Abstract
The cerebellum has recently been recognized for its role in non-motor functions, including classical fear conditioning. However, the molecular mechanisms underlying non-motor learning and memory remain largely unknown. Here, we investigate the transcriptional changes in the cerebellum associated with auditory fear conditioning. Spatial transcriptomic analysis revealed that in the deep cerebellar nuclei (DCN), an output region of the cerebellum, the expression of immediate early genes increased following fear learning and retrieval, suggesting that DCN may contribute to fear memory processing. As for the cerebellar cortex, robust and region-specific transcriptional changes were observed, with distinct expression patterns emerging across the Purkinje cell layer of vermis region. To further elucidate transcriptional changes in specific DCN cell types involved in fear processing, we performed single-nucleus RNA sequencing and identified prominent gene expression changes in Kit + inhibitory neurons. Collectively, our findings highlight region- and cell-type-specific molecular adaptations in the cerebellum, providing insights into its contribution to non-motor learning.
Keywords: spatial transcriptomics, single-nucleus RNA-seq, cerebellum, deep cerebellar nuclei, Purkinje cell layer, fear memory
Introduction
The cerebellum is a key brain region involved in modulating a wide range of behavioral outputs, including not only motor but also non-motor functions, such as fear learning and social behaviors1–8. Although traditionally thought to process sensorimotor information through cerebellar neuron types (e.g., Purkinje cells and granule cells) that are seemingly uniform across the cerebellum, recent studies reveal substantial molecular, structural, and physiological heterogeneity within the cerebellum9–17. This heterogeneity suggests that spatially distinct cerebellar cell populations exhibit different activity patterns in response to learning, depending on their anatomical location and molecular characteristics.
Classical fear conditioning is a well-established form of non-motor associative learning that involves the cerebellum4,6,7,18–20. Previous studies have demonstrated that different cerebellar circuits and subregions regulate various aspects and temporal stages of fear learning6,18,20–25 and that fear conditioning induces synaptic plasticity, requiring molecular modifications at multiple cerebellar synapses18. However, how classical fear conditioning drives molecular changes in the cerebellum remains unclear.
In contrast, it is well known that cerebellum-dependent motor learnings induce transcriptional and translational changes in cerebellar circuits13,26,27. For instance, oculomotor learning tasks—such as the vestibulo-ocular reflex and optokinetic response —promote learning-dependent protein synthesis in the flocculus26, while delayed eyeblink conditioning alters transcriptional profiles in the anterior interpositus nucleus across different learning stages27. Moreover, a subset of cerebellar Purkinje cells expressing Plcb4 + undergoes transcriptional plasticity during motor learning and is essential for both associative and motor learning through fibroblast growth factor signaling13. Despite accumulating evidence suggesting that the cerebellum also plays a role in non-motor learning and memory, such as classical fear conditioning, how non-motor stimuli reshape the cerebellar molecular architecture remains unknown.
To address this question, we employed spatial transcriptomics and single-nucleus RNA sequencing (snRNA-seq) to investigate how classical auditory fear conditioning modulates cerebellar gene expression across different learning phases in male mice. Our analysis revealed distinct transcriptional responses in the cerebellar vermis, robust induction of immediate early genes in the DCN (deep cerebellar nuclei) after fear memory acquisition and retrieval. Furthermore, a specific subset of Kit + inhibitory DCN neurons displayed pronounced transcriptional alterations, including upregulation of mGluR5, suggesting the formation of an engram through inhibitory signaling. These findings uncover a heterogeneous molecular landscape underlying non-motor associative learning in the cerebellum.
Results
Spatial transcriptomic analysis in the cerebellum after classical fear conditioning
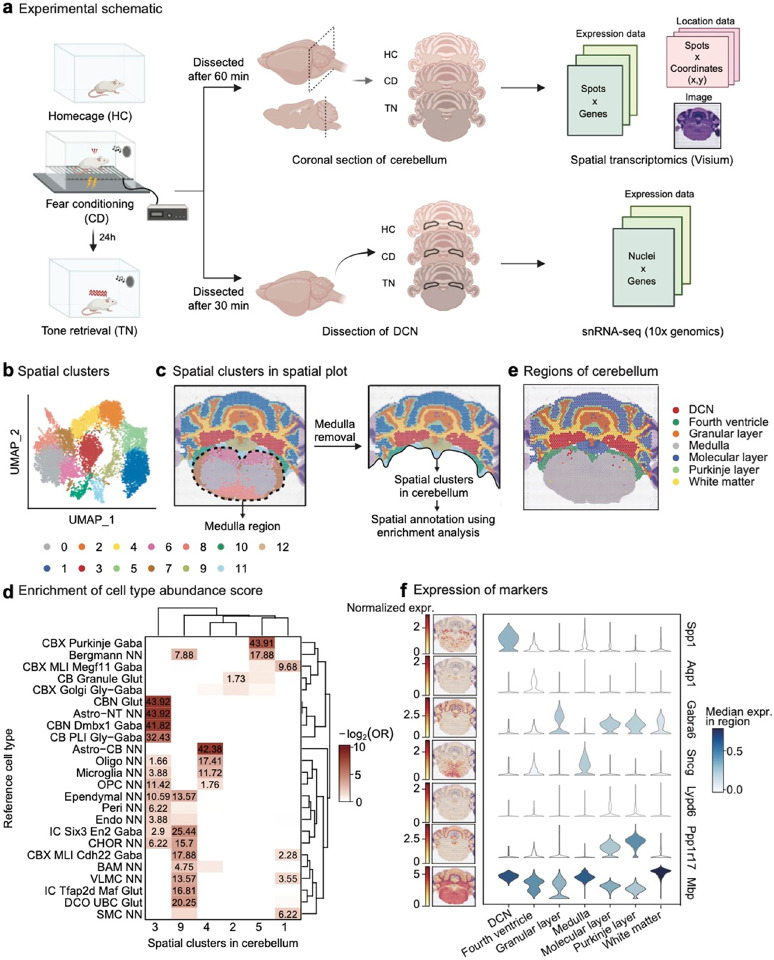
To investigate region-specific gene expression changes in the cerebellum across different phases of auditory fear conditioning, we performed spatial transcriptomics (10X Visium) on coronal sections of the mouse cerebellum, collected 1 h after fear conditioning (conditioned, CD) or 1 h after auditory memory retrieval (tone retrieval, TN). Mice that remained in their home cage (HC) were sacrificed at a similar circadian time and served as controls (Fig. 1a). Each sample contained between 3,000 and 3,500 spatial spots, yielding an integrated dataset of 9,780 spots and 18,779 genes. Unsupervised clustering, based on both gene expression profiles and spatial coordinate values identified 13 distinct clusters (Fig. 1b, c). Due to the complexity of the dissection process, our samples included portions of the medulla. To refine our analysis and focus specifically on the cerebellum, we selected only the clusters corresponding to cerebellar regions (Fig. 1c) for downstream analysis.
Figure 1. Spatial transcriptomic profile of the mouse cerebellum after fear conditioning.
a, Experimental overview. During classical fear conditioning, mice underwent five tone-footshock pairings (2.8 kHz, 80 dB, 30 s tone; 0.5 mA, 2 s footshock). Brain samples were obtained 60 min after behavior tests for spatial transcriptomics, and 30min after for single nucleus sequencing. A coronal section from a single mouse per condition was used for spatial transcriptomic analysis, while DCNs from five mice per condition were pooled for snRNA-seq. b, UMAP representation of the dataset, colored by spatial clusters (9,780 spots, n=1 mouse per condition). c, Spatial plot of the dataset, colored by spatial clusters, illustrating the schematic process of medulla removal to retain only the cerebellar regions. d, Heatmap of enrichment analysis comparing spatial clusters to reference snRNA-seq cerebellar cell types. Cell type abundance scores for each spot, estimated using cell2location, were binarized and analyzed using Fisher’s exact test. Significance is represented as −log10(FDR) values for results meeting the FDR < 0.05 threshold, with Log2(OR) indicated by color. Abbreviations: CBX, cerebellar cortex; MLI, molecular layer interneurons; CB, cerebellum; PLI, Purkinje cell layer interneuron; OPC, oligodendrocyte precursor cells; Peri, pericytes; Endo, endothelial cells; IC, inferior colliculus; CHOR, choroid plexus; BAM, border-associated macrophages; VLMC, vascular leptomeningeal cells; DCN, deep cerebellar nuclei; UBC, unipolar brush cells; SMC, smooth muscle cells; NN, non-neuronal. e, Spatial plot of the dataset, colored by annotated regions. f, Expression of marker genes of each cerebellar region, visualized using a spatial plot (left) and violin plot (right).
We then performed cell type enrichment analysis using a well-annotated reference scRNA-seq dataset28 (Fig. 1d, Extended Data Fig. 1a, Supplementary Table 1a). Additionally, we manually compared our data with an in situ hybridization reference dataset29 to identify non-cerebellar regions and those not clearly defined using the enrichment analysis (Extended Data Fig. 1b). Our 13 spatial clusters were classified into seven distinct regions: three cerebellar cortical regions (molecular layer, granular layer and Purkinje cell layer), DCN, white matter, fourth ventricle, and medulla (Fig. 1e). The expression patterns of well-known cell type markers confirmed the accuracy of our regional annotations (Fig. 1f). Furthermore, all three experimental conditions exhibited similar distributions across these regions (Extended Data Fig. 1c, d), suggesting that fear conditioning does not alter the overall regional composition of cerebellar cell populations.
