Abstract
Background
Sarcopenia, characterized by progressive muscle mass and strength decline, poses a significant health challenge among older adults, especially those with chronic diseases. Our study aims to evaluate the combined diagnostic potential of irisin, 25(OH)D3, and albumin for sarcopenia in older patients with chronic conditions.
Methods
A cohort of 393 older patients with chronic diseases, including 117 diagnosed with sarcopenia were included. Fasting blood samples were collected, and serum biomarkers (25(OH)D3, albumin, and irisin) were measured using automated biochemical analyzers and enzyme-linked immunosorbent assay to evaluate nutritional and muscle-related parameters.
Results
The prevalence of sarcopenia was higher among patients aged 80 or older compared to younger age groups in our study population. Strong associations were observed between sarcopenia and osteoporosis, tumors, and risk of malnutrition. Serum irisin, 25(OH)D3, and albumin levels were significantly lower in sarcopenic patients. Individual biomarkers displayed diagnostic potential, and a combined biomarker test showed superior accuracy. Multivariate logistic regression identified age, osteoporosis, malnutrition, and fatigue as independent risk factors, while higher serum biomarker levels correlated with reduced sarcopenia risk. Positive correlations were observed between serum biomarkers and sarcopenia severity indicators.
Conclusions
This study highlights the potential of irisin, 25(OH)D3, and albumin as diagnostic and prognostic tools for sarcopenia in older patients with chronic diseases, contributing to early detection and intervention strategies to enhance their quality of life.
Keywords: Sarcopenia, Older adults, Irisin, 25(OH)D3, Albumin
Introduction
Sarcopenia, clinically recognized as a syndrome characterized by the progressive and generalized loss of skeletal muscle mass and strength, poses a substantial health concern in geriatric populations, particularly for those contending with chronic disease burdens [1, 2]. This condition is characterized not merely by a reduction in muscle quantity but also by a decline in muscle quality and functional performance [3]. While estimates vary based on the diagnostic criteria used, sarcopenia affects approximately 10–16% of older adults globally and occurs at a higher incidence in clinical settings than in the general older population [4, 5]. The syndrome is associated with a range of detrimental health consequences, including a decline in survival rates, an increase in postoperative complications, prolonged hospitalization, and a higher incidence of falls, fractures, and metabolic and cognitive impairments, leading to elevated mortality rates. Traditional diagnostic techniques for sarcopenia, which include evaluations of muscle power, ambulatory speed, and imaging studies, are criticized for their intricate nature and a shortfall in uniform, early detection methods [6, 7]. This deficiency accentuates the pressing necessity for novel, more user-friendly diagnostic approaches to effectively combat the rising incidence of sarcopenia and its associated health ramifications in the aging sector.
In the realm of sarcopenia research, serum biomarkers such as Irisin, 25-hydroxyvitamin D3 (25(OH)D3), and albumin have garnered attention as potential indicators of the condition [8–11], linked to their roles in exercise physiology and nutritional risk. Irisin, a myokine secreted by skeletal muscles in response to physical activity, acts as a messenger molecule to various tissues and has been shown to correlate with muscle health [12]. Its levels in serum rise with exercise, suggesting a positive relationship with muscle mass and function [13]. Vitamin D, traditionally associated with bone metabolism, also affects muscle cell signaling pathways, with deficiencies potentially exacerbating muscle atrophy and weakness [14]. Albumin, indicative of nutritional status, further ties sarcopenia to nutritional intake and malnutrition, as seen in populations with abdominal obesity and sarcopenia [15]. These associations underscore the complex interplay between physical activity, nutrition, and muscle integrity, framing these biomarkers as critical to understanding and diagnosing sarcopenia.
Previous meta-analyses have demonstrated that patients with sarcopenia exhibit lower levels of blood irisin, 25 hydroxyvitamin D (25(OH)D), and albumin [16, 17]. However, the specific combination of these three biomarkers in relation to sarcopenia has not been comprehensively investigated in older patients with sarcopenia. We hypothesize that the combined analysis of irisin, 25(OH)D3, and albumin may provide enhanced diagnostic and prognostic value for sarcopenia in this population. Our objectives were to evaluate the combined diagnostic potential of the three biomarkers for sarcopenia, assess their relationship with sarcopenia severity, and investigate their prognostic utility in identifying sarcopenia risk. By focusing on these objectives, we aim to contribute to the development of more accessible and efficient diagnostic approaches for sarcopenia in the geriatric population with chronic diseases, potentially improving early detection and treatment strategies.
Methods
Study population
This retrospective study was conducted at Longgang Central Hospital of Shenzhen from Jan 5thth 2023 to Jan 5th 2025. The medical documents of a cohort of 393 individuals were reviewed and analyzed, with 117 participants diagnosed with sarcopenia based on the criteria established by the Asian Working Group for Sarcopenia (AWGS) in 2014 [18]. Two trained researchers independently reviewed the medical records and extracted the relevant data using a standardized data collection form. Sarcopenia was diagnosed using the following AWGS criteria. An appendicular skeletal muscle mass index (ASMI) calculated by dividing appendicular muscle mass (measured by dual-energy X-ray absorptiometry) by height squared, with thresholds of less than 7.0 kg/m² for men and less than 5.4 kg/m² for women. Grip strength below 26 kg for men and 18 kg for women. Physical performance measured by walking speed, with a 6-meter walk test speed of less than 0.8 m/s. Participants meeting the first criterion, in addition to either or both second and third criteria, were diagnosed with sarcopenia.
