Abstract
BACKGROUND
Elevated levels of tropospheric ozone (O3) pose a significant threat to plant health and productivity. Developing ozone‐tolerant varieties is crucial for mitigating these environmental stresses. This study investigates the effects of ascorbic acid (AA) and silicic acid (SA) treatments on 12 different mung bean varieties under elevated O3 conditions.
RESULTS
A controlled pot experiment was conducted with four treatments: ambient O3 (40–45 ppb), elevated O3 (120 ppb), elevated O3 with silicic acid (0.1 mmol L−1), and high O3 with ascorbic acid (10 mmol L−1). High O3 stress negatively impacted growth attributes across all mung bean cultivars. However, both AA and SA treatments significantly alleviated O3‐induced growth reductions. Under O3 stress, osmotic potential, water potential, relative water content, turgor potential, sugars, pod number, amino acids, 100‐seed weight, and grain carbohydrates all decreased. In contrast, antioxidant enzymes (ascorbate peroxidase, peroxidase, catalase, and superoxide dismutase), flavonoids, tannins, and grain protein content increased.
CONCLUSION
The NIAB Mung 20‐21, NIAB Mung 2006, and NIAB Mung varieties exhibited O3 resistance. Silicic acid proved to be more effective than ascorbic acid in mitigating O3 damage, though a combination of both treatments was the most beneficial for enhancing plant resilience under elevated O3 conditions. © 2025 The Author(s). Journal of the Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: plant biomass, tropospheric ozone stress, plant growth regulators, mung bean
INTRODUCTION
In the context of increased food demand due to an ever‐increasing population, environmental factors that affect plant development and growth are a major source of concern worldwide. The effects of biotic and abiotic stresses on crop productivity are extremely harmful. For a long time, agricultural science specialists have been concerned about the constancy of crop output and how it develops in harsh environments, including elevated levels of ozone (O3) in the troposphere. 1 Volatile organic molecules generated by industry embrace the potential to contribute to O3 pollution. During summer, when the temperature is high and UV exposure is significant, they play a substantial role in atmospheric photochemical reactions, producing O3 explosions close to the surface. 2 Surface ozone poses a threat to ecosystems and has a negative effect on the physiology, development, and sustainability of plants. Reactive oxygen species (ROS) are produced when this oxidative pollutant enters a plant through open stomata and initiates a series of processes inside the plant cells. 3 , 4 , 5
ROS and the products of their reactions damage cell membranes and interfere with the metabolism of carbohydrates, lipids, nucleic acids, and proteins, according to numerous studies. Agricultural yield is reduced because it alters the structural characteristics of leaves and eventually causes cell malfunction or death. 6 Enzymatic (superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and peroxidase (POD)) and non‐enzymatic antioxidants (ascorbic acid, tocopherol, glutathione, flavonoids, and carotenoids), which are the primary components of the plant's natural defense system, are among the compounds that plants produce as a line of defense under stress conditions. 7 , 8 In emerging countries like Pakistan, Indonesia, and India, as well as in the peri‐urban and urban regions of major Asian cities, elevated tropospheric O3 concentration has been rising repeatedly. Due to ambient air pollution, Pakistan's agricultural production appears to be significantly declining. 9 As background O3 concentration has increased globally, scientists have rigorously monitored the impact of rising O3 concentration on diverse agricultural plant species in the subtropical zone. 10
As an unimpeachable source of protein, which is crucial for human growth and development, mung bean (Vigna radiata L.) is a better choice for examination of O3 effects, 11 as it is high in bioactive components such as peptides and polysaccharides 1 and it is included in crops that are most sensitive to ozone (in terms of yield) like wheat, peas, beans, and onion. 12 Mung bean production was 122.100 thousand tons in 2018 13 and its production is estimated to be 1.43 million tonnes from 198 000 ha for rabi season 2023–2024 (Federal Committee on Agriculture, Dawn, October 12, 2023). Pakistan was South Asia's second most renowned producer of mung bean in 2009–2010. Numerous biological and physiological characteristics like seed yield, harvest index, days to maturity, and characteristics related to gas exchange such as transpiration rate, stomatal conductance, net photosynthetic activity, and water‐use efficiency of different crops, including mung bean, have been affected by the high levels of tropospheric O3 observed in Pakistan's suburban agricultural regions. 14 , 15 Numerous studies have demonstrated the importance of micronutrients in supporting plants to tolerate various stresses. 16 One such nutrient – silicic acid, a silicon derivative – is rising in popularity 17 because of its ability to increase resistance to both biotic and abiotic stresses in many plants such as cucumbers 18 and rice. 19 Silicic acid, when used sparingly as a foliar spray, boosts crop yield under stress. 20 While ascorbic acid represents a ROS scavenger, it improves the plants' oxidative defense capability, resulting in more massive development and growth under stress conditions. 21 The present study was aimed at evaluating the effectiveness of silicic and ascorbic acid foliar applications in mitigating the detrimental impacts of increased O3 on mung bean growth, biochemical attributes, water relation attributes, antioxidant enzymes, and yield.
MATERIALS AND METHODS
Seeds of 12 mung bean varieties (V1 = NM‐28, V2 = NM‐13‐1, V3 = NM‐19‐19, V4 = NM‐20‐21, V5 = NM‐121‐25, V6 = NM‐51, V7 = NM‐54, V8 = NM‐92, V9 = NM‐98, V10 = NM‐2006, V11 = NM‐2011, and V12 = NM‐2016) with diverse parentages used in this investigation were collected from the Nuclear Institute for Agriculture and Biology (NIAB).
The experiment was carried out from April 2021 to July 2021, and seeds were sown under controlled conditions in a glasshouse. The experiment was conducted in an environment with an average temperature of 30 °C and a relative humidity of 49%. The seeds were planted in soil‐filled plastic pots with four treatments and three replicates. Ascorbic acid (10 mmol L−1) and silicic acid (0.1 mmol L−1) (selected from pretrial experiment, data not published) were sprinkled on the plants 3 weeks after germination for 1 week, together with Tween‐20 (0.1%) as a surfactant. A pressure pump sprayer bottle was used for this purpose. The elevated ozone levels were exposed to plants (120 ppb) for 4 h per day, except for control (O3 untreated). The O3 generator (AOT‐MD‐500 model, AQUAPURE (Shenzhen) Ozone Technology Co., LTD) was operated for O3 generation. Monitoring was performed using a UV photometry O3 analyzer (model 0342e). The elevated O3 level was maintained for 15 days. Normal air was considered as the control treatment. After 1 week of O3 treatment, leaf samples were collected for estimation of growth and biochemical attributes. Grain quality attributes were studied at maturity.
Number of leaves
The total number of leaves on each plant were counted manually.
