Abstract
BACKGROUND:
Patients discharged after acute coronary syndrome experience a high risk of major adverse cardiovascular events (MACE) within the first 6 months. We examined whether a quality of care improvement initiative implemented in hospitals affects clinical preventive management and outcomes after discharge.
METHODS:
We used data from the third phase of the CPACS-3 study (Clinical Pathways for Acute Coronary Syndromes in China), a large stepped wedge- and cluster-randomized trial conducted from 2011 to 2015, to evaluate the effectiveness of an in-hospital quality of care improvement program on the composite score of preventive medication use and the risk of MACE in 6 months after discharge among acute coronary syndrome survivors. The intervention included establishing a quality of care improvement team, training clinical staff, implementing acute coronary syndrome clinical pathways, performance assessment and feedback, online technical support, and patient education. A total of 101 hospitals were randomized into 4 wedges, and the intervention was initiated randomly by wedge and step. Participants recruited before (control) and after (intervention) the intervention initiation were compared with generalized estimating equations, adjusting for clustering and time trend.
RESULTS:
A total of 23 258 patients (11 224 in the intervention group and 12 034 in the control group), with a mean age of 63.6±11.6 years and 39% women, had available follow-up data on MACE and 14 826 patients (6813 in the intervention group and 8013 in the control group) had available data on preventive medication use at 6 months were analyzed. Compared with the control period, the mean preventive medication use score during the intervention period was higher at 6 months (65.8 versus 60.4 for intervention and control periods, adjusted mean difference, 3.7 [95% CI, 0.3–7.0]), but the 6-month incidence of MACE showed no difference (5.8% versus 6.6%, adjusted odds ratio, 1.04 [95% CI, 0.83–1.29]).
CONCLUSIONS:
The in-hospital multifaceted quality of care improvement intervention in resource-constrained Chinese hospitals increased preventive medication use among acute coronary syndrome survivors in the 6 months after discharge, but this did not translate into a reduction in MACE.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01398228.
Keywords: acute coronary syndrome, clinical trial, quality improvement, secondary prevention
What is Known
In-hospital quality of care improvement strategies have been associated with lower risks of both in-hospital and post-hospital major adverse cardiovascular events in observational studies among patients with acute coronary syndrome.
Randomized trials have demonstrated that in-hospital quality of care improvement strategies improve preventive medication use at discharge. However, whether this improvement would persist and translate into significant clinical benefits after discharge remains unknown.
What the Study Adds
The present study showed that the CPACS-3 (Clinical Pathways for Acute Coronary Syndromes in China) in-hospital multifaceted quality of care improvement intervention for acute coronary syndrome management increased preventive medication use at 6 months after discharge but did not reduce the risk of major adverse cardiovascular events within 6 months.
It is estimated that 5% to 25% of patients discharged with acute coronary syndrome (ACS) experience a major adverse cardiovascular events (MACE) within the first 6 months after hospitalization, which rapidly decreases to about 1% to 2% in the second 6 months.1–3 In China, mortality in the first 6 months post-discharge following ACS has been reported to be between 4.5% and 6.5%.3 The risk of MACE after discharge has been reported to be significantly associated with the quality of clinical care during hospitalization, the use of secondary preventive medications at discharge, and subsequent adherence to treatment.2,4 It is critical to identify effective intervention strategies that focus on these factors to reduce the first 6-month risk of MACE among the survivors of ACS.
Clinical pathway-based quality of care improvement (QCI) strategies have been associated with lower risks of both in-hospital and post-hospital MACE in observational studies.5,6 However, 2 large stepped wedge- and cluster-randomized controlled trials, the third phase of the CPACS-3 (Clinical Pathways for Acute Coronary Syndromes in China)7 and the ACS QUIK (Acute Coronary Syndrome Quality Improvement in Kerala),8 did not demonstrate reductions in MACE in hospitals in China and India, respectively, with such quality improvement approaches. However, CPACS-3 and other randomized trials consistently reported significant improvements in nearly all evidence-based preventive medication use at discharge. In CPACS-3, appropriate preventive medication use at discharge increased in absolute terms by between 4.6% and 7.6% for each medication.7 Whether these improvements would translate to significant benefits in clinical outcomes after discharge remains unknown, particularly outside the context of high-income countries.9 Thus, we evaluated the effects of the in-hospital clinical pathway-based QCI initiative intervention in China on preventive medication use and clinical outcomes in the 6 months after discharge among patients hospitalized with ACS in CPACS-3.
