Abstract
The intensity and duration of exposure can influence vaccine effectiveness (VE). For “leaky” vaccines such as SARS-CoV-2 vaccines, which reduce but do not entirely prevent infections, repeated or prolonged exposures may increase breakthrough infection likelihood. To test this hypothesis, we conducted a systematic review and meta-analysis of 76 test-negative design studies reporting VE against SARS-CoV-2 infection or severe disease. Exposure intensity was approximated using Oxford COVID-19 Government Response Tracker indices: Stringency Index (SI), Containment and Health Index (CHI), and Government Response Index (GRI). Based on 1,419 VE estimates, pooled VE against infection was significantly higher in settings with higher index values (lower exposure intensity): 82% (95% CI: 80–83%) in high-SI settings versus 39% (95% CI: 35–43%) in low-SI settings. Similar patterns appeared for other indices and severe disease outcomes. These associations persisted in meta-regression models adjusting for viral variant, vaccine type, time since vaccination, prior infection status, and enrollment criteria. Correlation analyses showed moderate-to-strong positive correlations between VE estimates and exposure indices (Spearman’s correlation: 0.50–0.62). These findings establish exposure intensity as a critical effect modifier of SARS-CoV-2 VE, demonstrating the leaky nature of COVID-19 vaccines and explaining heterogeneity in real-world effectiveness estimates. Future VE evaluations and vaccination strategies should account for exposure intensity to ensure accurate, context-specific estimates.
Keywords: COVID-19, SARS-CoV-2, vaccination, vaccine effectiveness, test-negative design, pre-existing immunity
INTRODUCTION
The COVID-19 pandemic, caused by SARS-CoV-2, has underscored the importance of effective vaccination strategies to reduce the burden of disease and safeguard public health. While vaccines have demonstrated significant efficacy in controlled clinical trials, their effectiveness in real-world conditions often varies due to differences in exposure, study design, and population characteristics. Vaccine effectiveness (VE) against COVID-19 is influenced by the level and duration of exposure to SARS-CoV-2, which is shaped by public health measures and individual behaviors. Observational studies, particularly test-negative design (TND) studies, have been widely used to evaluate VE in real-world settings (1). However, substantial variability in VE against COVID-19 has been reported both between and within populations (2–5). Some of this heterogeneity may be attributable to differences in the vaccines used and study design choices, such as the use of clinical symptoms criteria (6, 7), which we have previously shown can influence VE (8).
VE is also influenced by underlying population susceptibility, which itself may depend on pre-existing population immunity arising from prior infections (9) as well as behavioral differences affecting the amount of contact between potentially infectious and susceptible individuals (10). Directly measuring whether an individual has been exposed to SARS-CoV-2 (sometimes called the exposure-necessity assumption (11)) is challenging due to the dynamic nature of viral transmission and the complexity of population behaviours (12–15). Consequently, VE studies typically assume that vaccinated and unvaccinated individuals have equal risk of infection (16). However, it has previously been shown that vaccines with incomplete or “leaky” protection may demonstrate reduced effectiveness in high-exposure settings, where more frequent or more intense viral encounters could overcome vaccine-induced immunity (17) (18). Conversely, in settings with stringent public health measures, lower exposure levels may allow vaccines to perform more effectively by reducing the likelihood of breakthrough infections. In contrast, “all-or-nothing” vaccines provide complete immunity to some individuals while leaving others entirely susceptible (18, 19). In this model, VE is determined by the proportion of fully immune individuals and remains unaffected by exposure intensity.
Uniquely, during the COVID-19 pandemic several indicators of public health control measures to limit viral transmission were maintained which may make it possible to examine the role of differences in duration and intensity of exposure on COVID-19 VE (20). Such indices systematically quantify public health interventions such as lockdowns, mask mandates, and travel restrictions. Higher values reflect stricter measures and reduced opportunity for exposure, while lower values indicate weaker measures and greater opportunities for prolonged or frequent viral encounters (11, 17). In this study, we use three indices as proxies to investigate the relationship between exposure intensity to SARS-CoV-2 and estimated VE: the Stringency Index (SI); Containment and Health Index (CHI); and Government Response Index (GRI). We hypothesize that higher exposure intensity, indicated by lower index values, are associated with lower VE, reflecting the potential for COVID-19 vaccines to exhibit “leaky” behaviour in high-exposure settings. By examining how these indices act as effect modifiers and confounders, this study provides critical insights into the context-dependent nature of VE and offers guidance for optimizing vaccination strategies in diverse public health environments.
RESULTS
Overview
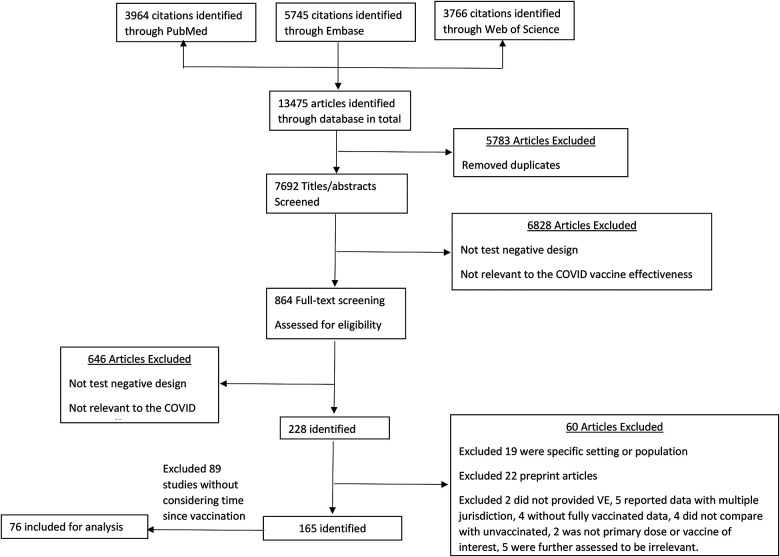
We systematically searched PubMed, Embase, and Web of Science for test-negative design studies reporting vaccine effectiveness (VE) against SARS-CoV-2 infection or severe disease following primary vaccination series. A total of 13,475 studies were identified, of which 5,783 were duplicates. Following title and abstract screening of the remaining articles, 864 studies were selected for full-text review. From these, 76 studies met the inclusion criteria (3, 4, 21–94). (Figure 1; Table S2–S3). Across these studies, 924 VE estimates against infection were extracted from 63 studies, while 495 estimates against severe disease were derived from 49 studies (Table S4). A detailed summary of study characteristics and the distribution of VE estimates across various subgroups, such as prior infection status, enrollment criteria, vaccine types, and circulating virus variants, is provided in Table S5.
Figure 1.
Selection of studies for the systematic review
The primary focus of the study was to examine the relationship between VE and potential exposure intensity. For a leaky vaccine, we hypothesized that more frequent or more intense exposures would be associated with reduced VE (Figure S1). Exposure intensity was approximated using indices from the Oxford COVID-19 Government Response Tracker as proxies for exposure intensity: the Stringency Index (SI), Containment and Health Index (CHI), and Government Response Index (GRI). Higher values of these indices (scale 0–100) reflect stricter public health measures and consequently lower exposure intensity.
We analyzed 1,419 VE estimates from 76 eligible studies, stratified by outcome (infection or severe disease), circulating variant, vaccine type, and time since vaccination. We conducted random-effects meta-analyses to estimate pooled VE across tertiles of each index and performed meta-regression to assess the relationship between indices and VE while adjusting for potential confounders. Correlation analyses using Pearson and Spearman coefficients quantified the association between exposure indices and VE estimates.
