Abstract
This study presents the design of a gas-generating formulation with a stoichiometric 5-aminotetrazole/sodium periodate. TG-FTIR-MS analysis was used to investigate the thermal decomposition behavior of the gas generator. The thermal kinetic parameters were calculated by non-isothermal kinetic methods to gain insight into the reaction mechanism. The results of this study demonstrate a distinct decomposition process for this gas-generating agent compared to conventional 5AT-based formulations. Specifically, it eliminates the formation of melamine through deamination polymerization and subsequent deamination polymerization processes, directly decomposing into amino cyanic acid (NH2CN), hydrogen cyanide (HCN), ammonia (NH3), and nitrogen gas (N2). Kinetic calculations were performed using model-free methods and model-fitting methods. With the addition of NaIO4, the decomposition activation energy of the first stage was reduced to 180 kJ/mol by 100 kJ/mol, making the decomposition of 5AT easier. The reaction process in the first stage followed a reaction order model of F3/2. The results of this study contribute to better understanding of the thermal kinetics and reaction mechanism of 5AT/NaIO4 system, which is significant for improving the decomposition efficiency of 5AT and for gas generator design.
Subject terms: Catalysis, Energy, Environmental chemistry, Green chemistry, Materials for energy and catalysis, Theory and computation
Introduction
High-nitrogen energetic materials have attracted considerable attention due to their significant potential in gas-generating applications1. Among them, the green energetic material 5-aminotetrazole (5AT) has gradually replaced azides as an important raw material for gas generators today, thanks to its high energy density and low sensitivity requirements for environmental protection and production safety2,3. The 5AT molecule contains multiple high-energy N–N bonds, C–N bonds, and a larger tetrazole ring strain, and it is free of nitro groups. It possesses a high energy density (nitrogen content of 85.35%) and low sensitivity4, good thermal stability, and produces nontoxic gaseous products of nitrogen, carbon dioxide, and water vapor upon complete oxidation, making it a green energetic material5.
5-AT is studied as a burn rate modifier, fire suppressor, green and clean gas-generating agent and propellant6. Studies on the thermal decomposition process of 5AT have shown that the process and gas-generating characteristics of 5AT are mainly influenced by oxidizers, coolants, and their reactions with hydrogen halide salts7. Zhang et al.8 found that the activation energy of 5AT particles decreases with decreasing particle size, indicating increasing thermal sensitivity. Kinetic studies suggest that the decomposition of 5AT follows a third-order reaction kinetics model. In recent years, research on gas-generating formulations based on 5AT as the fuel has been conducted. Poole et al.9 studied a gas-generating formulation based on 5AT/Sr(NO3)2/NaNO3 and found that the addition of SiO2 as an additive improves the gas-generating performance of 5AT. Lund et al.10 further enhanced the gas generation rate by incorporating Zn and Cu as additives. Mei et al.6 evaluated the combustion characteristics of 5AT/KNO3 gas-generating formulations and calculated the reaction activation energy. Cao et al.11 investigated the reaction mechanism during the decomposition of 5AT/Sr(NO3)2 gas-generating formulations by adding coolants Al2O3 and MgO. It can be observed that most gas-generating formulations using 5AT as the raw material utilize nitrates as oxidizers. Although nitrates are non-corrosive and thermally stable in conventional usage12, their use as easily explosive oxidizers has gradually received attention in terms of environmental regulations13.
Sodium periodate possesses advantages such as moisture resistance and non-toxicity13. Its decomposition and involvement in combustion release a large amount of gas14. Additionally, the combustion products are clean and do not contain harmful gases like NO, making it environmentally friendly15. Moretti et al.16 introduced sodium periodate into pyrotechnic formulations to address environmental concerns. Jian et al.17 combined iodates with metals and found that the oxygen released from iodate decomposition positively affects ignition and combustion processes. Considering the above research, sodium periodate, which is green, clean, and exhibits high energy density and gas production, was chosen as the oxidizer component in the gas-generating formulation.
Since the full potential of 5AT as a nitrogen-rich gas-generating agent has not been fully exploited, it is meaningful and necessary to explore an efficient formulation for gas generation. In this work, a novel non-azide, non-toxic gas-generating agent was studied, composed of 5AT and NaIO4. The stoichiometric ratio of 5AT/NaIO4 was designed, and thermal analysis experiments were conducted on the raw materials and mixtures to analyze the weight loss rate during the reaction process. Non-isothermal kinetic studies were performed on the 5AT/NaIO4 mixture at different heating rates. The obtained kinetic parameters such as pre-exponential factor and activation energy have a theoretical guiding role in the design of gas generator based on 5AT/NaIO4. For the first-stage reaction, the most likely reaction model was determined using a model-free method. Combined with TG-FTIR-MS analysis, a decomposition scheme for the first step of the reaction was proposed and a thermal safety analysis was performed. The experimental results contribute to the simulation and understanding of gas-generating behavior and thermal hazards of gas-generating agents, aiding their practical application.
Experimental section
Material preparation
The 5AT and NaIO4 used in this experiment were of reagent grade, purchased from Sinopharm Chemical Reagent Co., Ltd. The gas-producing pharmaceutical powder prepared in the experiment was a mixture of 5AT/NaIO4 in stoichiometric ratio, denoted as sample S1, with the mass composition of 5AT/NaIO4 = 31.22/68.78. The raw material 5AT, due to its large particle size, was first ball-milled for 12 h at 200 r/min in a horizontal planetary ball mill with ethanol as the solvent, and then dried in an oven at 80 °C for 4 h. The powder was sieved through 180 mesh metal sieve. The obtained powder was sieved through 180 mesh and screened with a diameter of 60–80 μm for the preparation of 5AT/NaIO4 mixture with stoichiometric ratio. The mixture was prepared by the same process as the preparation of 5AT. Figure S1 is the schematic diagram of the preparation process of sample S1. The ball milling process resulted in a higher degree of mixing of 5AT with NaIO4.
Sample characterization
The surface morphology of the samples was characterized using a JEOLISM-6380LV scanning electron microscope from Japan Electronics Corporation to observe the particle size and dispersion uniformity of the prepared gas-producing sample S1. During the experiment, a vacuum pressure of 1 Pa and an acceleration voltage of 10 kV were maintained. The GENESIS 2000 X-ray spectrometer from EDAX, USA was used as a complementary equipment to characterize the uniformity of sample dispersion. A FTIR-MS spectrometer (FTIR Nicolet iS20, Thermo Fisher Scientific, Inc, USA and QMS 403, NETZSCH, Germany) was coupled with TG analyzer to track the evolution of volatiles and identify gas product compositions in the range of room temperature to 800 °C. The heating rate was 10 °C/min and the transfer line was heated to 200 °C to prevent gas condensation. The air flow rate was maintained at 60 ml min−1. In order to illustrate the decomposition process of 5AT/NaIO4 more clearly, especially the specifics of the reaction occurring in the first stage, the 5AT/NaIO4 mixture was heated to 200 °C in a muffle furnace and held for 4 h to determine the complete conversion of the reaction. The reaction products at 200 °C were subjected to XRD/FTIR tests and combined with relevant literature studies to deduce the reaction process.
