Abstract
Objective: To identify the risk factors for vascular complications after total cerebral angiography by trans-radial access (TRA) in elderly cerebrovascular accident (CVA) patients and develop a predictive model. Methods: Data from 248 elderly CVA patients at Lianyungang Affiliated Hospital from December 2021 to March 2024 were retrospectively analyzed. The patients were divided into two groups: complicated (those with vascular complications) and non-complicated (those without vascular complications). Clinical data were collected and analyzed. Risk factors were identified using multifactorial logistic regression. Results: A total of 62 patients experienced vascular complications. Independent risk factors included intraoperative heparin dosage, number of radial artery punctures, timing of surgery, surgical time, mode of pressure hemostasis, duration of pressure hemostasis, and postoperative HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly) score (P<0.05). The nomogram model for predicting complications showed a good Hosmer-Lemeshow fit (χ2=2.099, P=0.978). Receiver operating characteristic analysis revealed an area under the curve of 0.868 [95% confidence interval (CI) (0.800, 0.936)] for the training set and 0.822 [95% CI (0.723, 0.921)] for the validation set. Decision curve analysis showed a net benefit >0 in both sets within certain risk thresholds. Conclusions: Vascular complications following total cerebral angiography by TRA in elderly CVA patients are associated with multiple factors. The nomogram model developed from these risk factors has significant predictive value.
Keywords: Cerebrovascular accident, trans-radial access, total cerebral angiography, vascular complications, risk factors
Introduction
Acute cerebrovascular accident (CVA), commonly referred to as “stroke”, is a highly debilitating neurological disorder that represents a major public health threat [1]. It can be broadly categorized into ischemic and hemorrhagic types. Ischemic stroke occurs due to vascular embolism, obstructing blood supply to the brain, while hemorrhagic stroke results from blood vessel rupture, causing bleeding within brain tissue. The high morbidity, disability, and mortality rates associated with stroke are significant global concerns [1].
Acute stroke is the second leading cause of death worldwide, affecting more than 15 million individuals annually. In the United States, it causes long-term disabilities in over 800,000 people, imposing an economic burden of approximately $46 billion annually [2]. In China, the 2019 Global Burden of Disease Study reported 3.94 million new stroke cases, 28.76 million prevalent cases, and 2.19 million stroke-related deaths [3]. These alarming statistics underscore the urgent need for effective prevention, diagnosis, and treatment strategies.
In cerebrovascular interventions, the choice of access route is critical. Initially, trans-radial access (TRA) was less commonly used compared to trans-femoral access (TFA) due to anatomic and technical challenges, such as the smaller diameter of the radial artery, its tortuous path, the complexity of selective intubation, and limited catheter flexibility. However, with advancements in medical technology, particularly the development of more durable and flexible catheters, TRA has become more widely adopted in neurological interventions. TRA has demonstrated advantages, such as reduced mortality, morbidity, and access site complications [4-7]. Additionally, it is associated with lower hospitalization costs and shorter stays, while significantly improving patient satisfaction.
Several studies have compared the therapeutic efficacy of TFA and TRA in mechanical resection for acute ischemic stroke, consistently showing that TRA effectively reduces the incidence of vascular complications at the access site without compromising clinical outcomes or surgical results [8-13]. As a result, TRA has become an increasingly preferred approach in cerebrovascular interventions.
Total cerebral angiography using TRA is a valuable diagnostic procedure in elderly CVA patients, offering detailed insight into the cerebral arterial vasculature and the severity of injury [14,15]. However, as an invasive procedure, it carries inherent risks of complications related to the access site. These complications may include radial artery spasm, radial artery occlusion, hematoma, pseudoaneurysm, or even neurological injury. Such complications can cause bleeding, significant pain, and discomfort, which may adversely affect the patient’s prognosis and quality of life.
Despite the growing use of TRA in cerebrovascular interventions, there remains a lack of comprehensive understanding regarding the specific risk factors for vascular complications in elderly CVA patients undergoing total cerebral angiography. Furthermore, there is a need for reliable risk prediction models that can help clinicians identify high-risk patients in advance. These models would enable the implementation of preventive measures and personalized treatment plans, ultimately reducing vascular complications and improving patient outcome. This knowledge gap and the clinical need for better risk assessment tools motivated this study.
Our study aims to systematically investigate the risk factors for vascular complications in elderly CVA patients following total cerebral angiography by TRA. Additionally, we aim to construct a risk prediction model to identify key factors contributing to vascular complications. By doing so, we hope to reduce the incidence of such complications in elderly CVA patients undergoing TRA angiography, providing a reference for more standardized and reasonable clinical treatment plans, and ultimately improving patient management.
Materials and methods
Research design
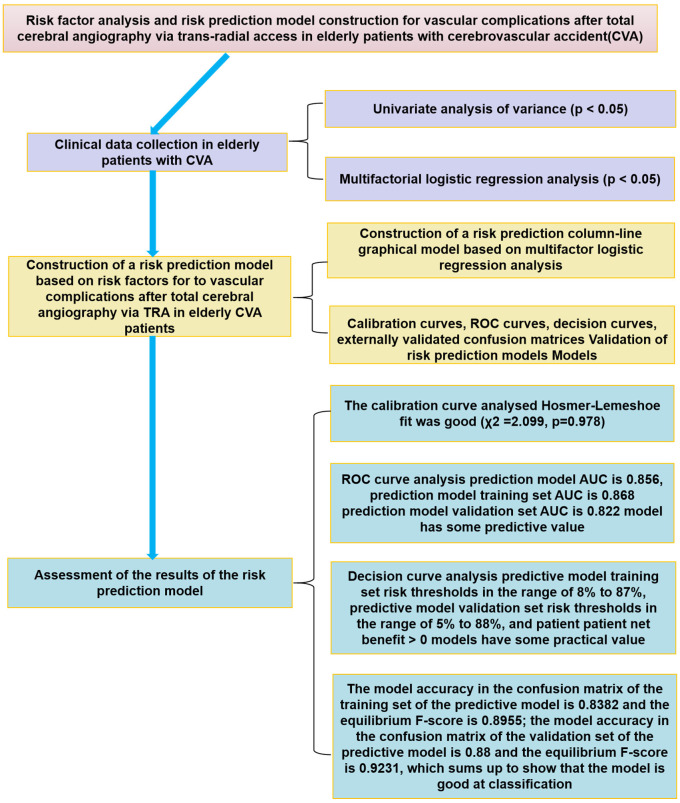
The flowchart of this study outlines the process used to analyze the risk factors associated with elderly patients who have experienced a CVA after total cerebral angiography by TRA. It also details the development of a risk prediction model, its subsequent validation, and the assessment of the results in elderly CVA patients. This is presented in Figure 1.
