Abstract
Purpose
The potential benefits of robotic surgery (RS) for rectal cancer (RC) remain uncertain. The objective of this study was to evaluate the short- and long-term outcomes of RS compared to conventional laparoscopic surgery (LS) for stage I–III middle or lower RC.
Methods
This study retrospectively analyzed 350 consecutive patients with stage I–III middle or lower RC who underwent curative surgery from 2017 to 2021, employing propensity score matching (PSM) analysis.
Results
Of 350 patients, 128 patients underwent RS. After PSM, we enrolled 256 patients. Median follow-up was 59.8 months. Before PSM, significant differences were observed between groups regarding primary tumor site (p = 0.02). After PSM, no significant differences between groups were observed in terms of operative time, blood loss, conversion rate, intra-operative and postoperative complications, or number of lymph nodes harvested. After PSM, 3- and 5-year cumulative LR rates were 3.2% and 3.2% in the RS group, and 2.8% and 3.2% in the LS group, respectively. The cumulative distant recurrence (DR) rates in the RS group were 13.4% at 3-year and 15.1% at 5-year, whereas in the LS group, they were 14.9% and 18.7%, respectively. No notable differences in cumulative LR or DR rates were evident between groups. Furthermore, no notable differences were observed between groups regarding overall, cancer-specific, or recurrence-free survival according to stage.
Conclusions
RS appears to be viable and safe treatment approach for patients with middle or lower RC, offering short- and long-term outcomes comparable to those of LS.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00384-025-04888-9.
Keywords: Rectal cancer, Robotic surgery, Laparoscopic surgery, Propensity score matching, Short-term outcome, Long-term outcome
Introduction
Rectal cancer (RC) constitutes 38.9% of all colorectal cancer cases, with 729,833 new cases reported globally in 2022 [1]. Laparoscopic surgery (LS) for RC is commonly performed in the era of minimally invasive surgery; however, it has several limitations. These include the two-dimensional view, restricted instrument maneuverability due to fixed tips and suboptimal visualization caused by camera instability or insufficient traction from assistants. Additionally, in RC patients with a deep and narrow pelvis, performing total mesorectal excision (TME) with straight laparoscopic instruments presents a significant technical challenge. On the other hand, robotic surgery (RS) offers solutions to some of these drawbacks by offering a stable three-dimensional camera platform, articulated instruments, and the potential for the surgeon to independently control both the camera and assist arm [2]. Although a phase III randomized clinical trial (RCT) comparing robotic and laparoscopic resection for RC (ROLARR) [3] did not show a significant difference in conversion rates between RS and LS, several other clinical studies and smaller RCTs have reported positive outcomes, highlighting the clinical advantages of RS [4–8]. However, long-term oncological outcomes have only been shown from some single-institution retrospective studies and one meta-analysis with inconsistent conclusions. Whether RS can address some of the limitations of LS in patients with stage I–III middle or lower RC thus remains contentious.
The purpose of this retrospective study was to evaluate short- and long-term outcomes for RS compared with those of conventional LS among patients with stage I–III middle or lower RC. Long-term outcomes in this study were overall survival (OS), cancer-specific survival (CSS), recurrence-free survival (RFS), cumulative incidence of local recurrence (LR), and cumulative incidence of distant recurrence (DR).
Methods
Study design
A total of 389 consecutive patients underwent elective RS for middle or lower RC between January 2017 and December 2021 at Osaka International Cancer Institute, Japan. Of these, we conducted a retrospective analysis of 350 consecutive patients with stage I–III middle or lower RC. Patients who underwent RS were categorized as the RS group, and those who underwent LS as the LS group. To enhance comparability, patients in the RS group were matched with those in the LS group through propensity score matching (PSM). This study adhered to the reporting recommendation (STROBE).
Data source
The data for this study were sourced from our hospital’s medical records. All documented recorded clinical (c) and pathological (p) data were revalidated based on the medical and pathology records. During the perioperative period (within 30 days of surgery), the following demographic details were collected: age, sex, body mass index (BMI), American Society of Anesthesiologists physical status classification (ASA-PS), level of primary RC, carcinoembryonic antigen level (CEA), carbohydrate antigen 19–9 level (CA19 - 9), cTNM stage (as classified by the Union for International Cancer Control (UICC) classification, 8 th edition) [9], preoperative treatment, surgical procedure, lateral lymph node dissection (LLND), combined resection, tumor size, histological grade, lymphovascular invasion, distal margin (DM), radial margin (RM), pTNM stage (as classified by the UICC classification, 8 th edition) [9], residual tumor, and adjuvant therapy. There were no missing data for any of the analyzed variables.
Perioperative procedures and follow-up schedule
Perioperative procedures and follow-up schedules were conducted in accordance with the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines [10]. Additionally, postoperative complications were categorized according to the Clavien–Dindo (CD) classification [11]. The details are provided in the supplementary materials.
Recurrence classification
Recurrence patterns were determined based on clinical assessments, radiological findings (primarily through CT, MRI, positron emission tomography, or colonoscopy), or pathological findings. LR was defined as tumor recurrence within the pelvic region, including central pelvic recurrence, LLN recurrence, or anastomotic recurrence. DR was classified as any occurring outside the LR category.
Study outcomes
The primary outcomes were long-term outcomes (incidence of cumulative LR, incidence of cumulative DR, OS, CSS, and RFS) in the RS and LS groups. OS was measured as the duration from the surgery date to death from any cause. CSS was defined as the interval from the date of surgery to the day of cancer-specific death. RFS was defined as the interval from the date of surgery to the date of identification of any radiological or histological recurrence, or death from any cause. The secondary outcomes included short-term parameters in the RS and LS groups, such as operative time, blood loss, conversion rate, number of harvested lymph nodes (LNs), intraoperative and postoperative complications, reoperation rate, 30-day postoperative mortality, and length of hospital stay after surgery.