Thermal analysis experiments
The thermal decomposition behavior of the samples was investigated using a thermal analysis instrument. The TG curves of the samples were tested using the HCT-2 microcomputer differential thermal balance (Beijing Heng Jiu Scientific Instruments Factory) measured under air atmosphere. The TG-DTG-DSC curves of the samples were recorded by heating up the samples from room temperature to 900 °C at a rate of 10 °C/min. To minimize the experimental error, each sample was repeated three times under the same conditions.
To systematically analyze the decomposition process of the 5-AT/NaIO4 mixture and perform kinetic calculations, about 3 mg sample S1 was subjected to a thermogravimetric analysis (TGA) at scanning rates of 5, 10, 15, and 20 °C/min over a temperature range from room temperature to 800 °C with the TG-DTG data were recorded. TGA measurements allowed for the recording of weight changes as a function of temperature, while the derivative thermogravimetry (DTG) provided information about the rate at which weight loss occurred. For detailed analysis of the reaction before 200 °C, the samples were heated from room temperature to 200 °C at a constant rate of 1 °C/min. The TGA and DTG data were recorded during this specific heating process. All of the above tests were carried out in an atmospheric environment.
Results
Thermal decomposition of 5AT
The TG-DTG and DSC curves of 5AT under air atmosphere with a heating rate of 10 °C/min are shown in Fig. 1a. From the DTG curve, it can be observed that there are four weight loss reaction stages during the entire decomposition process, which occur at 200–310 °C, 310–400 °C, 400–560 °C, and 560–630 °C, respectively. The thermal decomposition of 5AT begins with an endothermic melting process at 206 °C, followed by weight loss starting from 210 °C. The first decomposition stage reaches its peak weight loss at 223.53 °C, accounting for approximately 58% of the total weight loss. The second decomposition stage occurs between 310 and 400 °C, with a gradual weight loss of about 5%. The third stage takes place between 400 and 560 °C, exhibiting a relatively fast decomposition rate with a weight loss of 25%. Finally, the fourth stage occurs between 560 and 630 °C, resulting in a weight loss of 10%. After the complete pyrolysis process, the residual mass is approximately 2%. These thermal decomposition behaviors of 5AT are consistent with previous reports11. The DSC curve indicates that the thermal decomposition of 5AT is an endothermic process. A weak endothermic peak appears at 198.26 °C, possibly indicating an absorption of heat due to a phase transition before the melting of 5AT18. The DSC curve also shows that the melting enthalpy in the first stage reaches its peak at 210.24 °C, ΔH = − 133.2J/g. Throughout the subsequent decomposition process, there is a slow endothermic decomposition. This may be due to the polymerization of the decomposition product NH2CN to form melamine, followed by continued endothermic decomposition. This means that the decomposition process after 400 °C may be hindered if there is no continuous energy input from the environment11.
Fig. 1.
Thermal analysis data obtained at a scanning rate of 10 °C/min. (a) TG-DTG-DSC curves of 5AT decomposition process. (b) TG-DTG-DSC curves of NaIO4 decomposition process. (c) TG-DTG-DSC curves of sample S1 decomposition process.
Thermal decomposition of NaIO4
The TG-DTG and DSC curves of NaIO4 under air atmosphere with a heating rate of 10 °C/min are shown in Fig. 1b. The DSC curve indicates that the thermal decomposition of NaIO4 consists of both exothermic and endothermic processes. There are four weight loss reaction stages throughout the entire decomposition process, as observed from the DTG curve. The first reaction stage is an exothermic process occurring at 250–308 °C, with a weight loss rate of 13%. The exothermic peak reaches its maximum temperature at 300.01 °C, ΔH = 134.3J/g, which is consistent with previous literature14. The second reaction stage exhibits an endothermic process with a weight loss of 38% in the temperature range of 460–607 °C. The DTG curve shows a double-peaked pattern during this stage, indicating an endothermic reaction. Additionally, the DSC curve shows an endothermic peak at 423.13 °C without any corresponding mass change, which may correspond to a phase transition. The third reaction stage is an endothermic process occurring at 607–730 °C, with a weight loss of 43%. The endothermic peak reaches its maximum temperature at 661.44 °C. Beyond 730 °C, there is a slow endothermic decomposition accompanied by a further weight loss of approximately 4%, attributed to the volatilization of residual NaI. At the end of the heating process, there is a remaining mass of around 2%. Although no specific literature on the thermal decomposition of NaIO4 was found, considering the similarity of Na with K as main group elements, the decomposition process of KIO4 described in reference13 corroborates the findings of this study on the thermal decomposition of NaIO4.
Thermal decomposition of sample S1
The TG-DSC curve of sample S1 at a heating rate of 10 °C/min is shown in Fig. 1c, indicating distinctive thermal decomposition behavior. In contrast to the multi-step decomposition processes observed for the individual raw materials 5AT and NaIO4, the 5AT/NaIO4 mixture (sample S1) designed in this experiment undergoes almost complete decomposition in a single step reaction. The thermal decomposition of the gas-generating agent can be divided into two weight loss stages. The first weight loss stage occurs before 200 °C (approximately 150–190 °C), exhibiting a weight loss of approximately 73%. This stage is accompanied by an exothermic effect, with the exothermic peak reaching its maximum value at 174.06 °C, ΔH = 159.37J/g. The second stage takes place between 450 and 600 °C and involves a slow and continuous decomposition process, resulting in a weight loss of approximately 12%. Subsequently, beyond 650 °C, NaI undergoes melting and gradually loses mass. Throughout the entire reaction process, the total weight loss rate is approximately 95%, with a residual mass of about 5%. After the addition of NaIO4, the decomposition process of 5AT transitions from a four-step process to a single dominant step. The process of monomeric decomposition followed by polymerization to form melamine and other polymers, which occurs in the temperature range of 310–560 °C, is eliminated. This significant improvement in the reaction efficiency of 5AT greatly enhances its overall performance.
Characterization of sample S1
During the thermal decomposition process, a distinct decomposition behavior was observed for sample S1, which differed from the stepwise decomposition processes of 5AT and NaIO4 individually. Instead, it exhibited a significant mass loss during a single-step reaction process before reaching 200 °C, followed by minimal mass loss during the subsequent heating process at 250 °C (200–450 °C). Therefore, an experiment was designed to heat the stoichiometric ratio of 5AT/NaIO4 in a muffle furnace to 200 °C and maintain it for 4 h. The remaining solid residue obtained from this process was then characterized to elucidate the reaction process. The remaining solid residue obtained from the sample is designated as 5AT/NaIO4-200 °C.