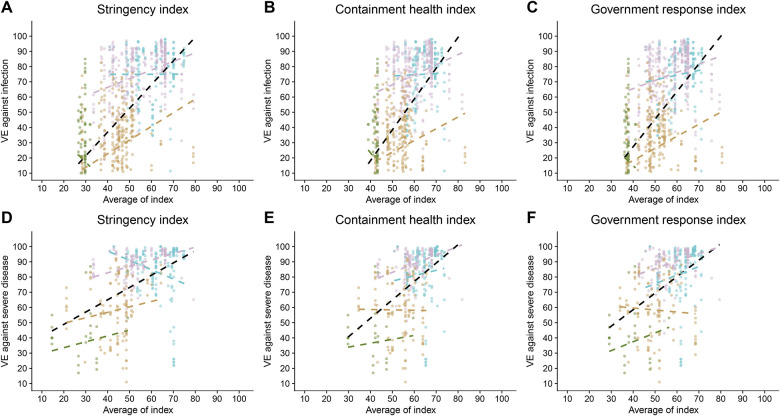
Overall, we observed moderate to high positive correlations between VE against infection and severe disease with SI, CHI and GRI, except in the case of the Omicron BA.4/BA.5 subvariant (Figure 2, Table S1). These suggested that lower exposure intensity, proxied by these indices, was associated with higher VE estimates.
Figure 2.
Association between vaccine effectiveness (VE) and public health indices. Scatterplots illustrating the relationship between VE and the Stringency Index (SI), Containment and Health Index (CHI), and Government Response Index (GRI) for different outcomes (infection and severe disease). Correlation coefficients (r and ρ) are shown for the overall dataset and stratified by variant periods (pre-Delta and Delta, late-Delta, Omicron BA.1/BA.2, Omicron BA.4/BA.5). The figures demonstrate positive correlations between VE and higher index values, indicating that stricter public health measures are associated with increased VE.
Vaccine effectiveness against infection and severe disease
The 924 VE point estimates against infection exhibited considerable heterogeneity, spanning from −38% to 98%, with an I2 value of 100% (Figure 3). The 495 VE estimates against severe disease also demonstrated significant variability, with I2=100% and point estimates ranging between −4% and 100% (Figure 3).
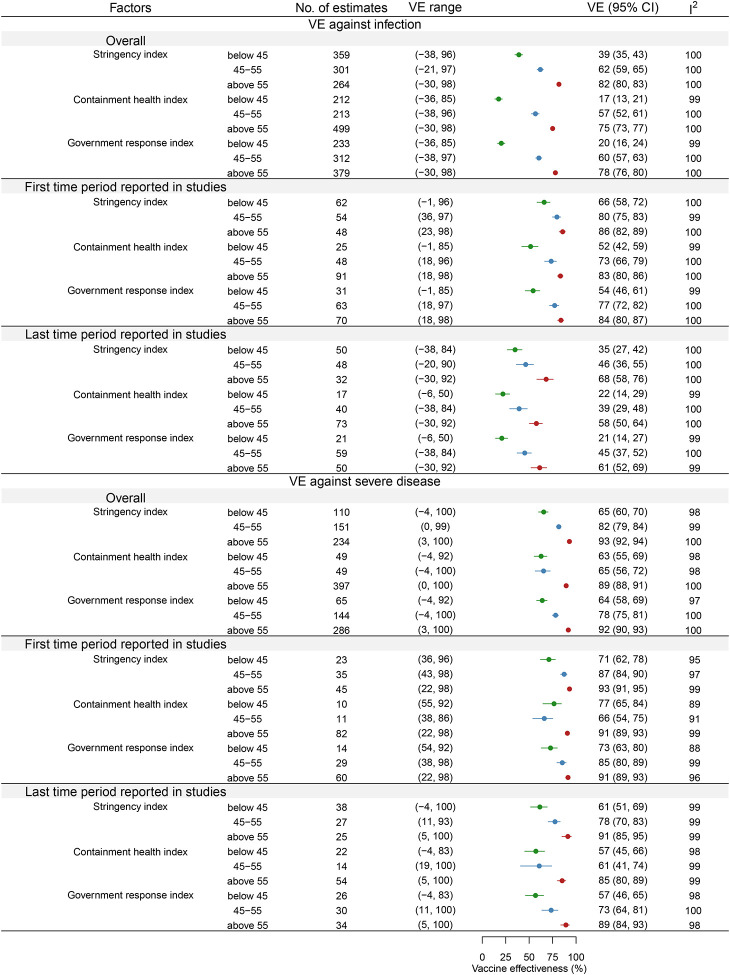
Figure 3.
Impact of public health indices on pooled vaccine effectiveness (VE). Meta-analysis results comparing VE across tertiles of the Stringency Index (SI), Containment and Health Index (CHI), and Government Response Index (GRI). The pooled VE is significantly higher in the highest tertile of each index, suggesting that stricter control measures, and consequently lower exposure levels, are associated with improved VE against both infection and severe disease.
In the earliest post-vaccination period (Figure 3), when waning effects were minimal, VE estimates against (n=164) ranged from −0.97% to 97.7%(I2=100%), while severe disease estimates (n=103) ranged from 22% to 98% (I2=99%). In the latest period (Figure 3), as waning effects became pronounced, VE estimates against infection (n=130) varied from −38% to 92% (I2=100%), compared to severe disease estimates (n=90) that ranged from −4% to 100% (I2=100%).
Impact of intensity of control measure on vaccine effectiveness
Our meta-analysis (Figure 3) demonstrated that higher intensity of control measures and hence lower exposure intensity, measured by SI, CHI and GRI, was associated with higher pooled VE. For VE against infection, high stringency measures resulted in a pooled VE of 82% (95% CI: 80%, 83%), compared to 62% (95% CI: 59%, 65%) for moderate SI and 39% (95% CI: 35%, 43%) for low SI. Similarly, high CHI yielded a pooled VE of 75% (95% CI: 73%, 77%), compared to 57% (95% CI: 52%, 61%) for moderate and 17% (95% CI: 13%, 21%) for low CHI. For GRI, VE against infection was 78% (95% CI: 76%, 80%) for high intensity, 60% (95% CI: 57%, 63%) for moderate intensity, and 20% (95% CI: 16%, 24%) for low intensity.
A similar trend was observed for VE against severe disease. High-intensity SI were associated with a pooled VE of 93% (95% CI: 92%, 94%), compared to 82% (95% CI: 79%, 84%) for moderate SI and 65% (95% CI: 60%, 70%) for low SI. For CHI, VE against severe disease was 89% (95% CI: 88%, 91%) for high intensity, 65% (95% CI: 56%, 72%) for moderate intensity, and 63% (95% CI: 55%, 69%) for low intensity. Similarly, GRI yielded a pooled VE of 92% (95% CI: 90%, 93%) for high intensity, 78% (95% CI: 75%, 81%) for moderate intensity, and 64% (95% CI: 58%, 69%) for low intensity. These patterns were consistent when further analysed for vaccine effectiveness reported in the earliest and the latest period post-vaccination (Figure 3).
In the meta-regression analysis, which adjusted for variables including vaccine type, circulating virus, enrolment criteria, time since vaccination, and prior infection (Table 1, S6), the relative odds ratios (RORs) for infection were 0.84 (95% CI: 0.81, 0.87) for the SI, 0.83 (95% CI: 0.79, 0.88) for the CHI, and 0.85 (95% CI: 0.81, 0.89) for the GRI. These findings suggest that higher control measure intensity and hence lower exposure intensity was associated with improved VE. For instance, in a setting with a baseline VE of 50% against infection, a 10-unit increase in SI, CHI, and GRI would correspond to increases in VE to 58% (95% CI: 56.5%, 59.5%), 58.5% (95% CI: 56%, 60.5%), and 57.5% (95% CI: 55.5%, 59.5%), respectively.
Table 1.