Participants
Participants were eligible for the study if they were aged 60 years or older, had at least one chronic disease, possessed good general condition and cardiopulmonary function, and provided informed consent. Patients were excluded if they had acute illnesses or severe diseases, comorbid conditions like severe osteoarthritis or neuromuscular disorders affecting functional capacity, Parkinson’s disease, motor neuron disease, post-stroke conditions, rheumatoid arthritis affecting grip strength, or if they had used glucocorticoids, immunosuppressants, or vitamin D supplements in the past six months. Specifically, severe diseases encompassed acute conditions such as acute myocardial infarction, acute heart failure, acute respiratory distress syndrome, septic shock, intracranial hemorrhage, acute poisoning, and cerebral hemorrhage. The study was approved by the ethics committee of Longgang Central Hospital of Shenzhen, and all participants provided written informed consent. All methods were carried out in accordance with relevant guidelines and regulations.
Biochemical measurements
Participants were instructed to fast and avoid drinking water after 10 PM, with blood samples taken the following morning from the antecubital vein. Samples were collected and immediately processed in our laboratory. Whole blood was allowed to clot at room temperature for 15–30 min. Serum was separated by centrifugation at 2000 rpm for 10 min at 4 °C. Serum 25(OH)D3 and albumin levels were determined using a fully automated biochemical analyzer (Beckman, Germany) and tandem mass spectrometry. Serum irisin concentrations were measured using an enzyme-linked immunosorbent assay according to instruments (ELISA, Shanghai Huaying Corporation DECO). All assays were performed in triplicate, and the mean value was used for analysis.
Nutritional and fatigue assessment
Nutritional risk and fatigue assessments were conducted by trained research nurses during face-to-face interviews with participants. Nutritional risk was evaluated using the Short-Form Mini-Nutritional Assessment (MNA-SF) developed by Rubenstein et al. [19]. comprising items on food intake reduction, weight loss, mobility, psychological stress or acute disease, neuropsychological problems, and body mass index. Scores ranged from 0 to 14, with 0–7 indicating malnutrition, 8–11 suggesting risk of malnutrition, and 12–14 corresponding to normal nutritional status. Fatigue levels were assessed using the Chalder Fatigue Scale [20], consisting of 11 items with items 1 to 7 representing physical fatigue (PF), and the remaining items assessing mental fatigue (MF). Scores for PF ranged from 0 to 7, and MF from 0 to 4, with a total score range of 0 to 11. A total score of 4 or higher indicated a diagnosis of fatigue.
Statistical analysis
Data analysis was performed using GraphPad Prism (version 6.01) and SPSS (version 28.01.0). The Kolmogorov-Smirnov test was used to assess the normality of data distribution. For comparisons between groups where data followed a normal distribution, the unpaired t-test with Welch’s correction was applied. Spearman’s rank correlation was used to evaluate the association between serum biomarkers and measures of sarcopenia severity (ASMI and grip strength). To identify independent risk and protective factors for sarcopenia, multivariate logistic regression analysis was conducted. The diagnostic value of individual serum biomarkers for sarcopenia was assessed using Receiver Operating Characteristic (ROC) analysis. To evaluate the diagnostic performance of combined biomarkers, a composite score was derived from the individual effects of the biomarkers. Statistical significance was set at p < 0.05.
Results
Demographic and clinical correlates of sarcopenia
Our study included a cohort of 393 older individuals with chronic diseases such as diabetes mellitus, hypertension, coronary heart disease, osteoporosis, chronic obstructive pulmonary disease, and various types of tumors. Among these participants, 117 were diagnosed with sarcopenia according to the Asian Working Group for Sarcopenia criteria (Table 1). We compared the demographic and clinical characteristics among the patients with and without sarcopenia. It was found that those aged 80 or older had a higher incidence of sarcopenia (35.9%) compared to younger counterparts, and significant associations were found with osteoporosis (56.4% in sarcopenic patients), tumors (17.1%), and nutritional deficits, with 36.8% of sarcopenic patients being at risk of malnutrition (p < 0.001 for all). Additionally, sarcopenic patients exhibited higher fatigue levels (68.4%, p < 0.001). Other factors such as gender, smoking, alcohol consumption, and comorbidities including diabetes mellitus, hypertension, COPD, and peptic ulcer disease did not show statistically significant differences between the sarcopenic and non-sarcopenic groups.
Table 1.