Methods
The data that support the findings of this study are available from the corresponding author (Y.W.) upon reasonable request.
Study Design
The CPACS-3 was a large, stepped wedge- and cluster-randomized controlled trial among nontertiary nonpercutaneous coronary intervention regional hospitals (as clusters) in China.9 The protocol, sample size, and main results have been published elsewhere.7,9 Briefly, 101 eligible hospitals were randomly assigned into 4 wedges, and 50 eligible patients were recruited consecutively in each hospital during each of 5 steps, with each step continued for 6 months. No intervention was applied in the first step in all participating hospitals. Each wedge commenced the intervention in one of the 4 remaining steps. All hospitals received the intervention in the last step. The intervention was applied at the hospital level, with outcomes measured at the patient level. The Peking University institutional review board reviewed and approved the study and all participating patients provided written informed consent. The study was registered at ClinicalTrials.gov (NCT01398228). The trial protocol and statistical analysis plan have been published.9
Patients
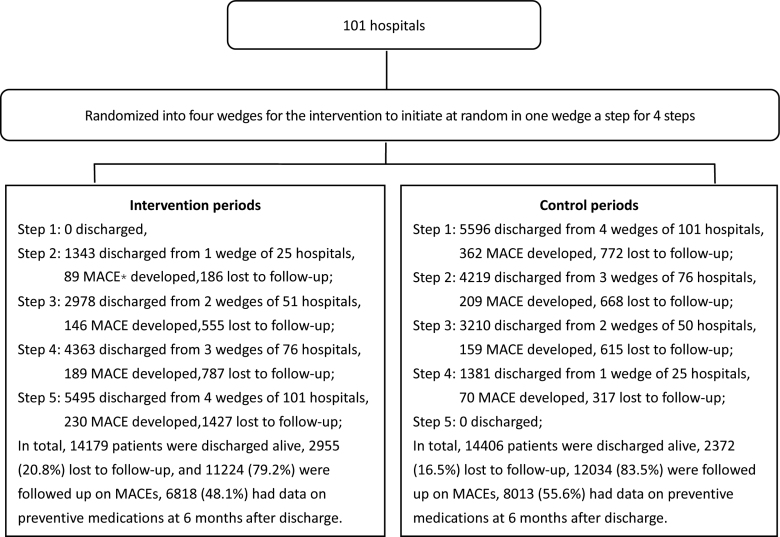
Up to 50 ACS patients older than 18 years with a final diagnosis of ACS at discharge or death were consecutively enrolled in each step in each eligible hospital. The recruitment of patients was scheduled in 6-month cycles following a set date. All hospitals started recruiting patients at the same time for each cycle.7 Study patients were recruited from October 9, 2011, to December 29, 2014, and followed-up until July 12, 2015. A total of 29 346 patients were recruited from these 101 eligible hospitals, of which 761 patients died during hospitalization.7 For the 28 585 patients who were discharged alive, the process of discharge and follow-up in each step of the intervention and control periods is shown in the Figure.
Figure.
Study flowchart. *MACE indicates major adverse cardiovascular event.