Induction of immediate early genes in DCN
Immediate early genes (IEGs), such as cFos and Egr1, are well-established markers of neuronal activity30,31. Although prior studies have explored behavior-induced IEGs in the cerebellum32, which IEGs consistently serve as reliable markers of activity within this brain region remains unclear.
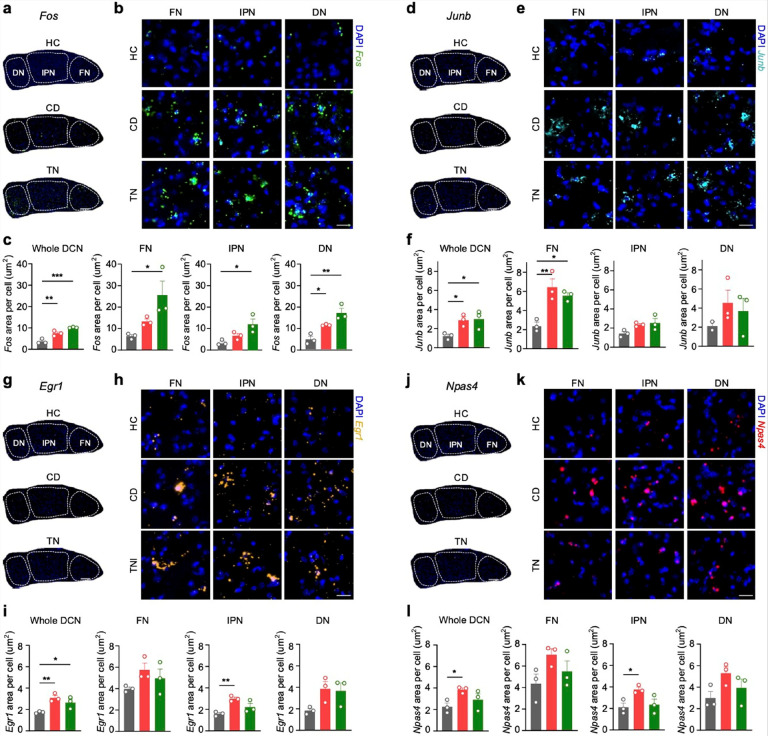
To investigate the transcriptional signature of fear learning in each cerebellar region, we conducted a region-specific differentially expressed genes (DEG) analysis in the cerebellum across conditions HC, CD, and TN. Likelihood ratio test (LRT) revealed that the molecular layer exhibited the most significant gene expression changes across all conditions, followed by the DCN (Extended Data Fig. 2, Supplementary Table 2a). We next performed pairwise comparisons between the three conditions (CD vs. HC, TN vs. HC, and TN vs. CD) to identify genes regulated during specific phases of fear learning. Notably, the DCN was the only region where IEGs Fos, Junb, Egr1, and Npas4 were significantly upregulated during fear learning (Fig. 2a). Among these, Fos, Junb, and Egr1 were the most significantly upregulated DEGs during both the CD and TN phases (Fig. 2b). This indicates enhanced neural activation in the DCN during both fear memory acquisition and consolidation. Interestingly, Npas4 was identified as the top upregulated DEG in the CD vs. HC comparison but did not show significant changes in the TN vs. HC comparison (Fig. 2b). This suggests that Npas4 may play a selective role in fear memory acquisition but not in memory retrieval within the DCN. The enriched expression patterns of IEGs during fear learning were further confirmed through spatial visualization (Fig. 2c).
Figure 2. Different gene expression signatures in the DCN during fear learning.
a, UpSet plots showing unique and shard DEGs upregulated during the CD vs. HC comparison (top) and the TN vs. HC comparison (bottom) across cerebellar regions. The column highlighting DCN-specific DEGs is marked in red. b, Quadrant plot illustrating log2FC values for DCN DEGs in the CD vs. HC (x-axis) and TN vs. HC comparisons (y-axis). Genes are colored based on the comparison in which they are significant. Violin plots show normalized expression patterns of the top “both significant” DEGs in quadrant I (left) and quadrant III (right), separated by condition. c, Spatial plots showing normalized expression of IEGs Fos, Junb, Egr1, and Npas4 in the DCN. d, Gene Ontology (GO) enrichment analysis results for the CD vs. HC (top) and TN vs. HC comparisons (bottom) in oligodendrocytes. Bar colors indicate the direction of enrichment, and false discovery rate (FDR) values denote significance.
Functional analysis of the DEGs whose expression was higher in the DCN during the CD phase than during the HC phase revealed enrichment for fear memory-related processes, including memory, cognition, long-term memory, and regulation of synaptic plasticity (Fig. 2d, Supplementary Table 2b). CD-upregulated DEGs involved in memory and cognition included Calb1, Egr1, Npas4, S100b, and Slc61a. In contrast, the DEGs whose expression was higher during the TN phase than during the HC phase were enriched in processes related to cytoplasmic translation at the synapse (Fig. 2d, Supplementary Table 2b).
Overall, our findings demonstrate region-specific neuronal activation within the DCN following fear conditioning and reveal distinctive transcriptional patterns throughout the progressive stages of fear memory formation and retrieval.
Validation of IEG expression in the DCN
To validate the IEG data obtained from spatial transcriptomics, we performed fluorescence in situ hybridization (ISH) after fear conditioning and retrieval testing. The expression of IEGs Fos, Junb, Egr1, and Npas4, known markers of neuronal plasticity, was significantly increased in the DCN following fear learning or retrieval tests (Fig. 3). Interestingly, the expression of Fos, Junb, and Egr1 was elevated after both fear conditioning and retrieval (Fig. 3a–i), whereas that of Npas4 was elevated only after fear conditioning (Fig. 3j–l). These results align with the patterns observed in the spatial transcriptomics data. The DCN is composed of three subnuclei: the fastigial nucleus (FN), interpositus nucleus (IPN), and dentate nucleus (DN). We compared the IEG expression levels among these subnuclei and found distinct expression patterns. For instance, Fos was upregulated across all three subnuclei (Fig. 3a–c), whereas Junb showed a significant increase only in the FN (Fig. 3d–f). These findings suggest that IEGs may play distinct roles in fear learning through their respective downstream targets. Interestingly, such increased expression of IEGs was not observed in the cerebellar cortex (Extended Data Fig. 7).
Figure 3. Immediate early genes are upregulated in the DCN during fear conditioning.
a, ISH results showing the expression of Fos in the DCN subnuclei. (FN, fastigial nucleus; IPN, interpositus nucleus; and DN, dentate nucleus). Scale bar: 150 μm. b,Representative ISH images of Fos expression in each subnucleus. Scale bar: 20 μm. c, Quantification of ISH data for Fos (n = 3 per group). Whole DCN: One-way ANOVA: F(2,6) = 33.12, P = 0.0006. Dunnett’s multiple comparisons test: **P = 0.0059, ***P = 0.0004. FN: One-way ANOVA: F(2,6) = 6.258, P = 0.0340. Dunnett’s multiple comparisons test: P = 0.4005 (HC vs. CD), *P = 0.0229 (HC vs. TN). IPN: One-way ANOVA: F(2,6) = 7.529, P = 0.0231. Dunnett’s multiple comparisons test: P = 0.3211 (HC vs. CD), *P = 0.0152 (HC vs. CD). DN: One-way ANOVA: F(2,6) = 17.83, P = 0.0030. Dunnett’s multiple comparisons test: *P = 0.0327 (HC vs. CD), **P = 0.0018 (HC vs. TN). d, ISH results showing Junb expression in the DCN. Scale bar: 150 μm. e, Representative ISH images for Junb in each subnucleus. Scale bar: 20 μm. f, Quantification of ISH data for Junb: Whole DCN: One-way ANOVA: F(2,6) = 6.243, P = 0.0342. Dunnett’s multiple comparisons test: *P = 0.0452 (HC vs. CD), *P = 0.0341 (HC vs. TN). FN: One-way ANOVA: F(2,6) = 13.50, P = 0.0060. Dunnett’s multiple comparisons test: **P = 0.0046 (HC vs. CD), *P = 0.0151 (HC vs. TN). IPN: One-way ANOVA: F(2,6) = 3.999, P = 0.0788. Dunnett’s multiple comparisons test: P = 0.1167 (HC vs. CD), P = 0.0677 (HC vs. TN). DN: One-way ANOVA: F(2,5) = 0.8278, P = 0.4892. Dunnett’s multiple comparisons test: P = 0.3871 (HC vs. CD), P = 0.6351 (HC vs. TN). g, ISH results showing Egr1 expression in the DCN. Scale bar: 150 μm. h, Representative RNAscope images of Egr1 expression in each subnucleus. Scale bar: 20 μm. I, Quantification of ISH data for Egr1expression: Whole DCN: One-way ANOVA: F(2,6) = 9.602, P = 0.0135. Dunnett’s multiple comparisons test: **P = 0.0091 (HC vs. CD), *P = 0.0476 (HC vs. TN). FN: One-way ANOVA: F(2,6) = 2.043, P = 0.2105. Dunnett’s multiple comparisons test: P = 0.1520 (HC vs. CD), P = 0.4686 (HC vs. TN). IPN: One-way ANOVA: F(2,6) = 10.76, P = 0.0104. Dunnett’s multiple comparisons test: **P = 0.0064 (HC vs. CD), P = 0.1345 (HC vs. TN). DN: One-way ANOVA: F(2,6) = 3.630, P = 0.0927. Dunnett’s multiple comparisons test: P = 0.0863 (HC vs. CD), P = 0.1185 (HC vs. TN). j, ISH results showing Npas4 expression in the DCN. Scale bar: 150 μm. k, Representative ISH images of Npas4expression in each subnucleus. Scale bar: 20 μm. l Quantification of ISH data for Npas4: Whole DCN: One-way ANOVA: F(2,6) = 4.204, P = 0.0722. Dunnett’s multiple comparisons test: *P = 0.0486 (HC vs. CD), P = 0.4340 (HC vs. TN). FN: One-way ANOVA: F(2,6) = 2.685, P = 0.1469. Dunnett’s multiple comparisons test: P = 0.1030 (HC vs. CD), P = 0.5437 (HC vs. TN). IPN: One-way ANOVA: F(2,6) = 5.519, P = 0.0437. Dunnett’s multiple comparisons test: *P = 0.0380 (HC vs. CD), P = 0.8627 (HC vs. TN). DN: One-way ANOVA: F(2,6) = 2.836, P = 0.1358. Dunnett’s multiple comparisons test: P = 0.0955 (HC vs. CD), P = 0.5664 (HC vs. TN).