Demographic and clinical characteristics of older patients with chronic disease with sarcopenia (SP) or not (NSP)
| Characteristics | NSP (n = 276) | SP (n = 117) | p value |
|---|---|---|---|
| Age (years, n, %) | |||
| 60–69 | 125 (45.3%) | 29 (24.8%) | < 0.001 |
| 70–79 | 101 (36.6%) | 46 (39.3%) | |
| ≥ 80 | 50 (18.1%) | 42 (35.9%) | |
| Gender (n, %) | |||
| Male | 143 (51.8%) | 54 (46.2%) | 0.322 |
| Female | 133 (48.2%) | 63 (53.8%) | |
| Smoke (n, %) | |||
| Yes | 65 (23.6%) | 32 (27.4%) | 0.444 |
| No | 211 (76.4%) | 85 (72.6%) | |
| Alcohol drinking (n, %) | |||
| Often | 57 (20.7%) | 29 (24.8%) | 0.423 |
| Rarely or never | 219 (79.3%) | 88 (75.2%) | |
| Diabetes mellitus (n, %) | |||
| Yes | 114 (41.3%) | 57 (48.7%) | 0.183 |
| No | 162 (58.7%) | 60 (51.3%) | |
| Hypertension (n, %) | |||
| Yes | 201 (72.8%) | 82 (70.1%) | 0.624 |
| No | 75 (27.2%) | 35 (29.9%) | |
| Coronary heart disease (n, %) | |||
| Yes | 69 (25%) | 33 (28.2%) | 0.531 |
| No | 207 (75%) | 84 (71.8%) | |
| Osteoporosis (n, %) | |||
| Yes | 110 (39.9%) | 66 (56.4%) | 0.003 |
| No | 166 (60.1%) | 51 (43.6%) | |
| Chronic obstructive pulmonary disease (n, %) | |||
| Yes | 22 (8%) | 12 (10.3%) | 0.441 |
| No | 254 (92%) | 105 (89.7%) | |
| Peptic ulcer (n, %) | |||
| Yes | 27 (9.8%) | 15 (12.8%) | 0.376 |
| No | 249 (90.2%) | 102 (87.2%) | |
| Tumor (n, %) | |||
| Yes | 23 (8.3%) | 20 (17.1%) | 0.014 |
| No | 253 (91.7%) | 97 (82.9%) | |
| Malnutrition (n, %) | |||
| Eutrophy | 172 (62.3%) | 48 (41%) | < 0.001 |
| Risk of malnutrition | 48 (17.4%) | 26 (22.2%) | |
| Malnutrition | 56 (20.3%) | 43 (36.8%) | |
| Fatigue | |||
| Yes | 129 (46.7%) | 80 (68.4%) | < 0.001 |
| No | 147 (53.3%) | 37 (31.6%) | |
The data are presented as n (percentage). The comparisons of data were done by Fisher’s exact test or Chi-square test
Serum biomarker levels in sarcopenia
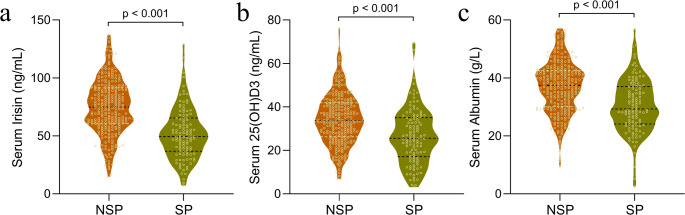
Upon assessing serum biomarker levels among older patients with chronic disease, we identified significant disparities between those with sarcopenia (SP, n = 117) and without (NSP, n = 276). Our analysis demonstrated that patients with sarcopenia had notably lower levels of serum irisin, 25(OH)D3, and albumin. Serum irisin levels were substantially decreased in sarcopenic patients (p < 0.001, Fig. 1a), a finding that was echoed in the serum 25(OH)D3 levels, with sarcopenic patients also exhibiting a significant reduction as compared to their non-sarcopenic counterparts (p < 0.001, Fig. 1b). Additionally, serum albumin concentrations were significantly diminished in patients suffering from sarcopenia (p < 0.001, Fig. 1c). These results highlight pronounced differences in the serum biomarker profiles between sarcopenic and non-sarcopenic older individuals with chronic diseases, suggesting a potential correlation between these biomarkers and the manifestation of sarcopenia.
Fig. 1.
Comparative analysis of serum biomarker levels between older chronic disease patients with or without sarcopenia. The violin plots depict the distribution and median values of serum irisin (a), 25-hydroxyvitamin D3 (25(OH)D3) (b), and albumin (c). Statistically significant lower levels of these biomarkers are evident in the sarcopenic group (SP), as compared to the non-sarcopenic group (NSP), with p-values < 0.001 obtained from an unpaired t-test with Welch’s correction, indicating a strong association between reduced levels of these biomarkers and the presence of sarcopenia in this population
Enhanced diagnostic accuracy of combined serum biomarkers for sarcopenia
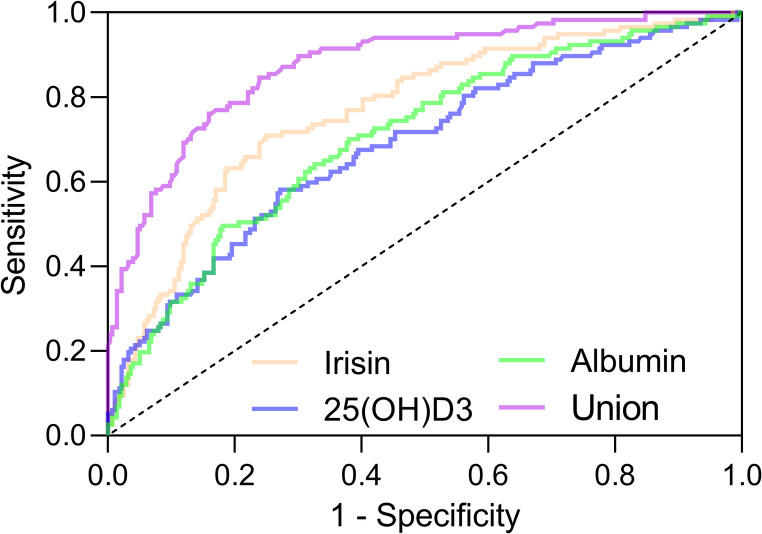
The ROC analysis was employed to determine the diagnostic value of serum biomarkers irisin, 25(OH)D3, and albumin for sarcopenia in older patients with chronic diseases (Fig. 2). Additionally, a composite biomarker test was devised, which combined the biomarkers using a formula that accounted for the individual effects of irisin, 25(OH)D3, and albumin, with respective weights of -0.042, -0.051, and − 0.087.