Figure 1.
Flow chart of risk factor analysis. CVA: cerebrovascular accident; TRA: trans-radial access; ROC: receiver operating characteristic; AUC: area under the curve.
Case selection
A total of 248 elderly CVA patients who were admitted to Lianyungang Affiliated Hospital of Nanjing University of Chinese Medicine from December 2021 to March 2024 were retrospectively selected for this study.
Inclusion Criteria [16]: All patients met the diagnostic criteria for CVA. Patients were aged ≥60 years.
Exclusion criteria: (1) Patients with impaired consciousness. (2) Patients with vasculitis, cerebral embolism, or other non-atherosclerotic diseases. (3) Patients with liver or kidney dysfunction, or malignant tumors. (4) Patients with incomplete follow-up data or unavailable clinical information. (5) Patients who withdrew from the study or had poor adherence. (6) Patients who had craniocerebral trauma within the last three months.
This study was approved by the Ethics Committee of Lianyungang Affiliated Hospital of Nanjing University of Chinese Medicine.
Diagnostic Criteria for Vascular Complications [15]: (1) The appearance of a dark red or red bloody mass under the skin at the puncture site, which does not fade when pressed, accompanied by pain, tenderness, or other local hematoma signs. (2) If the circumference of the operated limb is more than 5 mm larger than the preoperative limb, it indicates the occurrence of limb swelling. (3) Patients may experience concurrent vascular complications such as radial artery occlusion, radial artery spasm, or bleeding at the puncture site.
Within three days after total cerebral angiography by TRA, patients were categorized into two groups based on the occurrence of vascular complications: the complicated group and the non-complicated group.
Intervening methods
During the TRA procedure, the patient was placed in a horizontal position, and local anesthesia was administered.
Puncture method: The puncture site was selected approximately two cm above the transverse carpal tunnel, and the radial artery was punctured using Seldinger’s method [17]. After successful puncture, a soft-tipped straight guidewire was inserted, the needle core was withdrawn, and a 6F radial artery sheath was placed [18]. Heparin was then injected into the sheath at a dosage ranging from 50 IU/kg to 80 IU/kg. To prevent vasospasm, 100 μg of nitroglycerin was injected into the sheath [15].
Cerebral angiography: A guidewire was used for aortic arch imaging. Then, a 5F Simon III contrast catheter was inserted under the guidance of a 0.035-in guidewire. The catheter crossed the aortic arch and entered the descending aorta. After withdrawing the guidewire, the ultra-smooth guidewire catheter was pulled back, rotated, and shaped in the ascending aorta. The catheter was then rotated and retracted to identify the openings of arteries in the upper arch. Subsequently, the catheter was advanced into the right and left common carotid arteries for angiography of the internal carotid arteries. The catheter was further advanced into the left subclavian artery for left vertebral basilar artery angiography. Finally, the catheter was retracted from the right vertebral artery, where angiography was performed. The vessel alignment and radiation source placement were determined, and based on vessel alignment and arterial lesions, the appropriate balloon and stent were selected. Stent placement was determined, the catheter was removed, and pressure was applied to stop bleeding after completing the TRA procedure.
Data collection and outcome measurement
(1) The primary indicators included gender, age, intraoperative heparin dosage, number of implanted stents, number of radial artery punctures, timing of surgery, surgical time, mode of pressure hemostasis, duration of pressure hemostasis, and the postoperative hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly (HAS-BLED) score.
Postoperative HAS-BLED score: This scoring system is used to assess the bleeding risk after surgery. It incorporates factors such as hypertension, abnormal renal and liver function, stroke history, bleeding history or predisposition, labile international normalized ratio, age over 65 years, and concomitant use of drugs or alcohol. A higher score indicates a greater bleeding risk and may be associated with vascular complications.
(2) The secondary indicators included body mass index (BMI), white blood cell count, hemoglobin, platelet count, creatinine, total cholesterol, and a history of smoking, alcohol consumption, diabetes mellitus, hypertension, hyperlipidemia, atrial fibrillation, or acute coronary syndrome (ACS) type.
Statistical methods
The data were analyzed using SPSS 27.0 statistical software. For counted data, the results were presented as relative frequencies, and for continuous data that followed a normal distribution, the results were expressed as mean (x̅±s, standard deviation). The chi-square test (χ2-test) was used for intergroup comparisons of counted data, while the independent samples t-test was used for comparisons between two groups of measured data. Multifactorial logistic regression analysis was conducted to identify the risk factors for vascular complications in elderly CVA patients after total cerebral angiography by TRA. Based on the results of multifactorial analysis, statistically significant data were split into a training set and a validation set in a 7:3 ratio, with sample sizes of 173 and 75 cases, respectively. A nomogram model for predicting the risk of vascular complications in elderly CVA patients undergoing total cerebral angiography by TRA was constructed using R 4.2.2 software. The calibration curve was used to assess the goodness-of-fit of the nomogram model. The receiver operating characteristic (ROC) curve was employed to analyze the predictive value of the nomogram model for vascular complications in elderly CVA patients. A decision curve was plotted to evaluate the clinical validity of the nomogram model. A P-value of less than 0.05 was considered significant.