Determination of total leaf area (cm2)
The total leaf area was determined using the following formula: 22
where 0.75 is the correction factor.
Shoot and root
Mung bean plants were measured individually for growth characteristics such as shoot and root length.
Enzymatic antioxidant activity
Leaf samples were collected to estimate enzymatic antioxidant activity. SOD was evaluated using the method executed by Giannopolitis and Ries, 23 and the reading was recorded at 560 nm. The enzyme extract was processed according to the Chance and Maehly 24 method, and the activity of CAT enzyme was determined at 240 nm. The mixture of reactions was prepared according to the methodology of Chance and Maehly, 24 and the absorbance for POD efficiency was measured at 470 nm. The activity of APX was assessed using the methodology of Asada. 25 Enzyme activity was detected by a drop in absorbance at 290 nm and represented as units/g F.wt.
Plant–water relationship attributes
Leaf water potential (Ψw)
From each treatment, fully expanded and young second leaves from the top of the plants were utilized to evaluate leaf water potential. From 8 a.m. until 10 a.m., measurements were obtained using a Scholander‐type pressure chamber.
Leaf osmotic potential (Ψs)
The leaf that was used to determine Ψw was also used to determine the osmotic potential, as described by Hussain et al. 26 Leaf samples were frozen at 20 °C and then thawed and crushed with a glass rod to collect cell sap. The sap was then sucked using a disposable syringe and was immediately measured with an osmometer (Wescor 5500, Champaign, Illinois) for Ψs determination.
Turgor potential (Ψp)
The difference between Ψw and Ψs values was used to compute the turgor potential as reported by Hussain et al: 26
Relative water content
Three plants from each treatment were sampled for flag leaf samples. Each sample was weighed (w F) and stored for 24 h in a test tube containing distilled water. Tissue paper was then wiped over and used for its turgid weight (w T). The samples were dried for 72 h at 65 °C, after which their dry weight (w D) was calculated. Relative water content (RWC) was calculated for each treatment using the following formula: 27
Reducing and non‐reducing sugars (sugar content)
Dinitrosalicylic acid was used to measure the total reducing sugars in the plant (Miller 1959). Total soluble sugars were measured following. 28 The difference between soluble and reducing sugars was used to compute non‐reducing sugars.
Total flavonoid content
A colorimetric approach was implemented to determine the analysis, with quercetin serving as the standard. 29 10 g leaf tissue was used to make 200 μL filtrate, which was prepared using 95% methanol extract and 40 mmol L−1 phosphate buffer (pH 6.8). After adding 50 μL AlCl2 (10%) and 1 mol L−1 potassium acetate (50 μL) the mixture was incubated for 40 min at room temperature before absorbance at 415 nm.
Tannin
After keeping 0.5 g leaf sample in 95% methanol for 48 h in the dark, the supernatant was collected and mixed with 150 μL of 100% Folin–Ciocâlteu reagent and 1.2 mL of 700 mmol L −1 sodium carbonate. This mixture was allowed to cool for 1 h at room temperature. 30 Then, in the above‐prepared sample, 0.1 g polyvinylpolypyrrolidone was added; the mixture was vortexed and centrifuged at 14 000 × g, and the absorbance was measured at 765 nm.
Number of pods and 100‐seed weight
The mean number of pods per plant was calculated after counting the number of pods from ten random plants of each variety from each replicate. The 100‐seed average weight was recorded using a digital weight balance after taking 100 seeds from ten random plants of each replicate. 15
Grain quality attributes
Grain protein
The micro‐Kjeldhal technique was used to quantify nitrogen of grains as described by Bremner. 31 In order to determine the quantity of grain protein (GP), the nitrogen percentage was multiplied by a factor of 6.25.
Grain carbohydrates
Determination of starch was performed according to the method of Malik and Srivastava. 32 1 mL of the extract was placed in a 25 mL test tube, followed by 10 mL anthrone solution. It was heated for 12 min in boiling water, then cooled and measured absorbance at 625 nm.
Free amino acids
Hamilton's 33 method was used to determine total free amino acids (FAA). For this, 1 mL of the extract used in the antioxidant enzyme activity was combined with 1 mL ninhydrin and 1 mL pyridine. The mixture was heated at 95 °C for 35 min and absorbance was measured spectrophotometrically at 570 nm.
Statistical analysis
Each experimental unit had three replications during split‐plot analysis. Statistical analysis of the obtained data was performed using Co‐Stat version 6.4.5.1 (2003; Cohorts Software, Monterey, CA, USA) to assess the significance of differences among mean values. The correlations and principal component analysis of the mean values of all variables were performed using R Studio.
RESULTS
The application of O3 stress (120 ppb) resulted in a significant reduction in the shoot and root lengths of all mung bean varieties. However, because of genetic diversity among varieties, there was a difference in the rate of decrease among them. Foliar treatment with ascorbic acid significantly (P ≤ 0.001) increased the shoot and root lengths of the varieties NIAB Mung‐28 and NIAB Mung‐98. Silicic acid application (0.1 mmol L−1) as a foliar spray significantly (P ≤ 0.001) increased shoot and root length under O3 stress conditions in NIAB Mung‐28, followed by NIAB Mung‐98. It was found that NIAB Mung‐2011 was less affected by silicic acid foliar application (Table 1 and Fig. 1).
Table 1.
Data from analysis of variance (ANOVA) demonstrating changes in root length, shoot length, leaf area, number of leaver, number of pods per plant, and seed weight by foliar spray of silicic acid and ascorbic acid on 12 mung bean varieties under elevated ozone stress and non‐stress conditions
| Root length (cm) | Shoot length (cm) | Leaf area (cm2) | No. of leaves per plant | No. of pods per plant | 100‐Seed weight (g) | ||
|---|---|---|---|---|---|---|---|
| Main effects | df | MS | MS | MS | MS | MS | MS |
| Variety (V) | 11 | 60.08*** | 597.69*** | 280.00*** | 121.52*** | 629.39*** | 1.043*** |
| Treatment (T) | 5 | 96.95*** | 718.21*** | 777.14*** | 319.45*** | 879.93*** | 15.10*** |
| V × T | 55 | 1.64*** | 14.88*** | 11.34*** | 9.21*** | 16.21*** | 0.29*** |
| Error | 144 | 0.99 | 2.37 | 3.26 | 1.99 | 1.78 | 0.21 |
| Total | 215 | ||||||
Abbreviation: df, degree of freedom; MS, Mean square values.
***highly significant at P≤0.001.
Figure 1.