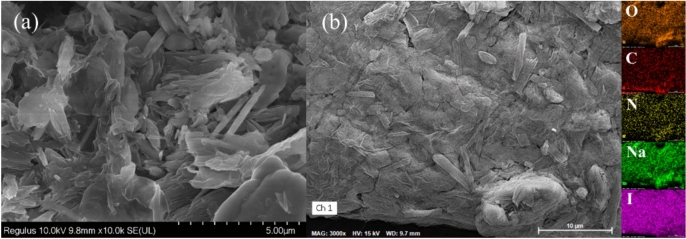
The samples were characterized using a scanning electron microscope. Figure 2a shows that there are smaller-sized flaky substances (approximately 15 μm) as well as polymers formed after melting and solidification. The close bonding indicates that polymers or copolymers were formed during the reaction process before reaching 200 °C. Figure 2b shows the EDS spectrum of O/C/N/Na/I. It can be seen that the compound content of Na/I is relatively high, while the content of C/N is lower. The normalized mass obtained from the spectrum scan also confirms this observation. The C content is 3.92%, N content is 0.37%, O content is 10.15%, Na content is 14.23%, and I content is 71.33%. 5AT decomposes in large quantities in this step of the reaction. Since the N content is almost zero, it can be concluded that 5AT is basically completely decomposed, with only a very small amount remaining as a polymer residue in the solid product. Combined with the thermal decomposition of 5AT and NaIO414,19, it can be inferred that the 5AT in sample S1 was decomposed in one step before 200 °C, resolving the gaseous products NH2CN, HN3, N2, NH3, and HCN, with only a very small portion of NH2CN remaining in the solid product. The addition of NaIO4 accelerated the decomposition process of 5AT, effectively eliminating the step of deamination polymerization during the reaction, transforming the four-step decomposition process of the organic combustible 5AT into a one-step decomposition process.
Fig. 2.
Sample 5AT/NaIO4-200 °C test results. (a) SEM image, (b) EDS energy spectrum scan.
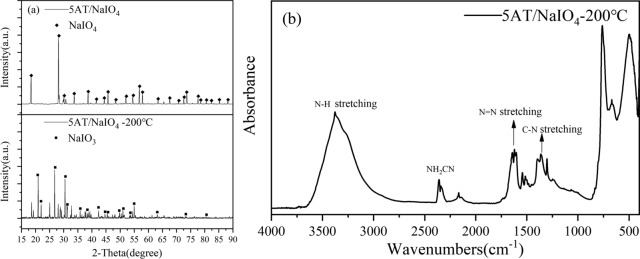
The XRD spectrum of sample S1 and the XRD test results for 5AT/NaIO4-200 °C are shown in Fig. 3a. From the graph, it can be observed that during the first-step reaction, NaIO4 is completely transformed into NaIO3. In the XRD spectrum of 5AT/NaIO4-200 °C, some unknown diffraction peaks at 2θ angles less than 40° may represent a small amount of products formed after the decomposition of the organic compound 5AT or the combination of multiple phases of the products20.
Fig. 3.
Characterization results of mixture sample. (a) XRD spectrum of sample S1 and 5AT/NaIO4-200 °C. (b) FTIR spectra of 5AT/NaIO4-200 °C.
The FTIR spectrum of sample 5AT/NaIO4-200°C is shown in Fig. 3b and the corresponding attribution of the bond peaks are presented in Table 1. The peak in the range of 3100–3500 cm−1 come from isotropic as well as anisotropic stretching vibrations of the N–H groups, and the reason for the large peak area may be due to the superimposition of the N–H stretching vibration peaks of a small amount of decomposition products NH2CN/NHCNH/CH3N3. The stretching vibrational band peak of cyanide group (–CN) often appears around 2240 cm−1, and together with the C–N bond vibrational peak at 1365 cm−1, it can be inferred that NH2–CN is present in the solid product21,22. In fact, the direct product at this stage is HN=C=NH, which is unstable and isomerized to NH2–CN.
Table 1.
Characteristic peaks and their corresponding structures.
| Wavenumber (cm−1) | Groups | Corresponding chemical structure |
|---|---|---|
| 3400–3300 | ν(N–H) | –NH2 |
| 2410–2270 | ν(C = O) | CO2 |
| 2410–2200 | ν(C≡N) | –C≡N |
| 2150–2000 | ν(N = N) | HN3 |
| 1700–1600 | ν(N = N) | –N = N– |
| 1570–1500 | δ(N–H) | –N–H |
| 1365 | ν(C–N) | –C–N |
| 1335–1193 | ν(N–O) | NO2 |
| 1250–1050 | ν(N = N) | HN3 |
| 960 | δ(N–H) | NH3 |
| 720 | δ(C≡N) | HCN |
As shown in Fig. 4, for sample S1, it can be seen from the spectral signal that the main decomposition phase is concentrated in the range of 170–200 °C. During the first step of decomposition, HN3 (2150–2000 cm−1) and -NH2 (3400–3250 cm−1) bands can be observed. The double peak at 3400–3250 cm−1 is considered to be the symmetrical stretching vibration of the primary amine group (–NH2)23. The absorption bands at 2150–2000 cm−1 and 1220–1060 cm−1 belong to asymmetric and symmetric NNN stretching vibrations, respectively24. The spectral band at 2410–2200 cm−1 is associated with the cyano group (–CN). The production of cyano is mainly attributed to HCN produced by pyrolysis of 5AT. It can be speculated that during this stage, 5AT decomposes to produce HCN and NH2CN. During the decomposition process at 170–200 °C, weak NO2 and CO2 peaks can be observed, indicating the occurrence of redox reactions. After the temperature exceeds 200 °C, the spectrum of the precipitated gas is not obvious, indicating that the reaction before 200 °C is the main reaction temperature range of sample S1. During the decomposition of the pure substance 5AT at 400–780 °C, it shows distinct peaks of –CN and –NH225. With the addition of NaIO4, the absorption peaks of –CN and –NH2 are barely visible above 300 °C. It is proved that during the decomposition of sample S1, the monomer NH2CN produced by decomposition did not polymerize to form melamine, but was produced and subsequently decomposed during the first step reaction before 200 °C.
Fig. 4.
FTIR spectra (2D) of sample S1 in the range of room temperature to 800 °C at a heating rate of 10 °C/min.
Figure 5 shows the ion abundance distribution of four specific gas fragments of sample S1 during decomposition. The peaks at m/z = 17, 28, 42 and 43 mainly represent NH3, N2, NH2CN and HN3, respectively. The presence of these gaseous products was further confirmed by MS analysis. A very distinct peak can be observed in the gas-producing agent sample throughout the test, with a concentration distribution of 170–200 °C, which consistent with the temperature range of the IR bond of -NH/-CN/-NN in Fig. 4. The formation time of HN3, NH2CN and HN3 in the mass spectrum is around 170 °C, which is highly consistent with the infrared spectrum, and only occurs in the range of 170–200 °C over the entire test temperature range. The peaks of NH3, N2, NH2CN and HN3 were only observed in the initial phase, indicating that no polymer formation and decomposition occurred during the temperature rise above 300 °C. It can be seen that the gas generator designed in this paper greatly advances the temperature of complete decomposition of 5AT, with almost complete decomposition before 200 °C.