Results
Vascular complication profile
A total of 248 elderly patients who had suffered a CVA were enrolled in the study, with 62 patients (25%) experiencing vascular complications. These complications included 11 cases of puncture-site bleeding, 9 cases of puncture-site hematoma, 14 cases of upper extremity swelling, 16 cases of subcutaneous petechiae, and 12 cases of radial artery occlusion.
Comparison of general data
There were no statistically significant differences between the two groups in terms of gender, age, BMI, number of stents implanted, smoking history, alcohol consumption history, diabetes history, hypertension history, hyperlipidemia history, atrial fibrillation history, or ACS type history (all P>0.05). However, the intraoperative heparin dosage in the complicated group was higher than in the non-complicated group. The proportion of patients with ≥3 radial artery punctures, emergency surgery, surgical duration ≥2 hours, use of an elastic compression bandage for hemostasis, duration of pressure hemostasis ≥4 hours, and postoperative HAS-BLED score ≥3 were significantly higher in the complicated group compared to the non-complicated group (all P<0.05), as shown in Table 1.
Table 1.
Comparison of general data, univariate analysis
| Variable | Complicated group (n=62) | Non-complicated group (n=186) | χ2 (t) | P |
|---|---|---|---|---|
| Gender (male/female) | 42/20 | 145/41 | 2.616 | 0.106 |
| Age (x̅±s) | 71.34±8.21 | 72.30±8.26 | 0.801 | 0.424 |
| BMI (Kg/m2) (x̅±s) | 21.68±2.46 | 21.73±2.59 | 0.125 | 0.901 |
| Intraoperative heparin dosage (x̅±s, IU) | 4766±654 | 4086±897 | 5.496 | <0.001 |
| Number of implanted stents (x̅±s) | 1.82±0.67 | 1.80±0.69 | 0.213 | 0.832 |
| History of smoking [n (%)] | 25 (40.32) | 78 (41.94) | 0.050 | 0.823 |
| Alcohol consumption [n (%)] | 22 (32.48) | 76 (40.86) | 0.562 | 0.453 |
| Diabetes mellitus [n (%)] | 10 (16.13) | 36 (19.35) | 0.320 | 0.571 |
| Hypertension [n (%)] | 20 (32.26) | 55 (29.56) | 0.159 | 0.690 |
| Hyperlipidaemia [n (%)] | 14 (22.58) | 35 (18.82) | 0.415 | 0.519 |
| Atrial fibrillation [n (%)] | 26 (41.94) | 77 (41.40) | 0.006 | 0.941 |
| White blood cell count (×10^9/L) | 8.69±1.18 | 8.50±1.01 | 1.249 | 0.231 |
| Hemoglobin (g/L) | 153.93±6.47 | 154.24±5.33 | 0.373 | 0.709 |
| Platelet count (×10^9/L) | 125.04±12.71 | 122.21±11.63 | 1.616 | 0.107 |
| Creatinine (μmol/L) | 70.75±11.65 | 70.41±10.00 | 0.219 | 0.827 |
| Total Cholesterol (mmol/L) | 5.93±1.16 | 5.71±0.88 | 1.539 | 0.125 |
| Type of ACS [n (%)] | 5.523 | 0.063 | ||
| Unstable angina | 15 (24.19) | 74 (39.78) | ||
| ST-segment elevation myocardial infarction | 40 (64.52) | 100 (53.76) | ||
| Non-ST-segment elevation myocardial infarction | 7 (11.29) | 12 (6.45) | ||
| Timing of surgery [n (%)] | 25.828 | <0.001 | ||
| Emergency | 42 (67.74) | 58 (31.18) | ||
| Schedule | 20 (32.26) | 128 (68.82) | ||
| Surgical time [n (%)] | 10.240 | 0.006 | ||
| <1 h | 7 (11.29) | 26 (13.98) | ||
| 1-2 h | 28 (45.16) | 118 (63.44) | ||
| ≥2 h | 27 (43.55) | 42 (22.58) | ||
| Mode of pressure hemostasis [n (%)] | 18.727 | <0.001 | ||
| Elastic compression bandage | 24 (38.71) | 25 (13.44) | ||
| Pressure device | 38 (61.29) | 161 (86.56) | ||
| Duration of pressure hemostasis [n (%)] | 6.549 | 0.010 | ||
| <4 h | 41 (66.13) | 152 (81.72) | ||
| ≥4 h | 21 (33.87) | 34 (18.28) | ||
| Postoperative HAS-BLED bleeding score [n (%)] | 6.930 | 0.008 | ||
| <3 | 47 (75.80) | 166 (89.24) | ||
| ≥3 | 15 (24.19) | 20 (10.75) | ||
| Number of radial artery punctures | 4.333 | 0.037 | ||
| <3 | 48 (77.42) | 164 (88.17) | ||
| ≥3 | 14 (22.58) | 22 (11.83) |
CVA: cerebrovascular accident; TRA: trans-radial access; BMI: body mass index; IU: international units; ACS: acute coronary syndrome; HAS-BLED: Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly, Drugs/alcohol concomitantly.
Multifactorial logistic regression analysis of risk factors for vascular complications
The occurrence of vascular complications after total cerebral angiography by TRA in elderly CVA patients was treated as the dependent variable (0= no complication, 1= complication). The items from Table 1 that showed statistically significant differences, including intraoperative heparin dosage, number of radial artery punctures, timing of surgery, surgical time, mode of pressure hemostasis, duration of pressure hemostasis, and postoperative HAS-BLED score, were selected as independent variables for the logistic regression analysis. The assignment details of each indicator are provided in Table 2. The results showed that intraoperative heparin dosage (P<0.001), number of radial artery punctures (P=0.015), timing of surgery (P=0.015), surgical time (P=0.002), mode of pressure hemostasis (P<0.001), duration of pressure hemostasis (P=0.002), and postoperative HAS-BLED score (P=0.004) were independent risk factors for vascular complications after total cerebral angiography via TRA in elderly CVA patients (P<0.05). See Table 3.
Table 2.