Relationship between government response index and estimates of risk ratios against infection or severe disease.
| Endpoint | Infection | Infection | Infection | Severe disease | Severe disease | Severe disease |
|---|---|---|---|---|---|---|
| Index | Stringency index | Containment health index | Government response index | Stringency index | Containment health index | Government response index |
| Model adjusted for vaccine type, circulating virus and recruitment criteria + day since vaccination + study including participants with COVID infection history | ||||||
| Stringency index | 0.84 (0.81, 0.87) | NA | NA | 0.84 (0.77, 0.92) | NA | NA |
| Containment health index | NA | 0.83 (0.79, 0.88) | NA | NA | 0.90 (0.79, 1.02) | NA |
| Government response index | NA | NA | 0.85 (0.81, 0.89) | NA | NA | 0.95 (0.84, 1.07) |
Similarly, the RORs for severe disease were 0.84 (95% CI: 0.77, 0.92) for SI, but did not reach statistical significance for CHI (0.90; 95% CI: 0.79, 1.02), and for GRI (0.95; 95% CI: 0.84, 1.07). For a baseline VE of 50% against severe disease, a 10-unit increase in SI would result in increases to 58% (95% CI: 54%, 61.5%) respectively. These results remained consistent after excluding estimates from studies identified as having a serious risk of bias (Table S7), and excluding estimates from studies not using a clinical case definition or excluding participants with prior infections (Table S8).
Impact of type of vaccine, circulating viruses, prior infection and enrolment criteria
Our meta-analysis revealed that pooled VE against infection varied by vaccine type: 63% (95% CI: 61%, 66%) for mRNA vaccines, 66% (95% CI: 61%, 71%) for adenovirus vector vaccines, and 40% (95% CI: 34%, 45%) for inactivated virus vaccines (Figure S3–5). Moreover, VE against infection was markedly lower during Omicron periods: 28% (95% CI: 25–31%) during BA.1/2 and 19% (95% CI: 14–24%) during BA.4/5, compared with 80–81% during the pre-Delta/Delta and late-Delta periods. A similar decline was observed for VE against severe disease, which decreased from 91–93% in the pre-Delta/Delta and late-Delta periods to 61% (95% CI: 57–65%) during BA.1/2 and 33% (95% CI: 27–38%) during BA.4/5. These trends persisted when analyses were stratified by prior infection status and using fixed-effects models (Figure S6). Additionally, studies including participants with prior COVID-19 infection reported higher pooled VE estimates against infection (68% vs. 55%) and severe disease (88% vs. 84%), with a similar pattern observed based on enrollment criteria. Further details of analyses of these factors could be found in Supplementary Note 1.
Risk of bias
The majority of studies included in the meta-analysis were assessed as having a moderate risk of bias. Specifically, 58 out of 63 studies (92%) analyzing VE against infection and 48 out of 49 studies (98%) evaluating VE against severe disease were categorized as moderate risk (Figure S7–S8). A smaller proportion of studies were judged to have a serious risk of bias: five studies (8%) in the VE against infection analysis and one study (2%) in the VE against severe disease analysis. The primary sources of bias included potential confounding, misclassification of interventions due to self-reported vaccination status, and participant selection bias (Figure S7–S8).
Sensitivity analyses excluding estimates from studies with serious or critical bias (Figure S9; Table S7) or those that excluded participants with prior infections or that did not use a clinical case definition yielded results consistent with the main findings (Figure S10; Table S8). This suggests that the observed impact of control measure intensity on VE estimates remained robust despite the exclusion of higher-risk studies.
DISCUSSION
In this study, we hypothesized that higher exposure intensity to SARS-CoV-2 would be associated with lower VE, consistent with the related hypothesis that COVID-19 vaccines provide “leaky” protection rather than “all-or-nothing” protection (18, 19). We used three indices (SI, CHI, GRI), to approximate the intensity of public health and social measures (PHSMs) and hence the exposure intensity to SARS-CoV-2. Our findings indicate that higher values of these indices, reflecting stricter public health measures and hence lower exposure intensity, were associated with higher VE estimates. This suggests that regions with stricter control measures may experience reduced exposure intensity, with higher associated VE that would decline when PHSMs are lifted. In addition, vaccine trials conducted in locations and periods with stricter public health measures would likely produce higher estimates of vaccine efficacy than trials or observational studies done in other locations or periods.
The most likely explanation for this relationship is that the exposure intensity to SARS-CoV-2, shaped by PHSMs, acts as an effect modifier of vaccine effectiveness (VE). VE may vary depending on the exposure intensity an individual has to the virus, such as their engagement in high-risk behaviors (95). Another possible explanation is that lower exposure intensity reduces opportunities for the virus to cause an infection in vaccinated individuals, giving their immune systems sufficient time to respond effectively to each encounter and a greater chance to neutralize the virus and prevent infection (96–98), so that the infectious dose is not achieved.
In addition to acting as effect modifiers, SI, CHI, and GRI could also function as confounders in the relationship between COVID-19 vaccination and outcomes. These indices are often correlated with both vaccination strategies and infection risk. For instance, regions with higher levels of PHSMs (higher SI, GRI or CHI) are likely to have more extensive vaccination campaigns, with higher coverage and better access to vaccines. These regions often experienced lower levels of virus circulation due to the stringent measures in place, independent of reduced exposure intensity associated with interventions such as mask wearing that reduces the infection risk after exposures (99, 100) or travel restrictions that prevent virus introduction to a region (101–104).
The relationship between VE and exposure intensity underscores the need to consider PHSMs when interpreting VE data. An important implication of our findings is that cross-country comparisons of VE may be inherently limited due to differing levels of PHSMs. Our analysis indicates that regions with stringent control measures tended to report higher VE, likely reflecting reduced exposure intensity rather than intrinsic differences in vaccine performance. Consequently, for policy makers, it is critical to contextualize VE estimates by considering data from settings with comparable levels of restrictions. This targeted approach not only ensures a more accurate interpretation of VE but also provides valuable insights for guiding the implementation or relaxation of restrictions. These results also support previous assertions that COVID-19 vaccines are leaky (18, 19). This may impact interpretation of VE estimates, For example, depletion of susceptibles bias is a concern for leaky vaccines but not all-or-nothing vaccines, and should therefore be taken into consideration in VE estimation (105). Moreover, the optimal vaccine target group could change depending on whether a vaccine affords all-or-nothing or leaky protection (106) even when the VE is the same.
It is important to recognize that these exposure indices are proxies for the complex reality of exposure intensity. Not all of the heterogeneity in VE can be explained by differences in exposure intensity. We considered additional sources of variation, including vaccine type, circulating virus variant, enrolment criteria, prior infection status, and time since vaccination. Some differences in study design, population demographics, behavioural factors also play critical roles. Therefore, while our findings underscore the importance of accounting for exposure intensity when interpreting VE, they also highlight the need for a cautious interpretation that acknowledges the multifactorial nature of these relationships.
This study has several limitations. First, we categorized VE against infection from both surveillance-based test-negative design (TND) studies and database-based studies as VE against infection. These two study designs are not entirely comparable, which may affect interpretation. Second, the TND studies reviewed were observational in nature. Although many studies adjusted for confounders such as age, sex, healthcare worker status, and pre-existing conditions, residual confounding cannot be ruled out. While we conducted bias assessments to evaluate whether confounding, measurement errors, or selection bias were adequately addressed, unidentified biases may still be present. Lastly, we used the values of three indices (SI, CHI, and GRI) in the study countries as proxies for the intensity of PHSMs and exposure levels. These indices are unlikely to capture the full complexity of PHSMs, resulting in measurement errors and subsequent residual confounding.