Effects of applying ascorbic acid and silicic acid on mung bean varieties under elevated level of O3. Root length (A), shoot length (B), leaf area (C), number of leaves (D), 100‐seed weight (E), and number of pods per plant (F). T1 = ambient ozone level; T2 = control + silicic acid + ascorbic acid; T3 = elevated ozone level 120 ppb; T4 = elevated ozone level + silicic acid; T5 = elevated ozone level + ascorbic acid; T6 = elevated ozone level + silicic acid + ascorbic acid.
Both the number of leaves and the leaf area per plant considerably decreased (P ≤ 0.01) at elevated O3 levels and NIAB Mung‐98 exhibited the maximum decline in the said attributes. In this study, foliar treatments with silicic and ascorbic acids on the NIAB Mung 13‐1 under O3 stress were more effective (Table 1 and Fig. 1).
Elevated O3 significantly decreased (P ≤ 0.001) the weight of 100 seeds and the number of pods per plant. This drastic decline in yield was observed in the mung bean varieties NIAB Mung 13‐1 and NIAB Mung‐51. The varieties NIAB Mung‐51 and NIAB Mung 13‐1 treated with ascorbic acid and silicic acid produced a greater number of pods per plant, and 100‐seed weight increased in NIAB Mung‐51 and NIAB Mung‐28 as compared with the untreated control (Table 1 and Fig. 1).
Results of the current study revealed that elevated O3 significantly (P ≤ 0.001) increased the activities of SOD, APX, CAT, and POD in mung bean varieties under investigation. Ascorbic acid treatment significantly improved (P ≤ 0.001) activities of different antioxidant enzymes, namely SOD (NIAB Mung 13‐1), POD (NIAB Mung‐98), CAT (NIAB Mung‐51), and APX (NIAB Mung 13‐1) in different varieties. Silicic acid application also augmented the activities of enzymatic antioxidants, namely SOD (NIAB Mung‐28), POD (NIAB Mung‐51), CAT (NIAB Mung‐51), and APX (NIAB Mung‐98) in the different varieties under investigation (Table 2 and Fig. 2).
Table 2.
Analysis of variance (ANOVA) data demonstrating changes in catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), superoxide dismutase (SOD), osmotic potential (OP), water potential (WP), turgor potential (TP), and relative water content (RWC) by foliar spraying of silicic acid and ascorbic acid on 12 mung bean varieties under elevated O3 stress and non‐stress conditions
| CAT | POD | APX | SOD | OP (−MPa) | WP (−MPa) | TP (MPa) | RWC (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Main effects | df | MS | MS | MS | MS | MS | MS | MS | MS |
| Variety (V) | 11 | 495 454.3 *** | 29 946 483*** | 147 997.9 *** | 7292.742*** | 0.78317*** | 1.27230*** | 0.675469*** | 307.8903*** |
| Treatment (T) | 5 | 788 076.7*** | 3.08812e8*** | 1 412 117.4*** | 28 511.40*** | 1.28143*** | 1.02560*** | 0.607309*** | 4350.770*** |
| V × T | 55 | 13 211.39*** | 5 559 703*** | 18 890.636*** | 638.0869*** | 0.02299*** | 0.02798*** | 0.037693*** | 52.20175*** |
| Error | 144 | 1453.643 | 352 197.6 | 1397.6713 | 22.10572 | 0.00318 | 0.00210 | 0.00125 | 57.48307 |
| Total | 215 | ||||||||
Abbreviation: df, degree of freedom; MS, Mean square values.
***highly significant at P≤0.001.
Figure 2.

Effects of applying ascorbic acid and silicic acid on mung bean varieties under elevated level of O3. CAT (A), POD (B), APX (C), SOD (D), osmotic potential (E), water potential (F), turgor potential (G), and relative water content (H). T1 = ambient ozone level; T2 = control + silicic acid + ascorbic acid; T3 = elevated ozone level 120 ppb; T4 = elevated ozone level + silicic acid; T5 = elevated ozone level + ascorbic acid; T6 = elevated ozone level + silicic acid + ascorbic acid.
Elevated O3 stress caused a significant (P ≤ 0.001) decrease in the water potential of mung bean varieties. A significant (P ≤ 0.001) difference among all varieties was found regarding water potential under elevated O3 stress. The variety NIAB Mung‐51 had a greater reduction in water potential, but the variety NIAB Mung‐2016 was found to be superior in this regard. This variable was significantly influenced (P ≤ 0.001) by foliar treatment with both ascorbic and silicic acids. Plants treated with 10 mmol L−1 ascorbic acid and 0.1 mmol L−1 silicic acid under O3 stress, for example, generated several‐fold increases in water potential of NIAB Mung 13‐1 and NIAB Mung‐51, respectively (Table 2 and Fig. 2). The results revealed a significant (P ≤ 0.001) reduction in the osmotic potential of mung bean varieties. NIAB Mung‐51 demonstrated a concentration‐dependent decrease in osmotic potential under O3 stress conditions.
Exogenous foliar treatments had a significant (P ≤ 0.001) effect on the osmotic potential of mung bean plants exposed to elevated levels of O3. Ascorbic and silicic acids displayed several‐fold increases in osmotic potential in NIAB Mung‐98 (Table 2 and Fig. 2). The turgor potential dropped even more markedly (P ≤ 0.001) in NIAB Mung‐98 under O3 stress. Foliar treatment with ascorbic and silicic acids significantly enhanced turgor potential in NIAB Mung‐98 and NIAB Mung 13‐1, respectively. Under O3 stress, the overall RWC exhibited a non‐significant outcome, whereas NIAB Mung 13‐1 showed a greater reduction. Exogenous applications of ascorbic and silicic acid significantly increased the RWC of NIAB Mung‐92 and NIAB Mung‐28 under O3 stress (Table 2 and Fig. 2).
The findings of this study demonstrated that both reducing and non‐reducing sugars decreased significantly (P ≤ 0.001). The elevated O3 level (120 ppb) caused a drastic decrease in both reducing and non‐reducing sugars in NIAB Mung‐98 and NIAB Mung‐28. In varieties NIAB Mung‐51 and NIAB Mung 13‐1, the foliar treatment with ascorbic and silicic acid resulted in a significant increase (P ≤ 0.001) in reducing and non‐reducing sugars under O3 stress (Table 3 and Fig. 3).
Table 3.
Analysis of variance (ANOVA) demonstrating changes in reducing and non‐reducing sugar, total flavonoids, and tannin by foliar spray of silicic acid and ascorbic acid on 12 mung bean varieties under elevated O3 stress and non‐stress conditions
| Reducing sugar (mg g−1 FW) | Non‐reducing sugar (mg g−1 FW) | Total Flavonoids | Tannin (μmol L−1 g−1 FW) | ||
|---|---|---|---|---|---|
| Main effects | df | MS | MS | MS | MS |
| Variety (V) | 11 | 4303.84*** | 1520.10*** | 61 569.20*** | 1.22698e9*** |
| Treatment (T) | 5 | 4516.28*** | 733.36*** | 32 807.70*** | 2.70105e8*** |
| V × T | 55 | 205.89*** | 42.50*** | 2449.80*** | 17 458 148*** |
| Error | 144 | 15.30 | 2.15 | 349.99*** | 1 300 231.1*** |
| Total | 215 | ||||
Abbreviation: df, degree of freedom; FW, fresh weight; MS, Mean square values.