Statistical analysis using propensity score matching
Prior to applying PSM, baseline patient characteristics were assessed using bivariate analyses to detect any imbalances in covariates. PSM was subsequently utilized to reduce potential selection biases and account for significant differences in baseline characteristics between patient groups (Fig. 1). The initial step in PSM involved conducting a multivariate logistic regression analysis to generate propensity scores. The model for calculating the propensity score incorporated eleven covariates that could impact surgical complexity or prognosis. These included sex, age, BMI, ASA-PS, primary rectal tumor location, cTNM stage, preoperative treatment, surgical approach, LLND, combined resection, and adjuvant therapy. Next, 1:1 matching was procedure was conducted using a caliper width of 0.2. This PSM was employed to evaluate the effect of RS on both short- and long-term outcomes. Additionally, baseline characteristics, including variables not included in the propensity score model, were analyzed using bivariate methods to compare differences between groups.
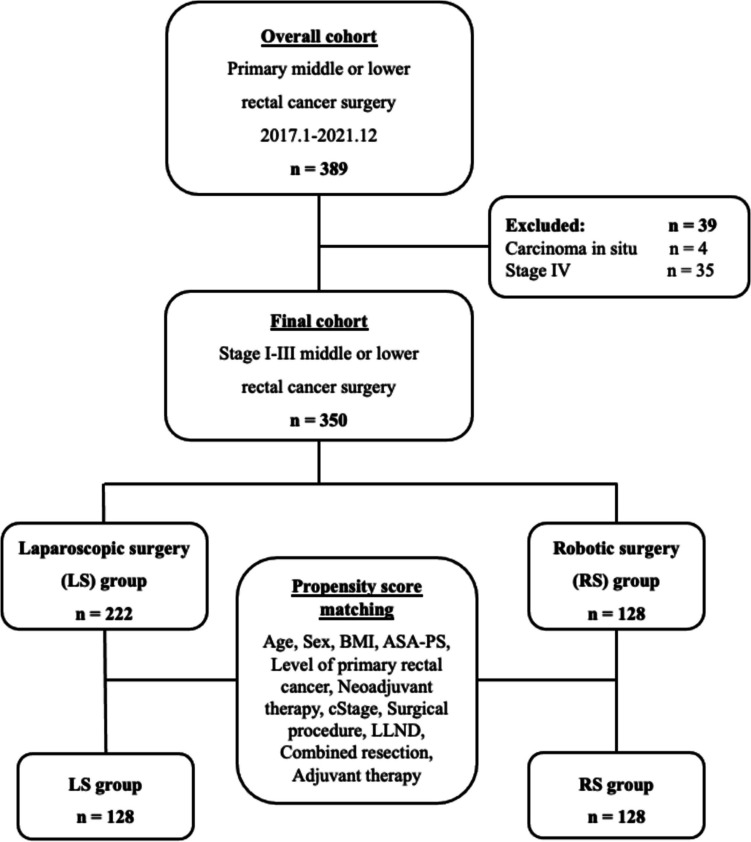
Fig. 1.
Flow diagram describing the patient-matching process
Continuous variables are presented as the mean with standard deviation, while categorical variables are reported as counts with corresponding percentages. Categorical variable comparisons were conducted using Pearson’s chi-squared test, while the Wilcoxon rank-sum test was applied for continuous variables. The cumulative incidence of LR and survival curves were calculated using the Kaplan–Meier method and were then compared by log-rank testing. Values of p < 0.05 were considered statistically significant. Statistical analyses were carried out using JMP Pro version 17 (SAS Institute, Cary, NC).
Results
Baseline patient characteristics
Figure 1 provides an overview of our study. Among the 389 consecutive patients with resection of middle or lower RC, 39 patients with carcinoma in situ or distant metastasis were excluded. Consequently, the final study cohort consisted of 350 patients, including 128 in the RS group and 222 in the LS group. The average patient age was 61.2 years, with males comprising 59.1% of the study population.
Table 1 presents the clinicopathological characteristics before and after PSM. Prior to PSM, lower RC was more common in the RS group (66.4%) than in the LS group (54.0%, p = 0.02). Compared to the LS group, super-low anterior resection (sLAR) was more common than intersphincteric resection (ISR) in the RS group. The LS group showed a higher tendency for combined resection compared to the RS group (p = 0.07). Following PSM, 128 matched pairs were identified. No significant group-dependent differences in patient characteristics were apparent. The two matched groups exhibited comparable baseline characteristics (Table 1).
Table 1.