In conclusion, our study suggests that exposure intensity to SARS-CoV-2, as reflected by PHSMs (SI, CHI, and GRI), substantially influence observed VE estimates. Stricter PHSMs correlated with higher VE estimates, while VE tended to be lower in settings with intense exposure. To provide more reliable VE estimates, future studies should account for exposure intensity and adjust for the impact of PHSMs, particularly as the virus continues to evolve and new variants emerge. For policy makers, this implies that VE estimates from areas with stringent PHSMs may overstate the vaccine’s true protective effect compared to regions with higher exposure intensity. Therefore, it is crucial to adjust VE estimates for local exposure conditions when developing vaccine strategies.
METHODS
Search strategy and selection criteria
This systematic review adhered to the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (107). We conducted a standardized search in PubMed, Embase and Web of Science, using the search term “(“test negative” OR “effectiveness”) AND (“vaccine”) AND (“COVID-19” OR “SARS-CoV-2”)”. Duplicates identified across the databases were removed. The search was performed on 04 Sept 2023, without language restrictions. Additionally, we examined the reference lists of identified articles to locate further relevant studies. Two independent reviewers (XH and ZC) completed title and full-text screening and extracted data from the included studies. Any disagreements were resolved through consensus with a third reviewer (TKT).
The inclusion criteria focused on studies employing a test-negative design (TND) where all cases and controls were tested (108, 109), and where vaccine effectiveness (VE) was estimated for at least two distinct time periods to assess potential waning effects. We included published TND studies which drew participants from the general population that received a complete primary vaccination series (two doses for most vaccines; one dose for Janssen). We considered the following outcomes: (1) positive test result, (2) symptomatic disease, (3) hospitalization, (4) ICU admission, (5) severe COVID-19, or (6) death. Studies were excluded if they met any of the following criteria: (1) participants were recruited from specific sub-populations, such as healthcare professionals; (2) VE estimates were reported for only single time period; (3) studies that merely summarized or reanalysed previously-published data; (4) studies that reported only pooled VE estimates across different vaccines; (5) the study was a preprint, as these are not peer-reviewed; or (6) the full text was not available.
Data were extracted from the included studies using a standardized data collection form (Table S9), which gathered information on the following aspects: (1) study period; (2) geographic region(s); (3) study population; (4) use of clinical criteria for participant enrolment; (5) inclusion of individuals with prior SARS-CoV-2 infection; and (6) time intervals used to assess vaccine effectiveness (VE) after vaccination. For each study, we extracted confounder-adjusted VE estimates with confidence intervals separately for each endpoint (e.g., infection, hospitalization), as well as for specific vaccines and circulating virus variants. VE estimates were collected for the earliest available time interval, starting at least 14 days post-vaccination, given that antibody levels have been shown to peak by that time in previously unexposed individuals (110). In cases where studies reported multiple estimates (e.g., by age group or vaccine type), all subgroupspecific estimates were included, while overall estimates were excluded.
The quality of the studies was assessed using the Risk of Bias in NonRandomized Studies of Interventions (ROBINS-I) tool (Sterne et al., 2016). Additionally, the certainty of the evidence for studies included in the meta-analysis was graded using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach (111). Sensitivity analyses were conducted by repeating all meta-analyses and meta-regressions while excluding studies classified as having a serious or critical risk of bias.
Our previous reviews (8, 9) indicated that studies excluding individuals with prior infections or not using clinical definitions may report artificially high VE estimates. Therefore, additional sensitivity analyses were performed by repeating all meta-analyses and meta-regressions while excluding such studies.
Exposure intensity
The primary focus of the study was to examine the relationship between VE and potential exposure intensity. For a leaky vaccine, we hypothesized that more frequent or more intense exposures would be associated with reduced VE (Figure S1). Exposure intensity was approximated using indices from the Oxford COVID-19 Government Response Tracker (OxCGRT, https://github.com/OxCGRT/covid-policy-tracker) (112). This tracker compiled publicly accessible data on various government responses to COVID-19 and aggregated them into systematic indices. Three specific indices were extracted: (1) the Stringency Index (SI), which captures changes in school and workplace closures, containment measures, and public information campaigns; (2) the Containment and Health Index (CHI), which includes SI data along with changes in health policy; and (3) the Government Response Index (GRI), which provides a comprehensive measure encompassing SI, CHI, and economic support measures to mitigate the pandemic’s impact on economic activities.
The scale of each index was 0 to 100, with higher scores reflecting stricter government policies, and thus, shorter durations of high exposure intensity. We used the average of these indices during the study period to measure the average control intensity and approximate the likely exposure intensity experienced by participants in each study (Figure S2).
Meta-analysis
In all the included studies, VE was defined as 100%*(1-OR). VE estimates were transformed to the odds ratio (OR) scale, analyzed via meta-analysis, and back-transformed to the VE scale for interpretation. The pooled OR was calculated using random-effects meta-analyses, employing the inverse variance method along with the restricted maximum likelihood estimator to account for heterogeneity (113–116). Heterogeneity was evaluated using Cochran’s Q and the I2 statistic (117). An I2 value exceeding 75% was interpreted as indicative of high heterogeneity (118). Additionally, sensitivity analysis was performed using fixed-effects meta-analysis.
Severe disease was defined as hospitalization, ICU admission, or death. VE estimates not limited to severe cases were classified as VE against infection, referring to VE against positive test results or symptomatic infection without hospitalization.
Pooled VE estimates were stratified by the tertile of each of the three indices, to explore their relationship with VE estimates. Pooled VE estimates were further stratified by the predominant circulating virus and the type of vaccine administered. Most studies did not provide variant-specific VE estimates but instead reported study periods and the general prevalence of variants during those times. Thus, VE estimates were grouped based on the predominant circulating virus as follows: (1) Omicron BA.4/BA.5 or later, (2) Omicron BA.1/BA.2, (3) late-Delta (a period with Delta and Omicron co-circulation), and (4) Delta and pre-Delta, which included ancestral strains and earlier variants. Vaccine types were categorized into: (1) mRNA vaccines (Moderna, Pfizer-BioNTech), (2) adenovirus vector vaccines (AstraZeneca, Janssen, Gamaleya), and (3) inactivated virus vaccines (Sinovac Biotech, Sinopharm).
Meta-regression
We used meta-regression to assess the impact of exposure intensity, approximated by the level of public health and social measures as indicated by the Government Response Index (GRI), on vaccine effectiveness (VE) estimates. Initially, correlation analyses were performed using Pearson (r) and Spearman (ρ) correlation coefficients, to determine the association between these indices and VE estimates. The meta-regression models were adjusted for several covariates: age group (age <65 or ≥65 years), vaccine types, predominant circulating virus strain, inclusion/exclusion of participants with prior infections, and the use of clinical criteria for participant enrolment.
The fitted meta-regression model estimated the ratio of odds ratios (ROR) for each 10-unit increase in the indices. On the OR scale, values closer to 0 indicated greater vaccine effectiveness, while values closer to 1 suggested reduced effectiveness. This interpretation contrasts with the VE scale, where values closer to 0 imply lower effectiveness. For instance, if the ROR for the GRI is less than 1, it suggests that studies with a higher average GRI during their study period reported lower ORs (indicating greater vaccine effectiveness) compared to studies with lower average GRI scores. On the VE scale, this corresponds to higher VE estimates for studies conducted in regions with more stringent government responses during the study period.
All statistical analyses were performed using R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria), employing the metafor package for meta-analyses and the robvis package for visualizing risk of bias assessments.
Supplementary Material
Funding
This project was supported by the National Institute of General Medical Sciences (grant no. R01 GM139926), and the Theme-based Research Scheme (Project No. T11-705/21-N) of the Research Grants Council of the Hong Kong SAR Government. BJC is supported by an RGC Senior Research Fellowship (grant number: HKU SRFS2021-7S03) and the AIR@innoHK program of the Innovation and Technology Commission of the Hong Kong SAR Government. The WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health and Aged Care.