Randomization, Intervention, and Data Collection During Hospitalization
Cluster randomization was computer-generated and concealed centrally, with stratification by province, after data collection for the first step and before initiating the intervention.7
The intervention included 6 components: the establishment of a QCI team; implementation of clinical pathways for managing different subtypes of ACS; regular reports provided every 6 months on key performance indicators; technical training and a compulsory test for medical staff engaged in ACS care; web-based online technical support to access advice from senior cardiologists; and patient educational materials on ACS clinical manifestation, treatment, secondary prevention, and lifestyle modification. The intervention was maintained or repeated during the intervention period at each hospital. Central training for the intervention protocol (2 days) took place within 2 weeks before initiating the new cycle for patient recruitment. The local training and the establishment of the QCI team at each hospital were required to be done immediately after the central training. There was not a transit period between the intervention and control periods.
Data collection during hospitalization included sociodemographic information, medical history, and treatments before admission, during hospitalization, and at death or hospital discharge.
Follow-Up in 6 Months After Discharge
All patients discharged alive from the hospital were followed-up at 6 months by face-to-face interview or telephone call. A standardized questionnaire was used to collect information from each patient or their relative or other contact if the patient had died. A trained physician or senior cardiac nurse from participating hospitals was responsible for the interview and telephone follow-up. Information collected included survival status, hospitalizations, reasons for re-hospitalization, and the use of evidence-based medications. For suspected cases of MACE, medical charts, including laboratory tests or death certificates were obtained. A central adjudication committee was responsible for the final diagnosis of all MACE.
Outcomes
The main outcomes in the present study include a patient-level composite preventive medications use score at discharge, the preventive medications use score at 6 months after discharge and the 6-month risk of MACE. The patient-level composite persistent preventive medications use score was derived as an exploratory outcome. Preventive medications considered in the present study include aspirin, clopidogrel, β-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, and statins. The composite preventive medications use score at discharge and at 6 months after discharge were calculated as the number of secondary prevention medications from each class (maximum 5) that were actually taken by the patient divided by the number of guideline-recommended secondary medications10–13 at the specified time point, then multiplied by 100. The persistent preventive medications use score was defined as number of preventive medications that were taken by the patient at both discharge and 6 months, divided by the number of guideline-recommended secondary medications, then multiplied by 100. MACE was defined as a composite outcome including all-cause mortality, nonfatal myocardial infarction, and nonfatal stroke. Nonfatal myocardial infarction was defined as re-hospitalization due to myocardial infarction diagnosed on symptoms/signs and at least one of electrocardiographic or cardiac biomarker change. Nonfatal stroke after discharge was defined as re-hospitalization due to stroke diagnosed on symptoms/signs and brain computed tomography/magnetic resonance imaging. All reported MACEs were adjudicated by an independent committee masked to the hospital’s randomization status.
Data Quality Control
A trained hospital staff member who was not involved in treating patients with ACS was responsible for collecting and entering data into a dedicated web-based data management system. Follow-up data were collected by trained staff using a standard questionnaire, referring to the medical charts if the patient was hospitalized, and blinded to hospital randomization. Data quality was maintained through in-person and online study monitoring activities.
Data Analysis
The analyses for all outcomes were conducted at the individual level while accounting for the clustering of patients at the hospital level. The primary model included a fixed effect for time trend (categorized by number of steps) and a binary variable to represent the effect of the intervention. To account for within-cluster correlations, generalized estimating equations were used with an exchangeable working correlation structure.
The intervention effects were summarized as odds ratios and differences of proportions for MACE or mean differences for preventive medications use.
For the purposes of the present study, participants who had incomplete follow-up data on clinical events and preventive medications use were excluded from the respective analyses. To understand the potential impact of differential loss to follow-up, we compared the patient baseline characteristics between intervention and control periods in the analyzed patients for MACE and preventive medications use, respectively.
All statistical tests were 2-tailed. The intervention effects on outcomes were considered significant at P=0.05. All analyses were conducted using SAS, version 9.4 (SAS Institute).
Data Availability Statement
Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article.