Assignment method
| Variable | Assignment |
|---|---|
| Intraoperative heparin dosage | measured value |
| Number of radial artery punctures | <3=0, ≥3=1 |
| Timing of surgery | 0= Schedule, 1= emergency |
| Surgical time | <1 h= 0, 1 h-2 h=1, >2 h=2 |
| Mode of pressure hemostasis | 0= pressure device, 1= elastic compression bandage |
| Duration of pressure hemostasis | <4 h=0, ≥4 h=1 |
| Postoperative HAS-BLED bleeding score | <3 scores =0, ≥3 scores =1 |
HAS-BLED: Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly, Drugs/alcohol concomitantly.
Table 3.
Multifactorial logistic regression analysis
| Variable | β | SE | Wald χ2 | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Intraoperative heparin dosage | 0.001 | 0.000 | 20.038 | <0.001 | 1.001 | (1.001, 1.002) |
| Number of radial artery punctures | 1.239 | 0.512 | 5.861 | 0.015 | 3.452 | (1.266, 9.414) |
| Timing of surgery | 1.318 | 0.378 | 12.174 | 0.015 | 3.738 | (1.782, 7.838) |
| Surgical time | 0.971 | 0.306 | 10.054 | 0.002 | 2.641 | (1.449, 4.814) |
| Mode of pressure hemostasis | 1.400 | 0.425 | 10.849 | <0.001 | 4.056 | (1.763, 9.332) |
| Duration of pressure hemostasis | 1.274 | 0.414 | 9.468 | 0.002 | 3.574 | (1.588, 8.045) |
| Postoperative HAS-BLED bleeding score | 1.418 | 0.492 | 8.326 | 0.004 | 4.130 | (1.576, 10.824) |
HAS-BLED: Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly, Drugs/alcohol concomitantly; SE: standard error; OR: odds ratio; CI: confidence interval.
Risk prediction nomogram model
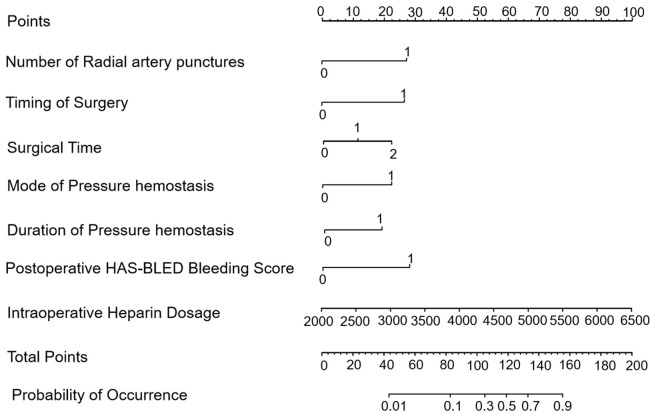
Based on multifactorial logistic regression analysis, a nomogram model was constructed to predict the risk of vascular complications after total cerebral angiography by TRA in elderly CVA patients, as shown in Figure 2.
Figure 2.
Risk-predictive columnar graphical model of the risk of vascular complications in elderly patients with cerebrovascular accident (CVA) after total cerebral angiography by trans-radial access (TRA). HAS-BLED: Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly, Drugs/alcohol concomitantly.
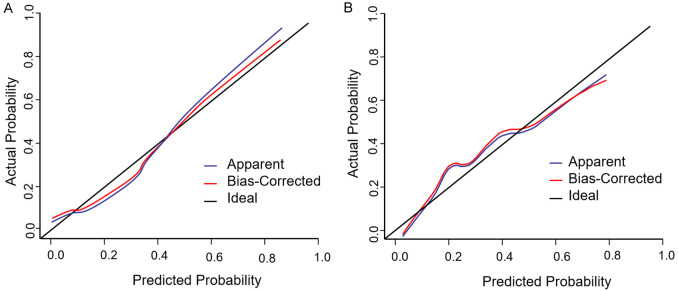
The results of the Hosmer-Lemeshow goodness-of-fit test indicated that the nomogram model exhibited a good fit (χ2=2.099, P=0.978), as shown in Figure 3A and 3B.
Figure 3.
Training set and validation set calibration curves of elderly cerebrovascular accident (CVA) patients to vascular complications after whole brain angiography by trans-radial access (TRA). A: Training set; B: Validation set.
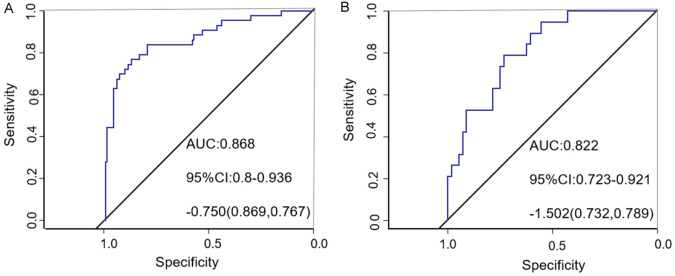
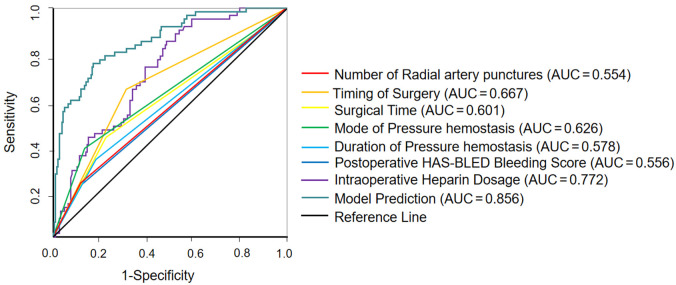
ROC curve analysis demonstrated that the area under the curve (AUC) for the nomogram model predicting vascular complications in elderly CVA patients undergoing cerebral angiography by TRA in the training set was 0.868 [95% confidence interval (CI) (0.800, 0.936)], as shown in Figure 4A. For the validation set, the nomogram model predicted an AUC of 0.822 [95% CI (0.723, 0.921)] for vascular complications after TRA in elderly CVA patients, as shown in Figure 4B. Additionally, the study compared the predictive efficiency of the single index and the prediction model. The results showed that the AUC of the prediction model was higher than that of the single index, as shown in Figure 5.
