Abstract
Background
Clinical trials have shown the efficacy of adding CDK4/6 inhibitors to standard endocrine therapy in hormone receptor (HR)-positive, human epidermal growth factor receptor II (HER2)-negative high-risk early breast cancer.
Materials and methods
HR+ /HER2− early breast cancers were retrieved from cancer registry. The primary endpoints were overall survival (OS) and recurrence-free survival (RFS) among trial-defined high-risk patients, as well as the impact of adjuvant chemotherapy.
Results
Among 2758 registered cases, 511 and 1207 patients met MonarchE (M) and NATALEE (N) high-risk criteria, respectively. OS was 94.8% for M-high/N-high, 96.8% for M-low/N-high, 90.7% for M-high/N-low, and 98.9% for M-low/N-low patients, with a hazard ratio (HR) of 2.3 and 2.8 for M-high and N-high, respectively. For RFS, chemotherapy reduced recurrence risk in M-high patients (HR: 0.24) but showed no benefit for N-high patients overall, except for stage III N-high cases (HR: 0.2).
Conclusion
Adjuvant chemotherapy significantly reduced recurrence risk in M-high patients with early breast cancer. Further stratification of M-low/N-high patients is needed to guide tailored chemotherapy approaches alongside CDK4/6 inhibition.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-02601-4.
Keywords: Early breast cancer, CDK4/6 inhibitors, Adjuvant chemotherapy, Risk stratification
Introduction
Cyclin-dependent kinases 4 and 6 (CDK4/6) are key enzymes that regulate cell division. CDK4/6 inhibitors (CDK4/6i) are designed to interrupt cancer cell proliferation by selectively inhibiting these enzymes, leading to cell cycle arrest and subsequent tumor growth inhibition [1]. When combined with endocrine therapy (ET), CDK4/6i enhance the effectiveness of ET and improves outcomes in hormone receptor-positive (HR+)/human epidermal growth factor receptor II-negative (HER2 −) breast cancer [2, 3]. These inhibitors have transformed the treatment landscape for HR+/HER2− breast cancer, demonstrating significant benefits in advanced/metastatic and early-stage settings by addressing treatment resistance and enhancing ET efficacy [1, 4–7].
Despite advances in therapy, patients with HR+ /HER2− early breast cancer (EBC) remain at risk of recurrence even after surgery, adjuvant chemotherapy and ET. Studies report a 3-year recurrence risk of 5–15% depending on lymph node involvement and disease stage [8]. The 5-year recurrence risk is approximately 12% for the overall HR+/HER2− EBC population, and the risk persists long-term, with recurrences occurring steadily up to 20 years post-treatment [9, 10]. Node-positive disease presents a particularly high risk, with 1 in 6 women experiencing recurrence or death within 5 years [9]. Factors such as lymph node involvement, tumor size, grade, and other clinical and pathological characteristics significantly influence recurrence risk [11]. Addressing this persistent risk following curative surgery, adjuvant chemotherapy, and ET is still a critical challenge in the management of HR+/HER2− EBC.
Recent phase III clinical trials, MonarchE and NATALEE, have demonstrated the efficacy of adding CDK4/6i to standard ET in trial-defined high-risk populations [12, 13]. Identifying high-risk patients who may benefit from more aggressive adjuvant therapies is crucial for improving long-term outcomes. Clinical trials like MonarchE and NATALEE have established important criteria for defining high-risk early breast cancer and demonstrated the efficacy of adding CDK4/6i to standard endocrine therapy in these populations. However, questions remain regarding the optimal use of adjuvant chemotherapy in conjunction with CDK4/6i, and the applicability of these trial-derived risk criteria in diverse real-world settings. Therefore, this study aims to utilize real-world data from a single institution in Taiwan to evaluate treatment outcomes for patients meeting the high-risk criteria defined in the MonarchE and NATALEE trials, and to investigate the impact of adjuvant chemotherapy in this patient population.
In this study, we utilized real-world data from a single institution to evaluate treatment outcomes for patients meeting the risk criteria defined in these trials. Additionally, as the necessity of prior chemotherapy before CDK4/6 inhibition remains uncertain, we also investigated the impact of adjuvant chemotherapy in this high-risk population [14].