Our validation experiments confirm that IEGs are prominently induced in the DCN during fear conditioning4,6,21,33, with distinct expression patterns among DCN subnuclei. The differential regulation of IEGs such as Fos, Junb, Egr1, and Npas4 underscores their unique contributions to fear learning and memory processes within the cerebellum. These findings highlight region-specific neuronal activation in the DCN and suggest that IEGs orchestrate distinct molecular pathways critical for the formation and retrieval of fear memories.
snRNA-seq in the DCN reveals cell type- and learning phase-specific transcriptional changes
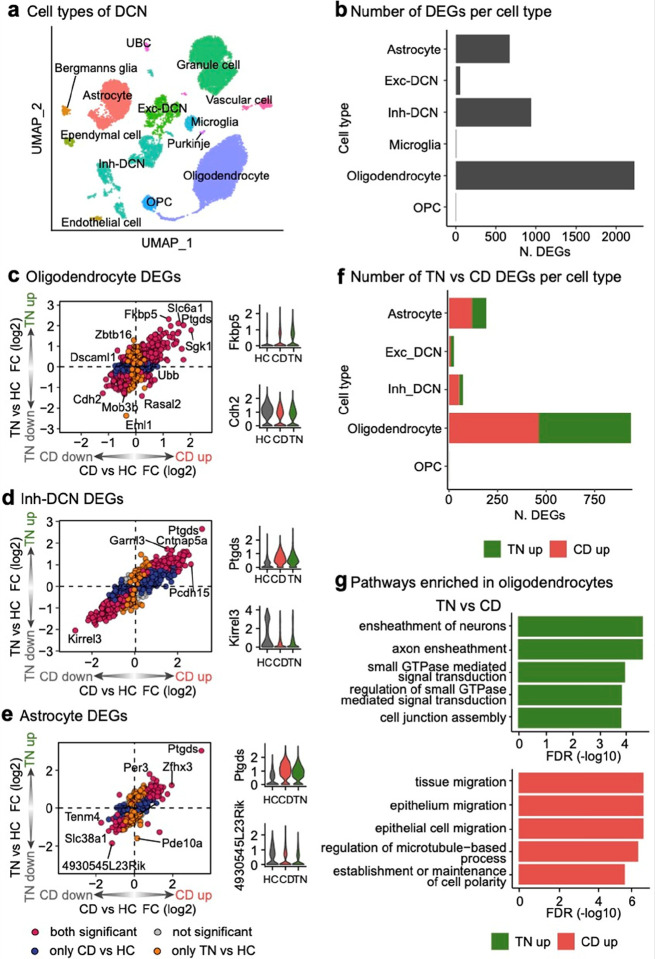
The DCN consists of various cell types (Fig. 1c) that contribute to complex neurological processes such as fear learning. To better understand the role of individual cell types in the learning and retrieval of fear memories, we performed snRNA-seq on DCN samples collected from mice under the same experimental conditions as before (HC, CD, and TN). Considering the rapid translocation of mRNA to the cytoplasm, the DCN was dissected 30 min after either fear conditioning or tone retrieval. After excluding cell types predicted to be contaminants from adjacent regions, six distinct cell types were identified based on the expression of canonical marker genes (Fig. 5a, Extended Data Fig. 4a).
Figure 5. Cell type-specific transcriptional changes within the DCN during fear learning.
a, UMAP representation of the snRNA-seq dataset, colored by cell types (24,290 nuclei). Exc-DCN, excitatory neurons in DCN; Inh-DCN, inhibitory neurons in DCN. b, Bar plot showing the number of significant LRT DEGs per cell type (adjusted P < 0.05). c–e, Quadrant plots showing log2FC values for oligodendrocyte (c), Inh-DCN (d), and astrocyte (e) DEGs in the CD vs. HC (x-axis) and TN vs. HC comparisons (y-axis). Genes are colored based on the comparison in which they are significant. Violin plots display the normalized expression patterns of a “both significant” DEG with the highest log2FC in each quadrant I (left) and quadrant III (right), separated by condition. f, Bar plot showing the number of significant TN vs. CD DEGs per cell type (adjusted P < 0.05). g, GO enrichment analysis results for TN vs. CD DEGs in oligodendrocytes, categorized by biological processes. Bar colors indicate the direction of enrichment, and FDR values represent significance.
Among these, oligodendrocytes exhibited the highest number of significant LRT DEGs (n = 2,106), followed by inhibitory neurons (n = 944) and astrocytes (n = 728). (Fig. 5b). Pairwise comparisons for each cell type revealed distinct DEG patterns, highlighting that fear learning involves interactions between various cell types with specialized roles (Fig. 5c–e, Supplementary Table 4a).
Interestingly, DEG analysis comparing the TN vs. CD conditions revealed that oligodendrocytes exhibited a significantly higher number of DEGs than other cell types, such as inhibitory neurons and astrocytes, which showed relatively few DEGs (Fig. 5f, Supplementary Table 4a). This pronounced transcriptional activity in oligodendrocytes was much less evident in the CD vs. HC and TN vs. HC comparisons (Extended Data Fig. 4b), suggesting that oligodendrocytes are primarily involved during fear memory storage. This observation contrasts with previous assumptions that memory retrieval involves substantial glial-neuron interactions45. GO enrichment analysis of the upregulated DEGs in the TN phase compared to the CD phase showed strong associations with myelination-related processes (Fig. 5g, Supplementary Table 4b), consistent with recent studies demonstrating that new myelin formation supports the preservation of remote fear memory recall46,47. To identify the transcriptional regulators driving these gene expression changes, we conducted a transcription factor (TF) analysis. The most highly activated TF in oligodendrocytes during the TN phase compared to the CD phase was Smad3 (Extended Data Fig. 4c, Supplementary Table 4c). Smad3, known to regulate the timing of central nervous system myelination via TFG-beta signaling48, emerged as a key regulator of fear memory retrieval in oligodendrocytes.
Specific inhibitory neuron subtype expressing Kit is highly associated with fear conditioning
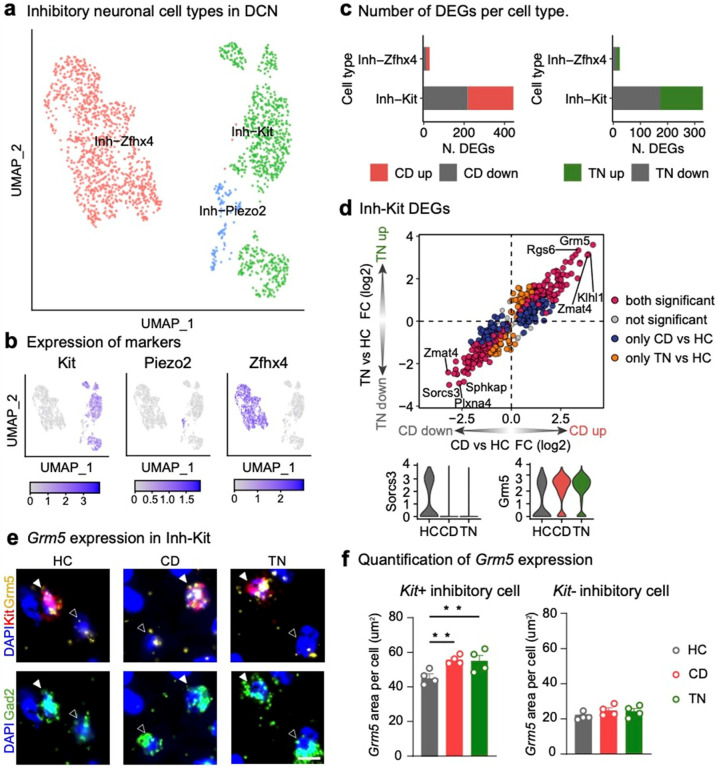
In our analysis, inhibitory neurons (Inh-DCN) exhibited more DEGs than excitatory neurons (Exc-DCN) (Fig. 5b). Given the significant heterogeneity within inhibitory neurons49, we further analyzed these cells by categorizing their subtypes. Based on the classification by Kebschull et al., inhibitory neurons in the DCN can be grouped into three subtypes characterized by specific marker genes: Zfhx4, Kit, and Piezo210. To explore this heterogeneity, we performed unsupervised clustering of inhibitory neurons in our snRNA-seq dataset, identifying eight clusters that predominantly expressed one of these three marker genes (Extended Data Fig. 5a, b). Using this expression pattern, we classified the clusters into three subtypes: Inh-Zfhx4, Inh-Kit, and Inh-Piezo2 (Fig. 6a, b).