Figure 4.
Training set and validation set receiver operating characteristic of elderly cerebrovascular accident (CVA) patients to vascular complications after whole brain angiography by trans-radial access (TRA). A: Training set; B: Validation set.
Figure 5.
Receiver operating characteristic of predictive models versus risk factors alone for predicting vascular complications in elderly patients with cerebrovascular accident (CVA) after total cerebral angiography by trans-radial access (TRA). AUC: area under the curve.
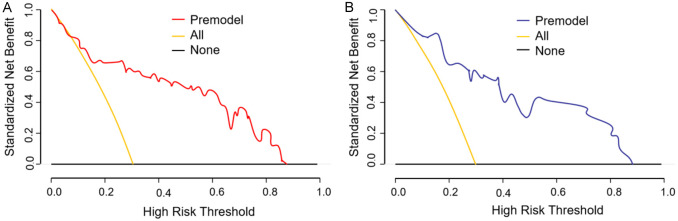
The results of decision curve analysis showed that when the risk threshold for vascular complications after total cerebral angiography by TRA in elderly CVA patients was predicted in the range of 8% to 87% for the training set, the net benefit rate was >0. Similarly, for the validation set, when the risk threshold was predicted in the range of 5% to 88%, the net benefit rate was >0, as shown in Figure 6.
Figure 6.
Training set and validation set decision curves of elderly cerebrovascular accident (CVA) patients for vascular complications after whole brain angiography by trans-radial access (TRA). A: Training set; B: Validation set.
Based on the established prediction model for vascular complications after cerebral angiography by TRA in elderly CVA patients, both the training set and the validation set were validated and evaluated. As shown in Figure 7, for the training set’s confusion matrix, among the cases where vascular complications actually occurred (observed as 1), the model correctly predicted 25 cases as having complications and wrongly predicted 20 cases as not having complications. Among the cases where vascular complications did not occur (observed as 0), the model wrongly predicted 8 cases as having complications and correctly predicted 120 cases as not having complications. For the validation set’s confusion matrix, among the cases where vascular complications actually occurred (observed as 1), the model correctly predicted 12 cases as having complications and wrongly predicted 5 cases as not having complications. Among the cases where vascular complications did not occur (observed as 0), the model wrongly predicted 4 cases as having complications and correctly predicted 54 cases as not having complications.
Figure 7.
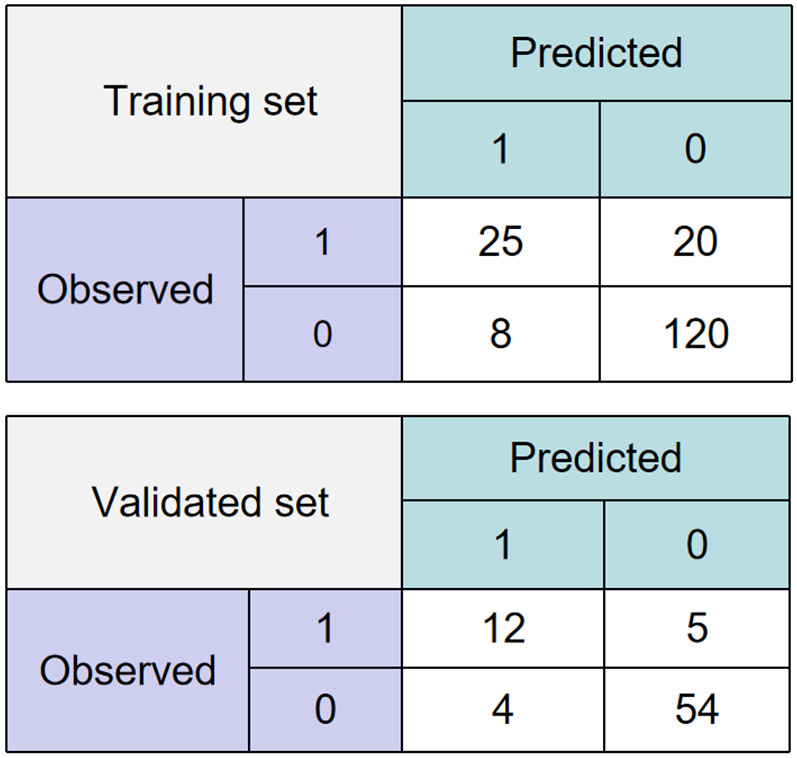
Confusion matrix for elderly cerebrovascular accident (CVA) patients for vascular complications after total cerebral angiography by trans-radial access (TRA).
Discussion
In this study, we found that the prevalence of vascular complications following total cerebral angiography by TRA in elderly CVA patients was 25%. These complications typically manifested as hematomas, subcutaneous bruising, localized limb pain, and radial artery occlusion. Several factors contributed to the occurrence of these complications.
Intraoperative heparin dosage was identified as a key factor [19]. Insufficient intraoperative anticoagulation may lead to radial artery occlusion, which is the most common complication of radial artery access, with a reported prevalence ranging from 1% to 30% [20-24]. In our study, the heparin dosage in the complicated group was approximately 4,700 IU, compared to 4,000 IU in the non-complicated group. The Society of Cardiovascular Angiography and Interventions recommends a minimum of 50 IU/kg or 5,000 IU of heparin during radial artery access [25]. Multifactorial analysis indicated that intraoperative heparin dosage was an independent risk factor. Thus, adjusting the heparin dosage to 5,000 IU based on the patient’s condition during radial artery total cerebral angiography is essential for minimizing the risk of vascular complications [26].
In addition to heparin dosage, the number of radial artery punctures was also an independent risk factor. Each additional puncture increased the risk of vascular complications by 2.452-fold. Multiple punctures are associated with higher risks of complications, such as radial artery occlusion, spasm, and loss of pulsation [27]. To improve access success, methods like subcutaneous injection of nitrates to dilate the radial artery can be used [28]. In our study, an intrathecal injection of 100 μg nitroglycerin was employed to mitigate puncture failure and reduce complication incidence.