Fig. 2.
Receiver Operating Characteristic (ROC) analysis for sarcopenia biomarkers. The ROC curves illustrating the diagnostic efficacy of serum biomarkers—irisin, 25(OH)D3, albumin, and their combined form (‘Union’) for identifying sarcopenia in older patients with chronic diseases in older patients with chronic diseases. The union test formula is- 0.042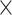 Irisin − 0.051
Irisin − 0.051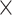 25(OH)D3–0.087
25(OH)D3–0.087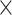 Albumin. The curves demonstrate the diagnostic capability of each biomarker alone and in combination to differentiate between sarcopenic (SP) and non-sarcopenic (NSP) states.
Albumin. The curves demonstrate the diagnostic capability of each biomarker alone and in combination to differentiate between sarcopenic (SP) and non-sarcopenic (NSP) states.
In sarcopenia diagnosis among older patients with chronic disease, serum irisin, 25(OH)D3, and albumin showed diagnostic potential. Irisin had an AUC of 0.77 at a 60.59 ng/mL cut-off, indicating good diagnostic capability with 70.94% sensitivity and 75% specificity (Table 2). 25(OH)D3 at a 27.66 ng/mL cut-off had a moderate AUC of 0.69, with 58.12% sensitivity and 72.83% specificity. Albumin showed an AUC of 0.71 at a 34.96 g/L cut-off, with 70.09% sensitivity and 61.96% specificity. The combined biomarker test with an AUC of 0.87 provided superior diagnostic accuracy (p < 0.001), 84.62% sensitivity, and 76.09% specificity, surpassing individual biomarkers in detecting sarcopenia. This suggests the potential of combined test as an effective clinical tool for sarcopenia screening in this patient group.
Table 2.
Diagnostic values of serum irisin, 25(OH)D3, albumin and their union test for sarcopenia (SP) in older patients with chronic disease
| Cut off | AUC (95% CI) | p | Sensitivity (%) | Specificity (%) | Youden index | |
|---|---|---|---|---|---|---|
| Irisin | 60.59 ng/mL | 0.77 (0.72 to 0.82) | < 0.001 | 70.94 | 75 | 0.46 |
| 25(OH)D3 | 27.66 ng/mL | 0.69 (0.63 to 0.74) | < 0.001 | 58.12 | 72.83 | 0.31 |
| Albumin | 34.96 g/L | 0.71 (0.65 to 0.76) | < 0.001 | 70.09 | 61.96 | 0.32 |
| Union* | - | 0.87 (0.84 to 0.91) | < 0.001 | 84.62 | 76.09 | 0.61 |
CI: confidence interval
*COMPUTE = − 0.042 * Irisin − 0.051 * 25(OH)D3–0.087 * Albumin
Independent risk and protective factors for sarcopenia in older patients with chronic disease
Multivariate logistic regression analysis identified age, osteoporosis, malnutrition, and fatigue as independent risk factors for sarcopenia in older patients with chronic disease, with individuals over 80 years exhibiting more than double the risk compared to younger counterparts (OR = 2.479, p < 0.001, Table 3). Tumor presence was not a significant risk factor. Conversely, higher serum levels of irisin (> 60.59 ng/mL), 25(OH)D3 (> 27.66 ng/mL), and albumin (> 34.96 g/L) were associated with reduced sarcopenia risk (ORs ranging from 0.809 to 0.915, p ≤ 0.035), indicating their potential role as protective biomarkers.
Table 3.