Comparison of baseline characteristics between LS and RS groups
| Total | Overall (n = 350) | Propensity score-matched pairs (n = 256) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LS group (n = 222) | RS group (n = 128) | p | LS group (n = 128) | RS group (n = 128) | p | |||||||
| n or mean | % or SD | n or mean | % or SD | n or mean | % or SD | n or mean | % or SD | n or mean | % or SD | |||
| Sex, n (%) | 0.946 | 0.898 | ||||||||||
| Male | 207 | 59.1 | 131 | 59.0 | 76 | 59.4 | 77 | 60.2 | 76 | 59.4 | ||
| Female | 143 | 40.9 | 91 | 41.0 | 52 | 40.6 | 51 | 39.8 | 52 | 40.6 | ||
| Age, mean ± SD, y | 61.2 | 11.2 | 61.6 | 11.4 | 60.4 | 10.8 | 0.207 | 61.8 | 11.1 | 60.4 | 10.8 | 0.129 |
| ASA-PS, n (%) | 0.196 | 0.117 | ||||||||||
| 1, 2 | 329 | 94.0 | 206 | 92.8 | 123 | 96.1 | 117 | 91.4 | 123 | 96.1 | ||
| ≥ 3 | 21 | 6.0 | 16 | 7.2 | 5 | 3.9 | 11 | 8.6 | 5 | 3.9 | ||
| BMI, mean ± SD, kg/m2 | 22.6 | 3.7 | 22.6 | 3.5 | 22.8 | 4.1 | 0.952 | 22.8 | 3.5 | 22.8 | 4.1 | 0.532 |
| Level of primary RC, n (%) | 0.023 | 0.244 | ||||||||||
| Middle | 145 | 41.4 | 102 | 46.0 | 43 | 33.6 | 52 | 40.6 | 43 | 33.6 | ||
| Lower | 205 | 58.6 | 120 | 54.0 | 85 | 66.4 | 76 | 59.4 | 85 | 66.4 | ||
| CEA, n (%), ng/ml | 0.818 | 0.782 | ||||||||||
| ≤ 5.0 | 250 | 71.2 | 157 | 70.7 | 92 | 71.9 | 90 | 70.3 | 92 | 71.9 | ||
| > 5.0 | 101 | 28.8 | 65 | 29.3 | 36 | 28.1 | 38 | 29.7 | 36 | 28.1 | ||
| CA19 - 9, n (%), U/ml | 0.142 | 0.299 | ||||||||||
| ≤ 37 | 331 | 94.6 | 213 | 96 | 118 | 92.2 | 122 | 95.3 | 118 | 92.2 | ||
| > 37 | 19 | 5.4 | 9 | 4 | 10 | 7.8 | 6 | 4.7 | 10 | 7.8 | ||
| cStage, n (%) | 0.121 | 0.423 | ||||||||||
| I | 149 | 42.6 | 86 | 36.5 | 63 | 49.2 | 54 | 42.2 | 63 | 49.2 | ||
| II | 85 | 24.3 | 55 | 24.8 | 30 | 23.4 | 30 | 23.4 | 30 | 23.4 | ||
| III | 116 | 33.1 | 81 | 38.7 | 35 | 27.3 | 44 | 34.4 | 35 | 27.3 | ||
| Preoperative treatment, n (%) | 0.749 | 1.000 | ||||||||||
| Yes | 21 | 6.0 | 14 | 6.3 | 7 | 5.5 | 7 | 5.5 | 7 | 5.5 | ||
| No | 329 | 94.0 | 208 | 93.7 | 121 | 94.5 | 121 | 94.5 | 121 | 94.5 | ||
| Surgical procedure, n (%) | 0.070 | 0.229 | ||||||||||
| LAR | 174 | 49.7 | 113 | 50.9 | 61 | 47.7 | 58 | 45.3 | 61 | 47.7 | ||
| sLAR | 105 | 30.0 | 57 | 25.7 | 48 | 37.5 | 39 | 30.5 | 48 | 37.5 | ||
| ISR | 17 | 4.9 | 13 | 5.9 | 4 | 3.1 | 9 | 7.0 | 4 | 3.1 | ||
| APR | 54 | 15.4 | 39 | 17.6 | 15 | 11.7 | 22 | 17.2 | 15 | 11.7 | ||
| LLND, n (%) | 0.974 | 0.592 | ||||||||||
| Yes | 81 | 23.1 | 68 | 30.6 | 39 | 30.4 | 43 | 33.6 | 39 | 30.4 | ||
| No | 270 | 76.9 | 154 | 69.4 | 89 | 69.6 | 85 | 66.4 | 89 | 69.6 | ||
| Combined resection, n (%) | 0.079 | 1.000 | ||||||||||
| Yes | 9 | 2.6 | 8 | 3.6 | 1 | 0.8 | 1 | 0.8 | 1 | 0.8 | ||
| No | 341 | 97.4 | 214 | 96.4 | 127 | 99.2 | 127 | 99.2 | 127 | 99.2 | ||
| Tumor size, mean ± SD, mm | 38.2 | 18.9 | 38.1 | 18.1 | 38.3 | 20.3 | 0.946 | 39.1 | 17.9 | 38.3 | 20.3 | 0.643 |
| LVI, n (%) | 0.775 | 0.791 | ||||||||||
| Present | 233 | 66.6 | 149 | 67.1 | 84 | 65.6 | 86 | 67.2 | 84 | 65.6 | ||
| Absent | 117 | 33.4 | 73 | 32.9 | 44 | 34.4 | 42 | 32.8 | 44 | 34.4 | ||
| Histological grade, n (%) | 0.539 | 0.470 | ||||||||||
| Pap/tub | 339 | 96.9 | 216 | 97.3 | 123 | 96.1 | 125 | 97.7 | 123 | 96.1 | ||
| Muc/por/sig | 11 | 3.1 | 6 | 2.7 | 5 | 3.9 | 3 | 2.3 | 5 | 3.9 | ||
| DM positivity, n (%) | 0.155 | 0.238 | ||||||||||
| Yes | 1 | 0.2 | 0 | 0.0 | 1 | 0.8 | 0 | 0.0 | 1 | 0.8 | ||
| No | 349 | 99.7 | 222 | 100 | 127 | 99.2 | 128 | 100 | 127 | 99.2 | ||
| RM positivity, n (%) | 0.867 | 1.000 | ||||||||||
| Yes | 6 | 1.7 | 4 | 1.8 | 2 | 1.6 | 2 | 1.6 | 2 | 1.6 | ||
| No | 344 | 98.3 | 218 | 98.2 | 126 | 98.4 | 126 | 98.4 | 126 | 98.4 | ||
| pTNM stage, n (%) | 0.530 | 0.844 | ||||||||||
| I | 122 | 34.9 | 74 | 33.3 | 48 | 37.5 | 44 | 34.4 | 48 | 37.5 | ||
| II | 86 | 24.6 | 53 | 23.9 | 33 | 25.8 | 33 | 25.8 | 33 | 25.8 | ||
| III | 142 | 40.5 | 95 | 42.8 | 47 | 36.7 | 51 | 39.8 | 47 | 36.7 | ||
| Residual tumor, n (%) | 0.867 | 1.000 | ||||||||||
| R0 | 344 | 98.3 | 218 | 98.2 | 126 | 98.4 | 126 | 98.4 | 126 | 98.4 | ||
| R1 | 6 | 1.7 | 4 | 1.8 | 2 | 1.6 | 2 | 1.6 | 2 | 1.6 | ||
| Adjuvant therapy, n (%) | 0.706 | 0.899 | ||||||||||
| Yes | 122 | 34.9 | 79 | 35.6 | 43 | 33.6 | 42 | 32.8 | 43 | 33.6 | ||
| No | 228 | 65.1 | 43 | 64.4 | 85 | 66.4 | 86 | 67.2 | 85 | 66.4 | ||
LS, laparoscopic surgery; RS, robotic surgery; SD, standard deviation; ASA-PS, American Society of Anesthesiologists physical status classification; BMI, body mass index; RC, rectal cancer; CEA, carcinoembryonic antigen; CA19 - 9, carbohydrate antigen 19–9; C, clinical; LAR, low anterior resection; sLAR, super-low anterior resection; ISR, intersphincteric resection; APR, abdominoperineal resection; LVI, lymphovascular invasion; DM, distal margin; RM, radial margin; P, pathological
Comparison of short-term outcomes between RS and LS groups
Short-term outcomes for both the overall and matched cohorts are shown in Table 2. After PSM, no significant differences were observed in operative time, blood loss, conversion rate, intraoperative complication, or number of LNs harvested.
