Abstract
Vitamin K has been implicated in skeletal health because vitamin K-dependent proteins are present in bone tissue. While there are multiple forms of vitamin K, most research has focused on phylloquinone, which is found mainly in plant-based foods, and its metabolite menaquinone-4 (MK4). However, there are additional forms of vitamin K that are bacterially produced that appear to influence bone health but have not yet been studied extensively. Herein, we evaluated the effects of menaquinone-9 (MK9), a bacterially produced form of vitamin K, on bone tissue quality and density in young mice. Four-week-old male (n = 32) and female (n = 32) C57BL/6 mice were supplemented with 0.06 mg/kg diet or 2.1 mg/kg diet of MK9 for 12 wk. During week 11, a subgroup of mice (n = 7/sex/group) received daily deuterium-labeled MK9 to trace its metabolic fate in bone. Liver MK4 and MK9 were significantly higher in mice fed 2.1 mg MK9/kg compared to those receiving 0.06 mg MK9/kg, regardless of sex (all p ≤ .017). MK4 was the only vitamin K form detected in bone, with 63%-67% of skeletal MK4 in mice fed 2.1 mg MK9/kg derived from deuterium-labeled MK9. Femoral tissue strength, maximum bending moment, section modulus, and BMD did not differ significantly between diet groups in either sex (all p ≥ .083). Cross-sectional area (p = .003) and moment of inertia (p = .001) were lower in female mice receiving 2.1 mg MK9/kg compared to those receiving 0.06 mg MK9/kg, but no differences were found in male mice. Higher bone MK4 concentrations did not correlate with higher bone tissue quality or density. Despite dietary MK9 being a dietary precursor to MK4 in bone, dietary MK9 supplementation did not affect bone tissue quality or BMD during skeletal development.
Keywords: skeletal health, bone tissue quality, bone mineral density, vitamin K, menaquinone
Introduction
Vitamin K, an essential fat-soluble nutrient, has been implicated in bone health1based on its role as an enzymatic cofactor required for the post-translational carboxylation of certain proteins (vitamin K-dependent proteins).2One such protein is osteocalcin, the predominate noncollagenous protein in bone. Once carboxylated, osteocalcin binds to calcium in the bone matrix.3There are multiple naturally occurring forms of vitamin K that are capable of carboxylation. The plant-based form, phylloquinone (PK), is found in green leafy vegetables and vegetable oils.4Menaquinones (MKs) are a class of vitamin K compounds that are primarily bacterially produced and are therefore abundant in the intestinal microbiome. At least 13 MK forms have been identified that differ structurally from PK in the length and saturation of their prenylated side chain, with the number of prenyl units differentiating among the specific MKs (MKn).5,6
Among the MKs, MK4 is unique because it is not known to be made by bacteria. Instead, it is synthesized in several extra-hepatic tissues from other forms of vitamin K, including MKs that are bacterially produced.7–9It was recently demonstrated that MK4 is found in bone,9but the role of dietary vitamin K as a precursor to MK4 in bone has not yet been fully elucidated. This is an important gap to address because MK4 signaling pathways have been implicated in bone tissue homeostasis.10,11
Most observational studies and randomized clinical trials evaluating the role of vitamin K in skeletal health have focused primarily on PK and MK4, with age-related bone loss measured using BMD as the main outcome. However, these studies have yielded inconsistent results.12A systematic review and meta-analysis of randomized controlled trials that tested the effect of PK or MK4 supplementation on age-related bone loss concluded there is little evidence that vitamin K affects BMD or vertebral fractures. The authors reported a potentially beneficial effect of PK or MK4 supplementation on clinical fracture risk but concluded additional studies are needed to confirm this.13While fragility fractures are often associated with low BMD, they also occur in individuals with normal BMD.14Bone tissue quality—encompassing bone strength and mechanical properties—is another critical aspect of bone health not captured by BMD.15–18An impairment in bone tissue quality can reduce whole bone strength without altering BMD.19In mice, knockout of the vitamin K-dependent protein osteocalcin results in impaired bone tissue composition and quality with only minor changes in bone geometry or BMD.20,21Hence, vitamin K-induced alteration of bone tissue quality may not be reflected by BMD or other indices of bone geometry and mass. Furthermore, there are limited data on the potential effects of bacterially-produced MKs on bone health outcomes. Addressing this gap is essential for understanding the broader role of vitamin K in skeletal health.