Multivariate logistic analysis for sarcopenia (SP) in older patients with chronic disease
| OR | 95% CI | p value | |
|---|---|---|---|
| Age more than 70 | 1.242 | 1.091 to 1.683 | 0.021 |
| Age more than 80 | 2.479 | 1.845 to 4.217 | < 0.001 |
| Osteoporosis | 1.307 | 1.158 to 1.982 | 0.008 |
| Tumor | 1.195 | 0.966 to 2.037 | 0.093 |
| Malnutrition | 1.751 | 1.093 to 3.205 | 0.005 |
| Fatigue | 2.116 | 1.584 to 3.962 | < 0.001 |
| Serum Irisin level more than 60.59 ng/mL | 0.809 | 0.647 to 0.916 | 0.007 |
| Serum 25(OH)D3 level more than 27.66 ng/mL | 0.915 | 0.833 to 0.971 | 0.035 |
| Serum Albumin level more than 34.96 g/L | 0.858 | 0.796 to 0.934 | 0.018 |
OR: Odds Ratio, CI: confidence interval
Serum biomarkers correlation with sarcopenia severity
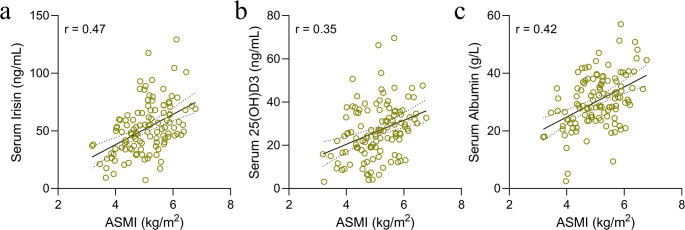
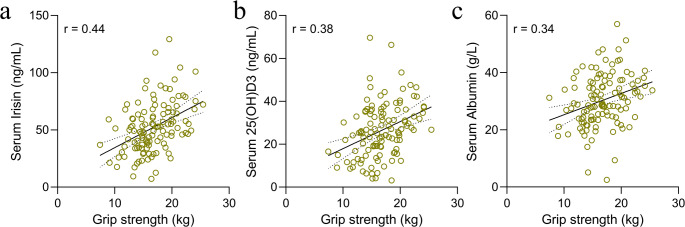
In terms of sarcopenia severity, positive correlations were observed between the ASMI and serum biomarkers. Specifically, serum irisin (r = 0.47) (Fig. 3a), 25(OH)D3 (r = 0.35) (Fig. 3b), and albumin (r = 0.42) (Fig. 3c) levels were positively associated with ASMI. Grip strength, another measure of sarcopenia severity, similarly demonstrated positive correlations with these biomarkers, irisin (r = 0.44) (Fig. 4a), 25(OH)D3 (r = 0.38) (Fig. 4b), and albumin (r = 0.34) (Fig. 4c). These findings indicate that serum levels of irisin, 25(OH)D3, and albumin could serve as valuable indicators for monitoring sarcopenia severity, offering insights beyond mere diagnostic capabilities.
Fig. 3.
Correlation Analysis between ASMI and Serum Biomarkers in Sarcopenic Patients. The results of a Spearman correlation coefficient analysis between appendicular skeletal mass index (ASMI) and serum biomarkers in a cohort of 117 older patients with chronic diseases and diagnosed with sarcopenia. The correlation of ASMI with serum irisin (a), serum 25(OH)D3 levels (b), and serum albumin levels
Fig. 4.
Correlation analysis between grip strength and serum biomarkers in sarcopenic patients. The Spearman correlation coefficient analysis illustrating the association between grip strength and serum biomarkers in 117 older chronic disease patients with sarcopenia. The grip strength in relation to serum irisin levels (a), serum 25(OH)D3 levels (b), and serum albumin levels (c)
Discussion
The phenomenon of sarcopenia, characterized by a progressive loss of skeletal muscle mass and strength, poses a significant health concern for the older adults, particularly those with chronic diseases. Our study encompassed a cohort of 393 older patients with chronic diseases, providing a comprehensive analysis of demographic, clinical, and biochemical factors associated with sarcopenia. Notably, we observed significantly reduced levels of irisin, 25(OH)D3, and albumin in older patients with sarcopenia. The combined serum levels of these three biomarkers demonstrated potential as an accurate diagnostic test for sarcopenia. Furthermore, the serum concentrations of irisin, 25(OH)D3, and albumin could serve as valuable indicators for monitoring sarcopenia severity. Our findings also identified age, osteoporosis, malnutrition, and fatigue as independent risk factors for sarcopenia in geriatric patients with chronic diseases. These results contribute to the growing body of knowledge surrounding sarcopenia and may inform future diagnostic and therapeutic strategies for this condition in the older population with chronic illnesses.
Our study provides insights into the complex interplay of factors associated with sarcopenia. While age emerged as a significant factor, the strength of this association varied notably across age groups. Patients aged 80 and above demonstrated a markedly stronger association with sarcopenia (OR = 2.479, 95% CI: 1.845–4.217) compared to those aged 70–79 (OR = 1.242, 95% CI: 1.091–1.683). This substantial difference in effect size suggests that advanced age may have a more pronounced impact on sarcopenia risk, aligning with previous research identifying age as a factor associated with sarcopenia [21]. Age-related changes in muscle composition, neuromuscular integrity, and hormonal balance are well-recognized contributors to sarcopenia [3, 22], and our data support these established paradigms.
The association between osteoporosis and sarcopenia in our population aligns with the concept of osteosarcopenia, highlighting the interconnected nature of bone and muscle health. This relationship underscores the potential clinical importance of addressing both conditions concurrently to mitigate the risk of adverse outcomes such as falls and fractures [23–25]. However, it is important to note that our study design did not allow for the establishment of a causal relationship between these conditions.
Nutritional deficits and fatigue emerged as independent correlates of sarcopenia in our analysis. The association between malnutrition and sarcopenia suggests a clinically relevant relationship that may have implications for preventive strategies and treatment approaches. Inadequate intake of protein and other nutrients can directly affect muscle protein synthesis, potentially contributing to the progressive weakness seen in sarcopenia [26–29]. However, our results were insufficient to determine the directionality of this relationship. There is a strong association between fatigue and sarcopenia in our study. Fatigue is a common symptom of sarcopenia, and studies have shown a higher prevalence of fatigue in individuals with sarcopenia [30]. A previous study on patients with osteoarthritis or rheumatoid arthritis found no significant difference in fatigue levels between groups with and without sarcopenia [31]. However, several studies on geriatric patients have identified significant relationships not only between fatigue and sarcopenia components (such as hand grip strength and walking speed) but also between fatigue and sarcopenia itself [30].