Funding Statement
This project was supported by the National Institute of General Medical Sciences (grant no. R01 GM139926), and the Theme-based Research Scheme (Project No. T11-705/21-N) of the Research Grants Council of the Hong Kong SAR Government. BJC is supported by an RGC Senior Research Fellowship (grant number: HKU SRFS2021-7S03) and the AIR@innoHK program of the Innovation and Technology Commission of the Hong Kong SAR Government. The WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health and Aged Care.
Footnotes
Competing Interests
BJC reports honoraria from AstraZeneca, Fosun Pharma, GSK, Haleon, Moderna, Pfizer, Roche and Sanofi Pasteur. SGS reports consulting for AstraZeneca, CSL Seqirus, GSK, Moderna, Novavax, Pfizer, and Sanofi. The authors report no other potential conflicts of interest.
Ethics approval
This is a systematic review and meta-analysis. No ethical approval is required.
REFERENCES
- 1.Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942–51. [DOI] [PubMed] [Google Scholar]
- 2.Bruxvoort KJ, Sy LS, Qian L, Ackerson BK, Luo Y, Lee GS, et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study. Bmj. 2021;375:e068848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews1 N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chemaitelly2 H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27(9):1614–21. [DOI] [PubMed] [Google Scholar]
- 6.Higdon MM, Wahl B, Jones CB, Rosen JG, Truelove SA, Baidya A, et al. A Systematic Review of Coronavirus Disease 2019 Vaccine Efficacy and Effectiveness Against Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Disease. Open Forum Infect Dis. 2022;9(6):ofac138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewnard JA, Patel MM, Jewell NP, Verani JR, Kobayashi M, Tenforde MW, et al. Theoretical Framework for Retrospective Studies of the Effectiveness of SARS-CoV-2 Vaccines. Epidemiology. 2021;32(4):508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan SG, Khvorov A, Huang X, Wang C, Ainslie KEC, Nealon J, et al. The need for a clinical case definition in test-negative design studies estimating vaccine effectiveness. npj Vaccines. 2023;8(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsang TK, Sullivan SG, Huang X, Wang C, Wang Y, Nealon J, et al. Prior infections and effectiveness of SARS-CoV-2 vaccine in test-negative studies: a systematic review and meta-analysis. American Journal of Epidemiology. 2024;193(12):1868–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikas A, Ahmed H, Zarnitsyna VI. Competing Heterogeneities in Vaccine Effectiveness Estimation. Vaccines (Basel). 2023;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stensrud MJ, Smith L. Identification of Vaccine Effects When Exposure Status Is Unknown. Epidemiology. 2023;34(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S, Flegg JA, White LJ, Aguas R. Levels of SARS-CoV-2 population exposure are considerably higher than suggested by seroprevalence surveys. PLoS Comput Biol. 2021;17(9):e1009436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindeboom RGH, Worlock KB, Dratva LM, Yoshida M, Scobie D, Wagstaffe HR, et al. Human SARS-CoV-2 challenge uncovers local and systemic response dynamics. Nature. 2024;631(8019):189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang B, Lin Y, Xiong W, Liu C, Gao H, Ho F, et al. Comparison of control and transmission of COVID-19 across epidemic waves in Hong Kong: an observational study. Lancet Reg Health West Pac. 2024;43:100969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang B, Wong IOL, Xiao J, Tsang TK, Liao Q, Cowling BJ. Effectiveness of CoronaVac and BNT162b2 Vaccines Against Severe Acute Respiratory Syndrome Coronavirus 2 Omicron BA.2 Infections in Hong Kong. J Infect Dis. 2022;226(8):1382–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halloran ME, Struchiner CJ, Longini IM Jr., Study Designs for Evaluating Different Efficacy and Effectiveness Aspects of Vaccines. American Journal of Epidemiology. 1997;146(10):789–803. [DOI] [PubMed] [Google Scholar]
- 17.Struchiner CJ, Halloran ME. Randomization and baseline transmission in vaccine field trials. Epidemiol Infect. 2007;135(2):181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lind ML, Dorion M, Houde AJ, Lansing M, Lapidus S, Thomas R, et al. Evidence of leaky protection following COVID-19 vaccination and SARS-CoV-2 infection in an incarcerated population. Nat Commun. 2023;14(1):5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halloran ME, Struchiner CJ. Invited Commentary: Thirty-five Years of Leaky Vaccines. American Journal of Epidemiology. 2024:kwae379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kishore K, Jaswal V, Pandey AK, Verma M, Koushal V. Utility of the Comprehensive Health and Stringency Indexes in Evaluating Government Responses for Containing the Spread of COVID-19 in India: Ecological TimeSeries Study. JMIR Public Health Surveill. 2023;9:e38371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abu-Raddad1 LJ, Chemaitelly H, Ayoub HH, Yassine HM, Benslimane FM, Al Khatib HA, et al. Waning of mRNA-1273 vaccine effectiveness against SARS-CoV-2 infection in Qatar. N Engl J Med. 2022;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Accorsi1 EK, Britton A, Shang N, Fleming-Dutra KE, Link-Gelles R, Smith ZR, et al. Effectiveness of Homologous and Heterologous Covid-19 Boosters against Omicron. New England Journal of Medicine. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrejko2 KL, Pry JM, Myers JF, Mehrotra M, Lamba K, Lim E, et al. Waning of 2-Dose BNT162b2 and mRNA-1273 Vaccine Effectiveness Against Symptomatic SARS-CoV-2 Infection Accounting for Depletion-of-Susceptibles Bias. American Journal of Epidemiology. 2023;192(6):895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arashiro1 T, Arima Y, Kuramochi J, Muraoka H, Sato A, Chubachi K, et al. Effectiveness of BA.1- and BA.4/BA. 5-Containing Bivalent COVID-19 mRNA Vaccines Against Symptomatic SARS-CoV-2 Infection During the BA.5-Dominant Period in Japan. Open Forum Infect Dis. 2023;10(6):ofad240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arashiro2 T, Arima Y, Muraoka H, Sato A, Oba K, Uehara Y, et al. Coronavirus Disease 19 (COVID-19) Vaccine Effectiveness Against Symptomatic Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection During Delta-Dominant and Omicron-Dominant Periods in Japan: A Multicenter Prospective Case-control Study (Factors Associated with SARS-CoV-2 Infection and the Effectiveness of COVID-19 Vaccines Study). Clin Infect Dis. 2023;76(3):e108–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belayachi J, Obtel M, Mhayi A, Razine R, Abouqal R. Long term effectiveness of inactivated vaccine BBIBP-CorV (Vero Cells) against COVID-19 associated severe and critical hospitalization in Morocco. PLoS One. 2022;17(12):e0278546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brazete C, Brazete J, Alves F, Aguiar A, Gonçalves AM, Cardoso M, et al. COVID-19 vaccines effectiveness against symptomatic disease and severe outcomes, 2021–2022: a test-negative case-control study. Public Health. 2023;218:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchan SA, Chung H, Brown KA, Austin PC, Fell DB, Gubbay JB, et al. Estimated Effectiveness of COVID-19 Vaccines Against Omicron or Delta Symptomatic Infection and Severe Outcomes. JAMA Netw Open. 2022;5(9):e2232760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchan2 SA, Nguyen L, Wilson SE, Kitchen SA, Kwong JC. Vaccine effectiveness of BNT162b2 against Omicron and Delta outcomes in adolescents. Pediatrics. 2022. [DOI] [PubMed] [Google Scholar]
- 30.Carazo2 S, Skowronski DM, Brisson M, Sauvageau C, Brousseau N, Fafard J, et al. Effectiveness of previous infection-induced and vaccine-induced protection against hospitalisation due to omicron BA subvariants in older adults: a test-negative, case-control study in Quebec, Canada. Lancet Healthy Longev. 2023;4(8):e409–e20. [DOI] [PubMed] [Google Scholar]