Results
Patient Characteristics
For the 28 585 patients who were discharged alive, 5327 (18.6% of 28 585) patients were lost to follow-up for MACE, and an additional 8427 (29.5% of 28 585) did not attend the face-to-face follow-up visit at 6 months, where information on secondary preventive medication use was collected, who were excluded from the analyses (Figure). Among the 23 258 participants analyzed for 6-month MACE, 11 224 (48.3%) and 12 034 (51.7%) were recruited during the intervention and control periods, respectively. The mean age of the study participants was 63.6 (±11.6) years, with a range from 19 to 99 years. About 61% were men, 59% were farmers, 33% were hospitalized with ST-segment–elevation myocardial infarction, and 50% with unstable angina pectoris. No apparent differences were found between all eligible patients randomized to the control and intervention periods for the analyses on MACE and those included in the cohorts for analyses on preventive medication use scores (Table 1).
Table 1.
Characteristics of Analyzed Participants by Randomized Allocation in CPACS-3 Study
Effects on Preventive Medications Use
The mean composite preventive medication use score at 6 months after discharge was significantly higher in the intervention period compared with the control period (65.8±34.0 versus 60.4±32.7, the cluster- and time-adjusted difference, 3.7 [95% CI, 0.3–7.0]; P=0.031; Table 2). The difference also presented for the mean composite preventive medications use score at discharge (78.3±28.8 versus 69.2±31.5, the cluster- and time-adjusted difference, 8.2 [95% CI, 4.3–12.1]; P<0.001) and the exploratory outcome of the mean persistent preventive medication use score during the first 6 months (58.1±36.0 versus 50.6±34.8, the cluster-and time-adjusted difference, 7.5 [95% CI, 3.6–11.4]; P<0.001).
Table 2.
Effects of Intervention on Preventive Medications Use* at Discharge and 6 months and the Persistence Use in 6 Months
Effects on Risk of MACE
The 6-month incidence of MACE was 5.8% in the intervention group and 6.7% in the control group (Table 3). The cluster- and time-adjusted odds ratio was not significant (1.04 [0.83–1.29], P=0.737).
Table 3.
MACEs in 6 Months After Discharge by Randomized Allocation
Discussion
To the best of our knowledge, this is the first randomized clinical trial to report the effect of an in-hospital QCI intervention on post-discharge clinical outcomes among ACS survivors.14–22 In this large stepped wedge- and cluster-randomized controlled trial in China, we found that QCI implemented during hospitalization not only increased preventive medication use at discharge but that some of this improvement persisted to 6 months post-discharge, indicating a sustained impact of the in-hospital QCI intervention on use of secondary prevention therapies. However, the magnitude of the intervention effect might be not large enough to be translated into significant changes in the risk of MACE at 6 months. The study would be helpful to address the paucity of high-quality evidence on quality improvement initiatives in non-high-income settings what was indicated by Rowe et al.23
Several large trials have found that QCI interventions increased nearly all evidence-based medications in the hospital and at discharge.5–8 The possible reasons for the effect persistence after discharge in the present study may be that the QCI intervention improved the knowledge level of physicians who were usually not just responsible for in-hospital treatment but also for the clinical follow-up after patient discharge. It is worth noting that the improvement in medication use after discharge was not equally observed across all therapeutic classes. The largest improvement at 6 months after discharge occurred with clopidogrel use (9.4%, P<0.001), for which the largest improvement was also observed in terms of persistent use both at discharge and follow-up (11.1%, P<0.001). This may be due to low rates of use of clopidogrel in control periods and therefore greatest potential to improve. Rates of post-discharge use of aspirin and statin were high (>70%) and similar between groups. These results indicate that intervention may be more effective for those medications where the largest evidence-practice gaps lie.
It is worth noting that the dual antiplatelet therapy, β-blockers, statins, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have been considered as part of quality indicators by the European Society of Cardiology.24 Unfortunately, the proportion of patients who used preventive medications was still greatly less than the number who were eligible for these medications in the present randomized study. Similar findings also presented in those real-world studies shown by Mas-Llado et al.25 These findings suggest that more and stronger intervention strategies should be developed to improve the proportion of secondary preventive medication use among patients discharged with ACS.