Materials and methods
This study was reviewed and approved by the Institutional Review Board of Taipei Veterans General Hospital (protocol number: 2024-06-007B), which granted a waiver of informed consent due to the exclusive use of anonymized data.
Patient inclusion/exclusion criteria
This study included women diagnosed with breast cancer at Taipei Veterans General Hospital, a tertiary referral medical center in North Taiwan. Patient data were recorded in the Taiwan Cancer Registry (TCR), which includes a long-form cancer registry [15]. The long-form database provided detailed information, including the date of initial diagnosis, pathology, clinical TNM stage, surgical procedures, treatment dates, and performance status [16, 17]. Since 2011, site-specific factors (SSFs), such as estrogen and progesterone receptor status and axillary lymph node status, have been recorded. The enrollment criteria included patients with stage I to III HR+/HER2− negative EBC. Patients with incomplete follow-up or missing data regarding HR, grade, tumor size or axillary lymph node status were excluded. As Ki-67 status became mandatory only after 2018, additional sources were utilized to obtain missing Ki-67 data for earlier cases. Specifically, local cancer case management system was used to supplement Ki-67 information. Using unique patient IDs, clinical characteristics, treatment patterns, and outcomes were compiled for analysis. Patients with incomplete or unreliable Ki-67 values were excluded.
Risk stratification of HR+/HER2− early breast cancer
Risk stratification was based on criteria from the MonarchE and NATALEE trials. The MonarchE trial defined high-risk HR+/HER2− EBC as node-positive (N+) disease with either four or more positive lymph nodes, or one to three positive nodes combined with at least one risk factor such as tumor size ≥ 5 cm, histologic grade III, or Ki-67 ≥ 20% (Cohort 2) [12, 18]. The NATALEE trial expanded high-risk criteria to include node-negative (N0) patients with grade III disease or grade II disease with a high genomic risk profile (e.g., MammaPrint/Oncotype) or Ki-67 ≥ 20% [19, 20]. Both trials aimed to reduce the risk of recurrence by identifying patients likely to benefit from additional therapy beyond standard ET. These high-risk factors guided the stratification of risk in this study.
Outcome variables
Patients were followed for up to 80 months to compare survival outcomes across risk stratification groups and evaluate the impact of adjuvant chemotherapy. Outcome variables included overall survival (OS) and recurrence-free survival (RFS). OS was defined as the time from diagnosis to death, while RFS was the time from diagnosis to the first recurrence or death. Clinical factors such as cancer stage and grade were included as covariates in multivariable regression models.
Statistical analysis
Categorical variables were summarized as counts and percentages, and their distributions were compared using the Chi-Square test. A two-sided P-value of < 0.05 was considered statistically significant. Survival periods were estimated using the Kaplan–Meier method and compared using the log-rank test. Multivariable analysis was conducted using the Cox proportional hazards model, with statistical significance set at P < 0.05. Survival times beyond 80 months were right-censored.
Results
Risk group distributions
A total of 2758 HR+/HER2− EBC patients with complete clinical data were included in the study. The distribution of pathological stages based on the 8th AJCC staging system was as follows: IA (45.8%, n = 1263), IIA (28.9%, n = 798), IIB (10.8%, n = 297), IIIA (7.5%, n = 207), IIIB (1.1%, n = 30), and IIIC (5.9%, n = 163) [21]. Tumor size distributions were T1 (58%), T2 (35%), T3 (2%), and T4 (1%), while nodal status was N0 (66%), N1 (21%), N2 (7%), and N3 (6%). Among these patients, 18.5% (n = 511) and 43.8% (n = 1207) met the high-risk criteria for MonarchE and NATALEE trials, respectively.
Overall survival
The mean OS was 5.6 years (SD: 0.4) for NATALEE high-risk (N-H) patients and 6.7 years (SD: 0.1) for NATALEE low-risk (N-L) patients, with the median survival not reached. At 8 years, the OS rate was 96% for N-H patients and 98.7% for N-L patients, with a hazard ratio (HR) of 2.3 (95% CI 1.4–3.8) for N-H.
For MonarchE high-risk (M-H) patients, the mean survival was 5.3 years (SD: 0.1) compared to 6.7 years (SD: 0) for MonarchE low-risk (M-L) patients. The HR for M-H patients was 2.8 (95% CI 1.8–4.5) compared to the M-L group. Figure 1A and B illustrate OS stratified by both trial criteria, demonstrating strong prognostic value in the Taiwanese population.