The significance of serum biomarkers, particularly irisin, 25(OH)D3, and albumin, in relation to sarcopenia was another key finding. Lower levels of these biomarkers were notably present in sarcopenic patients, providing a biochemical signature of the condition. Irisin, a myokine known to influence muscle metabolism and energy homeostasis [32, 33]. Our findings of diminished irisin levels in sarcopenic patients align with previous research, including a systematic review and meta-analysis that reported significantly lower irisin levels in sarcopenic individuals [17]. The importance of irisin in sarcopenia extends beyond its role as a biomarker. Animal studies have demonstrated its potential therapeutic effects, with intraperitoneal administration of irisin protein to aging mice resulting in significant improvements in sarcopenia-related parameter [34]. However, a study conducted in South Korea found no significant association between serum irisin levels and sarcopenia status in older adults [10]. This discrepancy might be attributed to the limited sample size (23 sarcopenic patients, mean age 71 years), which may not fully represent the broader population of sarcopenic individuals. These conflicting findings underscore the need for larger, more diverse studies to definitively establish the relationship between irisin and sarcopenia across different populations.
The association of sarcopenia with low levels of 25(OH)D3 is particularly notable, given the well-documented role of vitamin D in muscle function and strength [35]. Multiple studies have reinforced this connection, with one study concluding that older individuals with low serum 25(OH)D concentrations are more susceptible to sarcopenia [36]. This relationship appears to be complex and potentially synergistic with other factors. Yang et al. found that insufficient serum vitamin D levels and physical inactivity may synergistically contribute to the development of sarcopenia in older individuals [37]. However, it is crucial to consider that vitamin D levels could be influenced by various factors, including sunlight exposure and dietary habits, which were not fully accounted for in our study.
Albumin, often used as a marker of nutritional risk [38], was also found to be lower in sarcopenic individuals. While this could reflect reduced protein availability for muscle maintenance and repair, it is important to note that albumin levels could be affected by numerous factors beyond nutritional status, including inflammation and liver function. Our study employed a composite biomarker test, amalgamating individual markers into a single diagnostic tool. This approach yielded a robust AUC of 0.87, suggesting enhanced accuracy for sarcopenia diagnosis compared to individual biomarkers. While this is promising, it is crucial to validate these findings in larger, diverse populations before considering widespread clinical application. The sensitivity and specificity achieved by the composite test surpass that of individual biomarkers, highlighting the potential for a more nuanced and accurate diagnostic model. The serum irisin, 25(OH)D3, and albumin as biomarkers could be integrated into routine blood tests for older patients in geriatric department, particularly those with chronic diseases.
Our results also shed light on the relationship between sarcopenia severity and physical manifestations. The positive correlations of ASMI and grip strength with serum biomarkers emphasize the clinical relevance of these biochemical indicators. ASMI has been recognized as a reliable metric for muscle mass, while grip strength is a practical and effective measure of muscle function and has been linked to health outcomes in the older adults [39]. The positive correlation between these clinical measures and serum biomarker levels suggests that these biomarkers could serve not only in diagnosing sarcopenia but also in monitoring its progression and response to treatment. Despite the significant insights provided by our study, limitations must be acknowledged. The cross-sectional design limits causal inferences, and longitudinal studies are required to understand the temporal dynamics of sarcopenia development. Additionally, the study population was limited to those with chronic diseases, which may not be representative of the general older population. Future research should explore these associations in broader populations and investigate the potential for interventional studies to assess the impact of targeted therapies on serum biomarkers and sarcopenia outcomes.
Conclusions
Our investigation into the demographic, clinical, and biochemical facets of sarcopenia has illuminated critical insights into its etiology and potential diagnostic markers in older individuals with chronic diseases. The findings suggest that advancing age, osteoporosis, malnutrition, and fatigue are significant independent risk factors for sarcopenia. Importantly, the study underscores the protective potential of higher serum levels of irisin, 25(OH)D3, and albumin against the development of sarcopenia. The positive correlation of these biomarkers with ASMI and grip strength, integral indicators of sarcopenia severity, further validates their relevance. The composite biomarker test, harnessing the synergistic effects of irisin, 25(OH)D3, and albumin, demonstrated superior diagnostic precision, heralding a promising stride toward an efficacious, non-invasive screening tool for sarcopenia, which could facilitate timely intervention and potentially curb the syndrome progression in this vulnerable population.
Acknowledgements
None.
Author contributions
Yuxia Ma: Writing– original draft, Validation, Data curation, Writing– review & editing, Supervision. Yi Liu: Writing– original draft, Validation, Data curation and analysis, Writing– review & editing. Jiachuang Zheng: Writing– original draft, Validation, Data curation and analysis, Writing– review & editing. Zhixia Zheng: Writing– original draft, Validation, Data curation and analysis, Writing– review & editing. Jingjing Li: Writing– original draft, Validation, Data curation, Writing– review & editing, Supervision.
