Abstract
Silver(I) phosphine complexes have attracted significant attention recently due to their structural versatility and promising anticancer properties. These complexes exhibit diverse coordination geometries—ranging from tetrahedral and trigonal planar to linear—depending on the ligand environment and metal-to-ligand ratio, directly influencing their biological activity. Notably, they demonstrate substantial cytotoxicity against various cancer cell lines, including oesophageal (SNO), breast (MCF-7), and lung (A549) cancers, with IC₅₀ values in the low micromolar range. A key advantage of these complexes is their selective toxicity toward malignant cells while sparing healthy ones, positioning them as potential alternatives to traditional chemotherapeutics like cisplatin, often associated with severe side effects and drug resistance. The anticancer mechanism of silver(I) phosphine complexes primarily involves apoptosis induction through mitochondrial disruption, phosphatidylserine externalisation, and caspase activation. Additionally, these complexes can overcome common resistance mechanisms encountered in conventional cancer treatments by targeting alternative cellular pathways. This review critically evaluates the structural chemistry, synthesis, and characterisation of silver(I) phosphine complexes and recent advancements in their biological applications. Furthermore, we discuss their potential to address critical limitations in cancer therapies, particularly in overcoming drug resistance and toxicity, while exploring opportunities for ligand optimisation and progress toward clinical applications.
Keywords: Silver(I) phosphine complexes, Apoptosis, Cancer therapy, Structural chemistry, Chemotherapy resistance, Anticancer agents
Introduction
Cancer remains a major global health concern, responsible for over 19 million new cases and 10 million deaths in 2020 alone [1]. While conventional therapies such as chemotherapy, radiotherapy, and immunotherapy have improved patient outcomes, these approaches are often associated with significant limitations, including systemic toxicity, poor selectivity, and the emergence of drug resistance [2–4]. Metal-based chemotherapeutics, particularly platinum(II) complexes like cisplatin and its analogues (e.g., carboplatin and oxaliplatin), have become cornerstones in cancer treatment [5–8]. However, their widespread use is hindered by dose-limiting side effects such as nephrotoxicity, hepatotoxicity, and ototoxicity [8–10], prompting the search for less toxic and more selective metal-based alternatives.
Silver(I) complexes have recently attracted attention as promising anticancer agents due to their distinctive modes of action and comparatively lower systemic toxicity [11–13]. While silver has a long history of medicinal use, its anticancer properties have only recently been systematically explored [14–18]. The cytotoxic activity of silver compounds is largely attributed to the controlled release of Ag⁺ ions, which interact with cellular targets to induce apoptosis and oxidative stress [14, 17, 18]. Importantly, the choice of ligands significantly influences the biological profile of silver(I) complexes by modulating their solubility, stability, and bioavailability.
Phosphine ligands, in particular, have shown promise in coordinating silver(I) centres to form structurally diverse complexes with potent anticancer activity [8, 14, 18]. These complexes exhibit various geometries—most commonly linear, but also trigonal planar, tetrahedral, and others—each influencing their mechanism of action [19–21]. Despite their potential, silver(I) phosphine complexes remain underexplored in oncology, and their therapeutic implications are not yet fully realized.
This review critically examines recent advances in the design, structural features, and biological performance of silver(I) phosphine complexes in cancer therapy. It highlights their ability to overcome resistance mechanisms, induce apoptosis, and selectively target cancer cells. The paper also outlines key challenges, including stability and ligand optimization, and proposes future research directions to facilitate the translation of these compounds into clinically viable chemotherapeutic agents. Bioactive complexes based on N-heterocyclic carbenes, 5-fluorouracil, Schiff bases, and other ligand systems are excluded, as they have been extensively reviewed elsewhere [22–29].
Metal-based anticancer compounds
The escalating prevalence of drug-resistant cancers has critically reduced the effectiveness of existing anticancer therapies, underscoring the urgent need for the development of safe, potent, and cost-effective chemotherapeutic agents. This challenge has accelerated research into alternative metal-based drugs beyond platinum, focusing on metals such as gold, ruthenium, copper, and silver as promising candidates [12, 15, 30–33]. These metals exhibit considerable structural diversity when coordinated with ligands due to the variable binding ratios and the ability to replace existing ligands on the transition metal centre. This chemical versatility enhances their redox activity and catalytic properties, making them attractive for therapeutic applications. As a result, significant progress has been made in developing metal–ligand complexes to address the global health burden of cancer [12, 15, 30–33].
Platinum-based anticancer complexes
Platinum-based anticancer agents remain the most successful metal-derived chemotherapeutics, having been employed extensively in treating various cancers for several decades. Among these, cisplatin (cis-diamminedichloroplatinum; CDDP) and its derivatives—including carboplatin, oxaliplatin, lobaplatin, nedaplatin, and heptaplatin, as illustrated in Fig. 1—are the most widely prescribed [33, 34]. These drugs have been approved by the United States Food and Drug Administration (US-FDA) and have gained global acceptance in cancer treatment protocols [34, 35]. Notably, carboplatin and oxaliplatin are associated with reduced toxicity at higher doses. Their mechanisms of action involve disrupting DNA (Deoxyribonucleic acid) replication and RNA (Ribonucleic Acid) transcription, leading to the formation of DNA adducts, which arrest the cell cycle and induce apoptosis [33–35].
Fig. 1.
Structural representations of the most commonly utilised platinum-based compounds.