Figure 6. Inh-Kit neurons show significant changes in response to fear conditioning.
a, UMAP representation of inhibitory neurons in the DCN, colored by subtype (1,980 nuclei). b, UMAP representation of subtype marker gene expression (Kit, Piezo2, and Zfhx4). c, Bar plots showing the number of significant DEGs per inhibitory neuron subtype for the CD vs. HC (right) and TN vs. HC comparisons (right). Significant DEGs were defined as adjusted P < 0.05 and log2FC > ±0.1. d, Quadrant plot showing log2FC values for Inh-Kit DEGs in the CD vs. HC (x-axis) and TN vs. HC comparisons (y-axis). Genes are colored based on the comparison in which they are significant. Violin plots display the normalized expression patterns of a “both significant” DEG with the highest log2FC in each quadrant I (left) and quadrant III (right), separated by condition. e, ISH results showing the expression of Grm5 in the DCN after fear learning. Filled arrows indicate Gad2+ Kit+ cells (Kit+ inhibitory cells) and open arrows indicate Gad2+ Kit− cells (Kit− inhibitory cells). Scale bar: 10 μm. f, Quantification of ISH results (n = 4 per group). Kit+ inhibitory cells: One-way ANOVA: F(2,9) = 6.007, P = 0.0220. Dunnett’s multiple comparisons test: *P = 0.0233 (HC vs. CD), *P = 0.0314 (HC vs. TN). Kit− inhibitory cells: One-way ANOVA: F(2,9) = 1.526, P = 0.2687. Dunnett’s multiple comparisons test: P = 0.2320 (HC vs. CD), *P = 0.3323 (HC vs. TN).
We performed subtype-specific DEG analyses across three conditions (CD vs. HC, TN vs. HC, and TN vs. CD). Among the subtypes, Inh-Kit displayed a significantly higher number of DEGs in both the CD vs. HC and TN vs. HC comparisons (Fig. 6c, Supplementary Table 5a). Despite the similar proportions of the Inh-Kit and Inh-Zfhx4 subtypes among inhibitory neurons in the DCN (Extended Data Fig. 5c), the pronounced transcriptional activity in Inh-Kit suggests a critical role in the fear learning process. Interestingly, the ratio of Kit + inhibitory neurons (Kit + Gad2 + cells) among total inhibitory neurons (Gad2+) varied across DCN subnuclei. However, fear conditioning did not affect the overall population of inhibitory neurons or Kit + inhibitory neurons (Extended Data Fig. 6a–d). This indicates that the three subnuclei have distinct cell type compositions, suggesting specialized roles for each subnucleus in fear memory processing. Among the top DEGs, Grm5 was the most upregulated gene in Inh-Kit during both the CD and TN phases compared to the HC phase (Fig. 6d). Grm5 encodes the metabotropic glutamate receptor mGluR5, which is crucial for neuronal plasticity50,51. Fear acquisition and extinction are impaired in mGluR5 knockout mice52, underscoring its role as a key regulator of fear learning and memory53,54.
To validate the subtype-specific expression of Grm5, we performed ISH and confirmed that Grm5 was significantly upregulated in Kit + inhibitory cells, but not in Kit− inhibitory cells, following fear conditioning (Fig. 6e, f, Extended Data Fig. 6e). Notably, this upregulation persisted during the retrieval phase, indicating the sustained involvement of Grm5 in fear learning and memory processes.
TF analysis revealed highly cell type-specific TF activity changes in Inh-Kit (Extended Data Fig. 5d, Supplementary Table 5b). Among the top TFs activated during both the CD and TN phases, Thrb was identified as a key regulator of Grm5. Thyroid hormones, encoded by Thrb, are essential for normal brain development, and their deficiency has been linked to intellectual and neurological impairments55. Our results highlight the unique role of Inh-Kit inhibitory neurons in fear learning and suggest key transcriptional changes underlying their function.
Discussion
In this study, we demonstrate that classical fear conditioning induces region-, cell type-, and learning phase-specific transcriptomic changes in the cerebellum. Spatial transcriptomic analysis revealed distinct transcriptional profiles across different cerebellar regions during various stages of fear learning. Our findings underscore that the role of the cerebellum in fear learning is not uniform but instead involves specialized and coordinated transcriptional changes in specific circuits. Notably, the robust induction of IEGs (Fos, Junb, and Egr1) in the DCN was observed, highlighting the significance of this output hub in shaping fear memory processes. Interestingly, vermis displayed broader transcriptional changes than the hemisphere, consistent with its established role in integrating conditioned and unconditioned stimuli. In the DCN, where region-specific activation appeared, oligodendrocytes and specific inhibitory neuron subtypes, such as Kit + neurons, exhibited unique transcriptional responses, suggesting that glial and inhibitory circuits contribute distinctively to encoding and retrieving fear memories. These findings expand our understanding of cerebellar functions beyond motor coordination and suggest potential molecular targets for exploring cellular mechanisms underlying fear learning.
IEGs such as c-Fos, Junb, Egr1, and Npas4 are well-established memory tags in brain regions such as the hippocampus, medial frontal cortex, and amygdala56–58; however, their roles in cerebellar learning mechanisms remain underexplored4,32. Here, we observed robust fear-conditioning-dependent upregulation of IEGs in the DCN, suggesting their role as memory tags in cerebellar circuits59. Each IEG exhibited distinct expression patterns across fear learning stages and DCN subnuclei. Given that IEGs also function as transcription factors targeting specific genes60, our findings suggest they may play specialized roles in fear learning61–64. However, IEG expression was not detected in our snRNA-seq dataset, likely due to technical limitations; the Visium platform captures transcripts in tissue spots, whereas snRNA-seq focuses on nuclear RNA. By the time of brain dissection, IEG mRNAs may have been exported to the cytoplasm, leaving their cell type of origin unresolved.
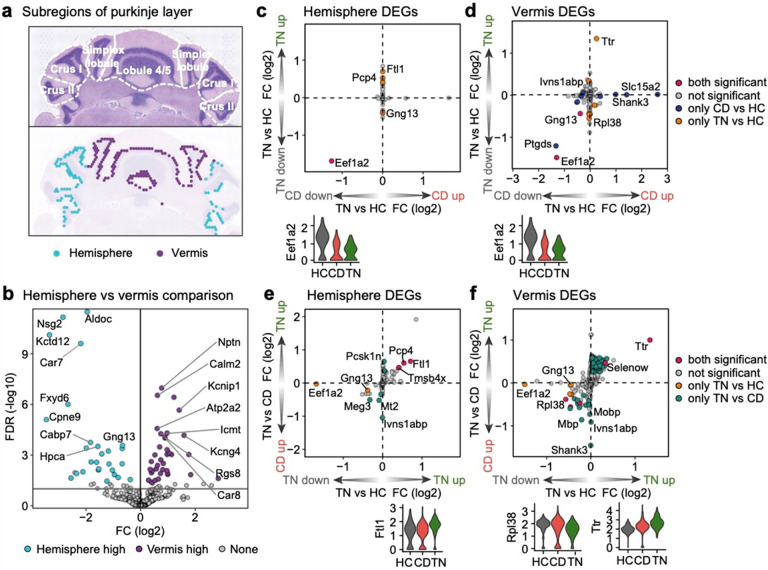
The cerebellar vermis exhibited more extensive transcriptional changes than the hemisphere across all stages of fear learning. This finding aligns with that of previous studies identifying the vermis as a core site for the convergence of conditioned and unconditioned stimuli during classical fear conditioning, regulating fear-evoked freezing behavior and bradycardia6,23–25,65,66. Our findings extend this understanding by showing that the vermis undergoes distinct transcriptomic remodeling throughout multiple learning phases.
We identified Ttr and Selenow, genes implicated in oxidative stress and energy metabolism67–70, as highly upregulated in the vermis following tone memory retrieval. These genes may regulate the metabolic states required for learning-induced changes. Interestingly, their upregulation appeared primarily during the memory consolidation phase, as their expression was significantly higher during tone retrieval than during training. However, our analysis focused primarily on coronal sections encompassing lobules V and VI, leaving the possibility of transcriptional changes in other cerebellar regions, such as lobule VIII71,72, unexplored.