To clarify the role of bacterially-produced MK forms in bone tissue, we supplemented male and female C57BL/6 mice with 2 different doses of menaquinone-9 (MK9) in their diet for 12 wk. We chose MK9 because (1) MK9 is an MK form produced by bacteria. (2) Of the MK forms currently characterized in the food supply, MK9 is the most abundant, especially in dairy and fermented products.6(3) Through the use of stable isotopes, we have already characterized the metabolic fate of dietary MK9 in C57BL/6 mice, including the conversion of MK9 to MK4 in extra-hepatic tissues other than bone,9which serves as a quality control from which to compare our findings. We also evaluated the conversion of dietary MK9 to MK4 in skeletal tissue using stable isotopes. We hypothesized that dietary supplementation with MK9 would positively influence bone tissue quality and density and serve as a precursor to MK4 in bone tissue.
Materials and methods
Study design and experimental diets
Three-week-old male (n = 32) and female (n = 32) C57BL/6 mice were acquired from Charles River Laboratories and housed in single cages at the Tufts University Human Nutrition Research Center on Aging (HNRCA) Comparative Biology Unit. Upon arrival, mice were acclimated to an AIN-93G diet (Inotiv, TD.94045), which contained 1 mg PK/kg diet (per manufacturer), for 1 wk. At 4 wk of age, mice were divided at random into 2 experimental groups to receive diets containing varying amounts of MK9 as the sole source of vitamin K for 12 wk.22Males and females were randomized separately to one of the 2 experimental groups (Figure 1): (1) diet containing 2.1 mg/kg MK9 and (2) diet containing 0.06 mg/kg MK9. Diets were composed of a mixture of the vitamin K-free basal diet (Inotiv, TD.120060) with 2 different amounts of MK9. A MK9 content of 2.1 mg/kg corresponds to the recommended vitamin K intake, which was based on the molar equivalent of MK9 to the currently recommended concentration of vitamin K for rodents (1 mg/kg PK), following a protocol developed by the Vitamin K Laboratory at the HNRCA.23In contrast, an MK9 content of 0.06 mg/kg indicates a vitamin K intake below the recommended level, which was molar equivalent to 31 ± 0.45 μg PK/kg contained in vitamin K-free basal diet.9,24The Vitamin K laboratory previously demonstrated that feeding mice a diet containing MK9 (as the only form of vitamin K in the diet) in an equal molar amount to the current rodent diet recommendations showed no sign of vitamin K-deficiency.9,24Mice were pair-fed between diet groups to minimize the possibility that any outcome differences would be due to differences in the total amount of food consumed.
Figure 1.
Animal study design. Four-week-old male (n = 32) and female (n = 32) C57BL/6 mice were supplemented with 0.06 mg/kg and 2.1 mg/kg of MK9 for 12 wk. during the final week, a sub-group of mice (n = 7/sex/group) received deuterium-labeled MK9 to trace its metabolic fate in bone.
To determine whether MK9 from the diet serves as a precursor to MK4 present in bone a subset of mice (n = 14, 7 males and 7 females) from each group were randomly selected to receive daily diets containing deuterium-labeled MK9 (2H7MK9) (in the same amount as in the initial diet, mixed with vitamin K-free basal diet using the protocol developed by the Vitamin K Laboratory) during week 11 of the experiment (the last week prior to sacrifice) (Figure 1). MK9 concentrations in study diets were measured by liquid chromatography-mass spectrometry,25and diet MK9 concentrations were 0.06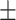 0.004 mg MK9/kg, 0.07
0.004 mg MK9/kg, 0.07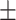 0.006 mg2H7MK9/kg, 2.03
0.006 mg2H7MK9/kg, 2.03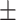 0.12 mg MK9/kg, and 2.47
0.12 mg MK9/kg, and 2.47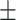 0.18 mg 2H7MK9/kg, respectively.
0.18 mg 2H7MK9/kg, respectively.
Animals were euthanized at 16 wk of age. After sacrifice, mice carcasses were scanned for BMD measurement, and then femurs, tibias, and liver were collected immediately after euthanasia and stored and maintained at −80  for bone biomechanical testing and vitamin K analyses. All animal experiments and procedures were approved by the Institutional Animal Care and Use Committee at Tufts University.
for bone biomechanical testing and vitamin K analyses. All animal experiments and procedures were approved by the Institutional Animal Care and Use Committee at Tufts University.