Fig. 5.
Ion abundance of four evolved gas fragments generated during the decomposition of sample S1 (room temperature to 800 °C). The peaks at m/z = 17, 28, 42 and 43 mainly represent NH3, N2, NH2CN and HN3, respectively.
From the above analysis of the condensed phase and gas phase products, it can be speculated that sample S1 firstly undergoes a solid–solid phase pre-reaction at the beginning of the reaction, where O2 is resolved from NaIO4 and energy is released, accelerating the splitting of 5AT. Next, N2, HN3, and NH2CN begin to form due to the destruction of N–N and N–C bonds in the tetrazole ring of CH3N5. Then, the interaction of O2 with the pyrolysis products of 5AT favors redox reactions, accelerates the decomposition of NH2CN, and hinders the formation of melamine. Finally, the C–N and N–H bonds are broken to produce HCN and NH323. The interaction between NaIO4 and the dissociation products of 5AT suggests that NaIO4 has a catalytic effect on the pyrolysis and phase transition of 5AT. The key reason for the dramatic acceleration of the pyrolysis process is the occurrence of redox reactions and the absence of polyaddition reactions.
Calculation of pyrolysis kinetic parameters for sample S1
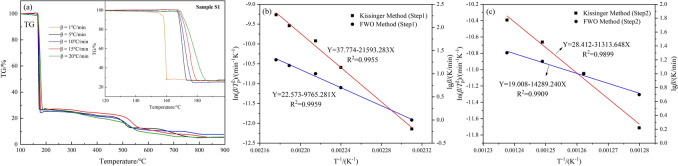
The TG curves of sample S1 obtained at different heating rates are shown in Fig. 6a. The weight loss in the temperature range of 120–200 °C is shown in particular magnification in Fig. 6a. In this study, the activation energy (Eα) and pre-exponential factor (A) of sample S1 were calculated using the Flynn–Wall–Ozawa (FWO) method and Kissinger method, respectively. DTG data for sample S1 and its weight loss rates of TG curves at different scanning rates in each stage were listed in Table 2. The data were plotted with 1/Tp as the x-axis and ln(β/Tp2) or lgβ as the y-axis, and linear regression was performed to obtain the straight lines as shown in Fig. 6b,c. The calculated kinetic parameters are presented in Table 3. The activation energy values obtained from both methods are nearly identical, and the correlation coefficients of the fitted curves are close to 0.99, indicating a good fit. The activation energy of the second-step reaction is significantly higher than that of the first-step reaction, suggesting that the initial stage of the pyrolysis reaction is more prone to occur. Additionally, the first stage accounts for approximately 75% of the mass loss and represents the main decomposition stage of the combustible material. Therefore, a detailed analysis of the first stage of the pyrolysis reaction is required.
Fig. 6.
Results of kinetic analysis of sample S1. (a) TG curves for sample S1 with gradient heating rates. (b) Fitted curves of decomposition kinetics of sample S1 by Kissinger and FWO methods: first step decomposition. (c) Fitted curves of decomposition kinetics of S1 samples by Kissinger and FWO methods: second step decomposition.
Table 2.
DTG data for sample S1 and its weight loss rates of TG curves at different scanning rates in each stage.
| Heating rate β °C/min |
peak temperature Tp1 (step 1) K |
Peak temperature Tp2 (step 2) K |
Step1 weight loss % | Weight loss between step 1 and 2% | Step 2 weight loss % |
Weight loss after step 2% | Residual mass% |
|---|---|---|---|---|---|---|---|
| 1 | 432.94 | – | 72.52 | – | – | – | – |
| 5 | 446.36 | 781.30 | 72.96 | 5.53 | 8.84 | 7.28 | 5.39 |
| 10 | 451.47 | 792.28 | 75.41 | 3.87 | 9.52 | 3.51 | 7.69 |
| 15 | 456.90 | 800.68 | 73.25 | 3.49 | 10.97 | 6.54 | 5.75 |
| 20 | 459.60 | 808.00 | 73.58 | 5.49 | 9.27 | 5.95 | 5.71 |
Table 3.
Kinetic parameters calculated by Kissinger method and FWO method.
| Kinetic parameter | Step 1 | Step 2 | ||
|---|---|---|---|---|
| Kissinger method | FWO method | Kissinger method | FWO method | |
| Ea(kJ/mol) | 179.59 | 177.77 | 260.34 | 260.13 |
| A(min−1) | lnA = 47.75 | lgA = 20.56 | lnA = 38.76 | lgA = 16.83 |
| R2 | 0.9955 | 0.9959 | 0.9899 | 0.9909 |
Model-free analysis for sample S1
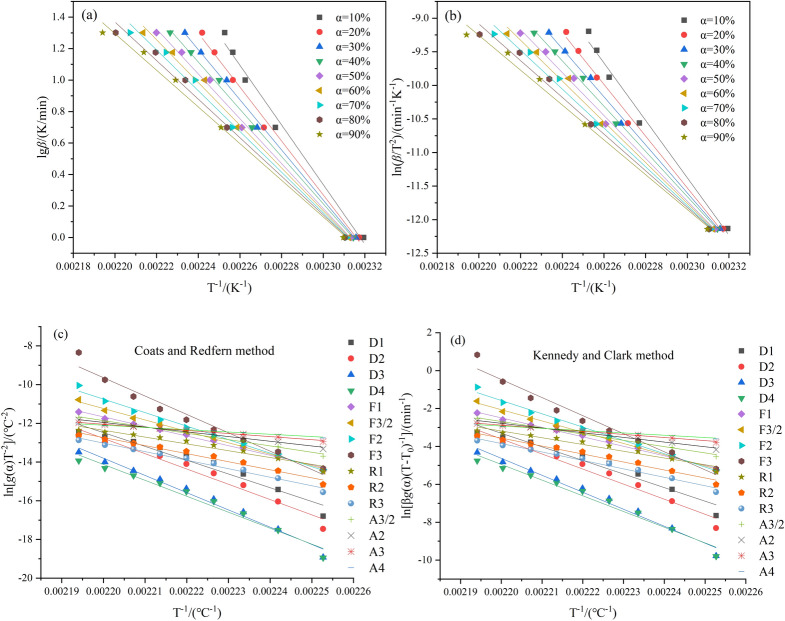
In order to calculate the activation energy at different conversions in the first stage of the pyrolysis process, two different iso-conversional kinetic methods, FWO method and KAS method, were applied and compared in thermal analysis. The fitting curves calculated using the FWO method and KAS method at conversion rates ranging from 0.1 to 0.9 are shown in Fig. 7a,b, respectively. Table 4 records the calculated Eα values and the corresponding correlation coefficients (R2) obtained from both methods. Both the KAS and FWO methods exhibit a similar trend in the average Eα values, which decrease as the conversion rate increases. The average activation energy calculated by the KAS method is 268.96 kJ/mol, while that calculated by the FWO method is 266.35 kJ/mol. These results from the two iso-conversional methods are very close. Except for the data point at α = 0.1, the correlation coefficients of the fitting curves at other conversion rates are above 0.99, confirming the reliability of the Eα calculation. It is worth noting that the highest Eα value is observed at α = 0.1, which may be attributed to the presence of tetrazole rings, leading to relatively lower reaction rates. As the reaction progresses, the temperature increases, and Eα gradually decreases. This is accompanied by the gradual breaking of the tetrazole rings, resulting in the formation of free amino groups (-NH2) and cyanide groups (–CN), further catalyzing the decomposition reaction.