- 31.Cerqueira-Silva T, Katikireddi SV, de Araujo Oliveira V, Flores-Ortiz R, Júnior JB, Paixão ES, et al. Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil. Nat Med. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerqueira-Silva3 T, Katikireddi SV, Oliveira VD, Flores-Ortiz R, Bertoldo J, Paixao ES, et al. Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil. Nature Medicine. 2022;28(4):838-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerqueira-Silva4 T, de Araujo Oliveira V, Paixão ES, Júnior JB, Penna GO, Werneck GL, et al. Duration of protection of CoronaVac plus heterologous BNT162b2 booster in the Omicron period in Brazil. Nat Commun. 2022;13(1):4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatzilena A, Hyams C, Challen R, Marlow R, King J, Adegbite D, et al. Effectiveness of BNT162b2 COVID-19 vaccination in prevention of hospitalisations and severe disease in adults with SARS-CoV-2 Delta (B.1.617.2) and Omicron (B.1.1.529) variant between June 2021 and July 2022: A prospective test negative case-control study. Lancet Reg Health Eur. 2023;25:100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chemaitelly1 H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N Engl J Med. 2021;385(24):e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chemaitelly3 H, Ayoub HH, AlMukdad S, Coyle P, Tang P, Yassine HM, et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun. 2022;13(1):3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung3 H, Austin PC, Brown KA, Buchan SA, Fell DB, Fong C, et al. Effectiveness of COVID-19 Vaccines Over Time Prior to Omicron Emergence in Ontario, Canada: Test-Negative Design Study. Open Forum Infect Dis. 2022;9(9):ofac449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciesla AA, Wiegand RE, Smith ZR, Britton A, Fleming-Dutra KE, Miller J, et al. Effectiveness of Booster Doses of Monovalent mRNA COVID-19 Vaccine Against Symptomatic Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Children, Adolescents, and Adults During Omicron Subvariant BA.2/BA.2.12.1 and BA.4/BA.5 Predominant Periods. Open Forum Infect Dis. 2023;10(5):ofad187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collie2 S, Nayager J, Bamford L, Bekker LG, Zylstra M, Gray G. Effectiveness and Durability of the BNT162b2 Vaccine against Omicron Sublineages in South Africa. N Engl J Med. 2022;387(14):1332–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ella R, Km V, Jogdand H. BNT162b2 vaccine effectiveness against SARS-CoV-2 omicron BA. 4 and BA.5 (vol 22, pg 1663, 2022). Lancet Infectious Diseases. 2023;23(3):E80–E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Embi2 PJ, Levy ME, Patel P, DeSilva MB, Gaglani M, Dascomb K, et al. Effectiveness of COVID-19 vaccines at preventing emergency department or urgent care encounters and hospitalizations among immunocompromised adults: An observational study of real-world data across 10 US states from August-December 2021. Vaccine. 2023;41(37):5424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferdinands JM, Rao S, Dixon BE, Mitchell PK, DeSilva MB, Irving SA, et al. Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance - VISION Network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferdinands2 JM, Rao S, Dixon BE, Mitchell PK, DeSilva MB, Irving SA, et al. Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: test negative, case-control study. Bmj. 2022;379:e072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleming-Dutra KE, Britton A, Shang N, Derado G, Link-Gelles R, Accorsi EK, et al. Association of Prior BNT162b2 COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance. Jama-Journal of the American Medical Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleming-Dutra2 KE, Ciesla AA, Roper LE, Smith ZR, Miller JD, Accorsi EK, et al. Preliminary Estimates of Effectiveness of Monovalent mRNA Vaccines in Preventing Symptomatic SARS-CoV-2 Infection Among Children Aged 3–5 Years - Increasing Community Access to Testing Program, United States, July 2022-February 2023. MMWR Morb Mortal Wkly Rep. 2023;72(7):177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Florentino2 PTV, Millington T, Cerqueira-Silva T, Robertson C, de Araújo Oliveira V, Júnior JBS, et al. Vaccine effectiveness of two-dose BNT162b2 against symptomatic and severe COVID-19 among adolescents in Brazil and Scotland over time: a test-negative case-control study. Lancet Infect Dis. 2022;22(11):1577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grewal2 R, Nguyen L, Buchan SA, Wilson SE, Nasreen S, Austin PC, et al. Effectiveness of mRNA COVID-19 vaccine booster doses against Omicron severe outcomes. Nat Commun. 2023;14(1):1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heidarzadeh A, Amini Moridani M, Khoshmanesh S, Kazemi S, Hajiaghabozorgi M, Karami M. Effectiveness of COVID-19 vaccines on hospitalization and death in Guilan, Iran: a test-negative case-control study. Int J Infect Dis. 2023;128:212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Husin M, Tok PSK, Suah JL, Thevananthan T, Tng BH, Peariasamy KM, et al. Real-world effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection among adolescents (12 to 17-year-olds) in Malaysia. Int J Infect Dis. 2022;121:55–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ionescu IG, Skowronski DM, Sauvageau C, Chuang E, Ouakki M, Kim S, et al. BNT162b2 Effectiveness Against Delta and Omicron Variants of Severe Acute Respiratory Syndrome Coronavirus 2 in Adolescents Aged 12–17 Years, by Dosing Interval and Duration. J Infect Dis. 2023;227(9):1073–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan FL, Nguyen JL, Singh TG, Puzniak LA, Wiemken TL, Schrecker JP, et al. Estimated BNT162b2 Vaccine Effectiveness Against Infection With Delta and Omicron Variants Among US Children 5 to 11 Years of Age. JAMA Netw Open. 2022;5(12):e2246915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim2 SS, Chung JR, Talbot HK, Grijalva CG, Wernli KJ, Kiniry E, et al. Effectiveness of two and three mRNA COVID-19 vaccine doses against Omicron- and Delta-Related outpatient illness among adults, October 2021-February 2022. Influenza and Other Respiratory Viruses. 2022;16(6):975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein NP, Demarco M, Fleming-Dutra KE, Stockwell MS, Kharbanda AB, Gaglani M, et al. Effectiveness of BNT162b2 COVID-19 Vaccination in Children and Adolescents. Pediatrics. 2023;151(5). [DOI] [PubMed] [Google Scholar]
- 54.Klein1 NP, Stockwell MS, Demarco M, Gaglani M, Kharbanda AB, Irving SA, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA Vaccination in Preventing COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Nonimmunocompromised Children and Adolescents Aged 5–17 Years - VISION Network, 10 States, April 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(9):352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewis2 NM, Self WH, Gaglani M, Ginde AA, Douin DJ, Keipp Talbot H, et al. Effectiveness of the Ad26.COV2.S (Johnson & Johnson) COVID-19 Vaccine for Preventing COVID-19 Hospitalizations and Progression to High Disease Severity in the United States. Clin Infect Dis. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim AH, Ab Rahman N, Ong SM, Paraja J, Rashid R, Parmar IS, et al. Evaluation of BNT162b2 vaccine effectiveness in Malaysia: test negative case-control study. Vaccine. 2022;40(39):5675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lind1 ML, Copin R, McCarthy S, Coppi A, Warner F, Ferguson D, et al. Use of Whole-Genome Sequencing to Estimate the Contribution of Immune Evasion and Waning Immunity on Decreasing COVID-19 Vaccine Effectiveness. Journal of Infectious Diseases. 2023;227(5):663–74. [DOI] [PubMed] [Google Scholar]