The timing of surgery and surgical time also significantly influenced the risk of vascular complications. The risk of complications was 3.738 times higher in patients undergoing emergency total cerebral angiography by TRA compared to those undergoing elective procedures. Elective surgery in CVA patients was associated with lower perioperative mortality and fewer major cardiovascular events during the first nine months post-stroke, while emergency surgery increased the risk of these events [29]. Adequate preoperative observation and preparation are crucial for determining the optimal surgical time and minimizing vascular complication risks [30].
Inexperience of the interventionalist and improper technique can result in longer surgical times, increased contrast use, and higher radiation exposure. Our analysis showed that prolonged surgical time was an independent risk factor, with longer surgical times increasing the risk of vascular complications. Extended procedures not only increase patient discomfort but also pose higher risks of adverse events due to prolonged exposure to invasive procedures and potential damage to the vascular endothelium.
The mode and duration of pressure hemostasis, as well as the postoperative HAS-BLED bleeding score, were significant factors in the occurrence of vascular complications after total cerebral angiography by TRA. A four-hour cycle of intermittent deflation after radial artery puncture has been shown to reduce the incidence of vascular complications at the puncture site [31]. However, smaller radial arteries and longer hemostasis times in TRA compression occlusion can lead to a higher incidence of radial artery occlusion. This is likely due to compromised blood flow and increased pressure on the arterial wall during prolonged compression [32].
The HAS-BLED score, which assesses bleeding risk in atrial fibrillation patients on anticoagulants [33], was also identified as an independent risk factor in our study. A score of ≥3 indicates a higher bleeding risk, and this score can help predict the likelihood of vascular complications following total cerebral angiography by TRA [34]. Clinicians can use this score to stratify patients based on their bleeding risk and implement personalized preventive strategies, such as adjusting anticoagulation intensity or closely monitoring for bleeding signs.
A nomogram is a visualized clinical prediction model that provides a scientific basis for clinical decision-making [35]. In this study, a nomogram model was developed to predict the risk of vascular complications following total cerebral angiography by TRA in elderly CVA patients. Each risk factor was assigned a score on a scale of 0-100, and the total score was used to calculate the corresponding risk probability. The Hosmer-Lemeshow goodness-of-fit test showed a high degree of fit. ROC analysis revealed that the AUCs for the training set, validation set, and the prediction model were 0.868, 0.822, and 0.856, respectively, indicating good predictive value. Decision curve analysis suggested that the model was of practical value, with positive net benefits within certain risk thresholds. High accuracy, precision, recall, and F1-scores in both the training and validation sets further supported the model’s favorable predictive efficacy.
A major limitation of this study was the relatively small sample size, which may have affected the accuracy and stability of the constructed predictive model. A small sample size may not fully represent the entire population of elderly CVA patients undergoing total cerebral angiography by TRA, possibly introducing biases in the identification of risk factors and the performance of the predictive model. Additionally, the single-center nature of the study could have led to selection biases, as the patient population in one center may not be fully representative of the broader population.
In the future, multi-center joint clinical studies should be conducted to significantly expand the sample size and include a more diverse patient population. By integrating diverse data from multiple centers, the generalizability of the results can be improved, enabling construction of a more accurate, stable, and clinically relevant prediction model. This model could better guide clinical decision-making, such as optimizing surgical procedures, adjusting anticoagulant dosages, and improving hemostasis methods, ultimately reducing the incidence of vascular complications in elderly CVA patients undergoing total cerebral angiography by TRA. Furthermore, future studies could explore the potential interactions between different risk factors and their combined effect on vascular complications, providing deeper insight into the underlying mechanisms and leading to more effective preventive strategies.
In conclusion, vascular complications after total cerebral angiography by TRA in elderly CVA patients are associated with factors such as intraoperative heparin dosage, number of radial artery punctures, timing of surgery, surgical time, the mode of pressure hemostasis, and postoperative HAS-BLED bleeding score. The nomogram model developed based on these risk factors demonstrated excellent predictive ability for vascular complications after TRA cerebral angiography in elderly CVA patients. This model exhibits a high degree of goodness-of-fit, strong predictive value, practical significance, and high-precision metrics, making it a valuable tool for guiding clinical decision-making.
Disclosure of conflict of interest
None.