In the present report, we found that QCI intervention was not associated with a reduction in 6-month MACE. While the intervention increased sustained preventive medications use, the magnitude of that effect may not be sufficient to translate to clinical differences with relatively shorter follow-up (only 6 months). First factor may be the influence of secular trends that influenced the comparison. The China National Health Commission issued clinical pathways for managing various diseases,26,27 including ACS, in 2009. During the CPACS-3 study period, the Chinese Society of Cardiology had issued several clinical guidelines on the management of ACS.11,28 These guidelines were disseminated through numerous conferences, meetings, symposiums, and workshops across the country, which could have led to an improvement in the management of ACS. The outside intervention should have diminished the effect of the QCI intervention on MACE, which was demonstrated by comparing models adjusting for clustering only (significant but data were not shown) with models further adjusting for the time trend (not significant, Table 3). Second factor was that the most of the preventive medication use difference was driven by the higher use of clopidogrel in the intervention arm and for the other agents (statins, β-blockers, aspirin, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers) the difference between arms was really modest and not statistically significant. Third factor was that due to no transit period existed between intervention and control periods by design part of patients who were recruited close to the end of control period might actually be intervened during the follow-up visits by the same physicians who had received the intervention, resulting in study outcomes improved in patients recruited in control period and diminished intervention effect. Fourth factor was that our post hoc power analyses indicates that the study had only 71% power, which is not high enough for claiming a true no effect of the CPACS-3 intervention on 6-month MACE. The loss to follow-up (18.6%), and the higher proportion of participants with unstable angina (50%) should both reduced the power of the statistical analysis.
Strengths of This Study
This study has several strengths. First, the study is the first large randomized trial to evaluate the effect of a clinical pathway-based QCI initiatives on post-discharge clinical outcomes among ACS survivors. Second, the study design, conduct, and data analyses were overseen by an experienced steering committee composed of international experts in cardiology, epidemiology, and biostatistics. Third, the study used a central adjudication committee for MACE event diagnosis and classification, and a professional project management team monitored the study process. Last, around 40% of the study population were women, which is higher than that reported in previous studies29 and the results should be more generalizable to women patients.
Limitations of the Study
The study also has limitations. First, due to resource constraints the time of follow-up was only 6 months, the generalization of the intervention effect to longer term should be made with caution. Second, there were nearly 20% of participants lost to the follow-up for MACE. Fortunately, number and clinical characteristics of participants remained in the cohort were similar between the intervention and control groups (Table 1). Thus, we do not think that the loss to follow-up would introduce significant systematic bias. Third, the study was done in Chinese resource-constrained hospitals. The generalizability to other countries and settings should be considered with caution. Fourth, about 50% of the study population had unstable angina, which was higher than that reported previously30 and should reduce the expected risk of MACE in follow-up and hence the statistical power. Thus, our study might underestimate the effect of intervention. Fifth, the feedback reports of key performance indicators every 6 months should help to strengthen the intervention effect and that effect could be unequal between the wedges. We did not consider this possible heterogeneity in our analyses. Instead, we followed our statistical plan, which assumed a constant intervention effect from all intervention components.
Conclusions
In resource-constrained regional hospitals in China, the introduction of a multifaceted, clinical pathway-based QCI intervention program led to an increase in the use of ACS secondary prevention medications at discharge and at 6 months post-discharge, and also an increase in the persistent use of these medications during the 6 months post-discharge, but did not result in a significant decrease in the risk of 6-month MACE.
Article Information
Acknowledgments
The article used data from CPACS-3 study (Clinical Pathways for Acute Coronary Syndromes in China) in China. The authors thank all researchers, and patients who participated in the CPACS-3 study. Dr Wu had full access to all study data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Y. Wu, A. Patel, and R. Gao. Acquisition of data: Y. Wu, X. Du, and Z. Hao. Data analysis: G. Xie, X. Li, T. Wu. Drafting of the article and data interpretation: G. Xie, A Patel, and Y Wu. Critical revision of the article for important intellectual content: Y. Wu, A. Patel, X. Du, Y. Sun, E. Peterson, and R. Gao. Obtained funding: Y. Wu, R. Gao.
