Abstract
Objective:
Oxidative stress prompts breast cancer cells to adapt by raising the lethal threshold and enhancing the antioxidant mechanism, thereby enabling survival and continuous proliferation that facilitates tumor progression. Nrf2 and 8-OHdG are indicative of oxidative stress activity and impact the progression of breast cancer. We aimed to analyze the expression of Nrf2 and 8-OHdG in various T stages of breast cancer in our hospital.
Methods:
This observational study employed a cross-sectional design and included patients with invasive breast carcinoma of no special type diagnosis from histopathology examination who underwent modified radical mastectomy without neoadjuvant chemotherapy at Dr. Soetomo General Academic Hospital between January 2019 and December 2022. Medical records and paraffin blocks that met these criteria were obtained. 8-OHdG and Nrf2 were assessed using immunohistochemistry.
Result:
There was no significant difference and correlation between 8-OHdG (p=0.578) and Nrf2 (p=0.694) expression with various T stages of IBC-NST and no significant correlation between 8-OHdG/Nrf2 expression and T stage (p=0.242 and 0.625 respectively).
Conclusion:
Consistent expression of 8-OHdG and Nrf2 in various T stages of breast cancer represents a continuation of the oxidative stress process in breast cancer that is not influenced by the tumor size. The existence of consistent oxidative stress at all tumor sizes (T stage) stimulates breast cancer cells to continue proliferating.
Key Words: Breast cancer, invasive breast carcinoma of no special type, 8-OHdG, Nrf2
Introduction
Breast cancer is a prevalent malignancy in women, with 66,271 new cases recorded in Indonesia in 2022 [1]. The increased proliferation of cancer cells results in the consequent accumulation of elevated reactive oxygen species (ROS) and oxidative stress, which are harmful to the cells. Increased oxidative stress and hypoxic conditions prompt breast cancer cells to adapt by raising the threshold for lethal levels of oxidative stress, enhancing antioxidant mechanisms and angiogenesis. These adaptations enable breast cancer cells to withstand elevated oxidative stress and continue to proliferate [2-5].
Both normal and cancer cells undergo cellular metabolism, which induces an imbalance between pro-oxidant and antioxidant, a phenomenon of oxidative stress [6]. Oxidative stress affects carcinogenesis, including the promotion, progression, and aggressiveness of breast cancer, through increased cell proliferation. Oxidative stress accumulates at a significant level in the late stage of breast cancer [7, 8]. A practical method to detect oxidative stress is the detection of 8-hydroxy-2-deoxyguanosine (8-OHdG), the product of DNA damage by ROS [9]. Low expression of 8-OHdG, a prognostic factor for poor prognosis, is a robust indicator of breast cancer invasion and is more prevalent in ductal carcinoma type [10-13]. This phenomenon can be attributed, in part, to a higher antioxidant activity in breast cancer tissue [14]. The primary regulator of the antioxidant enzyme, nuclear factor erythroid 2-related factor 2 (Nrf2), is highly expressed in breast cancer. Upregulation of Nrf2 and its associated enzymes reduce 8-OHdG expression and counteract the deleterious effects of oxidative stress, leading to the progression and metastatic potential of breast cancer [11, 14].
A few studies analyzed the different amounts of oxidative stress in various tumor sizes, whether higher or lesser in bigger tumor sizes. We aimed to analyze the expression of Nrf2 and 8-OHdG in various T stages of breast cancer.
Materials and Methods
Sample collection
Samples from 49 patients who had not received neoadjuvant chemotherapy, underwent modified radical mastectomy (MRM) and were identified as having invasive breast carcinoma of no special type (IBC-NST) by histopathology examination at Dr. Soetomo General Academic Hospital between January 2019 – December 2022 were used in this study. Using the breast cancer T stage categorization by the World Health Organization (WHO), samples were divided into four groups: T1 (14 cases), T2 (14 cases), T3 (14 cases), and T4 (7 cases). Before the study, all sample re-examination was conducted.