Reproduced from ref [8]. licensed under the CC BY 4.0. Copyright 2022, German Journal of Pharmaceuticals & Biomaterials
Despite their therapeutic efficacy in treating cancers such as cervical, ovarian, testicular, bladder, and small-cell lung cancers, platinum-based drugs like CDDP present significant side effects. These include nephrotoxicity (which may develop within ten days), neurotoxicity, ototoxicity (affecting hearing and balance), hypertension, vascular damage, and myocardial injury, often linked to mitochondrial dysfunction in cardiomyocytes. Furthermore, platinum accumulation in paediatric patients has been associated with fatal gastrointestinal toxicity, cardiovascular disease, and respiratory complications [8, 36–38]. The increasing resistance of cancer cells to CDDP-based therapies is thought to result from the suppression of apoptosis and disruptions in redox homeostasis. However, the precise mechanisms underlying this resistance remain the subject of ongoing research [36].
Gold-based anticancer complexes
Gold (Au) complexes have increasingly drawn attention for their potential as cytotoxic and anticancer agents. Epidemiological and experimental studies have demonstrated that gold-based metallodrugs exhibit notable anticancer properties [38]. An earlier study revealed that the complex [Au(DPPE)₂]Cl (DPPE: bis(diphenylphosphine)ethane) was effective in treating in vivo B16 melanoma and P388 leukaemia while also showing cytotoxic effects against these cancer cell lines in vitro. [39] The lipophilic nature of gold complexes facilitates their selective targeting of cellular and mitochondrial membranes, allowing [Au(DPPE)₂]Cl to access critical intracellular targets. This lipophilicity-driven targeting was pivotal in developing gold complexes, given their pronounced toxicity and mitochondrial accumulation in various cancer cell lines [40]. Auranofin, initially introduced as a treatment for rheumatoid arthritis, was the first gold(I) monophosphine derivative identified to possess antiproliferative properties in both in vivo (P388 murine leukaemia) and in vitro (B16 melanoma and P388 leukaemia) models [41]. It has also shown efficacy against microbial infections [42]. Furthermore, gold(I) complexes bearing phosphine ligands induced high levels of reactive oxygen species (ROS) in OVCAR8 (ovarian cancer) cells and significantly increased caspase activity [42].
Gold(I) complexes with diphosphine donor ligands are potent anticancer agents, mainly through their inhibition of DNA-topoisomerase II (Topo II) [43]. Topoisomerases I and II are critical for DNA replication, maintaining genomic stability, and ensuring accurate chromosome segregation during mitosis [44]. Elsewhere, synthesised Au(I) complexes with phosphine sulphide ligands were appraised on their effects on A549 (lung cancer) and SKOV3 (ovarian cancer) cell lines. The complexes exhibited potent cytotoxicity, with IC₅₀ values below 50 μM for A549 and below 5 μM for SKOV3 cells. These compounds inhibited Topo I enzymatic activity by preventing DNA relaxation and promoting DNA supercoiling, thereby impairing cell division [45]. The effects of Au(III) tetraphenyl-porphyrin chloride [Au(TPP)]Cl on colorectal cancer cells (HT-29 and HCT-116) indicated that apoptosis was triggered via the intrinsic pathway, with the activation of caspases −3 and −9 and the cleavage of poly ADP-ribose polymerase (PARP), alongside the upregulation of the pro-apoptotic Bcl-2-associated X protein (Bax) [46].
Despite the promising anticancer activities of gold-based complexes, their clinical application remains limited. A significant drawback is the tendency of gold to accumulate within cells, leading to adverse side effects. This bioaccumulation can cause dermatological conditions such as dermatitis, nephropathy, and thrombocytopenia (platelet deficiency). These toxicities, coupled with an incomplete understanding of their long-term effects and mechanisms of action, have hindered the widespread adoption of gold complexes in cancer treatments. Ongoing research addresses these limitations and optimises the therapeutic potential of gold-phosphine complexes [47].
Ruthenium-based anticancer complexes
Ruthenium (Ru), located at the centre of the second row of transition metals, demonstrates characteristics of both early and late transition metals [48]. Its Lewis acidic yet less oxophilic nature gives rise to unique properties widely applied in industries such as solar energy, electronics, alloys, catalysis, and diagnostic and therapeutic medicines [32, 49]. Ru complexes have gained recognition in medicinal chemistry for their bioactivity and potential anticancer effects, particularly in addressing chemo-resistant tumours. Possessing chemical stability in two primary oxidation states, Ru(II) and Ru(III), contributes to its versatility in therapeutic applications. Ru-based complexes generally adopt an octahedral molecular geometry, with good water solubility and slow ligand exchange kinetics, that enables prolonged interaction with biological targets [32, 48, 49].