References
- 1.Lee EC, Ha TW, Lee DH, Hong DY, Park SW, Lee JY, Lee MR, Oh JS. Utility of exosomes in ischemic and hemorrhagic stroke diagnosis and treatment. Int J Mol Sci. 2022;23:8367. doi: 10.3390/ijms23158367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shehjar F, Maktabi B, Rahman ZA, Bahader GA, James AW, Naqvi A, Mahajan R, Shah ZA. Stroke: molecular mechanisms and therapies: update on recent developments. Neurochem Int. 2023;162:105458. doi: 10.1016/j.neuint.2022.105458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang YJ, Li ZX, Gu HQ, Zhai Y, Zhou Q, Jiang Y, Zhao XQ, Wang YL, Yang X, Wang CJ, Meng X, Li H, Liu LP, Jing J, Wu J, Xu AD, Dong Q, Wang D, Wang WZ, Ma XD, Zhao JZ China Stroke Statistics Writing Committee. China stroke statistics: an update on the 2019 report from the national center for healthcare quality management in neurological diseases, China national clinical research center for neurological diseases, the Chinese stroke association, national center for chronic and non-communicable disease control and prevention, Chinese center for disease control and prevention and institute for global neuroscience and stroke collaborations. Stroke Vasc Neurol. 2022;7:415–450. doi: 10.1136/svn-2021-001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khanna O, Velagapudi L, Das S, Sweid A, Mouchtouris N, Al Saiegh F, Avery MB, Chalouhi N, Schmidt RF, Sajja K, Gooch MR, Tjoumakaris S, Rosenwasser RH, Jabbour PM. A comparison of radial versus femoral artery access for acute stroke interventions. J Neurosurg. 2020;135:727–732. doi: 10.3171/2020.7.JNS201174. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman H, Bunch KM, Mikhailova T, Cote JR, Kumar AA, Masoud HE, Gould GC. Comparison of the safety, efficacy, and procedural characteristics associated with proximal and distal radial access for diagnostic cerebral angiography. J Stroke Cerebrovasc Dis. 2022;31:106204. doi: 10.1016/j.jstrokecerebrovasdis.2021.106204. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia K, Guest W, Lee H, Klostranec J, Kortman H, Orru E, Qureshi A, Kostynskyy A, Agid R, Farb R, Radovanovic I, Nicholson P, Krings T, Pereira VM. Radial vs. femoral artery access for procedural success in diagnostic cerebral angiography: a randomized clinical trial. Clin Neuroradiol. 2021;31:1083–1091. doi: 10.1007/s00062-020-00984-1. [DOI] [PubMed] [Google Scholar]
- 7.Sweid A, Das S, Weinberg JH, E L Naamani K, Kim J, Curtis D, Joffe D, Hiranaka CG, Vijaywargiya D, Sioka C, Oneissi M, El Hajjar AH, Gooch MR, Herial N, Tjoumakaris SI, Rosenwasser RH, Jabbour P. Transradial approach for diagnostic cerebral angiograms in the elderly: a comparative observational study. J Neurointerv Surg. 2020;12:1235–1241. doi: 10.1136/neurintsurg-2020-016140. [DOI] [PubMed] [Google Scholar]
- 8.Munich SA, Vakharia K, McPheeters MJ, Waqas M, Tso MK, Levy EI, Snyder KV, Siddiqui AH, Davies JM. Transition to transradial access for mechanical thrombectomy-lessons learned and comparison to transfemoral access in a single-center case series. Oper Neurosurg (Hagerstown) 2020;19:701–707. doi: 10.1093/ons/opaa230. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez D, Requena M, Olivé-Gadea M, de Dios M, Gramegna LL, Muchada M, García-Tornel Á, Diana F, Rizzo F, Rivera E, Rubiera M, Piñana C, Rodrigo-Gisbert M, Rodríguez-Luna D, Pagola J, Carmona T, Juega J, Rodríguez-Villatoro N, Molina C, Ribo M, Tomasello A. Radial versus femoral access for mechanical thrombectomy in patients with stroke: a noninferiority randomized clinical trial. Stroke. 2024;55:840–848. doi: 10.1161/STROKEAHA.124.046360. [DOI] [PubMed] [Google Scholar]
- 10.Waqas M, Monteiro A, Cappuzzo JM, Kruk MD, Almayman F, Housley SB, Lim J, Dossani RH, Snyder KV, Siddiqui AH, Davies JM, Levy EI. Mechanical thrombectomy with a balloon-guide catheter: sheathless transradial versus transfemoral approach. J Neurointerv Surg. 2024;16:187–191. doi: 10.1136/jnis-2022-019607. [DOI] [PubMed] [Google Scholar]
- 11.Siddiqui AH, Waqas M, Neumaier J, Zhang JF, Dossani RH, Cappuzzo JM, Van Coevering Iii RJ, Rai HH, Monteiro A, Sonig A, Davies JM, Snyder KV, Levy EI. Radial first or patient first: a case series and meta-analysis of transradial versus transfemoral access for acute ischemic stroke intervention. J Neurointerv Surg. 2021;13:687–692. doi: 10.1136/neurintsurg-2020-017225. [DOI] [PubMed] [Google Scholar]
- 12.Barranco-Pons R, Caamaño IR, Guillen AN, Chirife OS, Quesada H, Cardona P. Transradial versus transfemoral access for acute stroke endovascular thrombectomy: a 4-year experience in a high-volume center. Neuroradiology. 2022;64:999–1009. doi: 10.1007/s00234-021-02850-4. [DOI] [PubMed] [Google Scholar]
- 13.Verhey LH, Orozco AR, Oliver M, Lyons L, Sewell AP, Tsai JP, Mazaris P, Khan M, Singer JA. Transradial versus transfemoral access for mechanical thrombectomy in acute ischemic stroke: a retrospective cohort study. J Stroke Cerebrovasc Dis. 2023;32:107282. doi: 10.1016/j.jstrokecerebrovasdis.2023.107282. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia V, Kesha M, Kumar A, Prabhakar A, Chauhan R, Singh A. Diagnostic cerebral angiography through distal transradial access: early experience with a promising access route. Neurol India. 2023;71:453–457. doi: 10.4103/0028-3886.378692. [DOI] [PubMed] [Google Scholar]
- 15.Andò G, Costa F, Trio O, Oreto G, Valgimigli M. Impact of vascular access on acute kidney injury after percutaneous coronary intervention. Cardiovasc Revasc Med. 2016;17:333–8. doi: 10.1016/j.carrev.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, Lennon O, Meschia JF, Nguyen TN, Pollak PM, Santangeli P, Sharrief AZ, Smith SC Jr, Turan TN, Williams LS. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American heart association/American stroke association. Stroke. 2021;52:e364–e467. doi: 10.1161/STR.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 17.François K, De Clerck D, Robberechts T, Van Hulle FO, Luyten I, Jacobs-Tulleneers-Thevissen D, Van Cauwelaert SE. Let’s not be blind for alternatives: percutaneous insertion of peritoneal dialysis catheters by the nephrologist (modified Seldinger technique) Bulletin de la Dialyse à Domicile. 2023:6. [Google Scholar]