Immunohistochemistry
Formalin-fixed paraffin-embedded blocks (FFPE) were cut 4-6 µm thick. Deparaffinized using xylol and rehydrated using absolute, 96%, 80%, and 70% alcohol for 5 minutes were performed. Slides were washed in running and distilled water for 5 minutes, followed by 15 minutes of placing at room temperature in 3% H2O2 in methanol and rewashed for 5 minutes with distilled water. Slides were then warmed for 30 minutes at 95 °C in a deep decloaking chamber using a Diva Decloaker with PH 7 for 8-OHdG and Nrf2. PBS was used for 5-minute washing. The background snipper was dripped for 15 minutes, followed by the primary antibodies. The primary antibodies were mouse monoclonal antibody against 8-OHdG (e-8) sc-393871 (Santa Cruz Biotechnology) at 1:100 dilution and rabbit monoclonal antibody IgG against Nrf2 bsm-52179R (Bioss Antibodies) at 1:100 dilution. After 60 minutes of incubation at room temperature, slides were washed with PBS for 5 minutes. The universal link was dripped for 20 minutes, washed with PBS, dripped with the HRP Trekavidin label for 15 minutes, and washed again with PBS. Then, diaminobenzidine (DAB) was dripped. Slides were incubated at room temperature for five minutes, washed with running water, and then Mayer’s Hematoxylin was added. Slides were then washed with running water and soaked in water for 5 minutes. Dehydration in 80%, 96%, and absolute alcohol was performed then. The slides were then placed in xylol and continued by covering each slide with cover glass. Two pathologists, unaware or blinded to the T stage of the samples, independently assessed the immunostaining results using the Olympus CX31 binocular microscope. Assessment and settlement of disagreements between observers were performed using a double-headed microscope.
Scoring
The nuclei of tumor cells were examined for 8-OHdG and Nrf2 immunostaining localization. There were four staining intensity levels: negative, weak, moderate, and strong. Histochemical scoring assessment (H-score), which was the result of combining the values of all intensity levels that were overlaid on the tumor cells (negative, weak, moderate, and strong) multiplied by the percentage of overlaid tumor cells (1-100%). The H-score formula is stated below:
H-score=(0 x P0)+(1 x P1)+(2 x P2)+(3 x P3)
The results were then summed to obtain a range of H-score values from 0 to 300. The H-score ranges were grouped into 4: negative = 0 (0-49), weak = 1 (50-99), moderate = 2 (100-199), and strong = 3 (200-300) [15-18].
Statistical analysis
IBM SPSS Statistics 26.0.0.0 was used for statistical analysis. Using the Kruskal-Wallis and Spearman’s correlation tests, the difference and correlation of 8-OHdG and Nrf2 expression in various T stages of breast cancer were assessed. Results were significant if the p-value is < 0.05.
Results
Patient characteristics
Patients with IBC-NST in this study had an average age of 52.69 years. The oldest patient was 84 years old, and the youngest was 28 years old. Patients’ ages were divided into seven groups with 10-year time intervals. The highest prevalence of 18 patients (36.73%) was in the age group of 41-50 years old. The T2 stage in the population of this study had 89 cases, followed by the T4 stage with 42 cases, the T3 stage with 36 cases, and the T1 stage with 29 cases. T1 stage in this study was found most in the 41-50 years old age group with 6 cases (12.24%), T2 stage in the 51-60 years old age group with 7 cases (14.29%), T3 stage in the 41-50 years old age group with 5 cases (10.20%), and T4 stage in the 41-50 years old age group with 4 cases (8.16%) (Table 1).
Table 1.
Clinicopathology Characteristics
| Characteristics | T1 | T2 | T3 | T4 | Total n (%) |
|---|---|---|---|---|---|
| Population | 29 | 89 | 36 | 42 | 196 |
| Sample | 14 | 14 | 14 | 7 | 49 |
| Age | |||||
| 21-30 | 0 | 0 | 0 | 1 | 1 (2.04) |
| 31-40 | 3 | 1 | 2 | 0 | 6 (12.24) |
| 41-50 | 6 | 3 | 5 | 4 | 18 (36.73) |
| 51-60 | 1 | 7 | 3 | 1 | 12 (24.49) |
| 61-70 | 3 | 2 | 2 | 1 | 8 (16.33) |
| 71-80 | 1 | 1 | 1 | 0 | 3 (6.12) |
| 81-90 | 0 | 0 | 1 | 0 | 1 (2.04) |
| Tumor location | |||||
| Right breast | 5 | 8 | 9 | 2 | 24 (48.98) |
| Left breast | 9 | 6 | 5 | 5 | 25 (51.02) |
| Grade | |||||
| 1 | 1 | 0 | 1 | 0 | 2 (4.08) |
| 2 | 9 | 6 | 1 | 3 | 19 (38.78) |
| 3 | 4 | 8 | 12 | 4 | 28 (57.14) |
Using the Nottingham modification Bloom and Richardson grading system, the histology grading of breast cancer in this study was divided into three grades: grade 1 – grade 3. The most common grade in this study was grade 3, with 28 cases (57.14%), followed by grade 2, with 19 cases (38.78%), and grade 1, with 2 cases (4.08%) (Table 1).