Funding
This study was supported by Research Topics for Health Education and Promotion Work in Shenzhen in 2024 (SJC202426), and Shenzhen Longgang District Science and Technology Innovation Special Fund Medical and health science and technology project in 2024 (LGWJ2024-7).
Data availability
The raw data could be obtained upon reasonable request to the corresponding author.
Declarations
Ethical approval
The study was approved by the ethics committee of Longgang Central Hospital of Shenzhen, and all participants provided written informed consent.
Informed consent
All patients signed the informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuxia Ma and Yi Liu contributed equally to this work.
Contributor Information
Yuxia Ma, Email: DrMayuxia@foxmail.com.
Jingjing Li, Email: ljj786086840@126.com.
References
- 1.Cruz-Jentoft AJ, Landi F (2014) Sarcopenia. Clin Med (Lond) 14:183–186. 10.7861/clinmedicine.14-2-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santilli V, Bernetti A, Mangone M, Paoloni M (2014) Clinical definition of sarcopenia. Clin Cases Min Bone Metab 11:177–180 [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M (2019) Sarcopenia: Aging-Related loss of muscle mass and function. Physiol Rev 99:427–511. 10.1152/physrev.00061.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan S, Larsson SC (2023) Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism 144:155533. 10.1016/j.metabol.2023.155533 [DOI] [PubMed] [Google Scholar]
- 5.Kitamura A, Seino S, Abe T, Nofuji Y, Yokoyama Y, Amano H, Nishi M, Taniguchi Y, Narita M, Fujiwara Y, Shinkai S (2021) Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle 12:30–38. 10.1002/jcsm.12651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teraz K, Marusic U, Kalc M, Simunic B, Pori P, Grassi B, Lazzer S, Narici MV, Blenkus MG, di Prampero PE, Reggiani C, Passaro A, Biolo G, Gasparini M, Pisot R (2023) Sarcopenia parameters in active older adults - an eight-year longitudinal study. BMC Public Health 23:917. 10.1186/s12889-023-15734-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isaka M, Sugimoto K, Yasunobe Y, Akasaka H, Fujimoto T, Kurinami H, Takeya Y, Yamamoto K, Rakugi H (2019) The usefulness of an alternative diagnostic method for sarcopenia using thickness and echo intensity of lower leg muscles in older males. J Am Med Dir Assoc 20. 10.1016/j.jamda.2019.01.152.:1185 e1181-1185 e1188 [DOI] [PubMed]
- 8.Wang Y, Gu Y, Huang J, Wu H, Meng G, Zhang Q, Liu L, Zhang S, Wang X, Zhang J, Sun S, Wang X, Zhou M, Jia Q, Song K, Huo J, Zhang B, Ding G, Du P, Niu K (2022) Serum vitamin D status and Circulating Irisin levels in older adults with sarcopenia. Front Nutr 9:1051870. 10.3389/fnut.2022.1051870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JS, Kim TH, Nguyen TT, Park KS, Kim N, Kong ID (2017) Circulating Irisin levels as a predictive biomarker for sarcopenia: A cross-sectional community‐based study. Geriatr Gerontol Int 17:2266–2273 [DOI] [PubMed] [Google Scholar]
- 10.Baek JY, Jang I-Y, Jung H-W, Park SJ, Lee JY, Choi E, Lee YS, Lee E, Kim B-J (2022) Serum Irisin level is independent of sarcopenia and related muscle parameters in older adults. Exp Gerontol 162:111744 [DOI] [PubMed] [Google Scholar]
- 11.Silva-Fhon JR, Rojas-Huayta VM, Aparco-Balboa JP, Cespedes-Panduro B, Partezani-Rodrigues RA (2021) Sarcopenia and blood albumin: A systematic review with meta-analysis. Biomedica 41:590–603. 10.7705/biomedica.5765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grygiel-Górniak B, Puszczewicz M (2017) A review on Irisin, a new protagonist that mediates muscle-adipose-bone-neuron connectivity. Eur Rev Med Pharmacol Sci 21 Process:4687–4693. [PubMed]
- 13.Ma C, Ding H, Deng Y, Liu H, Xiong X, Yang Y (2021) Irisin: A new code uncover the relationship of skeletal muscle and cardiovascular health during exercise. Front Physiol 12:620608. 10.3389/fphys.2021.620608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laird E, Ward M, McSorley E, Strain JJ, Wallace J (2010) Vitamin D and bone health: potential mechanisms. Nutrients 2:693–724. 10.3390/nu2070693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasimi N, Dabbaghmanesh MH, Sohrabi Z (2019) Nutritional status and body fat mass: determinants of sarcopenia in community-dwelling older adults. Exp Gerontol 122:67–73. 10.1016/j.exger.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 16.Luo J, Quan Z, Lin S, Cui L (2018) The association between blood concentration of 25- hydroxyvitamin D and sarcopenia: a meta-analysis. Asia Pac J Clin Nutr 27:1258–1270. 10.6133/apjcn.201811_27(6).0013 [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Peng Y, Kong Y, Zhang X, Li Z, Jia H (2025) Circulating Irisin levels in patients with sarcopenia: a systematic review and meta-analysis. Eur Geriatr Med 16:5–13. 10.1007/s41999-024-01097-5 [DOI] [PubMed] [Google Scholar]