***highly significant at P≤0.001.
Figure 3.
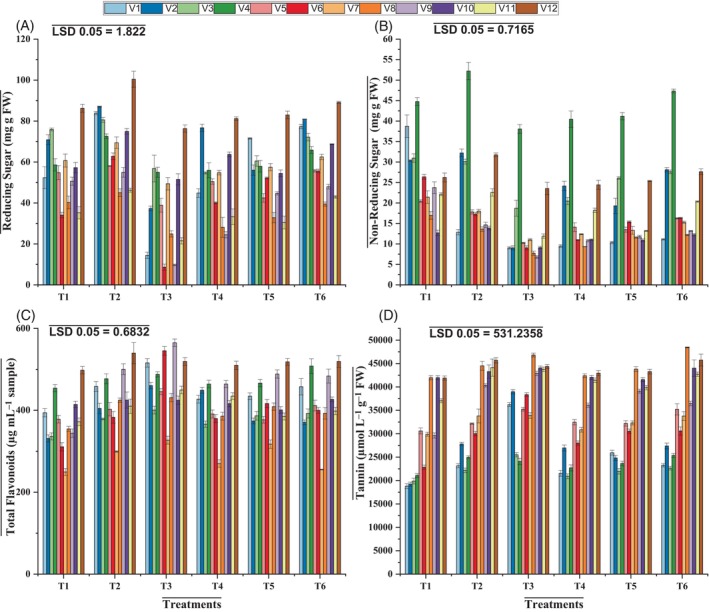
Effects of applying ascorbic acid and silicic acid on reducing sugar (A), non‐reducing sugar (B), total flavonoids (C), and tannin (D) in mung bean varieties under elevated O3. T1 = ambient ozone level; T2 = control + silicic acid + ascorbic acid; T3 = elevated ozone level 120 ppb; T4 = elevated ozone level + silicic acid; T5 = elevated ozone level + ascorbic acid; T6 = elevated ozone level + silicic acid + ascorbic acid.
Total flavonoid (TF) and tannin contents in all mung bean varieties significantly increased (P ≤ 0.001) under elevated O3 levels. Ascorbic and silicic acid applied topically significantly (P ≤ 0.001) boosted the flavonoids and tannin levels. Overall, NIAB Mung‐51 was more sensitive to O3 stress for flavonoid content and NIAB Mung 13‐1 for tannin content. Application of ascorbic acid proved to be more effective in NIAB Mung 13‐1 for flavonoids and tannins, whereas silicic acid showed a more positive response in NIAB Mung‐98 and NIAB Mung‐28 for flavonoid and tannin contents (Table 3 and Fig. 3).
Elevated levels of O3 significantly (P ≤ 0.001) enhanced GP in all varieties, particularly in the variety NIAB Mung‐51, whereas both the treatments used in this study significantly (P ≤ 0.001) boosted GP in NIAB Mung 13‐1 and NIAB Mung‐51. O3 treatment had a detrimental effect (P ≤ 0.001) on grain carbohydrates in mung bean varieties. Both the applied treatments significantly (P ≤ 0.001) enhanced grain carbohydrates in NIAB Mung‐51. A significant (P ≤ 0.001) decrease in FAA of mung bean plants was observed under elevated levels of O3. NIAB Mung‐98 showed the maximum reduction in FAA under O3 stress. Treatments with foliar ascorbic and silicic acids increased maximal FAA in NIAB Mung 13‐1 and NIAB Mung‐98 (Tables 4 and 5). Principal component analysis showed that positive correlations were present among POD, GP, TF, SOD, APX, CAT, number of leaves, and tannin, but a negative correlation of these attributes was found with shoot length, root length, leaf area, number of pods per plant, reducing sugar, non‐reducing sugar, FAA, grain carbohydrates, water potential, osmotic potential, turgor potential, RWC, and 100‐seed weight (Fig. 4). However, POD, GP, TF, SOD, and APX are closely positively correlated, whereas shoot length is closely correlated with root length, leaf area, number of pods per plant, reducing sugar, non‐reducing sugar, FAA, and osmotic potential. Among the extracted components, the major contribution was Dim1 (39.3%), followed by Dim2 (14%), with a cumulative contribution of 53.3%. The same trend is depicted in the Pearson correlation plot (Fig. 5).
Table 4.
Analysis of variance (ANOVA) demonstrating changes in grain protein (GP), grain carbohydrates (GC), and free amino acids (FAA) by foliar spraying of ascorbic acid and silicic acid on 12 mung bean varieties under elevated O3 stress and non‐stress conditions
| GP | GC (mg g−1) | FAA (mg g−1) | ||
|---|---|---|---|---|
| Main effects | df | MS | MS | MS |
| Variety (V) | 11 | 13.43*** | 9.40*** | 0.26*** |
| Treatment (T) | 5 | 144.20*** | 0.00*** | 0.27*** |
| V × T | 55 | 2.53*** | 4.48*** | 0.02*** |
| Error | 144 | 0.36 | 1.07 | 9.37 |
| Total | 215 |
Abbreviation: df, degree of freedom; MS, Mean square values.
***highly significant at P≤0.001.
Table 5.