Oligodendrocytes in the DCN displayed the highest number of DEGs across fear conditioning phases, with pronounced transcriptional changes between memory consolidation and retrieval, a period during which other cell types showed minimal or no transcriptional changes. During the TN phase, the expression of genes related to myelination was significantly higher than that in the CD phase. Oligodendrocyte-mediated myelination supports rapid and stable neuronal communication, which is crucial for synaptic plasticity73. These findings align with those of a previous study showing that the DCN–lPBN circuit undergoes synaptic potentiation during auditory fear conditioning22.
A classical understanding of the interaction between the cerebellar cortex and DCN is that Purkinje cells in the cerebellar cortex alter their firing rates and synchronization, exerting inhibitory inputs to the DCN to modulate the firing rates of DCN neurons, which in turn regulate DCN output signals to frontal brain regions74,75. DCN neurons exhibit diverse projection patterns, and emerging evidence suggests the presence of multiple distinct cell types within the DCN10,76–81. Here, we identified Kit + inhibitory neurons in the DCN as undergoing prominent transcriptional changes during classical fear conditioning. Notably, the Grm5 gene, encoding mGluR5, was significantly upregulated in Kit + inhibitory neurons during both fear acquisition and retrieval phases. This suggests that, besides modulating DCN firing, these neurons engage in learning-dependent synaptic plasticity, potentially forming an inhibitory engram. Our work provides new insights into the role of the cerebellum in non-motor associative learning, highlighting region-, cell type-, and learning phase-specific transcriptional changes. The identification of IEGs in the DCN raises the exciting possibility of experimentally probing cerebellar fear memory engrams. Future research should focus on dissecting the glial and inhibitory circuits involved in cerebellar learning to further elucidate the contribution of the cerebellum to learning and memory.
Online Methods
Animals
6 to 8-week aged male wild-type mice (C57Bl/6N from Orient Bio Inc., Seongnam, Korea), were purchased and acclimated to the facility for at least one week before the experiment. All mice were maintained on a 12 h light/dark cycle with ad libitum access to food and water. All experiments were performed in accordance with the guidelines approved by the Institutional Animal Care and Use Committee of Seoul National University (SNU-221014–3).
Fear conditioning
For fear conditioning, mice were placed in fear conditioning chamber. After 2min exploration period, mice received five CS (30 s, 2800 Hz, 79 dB tone) and US (2 s, 0.5 mA, electrical foot shock) pairings. For tone retrieval test, mice were placed in a novel chamber (cylinder chamber) 24h after conditioning. 2 or 3 CS with a 30-s interval were represented. All behavior tests were performed between 09:00 and 16:00 during the light phase of the light/dark cycle.
Sample preparation for spatial transcriptomics
60 min after fear conditioning or retrieval test, mice were anesthetized with isoflurane and perfused with normal saline. Extracted brains were flash frozen in isopropyl alcohol at −70°C. Frozen brains were embedded in optimal cutting temperature (OCT) compound (VWR, Cat No. 25608–930) and were stored at −80°C until sectioning. Brains with a RIN (RNA integrity and Number) above 7 were used.
Tissue blocks were equilibrated to − 15°C and sectioned at a thickness of 10 μm using cryostat (Thermo, Cryostar NX70). The sections were mounted onto the active capture areas of 10x Genomics Visium slides (10x Genomics, PN 1000184) and stored in airtight containers at − 80°C until Visium library preparation. Prior to Visium library preparation, methanol fixation and H&E (hematoxylin and eosin) staining were performed following the Visium protocol (10x Genomics, CG000160).
Visium libraries were constructed following the manufacturer’s instructions (10x Genomics, CG000239). Sequencing was performed on a Novaseq 6000 platform (Illumina, Cat No. 20012850) using S1 sequencing reagents (Illumina, Cat No. 20028318), targeting 120 million reads per library using dual indexing. The resulting FASTQ files were aligned to the mm10 reference genome using Space Ranger (10x Genomics, v1.3.1).
In situ hybridization (RNAscope)
After fear conditioning or retrieval test, mice were anesthetized with isoflurane 60 min after for spatial transcriptomics validation and 30 min after for snRNA-seq. Whole brains were obtained and flash-frozen in isopentane equilibrated on dry ice. Frozen brains were embedded in OCT and maintained at −80°C until sectioning. 14-μm coronal sections including DCN were collected on Superfrost slides and stored at −80°C until processing. Multi-channel fluorescence in situ hybridization was performed using the RNAscope™ Multiplex Fluorescent Reagent Kit v2 (Advanced Cell Diagnostics) following manufacturer’s instructions. In brief, slides were fixed in 4% PFA and serial dehydration step was done in ethanol. Then, hydrogen peroxide treatment, followed by protease 4 treatment was done at room temperature. Hybridization was done at 40°C for 2h. Following probes were used: Fos (316921-C2), Junb (556651-C1), Egr1 (423371-C2), Npas4 (423431-C3), Eef1a2 (1262661-C1), Kit (314151), Grm5 (423631-C2), Gad2 (439371-C3). The slides were imaged using slide scanner (Zeiss, Axio Scan Z1). We used ABBA workflow provided by https://biop.github.io/ijp-imagetoatlas/ for ROI registration, then analyzed with ImageJ and Qupath82.
DCN sample preparation for snRNA-seq
30min after fear conditioning or retrieval test, mice were anesthetized and decapitated immediately. Brains were extracted and sectioned using a vibratome (VT1200S, Leica) in pre-chilled ACSF. The DCN were quickly micro-dissected from 300 μm of cerebellar coronal slices and stored at −80°C.
Visium spatial transcriptomics analysis
Spatial transcriptomics mapping and preprocess
Space Ranger (v1.3.1) analysis pipelines were used to align raw FASTQ files to mm10 reference genome and image files.
Output files were loaded onto Scanpy v1.10.083 as anndata objects for further preprocessing. We removed doublets using Scrublet v0.2.384. Gm42418 and genes expressed in less than 3 nuclei were removed. Nuclei that have fewer than 100 genes, more than 8,000 genes, and more than 50,000 UMI counts were filtered out. Spots with over 35% mitochondrial gene expression were also excluded. Raw gene read counts of each sample were normalized for library size correction and then log-transformed.
To account for spot similarity in both feature and spatial space, the clustering process was conducted in two main stages. First, single-cell variational inference (scVI) v1.1.285 was used to adjust batch effects between each sample. The top 2,000 most highly variable genes of the merged dataset were identified using sc.pp.highly_variable_genes() with “Seurat v3” flavor and used for scVI analysis. The resulting latent representation was used for neighbor identification, which estimates ‘connectivities’ of spots based on gene expression. Next, the spatial connectivity matrix of spots was calculated using Squidpy v.1.2.286. The two connectivity graphs were added to form a joint graph, with a 0.8 importance weight for the gene expression connectivity and 0.2 importance weight for the spatial connectivity. Clustering of spots was performed on the joint connectivity graph using the Leiden algorithm with a resolution of 0.8.
Annotation of spatial clusters
We used cell2location v.0.1.387 to map cerebellum cell types on our Visium data. To account for only cerebellum regions, we subset spatial clusters 1,2,3,4,5, and 9 that are within the cerebellum, as guided by anatomical structures. Cerebellum scRNA-seq data from the Allen Brain Cell Atlas28 was utilized as the reference data. For the reference data, only genes detected in more than 5 cells, more than 3 percent of cells, and average expression in non-zero cells higher than 1.12 were retained.
Cell2location estimates reference gene signatures of cell types from reference scRNA-seq using negative binomial regression and accounting for batch effects. Reference model was built using ‘donor_label’ as the batch key. By mapping our query Visium dataset on the model, the absolute spatial abundance of each reference cell type was estimated for each spot. For parameters, the expected cell abundance for each spot (N_cells_per_location) was set to 6, the detection alpha was set to 20, and the number of training epochs was set to 30,000.
To annotate each spatial cluster using cell2location cell type abundance results, the following approach was utilized: Cell abundance values for each cell type were binarized using the 99th quantile, and the binarized labels were assigned to each spot. Fisher’s exact test was conducted using the binary labels for each spatial cluster. Each cluster was annotated with the cell type exhibiting the highest odd ratio (OR) among the region-specific cell types, which included DCN, molecular layer, granular layer, and Purkinje cell layer.
Spatial clusters that could not be annotated to a particular region using the above analysis were further classified using anatomical knowledge. Cluster 4, located below the cerebellar cortex (Extended Data Fig. 1a) and showing high enrichment for glial cell types (Fig. 1c), was identified as white matter. Cluster 9, which exhibited the highest enrichment for inferior colliculus (IC) (Fig. 1c) - a region not present in our obtained image slices - was annotated as molecular layer through manual comparison with Allen Brain Atlas in situ hybridization data29. Clusters 0,6,7,8,10,11, and 12, located in the medulla region, were also annotated through manual comparison with in situ hybridization reference data.