Table 2.
Comparison of short-term outcomes between LS and RS groups
| Total | Overall (N = 350) | Propensity score-matched pairs (n = 256) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LS group (n = 222) | RS group (n = 128) | p | LS group (n = 128) | RS group (n = 128) | p | |||||||
| n or mean | % or SD | n or mean | % or SD | n or mean | % or SD | n or mean | % or SD | n or mean | % or SD | |||
| Surgical findings | ||||||||||||
| Estimated operation time, mean ± SD, min | 342.9 | 116.4 | 330.7 | 118.4 | 364 | 110.1 | 0.006 | 341.5 | 121.0 | 364.0 | 110.1 | 0.136 |
| Estimated blood loss, mean ± SD, ml | 70.1 | 178.8 | 67.1 | 168.4 | 75.2 | 196.2 | 0.295 | 79.6 | 192.3 | 75.2 | 196.2 | 0.173 |
| Number of lymph node, mean ± SD | 19.7 | 12.5 | 19.2 | 12.2 | 20.8 | 13.1 | 0.245 | 19.7 | 12.1 | 20.8 | 13.1 | 0.554 |
| Conversion to laparotomy, n (%) | 2 | 0.6 | 2 | 0.9 | 0 | 0.0 | 0.176 | 1 | 0.8 | 0 | 0.0 | 0.238 |
| Intraoperative complications, n (%) | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | - | 0 | 0.0 | 0 | 0.0 | - |
| Postoperative complications, n (%) | ||||||||||||
| Overall, CD classification all-grade | 43 | 12.3 | 24 | 10.8 | 17 | 13.3 | 0.492 | 15 | 11.7 | 17 | 13.3 | 0.705 |
| Anastomosis leakage | 6 | 2.0 | 4/184 | 2.2 | 2/113 | 1.8 | 0.808 | 4/107 | 3.7 | 2/113 | 1.8 | 0.366 |
| Ileus | 8 | 2.3 | 5 | 2.2 | 3 | 2.3 | 0.956 | 2 | 1.5 | 3 | 2.3 | 0.650 |
| Bleeding | 2 | 0.6 | 2 | 0.9 | 0 | 0 | 0.176 | 2 | 1.5 | 0 | 0 | 0.155 |
| Wound infection | 2 | 0.6 | 0 | 0 | 2 | 1.5 | 0.061 | 0 | 0.0 | 2 | 1.5 | 0.155 |
| Intra-pelvic abscess | 4 | 1.1 | 3 | 1.3 | 1 | 0.8 | 0.619 | 3 | 2.3 | 1 | 0.8 | 0.302 |
| Urinary dysfunction | 9 | 2.5 | 3 | 1.3 | 4 | 3.1 | 0.264 | 1 | 0.8 | 4 | 3.1 | 0.161 |
| Urinary complication | 11 | 3.1 | 6 | 2.7 | 5 | 3.9 | 0.539 | 4 | 3.1 | 5 | 3.9 | 0.734 |
| Obstructive neuropathy | 2 | 0.6 | 1 | 0.4 | 1 | 0.7 | 0.697 | 0 | 0.0 | 1 | 0.7 | 0.238 |
| Thrombosis/embolism | 4 | 1.1 | 2 | 0.9 | 2 | 1.5 | 0.582 | 1 | 0.8 | 2 | 1.5 | 0.557 |
| Pneumonia | 0 | 0.0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 0 | 0 | - |
| CD grade ≥ II | 41 | 11.7 | 23 | 10.3 | 16 | 12.5 | 0.542 | 14 | 10.9 | 16 | 12.5 | 0.697 |
| CD grade ≥ III | 12 | 3.4 | 9 | 4 | 3 | 2.3 | 0.383 | 6 | 4.7 | 3 | 2.3 | 0.304 |
| Reoperation, n (%) | 4 | 1.1 | 2 | 0.9 | 2 | 1.5 | 0.582 | 2 | 1.5 | 2 | 1.5 | 1.000 |
| Thirty-day postoperative mortality, n (%) | 0 | 0.0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 0 | 0 | - |
| Length of postoperative stay, mean ± SD, days | 12.9 | 6.9 | 13.1 | 6.3 | 12.4 | 7.9 | 0.110 | 13.4 | 6.5 | 12.4 | 7.9 | 0.070 |
LS, laparoscopic surgery; RS, robotic surgery; SD, standard deviation; CD, Clavien–Dindo
After PSM, the incidence of CD all-grade postoperative complications was 13.3% in the RS group and 11.7% in the LS group. The occurrence of CD grade ≥ II and CD grade ≥ III complications was comparable between the groups. No significant differences were observed in anastomotic leakage (AL), ileus, bleeding, wound infection, intra-pelvis abscess, urinary dysfunction, urinary complication, obstructive neuropathy, thrombosis/embolism, pneumonia, reoperation rate, or 30-day postoperative mortality. The RS group showed a trend toward a shorter postoperative stay (p = 0.07). Additionally, no patients in either group experienced mortality within 30 days of surgery.