Among the earliest studies on the biological activity of Ru was by Dwyer et al. [50] who developed a series of Ru polypyridyl complexes. These complex cations exhibited high biological activity, chemical stability, and coordination saturation, lacking specific active groups or centres. Their biological activity appeared to be a function of the entire cation rather than the metal atom alone. They behaved more like organic compounds due to their delocalised charge, though the charge magnitude was higher [50]. This pioneering work ignited significant interest in the role of Ru complexes in cancer therapy, leading to the synthesis and investigation of numerous Ru-based compounds over the years [51–53]. It is reported that Ru(II) polypyridyl complexes effectively induced apoptosis in several cancer cell lines, including A549 (lung carcinoma), MCF-7 (breast cancer), HeLa (cervical carcinoma), and Hs294 T (melanoma). The apoptotic mechanism involved the release of cytochrome c from mitochondria, followed by the activation of caspase-3 through autocatalytic cleavage [51]. Liang et al. [54] synthesised three Ru(II) complexes featuring ligands with imidazole rings. These complexes exhibited moderate cytotoxicity across four cancer cell lines, with the B16 melanoma cells demonstrating increased reactive oxygen species (ROS) production. The elevated ROS levels led to mitochondrial dysfunction and triggered apoptosis [54]. Another report argued that arene Ru(II) complexes, designated GA105 and GA113, that incorporated bis-amino-phosphine ligands effectively induced a dose-dependent reduction in the viability of A375 melanoma cells, with IC₅₀ values of 6.72 μM for GA105 and 8.76 μM for GA113, respectively. Importantly, these complexes showed no toxicity towards non-malignant HEK-293 embryonic kidney cells, thus highlighting their selective cytotoxicity [55]. GA113, in particular, caused morphological changes consistent with apoptosis, as evidenced by flow cytometry, where a significant proportion of cells shifted into the late apoptotic quadrant (28.83% at 10 μM; 73.97% at 20 μM) [55]. Ru(II) complexes exert these anticancer effects by disrupting key cellular processes and promoting programmed cell death when interacting with DNA and proteins within cancer cells [56].
While ruthenium complexes exhibit promising anticancer properties in preclinical studies, their widespread clinical application remains limited due to several challenges. One major limitation is the incomplete understanding of their pharmacokinetics and biodistribution, which raises concerns about potential accumulation in non-target tissues and long-term toxicity [57, 58]. Furthermore, the variability in efficacy observed across different tumour types and inconsistencies between in vitro and in vivo results hinder the translation of these compounds into clinical practice. Additionally, the precise molecular mechanisms through which ruthenium complexes induce apoptosis and interact with cellular components are not fully elucidated, necessitating further research to optimise their therapeutic potential. Addressing these challenges is crucial for advancing ruthenium-based therapies in cancer treatment [57, 58].
Miscellaneous metal-based anticancer complexes
Non-platinum metal-based anticancer agents such as iridium(III), osmium(II) (e.g., complexes 3a–e), and bismuth(III) complexes have recently gained traction as promising alternatives to platinum-based drugs due to their distinct mechanisms of action [59–61]. One notable study reports the development of twelve novel half-sandwich iridium(III) complexes incorporating imidazo-phenanthroline or phenanthrene ligands [61]. These complexes differ from the commonly studied cyclometalated analogues by exhibiting hydrolysis-resistant Ir–Cl bonds, strong coordination stability, and inherent luminescence. Among them, complex Ir6 displayed the most potent antiproliferative activity, with an IC₅₀ value nearly twice as effective as cisplatin against A549 lung cancer cells and improved selectivity over normal bronchial epithelial cells. Ir6 also demonstrated anti-migratory effects and operates via a non-energy-dependent uptake mechanism, accumulating preferentially in lysosomes [61]. This localization triggers a lysosomal-to-mitochondrial apoptotic cascade, characterized by lysosomal membrane disruption, cathepsin release, ROS elevation, NADH oxidation, mitochondrial membrane depolarization, and G1-phase cell cycle arrest. Western blot analysis confirmed this apoptotic pathway through the downregulation of LAMP-1 and upregulation of cathepsin B and cytochrome C, marking a mechanistic departure from cisplatin’s DNA-targeting action [61]. The study’s integration of DFT calculations further supports the correlation between low HOMO–LUMO gaps and high bioactivity, particularly for Ir6 and Ir12. Additionally, the complexes’ photoluminescent properties enable real-time intracellular imaging, offering dual therapeutic and diagnostic potential. Despite these strengths, some complexes (e.g., Ir5 and Ir11) were inactive, and Ir6 showed limited efficacy across other cancer cell lines [61]. The modest influence of ligand substituents on activity suggests a need for further structural refinement. Moreover, the absence of in vivo data limits current translational relevance. In conclusion, the report the study underscores the therapeutic promise of iridium(III) complexes with alternative apoptotic mechanisms and highlights their potential as next-generation non-platinum anticancer agents.
Another study report the prepared and characterized ten novel Ru(II) and Os(II) cyclometalated arene complexes (2a–e and 3a–e), incorporating 4-phenylthiazole C,N-chelating ligands [60]. These"piano-stool"complexes were confirmed via NMR, HRMS, and X-ray crystallography. The complexes exhibited low micromolar cytotoxicity in A549, SW480, and CH1/PA-1 cancer cell lines, with 2c and 3c showing the highest potency [60]. Unlike cisplatin, these compounds selectively stabilized G-quadruplex DNA, particularly the c-MYC motif without affecting duplex DNA, as shown through FRET assays, circular dichroism, mass spectrometry, and docking studies [60]. This indicates a distinct, non-covalent mechanism of action focused on groove binding. Additional analyses revealed modest cell cycle disruption and limited apoptosis at high concentrations, with Os(II) compounds generally more effective than Ru(II) analogs. Amino acid interaction studies showed selective binding to methionine and histidine, with potential thiol-induced ligand release from cysteine, suggesting metabolic sensitivity [60]. The work introduces structurally stable, G4-selective Ru(II)/Os(II) metalacycles with promising anticancer properties distinct from platinum drugs, offering a valuable foundation for developing next-generation metallodrugs with novel biological targets and mechanisms.