Effect of elevated O3 levels (120 ppb) on grain proteins, grain carbohydrates, and free amino acid content of mung bean varieties after foliar spraying with ascorbic acid and silicic acid
| Grain protein | Grain carbohydrates (mg g−1) | Free amino acid (mg g−1) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T1 | T2 | T3 | T4 | T5 | T6 | T1 | T2 | T3 | T4 | T5 | T6 | |
| NIAB Mung‐28 | 10.02 ± 0.32 | 11.87 ± 0.21 | 4.23 ± 0.27 | 8.56 ± 0.19 | 9.29 ± 0.49 | 10.88 ± 0.23 | 0.07 ± 0.001 | 0.10 ± 0.00 | 0.08 ± 0.00 | 0.09 ± 0.00 | 0.08 ± 0.00 | 0.09 ± 0.00 | 0.54 ± 0.01 | 0.54 ± 0.01 | 0.35 ± 0.01 | 0.44 ± 0.01 | 0.37 ± 0.01676 | 0.47 ± 0.01117 |
| NIAB Mung‐13‐1 | 9.63 ± 0.42 | 12.57 ± 0.47 | 3.29 ± 0.43 | 9.27 ± 0.60 | 8.04 ± 0.55 | 11.12 ± 0.22 | 0.07 ± 0.00 | 0.10 ± 0.00 | 0.07 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.09 ± 0.00 | 0.51 ± 0.02 | 0.75 ± 0.01 | 0.57 ± 0.02 | 0.62 ± 0.02 | 0.68 ± 0.01076 | 0.7 ± 0.00806 |
| NIAB Mung‐19‐19 | 10.77 ± 0.13 | 9.35 ± 0.22 | 5.75 ± 0.22 | 6.44 ± 0.38 | 7.02 ± 0.51 | 8.28 ± 0.22 | 0.07 ± 0.00 | 0.09 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.47 ± 0.02 | 0.82 ± 0.01 | 0.63 ± 0.02 | 0.65 ± 0.00 | 0.69 ± 0.00461 | 0.72 ± 0.01936 |
| NIAB Mung‐20‐21 | 10.65 ± 0.38 | 12.347 ± 0.451 | 9.02 ± 0.33 | 9.35 ± 0.63 | 9.71 ± 0.20 | 11.16 ± 0.11 | 0.08 ± 0.0008 | 0.09 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.08 ± 0.00 | 0.67 ± 0.02 | 0.69 ± 0.02 | 0.47 ± 0.01 | 0.53 ± 0.03 | 0.56 ± 0.01023 | 0.61 ± 0.01505 |
| NIAB Mung‐121‐25 | 11.08 ± 0.34 | 11.32 ± 0.07 | 5.56 ± 0.36 | 8.73 ± 0.36 | 7.42 ± 0.25 | 9.92 ± 0.24 | 0.07 ± 0.00 | 0.09 ± 0.00 | 0.07 ± 0.0 | 0.08 ± 0.00 | 0.07 ± 0.00 | 0.08 ± 0.00 | 0.39 ± 0.02 | 0.73 ± 0.01 | 0.53 ± 0.01 | 0.61 ± 0.02 | 0.58 ± 0.00869 | 0.69 ± 0.00164 |
| NIAB Mung‐51 | 10.12 ± 0.18 | 10.12 ± 0.29 | 3.04 ± 0.35 | 7.73 ± 0.27 | 8.42 ± 0.23 | 9.25 ± 0.06 | 0.07 ± 0.00 | 0.09 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.61 ± 0.01 | 0.82 ± 0.01 | 0.61 ± 0.01 | 0.63 ± 0.01 | 0.68 ± 0.00919 | 0.71 ± 0.0087 |
| NIAB Mung‐54 | 11.15 ± 0.36 | 12.05 ± 0.24 | 6.50 ± 0.34 | 8.79 ± 0.36 | 10.08 ± 0.22 | 10.95 ± 0.22 | 0.06 ± 0.00 | 0.09 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.06 ± 0.00 | 0.08 ± 0.00 | 0.32 ± 0.00 | 0.36 ± 0.01 | 0.05 ± 0.01 | 0.30 ± 0.00 | 0.22 ± 0.04879 | 0.32 ± 0.00696 |
| NIAB Mung‐92 | 9.41 ± 0.41 | 10.37 ± 0.23 | 5.79 ± 0.19 | 7.67 ± 0.38 | 8.73 ± 0.37 | 9.76 ± 0.08 | 0.06 ± 0.00 | 0.09 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.08 ± 0.00 | 0.38 ± 0.02 | 0.69 ± 0.01 | 0.51 ± 0.01 | 0.53 ± 0.01 | 0.56 ± 0.01439 | 0.61 ± 0.01223 |
| NIAB Mung‐98 | 11.31 ± 0.17 | 11.30 ± 0.11 | 4.46 ± 0.36 | 8.31 ± 0.38 | 8.91 ± 0.31 | 10.03 ± 0.21 | 0.06 ± 0.00 | 0.08 ± 0.00 | 0.04 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.29 ± 0.02 | 0.76 ± 0.01 | 0.34 ± 0.04 | 0.48 ± 0.01 | 0.57 ± 0.04917 | 0.71 ± 0.00467 |
| NIAB Mung‐2006 | 12.35 ± 0.35 | 11.41 ± 0.39 | 7.96 ± 0.52 | 9.23 ± 0.17 | 8.44 ± 0.59 | 10.14 ± 0.16 | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.05 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.49 ± 0.03 | 0.49 ± 0.01 | 0.36 ± 0.04 | 0.40 ± 0.01 | 0.41 ± 0.02362 | 0.46 ± 0.006 |
| NIAB Mung‐2011 | 9.25 ± 0.35 | 10.82 ± 0.33 | 4.84 ± 0.47 | 8.23 ± 0.38 | 6.62 ± 0.38 | 9.47 ± 0.20 | 0.05 ± 0.00 | 0.08 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.05 ± 0.01 | 0.58 ± 0.02 | 0.45 ± 0.03 | 0.48 ± 0.01 | 0.45 ± 0.00897 | 0.52 ± 0.00693 |
| NIAB Mung‐2016 | 10.99 ± 0.36 | 12.43 ± 0.43 | 8.77 ± 0.32 | 9.65 ± 0.15 | 10.02 ± 0.21 | 10.92 ± 0.19 | 0.06 ± 0.00 | 0.09 ± 0.00 | 0.06 ± 0.0 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.08 ± 0.00 | 0.46 ± 0.02 | 0.69 ± 0.01 | 0.54 ± 0.01 | 0.57 ± 0.01 | 0.59 ± 0.00362 | 0.64 ± 0.00557 |
Figure 4.

Principal component analysis showing the association among growth, antioxidants, water relation properties, biochemical parameters, and yield attributes in O3‐stressed mung bean plants treated with ascorbic acid and silicic acid. 100 SW, 100‐seed weight; APX, ascorbate peroxidase; CAT, catalase; FAA, free amino acids; GC, grain carbohydrates; GP, grain protein; LA, leaf area; NL, number of leaves; NPPP, number of pods per plant; NRS, non‐reducing sugar; OP, osmotic potential; POD, peroxidase; RL, root length; RS; reducing sugar; RWC, relative water content; SL, shoot length; SOD, superoxide dismutase; TF, total flavonoids; TP, turgor potential; WP, water potential.
Figure 5.

Pearson correlation among the growth, antioxidants, water relation properties, biochemical parameters, and yield attributes of mung bean treated with ascorbic acid and silicic acid under O3 stress. 100 SW, 100‐seed weight; APX, ascorbate peroxidase; CAT, catalase; FAA, free amino acids; GC, grain carbohydrates; GP, grain protein; LA, leaf area; NL, number of leaves; NPPP, number of pods per plant; NRS, non‐reducing sugar; OP, osmotic potential; POD, peroxidase; RL, root length; RS; reducing sugar; RWC, relative water content; SL, shoot length; SOD, superoxide dismutase; TANN, tannin; TF, total flavonoids; TP, turgor potential; WP, water potential.