Comparison of long-term outcomes between RS and LS groups
Recurrence
No significant difference was observed in terms of the proportion of patients who received adjuvant therapy between groups (p = 0.70 before PSM, p = 0.89 following PSM). The median follow-up duration was 59.8 months (range, 7–83 months). The recurrence patterns in both the overall and matched cohorts are shown in Table 3. Among the overall cohort, 18 patients (5.1%) died from RC, with 20 (5.7%) all-cause deaths during follow-up. The overall recurrence rate was observed in 72 patients (20.6%), while LR and DR were detected in 11 (3.1%) and 62 (17.7%) patients, respectively. After PSM, the cumulative LR rates at 3- and 5-year were both 3.2% in the RS group, while in the LS group, they were 2.8% at 3-year and 3.2% at 5-year. (Fig. 2A). The cumulative DR rates in the RS group were 13.4% at 3-year and 15.1% at 5-year, whereas in the LS group, they were 14.9% and 18.7%, respectively (Fig. 2B). No differences were observed in terms of cumulative LR or DR rates between groups (Fig. 2).
Table 3.
Comparison of recurrence patterns between LS and RS groups
| Total | Overall (n = 350) | Propensity score-matched pairs (n = 256) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LS group (n = 222) | RS group (n = 128) | p | LS group (n = 128) | RS group (n = 128) | p | |||||||
| n | % | n | % | n | % | n | % | n | % | |||
| Overall death, n (%) | 20 | 5.7 | 10 | 4.5 | 9 | 7.0 | 0.322 | 5 | 3.9 | 9 | 7.0 | 0.268 |
| RC death, n (%) | 18 | 5.1 | 10 | 4.5 | 7 | 5.5 | 0.688 | 5 | 3.9 | 7 | 5.5 | 0.553 |
| Overall recurrence, n (%) | 72 | 20.6 | 50 | 22.5 | 22 | 17.2 | 0.229 | 28 | 21.9 | 22 | 17.2 | 0.343 |
| Local recurrence, n (%) | 11 | 3.1 | 7 | 3.1 | 4 | 3.1 | 0.988 | 4 | 3.1 | 4 | 3.1 | 1.000 |
| Distant recurrence, n (%) | 62 | 17.7 | 44 | 19.8 | 18 | 14.1 | 0.168 | 25 | 19.5 | 18 | 14.1 | 0.241 |
| Lung | 36 | 10.2 | 23 | 10.4 | 13 | 10.2 | 0.951 | 11 | 8.6 | 13 | 10.2 | 0.667 |
| Liver | 16 | 4.6 | 13 | 5.8 | 3 | 2.3 | 0.111 | 8 | 6.3 | 3 | 2.3 | 0.116 |
| Peritoneum | 6 | 1.7 | 4 | 1.8 | 2 | 1.6 | 0.867 | 4 | 3.1 | 2 | 1.6 | 0.404 |
| Para-Ao LN | 8 | 2.3 | 6 | 2.7 | 2 | 1.6 | 0.479 | 3 | 2.3 | 2 | 1.6 | 0.650 |
| Ovary | 1 | 0.3 | 1 | 0.5 | 0 | 0.0 | 0.339 | 1 | 0,.8 | 0 | 0.0 | 0.238 |
| Adrenal gland | 2 | 0.6 | 1 | 0.5 | 1 | 0.8 | 0.697 | 0 | 0.0 | 1 | 0.8 | 0.238 |
| Bone | 3 | 0.8 | 2 | 0.9 | 1 | 0.8 | 0.906 | 2 | 1.6 | 1 | 0.8 | 0.557 |
LS, laparoscopic surgery; RS, robotic surgery; RC, rectal cancer; Para-Ao LN, para-aortic lymph node
Fig. 2.
Incidence of cumulative recurrence. A Local recurrence and B distant recurrence
Overall, cancer-specific, and recurrence-free survival
OS curves after PSM are shown in Fig. 3. After PSM, the RS group had 3- and 5-year OS rates of 96.8% and 92.5%, while the LS group reported rates of 97.6% and 96.7%, respectively. For OS, no differences were observed between the RS and LS groups. Three- and 5-year OS rates according to stage in the RS group were 100% and 100% in stage I, 96.7% and 91.3% in stage II, and 93.6% and 84.6% in stage III, respectively. In the LS group, 3- and 5-year OS rates according to stage in the LS group were 100% and 100% in stage I, 100% and 96.7% in stage II, and 94.0% and 94.0% in stage III, respectively. Five-year CSS rates according to stage were similar between groups (Fig. 3B–D).
Fig. 3.
Kaplan–Meier curves for overall survival according to stages. A All stages. B Stage I. C Stage II. D Stage III
CSS curves after PSM are shown in Fig. 4. After PSM, the CSS rates at 3- and 5-year were 97.5% and 93.2% in the RS group, while the LS group had rates of 97.6% and 96.7%, respectively. For CSS, no significant differences were seen between the RS and LS groups. Three- and 5-year CSS rates according to stage in the RS group were 100% and 100% in stage I, 98.4% and 91.3% in stage II, and 95.6% and 86.5% in stage III, respectively. In the LS group, 3- and 5-year CSS rates according to stage were 100% and 100% in stage I, 100% and 96.7% in stage II, and 94.0% and 94.0% in stage III, respectively. Five-year CSS rates according to stage were similar between groups (Fig. 4B–D).
Fig. 4.
Kaplan–Meier curves for cancer-specific survival according to stages. A All stages. B Stage I. C Stage II. D Stage III
RFS curves after PSM are shown in Fig. 5. After PSM, the RS group had RFS rates of 83.4% at 3-year and 81.8% at 5-year, whereas the LS group reported rates of 82.6% and 78.9%, respectively. Again, no significant differences in RFS were evident between the RS and LS groups. Three- and 5-year stage-specific RFS rates in the RS group were 93.7% and 93.7% in stage I, 78.7% and 78.7% in stage II, and 76.4% and 70.5% in stage III, respectively. In the LS group, 3- and 5-year RFS rates according to stage were 93.0% and 93.0% in stage I, 87.9% and 78.9% in stage II, and 70.2% and 66.1% in stage III, respectively. Five-year stage-specific RFS rates were again similar between groups (Fig. 5B–D).