We manually annotated the Purkinje layer into vermis and hemisphere subregions using an anatomical atlas based on the Allen Brain Atlas. Spots located in lobules 4/5, lobule 6, and the simplex lobule were classified as part of the vermis region, while spots in Crus I, Crus II, and the surrounding regions were categorized as the hemisphere region.
Differential gene expression analysis
Both single-cell based and pseudo-bulk method was conducted for DEG analysis. Results from the single-cell based method suggested gene expression variability within the sample. To mitigate this variability, we determined that the pseudo-bulk approach will be more appropriate for our dataset.
For Purkinje cell layer subregions, we used DeSeq2 v.1.40.1.88 on pseudo-bulk samples for DEG analysis. For each subregion and sample, we created 2 pseudo-replicate samples by dividing the spots within that group into 2 subsets of roughly equal sizes, then summing up transcript counts for each gene. Spatial cluster – sample pairs with less than 10 spots were excluded. Genes with expression in less than 3 pseudo-replicates and mitochondrial genes were removed for the analysis.
Initially, we tested the significance of genes at any level of the condition factor using “LRT” as the test parameter in the DESeq() function. Next, we determined the DEGs for each pairwise comparison - CD vs HC, TN vs HC, and TN vs CD - using the standard DESeq() function with parameters alpha as 0.05 and independentFiltering set to false. In consideration of the small number of spots in each subregion, we repeated this process 100 times, with different seeds for every pseudo-replicate creation. Genes that were significant (adjusted P < 0.05) in more than 80% of the LRT tests were considered significant LRT DEGs. Significant LRT DEGs with adjusted P < 0.05 and log2FC > ± 0.1 in more than 80% of the pairwise comparison tests were considered significant DEGs.
DEGs from the comparison between the vermis and hemisphere subregions were identified using the same method, excluding the LRT analysis.
The DEG analysis for each cerebellum region followed the same method as the Purkinje subregion but with adjusted criteria: 3 pseudo-replicates were created, a minimum of 10 spots was required for spatial cluster–sample pairs, and the gene expression threshold was set at 5 pseudo-replicates. The analysis process was conducted only once for each region. Genes that were significant in the LRT, with adjusted P < 0.05 and log2FC > ± 0.1, were considered significant DEGs.
Pathway enrichment analysis
GO biological processes enriched for region-specific DEGs were analyzed using clusterProfilter v4.10.089. Significantly enriched terms were identified using a threshold of Benjamini-Hochberg corrected P < 0.05.
snRNA-seq data analysis
Preprocess and annotation
We used cellranger (v7.1.10) analysis pipelines to align raw FASTQ files to mm10 reference genome.
To proceed with further preprocessing, the output files were imported into Scanpy v1.10.083 as an anndata object. Doublets were called and excluded using Scrublet84. Count matrix was filtered for genes expressed in less than 3 nuclei and the Gm42418 gene which is associated with ribosomal RNA contamination. Nuclei with fewer than 200 genes, more than 8,000 genes, more than 60,000 UMI counts, and mitochondrial contents over 20% were excluded. Normalization and log-transformation of the raw gene read counts were applied to stabilize variance across expression values.
scVI v1.1.285 was used to adjust batch effects between each sample, with top 2,000 most highly variable genes of the merged dataset identified using sc.pp.highly_variable_genes() with “Seurat v3” flavor and used for scVI analysis. The resulting latent representation was used for neighbor identification, and clustering of nuclei was performed using the Leiden algorithm with a resolution of 1. Annotation of each cluster was conducted by comparing marker genes with well-known cell-type marker genes from various references15,28,29,90.
Differential gene expression analysis
To identify DEGs between HC, CD, and TN conditions per cell type, we used MAST v1.28.091. Only genes expressed in more than 5% of the nuclei per cell type were included in the analysis. We used the zlm function to fit our gene expression data to a Hurdle model with the cellular detection rate (proportion of genes that have expression within a single nuclei) as a covariate. Initially, we ran an LRT using MAST’s latest function to test which genes are differentially expressed when we drop the ‘condition’ factor. Genes with adjusted P < 0.05 were considered significant LRT DEGs. Next, we conducted DEG analysis for each pairwise comparison of fear learning phases: CD vs. HC, TN vs. HC, TN vs. CD. Genes that are significant LRT DEGs and pass the adjusted P < 0.05 and log2FC > ± 0.1 threshold were considered significant DEGs for each pairwise comparison. GO biological processes enriched for cell type-specific DEGs were identified using clusterProfilter v4.10.089.
Transcription factor activity analysis
Python-based single-cell regulatory network inference and clustering (pySCENIC) package92 v0.12.1 was used to estimate TF activity and its target genes for each nuclei. Briefly, adjacency between a TF and its targets was calculated using the GRNBoost2 algorithm93. Next, to identify TF-enriched motifs and their target genes, two gene motif ranking datasets (mm10_10kbp_up_10kbp_down_full_txt_v10 and mm10_500bp_up_100bp_down_full_tx_v10) from the RcisTarget database were used. Finally, AUC enrichment scores were predicted for each nuclei to quantify the activity of TFs.
We evaluated the significant change of TF activity between two conditions for each cell type with a one-sided Wilcoxon rank-sum test on regulon activity scores (RAS), using rstatix v0.7.2. To exclude TFs with low activity, only TFs with an average RAS within the cell type higher than the dataset-wide average were considered. Bonferroni correction was applied, and TFs with adjusted P < 0.01 were considered significantly differentially regulated.
Figure 4. Subregion-specific analysis of the Purkinje cell layer across fear learning stages.
a, Anatomical (top) and spatial (bottom) plots of the Purkinje cell layer. The anatomical plot is divided based on cerebellar lobules, referencing the Allen Brain Atlas. Purkinje cell layer spots in the spatial plot were manually split according to the anatomical plot. The hemisphere includes Crus I and Crus II, while the vermis includes the simplex lobule, lobules 4/5, and lobule 6 (lobule 6 is included in the HC sample; see Extended Data Fig. 2b). b, Volcano plot showing differentially expressed genes (DEGs) in a hemisphere vs vermis comparison. Significant DEGs were defined as adjusted P < 0.05 and log2FC > ±0.1. All three condition samples were used for the analysis. c–f, Quadrant plots illustrating log2(fold change) (log2FC) values for the CD vs. HC (x-axis) and TN vs. HC (y-axis) comparisons in the hemisphere (c) and vermis (d) regions, and for the TN vs. HC (x-axis) and TN vs. CD (y-axis) comparisons in the hemisphere (e) and vermis (f) regions. Genes are colored based on the comparison in which they are significant. Violin plots display the normalized expression patterns of a “both significant” DEG with the highest log2FC in each quadrant I (left) and quadrant III (right), separated by condition.
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) Grant funded by the Ministry of Science and ICT (NRF-2022M3E5E8018049 to J.Y.A. and Y.-S.L.), NIH grant R01 MH 087463 (to T.A), Roy J. Carver Chair in Neuroscience (to T.A.), a grant of the Korea-US Collaborative Research Fund (KUCRF), funded by the Ministry of Science and ICT and Ministry of Health & Welfare, Republic of Korea (RS-2024-00467876 to J.Y.A.), Bio&Medical Technology Development Program of the NRF funded by the Korean government (MSIT) (RS-2024-00439474 to J.Y.A. and Y.-S.L.), and the Korea University Grant (to J.Y.A.). J.J. and J.B. received a scholarship from the Brain Korea (BK21) FOUR Education Program. Y.-S.L was also supported by KBRI basic research program through Korea Brain Research Institute funded by Ministry of Science and ICT(25-BR-07-01)
Footnotes
Competing interests
T.A. serves on the Scientific Advisory Board of EmbarkNeuro and is a scientific advisor to Aditum Bio and Radius Health. The other authors declare no conflicting interests.
Supplementary Files
Contributor Information
Yong-Seok Lee, Seoul National University College of Medicine.
Jungeun Ji, University of Iowa.
Jinhee Baek, University of Iowa.
Kyoung-Doo Hwang, University of Iowa.
Seunghwan Choi, University of Iowa.
Ted Abel, University of Iowa.
Joon-Yong An, Korea University.
Junko Kasuya, University of Iowa.