Fig. 1.
A, B Overall survival of the high- and low-risk groups defined by the NATALEE (A) and MonarchE (B) trials. The defined risk groups show a significant overall survival discrepancy (log-rank test: P = 0.001 and P < 0.001 for the NATALEE and MonarchE risk definition, os1: overall survival, Y-axis: survival time in months)
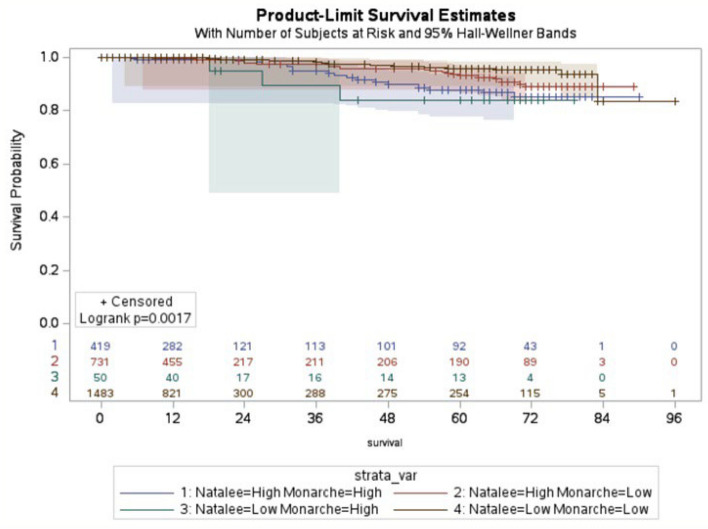
When stratifying patients by both trial risk groups, 16% (n = 457) were N-H/M-H, 26% (n = 750) were N-H/M-L, 2% (n = 53) were N-L/M-H, and 56% (n = 1584) were N-L/M-L. The corresponding 8-year OS rates were 94.8%, 96.8%, 90.6%, and 98.9%, with mean survival times of 5.4, 5.7, 3.1, and 6.7 years, respectively (Fig. 2, median not reached).
Fig. 2.
Overall survival for breast cancer risk groups defined by the NATALEE (N) and MonarchE (M) trial criteria. Patients were categorized into N-H/M-H, N-H/M-L, N-L/M-H, and N-L/M-L risk groups (log-rank test: P < 0.001, os1: overall survival, Y-axis: survival time in months)
The proportion of patients receiving adjuvant chemotherapy increased with stage: 34.9% for stage I, 71.9% for stage II, and 96.1% for stage III. Adjuvant chemotherapy improved OS for N-H patients from 93.4% to 96.6%, though this was not statistically significant (log-rank test: P = 0.09). For M-H patients, chemotherapy significantly improved OS from 71.4% to 95.5% (log-rank test: P < 0.001; Fig. 3A and B).
Fig. 3.
A, B Overall survival of the high-risk breast cancers defined by the NALATEE (A) and the MonarchE trial (B) with and without chemotherapy (ch_m: chemotherapy, os1: overall survival, Y-axis: survival time in months)
Recurrence-free survival
Pathological stage was a key prognostic factor for recurrence-free survival (RFS): HR for stage II vs I was 2.0 (95% CI 1.2–3.3), and HR for stage III vs I was 5.4 (95% CI 3.2–9.0; Supplementary Fig. 1). The 8-year recurrence-free rates were 98.2%, 96.3%, and 90.2% for stages I, II, and III, respectively. For N–H/M-H, N–H/M-L, N-L/M-H, and N-L/M-L patients, the 8-year RFS rates were 95.5%, 96.9%, 94%, and 98.9%, with mean RFS of 5.4, 5.7, 3.2, and 6.7 years, respectively (Fig. 4). The median RFS was not reached.
Fig. 4.