DISCUSSION
The results of this study revealed that altered assimilate partitioning across various plant portions may be the reason for significant reductions in plant development. Because of the uneven distribution of assimilates, plants had fewer and smaller leaves, which hindered shoot and root growth. 34 , 35 It may be possible to evaluate whether silicic acid promotes the growth and production of biomass in plants because silicon plays a role in cell replication and extension, as well as in improvements in photosynthesis and light absorption, 36 as demonstrated in the present work. In addition, it has been discovered that ascorbic acid functions as a cofactor for dioxygenases during the synthesis of auxins (indole‐3‐acetic acid) and gibberellins, which promotes greater plant development. 37 Ascorbic acid application enhances plant yield and development under normal and stressed conditions as it improves the antioxidant defense system and neutralizes lipid peroxidation of membranes. 35
In mung bean plants under high levels of O3 stress, POD, CAT, SOD, and APX activity was significantly higher, which led to a drop in cellular levels of ROS and the elimination of oxidative stress‐related negative consequences. 38 , 39 In the current study, foliar administration of ascorbic acid led to a significant increase in antioxidant enzyme activity. These findings substantiate recent research in which ascorbic acid enhanced the activity of SOD, CAT, POD, and APX enzymes in wheat. 40 Furthermore, it was found 41 that exogenous silicon can improve ROS scavenging by influencing antioxidant enzyme activity in wheat (Triticum aestivum) and similar findings have been reported recently. 42 , 43
The findings of the current research revealed a significant decrease in plant water relation attributes because elevated O3 may modify root architecture, limiting root water flow, and, as a result, water availability to above‐ground sections of the target plant. 1 However, Bybordi 44 reported that foliar ascorbic acid treatment might help improve plant water status by reducing transpiration water loss, preserving membrane integrity, and controlling osmotic adjustment. Plant defense mechanisms triggered by silicon include a series of signaling cascades that result in physiological and biochemical alterations. 45 The effect of silicon on root water absorption was studied by Zhu et al., 46 who reported that root hydraulic conductivity is a measure of a plant's capacity to absorb water. Silicon treatment has recently been shown to improve root hydraulic conductivity in sorghum. Under stress conditions, silicon spray was found to be involved in the modulation of root hydraulic conductivity by upregulating aquaporin gene expression by concentrating potassium inside the xylem sap. 47
It is assumed that increased mobilization resulted from a decrease in carbohydrate content (reducing and non‐reducing) in the presence of elevated O3. 48 Foliar treatment with ascorbic acid increased sugar deposition by acting as an activator of carbohydrate. 49 Silicon application tends to allocate more polysaccharides for grain formation compared with total dry matter. Silicon improved stressed flax plants by increasing endogenous phytohormone levels. 50 The oxidation of amino acids by elevated levels of O3 can change the nutritional and metabolic value of grains, although few studies have examined this (e.g., Martin et al.). 51 Additionally, the stimulatory effect of stress on the synthesis of FAA in different mung bean varieties was significantly increased by ascorbic acid application. The breakdown of protein into FAA, which serve as osmoprotectants in the mung bean varieties under study, is probably what led to tolerance against elevated O3 stress in the present study. Using silicon foliar treatment, Laane 20 reported similar results.
With an increase in O3 stress, all the investigated mung bean varieties' yield characteristics were noticeably reduced. Increased O3 stress, according to Burkey et al., 52 shortened plant development time due to decreased vegetative biomass production and reproductive growth, which decreased 100‐seed weight and pod number. It is well known that rising O3 levels in the atmosphere hinder plant growth and reproduction; 53 consequently, crop quality and yield decline. Crop yield has been reported to increase when drought‐stressed crops like maize, flax, and wheat are treated with exogenous ascorbic acid. 54 According to the study conducted by Arshad, 14 silicon treatment improved rice growth and development, which raised the component of plant yield and decreased elevated O3 stress.
Polyphenols, such as flavonoids and tannins, have a significant impact on plant–environment relationships. As reported in a previous study by Akram et al., 55 higher levels of tannins and flavonoids in the mung bean varieties were found in the current study. TF and tannins, which have a significant structural role in plants and are closely associated with antioxidant activity, are the main bioactive components. 56 , 57 The activation of non‐enzymatic antioxidants may account for the rise in polyphenols caused by the foliar application of ascorbic acid. 58 Similar to what was observed in the current investigation, silicon treatment enhanced secondary metabolism by increasing the generation of flavonoids and tannins. 46
It might be inferred from the findings of the present study that, under O3 stress, all sensitive mung bean varieties experienced significant decreases in their osmotic potential, water potential, RWC, turgor potential, reducing sugar, non‐reducing sugar, number of pods per plant, total FAA, 100‐seed weight, and grain carbohydrates. The results reveal that the varieties NIAB Mung‐2021 and NIAB Mung‐2006 are resistant to O3 and that the application of silicic acid is a more effective than ascorbic acid to reduce elevated O3 damaging effects. All of this is explained by the interaction of ascorbic acid and silicic acid at the primary and secondary metabolism levels in the tested mung bean varieties to deal with elevated O3 stress; it also reflects the genetic variation among the varieties that manipulates all the attributes at the back end.
CONCLUSIONS
All 12 mung bean varieties under O3 stress showed a decline in growth, water relation characteristics, and yield. Among the varieties studied, NM‐20‐21, NM‐2006, and NM‐2016 showed greater growth under O3 stress, but NM‐28, NM‐13‐1, NM‐51, and NM‐98 were found to be sensitive to O3 stress. All growth traits were less reduced in the O3‐tolerant mung bean varieties. In comparison with sensitive varieties, the O3 stress typically resulted in a significant change in the activities of SOD, POD, CAT, APX, and all other growth parameters in tolerant ones. To lessen the negative effects of O3 on mung bean, a combination of silicic acid and ascorbic acid application was the most effective.
CONFLICT OF INTEREST
The authors declare that there are no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Naeem Iqbal, Muhammad Nawaz, Fahad Khan, and Eram Shahzadi conceived and designed the study. Naeem Iqbal, Muhammad Shahid, and Muhammad Nawaz executed the experiment and compiled the data. Muhammad Shahid, Naeem Iqbal, and Eram Shahzadi helped in sample collection and chemical analysis. Naeem Iqbal and Fahad Khan statistically analyzed the data and helped in chemical analysis. Eram Shahzadi Fahad Khan wrote the manuscript. Muhammad Shahid and Muhammad Nawaz critically edited and revised the manuscript.