- 18.Wang H, Peng WJ, Liu YH, Ma GQ, Wang D, Su B, Liu YW. A comparison of the clinical effects and safety between the distal radial artery and the classic radial artery approaches in percutaneous coronary intervention. Ann Palliat Med. 2020;9:2568–2574. doi: 10.21037/apm-19-479. [DOI] [PubMed] [Google Scholar]
- 19.da Silva RL, de Andrade PB, Dangas G, Joaquim RM, da Silva TRW, Vieira RG, Pereira VC Jr, Sousa AGM, Feres F, Costa JR Jr. Randomized clinical trial on prevention of radial occlusion after transradial access using nitroglycerin: PATENS trial. JACC Cardiovasc Interv. 2022;15:1009–1018. doi: 10.1016/j.jcin.2022.02.026. [DOI] [PubMed] [Google Scholar]
- 20.Munir U, Khan R, Nazeer N, Akhter J, Hassan AU, Hanif B. Frequency and predictors of radial artery occlusion in patients undergoing percutaneous coronary intervention. Cureus. 2022;14:e25505. doi: 10.7759/cureus.25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dwivedi SK, Sharma AK, Nayak GR, Chaudhary GK, Chandra S, Pradhan A, Vishwakarma P, Bhandari M, Sethi R. Factors influencing radial artery occlusion after transradial coronary intervention in the Indian population. Anatol J Cardiol. 2022;26:105–111. doi: 10.5152/AnatolJCardiol.2021.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ognerubov DV, Sedaghat A, Provatorov SI, Tereshchenko AS, Bertrand OF, Bernat I, Arutyunyan GK, Pogorelova OA, Tripoten MI, Balakhonova TV, Samko AN, Merkulov EV. A randomized trial comparing short versus prolonged hemostasis with rescue recanalization by ipsilateral ulnar artery compression: impact on radial artery occlusion-the RESCUE-RAO trial. J Interv Cardiol. 2020;2020:7928961. doi: 10.1155/2020/7928961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohsen A, Alqasrawi M, Shantha GPS, DeZorzi C, Panaich S. Comparison of radial artery occlusion following transradial access for percutaneous coronary intervention using sheath-based versus sheathless technique. Sci Rep. 2018;8:12026. doi: 10.1038/s41598-018-30462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsigkas G, Papanikolaou A, Apostolos A, Kramvis A, Timpilis F, Latta A, Papafaklis MI, Aminian A, Davlouros P. Preventing and managing radial artery occlusion following transradial procedures: strategies and considerations. J Cardiovasc Dev Dis. 2023;10:283. doi: 10.3390/jcdd10070283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahal K, Sharma S, Yousuf A, Lee J, Azrin M, Jimenez E, Modi K, Tandon N. A comparison of standard versus low dose heparin on access-related complications after coronary angiography through radial access: a meta-analysis of randomized controlled trials. Cardiovasc Revasc Med. 2018;19:575–579. doi: 10.1016/j.carrev.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad GS, Zafarullah M, Anjum S, Arfeen S. Heparin low doses and standard doses effect on transradial catheterization. Pakistan Journal of Cardiovascular Intervention. 2022;2:11–19. [Google Scholar]
- 27.Bigler MR, Buffle E, Rappo MV, Grossenbacher R, Tschannen C, Seiler C. Association of palmar arch collateral function and radial artery occlusion after transradial access. Am J Cardiol. 2022;168:151–158. doi: 10.1016/j.amjcard.2021.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Doubell J, Kyriakakis C, Weich H, Herbst P, Pecoraro A, Moses J, Griffiths B, Snyman HW, Kabwe L, Du Toit R, Joubert L, Hassan K, Doubell A. Radial artery dilatation to improve access and lower complications during coronary angiography: the RADIAL trial. EuroIntervention. 2021;16:1349–1355. doi: 10.4244/EIJ-D-19-00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christiansen MN, Andersson C, Gislason GH, Torp-Pedersen C, Sanders RD, Føge Jensen P, Jørgensen ME. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology. 2017;127:9–19. doi: 10.1097/ALN.0000000000001685. [DOI] [PubMed] [Google Scholar]
- 30.Jones J, Rathod KS, Beirne AM, Hamshere SM, Choudry FA, O’Mahony C, Guttmann OP, Knight CJ, Amersey R, Wragg A, Baumbach A, Mathur A, Jones DA. An observational study assessing the predictors of procedural failure from the radial approach: is right radial access always the best? Cardiovasc Revasc Med. 2022;42:86–91. doi: 10.1016/j.carrev.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Jia D, Chen Y, Liu C, Ding S, Fang Y. Investigating optimal compression approach following radial artery puncture: a retrospective study. Am J Transl Res. 2024;16:2389–2397. doi: 10.62347/BWUP6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aminian A, Saito S, Takahashi A, Bernat I, Jobe RL, Kajiya T, Gilchrist IC, Louvard Y, Kiemeneij F, van Royen N, van Leeuwen M, Yamazaki S, Matsukage T, Iglesias JF, Rao SV. Impact of sheath size and hemostasis time on radial artery patency after transradial coronary angiography and intervention in Japanese and non-Japanese patients: a substudy from RAP and BEAT (Radial Artery Patency and Bleeding, Efficacy, Adverse evenT) randomized multicenter trial. Catheter Cardiovasc Interv. 2018;92:844–851. doi: 10.1002/ccd.27526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu J, Hutchens R, Hung J, Bennamoun M, McQuillan B, Briffa T, Sohel F, Murray K, Stewart J, Chow B, Sanfilippo F, Dwivedi G. Performance of multilabel machine learning models and risk stratification schemas for predicting stroke and bleeding risk in patients with non-valvular atrial fibrillation. Comput Biol Med. 2022;150:106126. doi: 10.1016/j.compbiomed.2022.106126. [DOI] [PubMed] [Google Scholar]
- 34.Vučić M, Lilić B. Clinical significance of bleeding scoring systems. Medicinski pregled. 2022;75(Suppl 1):133–142. [Google Scholar]
- 35.Wang X, Lu J, Song Z, Zhou Y, Liu T, Zhang D. From past to future: bibliometric analysis of global research productivity on nomogram (2000-2021) Front Public Health. 2022;10:997713. doi: 10.3389/fpubh.2022.997713. [DOI] [PMC free article] [PubMed] [Google Scholar]