Fig. 5.
Kaplan–Meier curves for recurrence-free survival according to stages. A All stages. B Stage I. C Stage II. D Stage III
Discussion
This study examined the available evidence regarding short- and long-term outcomes following RS for stage I–III middle or lower RC and identified two clinically significant findings. Firstly, short-term outcomes were similar between the RS and LS groups. Secondly, no significant difference in long-term outcomes was seen between the two groups. Further, no significant differences were found between groups in terms of OS, CSS, or RFS according to stage. No significant differences in cumulative LR or DR rates were apparent between groups. To reduce selection bias, our retrospective study utilized a propensity score-matched analysis, offering deeper insights into RS for patients with stage I–III middle or lower RC.
Our data were comparable to those in the literature in three main ways. The first is short-term outcomes. Regarding surgical procedure, interestingly, sLAR was more frequently performed than ISR in the RS group compared to the LS group, even though lower RC was more prevalent in the RS group and LAR rates were comparable between the two groups. These results indicate that RS could overcome certain limitations associated with LS in RC patients with a deep and narrow pelvis, potentially aiding in the preservation of anal function. Regarding conversion rate, several studies have shown that RS is associated with a significantly lower conversion rate compared with LS [4–6]. None of the patients in this study required conversion to laparotomy in either group. Regarding postoperative complications, four large RCTs [3, 12–14] previously indicated complication rates of around 33.1% in the RS group and 21.2–40.0% in the LS group. Compared to previous studies, CD all-grade postoperative complications in the present study exhibited a relatively low rate (13.3% in the RS group, 11.7% in the LS group). Additionally, previous studies including > 200 RS for RC reported AL rates ranging from 1.5 to 12.2% in RS and from 2.9 to 10.8% in LS [3, 15–21]. The rate of AL (1.8% in the RS group, 3.7% in the LS group) in this study was similar to rates reported in previous research [3, 15–21]. Regarding the length of postoperative stay, previous studies reported a shorter postoperative stay with RS [16, 22–24]. Postoperative stay for the RS group in this study also exhibited a trend toward a shorter postoperative hospital stay compared to the LS group (p = 0.07), although the difference between groups was not significant. Our finding suggested that RS is a viable and safe treatment approach for patients with middle or lower RC.
A second aspect is the resection margins (DM and RM) and number of harvested LNs. The pathological parameters of resection margins and number of harvested LNs reflect the surgical and oncological qualities of RC resection, which have significant effects on prognosis. According to two meta-analyses, the number of LNs retrieved during RC resection was comparable between the RS and LS groups, showing no significant differences [25, 26]. Likewise in the present study, both groups showed similar results, with no significant differences in positive resection margins (DM and RM) or the number of harvested LNs. The quality of oncological resection in this study was similar across both groups.
The final aspect is the long-term outcomes. Our analysis with PSM revealed no significant differences in OS, CSS, or RFS between RS and LS groups. Further, no significant between-group differences were evident in cumulative LR or DR rates. Reviewing the existing literature, several retrospective studies have compared long-term outcomes for robotic versus laparoscopic approaches. The findings have been reported in six propensity score analyses [15, 18, 27–30] and a systematic review and meta-analysis [31]. According to analyses with PSM [15, 18, 27–30] and retrospective cohorts [32–34], 3- and 5-year OS rates were 94.6–98.4% and 90.5–95.4% in the RS groups and 86.5–98.7% and 78.0–97.3% in the LS groups. Two previous studies showed 5-year CSS rates of 90.5–93.6% in the RS group and 79.5–95.5% in the LS group [15, 18]. Several other studies reported 3- and 5-year disease-free survival and RFS rates of 82.2–90.5% and 72.6–90.5% in the RS group and 77.9–89.2% and 68.0–88.5% in the LS group [15, 18, 27–30, 32, 33]. OS, CSS, and RFS rates in this study closely matched the results reported in previous studies [15, 18, 27–29, 32–34], despite the different study designs. Regarding cumulative LR or DR, some studies showed cumulative LR rates of 2.3–5.9% and cumulative DR rates of 7.7–16.3% [18, 27–30, 33]. The 5-year cumulative LR rate of 3.2% and cumulative DR rate of 15.1% in this study were comparable and acceptable. In addition, several studies [18, 29, 33–35] have shown long-term outcomes according to stage. Yamaguchi et al. [33] reported 3-year OS and RFS rates for stage III RC in the RS group of 95.5% and 71.8%, respectively. Four retrospective studies [18, 29, 34, 35] found 5-year OS, CSS, and RFS rates for stage III RC in the RS groups of 86.8–89.0%, 90–100%, and 63.2–77.6%, respectively. In this study, 5-year OS, CSS, and RFS rates for stage III RC were 93.6%, 95.6%, and 76.4%, similar to those previous findings [18, 29, 34, 35]. In alignment with findings from multiple previous studies, our results suggested that long-term outcomes of RS for stage I–III middle or lower RC were comparable to those from LS.
A notable strength of this study was the implementation of PSM, which helped mitigate selection bias and balance significant differences in baseline patient characteristics. Another strength was the focus on long-term outcomes according to stage. Nevertheless, our study had several limitations. First was the non-randomized, retrospective design. Second, our study cohort was from a single center. While the application of PSM helped balance the characteristics of the patient groups, this came at the cost of a smaller sample size. Third, while our propensity score-matched analysis adjusted for observed baseline characteristics, it was unable to account for unmeasured factors, such as the surgeon’s experience, the learning curve, or the complexity of the surgical cases. These unaccounted factors may have influenced both short- and long-term outcomes. Fourth, we analyzed DM and RM as pathological indicators to assess surgical and oncological quality in RC but did not examine circumferential resection margins. As a result, our pathological assessment may have been insufficient for comprehensive evaluation of TME quality. Finally, we did not evaluate functional outcomes, which are important when assessing the clinical benefits of a treatment option. Future studies are therefore necessary to fully examine long-term functional outcomes, including sexual, urinary, and anal functions, all of which affect postoperative quality of life.