Elsewhere, a study presents a rational investigation into the design, synthesis, and biological evaluation of nine cyclometalated bismuth(III) complexes (Bi1–Bi9) coordinated with C,O-bidentate ligands, as potential alternatives to traditional platinum-based chemotherapeutics [59]. The work is motivated by the need to overcome cisplatin’s clinical limitations, including off-target toxicity, drug resistance, and DNA-damaging mechanisms, and is built upon bismuth’s favorable properties—such as low toxicity, non-carcinogenicity, and a history of medicinal use [59]. The novelty of the study lies in its detailed exploration of the coordination environment around the bismuth center and how this directly influences anticancer efficacy, stability, and selectivity [59]. Structural analysis using NMR, HRMS, and X-ray crystallography revealed diverse hypervalent geometries (12-Bi-5 and 14-Bi-6 species), including chelate-induced chirality and hemidirected coordination geometries governed by the stereoactive 6 s2 lone pair [59].
Biological evaluation across four solid tumor cell lines (HCT-116, MDA-MB-231, A549, and SGC-7901) and one normal cell line (HEK-293) demonstrated that most complexes possess significantly higher cytotoxicity (IC₅₀ = 0.3–14.6 µM) and selectivity indices (SI = 3.0–71.3) compared to cisplatin (IC₅₀ ≈ 17–22 µM; SI ≈ 2.0). Particularly, complexes Bi1, Bi4, Bi6, and Bi8 showed excellent selectivity and potent activity against HCT-116 cells [59]. Mechanistic studies identified apoptosis as the principal mode of cytotoxicity. Complex Bi8 notably downregulated anti-apoptotic Bcl-2 expression and upregulated cleaved caspase-3 in HCT-116 cells, with caspase inhibition experiments confirming apoptosis as a caspase-dependent process. These results align with Annexin V/PI staining, which showed significant apoptosis rates for Bi6 and Bi8 (37.7% and 47.8%, respectively) [59]. Furthermore, the study demonstrates that these complexes can overcome platinum resistance, as evidenced by greater potency in A2780/cis cells compared to cisplatin. Resistance factors (Rf) for Bi1, Bi4, Bi6, and Bi8 ranged from 0.65 to 0.83, in contrast to cisplatin's Rf of 2.62, suggesting their potential in circumventing cisplatin-induced resistance [59]. The authors further established that anticancer activity correlates with coordination number and geometry, where higher-coordination (14-Bi-6) species, such as nitrates (Bi8, Bi9), were more cytotoxic than lower-coordination (12-Bi-5) analogs. Although lipophilicity (logP) played a role, the enhanced activity of nitrate-containing species was attributed to increased stability via stronger Bi–O bonding and reduced ligand exchange, preserving activity in biological environments [59]. This work contributes substantially to the field by linking the coordination environment of bismuth(III) complexes to anticancer potency and providing a strong scientific basis for their future development as non-platinum metallodrug candidates [59]. The findings set a precedent for rational metallodrug design that leverages geometric, electronic, and mechanistic properties to achieve targeted and selective cancer therapy.
Silver(I) phosphine complexes: an overview
In the 1860 s, through a series of reports, August Wilhelm von Hofmann described the synthesis of platinum and gold complexes with triethyl-phosphine, arsine, and stibine ligands [62–65]. Around the same time, Auguste André Thomas Cahours described the synthesis of palladium, platinum, and gold complexes with tertiary phosphines and arsines [66]. This began a field of chemistry that will expand enormously in the coming decades. In 1937, Mann et al. [67] published their work on silver(I) complexes of the type [AgLnX], where L represents a tertiary phosphine or arsine, n = 1–4, and X is either a coordinating or non-coordinating anion. These complexes were pioneering as they were the first metal-phosphine complexes to be characterised crystallographically [67]. The tendency of silver ions to form their most robust complexes with the second or a subsequent, rather than with the first, co-ordinating atom in each Group of the periodic table is very marked in Group V, i.e., the affinity of silver ions for the phosphine (ca. ~ 10 kcal/mol) is higher for arsine and anilines [68, 69]. Moreover, with transition-metal ions such as platinum (II) and silver (I), the tendency of coordinating atoms in their alkyls to form dπ-bonds decreases in the order phosphines > arsines > selenides ~ sulphides > amines [68, 69]. Tertiary phosphines exhibit distinct ligand properties that set them apart from amines, notably, unlike salt-like complexes typically formed by ammonia and amines (for example, ammonia and primary amines often form more ionic coordination complexes (e.g., [Cu(NH₃)₄]2⁺), they strongly tend to form non-ionic complexes easily soluble in organic solvents (a significant advantage in medicinal formulations) which is attributable to the enhanced steric hindrance [66, 68, 70]. The stability of these complexes is often attributed to the salts of elements positioned to the right of the transition metals in the periodic table, a property influenced by the unique characteristics of phosphine ligands [66, 68, 70].