References
- 1.Apps R. et al. Cerebellar Modules and Their Role as Operational Cerebellar Processing Units: A Consensus paper [corrected]. Cerebellum 17, 654–682 (2018). 10.1007/s12311-018-0952-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumann O. et al. Consensus paper: the role of the cerebellum in perceptual processes. Cerebellum 14, 197–220 (2015). 10.1007/s12311-014-0627-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carta I., Chen C. H., Schott A. L., Dorizan S. & Khodakhah K. Cerebellar modulation of the reward circuitry and social behavior. Science 363 (2019). 10.1126/science.aav0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang K. D. et al. Cerebellar nuclei neurons projecting to the lateral parabrachial nucleus modulate classical fear conditioning. Cell Rep 42, 112291 (2023). 10.1016/j.celrep.2023.112291 [DOI] [PubMed] [Google Scholar]
- 5.Ito M. & Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett 33, 253–258 (1982). 10.1016/0304-3940(82)90380-9 [DOI] [PubMed] [Google Scholar]
- 6.Sacchetti B., Baldi E., Lorenzini C. A. & Bucherelli C. Cerebellar role in fear-conditioning consolidation. Proc Natl Acad Sci U S A 99, 8406–8411 (2002). 10.1073/pnas.112660399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacchetti B., Scelfo B., Tempia F. & Strata P. Long-term synaptic changes induced in the cerebellar cortex by fear conditioning. Neuron 42, 973–982 (2004). 10.1016/j.neuron.2004.05.012 [DOI] [PubMed] [Google Scholar]
- 8.Schonewille M. et al. Purkinje Cell-Specific Knockout of the Protein Phosphatase PP2B Impairs Potentiation and Cerebellar Motor Learning. Neuron 67, 618–628 (2010). 10.1016/j.neuron.2010.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busch S. E. & Hansel C. Climbing fiber multi-innervation of mouse Purkinje dendrites with arborization common to human. Science 381, 420–427 (2023). 10.1126/science.adi1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kebschull J. M. et al. Cerebellar nuclei evolved by repeatedly duplicating a conserved cell-type set. Science 370 (2020). 10.1126/science.abd5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lackey E. P. et al. Specialized connectivity of molecular layer interneuron subtypes leads to disinhibition and synchronous inhibition of cerebellar Purkinje cells. Neuron 112, 2333–2348.e2336 (2024). 10.1016/j.neuron.2024.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voerman S., Urbanus B. H. A., Schonewille M., White J. J. & De Zeeuw C. I. Postsynaptic plasticity of Purkinje cells in mice is determined by molecular identity. Commun Biol 5, 1328 (2022). 10.1038/s42003-022-04283-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X. et al. Transcriptomic mapping uncovers Purkinje neuron plasticity driving learning. Nature 605, 722–727 (2022). 10.1038/s41586-022-04711-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khouri-Farah N., Guo Q., Morgan K., Shin J. & Li J. Y. H. Integrated single-cell transcriptomic and epigenetic study of cell state transition and lineage commitment in embryonic mouse cerebellum. Sci Adv 8, eabl9156 (2022). 10.1126/sciadv.abl9156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozareva V. et al. A transcriptomic atlas of mouse cerebellar cortex comprehensively defines cell types. Nature 598, 214–219 (2021). 10.1038/s41586-021-03220-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim C. H. et al. Lobule-specific membrane excitability of cerebellar Purkinje cells. J Physiol 590, 273–288 (2012). 10.1113/jphysiol.2011.221846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suvrathan A., Payne H. L. & Raymond J. L. Timing Rules for Synaptic Plasticity Matched to Behavioral Function. Neuron 92, 959–967 (2016). 10.1016/j.neuron.2016.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang K. D., Kim S. J. & Lee Y. S. Cerebellar Circuits for Classical Fear Conditioning. Front Cell Neurosci 16, 836948 (2022). 10.3389/fncel.2022.836948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tovote P., Fadok J. P. & Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16, 317–331 (2015). 10.1038/nrn3945 [DOI] [PubMed] [Google Scholar]
- 20.Frontera J. L. et al. Bidirectional control of fear memories by cerebellar neurons projecting to the ventrolateral periaqueductal grey. Nature Communications 11, 5207 (2020). 10.1038/s41467-020-18953-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frontera J. L. et al. The cerebellum regulates fear extinction through thalamo-prefrontal cortex interactions in male mice. Nature Communications 14 (2023). 10.1038/s41467-023-36943-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang K.-D. et al. Cerebellar nuclei neurons projecting to the lateral parabrachial nucleus modulate classical fear conditioning. Cell Reports 42 (2023). 10.1016/j.celrep.2023.112291 [DOI] [PubMed] [Google Scholar]
- 23.Supple W. F., Cranney J. & Leaton R. N. Effects of lesions of the cerebellar vermis on VMH lesion-induced hyperdefensiveness, spontaneous mouse killing, and freezing in rats. Physiology & Behavior 42, 145–153 (1988). 10.1016/0031-9384(88)90290-9 [DOI] [PubMed] [Google Scholar]
- 24.Supple W. F. & Leaton R. N. Cerebellar vermis: essential for classically conditioned bradycardia in the rat. Brain Research 509, 17–23 (1990). 10.1016/0006-8993(90)90303-S [DOI] [PubMed] [Google Scholar]
- 25.Supple W. F., Leaton R. N. & Fanselow M. S. Effects of cerebellar vermal lesions on species-specific fear responses, neophobia, and taste-aversion learning in rats. Physiology & Behavior 39, 579–586 (1987). 10.1016/0031-9384(87)90156-9 [DOI] [PubMed] [Google Scholar]
- 26.Kim Y. G. et al. Quantitative Proteomics Reveals Distinct Molecular Signatures of Different Cerebellum-Dependent Learning Paradigms. Journal of Proteome Research 19, 2011–2025 (2020). 10.1021/acs.jproteome.9b00826 [DOI] [PubMed] [Google Scholar]
- 27.Park J.-S., Onodera T., Nishimura S. i., Thompson R. F. & Itohara S. Molecular evidence for two-stage learning and partial laterality in eyeblink conditioning of mice. Proceedings of the National Academy of Sciences 103, 5549–5554 (2006). doi: 10.1073/pnas.0601150103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao Z. et al. A high-resolution transcriptomic and spatial atlas of cell types in the whole mouse brain. Nature 624, 317–332 (2023). 10.1038/s41586-023-06812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lein E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007). 10.1038/nature05453 [DOI] [PubMed] [Google Scholar]
- 30.Zangenehpour S. & Chaudhuri A. Differential induction and decay curves of c-Fos and zif268 revealed through dual activity maps. Brain Res Mol Brain Res 109, 221–225 (2002). 10.1016/s0169-328x(02)00556-9 [DOI] [PubMed] [Google Scholar]
- 31.Bisler S. et al. Expression of c-Fos, ICER, Krox-24 and Junb in the whisker-to-barrel pathway of rats: time course of induction upon whisker stimulation by tactile exploration of an enriched environment. Journal of Chemical Neuroanatomy 23, 187–198 (2002). 10.1016/S0891-0618(01)00155-7 [DOI] [PubMed] [Google Scholar]
- 32.Kim S. & Thompson R. F. c-Fos, Arc, and stargazin expression in rat eyeblink conditioning. Behav Neurosci 125, 117–123 (2011). 10.1037/a0022328 [DOI] [PubMed] [Google Scholar]
- 33.Frontera J. L. et al. Bidirectional control of fear memories by cerebellar neurons projecting to the ventrolateral periaqueductal grey. Nat Commun 11, 5207 (2020). 10.1038/s41467-020-18953-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blot F. G. C. et al. Purkinje cell microzones mediate distinct kinematics of a single movement. Nat Commun 14, 4358 (2023). 10.1038/s41467-023-40111-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerminara N. L. Cerebellar modules: individual or composite entities? J Neurosci 30, 16065–16067 (2010). 10.1523/jneurosci.4823-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apsley E. J. & Becker E. B. E. Purkinje Cell Patterning-Insights from Single-Cell Sequencing . Cells 11 (2022). 10.3390/cells11182918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brochu G., Maler L. & Hawkes R. Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J Comp Neurol 291, 538–552 (1990). 10.1002/cne.902910405 [DOI] [PubMed] [Google Scholar]
- 38.Carter R. A. et al. A Single-Cell Transcriptional Atlas of the Developing Murine Cerebellum. Curr Biol 28, 2910–2920.e2912 (2018). 10.1016/j.cub.2018.07.062 [DOI] [PubMed] [Google Scholar]
- 39.Hawkes R. & Leclerc N. Antigenic map of the rat cerebellar cortex: the distribution of parasagittal bands as revealed by monoclonal anti-Purkinje cell antibody mabQ113. J Comp Neurol 256, 29–41 (1987). 10.1002/cne.902560104 [DOI] [PubMed] [Google Scholar]
- 40.Drapeau E., Dorr N. P., Elder G. A. & Buxbaum J. D. Absence of strong strain effects in behavioral analyses of Shank3-deficient mice. Disease models & mechanisms 7, 667–681 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nunes A. F., Saraiva M. J. & Sousa M. M. Transthyretin knockouts are a new mouse model for increased neuropeptide Y. The FASEB Journal 20, 166–168 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Sousa J. C. et al. Transthyretin is involved in depression-like behaviour and exploratory activity. Journal of neurochemistry 88, 1052–1058 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Brouillette J. & Quirion R. Transthyretin: a key gene involved in the maintenance of memory capacities during aging. Neurobiology of aging 29, 1721–1732 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z.-H. & Song G.-L. Roles of selenoproteins in brain function and the potential mechanism of selenium in Alzheimer’s disease. Frontiers in Neuroscience 15, 646518 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Araque A., Parpura V., Sanzgiri R. P. & Haydon P. G. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22, 208–215 (1999). 10.1016/s0166-2236(98)01349-6 [DOI] [PubMed] [Google Scholar]