8-OHdG expression
8-OHdG expression was observed in all T1-T4 groups in this study. The expression of 8-OHdG was evaluated and examined using H-score (Figure 1). 8-OHdG expression varied across all T stage groups. The most prevalent score was 1 in 22 out of 49 samples. The T1, T2, and T4 stage group’s highest score was 1 (6, 8, and 4 cases, respectively), and the T3 stage group’s highest score was 2, with 5 cases (35.71%) (Table 2). There was no significant difference between 8-OHdG expression at various T stages of breast cancer (p-value = 0.578) (Table 2), and no significant correlation was observed between 8-OHdG expression and T stage (p-value = 0.242) in this study (Table 4).
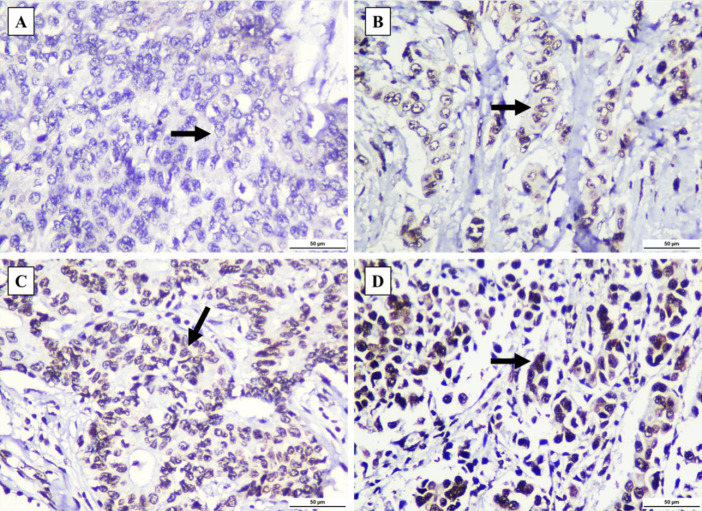
Figure 1.
8-OHdG Staining was Observed in Invasive Breast Carcinoma of No Special Type (IBC-NST) Tumor Cell’s Nuclei (black arrow). (A) Negative staining. (B) Weakly positive staining. (C) Moderately positive staining. (D) Strongly positive staining. All figures were captured at 400x magnification. Scale bar: 50 µm.
Table 2.
H-score for 8-OHdG Expression
| 8-OHdG | T stage | Total (n) | p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||||||||
| H-score | Interpretation | n | % | n | % | n | % | n | % | ||
| 0 | Negative | 2 | 14.29 | 1 | 7.14 | 4 | 28.57 | 2 | 28.57 | 9 | 0.578 |
| 1 | Weak | 6 | 42.86 | 8 | 57.14 | 4 | 28.57 | 4 | 57.14 | 22 | |
| 2 | Moderate | 5 | 35.71 | 5 | 35.71 | 5 | 35.71 | 1 | 14.29 | 16 | |
| 3 | Strong | 1 | 7.14 | 0 | 0 | 1 | 7.14 | 0 | 0 | 2 | |
| Total (n) | 14 | 100 | 14 | 100 | 14 | 100 | 7 | 100 | 49 | ||
*Kruskal-Wallis test, p <0.05 was considered statistically significant
Nrf2 expression
The H-score was used to evaluate Nrf2 expression of Nrf2 (Figure 2). The most prevalent score was 1 in 23 out of 49 samples. The T2 and T3 groups’ highest scores were 1 (9 and 7 cases, respectively), the T1 stage group’s highest score was 0 with 5 cases (35.71%), and the T4 stage group’s highest score was 1 with 3 cases (42.86%) (Table 3). There was no significant difference in Nrf2 expression between various T stages of breast cancer (p-value = 0.694) (Table 3), and no significant correlation was observed between Nrf2 expression and T stage (p-value = 0.625) in this study (Table 4).