Fig. 7.
Kinetic analysis of sample S1 by model-free and model-fitting methods. (a) Fitted curves at different conversion rates for major weight loss stage: FWO method. (b) Fitted curves at different conversion rates for major weight loss stage: KAS method. (c) CR curves of sample S1 in decomposition step I. (d) KC curves of sample S1 in decomposition step I. The numerical values at different conversion rates for analysis were obtained from the TG curves at a heating rate of 20 °C/min.
Table 4.
Eα and correlation coefficients at different conversion rate.
| α | KAS method | FWO method | ||
|---|---|---|---|---|
| Ea(kJ mol−1) | R2 | Ea(kJ mol−1) | R2 | |
| 0.1 | 351.79 | 0.9870 | 345.04 | 0.9875 |
| 0.2 | 316.66 | 0.9945 | 311.67 | 0.9947 |
| 0.3 | 293.90 | 0.9974 | 290.05 | 0.9975 |
| 0.4 | 276.34 | 0.9975 | 273.35 | 0.9977 |
| 0.5 | 261.58 | 0.9955 | 259.34 | 0.9959 |
| 0.6 | 248.55 | 0.9932 | 246.96 | 0.9936 |
| 0.7 | 236.62 | 0.9923 | 235.62 | 0.9927 |
| 0.8 | 223.38 | 0.9915 | 223.04 | 0.9920 |
| 0.9 | 211.80 | 0.9919 | 212.04 | 0.9925 |
| average | 268.96 | 266.35 | ||
Model fitting analysis for sample S1
After the model-free analysis, the CR and KC methods were introduced to estimate the reaction mechanism function of the first-stage reaction in the 5AT/NaIO4 mixture. The numerical values at different conversion rates for model fitting analysis were obtained from the TG curves at a heating rate of 20 °C/min. Figure 7c shows the CR plots of ln[g(α)/T2] against 1/T, while Fig. 7d presents the KC plots of ln[βg(α)/(T − T0)] against 1/T. The CR method and KC method are single curve methods, which may yield unreliable activation energy values; therefore, no comparison was attempted with the activation energies obtained through these methods. The most probable reaction mechanism function was determined based on the fitting curve with the highest correlation coefficient. Table 5 provides the correlation coefficients (R2) for different models. Clearly, the CR and KC plots based on the F3/2 model exhibit high linear correlation coefficients (R2 = 0.9963 for CR and R2 = 0.9964 for KC). It can be predicted that the decomposition rate in the first stage of sample S1 will be proportional to the concentration of reactants and the cube of the residual reactant.
Table 5.
Correlation coefficient R2 values based on CR and KC methods.
| Model | CR method | KC method |
|---|---|---|
| D1 | 0.9505 | 0.9511 |
| D2 | 0.9675 | 0.9678 |
| D3 | 0.9832 | 0.9834 |
| D4 | 0.9736 | 0.9739 |
| F1 | 0.9923 | 0.9924 |
| F3/2 | 0.9963 | 0.9964 |
| F2 | 0.9905 | 0.9907 |
| F3 | 0.9655 | 0.9658 |
| R1 | 0.9493 | 0.9505 |
| R2 | 0.9762 | 0.9766 |
| R3 | 0.9828 | 0.9832 |
| A3/2 | 0.9921 | 0.9923 |
| A2 | 0.9920 | 0.9922 |
| A3 | 0.9916 | 0.9921 |
| A4 | 0.9913 | 0.9919 |
Studies of thermal safety and thermodynamic parameters
Thermal safety and thermal kinetic parameters are crucial for high-nitrogen energy-containing materials. The activation energy, pre-exponential factor and correlation coefficient of sample S1 obtained by FWO method are listed in Table 6. Compared to the activation energy of the pure substance 5AT during the first step of decomposition, which is 276.4 kJ/mol8, there is a roughly 100 kJ/mol decrease in the activation energy of sample S1, indicating that the system is more reactive compared to the pure substance. Thermal kinetic data obtained by FWO method are used as input parameters here. As the heating rate approaches zero (β → 0), the values of T00, Te0, and Tp0 correspond to the values of T0, Te, and Tp26, which are obtained by linear regression as follows:
 |
1 |
where b, c, d, and e are coefficients. Te0, which equals to self-accelerating decomposition temperature, TSADT, referring to the lowest ambient temperature at which temperature increase of a chemical substance is at least 6°C in a specified commercial package during a period of seven days or less. During storage and handling process, TSADT is crucial for accessing safety management of self-reactive propellants, pyrotechnics, and explosives.
Table 6.
Thermal safety and thermal kinetic parameters of sample S1 based on FWO method.
| Parameters | Sample S1 |
|---|---|
| Ea/(kJ mol−1) | 177.77 |
| lgA/(s−1) | 20.56 |
| R2 | 0.9959 |
| T00/K | 426.25 |
| TSADT/K | 429.52 |
| Tp0/K | 431.74 |
| TTIT/K | 430.60 |
| Tb/K | 433.01 |
| ΔG≠/(kJ mol−1) | 114.94 |
| ΔH≠/(kJ mol−1) | 174.18 |
| ΔS≠/(J mol−1) | 137.21 |
Critical ignition temperature (TTIT) and thermal explosion temperature (Tb) are both vitally pivotal parameters for energetic materials26. There in, Tb is defined as the lowest temperature to which a specific charge might be heated without undergoing thermal run away. TTIT corresponds to the substitution of Ee0 and Te0, while Tb obtained by substituting of Ep0 and Tp0.
 |
2 |
As listed in Table 6, high values of TTIT and Tb represent that the occurrence of transition from thermal degradation to thermal explosion is not easy to happen. However, this temperature is still low compared to 5AT.
Gibbs free energy(ΔG≠), enthalpy(ΔH≠), and entropy of activation (ΔS≠) are critical thermodynamic parameters of activation, which could be obtained by Eqs. (3)–(5) when the values determined at T = Tp0, E = Ek, and A = Ak27,28.
 |
3 |
 |
4 |
 |
5 |
where kB is Boltzmann constant, and h is Plank constant. Computed values of ΔG≠ and ΔH≠ are all positive, indicating that the Sample S1 endothermic decomposition reaction could not proceed without heating, which belongs to the non-spontaneous reaction. It is noted that the temperature range here is less than 200°C. Therefore, attention should be paid to the design of the thermal insulation layer during the application process to prevent possible safety hazards due to temperature increase caused by the external environment.