Bone tissue quality
Right femurs were dissected and shipped to the Hernandez Laboratory at the University of California, San Francisco, where femur length, cross-sectional geometry, and maximum bending force were measured, as described elsewhere.22,26In brief, femur length was measured using digital calipers from the greater trochanter to the lateral condyle. The femoral diaphyseal cross-section was imaged with a micro-computed tomography scanner at a voxel size of 10 μm to assess femoral cross-sectional geometry. The measurements included cortical cross-sectional area, moment of inertia about the medial-lateral axis (I), the distance from the neutral axis to the bone surface (c), and section modulus (I/c). For mechanical testing, femurs were thawed to room temperature and kept hydrated. They were then subjected to 3-point bending tests in the anterior-posterior direction using the electromechanical testing instrument (ElectroForce 3200, TA instruments) until failure occurred (loading rate = 0.1 mm/s, span length = 8 mm between outer loading pins). The maximum bending force and displacement were recorded. The maximum bending force was used to calculate the maximum bending moment (max force × span length/4). Bone tissue strength was calculated using maximum bending moment and cross-sectional geometric measurements (maximum bending moment/section modulus).
Bone mineral density
After sacrifice, mice carcasses were scanned using the InAlyzer2 dual-energy X-ray absorptiometry (DEXA) system, Model M (Medikors Inc.) at HNRCA, with details described elsewhere.27In brief, a quality control phantom was scanned prior to each measurement. Each carcass was positioned in the center of the scanning area in a prone position, with its arms and legs extended outward and its tail aligned straight behind the body. Scans were performed with a pixel size of 103 × 106 μm and a pixel pitch of 48 μm (108 μm in analysis). Total body BMD (g/cm2) was obtained using InAlyzer software version 3.2.3.
Vitamin K analyses
Measurements of MKs in bone and liver were conducted in the Vitamin K Laboratory at HNRCA as previously described.25Briefly, liver tissues (0.15 g) were homogenized in phosphate-buffered saline (PBS) by use of a Powergen homogenizer (Fisher Scientific). Left and right tibias (in total of 0.1 g) collected at sacrifice were fragmented using a stainless-steel tissue pulverizer (Biospec) with the addition of liquid nitrogen to ensure thorough pulverization. The resulting bone powder was transferred into 2 mL tubes prefilled with beads, followed by the addition of 1 mL PBS. Samples were then homogenized using an Omni Bead Ruptor 24 Homogenizer, running 2 cycles of 1 min each. Homogenized tissue samples were extracted using hexane, further purified using solid-phase extraction, and then quantified using Quadrupole Time-of-Flight Mass Spectrometry (Q-TOF-MS). To differentiate deuterium-labeled vitamin K forms, a ProntoSil C30 column is required for the separation of stereoisomers.28
Statistical power and analyses
Sample size calculations were based on the work of Guss et al.,22who reported a decrease of 3.6 N/mm2in femoral peak moment bending/I/c in 4-wk-old C57BL/6 male mice treated with oral antibiotics for 12 wk compared to mice that received no antibiotic treatment (p < .05). The between-group standard deviation was 2.5.22Fifteen mice per group provided 92% power to detect a between-group difference of 3.6, using a 2-tailed alpha of 0.05. With 2 experimental groups, we had at least 85% power to detect differences comparable to 3.6 N/mm2in Peak Moment in bending/I/c, accounting for up to 6 pair-wise comparisons.
Data are presented as means ± SD. Statistical analyses were performed using RStudio (v.4.3.1, 2023). The Shapiro–Wilk test was used to assess normality, and Levene’s test was employed to evaluate equal variance. For outcome measures that satisfied parametric assumptions, an independent 2-sample t-test was conducted to compare femoral tissue strength (primary outcome), other skeletal outcomes, liver, and bone vitamin K concentrations between diet groups. When parametric assumptions were not met, data were log-transformed to achieve normality, and parametric assumptions were reassessed. The correlations between bone MK4 concentrations and bone biomechanical and geometry outcomes were assessed using the Pearson correlation coefficient. Analyses were conducted separately for males and females, with statistical significance set at p < .05. Normalization by body weight was achieved by dividing bone biomechanical measurements by body weight.
Results
Eight mice died prior to the end of the study (7 males and 1 female). The highest mortality rate, 38%, was observed among male mice receiving 0.06 mg/kg MK9. In contrast, all female mice receiving 0.06 mg/kg MK9 treatment survived. Additionally, one mouse of each sex was lost among those receiving 2.1 mg/kg MK9, corresponding to a mortality rate of 1% (see Figure S1).
