan L, Zhou J, Zou D et al (2022) Tislelizumab for relapsed/refractory classical Hodgkin lymphoma: 3-year follow-up and correlative biomarker analysis. Clin Cancer Res: Off J Am Assoc Cancer Res 28(6):1147–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bröckelmann PJ, Bühnen I, Meissner J, Trautmann-Grill K, Herhaus P, Halbsguth TV et al (2023) Nivolumab and doxorubicin, vinblastine, and dacarbazine in early-stage unfavorable hodgkin lymphoma: final analysis of the randomized German Hodgkin study group phase II NIVAHL trial. J Clin Oncol: Off J Am Soc Clin Oncol 41(6):1193–1199 [DOI] [PubMed] [Google Scholar]
- 29.Sun P, Yang H, Wang Y, Zhao B, Nie M, Huang K et al (2024) Tislelizumab monotherapy in patients with previously untreated early-stage classical Hodgkin lymphoma: a real-world study. Ann Hematol 103(3):793–801 [DOI] [PubMed] [Google Scholar]
- 30.Bröckelmann PJ, Müller H, Fuchs M, Gillessen S, Eichenauer DA, Borchmann S, Jacob AS, Behringer K, Momotow J, Ferdinandus J, Böll B (2025) Correlation between progression-free and overall survival in patients with Hodgkin lymphoma: a comprehensive analysis of individual patient data from randomized German Hodgkin study group (GHSG) trials. Ann Oncol 36(4):393–402 [DOI] [PubMed] [Google Scholar]
- 31.Bröckelmann PJ, Goergen H, Keller U, Meissner J, Ordemann R, Halbsguth TV et al (2020) Efficacy of nivolumab and AVD in early-stage unfavorable classic hodgkin lymphoma: the randomized phase 2 German Hodgkin study group NIVAHL trial. JAMA Oncol 6(6):872–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voltin CA, Mettler J, van Heek L, Goergen H, Müller H, Baues C et al (2021) Early response to first-line anti-PD-1 treatment in hodgkin lymphoma: a PET-based analysis from the prospective, randomized phase II NIVAHL trial. Clin Cancer Res: Off J Am Assoc Cancer Res 27(2):402–407 [DOI] [PubMed] [Google Scholar]
- 33.Mondello P, Musolino C, Dogliotti I, Bohn JP, Cavallo F, Ferrero S et al (2020) ABVD vs BEACOPP escalated in advanced-stage Hodgkin’s lymphoma: results from a multicenter European study. Am J Hematol 95(9):1030–1037 [DOI] [PubMed] [Google Scholar]
- 34.Wen Q, Ge J, Lei Y, Zhang Y, Kong X, Wang W et al (2023) Real-world evidence of ABVD-like regimens compared with ABVD in classical Hodgkin lymphoma: a 10-year study from China. J Cancer Res Clin Oncol 149(7):3989–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torka P, Feldman T, Savage KJ, Ganesan N, Drill E, Hancock H et al (2025) Phase II trial of nivolumab plus doxorubicin, vinblastine, dacarbazine as frontline therapy in older adults with hodgkin lymphoma. J Clin Oncol: Off J Am Soc Clin Oncol 43(8):985–993 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.