Discussion
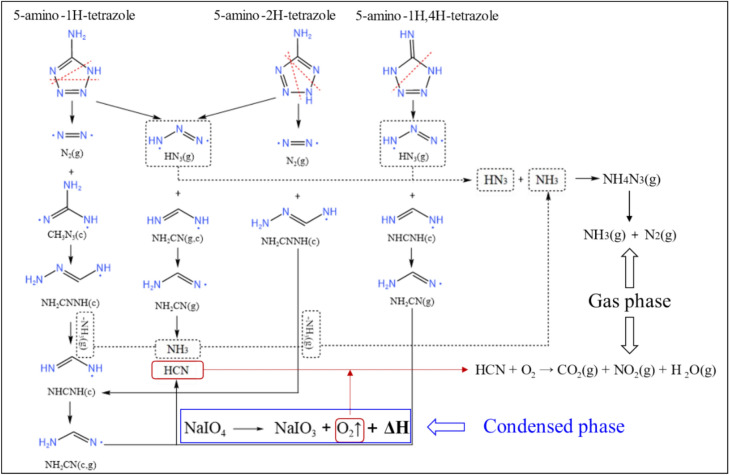
The thermal analysis results reveal that the raw material 5AT undergoes a four-step decomposition process, while NaIO4 undergoes a three-step decomposition process. This is the same as the pyrolysis process of the raw materials documented in references3,11,19,23,25. Reference11 shows that the pure substance 5AT has three structures, named 5-amino-1H-tetrazole, 5-amino-2H-tetrazole and 5-amino-1H,4H-tetrazole, respectively. Regardless of the structure, during the decomposition process, stable small molecule substances will be formed due to the break of N=N bond, the gas evolution of NH3, the rearrangement of CH3N3, NH2CNNH, and NHCNH, etc. Therefore, the substances produced by the decomposition of 5AT are: N2/HCN/NH2CN/NH3/HN3. References11,19,23,25 prove that during the thermal decomposition of 5AT, the polymerization reaction occurs in the second and third stages (310 °C − 590 °C). In the subsequent fourth reaction step, the polymer is decomposed into monomer species HCN/NH3/NH2CN, and the FTIR-MS test results corresponding to this process show the precipitation of NH2CN/HCN/NH3 in the temperature range above 600 °C. For the 5AT/NaIO4 system, the FTIR-MS test results showed that NH2CN/NH3/HCN/HN3 was precipitated during the decomposition process before 200 °C. At the same time, during the decomposition process above 200 °C, there is no characteristic spectral peak corresponding to the above-mentioned substances. Combined with the change of thermogravimetric process, it can be shown that in the decomposition process of the system, 5AT is decomposed into monomer substances in one step, and no polymerization reaction occurs. The novel gas generator designed in this study has greatly altered the decomposition process of 5AT, effectively enhancing its reaction efficiency. The decomposition scheme of sample S1 is shown in Fig. 8. Based on the FTIR-MS analysis and the combined thermal analysis results, the following reaction occurs as the initial step in the first-stage reaction between 5AT and NaIO4.
Fig. 8.
Scheme of the first-step thermal decomposition based on 5AT/NaIO4 gas generator.
Thermal decomposition of NaIO4 produces O2 and releases heat. Under the combined effect of exothermic and heating processes, the tetrazole ring cleaves and produces N2, HN3, CH3N3, NH2CN, NH2CNNH, and NHCNH. Due to the instability of the generated substances (CH3N3, NH2CN, NH2CNNH, and NHCNH), rearrangement forms stable NH2CN and releases NH3. Eventually, a small amount of NH2CN is left in the solid product, and N2, HN3, and NH3 precipitate as gaseous products. As the temperature continues to increase, the C-N bond in NH2CN breaks to form NH3 and HCN. In the gas-phase the decomposition product HN3 further reacts with the decomposition product NH3 of NH2CN11.
 |
6 |
Due to the decomposition of NaIO4, oxygen is released, which further reacts with HCN under heating conditions.
 |
7 |
Reaction (6) continuously consumes the decomposition product NH3 of NH2CN, while reaction (7) continuously consumes the decomposition product HCN of NH2CN. These two reactions facilitate the further decomposition of NH2CN, thereby reducing the likelihood of its polymerization to form the trimeric compound melamine. In the second-stage reaction process, the main reaction is the decomposition of NaIO314.
 |
8 |
 |
9 |
 |
10 |
The activation energy for the first-stage decomposition of sample S1 is approximately 180 kJ/mol, which is significantly lower compared to the activation energy of 276.4 kJ/mol8 for the pure decomposition of 5AT in the first stage. This indicates that the formula of the gas generator designed in this study has a distinct advantage, as it reduces the reaction process of 5AT and makes it more easily triggered. However, its lower initial reaction temperature makes it necessary to pay attention to the design of the insulation layer and the change in ambient temperature during storage.
Conclusion
This study systematically investigated the thermal decomposition kinetics of the 5AT/NaIO4 gas generator. TG-DTG analysis revealed that almost 95% of the mixture was decomposed at all scanning rates, with negligible residual mass. The significant decrease in activation energy highlights the potential value of this formula for gas generation. Model fitting analysis predicted that the decomposition rate of the gas generator in the first stage follows a reaction order model of F2/3. The addition of NaIO4 in the gas formula altered the decomposition pathway of 5AT, leading to nearly complete decomposition in a single step and eliminating the formation of polymers, which is favorable for the combustion of 5AT. The thermal-kinetic analysis provided the kinetic parameters of the 5AT/NaIO4 mixture based on stoichiometry. The experimental results can be used for designing simulation experiments. Based on this formula, environmentally friendly and non-toxic gas generators can be designed that exhibit rapid reactivity and enhance the activity of 5AT. In order to go one step further and quantify the full theoretical parameters of the reaction process, the theoretical foundation should be further deepened using DFT calculations.
Methods
The objective of studying kinetics is to obtain a kinetic model and to calculate the kinetic triple factors A, Eα and f(α). Where f(α) is the differential form of the kinetic reaction mechanism function, which represents the functional relationship between reaction rate and reaction conversion. The integral form of the reaction mechanism function is usually denoted as g(α)29.
According to the Arrhenius equation30, the kinetic equation under non-isothermal conditions can be expressed as Eq. (11):
 |
11 |
where A is the pre-exponential factor, Eα is the activation energy, R is the molar gas constant (8.314 Jmol−1 K−1), T is the thermodynamic temperature, α is the reaction conversion rate, and β is the linear heating rate. In this study, the pyrolysis kinetics of 5AT/NaIO4 will be analyzed using model-free and model fitting methods. The conversion rate α can be defined as Eq. (12)31
 |
12 |
where m0 is the initial mass; mt is the mass at a given temperature; mf is the final mass.
Model-free method
The advantage of the model-free method is that it can bypass the choice of the reaction mechanism function and directly find the reaction activation energy Eα, avoiding the possible errors due to the assumption of the reaction mechanism function.