Recurrence-free survival for breast cancer risk groups defined by the NATALEE (N) and the MonarchE (M) trial criteria. Patients were categorized into N-H/M-H, N-H/M-L, N-L/M-H, and N-L/M-L risk groups (log-rank test: P = 0.017, Y-axis: survival time in months)
Adjuvant chemotherapy had a significant impact on M-H patients, reducing the HR for recurrence to 0.2 (95% CI 0.1–0.5, P = 0.0002) and improving the recurrence-free rate from 81.3% to 93.3%. However, for N-H patients, the HR was 1.0 (95% CI 0.6–1.7), with recurrence-free rates of 94.5% with chemotherapy and 96% without. A post-hoc subgroup analysis revealed that only stage III N-H patients derived significant benefit from chemotherapy, with an HR of 0.2 (95% CI 0.1–0.7) and an improvement in recurrence-free rate from 72.7% to 92.4% (log-rank test: P = 0.004). Supplementary Table 1 summarizes main survival outcomes and key findings for clarity and accessibility.
Discussion
The clinical application of CDK4/6i has expanded significantly from their initial use in advanced/metastatic breast cancers to early-stage disease settings. Initially, CDK4/6i was used to manage HR+/HER2− metastatic breast cancer, where they demonstrated a remarkable ability to halt disease progression and prolong survival when combined with ET [22]. More recently, their use has extended to EBC, particularly in the adjuvant setting, to prevent recurrence by targeting residual microscopic disease. In these settings, CDK4/6i complement standard therapies to improve long-term outcomes. Clinical trials have provided strong evidence for their effectiveness in reducing relapse risk in patients with high-risk EBC [23].
In this study, we evaluated OS and RFS among a cohort of Taiwanese HR+/HER2− EBC patients treated with contemporary therapies. Among these patients, 18% met the M-H criteria, while 42% were categorized as N-H. Survival discrepancies were observed between the defined risk groups, with hazard ratios of 2.3 and 2.8, respectively, indicating the prognostic power of these stratifications. Our findings suggest that more than 40% of Taiwanese high-risk HR+/HER2− EBC patients could benefit from adjuvant CDK4/6i. The higher hazard ratio for the M-H group highlights a relatively higher baseline risk defined by the MonarchE criteria compared to NATALEE criteria.
When combining both criteria, we found that 56% of patients were categorized as low risk, 16% as concurrently high risk (N-H/M-H), and 28% as discordant risk (e.g., N-H/M-L or N-L/M-H). The worst overall survival was observed in the N-L/M-H subgroup (2%), while M-H patients consistently exhibited poorer survival outcomes regardless of NATALEE-defined risk. These results underscore the robust prognostic power of the MonarchE criteria, which may utilize a stricter risk definition. On the other hand, pT1N1micro (stage IB) patients, once with grade III or Ki67 ≥ 20% status, were destined to be N-L/M-H, while this worst minority deserves further evaluation with more cased enrolled.
It is widely accepted that adjuvant chemotherapy should generally precede the use of CDK4/6i in high-risk HR+/HER2− EBC. Chemotherapy addresses aggressive disease features, while CDK4/6i, such as abemaciclib or ribociclib, in combination with ET, suppresses residual cancer proliferation to reduce relapse risk [13, 24, 25]. Despite the better tolerability of CDK4/6i compared to chemotherapy, they cannot yet fully replace chemotherapy in the adjuvant setting for high-risk patients. For M-H patients in our study, chemotherapy significantly improved overall survival from 71.4% to 95.5%. Conversely, for N-H patients, the survival benefit was modest and statistically nonsignificant (93.4% to 96.6%), further underscoring the elevated baseline risk in M-H patients.
Analysis of RFS patterns revealed that the worst outcomes were observed in the N-L/M-H subgroup (94%), followed by N-H/M-H (95.5%). In contrast, M-L patients demonstrated better recurrence-free survival regardless of NATALEE risk status (96.9%-98.9%). These findings suggest that the MonarchE criteria may offer a more robust framework for risk stratification in Taiwanese HR+/HER2− EBC patients. Adjuvant chemotherapy remains crucial for M-H patients due to their high risk of recurrence and death, which can be improved significantly with chemotherapy. However, for some N-H patients, chemotherapy may be omitted without significantly compromising outcomes. For example, more N-H patients remained recurrence-free without chemotherapy compared to M-H patients who received chemotherapy (94.5% vs. 81.3%).