Bone tissue quality and density
Femoral tissue strength was not different across diet groups for either sex (male p = .725; female p = .302, Table 1). Similarly, there were no differences in maximum bending moment between diet groups for either male or female mice (p = .763 and 0.083, respectively, Table 1) or in sectional modulus in male or female mice between the diet groups (p = .873 and 0.277, respectively, Table 1).
Table 1.
Femoral biomechanics, geometry, and total body BMD measurements of male and female mice receiving diets supplemented with MK9.
| Male | Female | |||
|---|---|---|---|---|
| 0.06 mg/kg (n = 7) | 2.1 mg/kg (n = 11) | 0.06 mg/kg (n = 13) | 2.1 mg/kg (n = 11) | |
| Tissue strength (Mpa) | 153.3 ± 13.6 | 156 ± 16.3 | 171.8 ± 16.7 | 164.6 ± 14.8 |
| p-values | .725 | .302 | ||
| Maximum moment (N*mm) | 33.3 ± 2.4 | 33.7 ± 3.5 | 37.9 ± 3 | 35.4 ± 3.7 |
| p-values | .763 | .083 | ||
| Section modulus (mm3) | 0.22 ± 0.02 | 0.22 ± 0.01 | 0.22 ± 0.02 | 0.21 ± 0.02 |
| p-values | .873 | .277 | ||
| Moment of inertia (mm4) | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.13 ± 0.01 |
| p-values | .460 | .003 | ||
| Cross-sectional area (mm2) | 0.86 ± 0.07 | 0.9 ± 0.04 | 0.95 ± 0.07 | 0.87 ± 0.03 |
| p-values | .205 | .001 | ||
| Femur length (mm) | 15.6 ± 0.3 | 15.6 ± 0.5 | 15.6 ± 0.4 | 15.8 ± 0.3 |
| p-values | .774 | .288 | ||
Bone mineral density (g/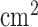 ) ) |
0.06 ± 0.004 | 0.06 ± 0.004 | 0.06 ± 0.002 | 0.06 ± 0.003 |
| p-values | .306 | .947 | ||
Values are mean ± SD. Differences between diet groups were determined using a parametric unpaired 2-sample t-test. Males and females were analyzed separately with statistical significance set as p < .05. Total body BMD is presented, with all other measurements analyzed using femurs.
Female mice receiving 0.06 mg/kg MK9 had higher moment of inertia and cross-sectional area compared to female mice receiving 2.1 mg/kg MK9 (both p 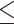 .003). No significant differences in these 2 measurements were observed among the male mice between diet groups (all p ≥ .205, Table 1). No significant differences were observed in femur length between diet groups for either male or female mice (p ≥ .288, Table 1). In addition, total body BMD did not differ between diet groups in either sex (all p ≥ .306, Table 1).
.003). No significant differences in these 2 measurements were observed among the male mice between diet groups (all p ≥ .205, Table 1). No significant differences were observed in femur length between diet groups for either male or female mice (p ≥ .288, Table 1). In addition, total body BMD did not differ between diet groups in either sex (all p ≥ .306, Table 1).
Body weight did not differ between diet groups for either male or female mice (all p ≥ .345, see Figure S2). When normalized to body weight, femoral tissue strength, maximum moment, section modulus, moment of inertia, and femur length in both male and female mice remained unchanged (see Table S1). The difference in femoral cross-sectional area for female mice was not statistically significant (p = .060, Table S1).
Tissue vitamin K content
We detected MK4 and MK9 in the liver, with significantly higher concentrations of both observed in mice fed 2.1 mg MK9/kg compared to those receiving 0.06 mg MK9/kg, regardless of sex (all p ≤ .017, Figure 2A-D). Furthermore, liver concentrations of MK9 were higher than those of MK4. MK4 was the only vitamin K form detected in bone. Mice receiving 0.06 mg/kg MK9 had significantly lower bone MK4 concentrations compared to mice receiving 2.1 mg/kg MK9 in both sexes (all p < .001; Figure 3A and B). In the subset of mice fed deuterium-labeled MK9 for the week prior to sacrifice, deuterium-labeled MK4 was detected in bone tissue of both male and female mice that received 2.1 mg/kg labeled MK9 (mean ± SD at 22 ± 9 pmol/g for males and 37 ± 21 pmol/g for females, Table 2). In addition, 63%-67% of MK4 measured in bone was derived from labeled MK9 (Table 2). In female mice, bone tissue MK4 concentrations were significantly inversely correlated with cross-sectional area (Pearson r = −0.55, p = .008) and nearly significantly inversely correlated with moment of inertia (Pearson r =−0.40, p = .066). Otherwise, bone tissue MK4 concentrations did not correlate with any other bone outcome analyzed (Pearson rcoefficients range −0.25 to +0.29, all p ≥ 0.25; Figure S3), and bone MK4 concentrations were not correlated with any bone outcome in male mice (Pearson rrange +0.01 to +0.30, all p ≥ .35; Figure S3).