Phosphines serve as both σ-bond donors and π-bond acceptors (which is significant in their biological activities), where the vacant 3 d-orbitals of phosphorus can interact with the filled nonbonding d-orbitals of transition metals [66, 68, 70]. In many cases, the π-acceptor properties of phosphines are as significant as their σ-donor capabilities. For instance, phosphorus trifluoride (PF₃) forms stable complexes despite its lower donor strength due to the increased electron affinity of the vacant d-orbitals of phosphorus [66, 68, 70]. When functioning as ligands, the extent of π-bonding in PF₃ makes its behaviour more akin to carbon monoxide than to other tertiary phosphines [66, 68, 70]. The well-known strong ligand-field strength of tertiary phosphines results in a considerable energy separation between the low-energy and high-energy d-orbitals of the metal [66, 68, 70]. Stability in these complexes is typically achieved when the low-energy orbitals are fully occupied while the high-energy orbitals remain unoccupied. This energy separation must also be sufficiently large to prevent electron promotion between the orbitals [70]. It is important to note that complex formation is often observed in reaction media with low donor capacity, which is crucial in successfully synthesising such complexes [66, 68, 70].
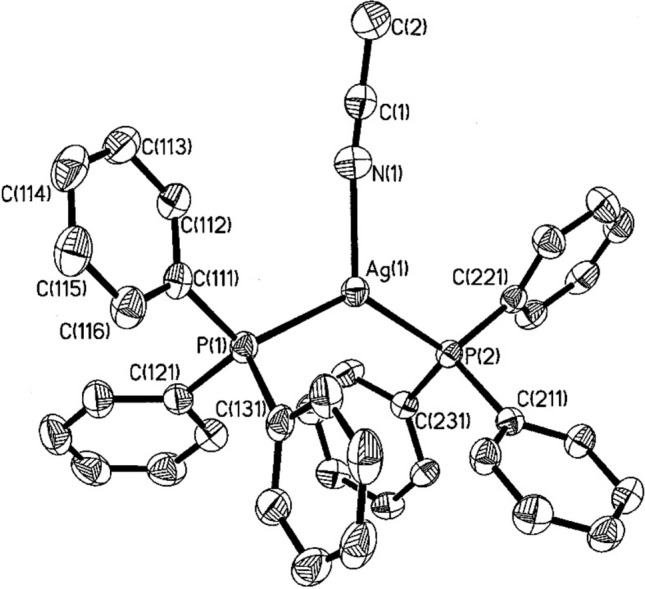
The typical preparation method for silver(I) complexes of the type [AgLnX] involves reacting stoichiometric amounts of a phosphine ligand with the appropriate silver(I) salt in a suitable solvent. For example, when silver(I) salts are reacted with monodentate tertiary phosphines in a 1:2 stoichiometric ratio, either monomeric complexes [AgX (PR₃)₂]/[Ag(PR₃)₂]·X or dimeric complexes [{AgX(PR₃)₂}₂] are generally formed. The outcome depends on factors such as the donor properties of the phosphine ligand, its bulkiness, substituents, and the donor properties of the anion [14, 20, 71]. Bachman et al. [72] report the preparation, isolation and crystallographic analysis of two new low-coordinate silver(I) complexes with triphenylphosphine, including the first structurally characterised two-coordinate phosphine complex of silver, [(Ph₃P)₂Ag]+ [72]. This complex was synthesised by reacting two equivalents of triphenylphosphine with silver tetrafluoroborate in acetonitrile, followed by recrystallisation from a mixture of dichloromethane and hexane [72]. This yielded colourless crystals of [(Ph₃P)₂AgNCCH₃][BF₄] (Fig. 2), as a dichloromethane solvate (I·0.5 CH₂Cl₂) with excellent yield. The resulting crystals were of sufficient quality for X-ray diffraction, allowing for direct structural confirmation. The unit cell contains eight pairs of cations and anions, along with four dichloromethane molecules positioned on two-fold axes. Although the solvent molecules do not form short contacts with the cations or anions, the ions are loosely associated with weak electrostatic interactions [72]. Elsewhere, Baranov et al. [73] report the synthesis of silver(I) complexes with tris[2-(2-pyridyl)ethyl]phosphine [73]. They showed that the coordination chemistry of tris[2-(2-pyridyl)ethyl]phosphine was first explored through reactions with Ag(I) and Au(I) salts [73]. Interaction with AgClO₄, AgNO₃, and AgOTf resulted in the formation of one-dimensional coordination polymers (CPs) of the type [Ag(L)X]ₙ (where X = ClO₄, NO₃, OTf), in which the ligand functions as a P,N, N'-bridging unit. When reacted with AgCl, this phosphine yields a CP, [Ag₄Cl₄(L)₂]ₙ, featuring stepped cubane-like [Ag₄Cl₄] modules, interconnected by “PyCH₂CH₂P” arms [73]. In the linear complex [Au(L)Cl], synthesised by treating the ligand (L) with Au(tht)Cl, the ligand coordinates in a P-monodentate manner [73].
Fig. 2.
Representation of the cationic species in the compound I·0.5 CH2Cl2. The cation, [(Ph3P)2Ag]+, consists of a central silver atom [Ag(I)] coordinated with two triphenylphosphine ligands. The phenyl rings are labelled according to the numbering scheme indicated for the reference ring in the figure.