- 58.Lind2 ML, Robertson AJ, Silva J, Warner F, Coppi AC, Price N, et al. Association between primary or booster COVID-19 mRNA vaccination and Omicron lineage BA.1 SARS-CoV-2 infection in people with a prior SARS-CoV-2 infection: A test-negative case-control analysis. Plos Medicine. 2022;19(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Link-Gelles1 R, Ciesla AA, Rowley EAK, Klein NP, Naleway AL, Payne AB, et al. Effectiveness of Monovalent and Bivalent mRNA Vaccines in Preventing COVID-19-Associated Emergency Department and Urgent Care Encounters Among Children Aged 6 Months-5 Years - VISION Network, United States, July 2022-June 2023. MMWR Morb Mortal Wkly Rep. 2023;72(33):886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Link-Gelles2 R, Levy ME, Gaglani M, Irving SA, Stockwell M, Dascomb K, et al. Effectiveness of 2, 3, and 4 COVID-19 mRNA Vaccine Doses Among Immunocompetent Adults During Periods when SARS-CoV-2 Omicron BA.1 and BA.2/BA.2.12.1 Sublineages Predominated - VISION Network, 10 States, December 2021-June 2022. MMWR Morb Mortal Wkly Rep. 2022;71(29):931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Link-Gelles3 R, Levy ME, Natarajan K, Reese SE, Naleway AL, Grannis SJ, et al. Estimation of COVID-19 mRNA Vaccine Effectiveness and COVID-19 Illness and Severity by Vaccination Status During Omicron BA.4 and BA.5 Sublineage Periods. JAMA Netw Open. 2023;6(3):e232598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu C, Zhang J, Zeng Y, Huang C, Chen F, Cao Y, et al. Effectiveness of SARS-CoV-2-inactivated vaccine and the correlation to neutralizing antibodies: A test-negative case-control study. J Med Virol. 2023;95(1):e28280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lutz CS, Hartman RM, Vigil DE, Britton A, Burrage AB, Campbell AP, et al. Effectiveness of COVID-19 mRNA Vaccines in Preventing COVID-19-Associated Outpatient Visits and Hospitalizations Among American Indian and Alaska Native Persons, January-November 2021: A Test-Negative Case-Control Analysis Using Surveillance Data. Open Forum Infect Dis. 2023;10(4):ofad172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maeda2 H, Saito N, Igarashi A, Ishida M, Terada M, Ito T, et al. Effectiveness of mRNA COVID-19 vaccines against symptomatic SARS-CoV-2 infections during the SARS-CoV-2 Omicron BA.1 and BA.2 epidemic in Japan: vaccine effectiveness real-time surveillance for SARS-CoV-2 (VERSUS). Expert Rev Vaccines. 2023;22(1):288–98. [DOI] [PubMed] [Google Scholar]
- 65.Nasreen2 S, Febriani Y, Garcia HAV, Zhang G, Tadrous M, Buchan SA, et al. Effectiveness of Coronavirus Disease 2019 Vaccines Against Hospitalization and Death in Canada: A Multiprovincial, Test-Negative Design Study. Clinical Infectious Diseases. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ng RWY, Sze RKH, Chong KC, Zhao S, Ling LW, Lui GC, et al. Effectiveness of mRNA and inactivated COVID-19 vaccines: A test-negative study in an infection-naive Hong Kong population. Journal of Infection. 2023;87(2):136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Powell AA, Kirsebom F, Stowe J, McOwat K, Saliba V, Ramsay ME, et al. Effectiveness of BNT162b2 against COVID-19 in adolescents. Lancet Infect Dis. 2022;22(5):581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Powell2 AA, Kirsebom F, Stowe J, Ramsay ME, Lopez-Bernal J, Andrews N, et al. Protection against symptomatic infection with delta (B.1.617.2) and omicron (B.1.1.529) BA.1 and BA.2 SARS-CoV-2 variants after previous infection and vaccination in adolescents in England, August, 2021-March, 2022: a national, observational, test-negative, case-control study. Lancet Infect Dis. 2023;23(4):435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Price AM, Olson SM, Newhams MM, Halasa NB, Boom JA, Sahni LC, et al. BNT162b2 Protection against the Omicron Variant in Children and Adolescents. N Engl J Med. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prunas O, Weinberger DM, Pitzer VE, Gazit S, Patalon T. Waning Effectiveness of the BNT162b2 Vaccine Against Infection in Adolescents in Israel. Clin Infect Dis. 2023;76(1):113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qassim SH, Chemaitelly H, Ayoub HH, Coyle P, Tang P, Yassine HM, et al. Population immunity of natural infection, primary-series vaccination, and booster vaccination in Qatar during the COVID-19 pandemic: an observational study. EClinicalMedicine. 2023;62:102102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ranzani3 OT, Hitchings MDT, de Melo RL, de Franca GVA, Fernandes CDR, Lind ML, et al. Effectiveness of an inactivated Covid-19 vaccine with homologous and heterologous boosters against Omicron in Brazil. Nature Communications. 2022;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roberts EK, Gu T, Wagner AL, Mukherjee B, Fritsche LG. Estimating COVID-19 Vaccination and Booster Effectiveness Using Electronic Health Records From an Academic Medical Center in Michigan. AJPM Focus. 2022;1(1):100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosa RG, Falavigna M, Manfio JL, de Araujo CLP, Cohen M, do Valle Barbosa GRG, et al. BNT162b2 mRNA COVID-19 against symptomatic Omicron infection following a mass vaccination campaign in southern Brazil: A prospective test-negative design study. Vaccine. 2023;41(37):5461–8. [DOI] [PubMed] [Google Scholar]
- 75.Self WH, Tenforde MW, Rhoads JP, Gaglani M, Ginde AA, Douin DJ, et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions - United States, March-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(38):1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skowronski DM, Febriani Y, Ouakki M, Setayeshgar S, El Adam S, Zou M, et al. Two-Dose Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Effectiveness With Mixed Schedules and Extended Dosing Intervals: Test-Negative Design Studies From British Columbia and Quebec, Canada. Clinical Infectious Diseases. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sritipsukho2 P, Khawcharoenporn T, Siribumrungwong B, Damronglerd P, Suwantarat N, Satdhabudha A, et al. Real-life effectiveness of COVID-19 vaccine during the Omicron variant-dominant pandemic: how many booster doses do we need? Emerg Microbes Infect. 2023;12(1):2174779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stowe J, Andrews N, Kirsebom F, Ramsay M, Bernal JL. Effectiveness of COVID-19 vaccines against Omicron and Delta hospitalisation, a test negative case-control study. Nat Commun. 2022;13(1):5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suarez Castillo M, Khaoua H, Courtejoie N. Vaccine-induced and naturally-acquired protection against Omicron and Delta symptomatic infection and severe COVID-19 outcomes, France, December 2021 to January 2022. Euro Surveill. 2022;27(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suphanchaimat R, Nittayasoot N, Jiraphongsa C, Thammawijaya P, Bumrungwong P, Tulyathan A, et al. Real-World Effectiveness of Mix-and-Match Vaccine Regimens against SARS-CoV-2 Delta Variant in Thailand: A Nationwide Test-Negative Matched Case-Control Study. Vaccines (Basel). 2022;10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Surie1 D, Bonnell L, Adams K, Gaglani M, Ginde AA, Douin DJ, et al. Effectiveness of Monovalent mRNA Vaccines Against COVID-19-Associated Hospitalization Among Immunocompetent Adults During BA.1/BA.2 and BA.4/BA.5 Predominant Periods of SARS-CoV-2 Omicron Variant in the United States - IVY Network, 18 States, December 26, 2021-August 31, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(42):1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tabak YP, Sun X, Brennan TA, Chaguturu SK. Incidence and Estimated Vaccine Effectiveness Against Symptomatic SARS-CoV-2 Infection Among Persons Tested in US Retail Locations, May 1 to August 7, 2021. JAMA Netw Open. 2021;4(12):e2143346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tamada Y, Takeuchi K, Kusama T, Maeda M, Murata F, Osaka K, et al. Effectiveness of COVID-19 vaccines against infection in Japan: A test-negative study from the VENUS study. Vaccine. 2023;41(37):5447–53. [DOI] [PubMed] [Google Scholar]