Figure 2.
Liver MK4 (A, B) and MK9 (C, D) concentrations (pmol/g) of male (A, C) and female (B, D) mice receiving diets supplemented with MK9. Differences between diet groups were determined using a parametric unpaired 2-sample t-test. Males and females were analyzed separately with statistical significance set as p < .05. Data are presented as mean ± SD.
Figure 3.
Bone MK4 concentrations (pmol/g) of male (A) and female (B) mice receiving diets supplemented with MK9. Differences between diet groups were determined using a parametric unpaired 2-sample t-test. Males and females were analyzed separately with statistical significance set as p < .05. Data are presented as mean ± SD.
Table 2.
Bone MK4 concentrations of male and female mice receiving diets supplemented with deuterium-labeled MK9.
| VK form | Male | Female | ||||
|---|---|---|---|---|---|---|
| 0.06 mg/kg (n = 4) | 2.1 mg/kg (n = 6) | p-values | 0.06 mg/kg (n = 7) | 2.1 mg/kg (n = 7) | p-values | |
| Unlabeled MK4 (pmol/g) | 4 ± 2 | 11 ± 3 | .009 | 12 ± 5 | 23 ± 15 | .066 |
| Labeled MK4 (pmol/g) | ND | 22 ± 9 | .002 | ND | 37 ± 21 | <.001 |
| Total MK4 (pmol/g) | 5 ± 2 | 32 ± 12 | .002 | 13 ± 5 | 60 ± 35 | <.001 |
| % of labeled MK4 (of total VK) | NC | 66.6 ± 3.4 | <.001 | NC | 62.6 ± 6.2 | <.001 |
Values are mean ± SD. Differences between diet groups were determined using a parametric unpaired 2-sample t-test. Males and females were analyzed separately with statistical significance set as p < .05. Abbreviations: NC, not calculable; ND, not detectable. The lowest detectable limit for both MK4 and deuterium-labeled MK4 was 1 pmol/g.
Discussion
Our findings did not support our hypothesis that dietary supplementation with MK9 will positively influence bone tissue quality and density in mice. In both sexes of mice, femoral tissue strength, section modulus, maximum bending moment, femur length, and BMD did not differ between those fed 0.06 mg MK9/kg diet and those fed 2.1 mg MK9/kg diet. In male mice, moment of inertia and cross-sectional area also did not differ between the diet groups. In female mice, the moment of inertia and cross-sectional area were higher in those receiving 0.06 mg MK9 /kg diet compared to those receiving 2.1 mg MK9 /kg diet. These findings further contradict our hypothesis that higher dietary MK9 intake improves skeletal health.
MK4 and MK9 were found in liver tissue of both sexes. MK9 detected in liver reflects the absorption of the vitamin K form supplemented in diet, aligning with a prior study that demonstrated that orally supplemented vitamin K forms accumulated in liver.9MK4 was the only form of vitamin K detected in bone tissue, with significantly higher concentrations observed in mice receiving the higher dose of dietary MK9. These findings suggest that bone MK4 concentrations respond to dietary MK9 intakes, consistent with what has been reported in other tissues. It was recently demonstrated using stable isotopes that multiple dietary vitamin K forms, including MK9, convert to MK4 in certain tissues.9However, bone was not measured in that experiment.9Here, we used stable isotopes to evaluate the conversion of dietary MK9 to MK4 in skeletal tissue and found 63%-67% of the MK4 in bone was derived from the labeled MK9—supporting our hypothesis that dietary MK9 serves as a precursor to MK4 in bone tissue. However, the MK4 concentrations in bone tissue were, for the most part, not correlated with bone tissue quality or density. Moreover, in female mice, higher bone MK4 correlated with a smaller femoral cross-sectional area and a lower moment of inertia (although statistical significance was borderline). Collectively, these findings challenge the premise that higher MK4 in bone is beneficial to skeletal health, and the importance of dietary vitamin K conversion to MK4 in bone is unclear.