Administrative, technical, or material support: Z. Hao. Sanofi and other funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Sources of Funding
The CPACS-3 study (Clinical Pathways for Acute Coronary Syndromes in China) is funded by Sanofi, China, through an unrestricted research grant. The George Institute for Global Health Beijing, China, sponsored the study and owns the data.
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- ACS
- acute coronary syndrome
- ACS QUIK
- Acute Coronary Syndrome Quality Improvement in Kerala
- CPACS-3
- Clinical Pathways for Acute Coronary Syndromes in China
- MACE
- major adverse cardiovascular event
- QCI
- quality of care improvement
For Sources of Funding and Disclosures, see pages 403 and 404.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCOUTCOMES.124.011441.
Contributor Information
Gaoqiang Xie, Email: gxie@bjmu.edu.cn.
Xin Du, Email: duxinheart@sina.com.
Yihong Sun, Email: yihongsun72@163.com.
Xian Li, Email: lxian@georgeinstitute.org.cn.
Tao Wu, Email: wuyf@bjmu.edu.cn.
Zhixin Hao, Email: 824825216@qq.com.
References
- 1.Berlin T, Rozenbaum E, Arbel J, Reges O, Erel J, Shetboun I, Leibovitch M, Mosseri M. Six- and twelve-month clinical outcomes after implantation of prokinetic BMS in patients with acute coronary syndrome. J Interv Cardiol. 2010;23:377–381. doi: 10.1111/j.1540-8183.2010.00550.x [DOI] [PubMed] [Google Scholar]
- 2.Xie G, Sun Y, Myint PK, Patel A, Yang X, Li M, Li X, Wu T, Li S, Gao R, et al. Six-month adherence to Statin use and subsequent risk of major adverse cardiovascular events (MACE) in patients discharged with acute coronary syndromes. Lipids Health Dis. 2017;16:155. doi: 10.1186/s12944-017-0544-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun Y, Feng L, Li X, Gao R, Wu Y. The sex difference in 6-month MACEs and its explaining variables in acute myocardial infarction survivors: data from CPACS-3 study. Int J Cardiol. 2020;311:1–6. doi: 10.1016/j.ijcard.2020.03.043 [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Xie G, Patel A, Li S, Zhao W, Yang X, Wu T, Li M, Li X, Du X, et al. Prescription of statins at discharge and 1-year risk of major clinical outcomes among acute coronary syndromes patients with extremely low LDL-cholesterol in clinical pathways for acute coronary syndromes studies. Clin Cardiol. 2018;41:1192–1200. doi: 10.1002/clc.23040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson ED, Roe MT, Mulgund J, DeLong ER, Lytle BL, Brindis RG, Smith SC, Jr, Pollack CV, Jr, Newby LK, Harrington RA, et al. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA. 2006;295:1912–1920. doi: 10.1001/jama.295.16.1912 [DOI] [PubMed] [Google Scholar]
- 6.Mathews R, Chen AY, Thomas L, Wang TY, Chin CT, Thomas KL, Roe MT, Peterson ED. Differences in short-term versus long-term outcomes of older black versus white patients with myocardial infarction: findings from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of American College of Cardiology/American Heart Association Guidelines (CRUSADE). Circulation. 2014;130:659–667. doi: 10.1161/CIRCULATIONAHA.113.008370 [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Li S, Patel A, Li X, Du X, Wu T, Zhao Y, Feng L, Billot L, Peterson ED, et al. ; CPACS-3 Investigators. Effect of a quality of care improvement initiative in patients with acute coronary syndrome in resource-constrained hospitals in china: a randomized clinical trial. JAMA Cardiol. 