A post-hoc analysis revealed that, within the N-H group, only pathological stage III patients benefited significantly from chemotherapy, with RFS improving from 81.3% to 93.3% (hazard ratio: 0.2). Pathological stage remains a key prognostic factor for recurrence, with survival rates decreasing from 98.2% (stage I) to 90.2% (stage III). Chemotherapy was commonly administered across stages I, II, and III (34.9%, 71.9%, and 96.1%, respectively). Among stage III patients classified as both N-H and M-H, chemotherapy improved recurrence-free survival from 78.6% to 94% (hazard ratio: 0.2; 95% CI 0.1–0.5; P = 0.009), though the primary driver of this benefit may have been the M-H population.
Chemotherapy remains indispensable for addressing micrometastatic disease, especially in patients with high-risk features. CDK4/6i is increasingly used in adjuvant therapy, often following chemotherapy, to improve disease control while minimizing the adverse effects typically associated with chemotherapy [5]. Although CDK4/6i offers a favorable toxicity profile, it currently serves as a complementary rather than a replacement therapy in adjuvant settings. In addition, higher serum levels of trace elements like selenium and zinc are associated with improved cancer survival, including in breast cancer, due to their roles in immune function, antioxidant defense, and apoptosis. However, both deficiencies and excesses can be harmful, highlighting the need for balanced levels [26].
The NATALEE and MonarchE trials used different criteria for defining high-risk HR+/HER2− EBC patients. The M-H group consistently demonstrated elevated recurrence rates, underscoring the necessity of adjuvant chemotherapy. The N-H criteria, which target a broader population, often include M-H patients, though the reverse is not always true. Multi-gene expression assays, such as Oncotype DX and Mammaprint, further inform risk stratification by providing insights into recurrence risk and chemotherapy benefit [27–32]. These tests may refine treatment plans, particularly in the context of CDK4/6i, potentially sparing some patients from unnecessary chemotherapy. Our study suggests that all M-H and stage III N-H patients should receive chemotherapy before initiating CDK4/6i.
To explore why MonarchE criteria predict poorer outcomes compared to NATALEE, a more detailed analysis is needed, focusing on the nuanced differences in risk factor definitions between the trials (as shown in Table 1). This should involve a granular breakdown of individual risk factors within each cohort, such as the compositions of specific nodal involvement, tumor size, grade, and Ki-67 index. MonarchE focuses heavily on nodal status and tumor size in conjunction with other factors while NATALEE incorporates node-negative patients with specific high-risk features like high genomic risk. Consequently, an inherited higher risk is prominent for MonarchE compared to NATALEE criteria.
Table 1.
Defined high-risk patients between clinical trials with CDK4/6 inhibitor for early hormone receptor (HR)+/human epidermal growth factor receptor II (HER2)-breast cancers
| Criteria | Natalee trial | MonarchE trial |
|---|---|---|
| Nodal status | Node-negative (N0) with high-risk features, or node-positive | 4 or more positive lymph nodes, or 1–3 positive lymph nodes with additional high-risk features |
| Tumor size | Not specified | Tumor size ≥ 5 cm for patients with 1–3 positive lymph nodes, or tumor size < 5 cm with additional high-risk features |
| Grade | N0 with Grade 2 and high-risk features (e.g., Ki67 ≥ 20%, or high genomic risk profile, or Oncotype Dx ≥ 26) or N0 with Grade 3 | Grade 3 for patients with 1–3 positive lymph nodes, or Grade < 3 with additional high-risk features |
| Ki67 Index | Ki67 ≥ 20% for node-negative patients with Grade 2 | Ki67 ≥ 20% for patients with 1–3 positive lymph nodes, or Ki67 < 20% with additional high-risk features |
| Genomic risk profile | High genomic risk profile (e.g., Oncotype DX ≥ 26) for node-negative patients with Grade 2 | Not specified |
This study is novel in its use of real-world data from a Taiwanese institution to compare the prognostic value of MonarchE and NATALEE trial criteria in HR+/HER2− early breast cancer patients, evaluating the impact of adjuvant chemotherapy and providing a detailed stratification of survival outcomes across combined risk groups; importantly it also performs a post-hoc analysis that reveals stage III N-high patients benefit from chemotherapy.
Limitations
This study utilized cancer registry data to provide real-world evidence, but its retrospective design was associated with potential limitations, such as selection bias, loss to follow-up, and data inaccuracies. While Taiwan’s cancer registry is recognized for its high reliability, with an accuracy rate exceeding 95% [33], the follow-up period (8 years) may be insufficient to fully assess late recurrences, a common concern in HR+/HER2− EBC [16, 34, 35]. The results were drawn from a single institution in Taiwan, which may limit their applicability to broader, ethnically diverse populations. Additionally, the study period predated the introduction of CDK4/6i, preventing an evaluation of its interaction with chemotherapy.