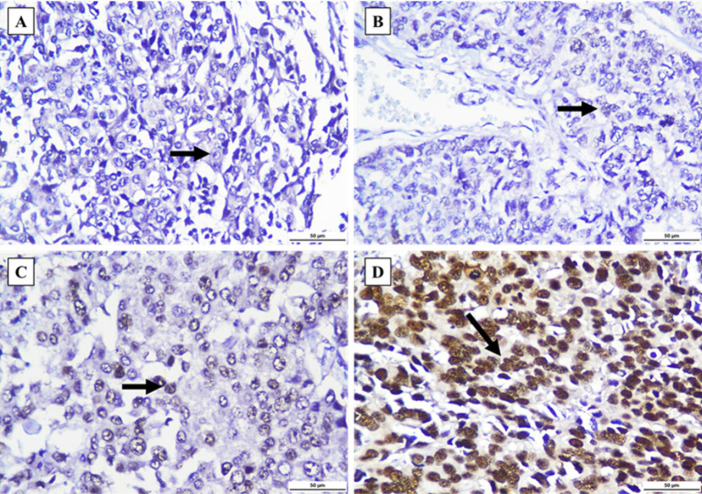
Figure 2.
Nrf2 Staining was Observed in Invasive Breast Carcinoma of No Special Type (IBC-NST) Tumor Cell’s Nuclei (black arrow). (A) Negative staining. (B) Weakly positive staining. (C) Moderately positive staining. (D) Strongly positive staining. All figures were captured at 400x magnification. Scale bar: 50 µm.
Table 3.
H-score of Nrf2 Expression
| Nrf2 | T stage | Total (n) | p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||||||||
| H-score | Interpretation | n | % | n | % | n | % | n | % | ||
| 0 | Negative | 5 | 35.71 | 3 | 21.43 | 4 | 28.57 | 1 | 14.29 | 13 | 0.694 |
| 1 | Weak | 4 | 28.57 | 9 | 64.29 | 7 | 50 | 3 | 42.86 | 23 | |
| 2 | Moderate | 4 | 28.57 | 1 | 7.14 | 3 | 21.43 | 2 | 28.57 | 10 | |
| 3 | Strong | 1 | 7.14 | 1 | 7.14 | 0 | 0 | 1 | 14.29 | 3 | |
| Total (n) | 14 | 100 | 14 | 100 | 14 | 100 | 7 | 100 | 49 | ||
*Kruskal-Wallis test, p <0.05 was considered statistically significant
Table 4.
8-OHdG and Nrf2 Expression Correlation Analysis
| Parameter | p-value | Correlation Coefficient |
|---|---|---|
| Correlation of 8-OHdG expression with T stage | 0.242 | -0.170 |
| Correlation of Nrf2 expression with T stage | 0.625 | 0.072 |
| Correlation of 8-OHdG and Nrf2 with T stage | 0.027 | -0.317 |
*Spearman’s correlation test, p <0.05 was considered statistically significant
Discussion
This study found that the T2 stage was the most common. Research conducted by Yahya et al. at Universitas Airlangga Hospital in 2022 found that the most common tumor size in their research was between 2-5 cm or stated as T2 stage [19]. Age is a significant risk factor, and the global incidence of breast cancer increases in conjunction with advancing age and subsequently decreases after menstruation [20, 21]. The incidence of breast cancer is higher in Asian women aged under 50 years old than in Western women, with the peak incidence occurring at 40-50 years old in Asia and 60-70 years old in Western countries [22]. The increasing incidence of breast cancer may be caused, among other things, by increased exposure to risk factors among younger individuals, as well as variations in quality of care and public awareness about breast cancer in Western countries [23]. The elevated risk of breast cancer observed in premenopausal women and the gradual decline in postmenopausal age may be attributed to estrogen and progesterone exposure [20].
The grade of breast cancer is classified based on Nottingham modification Bloom and Richardson grading system, which evaluates tumor cell aggressiveness by tubular formation, nuclear pleomorphism, and mitosis count; the histology grading of breast cancer in this study was divided into 3 grades [24]. The most common grade in this study was grade 3. A study by Anwar et al. in Sardjito Hospital in 2019 found that 78.5% of 1.259 cases of breast cancer in young women were grade 3, and a study by Syarti et al. in 2020 found that 44.8% of all breast cancer cases with spiculated mass in mammography examination were grade 3 [25, 26]. Grade has a high prognostic value, with grade 3 having 31% of the 10-year relapse risk, grade 1 being 7%, and grade 2 being 14% [27]. SEER data indicate that the 5-year survival rate in women with breast cancer limited to the breast and has size <1 cm is 99%, 89% in 1-3 cm, and 86% in 3-5 cm. Large tumor size significantly predicts regional lymph node involvement and skin or chest wall invasion correlates with poor prognosis [20].