2019;4:418–427. doi: 10.1001/jamacardio.2019.0897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huffman MD, Mohanan PP, Devarajan R, Baldridge AS, Kondal D, Zhao L, Ali M, Krishnan MN, Natesan S, Gopinath R, et al. ; Acute Coronary Syndrome Quality Improvement in Kerala (ACS QUIK) Investigators. Effect of a quality improvement intervention on clinical outcomes in patients in india with acute myocardial infarction: the ACS QUIK randomized clinical trial. JAMA. 2018;319:567–578. doi: 10.1001/jama.2017.21906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Wu Y, Du X, Li X, Patel A, Peterson ED, Turnbull F, Lo S, Billot L, Laba T, et al. ; CPACS-3 Investigators. Rational and design of a stepped-wedge cluster randomized trial evaluating quality improvement initiative for reducing cardiovascular events among patients with acute coronary syndromes in resource-constrained hospitals in China. Am Heart J. 2015;169:349–355. doi: 10.1016/j.ahj.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 10.Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM, et al. ; 2012 Writing Committee Members. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2012;126:875–910. doi: 10.1161/CIR.0b013e318256f1e0 [DOI] [PubMed] [Google Scholar]
- 11.Chinese Society of Cardiology, Chinese Medical Association, Editorial Committee of Chinese Journal of Cardiology. Guidelines for diagnosis and treatment of acute ST segment elevation myocardial infarction. Chine J Cardiol. 2010;38:675–690. doi: 10.3760/cma.j.issn.0253-3758.2010.08.002 [Google Scholar]
- 12.Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, et al. ; 2004 Writing Committee Members. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration With the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209 [DOI] [PubMed] [Google Scholar]
- 13.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, et al. ; American College of Cardiology. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:e148–e304. doi: 10.1161/CIRCULATIONAHA.107.181940 [DOI] [PubMed] [Google Scholar]
- 14.Alter DA, Iron K, Austin PC, Naylor CD; SESAMI Study Group. Influence of education and income on atherogenic risk factor profiles among patients hospitalized with acute myocardial infarction. Can J Cardiol. 2004;20:1219–1228 [PubMed] [Google Scholar]
- 15.Alter DA, Iron K, Austin PC, Naylor CD; SESAMI Study Group. Socioeconomic status, service patterns, and perceptions of care among survivors of acute myocardial infarction in Canada. JAMA. 2004;291:1100–1107. doi: 10.1001/jama.291.9.1100 [DOI] [PubMed] [Google Scholar]
- 16.Fors A, Gyllensten H, Swedberg K, Ekman I. Effectiveness of person-centred care after acute coronary syndrome in relation to educational level: subgroup analysis of a two-armed randomised controlled trial. Int J Cardiol. 2016;221:957–962. doi: 10.1016/j.ijcard.2016.07.060 [DOI] [PubMed] [Google Scholar]
- 17.Gerber Y, Benyamini Y, Goldbourt U, Drory Y; Israel Study Group on First Acute Myocardial Infarction. Neighborhood socioeconomic context and long-term survival after myocardial infarction. Circulation. 2010;121:375–383. doi: 10.1161/CIRCULATIONAHA.109.882555 [DOI] [PubMed] [Google Scholar]
- 18.Kämpfer J, Yagensky A, Zdrojewski T, Windecker S, Meier B, Pavelko M, Sichkaruk I, Kasprzyk P, Gruchala M, Giacomini M, et al. Long-term outcomes after acute myocardial infarction in countries with different socioeconomic environments: an international prospective cohort study. BMJ Open. 2017;7:e012715. doi: 10.1136/bmjopen-2016-012715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng Y, Du X, Li X, Ji J, Wu Y, Gao R, Patel A; Clinical Pathways for Acute Coronary Syndromes in China (CPACS) Investigators. Associations between education level and in-hospital treatment and outcomes among acute coronary syndrome in China. Am J Med Sci. 2021;361:253–260. doi: 10.1016/j.amjms.2020.09.015 [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen JN, Rasmussen S, Gislason GH, Buch P, Abildstrom SZ, Køber L, Osler M, Diderichsen F, Torp-Pedersen C, Madsen M. Mortality after acute myocardial infarction according to income and education. J Epidemiol Community Health. 