In conclusion, the present study with PSM indicates that RS offers a viable and safe treatment approach for patients with stage I–III middle or lower RC, yielding short- and long-term outcomes comparable to those of LS. However, given the current limitations in evidence, further prospective, multicenter RCTs assessing long-term outcomes are necessary to confirm the advantages of RS in RC.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Toshinori Sueda contributed to the conception and design, acquisition of data, analysis and interpretation of data, drafting of the article and has made final approval of the manuscript. Masayoshi Yasui, Junichi Nishimura, Yoshinori Kagawa, Masatoshi Kitakaze, Ryota Mori, Yoshitomo Yanagimoto, Takashi Kanemura, Kazuyoshi Yamamoto, Hiroshi Wada, Kunihito Gotoh, Hiroshi Miyata, and Masayuki Ohue contributed to the revision of the manuscript critically for important intellectual content and have made final approval of the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and informed consent
This retrospective study received approval from the institutional review board of Osaka International Cancer Institute (approval no. 18033). This study was exempt from the requirement for informed consent. Animal experimentation was not conducted in this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Agency for Research on Cancer (2022) World Health Organization. Cancer today. Cancer fact sheets. Available at: http://gco.iarc.fr/today. Accessed December 11, 2024
- 2.Corcione F, Esposito C, Cuccurullo D, Settembre A, Miranda N, Amato F, Pirozzi F, Caiazzo P (2005) Advantages and limits of robot-assisted laparoscopic surgery: preliminary experience. Surg Endosc 19:117–119. 10.1007/s00464-004-9004-9 [DOI] [PubMed] [Google Scholar]
- 3.Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, Tilney H, Gudgeon M, Bianchi PP, Edlin R, Hulme C, Brown J (2017) Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA 318:1569–1580. 10.1001/jama.2017.7219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simillis C, Tekkis PP (2019) Robotic versus laparoscopic surgery for rectal cancer: an evidence-based approach. Ann Surg 270:e57. 10.1097/sla.0000000000003156 [DOI] [PubMed] [Google Scholar]
- 5.Polat F, Willems LH, Dogan K, Rosman C (2019) The oncological and surgical safety of robot-assisted surgery in colorectal cancer: outcomes of a longitudinal prospective cohort study. Surg Endosc 33:3644–3655. 10.1007/s00464-018-06653-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuyama T, Kinugasa Y, Nakajima Y, Kojima K (2018) Robotic-assisted surgery for rectal cancer: current state and future perspective. Ann Gastroenterol Surg 2:406–412. 10.1002/ags3.12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sueda T, Tei M, Nishida K, Yoshikawa Y, Matsumura T, Koga C, Wakasugi M, Miyagaki H, Kawabata R, Tsujie M (2021) Hasegawa J (2022) Short-term outcomes of robotic-assisted versus conventional laparoscopic-assisted surgery for rectal cancer: a propensity score-matched analysis. J Robot Surg 16:323–331. 10.1007/s11701-021-01243-2 [DOI] [PubMed] [Google Scholar]
- 8.Han C, Yan P, Jing W, Li M, Du B, Si M, Yang J, Yang K, Cai H, Guo T (2020) Clinical, pathological, and oncologic outcomes of robotic-assisted versus laparoscopic proctectomy for rectal cancer: a meta-analysis of randomized controlled studies. Asian J Surg 43:880–890. 10.1016/j.asjsur.2019.11.003 [DOI] [PubMed] [Google Scholar]
- 9.Brierley JD, Gospodarowicz MK, Wittekind C, Fritz A (2017) Digestive system tumors. Colon and rectum. In: TNM Classification of Malignant Tumors, 8th edn. Wiley Blackwell, Oxford, pp 73–77 [Google Scholar]
- 10.Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K (2020) Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 25:1–42. 10.1007/s10147-019-01485-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS, Chang HJ, Lee HS, Kim SY, Jung KH, Hong YS, Kim JH, Sohn DK, Kim DH, Oh JH (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11:637–645. 10.1016/S1470-2045(10)70131-5 [DOI] [PubMed] [Google Scholar]
- 13.van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ, COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14:210–218. 10.1016/S1470-2045(13)70016-0 [DOI] [PubMed] [Google Scholar]
- 14.Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W, Simes J, ALaCaRT Investigators (2015) Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA 314:1356–1363. 10.1001/jama.2015.12009 [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Baek SJ, Kang DW, Roh YE, Lee JW, Kwak HD, Kwak JM, Kim SH (2017) Robotic resection is a good prognostic factor in rectal cancer compared with laparoscopic resection: long-term survival analysis using propensity score matching. Dis Colon Rectum 60:266–273. 10.1097/DCR.0000000000000770 [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi T, Kinugasa Y, Shiomi A, Tomioka H, Kagawa H, Yamakawa Y (2016) Robotic-assisted vs. conventional laparoscopic surgery for rectal cancer: short-term outcomes at a single center. Surg Today 46:957–962. 10.1007/s00595-015-1266-4 [DOI] [PubMed] [Google Scholar]
- 17.Law WL, Foo DCC (2017) Comparison of short-term and oncologic outcomes of robotic and laparoscopic resection for mid- and distal rectal cancer. Surg Endosc 31:2798–2807. 10.1007/s00464-016-5289-8 [DOI] [PubMed] [Google Scholar]