ACKNOWLEDGEMENTS
The authors greatly acknowledge the Department of Botany, Government College University, Faisalabad, Pakistan, for supporting this study. The findings of this study are a part of the Doctorate studies of Eram Shahzadi.
No external funding was obtained to conduct this study. The work was supported by the resources available within the research facilities of the Department of Botany, Government College University, Faisalabad, Pakistan. Open access publishing facilitated by University of Tasmania, as part of the Wiley ‐ University of Tasmania agreement via the Council of Australian University Librarians.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Young JW, Locke JCW, Altinok A, Rosenfeld N, Bacarian T, Swain PS et al., Measuring single‐cell gene expression dynamics in bacteria using fluorescence time‐lapse microscopy. Nat Protoc 7:80–88 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ran J, Qiu H, Sun S, Yang A and Tian L, Are ambient volatile organic compounds environmental stressors for heart failure? Environ Pollut 242:1810–1816 (2018). [DOI] [PubMed] [Google Scholar]
- 3. Agathokleous E, Feng Z, Oksanen E, Sicard P, Wang Q, Saitanis CJ et al., Ozone affects plant, insect, and soil microbial communities: a threat to terrestrial ecosystems and biodiversity. Sci Adv 6:eabc1176 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ge S, Wang S, Xu Q and Ho T, CAMx simulations of the control of anthropogenic emissions on the reduction of ozone formation in Southeast Texas of USA. Atmos Pollut Res. 12:101114 (2021). [Google Scholar]
- 5. Peng J, Shang B, Xu Y, Feng Z and Calatayud V, Effects of ozone on maize (Zea mays L.) photosynthetic physiology, biomass and yield components based on exposure‐ and flux‐response relationships. Environ Pollut 256:113466 (2020). [DOI] [PubMed] [Google Scholar]
- 6. Rathore D and Chaudhary IJ, Ozone risk assessment of castor (Ricinus communis L.) cultivars using open top chamber and ethylenediurea (EDU). Environ Pollut 244:257–269 (2019). [DOI] [PubMed] [Google Scholar]
- 7. Dumanović J, Nepovimova E, Natić M, Kuča K and Jaćević V, The significance of reactive oxygen species and antioxidant defense system in plants: a concise overview. Front Plant Sci 11:552969 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasanuzzaman M, Borhannuddin Bhuyan MHM, Zulfiqar F, Raza A, Mohsin SM, Al Mahmud J et al., Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahmad MN, Büker P, Khalid S, Van Den Berg L, Shah HU, Wahid A et al., Effects of ozone on crops in north‐west Pakistan. Environ Pollut 174:244–249 (2013). [DOI] [PubMed] [Google Scholar]
- 10. Pan L, Zou X‐j, Lie G‐w, Xue L and Chen H‐y, Ozone‐induced changes in physiological and biochemical traits in Elaeocarpus sylvestris and Michelia chapensis in South China. Atmos Pollut Res 11:973–980 (2020). [Google Scholar]
- 11. Turan V, Arbuscular mycorrhizal fungi and pistachio husk biochar combination reduces Ni distribution in mungbean plant and improves plant antioxidants and soil enzymes. Physiol Plant 173:418–429 (2021). [DOI] [PubMed] [Google Scholar]
- 12. Mills G and Harmens H, Ozone Pollution: A Hidden Threat to Food Security. Report prepared by the ICP Vegetation September 2011. NERC/Centre for Ecology and Hydrology, Bangor, UK: (2011). [Google Scholar]
- 13. Suleri AQ and Iqbal M, National Food Security Challenges and strategies in Pakistan: cooperation for technology and trade, in Regional Cooperation for Sustainable Food Security in South Asia. Routledge India, India: (2019). [Google Scholar]
- 14. Arshad A, A growth and biochemistry of ten high yielding genotypes of Pakistani rice (Oryza sativa L.) at maturity under elevated tropospheric ozone. Heliyon. 7:e08198 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shahzadi E, Nawaz M, Iqbal N, Ali B, Adnan M, Saleem MH et al., Silicic and ascorbic acid induced modulations in photosynthetic, mineral uptake, and yield attributes of mung bean (Vigna radiata L. Wilczek) under ozone stress. ACS Omega. 8:13971–13981 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bradáčová K, Weber NF, Morad‐Talab N, Asim M, Imran M, Weinmann M et al., Micronutrients (Zn/Mn), seaweed extracts, and plant growth‐promoting bacteria as cold‐stress protectants in maize. Chem Biol Technol Agric 3:19 (2016). [Google Scholar]
- 17. Ma JF, Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci Plant Nutr 50:11–18 (2004). [Google Scholar]
- 18. Menzies J, Bowen P, Ehret D and Glass ADM, Foliar applications of potassium silicate reduce severity of powdery mildew on cucumber, muskmelon, and zucchini squash. J Am Soc Hort Sci 117:902–905 (1992). [Google Scholar]
- 19. Ma JF, Tamai K, Ichii M and Wu GF, A rice mutant defective in Si uptake. Plant Physiol 130:2111–2117 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laane H‐M, The effects of foliar sprays with different silicon compounds. Plan Theory 7:45 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bilska K, Wojciechowska N, Alipour S and Kalemba EM, Ascorbic acid—the little‐known antioxidant in woody plants. Antioxidants 8:645 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carleton AE and Foote WH, A comparison of methods for estimating total leaf area of barley plants. Crop Sci 5:602–603 (1965). [Google Scholar]
- 23. Giannopolitis CN and Ries SK, Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chance B and Maehly AC, Assay catalase and peroxidase. Methods Enzymol 2:764–775 (1955). [Google Scholar]
- 25. Asada K, Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hussain N, Ghaffar A, Zafar ZU, Javed M, Shah KH, Noreen S et al., Identification of novel source of salt tolerance in local bread wheat germplasm using morpho‐physiological and biochemical attributes. Sci Rep 11:10854 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karrou M and Maranville JW, Response of wheat cultivars to different soil nitrogen and moisture regimes: III. Leaf water content, conductance, and photosynthesis 1. J Plant Nutr 18:777–791 (1995). [Google Scholar]
- 28. Yemm EW and Willis AJ, The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57:508–514 (1954). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chandra S, Khan S, Avula B, Lata H, Yang MH, ElSohly MA et al., Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: a comparative study. Evid Based Complement Alternat Med 2014:253875 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolfe K, Wu X and Liu RH, Antioxidant activity of apple peels. J Agric Food Chem 51:609–614 (2003). [DOI] [PubMed] [Google Scholar]