- 46.Pan S., Mayoral S. R., Choi H. S., Chan J. R. & Kheirbek M. A. Preservation of a remote fear memory requires new myelin formation. Nat Neurosci 23, 487–499 (2020). 10.1038/s41593-019-0582-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKenzie I. A. et al. Motor skill learning requires active central myelination. Science 346, 318–322 (2014). 10.1126/science.1254960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palazuelos J., Klingener M. & Aguirre A. TGFβ signaling regulates the timing of CNS myelination by modulating oligodendrocyte progenitor cell cycle exit through SMAD3/4/FoxO1/Sp1. J Neurosci 34, 7917–7930 (2014). 10.1523/jneurosci.0363-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fritschy J. M., Panzanelli P. & Tyagarajan S. K. Molecular and functional heterogeneity of GABAergic synapses. Cell Mol Life Sci 69, 2485–2499 (2012). 10.1007/s00018-012-0926-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheefhals N., Westra M. & MacGillavry H. D. mGluR5 is transiently confined in perisynaptic nanodomains to shape synaptic function. Nature Communications 14 (2023). ARTN244 10.1038/s41467-022-35680-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aloisi E. et al. Altered surface mGluR5 dynamics provoke synaptic NMDAR dysfunction and cognitive defects in Fmr1 knockout mice. Nat Commun 8, 1103 (2017). 10.1038/s41467-017-01191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu J., Zhu Y. L., Contractor A. & Heinemann S. F. mGluR5 Has a Critical Role in Inhibitory Learning. Journal of Neuroscience 29, 3676–3684 (2009). 10.1523/Jneurosci.5716-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matulewicz P., Ramos-Prats A., Gómez-Santacana X., Llebaria A. & Ferraguti F. Control of Theta Oscillatory Activity Underlying Fear Expression by mGlu(5) Receptors. Cells 11 (2022). 10.3390/cells11223555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xuan S. M. et al. mGluR5 in amygdala modulates fear memory generalization. Front Behav Neurosci 17, 1072642 (2023). 10.3389/fnbeh.2023.1072642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rivas M. & Naranjo J. Thyroid hormones, learning and memory. Genes, Brain and Behavior 6, 40–44 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Sun X. & Lin Y. Npas4: Linking Neuronal Activity to Memory. Trends in Neurosciences 39, 264–275 (2016). 10.1016/j.tins.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gallo F. T., Katche C., Morici J. F., Medina J. H. & Weisstaub N. V. Immediate Early Genes, Memory and Psychiatric Disorders: Focus on c-Fos, Egr1 and Arc. Frontiers in Behavioral Neuroscience 12 (2018). 10.3389/fnbeh.2018.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pelto-Huikko M. et al. Neuronal regulation of c-Fos, c-jun, and Junb immediate-early genes in rat adrenal medulla. J Neurosci 15, 1854–1868 (1995). 10.1523/jneurosci.15-03-01854.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012). 10.1038/nature11028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duclot F. & Kabbaj M. The Role of Early Growth Response 1 (EGR1) in Brain Plasticity and Neuropsychiatric Disorders. Front Behav Neurosci 11, 35 (2017). 10.3389/fnbeh.2017.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tischmeyer W. & Grimm R. Activation of immediate early genes and memory formation. Cellular and Molecular Life Sciences 55, 564–574 (1999). DOI 10.1007/s000180050315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abraham W. C. et al. Correlations between Immediate-Early Gene Induction and the Persistence of Long-Term Potentiation. Neuroscience 56, 717–727 (1993). Doi 10.1016/0306-4522(93)90369-Q [DOI] [PubMed] [Google Scholar]
- 63.Guzowski J. F., Setlow B., Wagner E. K. & McGaugh J. L. Experience-dependent gene expression in the rat hippocampus after spatial learning: A comparison of the immediate-early genes Arc, c-Fos, and zif268. Journal of Neuroscience 21, 5089–5098 (2001). Doi 10.1523/Jneurosci.21-14-05089.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abraham W. C., Dragunow M. & Tate W. P. The role of immediate early genes in the stabilization of long-term potentiation. Mol Neurobiol 5, 297–314 (1991). 10.1007/BF02935553 [DOI] [PubMed] [Google Scholar]
- 65.Leaton R. N. & Supple W. F. Cerebellar Vermis: Essential for Long-Term Habituation of the Acoustic Startle Response. Science 232, 513–515 (1986). 10.1126/science.3961494 [DOI] [PubMed] [Google Scholar]
- 66.Sebastiani L., La Noce A., Paton J. F. & Ghelarducci B. Influence of the cerebellar posterior vermis on the acquisition of the classically conditioned bradycardic response in the rabbit. Exp Brain Res 88, 193–198 (1992). 10.1007/bf02259141 [DOI] [PubMed] [Google Scholar]
- 67.Misra S. et al. Loss of selenoprotein W in murine macrophages alters the hierarchy of selenoprotein expression, redox tone, and mitochondrial functions during inflammation. Redox Biology 59, 102571 (2023). 10.1016/j.redox.2022.102571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jing J. et al. Selenoproteins synergistically protect porcine skeletal muscle from oxidative damage via relieving mitochondrial dysfunction and endoplasmic reticulum stress. Journal of Animal Science and Biotechnology 14, 79 (2023). 10.1186/s40104-023-00877-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eze F. N. Transthyretin Amyloidosis: Role of oxidative stress and the beneficial implications of antioxidants and nutraceutical supplementation. Neurochemistry International 179, 105837 (2024). 10.1016/j.neuint.2024.105837 [DOI] [PubMed] [Google Scholar]
- 70.Wieczorek E. & Ożyhar A. Transthyretin: From Structural Stability to Osteoarticular and Cardiovascular Diseases. Cells 10 (2021). 10.3390/cells10071768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koutsikou S. et al. Neural substrates underlying fear-evoked freezing: the periaqueductal grey-cerebellar link. J Physiol 592, 2197–2213 (2014). 10.1113/jphysiol.2013.268714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lange I. et al. The anatomy of fear learning in the cerebellum: A systematic meta-analysis. Neurosci Biobehav Rev 59, 83–91 (2015). 10.1016/j.neubiorev.2015.09.019 [DOI] [PubMed] [Google Scholar]
- 73.Bonetto G., Belin D. & Káradóttir R. T. Myelin: A gatekeeper of activity-dependent circuit plasticity? Science 374, eaba6905 10.1126/science.aba6905 [DOI] [PubMed] [Google Scholar]
- 74.Person A. L. & Raman I. M. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature 481, 502–505 (2012). 10.1038/nature10732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev 81, 1143–1195 (2001). 10.1152/physrev.2001.81.3.1143 [DOI] [PubMed] [Google Scholar]
- 76.Judd E. N., Lewis S. M. & Person A. L. Diverse inhibitory projections from the cerebellar interposed nucleus. Elife 10 (2021). 10.7554/eLife.66231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujita H., Kodama T. & du Lac S. Modular output circuits of the fastigial nucleus for diverse motor and nonmotor functions of the cerebellar vermis. Elife 9 (2020). 10.7554/eLife.58613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bagnall M. W. et al. Glycinergic projection neurons of the cerebellum. J Neurosci 29, 10104–10110 (2009). 10.1523/JNEUROSCI.2087-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Heijden M. E. et al. Glutamatergic cerebellar neurons differentially contribute to the acquisition of motor and social behaviors. Nat Commun 14, 2771 (2023). 10.1038/s41467-023-38475-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prekop H. T. et al. Sox14 Is Required for a Specific Subset of Cerebello-Olivary Projections. Journal of Neuroscience 38, 9539–9550 (2018). 10.1523/Jneurosci.1456-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang X. Y. et al. A role for the cerebellum in motor-triggered alleviation of anxiety. Neuron 112, 1165–1181 e1168 (2024). 10.1016/j.neuron.2024.01.007 [DOI] [PubMed] [Google Scholar]
- 82.Bankhead P. et al. QuPath: Open source software for digital pathology image analysis. Sci Rep 7, 16878 (2017). 10.1038/s41598-017-17204-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolf F. A., Angerer P. & Theis F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome biology 19, 1–5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolock S. L., Lopez R. & Klein A. M. Scrublet: computational identification of cell doublets in single-cell transcriptomic data. Cell systems 8, 281–291. e289 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lopez R., Regier J., Cole M. B., Jordan M. I. & Yosef N. Deep generative modeling for single-cell transcriptomics. Nature methods 15, 1053–1058 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palla G. et al. Squidpy: a scalable framework for spatial omics analysis. Nature methods 19, 171–178 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kleshchevnikov V. et al. Cell2location maps fine-grained cell types in spatial transcriptomics. Nature biotechnology 40, 661–671 (2022). [DOI] [PubMed] [Google Scholar]
- 88.Love M. I., Huber W. & Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biology 15, 1–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu G., Wang L.-G., Han Y. & He Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics: a journal of integrative biology 16, 284–287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeisel A. et al. Molecular architecture of the mouse nervous system. Cell 174, 999–1014. e1022 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Finak G. et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome biology 16, 1–13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Van de Sande B. et al. A scalable SCENIC workflow for single-cell gene regulatory network analysis. Nature protocols 15, 2247–2276 (2020). [DOI] [PubMed] [Google Scholar]
- 93.Moerman T. et al. GRNBoost2 and Arboreto: efficient and scalable inference of gene regulatory networks. Bioinformatics 35, 2159–2161 (2019). [DOI] [PubMed] [Google Scholar]