Image reproduced from ref [72]. Copyright 1998, American Chemical Society
Silver(I) complexes with two, three, and four coordination numbers, represented as AgX (X = CN⁻, and ), have been synthesised using triphenylphosphine, tricyclohexylphosphine, diphenylcyclohexylphosphine, and dicyclohexylphenylphosphine [71]. Using a metal salt-to-phosphine ligand ratio of 1:1, various compounds crystallised in 1:2, 1:3, and 1:4 ratios, yielding monomeric forms including [Ag(PPh₂Cy)₃(CN)]·5H₂O (1), [Ag(PCy₃)₂]·PF₆ (2), [Ag(PPh₃)₄]·SbF₆·CHCl₃ (3), [Ag(PCy₃)₂]·SbF₆ (4), [Ag(PPh₂Cy)₂(C₃H₆O)]·SbF₆ (5), and [Ag(PPhCy₂)₂]·SbF₆·CHCl₃ (6), exhibiting a range of coordination geometries around the central silver(I) atom [71]. As illustrated in Fig. 3, Compounds 1 and 3 feature slightly distorted tetrahedral geometries, while compounds 2, 4, and 6 display linear geometries around the metal centre. Compound 5 adopts a distorted trigonal planar geometry [71].
Fig. 3.
Structures of the prepared Silver(I) complexes 1, 2, 3, 5 and 6, with selected atom labelling scheme and displacement ellipsoids drawn at the 50% probability level (the anion has been omitted for clarity).
Reproduced from ref [71]. Copyright 2009, Elsevier B.V
As mentioned, silver(I) phosphine complexes exhibit remarkable structural diversity influenced by ligand bulk, metal-to-ligand ratios, and counterion identity. Standard geometries include tetrahedral, trigonal planar, and linear configurations. For instance, reactions between silver(I) and tertiary phosphines (e.g., cyclohexyldiphenylphosphine) yield complexes with either trigonal planar or tetrahedral geometries [18]. Adjusting the metal-to-ligand ratio or employing different halide or pseudo-halide counterions can modulate these structures. This interplay between phosphine properties and the coordination mode of supporting ligands often makes predicting the structural behaviour of silver-phosphine complexes, both in solution and solid state, challenging [18, 74, 75]. The steric and electronic properties of phosphine ligands significantly impact the stability and geometry of silver(I) complexes. Due to steric hindrance, bulky ligands, such as triphenylphosphine, often lead to lower coordination numbers (e.g., two- or three-coordinate structures). Conversely, smaller ligands may facilitate higher coordination numbers (e.g., tetrahedral geometries) [14, 71, 74]. Chelating ligands, such as bidentate phosphines, can stabilise silver(I) complexes by promoting intramolecular interactions [14, 71, 74].
This adaptable structural flexibility of silver(I) phosphine complexes enhances their efficient redox and catalytic performance, which is crucial for their biological activity. Therefore, complexes with flexible geometries, especially those containing bidentate ligands, can adopt multiple coordination modes, enhancing their ability to interact with various biological targets. Such flexibility enables fine-tuning of cytotoxic properties through ligand or counterion modifications [75, 76].
Silver(I) phosphine complexes in cancer treatments
Silver (I) phosphine complexes have garnered significant interest in the last decade as potential anticancer agents due to their ability to inhibit tumour cell growth across various cancer models [14, 75, 76]. These complexes are desirable because they can exhibit unique mechanisms of action compared to conventional platinum-based drugs (e.g., cisplatin) [14, 75, 76]. One of the primary mechanisms by which silver(I) phosphine complexes exert their anticancer effects is the induction of apoptosis. For example, interacting with mitochondrial membranes leads to the release of cytochrome c and the activation of caspase-dependent apoptotic pathways [14, 75, 76]. The anticancer activity of silver(I) phosphine complexes is influenced by the nature of the phosphine ligand, the coordination environment around the silver centre, and their overall structural properties [14, 75, 76].
Studies on SNO oesophagal cancer cells have shown that silver(I) complexes induce apoptosis via mitochondrial depolarisation, phosphatidylserine externalisation, and DNA fragmentation [77–79]. Silver(I) phosphine complexes exhibit selective cytotoxicity toward cancer cells, with minimal effects on healthy tissues. This selectivity is believed to be due to the increased metabolic activity and altered redox states of cancer cells, which make them more susceptible to oxidative stress induced by silver complexes [77–79]. For example, silver(I) thiocyanate with cyclohexyldiphenylphosphine complexes 1–3 (Complex 1: 1:1 molar ratio (Ag: ligand); Complex 2: 1:2 molar ratio (Ag: ligand); Complex 3: 1:3 molar ratio (Ag: ligand)) exhibited significantly lower IC₅₀ than cisplatin, improved ligand coordination, and enhanced potency [80]. Testing on SNO oesophagal cancer cells shows that apoptosis is the dominant mode of cell death. Unlike conventional drugs that induce necrosis (leading to inflammation), these silver(I) complexes primarily cause programmed cell death [80]. Furthermore, these complexes exhibited good stability with multiple ligands [80]. Although these silver(I) complexes are promising, the study only evaluates in vitro cytotoxicity against SNO esophageal cancer cells. Additionally, the study lacks data on the effect of these complexes on healthy cells and whether the SNO cells develop resistance to these silver(I) complexes. Moreover, using organic solvents in the study, such as pyridine and DMSO (dimethyl sulfoxide), raises environmental concerns. Elsewhere, silver (I) cyanide-phosphine complexes are proposed as an alternative metal-based agent because of good thermal stability and a different coordination environment, potentially improving effectiveness against resistant cancer cells [81]. The silver (I) complex is 10 times more potent than cisplatin against SNO cancer cells; however, it exhibited significant cytotoxicity against cancer and non-malignant cells [81] which limits the selectivity for cancer cells alone. Hence, modifications are needed to improve selectivity (e.g., targeted drug delivery systems employing nanoparticles or nanoencapsulation), or the complex could also be improved upon by employing cancer-targeting ligands (e.g., folic acid and antibodies) [82, 83].