- 31. Bremner JM, Chapter 83: Total nitrogen, in Methods of Soil Analysis, Vol. 2, ed. by Norman AG. American Society of Agronomy, Inc, Madison, USA: (1965). [Google Scholar]
- 32. Malik CP and Srivastava AK, Text Book of Plant Physiology. Kalyani Publishers, New Delhi: (1979). [Google Scholar]
- 33. Hamilton PB, Van Slyke DD and Lemish S, The gasometric determination of free amino acids in blood filtrates by the ninhydrin‐carbon dioxide method. J Biol Chem 150:231–250 (1943). [Google Scholar]
- 34. Ghosh A, Agrawal M and Agrawal SB, Examining the effectiveness of biomass‐derived biochar for the amelioration of tropospheric ozone‐induced phytotoxicity in the Indian wheat cultivar HD 2967. J Hazard Mater 408:124968 (2021). [DOI] [PubMed] [Google Scholar]
- 35. Wang K, Nan L‐L, Xia J, Wu S‐W and Yang L‐L, Metabolomics reveal root differential metabolites of different root‐type alfalfa under drought stress. Front Plant Sci. 15:1341826 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Farooq M, Saqib Z and Akhtar J, Silicon‐mediated oxidative stress tolerance and genetic variability in rice (Oryza sativa L.) grown under combined stress of salinity and boron toxicity. Turk J Agric For 39:718–729 (2015). [Google Scholar]
- 37. Mahmood AM and Dunwell JM, 2‐oxoglutarate‐dependent dioxygenases: a renaissance in attention for ascorbic acid in plants. PLoS One 15:e0242833 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu D, Shi M, Jia B, Yan Z, Gao L, Guan W et al., Effect of ozone on the activity of antioxidant and chlorophyll‐degrading enzymes during postharvest storage of coriander (Coriandrum sativum L.). J Food Process Preserv 43:e14020 (2019). [Google Scholar]
- 39. Bao J, Liu Z, Ding Z, Yisilam G, Wang Q and Tian X, Metabolomic analysis reveals key metabolites and metabolic pathways in Suaeda salsa under salt and drought stress. Funct Plant Biol 50:701–711 (2023). [DOI] [PubMed] [Google Scholar]
- 40. Hafez EM and Gharib HS, Effect of exogenous application of ascorbic acid on physiological and biochemical characteristics of wheat under water stress. Int J Plant Product 10:579–596 (2016). [Google Scholar]
- 41. Tripathi DK, Singh S, Singh VP, Prasad SM, Dubey NK and Chauhan DK, Silicon nanoparticles more effectively alleviated UV‐B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol Biochem 110:70–81 (2017). [DOI] [PubMed] [Google Scholar]
- 42. Nadeem M, Anwar‐ul‐Haq M, Saqib M, Maqsood M and He Z, Ameliorative effect of silicic acid and silicates on oxidative, osmotic stress, and specific ion toxicity in spring wheat (Triticum aestivum L.) Genotypes. J Soil Sci Plant Nutr 22:2334–2345 (2022). [Google Scholar]
- 43. Li W and Keller AA, Integrating targeted metabolomics and targeted proteomics to study the responses of wheat plants to engineered nanomaterials. ACS Agric Sci Technol 4:507–520 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bybordi A, Effect of ascorbic acid and Silicium on photosynthesis, antioxidant enzyme activity, and fatty acid contents in canola exposure to salt stress. J Integr Agri 11:1610–1620 (2012). [Google Scholar]
- 45. Zia A, Hegazy HS, Hassan NS, Naguib DM and Abdel‐Haliem MEF, Biochemical responses of wheat to silicon application under salinity. J Plant Nutr Soil Sci 184:255–262 (2021). [Google Scholar]
- 46. Zhu Y‐X, Xu X‐B, Hu Y‐H, Han W‐H, Yin J‐L, Li H‐L et al., Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep 34:1629–1646 (2015). [DOI] [PubMed] [Google Scholar]
- 47. Chen D, Wang S, Yin L and Deng X, How does silicon mediate plant water uptake and loss under water deficiency? Front Plant Sci. 9:281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Filha MSX, Rodrigues FA, Domiciano GP, Oliveira HV, Silveira PR and Moreira WR, Wheat resistance to leaf blast mediated by silicon. Australas Plant Pathol 40:28–38 (2011). [Google Scholar]
- 49. Meyer U, Köllner B, Willenbrink J and Krause GHM, Effects of different ozone exposure regimes on photosynthesis, assimilates and thousand grain weight in spring wheat. Agr Ecosyst Environ 78:49–55 (2000). [Google Scholar]
- 50. Hassanein RA, Bassuony FM, Baraka DM and Khalil RR, Physiological effects of nicotinamide and ascorbic acid on Zea mays plant grown under salinity stress. 1‐changes in growth, some relevant metabolic activities and oxidative defense systems. Res J Agric Biol Sci 5:72–81 (2009). [Google Scholar]
- 51. Martin TN, Nunes UR, Stecca JDL and Pahins DB, Foliar application of silicon on yield components of wheat crop. Rev Caatinga 30:578–585 (2017). [Google Scholar]
- 52. Burkey KO, Agathokleous E, Saitanis JC, Mashaheet AM, Koike T and Hung Y‐T, Ozone effects on vegetation: a walk from cells to ecosystems. handbook of environment and waste management, in Handbook of Environment and Waste Management, Vol. 3. World Scientific, Singapore: pp. 357–396 (2019). [Google Scholar]
- 53. Nowroz F, Hasanuzzaman M, Siddika A, Parvin K, Caparros PG, Nahar K et al., Elevated tropospheric ozone and crop production: potential negative effects and plant defense mechanisms. Front Plant Sci. 14:1244515 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mills G, Sharps K, Simpson D, Pleijel H, Frei M, Burkey K et al., Closing the global ozone yield gap: quantification and cobenefits for multistress tolerance. Glob Chang Biol 24:4869–4893 (2018) en. [DOI] [PubMed] [Google Scholar]
- 55. Akram NA, Shafiq F and Ashraf M, Ascorbic acid‐A potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci. 8:613 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ahmad MS, Liu C‐G, Nawaz M, Tawab A, Shen X, Shen B et al., Elucidating the pyrolysis reaction mechanism of Calotropis procera and analysis of pyrolysis products to evaluate its potential for bioenergy and chemicals. Bioresour Technol 322:124545 (2021). [DOI] [PubMed] [Google Scholar]
- 57. Šamec D, Karalija E, Šola I, Vujčić Bok V and Salopek‐Sondi B, The role of polyphenols in abiotic stress response: the influence of molecular structure. Plan Theory 10:118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kumari P, Kumar V, Kumar R and Pahuja SK, Sorghum polyphenols. Planta 254:1–14 (2021).34081200 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