2006;60:351–356. doi: 10.1136/jech.200X.040972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sulo G, Nygård O, Vollset SE, Igland J, Ebbing M, Sulo E, Egeland GM, Tell GS. Higher education is associated with reduced risk of heart failure among patients with acute myocardial infarction: a nationwide analysis using data from the CVDNOR project. Eur J Prevent Cardiol. 2016;23:1743–1750. doi: 10.1177/2047487316655910 [DOI] [PubMed] [Google Scholar]
- 22.Cannon CP, Brindis RG, Chaitman BR, Cohen DJ, Cross JT, Jr, Drozda JP, Jr, Fesmire FM, Fintel DJ, Fonarow GC, Fox KA, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards. 2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Acute Coronary Syndromes and Coronary Artery Disease Clinical Data Standards). Circulation. 2013;127:1052–1089. doi: 10.1161/CIR.0b013e3182831a11 [DOI] [PubMed] [Google Scholar]
- 23.Rowe AK, Rowe SY, Peters DH, Holloway KA, Chalker J, Ross-Degnan D. Effectiveness of strategies to improve health-care provider practices in low-income and middle-income countries: a systematic review. Lancet Global health. 2018;6:e1163–e1175. doi: 10.1016/S2214-109X(18)30398-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiele F, Aktaa S, Rossello X, Ahrens I, Claeys MJ, Collet JP, Fox KAA, Gale CP, Huber K, Iakobishvili Z, et al. ; ESC Scientific Document Group. 2020 update of the quality indicators for acute myocardial infarction: a position paper of the Association for Acute Cardiovascular Care: the study group for quality indicators from the ACVC and the NSTE-ACS guideline group. Eur Heart J Acute Cardiovasc Care. 2021;10:224–233. doi: 10.1093/ehjacc/zuaa037 [DOI] [PubMed] [Google Scholar]
- 25.Mas-Llado C, Rossello X, González-Del-Hoyo M, Pocock S, de Werf FV, Chin CT, Danchin N, Lee SW, Medina J, Huo Y, et al. Secondary prevention therapies in real-world patients with myocardial infarction: eligibility based on randomized trials supporting European and American Guidelines. Am J Med. 2024;137:137–146.e10. doi: 10.1016/j.amjmed.2023.09.021 [DOI] [PubMed] [Google Scholar]
- 26.General Administrative Office, Ministry of Health of China. Notice of the general office of the ministry of health on issuing clinical paths for 8 diseases. http://www.nhc.gov.cn/zwgk/wtwj/201304/e7f7c3fd5eca44e690d61701433ec90c.shtml. [Google Scholar]
- 27.General Administrative Office, Ministry of Health of China. Notice of the general office of the ministry of health on issuing clinical paths for six diseases of the cardiovascular system. http://www.nhc.gov.cn/yzygj/s3585u/201003/a002853653b8407c82d61f5e877d2a27.shtml. [Google Scholar]
- 28.Chinese Society of Cardiology, Chinese Medical Association, Editorial Committee of Chinese Journal of Cardiology. Guidelines for diagnosis and treatment of non ST segment elevation acute coronary syndromes. Chine J Cardiol. 2012;40:353–367. doi: 10.3760/cma.j.issn.0253-3758.2012.05.001 [Google Scholar]
- 29.Mas-Llado C, Gonzalez-Del-Hoyo M, Siquier-Padilla J, Blaya-Peña L, Coughlan JJ, García de la Villa B, Peral V, Rossello X. Representativeness in randomised clinical trials supporting acute coronary syndrome guidelines. Eur Heart J Qual Care Clin Outcomes. 2023;9:796–805. doi: 10.1093/ehjqcco/qcad007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossello X, Bueno H, Pocock SJ, Van de Werf F, Danchin N, Annemans L, Medina J, Zeymer U. Predictors of all-cause mortality and ischemic events within and beyond 1 year after an acute coronary syndrome: results from the EPICOR registry. Clin Cardiol. 2019;42:111–119. doi: 10.1002/clc.23116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article.