8-OHdG in this study was expressed in all breast cancer cells, regardless of the T stage. Liu and Liu, in 2021, stated that 8-OHdG expression in breast cancer cells was not significantly correlated with T stage but was instead correlated with lymph node involvement (N stage) [13]. On the contrary, Pande et al. [28] stated that there was a correlation between 8-OHdG and tumor size. Negative 8-OHdG staining in breast cancer tissue is an independent prognostic factor of poor prognosis and low survival rate [11, 12, 14]. A positive correlation exists between 8-OHdG levels and oxidative stress in breast cancer cells [13]. 8-OHdG is a biomarker of oxidative stress and oxidative DNA damage [29, 30]. The primary 8-OHdG repair enzyme is DNA glycosylase 1 (OGG1), which prevents G to T base pair mutation and further DNA damage [31]. ROS can inactivate OGG1, resulting in the inability to cleave the damaged guanine, reducing the amount of 8-OHdG [32]. Antioxidant enzyme activity can inhibit the interaction between ROS and DNA, thereby inhibiting the formation of 8-OHdG [33]. Compared with non-invasive breast lesions, 8-OHdG expression is significantly diminished in breast cancer [11].
Nrf2 in this study was also expressed in all breast cancer cells, regardless of the T stage. Lee et al. [34] found that Nrf2 expression was not correlated with the T stage. However, nuclear immunoreactivity of Nrf2 was correlated with histological grade and Ki67 index. Onodera et al. [35] found that Nrf2 expression was marginally correlated with T stage. The discrepancies in Nrf2 correlation may be caused by the activation of Nrf2 through alternative pathways, apart from the Keap1-Nrf2 pathway [34].
Nrf2 plays a significant role in maintaining cellular homeostasis and activating cytoprotective genes [36-38]. Kelch-like ECH-associated protein 1 (Keap1) interacts with Nrf2 and controls the stability of Nrf2 [39]. The keap1-Nrf2 pathway is a primary defense mechanism against oxidative stress and a significant hallmark of the regulation and development of breast cancer [38, 40]. The complexity and nature of cancer cell heterogeneity, along with the contribution of Nrf2, are currently being widely researched. Available data indicating the significant role of Nrf2 in the progression of breast cancer are still limited [41]. The activation of Nrf2 that activates HIF-1α increases the progression and angiogenesis in breast cancer. HIF-1α, the primary transcription factor for adaptive hypoxia condition, regulates several genes, including those involved in metabolic processes, angiogenesis (FGF and VEGF), and apoptosis (Bcl2, Bax, and p53). Reducing Nrf2 levels will decrease HIF-1α mRNA, reducing breast cancer cell proliferation [38]. Although not statistically significant, the expression of Nrf2 was lower in normal breast tissue than in breast cancer tissue. Nrf2 expression in the cytoplasm was identified in almost all cell lines, whereas Nrf2 expression in the nucleus was significantly higher in breast cancer cells [42]. The data demonstrated that all breast cancer types exhibited elevated Nrf2 levels in both the nucleus and cytoplasm. This finding shows that, regardless of the immunophenotype of breast cancer cells, tumor cells require augmented antioxidant protection [43]. The function of Nrf2 activity in breast cancer cells is reflected by nuclear Nrf2 immunoreactivity. In contrast, the importance of the Nrf2 activation pathway in breast cancer tissue is indicated by the wide distribution of Nrf2 immunoreactivity [35].
In breast cancer, a reduction in 8-OHdG levels was observed as tumor aggressiveness and progressivity increased. Enhanced antioxidant defenses facilitate cancer cell growth by inhibiting ROS-induced necrosis and apoptosis. Increased antioxidant enzyme activity impedes the interaction between ROS and DNA, thereby reducing 8-OHdG formation. A reduction in 8-OHdG levels in breast cancer cells indicates enhanced invasive properties, particularly in ductal carcinoma [13]. Nrf2 activation is increased in the tumor microenvironment, facilitating the accumulation of Nrf2 in the nucleus. This accumulation triggers tumor cell protection by transcribing protective genes that protect tumor cells against oxidative stress. In breast cancer, this often leads to tumor progression, resistance to anticancer treatment, especially under hypoxic conditions, and poor prognosis [42, 44]. Nuclei accumulation of Nrf2 will increase the transcription of genes involved in antioxidants, such as genes related to antioxidant response elements (ARE), namely glutathione reductase (GR), catalase (CA), NAD(P)H dehydrogenase quinone 1 (NQO1), superoxide dismutase (SOD), heme oxygenase-1 (HO-1), and glutathione peroxidase (GPX), as well as pro-proliferation, such as IGF1, NPNT, Notch1, VEGFC, SHMT, PSPH, PSAT1, and PHGDH, which will increase breast cancer cell proliferation. Nrf2 also degrades 8-OHdG through the upregulation of OGG1 [45].