Kissinger method
The Kissinger method can be expressed as Eq. (13), according to the peak temperature data obtained at different heating rates, make a plot of ln(β/Tp2) versus 1/Tp and fit it linearly to obtain a straight line with slope −E/R and intercept ln(AR/E). Thus, the activation energy Eα and the pre-exponential factor A are obtained32,33.
 |
13 |
Flynn–Wall–Ozawa (FWO) method
The Flynn–Wall–Ozawa (FWO) method is an integral method with an algebraic expression as shown in Eq. (14), which can be used to make a plot of lgβ versus 1/T from the peak temperature data or temperature data obtained at a certain conversion and fit it linearly. Based on a slope of 0.4567E/RT, the activation energy, Eα, is calculated34,35.
 |
14 |
Kissinger–Akahira–Sunose (KAS) method
The Kissinger–Akahira–Sunose (KAS) method is similar to the FWO method and is also an integral method with the algebraic expression shown in Eq. (15). By plotting ln(β/T2) against 1/T from data obtained at a certain conversion rate and fitting it linearly, the activation energy value can be calculated by the slope −Eα/R36.
 |
15 |
Model fitting methods
Coats-Redfern (CR) method
The Coats-Redfern (CR) method is an integral method that can be used for calculations at each heating rate37–40. For each kinetic model given, a plot of ln[g(α)/T2] against 1/T is made and the slope −Eα/R obtained from its linear fit can be calculated to give Eα.
 |
16 |
Kennedy-Clark (KC) method
In the Kennedy Clark (KC) method, considering the heating rate during the experimental process, T0 = 25 °C (298.15 K) is given. For the common kinetic model, Eα can be calculated by plotting ln[βg(α)/(T-T0)] against 1/T for each conversion. And Eα can be calculated from the slope -Eα/R obtained from its linear fit41.
 |
17 |
Supplementary Information
Acknowledgements
This work was supported by the Analysis and Testing Centre Nanjing University of Science & Technology.
Author contributions
C.S. designed the experiment and wrote the manuscript. H.G. provided guidance on the research direction of this study. Z.G., B.Z., Y.L., and J.H. participated in the experimental discussion and the discussion of the results. All the authors contributed to the further revision of this article. All authors reviewed the manuscript.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-00820-x.
References
- 1.Glück, J., Klapötke, T. M. & Küblböck, T. 5-Amino-1 H-tetrazole-based multi-colored smoke signals applying the concept of fuel mixes. New J. Chem.42, 10670–10675. 10.1039/C8NJ01786G (2018). [Google Scholar]
- 2.Miyata, Y., Date, S. & Hasue, K. Combustion mechanism of consolidated mixtures of 5-amino-1H-tetrazole with potassium nitrate or sodium nitrate. Propellants Explos. Pyrotech.29, 247–252. 10.1002/prep.200400048 (2004). [Google Scholar]
- 3.Zhang, D., Lu, S., Gong, L.-L., Cao, C.-Y. & Zhang, H.-P. Effects of calcium carbonate on thermal characteristics, reaction kinetics and combustion behaviors of 5AT/Sr(NO3)2 propellant. Energy Convers. Manag.109, 94–102. 10.1016/j.enconman.2015.11.051 (2016). [Google Scholar]
- 4.Ebeling, H., Schmid, H., Eisenreich, N. & Weiser, V. Development of gas generators for fire extinguishing. Propellants Explos Pyrotech.22, 170–175. 10.1002/prep.19970220314 (1997). [Google Scholar]
- 5.Talawar, M. B. et al. Studies on diaminoglyoxime (DAG): Thermolysis and evaluation as ballistic modifier in double base propellant. J. Hazard. Mater.125, 17–22. 10.1016/j.jhazmat.2005.05.026 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Mei, X., Yang, H., Li, X., Li, Y. & Cheng, Y. The effect of 5-amino-1H-tetrazole on the combustion performance and ignition capability of boron/potassium nitrate igniter. J. Therm. Anal. Calorim.120, 1749–1754. 10.1007/s10973-015-4512-5 (2015). [Google Scholar]
- 7.Brill, T. B. & Ramanathan, H. Thermal decomposition of energetic materials 76: Chemical pathways that control the burning rates of 5-aminotetrazole and its hydrohalide salts. Combust. Flame.122, 165–171. 10.1016/S0010-2180(00)00111-5 (2000). [Google Scholar]
- 8.Zhang, D., Jiang, L., Lu, S., Cao, C.-Y. & Zhang, H.-P. Particle size effects on thermal kinetics and pyrolysis mechanisms of energetic 5-amino-1h-tetrazole. Fuel217, 553–560. 10.1016/j.fuel.2017.12.052 (2018). [Google Scholar]
- 9.Poole, D. R. Azide-free gas generant composition with easily filterable combustion productions. USP: 5035757, 1991-07-30.
- 10.Lund, G. K., Stevens, M. R., Edwards, W. W., et al. Non-azide gas generant formulation, method and apparatus. USP: 005197758, 1993-03-30.