- 84.Tamandjou Tchuem CR, Auvigne V, Vaux S, Montagnat C, Paireau J, Monnier Besnard S, et al. Vaccine effectiveness and duration of protection of COVID-19 mRNA vaccines against Delta and Omicron BA.1 symptomatic and severe COVID-19 outcomes in adults aged 50 years and over in France. Vaccine. 2023;41(13):2280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tan CY, Chiew CJ, Pang D, Lee VJ, Ong B, Lye DC, et al. Vaccine effectiveness against Delta, Omicron BA.1, and BA.2 in a highly vaccinated Asian setting: a test-negative design study. Clin Microbiol Infect. 2023;29(1):101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tartof SY, Slezak JM, Puzniak L, Hong V, Xie F, Ackerson BK, et al. Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the omicron and delta variants in a large health system in the USA: a test-negative case-control study. Lancet Respir Med. 2022;10(7):689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tartof2 SY, Frankland TB, Puzniak L, Slezak JM, Hong VN, Takhar H, et al. BNT162b2 Against COVID-19-Associated Emergency Department and Urgent Care Visits Among Children 5–11 Years of Age: A Test Negative Design. Journal of the Pediatric Infectious Diseases Society. 2023;12(3):177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tartof3 SY, Frankland TB, Slezak JM, Puzniak L, Hong V, Xie F, et al. Effectiveness Associated With BNT162b2 Vaccine Against Emergency Department and Urgent Care Encounters for Delta and Omicron SARS-CoV-2 Infection Among Adolescents Aged 12 to 17 Years. JAMA Netw Open. 2022;5(8):e2225162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tartof4 SY, Slezak JM, Puzniak L, Hong V, Frankland TB, Ackerson BK, et al. BNT162b2 vaccine effectiveness against SARS-CoV-2 omicron BA.4 and BA.5. Lancet Infect Dis. 2022;22(12):1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tartof5 SY, Slezak JM, Puzniak L, Hong V, Frankland TB, Xie F, et al. Effectiveness and durability of BNT162b2 vaccine against hospital and emergency department admissions due to SARS-CoV-2 omicron sub-lineages BA.1 and BA.2 in a large health system in the USA: a test-negative, case-control study. Lancet Respir Med. 2023;11(2):176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tenforde3 MW, Self WH, Zhu Y, Naioti EA, Gaglani M, Ginde AA, et al. Protection of mRNA vaccines against hospitalized COVID-19 in adults over the first year following authorization in the United States. Clin Infect Dis. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thompson MG, Stenehjem E, Grannis S, Ball SW, Naleway AL, Ong TC, et al. Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings. N Engl J Med. 2021;385(15):1355–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thompson2 MG, Natarajan K, Irving SA, Rowley EA, Griggs EP, Gaglani M, et al. Effectiveness of a Third Dose of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance - VISION Network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Ewijk CE, Kooijman MN, Fanoy E, Raven SF, Middeldorp M, Shah A, et al. COVID-19 vaccine effectiveness against SARS-CoV-2 infection during the Delta period, a nationwide study adjusting for chance of exposure, the Netherlands, July to December 2021. Euro Surveill. 2022;27(45). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arashiro T, Arima Y, Kuramochi J, Muraoka H, Sato A, Chubachi K, et al. Letter to the editor: Importance of considering high-risk behaviours in COVID-19 vaccine effectiveness estimates with observational studies. Euro Surveill. 2023;28(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goyal A, Reeves DB, Cardozo-Ojeda EF, Schiffer JT, Mayer BT. Viral load and contact heterogeneity predict SARS-CoV-2 transmission and super-spreading events. Elife. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mishra B, Ranjan J, Purushotham P, Saha S, Payal P, Kar P, et al. High proportion of low cycle threshold value as an early indicator of COVID-19 surge. J Med Virol. 2022;94(1):240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rabaan AA, Tirupathi R, Sule AA, Aldali J, Mutair AA, Alhumaid S, et al. Viral Dynamics and Real-Time RT-PCR Ct Values Correlation with Disease Severity in COVID-19. Diagnostics (Basel). 2021;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Howard J, Huang A, Li Z, Tufekci Z, Zdimal V, van der Westhuizen HM, et al. An evidence review of face masks against COVID-19. Proc Natl Acad Sci U S A. 2021;118(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leung NHL, Chu DKW, Shiu EYC, Chan KH, McDevitt JJ, Hau BJP, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26(5):676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gwee SXW, Chua PEY, Wang MX, Pang J. Impact of travel ban implementation on COVID-19 spread in Singapore, Taiwan, Hong Kong and South Korea during the early phase of the pandemic: a comparative study. BMC Infect Dis. 2021;21(1):799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meng X, Guo M, Gao Z, Kang L. Interaction between travel restriction policies and the spread of COVID-19. Transp Policy (Oxf). 2023;136:209–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murano Y, Ueno R, Shi S, Kawashima T, Tanoue Y, Tanaka S, et al. Impact of domestic travel restrictions on transmission of COVID-19 infection using public transportation network approach. Sci Rep. 2021;11(1):3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quilty BJ, Diamond C, Liu Y, Gibbs H, Russell TW, Jarvis CI, et al. The effect of travel restrictions on the geographical spread of COVID-19 between large cities in China: a modelling study. BMC Med. 2020;18(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dean NE. RE: “MEASUREMENT OF VACCINE DIRECT EFFECTS UNDER THE TEST-NEGATIVE DESIGN”. American Journal of Epidemiology. 2019;188(4):806–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee DI, Nande A, Anderson TL, Levy MZ, Hill AL. Vaccine failure mode determines population-level impact of vaccination campaigns during epidemics. J R Soc Interface. 2025;22(223):20240689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical Basis of the Test-Negative Study Design for Assessment of Influenza Vaccine Effectiveness. Am J Epidemiol. 2016;184(5):345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31(17):2165–8. [DOI] [PubMed] [Google Scholar]
- 110.Yang B, Huang X, Gao H, Leung NH, Tsang TK, Cowling BJ. Immunogenicity, efficacy, and safety of SARS-CoV-2 vaccine dose fractionation: a systematic review and meta-analysis. BMC Med. 2022;20(1):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schünemann H, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group. https://gdt.gradepro.org/app/handbook/handbook.html (accessed June 1, 2022). [Available from: https://gdt.gradepro.org/app/handbook/handbook.html [Google Scholar]
- 112.Hale T, Angrist N, Goldszmidt R, Kira B, Petherick A, Phillips T, et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat Hum Behav. 2021;5(4):529–38. [DOI] [PubMed] [Google Scholar]
- 113.Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychological Methods. 1998;3(4):486–504. [Google Scholar]
- 114.Langan D, Higgins JPT, Jackson D, Bowden J, Veroniki AA, Kontopantelis E, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10(1):83–98. [DOI] [PubMed] [Google Scholar]
- 115.Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18(20):2693–708. [DOI] [PubMed] [Google Scholar]
- 116.Veroniki AA, Jackson D, Bender R, Kuss O, Langan D, Higgins JPT, et al. Methods to calculate uncertainty in the estimated overall effect size from a random-effects meta-analysis. Res Synth Methods. 2019;10(1):23–43. [DOI] [PubMed] [Google Scholar]
- 117.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 118.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.