Results of previous studies focused on MKs and bone health have been inconsistent. In ovariectomized rats, bone strength and BMD were not affected by 6 wk of dietary supplementation with 147 mg MK4/kg diet or 201 mg MK7/kg diet (equimolar doses).29(Like MK9, MK7 is also a bacterially produced form of vitamin K.) In aged female rats, bone formation and histomorphometry did not differ between those fed a diet containing 500 mg MK4/kg diet and those fed a control diet for 98 d.30However, bone resorption decreased, and bone formation increased in ovariectomized mice fed diets supplemented with 20 and 40 mg MK4/kg body weight.31
A meta-analysis of randomized clinical trials conducted primarily in postmenopausal women and older adults with osteoporosis found little evidence that vitamin K supplementation has beneficial effects on BMD and inconclusive evidence with respect to fracture risk.13Most of the included trials were supplemented with 45 mg/d of MK4, which is a pharmacological dose that has been prescribed as an antiosteoporotic medication in Japan.32Evidence is also mixed regarding the protective effects of MK7. In postmenopausal osteopenic women, BMD and bone microarchitecture did not differ between those who received 375 mcg/d MK7 with 800 mg/d calcium and 1500 IU/d vitamin D for 3 yr, and those who received calcium and vitamin D without MK7.33Supplementation with 360 μg/d MK7 alone for 1 yr also did not affect BMD in healthy women within 5 yr of menopause.34However, 180 μg/d MK7 supplementation for 3 yr reduced BMD loss at the lumbar spine and femoral neck, but not the total hip, in healthy postmenopausal women. Compression strength and impact strength were also favorably affected by MK7 supplementation.35This study also did not include calcium or vitamin D supplementation—2 nutrients that have been repeatedly shown to protect against age-related bone loss.36,37Overall, the available evidence regarding the clinical benefit of MKs with respect to bone health is weak and our findings provide further support for no protective role of MK9 in skeletal health.
Our study has several key strengths. We conducted a comprehensive evaluation of skeletal health in a mouse model, assessing bone tissue quality through multiple measurements, as well as BMD. The use of isotopically labeled MK9 allowed us to trace the origin of skeletal tissue MK4 back to dietary MK9, providing robust evidence that MK9 provided in the diet was a source of MK4 in skeletal tissue. However, we acknowledge certain limitations. We studied mice from 4 to 16 wk of age—a period during which skeletal development occurs. The generalizability of our findings to aging, when bone loss is more common, is uncertain. Some mice, most of which were male, did not survive for the 16-wk study. There were no signs of overt bleeding, and the cause of death is unknown. However, this is a consistent finding in male mice when fed low doses of vitamin K for prolonged periods of time.38Multiple MK forms are produced by bacteria, and it is possible that supplementation with other forms of MK would yield different results. However, previous studies found MK7, a form that has been extensively studied, and MK9 similarly convert to MK4 in extra-hepatic tissues,9there is no known unique biochemical mechanism for individual MK forms, and evidence regarding the skeletal benefit of MK7 is similarly inconclusive.29,33–35
In summary, these findings do not support the role of vitamin K in enhancing skeletal health during growth and development. Further research is needed to verify whether our results can be generalized to aging or other conditions when bone loss tends to occur.
Supplementary Material
Contributor Information
Minying Liu, USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA 02111, United States.
Chongshan Liu, Orthopaedic Surgery, University of California, San Francisco, CA 94143, United States; Sibley School of Mechanical and Aerospace Engineering, Cornell University, Ithaca, NY 14850, United States.
Nicolas Cevallos, Orthopaedic Surgery, University of California, San Francisco, CA 94143, United States.
Benjamin N Orbach, USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA 02111, United States.
Christopher J Hernandez, Orthopaedic Surgery, University of California, San Francisco, CA 94143, United States.
Xueyan Fu, USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA 02111, United States.
Jennifer Lee, USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA 02111, United States; Graduate School of Biomedical Sciences, Tufts University, Boston, MA 02111, United States.
Sarah L Booth, USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA 02111, United States.
M Kyla Shea, USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA 02111, United States.