Consistent with the findings and theory described above, our study showed that in breast cancer, 8-OHdG and Nrf2 were expressed in all T stages. This represents the continuation of the oxidative process in all stages of breast cancer, and oxidative stress is required for cancer cell growth. This has opened a new possibility that antioxidant interventions could potentially have beneficial effects on breast cancer patients by interfering with the oxidative stress process.
In conclusion, based on the results and data analysis, 8-OHdG and Nrf2 are consistently expressed in various T stages in breast cancer, indicating that the continuation of the oxidative stress process is not influenced by the size of the tumor (T stage). The existence of consistent oxidative stress at all tumor sizes (T stage) stimulates breast cancer cells to continue their proliferation.
Author Contribution Statement
All authors contributed to the conception and design of the study. Fira Soraya was responsible for data collection, analysis, interpretation, and manuscript writing. Willy Sandhika and Priangga Adi Wiratama developed the concept and critically revised the manuscript for important intellectual content, reviewed the text and manuscript writing, and approved the final version. All authors read and approved the final manuscript..
Acknowledgements
Funding Statement
This research was funded partly by Airlangga Research Fund Year 2023 batch 2.
Ethical Declaration
Approval from the Research Ethical Committee of Dr. Soetomo General Academic Hospital, dated November 4th, 2023, with number 0824/KEPK/XI/2023, was obtained.
Conflict of Interest
All of the authors declare no conflict of interest.
References
- 1.Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, et al. Available from: https://gco.iarc.who.int/today, authors. Global Cancer Observatory: Cancer Today [Internet] France: International Agency for Research on Cancer; c2024 [cited 2024 Aug 31]: [Google Scholar]
- 2.Huang Y, Yang Y, Xu Y, Ma Q, Guo F, Zhao Y, et al. Nrf2/HO-1 axis regulates the angiogenesis of gastric cancer via targeting VEGF. Cancer Manag Res. 2021;13:3155–69. doi: 10.2147/CMAR.S292461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Z, Mo Z. Keap1‐Nrf2 signaling pathway in angiogenesis and vascular diseases. J Tissue Eng Regen Med. 2020;14(6):869–83. doi: 10.1002/term.3053. [DOI] [PubMed] [Google Scholar]
- 4.Kirtonia A, Sethi G, Garg M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell Mol Life Sci. 2020;77(22):4459–83. doi: 10.1007/s00018-020-03536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarmiento-Salinas FL, Delgado-Magallón A, Montes-Alvarado JB, Ramírez-Ramírez D, Flores-Alonso JC, Cortés-Hernández P, et al. Breast cancer subtypes present a differential production of reactive oxygen species (ROS) and susceptibility to antioxidant treatment. Front Oncol. 2019;9:480. doi: 10.3389/fonc.2019.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tascioglu Aliyev A, Panieri E, Stepanić V, Gurer-Orhan H, Saso L. Involvement of NRF2 in breast cancer and possible therapeutical role of polyphenols and melatonin. Molecules. 2021;26(7):1853. doi: 10.3390/molecules26071853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zahra KF, Lefter R, Ali A, Abdellah EC, Trus C, Ciobica A, et al. The involvement of the oxidative stress status in cancer pathology: a double view on the role of the antioxidants. Oxid Med Cell Longev. 2021;2021(1):9965916. doi: 10.1155/2021/9965916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coughlin SS. Oxidative stress, antioxidants, physical activity, and the prevention of breast cancer initiation and progression. J Environ Health Sci. 2018;4(2):55–7. doi: 10.15436/2378-6841.18.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jelic MD, Mandic AD, Maricic SM, Srdjenovic BU. Oxidative stress and its role in cancer. J Cancer Res Ther. 2021;17(1):22–8. doi: 10.4103/jcrt.JCRT_862_16. [DOI] [PubMed] [Google Scholar]