- 18.Cho MS, Baek SJ, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2015) Short and long-term outcomes of robotic versus laparoscopic total mesorectal excision for rectal cancer: a case-matched retrospective study. Medicine (Baltimore) 94:e522. 10.1097/MD.0000000000000522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JC, Lee JL, Alotaibi AM, Yoon YS, Kim CW, Park IJ (2017) Robot assisted intersphincteric resection facilitates an efficient sphinctersaving in patients with low rectal cancer. Int J Colorectal Dis 32:1137–1145. 10.1007/s00384-017-2807-7 [DOI] [PubMed] [Google Scholar]
- 20.Tang B, Zhang C, Li C, Chen J, Luo H, Zeng D, Yu P (2017) Robotic total mesorectal excision for rectal cancer: a series of 392 cases and mid-term outcomes from a single center in China. J Gastrointest Surg 21:569–576. 10.1007/s11605-016-3335-4 [DOI] [PubMed] [Google Scholar]
- 21.Sammour T, Malakorn S, Bednarski BK, Kaur H, Shin US, Messick C, You YN, Chang GJ (2018) Oncological outcomes after robotic proctectomy for rectal cancer: analysis of a prospective database. Ann Surg 267:521–526. 10.1097/SLA.0000000000002112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, Xu H, Li Z, Han J, Song W, Wang J, Xu Z (2016) Robotic versus laparoscopic low anterior resection for rectal cancer: a meta-analysis. World J Surg Oncol 14:61. 10.1186/s12957-016-0816-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuyama T, Endo H, Yamamoto H, Takemasa I, Uehara K, Hanai T, Miyata H, Kimura T, Hasegawa H, Kakeji Y, Inomata M, Kitagawa Y, Kinugasa Y (2021) Outcomes of robot assisted versus conventional laparoscopic low anterior resection in patients with rectal cancer: propensity-matched analysis of the national clinical database in Japan. BJS Open 5:zrab083. 10.1093/bjsopen/zrab083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang J, Yoon KJ, Min BS, Hur H, Baik SH, Kim NK, Lee KY (2013) The impact of robotic surgery for mid and low rectal cancer: a case-matched analysis of a 3-arm comparison—open, laparoscopic, and robotic surgery. Ann Surg 257:95–101. 10.1097/SLA.0b013e3182686bbd [DOI] [PubMed] [Google Scholar]
- 25.Kim NK, Baik SH, Seong JS, Kim H, Roh JK, Lee KY, Sohn SK, Cho CH (2006) Oncologic outcomes after neoadjuvant chemoradiation followed by curative resection with tumor-specific mesorectal excision for fixed locally advanced rectal cancer: impact of postirradiated pathologic downstaging on local recurrence and survival. Ann Surg 244:1024–1030. 10.1097/01.sla.0000225360.99257.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trastulli S, Farinella E, Cirocchi R, Cavaliere D, Avenia N, Sciannameo F, Gullà N, Noya G, Boselli C (2012) Robotic resection compared with laparoscopic rectal resection for cancer: systematic review and meta-analysis of short-term outcome. Colorectal Dis 14:e134–e156. 10.1111/j.1463-1318.2011.02907.x [DOI] [PubMed] [Google Scholar]
- 27.Takamizawa Y, Tsukamoto S, Kato T, Nagata H, Moritani K, Kanemitsu Y (2024) Short- and long-term outcomes of robotic and laparoscopic surgery in rectal cancer: a propensity score-matched analysis. Surg Endosc 1. 10.1007/s00464-024-11374-w [DOI] [PubMed]
- 28.Mazaki J, Ishizaki T, Kuboyama Y, Udo R, Tago T, Kasahara K, Yamada T, Nagakawa Y (2024) Long-term outcomes of robot-assisted laparoscopic surgery versus conventional laparoscopic surgery for rectal cancer: single-center, retrospective, propensity score analyses. J Robot Surg 18:157. 10.1007/s11701-024-01894-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park EJ, Cho MS, Baek SJ, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2015) Long-term oncologic outcomes of robotic low anterior resection for rectal cancer: a comparative study with laparoscopic surgery. Ann Surg 261:129–137. 10.1097/SLA.0000000000000613 [DOI] [PubMed] [Google Scholar]
- 30.Kim JC, Yu CS, Lim SB, Park IJ, Kim CW, Yoon YS (2016) Comparative analysis focusing on surgical and early oncological outcomes of open, laparoscopy-assisted, and robot-assisted approaches in rectal cancer patients. Int J Colorectal Dis 31:1179–1187. 10.1007/s00384-016-2586-6 [DOI] [PubMed] [Google Scholar]
- 31.Qiu H, Yu D, Ye S, Shan R, Ai J, Shi J (2020) Long-term oncological outcomes in robotic versus laparoscopic approach for rectal cancer: a systematic review and meta-analysis. Int J Surg 80:225–230. 10.1016/j.ijsu.2020.03.009 [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi T, Kinugasa Y, Shiomi A, Kagawa H, Yamakawa Y, Furutani A, Manabe S, Yamaoka Y, Hino H (2018) Oncological outcomes of robotic-assisted laparoscopic versus open lateral lymph node dissection for locally advanced low rectal cancer. Surg Endosc 32:4498–4505. 10.1007/s00464-018-6197-x [DOI] [PubMed] [Google Scholar]
- 33.Yamanashi T, Miura H, Tanaka T, Watanabe A, Goto T, Yokoi K, Kojo K, Niihara M, Hosoda K, Kaizu T, Yamashita K, Sato T, Kumamoto Y, Hiki N, Naitoh T (2022) Short- and long-term outcomes of robotic-assisted laparoscopic surgery for rectal cancer: a single-center retrospective cohort study. Asian J Endosc Surg 15:794–804. 10.1111/ases.13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi T, Kinugasa Y, Shiomi A, Kagawa H, Yamakawa Y, Furuatni A, Manabe S, Yamaoka Y, Hino H (2018) Short- and long-term outcomes of robotic-assisted laparoscopic surgery for rectal cancer: results of a single high-volume center in Japan. Int J Colorectal Dis 33:1755–1762. 10.1007/s00384-018-3153-0 [DOI] [PubMed] [Google Scholar]
- 35.Hara M, Sng K, Yoo BE, Shin JW, Lee DW, Kim SH (2014) Robotic-assisted surgery for rectal adenocarcinoma: short-term and midterm outcomes from 200 consecutive cases at a single institution. Dis Colon Rectum 57:570–577. 10.1097/DCR.0000000000000088 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.