Engelbrecht et al. [78] prepared a novel silver (I) thiocyanate 4–methoxyphenyl phosphine complex [AgSCN{P(4-MeOC6H4)3}2]2 (Complex 1, shown in Fig. 4) as an efficient metal-based anticancer agent, which offers several key advantages. For example, it exhibits a lower IC₅₀ (3.5 µM) than cisplatin (42.89 µM), making it significantly more potent. In contrast to cisplatin, which can cause necrosis, this silver(I) complex triggers apoptosis via mitochondrial pathways as they selectively accumulate in mitochondria, leading to controlled cell death [78]. This silver(I) complex is over 10 times more potent than cisplatin and showed lower toxicity to healthy cells (HDF-a and HEK293) than malignant cells [78]. The mechanism of action was determined to be via mitochondrial-mediated apoptosis, with 70% of cancer cells treated with the complex undergoing early and late apoptosis with minimal necrotic cell death. It was shown that Caspase-3/7 levels increased more than six-fold after treatment, further confirming apoptotic induction [78]. In treated cells, ATP levels decreased significantly (15.71%), and ROS production increased (Fig. 5), leading to mitochondrial cytochrome c release [78]. Western blot analysis confirmed caspase-9 activation and PARP cleavage, marking the apoptosis pathway. However, the study was conducted only in vitro (cell culture) [78]. Moreover, because silver (I) complexes have solubility limitations, requiring DMSO for solubilisation, there is a need to reformulate the drug preparation methods (e.g., using nanoparticle-based delivery systems).
Fig. 4.
Structure of [AgSCN{P(4-MeOC6H4)3}2]2 (Complex 1).
Reproduced from ref [78]. Copyright 2018, Springer Nature
Fig. 5.
Total reactive oxygen species (ROS) generated in treated malignant SNO cells were measured using the Muse® Oxidative Stress Kit. The cells were exposed for 24 h to either 1% DMSO as a vehicle control (a), 10 µM of complex 1 (b), or 100 µM CDDP as a positive control (c). The results, expressed as the mean percentages of replicates, classify the cell populations into ROS-negative (M1) and ROS-positive (M2) groups, with each quadrant displaying the mean ± SEM (n = 3).
Reproduced from ref [78]. Copyright 2018, Springer Nature
The synthesis and characterisation of novel silver(I) cyclohexyldiphenylphosphine complexes (1–6) are reported [84]. These complexes were prepared using AgNO₃, AgCl, and AgBr salts with cyclohexyldiphenylphosphine in varying molar ratios (1:1, 1:2, 1:3), enabling the modulation of their coordination chemistry and properties [84]. Their cytotoxic effects were assessed against four malignant cell lines—SNO (oesophagal), MCF-7 (breast), A375 (melanoma), and A549 (lung)—as well as a non-malignant HEK293 (human embryonic kidney) cell line [84]. Notably, complexes with higher metal-to-ligand ratios (1:2, 1:3) exhibited increased cytotoxic potency [84]. The mechanism of action was associated with cell death induction, resulting in a significant reduction in cancer cell viability [84]. However, these complexes also displayed substantial cytotoxicity toward the non-malignant HEK293 cells, indicating a lack of selectivity [84]. Furthermore, while the findings suggest cytotoxic activity, the study does not establish whether the complexes specifically induce apoptosis, as observed with certain silver(I) drugs [84]. The absence of an investigation into the precise mode of cell death (apoptosis vs. necrosis) further limits conclusions regarding their potential as targeted anticancer agents [84].
To address the critical limitations of conventional chemotherapy—particularly cisplatin and 5-fluorouracil, the standard treatments for colorectal cancer—two novel silver(I) monodentate phosphine complexes were developed: Complex 1 ([Silver(I) diphenyl-2-pyridylphosphine]Br) and Complex 2 ([Silver(I) 4-(dimethylamino)phenyldiphenylphosphine]Br) [85]. These compounds were evaluated for their anticancer potential against HT-29 colorectal cancer cells. Complex 1 exhibited a significantly lower IC₅₀ value (7.49 µM) compared to cisplatin (IC₅₀ = 200.96 µM), demonstrating potent cytotoxicity against HT-29 cells while showing reduced toxicity toward non-malignant HEK-293 and MRHF cells, as illustrated in Fig. 6 [85]. Mechanistic studies revealed that these silver(I) complexes induce apoptosis via mitochondrial depolarisation (∆Ψm), leading to cytochrome c release, caspase activation, and reactive oxygen species (ROS) generation. Notably, Complex 1 significantly increased oxidative stress and apoptotic activity in HT-29 cells, distinguishing its mode of action from cisplatin, which primarily exerts its effect through DNA intercalation [85]. After 24 h, Complex 1 was found to be over 25 times more potent than cisplatin while exhibiting minimal toxicity to non-malignant cells. In contrast, cisplatin caused significant cytotoxicity in HEK-293 and MRHF cells [85]. The findings suggest that Complex 1, with its much lower IC₅₀ value and selective apoptotic mechanism via mitochondrial disruption, represents a promising alternative therapeutic strategy that minimises off-target toxicity [85]. Despite the study's robust spectroscopic and crystallographic characterisation confirming the chemical stability and reproducibility of the complexes, its scope is limited to in vitro cell culture experiments [85]. Additionally, the investigation was restricted to short-term (24–48 h) exposure, lacking an assessment of long-term effects or potential resistance development [85]. Furthermore, the study did not evaluate whether the silver(I) complex interacts with DNA in a manner similar to platinum-based drugs, leaving an essential aspect of its mechanism unexplored [85].