- 10.Eldin EE, El-Readi MZ, Eldein MM, Alfalki AA, Althubiti MA, Kamel HF, et al. 8-Hydroxy-2’-deoxyguanosine as a discriminatory biomarker for early detection of breast cancer. Clin Breast Cancer. 2019;19(2):e385–93. doi: 10.1016/j.clbc.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Karihtala P, Kauppila S, Soini Y. Oxidative stress and counteracting mechanisms in hormone receptor positive, triple-negative and basal-like breast carcinomas. BMC Cancer. 2011;11:1–6. doi: 10.1186/1471-2407-11-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakovcevic D, Dedic-Plavetic N, Vrbanec D, Jakovcevic A, Jakic-Razumovic J. Breast cancer molecular subtypes and oxidative DNA damage. Appl Immunohistochem Mol Morphol. 2015;23(10):696–703. doi: 10.1097/PAI.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Yi J, Liu FE. The expression and significance of 8-hydroxydeoxyguanosine in breast cancer patients’ blood, urine and cancer tissue. Trends Immunother. 2021;5(2.1):36–41. [Google Scholar]
- 14.Qing X, Shi D, Lv X, Wang B, Chen S, Shao Z. Prognostic significance of 8-hydroxy-2′-deoxyguanosine in solid tumors: a meta-analysis. BMC Cancer. 2019;19:1–5. doi: 10.1186/s12885-019-6189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saha M, Arun I, Ahmed R, Chatterjee S, Chakraborty C. HscoreNet: A Deep network for estrogen and progesterone scoring using breast IHC images. Pattern Recognition. 2020;102:107200. [Google Scholar]
- 16.Liu J, Xu B, Zheng C, Gong Y, Garibaldi J, Soria D, et al. An end-to-end deep learning histochemical scoring system for breast cancer TMA. IEEE Trans Med Imaging. 2018;38(2):617–28. doi: 10.1109/TMI.2018.2868333. [DOI] [PubMed] [Google Scholar]
- 17.Rai PD, Vagha S, Shukla S, Bhake A. Comparison of various scoring systems by immunohistochemistry for evaluating hormone receptors (Estrogen receptor and progesterone receptor) in carcinoma of breast. J Datta Meghe Inst Med Sci Univ. 2020;15(2):202–8. [Google Scholar]
- 18.Ruengwanichayakun P. Histochemical scoring assessment (H-score) Asian Arch Pathol. 2021;3:13–4. [Google Scholar]
- 19.Yahya ES, Mulawardhana P, Kurniasari N. The Relationship Between Bse Knowledge and Breast Tumor Size During Surgical Center Visit at Unair Hospital. Indonesian Midwifery and Health Sciences Journal. 2022;6(1):66–73. [Google Scholar]
- 20.Smolarz B, Nowak AZ, Romanowicz H. Breast cancer—epidemiology, classification, pathogenesis and treatment (review of literature) Cancers (Basel) 2022;14(10):2569. doi: 10.3390/cancers14102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Singla A. Epidemiology of breast cancer: current figures and trends. Preventive oncology for the gynecologist. 2019:335–9. [Google Scholar]
- 22.Lim YX, Lim ZL, Ho PJ, Li J. Breast cancer in Asia: incidence, mortality, early detection, mammography programs, and risk-based screening initiatives. Cancers (Basel) 2022;14(17):4218. doi: 10.3390/cancers14174218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J, Ngai CH, Deng Y, Tin MS, Lok V, Zhang L, et al. Cancer incidence and mortality in Asian countries: a trend analysis. Cancer Control. 2022;29:10732748221095955. doi: 10.1177/10732748221095955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. WHO classification of tumours series Breast tumours, 5th ed vol 2. France: International Agency for Research on Cancer; 2019. [Google Scholar]
- 25.Anwar SL, Raharjo CA, Herviastuti R, Dwianingsih EK, Setyoheriyanto D, Avanti WS, et al. Pathological profiles and clinical management challenges of breast cancer emerging in young women in Indonesia: a hospital-based study. BMC Womens Health. 2019;19(1):1–8. doi: 10.1186/s12905-019-0724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syarti A, Pasaribu U, Fauziah D, Mardiyana L, Wulanhandarini T. Characteristics and histopathological grading of malignant spiculated mass in regards to histopathological grading of breast cancer based on The Nottingham Grading System. Biomol Health Sci J. 2020;3:33–6. [Google Scholar]