- 11.Cao, C. et al. Investigation on the decomposition mechanism and kinetic behavior of 5-aminotetrazole with metal oxide produced by added coolants. Fuel303, 121315. 10.1016/j.fuel.2021.121315 (2021). [Google Scholar]
- 12.Zhu, C., Wang, J., Xie, W., Zheng, T. & Lv, C. Improving strontium nitrate-based extinguishing aerosol by magnesium powder. Fire Technol.51, 97–107. 10.1007/s10694-013-0361-6 (2015). [Google Scholar]
- 13.Aravind, S. L., Sivapirakasam, S. P., Balasubramanian, K. R. & Surianarayanan, M. Thermo-kinetic studies on azodicarbonamide/potassium periodate airbag gas generants. Process. Saf. Environ. Prot.144, 15–22. 10.1016/j.psep.2020.06.050 (2020). [Google Scholar]
- 14.Wan, Z. et al. Facile production of NaIO4-encapsulated nano-Al microsphere as green primary explosive and its thermodynamic research. Chem. Eng. J.360, 778–787. 10.1016/j.cej.2018.11.215 (2019). [Google Scholar]
- 15.Brusnahan, J. S., Shaw, A. P., Moretti, J. D. & Eck, W. S. Periodates as potential replacements for perchlorates in pyrotechnic compositions. Propellants Explos. Pyrotech.42, 62–70. 10.1002/prep.201600084 (2017). [Google Scholar]
- 16.Moretti, J. D., Sabatini, J. J. & Chen, G. Periodate salts as pyrotechnic oxidizers: Development of barium and perchlorate-ffree incendiary formulations. Angew. Chem. Int. Ed.51, 6981–6983. 10.1002/anie.201202589 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Jian, G., Feng, J., Jacob, R. J., Egan, G. C. & Zachariah, M. R. Super-reactive nano-energetic gas generators based on periodate Salts. Angew. Chem. Int. Ed.52, 9743–9746. 10.1002/anie.201303545 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Lesnikovich, A. I., Printsev, G. V., Ivashkevich, O. A., Lyutsko, V. A. & Kovalenko, K. K. Tetrazole combustion. Combust. Explos. Shock Waves24, 549–551. 10.1007/BF00755492 (1988). [Google Scholar]
- 19.Zhang, D., Jiang, L., Lu, S. & Zhang, H.-P. Insight into cooling agent addition on combustion activity and mechanism of catalyzed 5AT-Sr(NO3)2 based propellant. Combust. Flame196, 407–415. 10.1016/j.combustflame.2018.07.002 (2018). [Google Scholar]
- 20.Chen-guang, Z., Jun, W., Wan-xin, X., Ting-ting, Z. & Chun-xu, L. Improving strontium nitrate-based extinguishing aerosol by magnesium powder. Fire Technol.51, 97–107. 10.1007/s10694-013-0361-6 (2015). [Google Scholar]
- 21.Shuna, Z., Hao, L., Mi, L. & Lin, J. Elucidating the influence of metal oxides on pyrolytic behaviors and combustion performance of green gas generating agent 5-Aminotetrazole/Sr(NO3)2. J. Anal. Appl. Pyrol.179, 106474. 10.1016/j.jaap.2024.106474 (2024). [Google Scholar]
- 22.Siyuan, Z. et al. Catalytic pyrolysis of 5-Amino-1H-Tetrazole with copper and its oxide: A deep insight into the combustion mechanism for high nitrogen compound. Fuel334, 126764. 10.1016/j.fuel.2022.126764 (2023). [Google Scholar]
- 23.Dongsen, Z. et al. Experimental insight into co-pyrolysis mechanism of 5-amino 1H-tetrazole/nitrocellulose based pyrotechnic. J. Anal. Appl. Pyrol.167, 105669. 10.1016/j.jaap.2022.105669 (2022). [Google Scholar]
- 24.Chen, F. F. & Wang, F. Electronic structure of the azide group in 3¢-Azido-3¢-deoxythymidine (AZT) compared to small azide compounds. Molecules14, 2656–2668. 10.3390/molecules14072656 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, D., Cao, C.-Y., Lu, S., Cheng, Y. & Zhang, H.-P. Experimental insight into catalytic mechanism of transition metal oxide nanoparticles on combustion of 5-Amino-1H-Tetrazole energetic propellant by multi kinetics methods and TG-FTIR-MS analysis. Fuel245, 78–88. 10.1016/j.fuel.2019.02.007 (2019). [Google Scholar]
- 26.Pourmortazavi, S. M., Hosseini, S. G., Rahimi-Nasrabadi, M., Hajimirsadeghi, S. S. & Momenian, H. Effect of nitrate content on thermal decomposition of nitrocellulose. J. Hazard Mater.162, 1141–1144. 10.1016/j.jhazmat.2008.05.161 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Riyaz, N. S. & Badran, I. The catalytic thermo-oxidative decomposition of glimepiride using the isoconversional method. J. Therm. Anal. Calorim.147, 10755–10765. 10.1007/s10973-022-11304-9 (2022). [Google Scholar]
- 28.Badran, I., Hassan, A., Manasrah, A. D. & Nassar, N. N. Experimental and theoretical studies on the thermal decomposition of metformin. J. Therm. Anal. Calorim.138, 433–441. 10.1007/s10973-019-08213-9 (2019). [Google Scholar]
- 29.Zhang, J. et al. Pyrolysis characteristics, reaction mechanisms and gas emission of organic fireproof plugging materials by TG-FTIR-MS. J. Therm. Anal. Calorim.148, 12751–12760. 10.1007/s10973-023-12579-2 (2023). [Google Scholar]
- 30.Li, L. et al. Pyrolysis characteristic study on seat hard materials of China’s high-speed train. J. Therm. Anal. Calorim.134, 2107–2113. 10.1007/s10973-018-7757-y (2018). [Google Scholar]
- 31.Badran, I., Manasrah, A. D., Hassan, A. & Nassar, N. N. Kinetic study of the thermo-oxidative decomposition of metformin by isoconversional and theoretical methods. Thermochim. Acta694, 178797. 10.1016/j.tca.2020.178797 (2020). [Google Scholar]
- 32.Šesták, J., Fiala, J. & Gavrichev, K. S. Evaluation of the professional worth of scientific papers, their citation responding and the publication authority. J. Therm. Anal. Calorim.131, 463–471. 10.1007/s10973-017-6178-7 (2018). [Google Scholar]
- 33.Anca-Couce, A., Berger, A. & Zobel, N. How to determine consistent biomass pyrolysis kinetics in a parallel reaction scheme. Fuel123, 230–240. 10.1016/j.fuel.2014.01.014 (2014). [Google Scholar]
- 34.Hasani, S., Shamanian, M., Shafyei, A., Behjati, P. & Szpunar, J. A. Non-isothermal kinetic analysis on the phase transformations of Fe-Co-V alloy. Thermochim. Acta596, 89–97. 10.1016/j.tca.2014.09.020 (2014). [Google Scholar]
- 35.Chen, W. C., Lin, J. R., Liao, M. S., Wang, Y. W. & Shu, C. M. Green approach to evaluating the thermal hazard reaction of peracetic acid through various kinetic methods. J. Therm. Anal. Calorim.127, 1019–1026. 10.1007/s10973-016-5812-0 (2017). [Google Scholar]
- 36.Poletto, M., Zattera, A. J. & Santana, R. M. C. Thermal decomposition of wood: Kinetics and degradation mechanisms. Bioresour. Technol.126, 7–12. 10.1016/j.biortech.2012.08.133 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Ebrahimi-Kahrizsangi, R. & Abbasi, M. H. Evaluation of reliability of Coats-Redfern method for kinetic analysis of non-isothermal TGA. Trans. Nonferrous Met. Soc. China18, 217–221. 10.1016/S1003-6326(08)60039-4 (2008). [Google Scholar]
- 38.Coats, A. & Redfern, J. Kinetic parameters from thermogravimetric data. Nature201, 68–69. 10.1038/201068a0 (1964). [Google Scholar]
- 39.Trache, D., Abdelaziz, A. & Siouani, B. A simple and linear isoconversional method to determine the pre-exponential factors and the mathematical reaction mechanism functions. J. Therm. Anal. Calorim.128, 335–348. 10.1007/s10973-016-5962-0 (2017). [Google Scholar]
- 40.Pourmortazavi, S. M., Mirzajani, V. & Farhadi, K. Thermal behavior and thermokinetic of double-base propellant catalyzed with magnesium oxide nanoparticles. J. Therm. Anal. Calorim.137, 93–104. 10.1007/s10973-018-7904-5 (2019). [Google Scholar]
- 41.Janković, B., Adnađević, B. & Jovanović, J. Application of model-fitting and model-free kinetics to the study of non-isothermal dehydration of equilibrium swollen poly (acrylic acid) hydrogel: Thermogravimetric analysis. Thermochim. Acta452, 106–115. 10.1016/j.tca.2006.07.022 (2007). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.