Conclusion
Using a single-institution’s cancer registry for Taiwanese HR+/HER2− early breast cancers, we demonstrated that both the NATALEE and MonarchE high-risk criteria were prognostic. Adjuvant chemotherapy significantly reduced recurrence and mortality risks among M-H patients. For the N-H high-risk population, only those with pathological stage III benefited from chemotherapy. Further studies are needed to explore the interplay between chemotherapy and CDK4/6i in high-risk populations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wound like to thank the Taiwan Clinical Oncology Research Foundation, Melissa Lee Cancer Foundation, and Dr. Morris Chang for their kind assistance during the study.
Author contributions
Ta-Chung Chao: conceptualization; writing—original draft. Deanna Gracia, Chan-Heng Ho and Hao-Yang Chen: data curation; formal analysis; investigation. Ling-Ming Tseng: funding acquisition; investigation. Chi-Cheng Huang: conceptualization; formal analysis; methodology; supervision.
Funding
This work was supported in part by VGH-TPE (V114C-030).
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethical approval
The entire study protocol has been reviewed and approved by the Institute Review Board of Taipei Veterans General Hospital. Informed consent was waived as only anonymized cancer registry data were used.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chi-Cheng Huang, Email: chishenh74@gmail.com.
Ling-Ming Tseng, Email: lmtseng87@gmail.com.
References
- 1.Purohit L, Jones C, Gonzalez T, Castrellon A, Hussein A. The role of CD4/6 inhibitors in breast cancer treatment. Int J Mol Sci. 2024;25(2):1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barroso-Sousa R, Shapiro GI, Tolaney SM. Clinical development of the CDK4/6 inhibitors ribociclib and abemaciclib in breast cancer. Breast Care. 2016;11(3):167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwapisz D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib. Breast Cancer Res Treat. 2017;166(1):41–54. [DOI] [PubMed] [Google Scholar]
- 4.Fontanella C, Giorgi CA, Russo S, Angelini S, Nicolardi L, Giarratano T, Frezzini S, Pestrin M, Palleschi D, Bolzonello S, Parolin V, Haspinger ER, De Rossi C, Greco F, Gerratana L. Optimizing CDK4/6 inhibitors in advanced HR+/HER2- breast cancer: a personalized approach. Crit Rev Oncol Hematol. 2022;180: 103848. [DOI] [PubMed] [Google Scholar]
- 5.O’Sullivan CC, Suman VJ, Goetz MP. The emerging role of CDK4/6i in HER2-positive breast cancer. Ther Adv Med Oncol. 2019;27(11):1758835919887665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo T, Zhu K, Zhong X, He P, Yan X, Tian T. CDK4/6 inhibitors for primary endocrine resistant HR-positive/HER2-negative metastatic breast cancer: a case report. Transl Breast Cancer Res. 2023;7(4):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curigliano G, et al. Short-term risk of recurrence in patients (pts) with HR+/HER2− early breast cancer (EBC) treated with endocrine therapy (ET) in randomized clinical trials (RCTs): a meta-analysis. JCO. 2024;42:541–541. [Google Scholar]
- 8.Curigliano G, Ciruelos E, Kalinsky K, Proudman D, Nellesen D, Lopez P, Kaufhold S, Shane O, Kwok A, Ganapathy V, Amefule AQ, Lteif A, Fasching PA, Hamilton EP. Short-term risk of recurrence in patients (pts) with HR+/HER2− early breast cancer (EBC) treated with endocrine therapy (ET) in randomized clinical trials (RCTs): a meta-analysis. J Clin Oncol. 2024;42(16_suppl):541. [Google Scholar]