Author contributions
Minying Liu (Conceptualization, Formal analysis, Investigation, Writing—original draft, Writing—review & editing), Chongshan Liu (Formal analysis, Investigation, Writing—review & editing), Nicolas Cevallos (Investigation, Writing—review & editing), Benjamin N. Orbach (Investigation, Writing—review & editing), Christopher J. Hernandez (Conceptualization, Formal analysis, Investigation, Writing—review & editing), Xueyan Fu (Conceptualization, Formal analysis, Investigation, Writing—review & editing), Jennifer Lee (Conceptualization, Formal analysis, Writing—review & editing), Sarah L. Booth (Conceptualization, Formal analysis, Investigation, Writing—original draft, Writing—review & editing), and M. Kyla Shea (Conceptualization, Formal analysis, Investigation, Writing—original draft, Writing—review & editing)
Funding
This work was supported by NIA R01AG067997 and USDA Agricultural Research Service under Cooperative Agreement 58-8050-3-003. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Conflicts of interest
None declared.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
- 1. Booth SL, Dallal G, Shea MK, Gundberg C, Peterson JW, Dawson-Hughes B. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab. 2008;93(4):1217–1223. 10.1210/jc.2007-2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shearer MJ, Newman P. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis. J Lipid Res. 2014;55(3):345–362. 10.1194/jlr.R045559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berezovska O, Yildirim G, Budell WC, et al. Osteocalcin affects bone mineral and mechanical properties in female mice. Bone. 2019;128:115031. 10.1016/j.bone.2019.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Booth SL. Vitamin K: food composition and dietary intakes. Food Nutr Res. 2012;56(1):5505. 10.3402/fnr.v56i0.5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Booth SL. Roles for vitamin K beyond coagulation. Annu Rev Nutr. 2009;29(1):89–110. 10.1146/annurev-nutr-080508-141217 [DOI] [PubMed] [Google Scholar]
- 6. Walther B, Karl JP, Booth SL, Boyaval P. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Adv Nutr. 2013;4(4):463–473. 10.3945/an.113.003855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al Rajabi A, Booth SL, Peterson JW, et al. Deuterium-labeled phylloquinone has tissue-specific conversion to menaquinone-4 among Fischer 344 male rats. J Nutr. 2012;142(5):841–845. 10.3945/jn.111.155804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collins MD, Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev. 1981;45(2):316–354. 10.1128/mr.45.2.316-354.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellis JL, Fu X, Karl JP, et al. Multiple dietary vitamin K forms are converted to tissue menaquinone-4 in mice. J Nutr. 2021;152(4):981–993. 10.1093/jn/nxab332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tabb MM, Sun A, Zhou C, et al. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem. 2003;278(45):43919–43927. 10.1074/jbc.M303136200 [DOI] [PubMed] [Google Scholar]
- 11. Ichikawa T, Horie-Inoue K, Ikeda K, Blumberg B, Inoue S. Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J Biol Chem. 2006;281(25):16927–16934. 10.1074/jbc.M600896200 [DOI] [PubMed] [Google Scholar]
- 12. Shea MK, Booth SL. Update on the role of vitamin K in skeletal health. Nutr Rev. 2008;66(10):549–557. 10.1111/j.1753-4887.2008.00106.x [DOI] [PubMed] [Google Scholar]
- 13. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30(8):1543–1559. 10.1007/s00198-019-04949-0 [DOI] [PubMed] [Google Scholar]
- 14. Kimmel DB, Vennin S, Desyatova A, et al. Bone architecture, bone material properties, and bone turnover in non-osteoporotic post-menopausal women with fragility fracture. Osteoporos Int. 2022;33(5):1125–1136. 10.1007/s00198-022-06308-y [DOI] [PubMed] [Google Scholar]
- 15. Seeman E. Bone quality: the material and structural basis of bone strength. J Bone Miner Metab. 2008;26(1):1–8. 10.1007/s00774-007-0793-5 [DOI] [PubMed] [Google Scholar]
- 16. Wallach S, Feinblatt JD, Carstens JH Jr, Avioli LV. The bone “quality” problem. Calcif Tissue Int. 1992;51(3):169–172. 10.1007/BF00334542 [DOI] [PubMed] [Google Scholar]
- 17. Watts NB. Bone quality: getting closer to a definition. J Bone Miner Res. 2002;17(7):1148–1150. 10.1359/jbmr.2002.17.7.1148 [DOI] [PubMed] [Google Scholar]