- 18.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H (2014) Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc 15:95–101. 10.1016/j.jamda.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 19.Rubenstein LZ, Harker JO, Salva A, Guigoz Y, Vellas B (2001) Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol Biol Sci Med Sci 56:M366–372. 10.1093/gerona/56.6.m366 [DOI] [PubMed] [Google Scholar]
- 20.Wong WS, Fielding R (2010) Construct validity of the Chinese version of the Chalder fatigue scale in a Chinese community sample. J Psychosom Res 68:89–93. 10.1016/j.jpsychores.2009.05.008 [DOI] [PubMed] [Google Scholar]
- 21.Therakomen V, Petchlorlian A, Lakananurak N (2020) Prevalence and risk factors of primary sarcopenia in community-dwelling outpatient elderly: a cross-sectional study. Sci Rep 10:19551. 10.1038/s41598-020-75250-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gungor O, Ulu S, Hasbal NB, Anker SD, Kalantar-Zadeh K (2021) Effects of hormonal changes on sarcopenia in chronic kidney disease: where are we now and what can we do? J cachexia Sarcopenia Muscle 12:1380–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clynes MA, Gregson CL, Bruyere O, Cooper C, Dennison EM (2021) Osteosarcopenia: where osteoporosis and sarcopenia collide. Rheumatology (Oxford) 60:529–537. 10.1093/rheumatology/keaa755 [DOI] [PubMed] [Google Scholar]
- 24.Sjöblom S, Suuronen J, Rikkonen T, Honkanen R, Kröger H, Sirola J (2013) Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas 75:175–180 [DOI] [PubMed] [Google Scholar]
- 25.Gielen E, Dupont J, Dejaeger M, Laurent MR (2023) Sarcopenia, osteoporosis and frailty. Metabolism 145:155638. 10.1016/j.metabol.2023.155638 [DOI] [PubMed] [Google Scholar]
- 26.Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznaric Z, Nair KS, Singer P, Teta D, Tipton K, Calder PC (2014) Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN expert group. Clin Nutr 33:929–936. 10.1016/j.clnu.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, Ding P, Wu H, Yang P, Guo H, Tian Y, Meng L, Zhao Q (2023) Sarcopenia: molecular regulatory network for loss of muscle mass and function. Front Nutr 10:1037200. 10.3389/fnut.2023.1037200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickinson JM, Volpi E, Rasmussen BB (2013) Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc Sport Sci Rev 41:216–223. 10.1097/JES.0b013e3182a4e699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tome D (2017) Muscle protein synthesis and muscle mass in healthy older men. J Nutr 147:2209–2211. 10.3945/jn.117.263491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzan V, Kanat BB, Yavuzer H (2022) Fatigue and primary sarcopenia in geriatric patients. Rev Assoc Med Bras (1992) 68:1565–1570. 10.1590/1806-9282.20220662 [DOI] [PMC free article] [PubMed]
- 31.Vlietstra L, Stebbings S, Meredith-Jones K, Abbott JH, Treharne GJ, Waters DL (2019) Sarcopenia in osteoarthritis and rheumatoid arthritis: the association with self-reported fatigue, physical function and obesity. PLoS ONE 14:e0217462. 10.1371/journal.pone.0217462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sengupta P, Dutta S, Karkada IR, Akhigbe RE, Chinni SV (2021) Irisin, energy homeostasis and male reproduction. Front Physiol 12:746049. 10.3389/fphys.2021.746049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perakakis N, Triantafyllou GA, Fernandez-Real JM, Huh JY, Park KH, Seufert J, Mantzoros CS (2017) Physiology and role of Irisin in glucose homeostasis. Nat Rev Endocrinol 13:324–337. 10.1038/nrendo.2016.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo M, Yao J, Li J, Zhang J, Wang D, Zuo H, Zhang Y, Xu B, Zhong Y, Shen F, Lu J, Ding S, Hu C, Xu L, Xiao J, Ma X (2023) Irisin ameliorates age-associated sarcopenia and metabolic dysfunction. J Cachexia Sarcopenia Muscle 14:391–405. 10.1002/jcsm.13141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rejnmark L (2011) Effects of vitamin d on muscle function and performance: a review of evidence from randomized controlled trials. Ther Adv Chronic Dis 2:25–37. 10.1177/2040622310381934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visser M, Deeg DJ, Lips P (2003) Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the longitudinal aging study Amsterdam. J Clin Endocrinol Metabolism 88:5766–5772 [DOI] [PubMed] [Google Scholar]
- 37.Yang A, Lv Q, Chen F, Wang Y, Liu Y, Shi W, Liu Y, Wang D (2020) The effect of vitamin D on sarcopenia depends on the level of physical activity in older adults. J Cachexia Sarcopenia Muscle 11:678–689. 10.1002/jcsm.12545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keller U (2019) Nutritional laboratory markers in malnutrition. J Clin Med. 10.3390/jcm8060775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isaka M, Sugimoto K, Akasaka H, Yasunobe Y, Takahashi T, Xie K, Onishi Y, Yoshida S, Minami T, Yamamoto K (2022) The muscle thickness assessment using ultrasonography is a useful alternative to skeletal muscle mass by bioelectrical impedance analysis. Clin Interv Aging 17:1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data could be obtained upon reasonable request to the corresponding author.