- 9.Salvo EM, Ramirez AO, Cueto J, Law EH, Situ A, Cameron C, Samjoo IA. Risk of recurrence among patients with HR-positive, HER2-negative, early breast cancer receiving adjuvant endocrine therapy: a systematic review and meta-analysis. Breast. 2021;57:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M, Hayes DF. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Shaughnessy J, Yardley D, Hart L, Razavi P, Graff SL, Wogen J, McDermott C, Dionne PA, Haftchenary S, Pathak P, Tolaney S. Abstract P3–03–12: Risk of recurrence with adjuvant endocrine therapy in real world patients with hormone receptor positive/human epidermal growth factor receptor-negative early breast cancer: a US database analysis. Cancer Res. 2023;83(5_Supplement):P3-03. [Google Scholar]
- 12.Johnston SRD, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, Zhang QY, Martinez Rodriguez JL, Campone M, Hamilton E, Sohn J, Guarneri V, Okada M, Boyle F, Neven P, Cortés J, Huober J, Wardley A, Tolaney SM, Cicin I, Smith IC, Frenzel M, Headley D, Wei R, San Antonio B, Hulstijn M, Cox J, O’Shaughnessy J, Rastogi P, monarchE Committee Members and Investigators. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman RA, Caswell-Jin JL, Hassett M, Somerfield MR, Giordano SH, Optimal Adjuvant Chemotherapy and Targeted Therapy for Early Breast Cancer Guideline Expert Panel. Optimal adjuvant chemotherapy and targeted therapy for early breast cancer-cyclin-dependent kinase 4 and 6 inhibitors: ASCO guideline rapid recommendation update. J Clin Oncol. 2024;42(18):2233–5. [DOI] [PubMed] [Google Scholar]
- 14.Johnston SRD. Adjuvant CDK4/6 inhibitors: can they improve clinical outcomes in hormone receptor-positive (HR+) early breast cancer? Ann Palliat Med. 2023;12(2):421–6. [DOI] [PubMed] [Google Scholar]
- 15.Chiang CJ, Wang YW, Lee WC. Taiwan’s nationwide cancer registry system of 40 years: past, present, and future. J Formos Med Assoc. 2019;118:856–8. [DOI] [PubMed] [Google Scholar]
- 16.Kao CW, Chiang CJ, Lin LJ, Huang CW, Lee WC, Lee MY, Taiwan Society of Cancer Registry Expert Group, Senior Cancer Registrars. Accuracy of long-form data in the Taiwan cancer registry. J Formos Med Assoc. 2021;120:2037–41. [DOI] [PubMed] [Google Scholar]
- 17.Chiang CJ, You SL, Chen CJ, Yang YW, Lo WC, Lai MS. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jpn J Clin Oncol. 2015;45:291–6. [DOI] [PubMed] [Google Scholar]
- 18.Johnston SRD, Toi M, O’Shaughnessy J, Rastogi P, Campone M, Neven P, Huang CS, Huober J, Jaliffe GG, Cicin I, Tolaney SM, Goetz MP, Rugo HS, Senkus E, Testa L, Del Mastro L, Shimizu C, Wei R, Shahir A, Munoz M, San Antonio B, André V, Harbeck N, Martin M, monarchE Committee Members. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24(1):77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slamon DJ, Fasching PA, Hurvitz S, Chia S, Crown J, Martín M, Barrios CH, Bardia A, Im SA, Yardley DA, Untch M, Huang CS, Stroyakovskiy D, Xu B, Moroose RL, Loi S, Visco F, Bee-Munteanu V, Afenjar K, Fresco R, Taran T, Chakravartty A, Zarate JP, Lteif A, Hortobagyi GN. Rationale and trial design of NATALEE: a Phase III trial of adjuvant ribociclib + endocrine therapy versus endocrine therapy alone in patients with HR+/HER2- early breast cancer. Ther Adv Med Oncol. 2023;29(15):17588359231178124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yardley DA, Untch M, Barrios CH, Bardia A, Kalinsky K, Im S, Chia SKL, Ring AE, McAndrew NP, Ruiz-Borrego VVM, Fresco R, Sum E, Chattar Y, Waters S, Harbeck N. Baseline (BL) characteristics and efficacy endpoints for patients (pts) with node-negative (N0) HR+/HER2− early breast cancer (EBC): NATALEE trial. Journal of Clinical Oncology 2024;16_suppl, 512.