- 18. Hernandez CJ, Keaveny TM. A biomechanical perspective on bone quality. Bone. 2006;39(6):1173–1181. 10.1016/j.bone.2006.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castaneda M, Strong JM, Alabi DA, Hernandez CJ. The gut microbiome and bone strength. Curr Osteoporos Rep. 2020;18(6):677–683. 10.1007/s11914-020-00627-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moriishi T, Ozasa R, Ishimoto T, et al. Osteocalcin is necessary for the alignment of apatite crystallites, but not glucose metabolism, testosterone synthesis, or muscle mass. PLoS Genet. 2020;16(5):e1008586. 10.1371/journal.pgen.1008586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diegel CR, Hann S, Ayturk UM, et al. An osteocalcin-deficient mouse strain without endocrine abnormalities. PLoS Genet. 2020;16(5):e1008361. 10.1371/journal.pgen.1008361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guss JD, Horsfield MW, Fontenele FF, et al. Alterations to the gut microbiome impair bone strength and tissue material properties. J Bone Miner Res. 2017;32(6):1343–1353. 10.1002/jbmr.3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fu X, Booth SL, Smith DE. Vitamin K contents of rodent diets: a review. J Am Assoc Lab Anim Sci. 2007;46(5):8–12. [PubMed] [Google Scholar]
- 24. Harshman SG, Fu X, Karl JP, et al. Tissue concentrations of vitamin K and expression of key enzymes of vitamin K metabolism are influenced by sex and diet but not housing in C57Bl6 mice. J Nutr. 2016;146(8):1521–1527. 10.3945/jn.116.233130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karl JP, Fu X, Dolnikowski GG, Saltzman E, Booth SL. Quantification of phylloquinone and menaquinones in feces, serum, and food by high-performance liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;963:128–133. 10.1016/j.jchromb.2014.05.056 [DOI] [PubMed] [Google Scholar]
- 26. Cyphert EL, Liu C, Morales AL, et al. Effects of high dose aspartame-based sweetener on the gut microbiota and bone strength in young and aged mice. JBMR Plus. 2024;8(8). 10.1093/jbmrpl/ziae082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coulombe JC, Maridas DE, Chow JL, Bouxsein ML. Small animal DXA instrument comparison and validation. Bone. 2024;178:116923. 10.1016/j.bone.2023.116923 [DOI] [PubMed] [Google Scholar]
- 28. Fu X, Peterson JW, Hdeib M, et al. Measurement of deuterium-labeled phylloquinone in plasma by high-performance liquid chromatography/mass spectrometry. Anal Chem. 2009;81(13):5421–5425. 10.1021/ac900732w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fu X, Moreines J, Booth SL. Vitamin K supplementation does not prevent bone loss in ovariectomized Norway rats. Nutr Metab (Lond). 2012;9(1):12. 10.1186/1743-7075-9-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sakamoto W, Isomura H, Fujie K, et al. The effect of vitamin K2 on bone metabolism in aged female rats. Osteoporos Int. 2005;16(12):1604–1610. 10.1007/s00198-005-1881-9 [DOI] [PubMed] [Google Scholar]
- 31. Wang H, Zhang N, Li L, Yang P, Ma Y. Menaquinone 4 reduces bone loss in ovariectomized mice through dual regulation of bone remodeling. Nutrients. 2021;13(8). 10.3390/nu13082570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Orimo H, Nakamura T, Hosoi T, et al. Japanese 2011 guidelines for prevention and treatment of osteoporosis—executive summary. Arch Osteoporos. 2012;7(1-2):3–20. 10.1007/s11657-012-0109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rønn SH, Harsløf T, Oei L, Pedersen SB, Langdahl BL. The effect of vitamin MK-7 on bone mineral density and microarchitecture in postmenopausal women with osteopenia, a 3-year randomized, placebo-controlled clinical trial. Osteoporos Int. 2021;32(1):185–191. 10.1007/s00198-020-05638-z [DOI] [PubMed] [Google Scholar]
- 34. Emaus N, Gjesdal CG, Almås B, et al. Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trial. Osteoporos Int. 2010;21(10):1731–1740. 10.1007/s00198-009-1126-4 [DOI] [PubMed] [Google Scholar]
- 35. Knapen MH, Drummen NE, Smit E, Vermeer C, Theuwissen E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos Int. 2013;24(9):2499–2507. 10.1007/s00198-013-2325-6 [DOI] [PubMed] [Google Scholar]
- 36. Bischoff-Ferrari HA, Willett WC, Orav EJ, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40–49. 10.1056/NEJMoa1109617 [DOI] [PubMed] [Google Scholar]
- 37. Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657–666. 10.1016/S0140-6736(07)61342-7 [DOI] [PubMed] [Google Scholar]
- 38. Shea MK, Booth SL, Harshman SG, et al. The effect of vitamin K insufficiency on histological and structural properties of knee joints in aging mice. Osteoarthr Cartil Open. 2020;2(3):100078. 10.1016/j.ocarto.2020.100078 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.