- 27.Tsang JY, Gary MT. Molecular classification of breast cancer. Adv Anat Pathol. 2020;27(1):27–35. doi: 10.1097/PAP.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 28.Pande D, Negi R, Karki K, Khanna S, Khanna RS, Khanna HD. Oxidative damage markers as possible discriminatory biomarkers in breast carcinoma. Transl Res. 2012;160(6):411–8. doi: 10.1016/j.trsl.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Graille M, Wild P, Sauvain JJ, Hemmendinger M, Guseva Canu I, Hopf NB. Urinary 8-OHdG as a biomarker for oxidative stress: a systematic literature review and meta-analysis. Int J Mol Sci. 2020;21(11):3743. doi: 10.3390/ijms21113743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiorcea-Paquim AM. 8-oxoguanine and 8-oxodeoxyguanosine Biomarkers of Oxidative DNA Damage: A Review on HPLC–ECD Determination. Molecules. 2022;27(5):1620. doi: 10.3390/molecules27051620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yugawa K, Itoh S, Yoshizumi T, Yoshiya S, Takeishi K, Toshima T, et al. Prognostic impact of 8‐hydroxy‐deoxyguanosine and its repair enzyme 8‐hydroxy‐deoxyguanosine DNA glycosylase in hepatocellular carcinoma. Pathol Int. 2020;70(8):533–41. doi: 10.1111/pin.12952. [DOI] [PubMed] [Google Scholar]
- 32.Wang R, Hao W, Pan L, Boldogh I, Ba X. The roles of base excision repair enzyme OGG1 in gene expression. Cell Mol Life Sci. 2018;75:3741–50. doi: 10.1007/s00018-018-2887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pisoschi AM, Pop A, Iordache F, Stanca L, Predoi G, Serban AI. Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur J Med Chem. 2021;209:112891. doi: 10.1016/j.ejmech.2020.112891. [DOI] [PubMed] [Google Scholar]
- 34.Lee YS, Kang J, Jung ES, Lee A. High expression of NRF2 and low expression of KEAP1 predict worse survival in patients with operable triple-negative breast cancer. J Breast Cancer. 2023;26(5):461–78. doi: 10.4048/jbc.2023.26.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onodera Y, Motohashi H, Takagi K, Miki Y, Shibahara Y, Watanabe M, et al. NRF2 immunolocalization in human breast cancer patients as a prognostic factor. Endocr Relat Cancer. 2014;21(2):241–52. doi: 10.1530/ERC-13-0234. [DOI] [PubMed] [Google Scholar]
- 36.Silva-Islas CA, Maldonado PD. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol Res. 2018;134:92–9. doi: 10.1016/j.phrs.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Dodson M, de la Vega MR, Cholanians AB, Schmidlin CJ, Chapman E, Zhang DD. Modulating NRF2 in disease: timing is everything. Annu Rev Pharmacol Toxicol. 2019;59(1):555–75. doi: 10.1146/annurev-pharmtox-010818-021856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar H, Kumar RM, Bhattacharjee D, Somanna P, Jain V. Role of Nrf2 signaling cascade in breast cancer: strategies and treatment. Front Pharmacol. 2022;13:720076. doi: 10.3389/fphar.2022.720076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song MY, Lee DY, Chun KS, Kim EH. The role of NRF2/KEAP1 signaling pathway in cancer metabolism. Int J Mol Sci. 2021;22(9):4376. doi: 10.3390/ijms22094376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98(3):1169–203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oshi M, Angarita FA, Tokumaru Y, Yan L, Matsuyama R, Endo I, et al. High expression of NRF2 is associated with increased tumor-infiltrating lymphocytes and cancer immunity in ER-positive/HER2-negative breast cancer. Cancers (Basel) 2020;12(12):3856. doi: 10.3390/cancers12123856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bovilla VR, Kuruburu MG, Bettada VG, Krishnamurthy J, Sukocheva OA, Thimmulappa RK, et al. Targeted inhibition of anti-inflammatory regulator Nrf2 results in breast cancer retardation in vitro and in vivo. Biomedicines. 2021;9(9):1119. doi: 10.3390/biomedicines9091119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orešić T, Bubanović S, Ramić S, Šarčević B, Gašparović AČ. Nuclear localization of NRF2 in stroma of HER2 positive and triple-negative breast cancer. Pathol Res Pract. 2023;248:154662. doi: 10.1016/j.prp.2023.154662. [DOI] [PubMed] [Google Scholar]
- 44.Almeida M, Soares M, Ramalhinho AC, Moutinho JF, Breitenfeld L, Pereira L. The prognostic value of NRF2 in breast cancer patients: A systematic review with meta-analysis. Breast Cancer Res Treat. 2020;179:523–32. doi: 10.1007/s10549-019-05494-4. [DOI] [PubMed] [Google Scholar]
- 45.Ghareghomi S, Habibi-Rezaei M, Arese M, Saso L, Moosavi-Movahedi AA. Nrf2 modulation in breast cancer. Biomedicines. 2022;10(10):2668. doi: 10.3390/biomedicines10102668. [DOI] [PMC free article] [PubMed] [Google Scholar]