- 21.Giuliano AE, Edge SB, Hortobagyi GN. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol. 2018;25:1783–5. [DOI] [PubMed] [Google Scholar]
- 22.Pavlovic D, Niciforovic D, Papic D, Milojevic K, Markovic M. CDK4/6 inhibitors: basics, pros, and major cons in breast cancer treatment with specific regard to cardiotoxicity - a narrative review. Ther Adv Med Oncol. 2023;15:17588359231205848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gil-Gil M, Alba E, Gavilá J, de la Haba-Rodríguez J, Ciruelos E, Tolosa P, Candini D, Llombart-Cussac A. The role of CDK4/6 inhibitors in early breast cancer. Breast. 2021;58:160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agostinetto E, Arecco L, de Azambuja E. Adjuvant CDK4/6 inhibitors for early breast cancer: how to choose wisely? Oncol Ther. 2024;12:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanciu IM, Parosanu AI, Nitipir C. An overview of the safety profile and clinical impact of CDK4/6 inhibitors in breast cancer-a systematic review of randomized phase II and III clinical trials. Biomolecules. 2023;13:1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lubiński J, Lener MR, Marciniak W, Pietrzak S, Derkacz R, Cybulski C, Gronwald J, Dębniak T, Jakubowska A, Huzarski T, Matuszczak M, Pullella K, Sun P, Narod SA. Serum essential elements and survival after cancer diagnosis. Nutrients. 2023;15(11):2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markopoulos C, Hyams DM, Gomez HL, Harries M, Nakamura S, Traina T, Katz A. Multigene assays in early breast cancer: insights from recent phase 3 studies. Eur J Surg Oncol. 2020;46(4 Pt A):656–66. [DOI] [PubMed] [Google Scholar]
- 28.Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga JY, Brain E, Causeret S, DeLorenzi M, Glas AM, Golfinopoulos V, Goulioti T, Knox S, Matos E, Meulemans B, Neijenhuis PA, Nitz U, Passalacqua R, Ravdin P, Rubio IT, Saghatchian M, Smilde TJ, Sotiriou C, Stork L, Straehle C, Thomas G, Thompson AM, van der Hoeven JM, Vuylsteke P, Bernards R, Tryfonidis K, Rutgers E, Piccart M. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375(8):717–29. [DOI] [PubMed] [Google Scholar]
- 29.Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, Lin NU, Perez EA, Goldstein LJ, Chia SKL, Dhesy-Thind S, Rastogi P, Alba E, Delaloge S, Martin M, Kelly CM, Ruiz-Borrego M, Gil-Gil M, Arce-Salinas CH, Brain EGC, Lee ES, Pierga JY, Bermejo B, Ramos-Vazquez M, Jung KH, Ferrero JM, Schott AF, Shak S, Sharma P, Lew DL, Miao J, Tripathy D, Pusztai L, Hortobagyi GN. 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021;385(25):2336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbi M, Makower D, Sparano JA. The clinical utility of gene expression assays in breast cancer patients with 0–3 involved lymph nodes. Ther Adv Med Oncol. 2021;14(13):17588359211038468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blok EJ, Bastiaannet E, van den Hout WB, Liefers GJ, Smit VTHBM, Kroep JR, van de Velde CJH. Systematic review of the clinical and economic value of gene expression profiles for invasive early breast cancer available in Europe. Cancer Treat Rev. 2018;62:74–90. [DOI] [PubMed] [Google Scholar]
- 32.Mittendorf EA, King TA, Tolaney SM. Impact of RxPONDER and monarchE on the surgical management of the axilla in patients with breast cancer. J Clin Oncol. 2022;40(29):3361–4. [DOI] [PubMed] [Google Scholar]
- 33.Huang YC, Chen YH. Cancer incidence characteristic evolution based on the national cancer registry in Taiwan. J Oncol. 2020;22(2020):1408793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito M, Amari M, Sato A, Hikichi M, Sakamoto A, Yamazaki A, Saji S. Risk factors for late recurrence and postrelapse survival in estrogen receptor (ER)-positive, human epidermal growth factor receptor (HER) 2-negative breast cancer after 5 years of endocrine therapy. Breast. 2024;73: 103604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamashita H, Ogiya A, Shien T, Horimoto Y, Masuda N, Inao T, Osako T, Takahashi M, Endo Y, Hosoda M, Ishida N, Horii R, Yamazaki K, Miyoshi Y, Yasojima H, Tomioka N, Collaborative Study Group of Scientific Research of the Japanese Breast Cancer Society. Clinicopathological factors predicting early and late distant recurrence in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer. 2016;23(6):830–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].