Fig. 6.
Morphological alterations observed in two non-malignant cell lines, HEK-293 (top) and MRHF (middle), as well as one malignant cell line, HT-29 (bottom), following various treatments for 24 h. Cells were either untreated, exposed to 1% DMSO (vehicle control), treated with 100 µM CDDP (positive apoptotic control), or treated with 10 µM of complex 1 or 2. Hallmarks of apoptosis, including cell shrinkage, rounding, and apoptotic blebbing or body formation, are marked with red arrows.
Reproduced from ref [85]. Copyright 2023, MDPI (Basel Switzerland)
Recently, Roberts et al. [86] prepared and evaluated 13 silver(I) phosphine complexes across seven human malignant cell lines and two non-malignant cell lines. A summary of their findings is tabulated in Table 1.
Table 1.
Comparison with current metal-based drugs [86]
| Parameter | Silver(I) phosphine complexes | Platinum-based(cisplatin, carboplatin) | Gold-based complexes | Ruthenium-based complexes |
|---|---|---|---|---|
| Selectivity | High for cancer cells, lower toxicity to healthy cells | Less selective, affects healthy cells | Variable selectivity | Selective for some cancer types |
| Toxicity | Lower nephrotoxicity and neurotoxicity | Severe nephrotoxicity and neurotoxicity | Risk of gold bioaccumulation | Generally lower toxicity than platinum |
| Resistance | Less likely to induce resistance | Resistance is common in many cancers | Some resistance reported | Effective against cisplatin-resistant cancers |
| Mode of action | Induces apoptosis, targets mitochondria, affects ER signalling | DNA crosslinking, apoptosis | Inhibits DNA-topoisomerase II, induce apoptosis | ROS generation, mitochondrial dysfunction |
The research confirms selective cytotoxicity against breast cancer (MCF-7, MDA-MB-231), lung adenocarcinoma (A549), colorectal cancer (HT-29), and hepatocellular carcinoma (HEP-G2) [86]. Analysis of the molecular interactions of the silver(I) phosphine complexes using molecular docking and dynamics simulations reveal their binding to oestrogen receptors (ER-α and ER-β); this interaction with oestrogen receptors (ER-α and ER-β) in hormone-related cancers presents a novel treatment pathway, particularly relevant for breast cancer therapy [86]. Particularly, Complex 4 demonstrates intense anticancer activity in all malignancies tested, suggesting it could be a viable candidate for hormone therapy and combination chemotherapy regimens [86]. The structural differences in the ligands and their impact on activity were examined, showing higher selectivity for cancerous cells compared to traditional drugs such as cisplatin [86]. However, further research is needed to address possible long-term toxicity concerns, resistance mechanisms, and in vivo applicability before these complexes can move toward clinical trials.
A study comparing multiple structurally distinct silver(I) complexes: OHBT (4-hydroxybenzaldehyde thiosemicarbazone derivative containing a hydroxyl (–OH) group on the benzaldehyde ring), DOHBT (2,4-dihydroxy benzaldehyde thiosemicarbazone derivative containing a hydroxyl (–OH) groups at positions 2 and 4 on the benzaldehyde ring), BrOHBT (5-bromo-4-hydroxybenzaldehyde thiosemicarbazone derivative containing bromine (–Br) substituent at position 5 and a hydroxyl (–OH) group at position 4), OHMBT (4-hydroxy-2-methoxybenzaldehyde thiosemicarbazone derivative containing a hydroxyl (–OH) group at position 4 and a methoxy (–OCH₃) group at position 2) and BrOHMBT (5-bromo-2-hydroxy-3-methoxybenzaldehyde thiosemicarbazone derivative containing a bromine (–Br) at position 5, a hydroxyl (-OH) at position 2, and a methoxy (–OCH₃) at position 3), reinforces that ligand variations significantly impact cytotoxicity and apoptosis induction [87]. These modifications in functional groups and halogenation significantly improved their cytotoxicity and genotoxicity against malignant melanoma (SK-MEL-28) cells, with BrOHMBT exhibiting the highest cytotoxicity (IC50 = 0.64 μM) due to the bromine and methoxy functional groups enhancing anticancer activity [87]. The study shows that DNA damage is time-dependent, particularly with OHBT and BrOHMBT, distinct from conventional cisplatin-based therapies [87]. In contrast to most studies focusing only on cytotoxicity, this work goes further by using an alkaline comet assay to confirm DNA strand breaks, utilising Annexin V-FITC/PI flow cytometry to study apoptosis induction, confirmed by the Cytotoxicity and genotoxicity studies in Fig. 7 [87]. They investigated early-stage DNA damage before apoptosis, which helps map the molecular response essential for optimising treatment timing and dosage. It was argued that silver(I) complexes could be combined with DNA-targeting drugs (e.g., cisplatin) or redox-modulating agents to enhance therapeutic outcomes [87]. However, the study is limited to in vitro experiments on SK-MEL-28 melanoma cells; tumour microenvironments in living organisms differ significantly from cell culture conditions. Drug metabolism, immune response, and systemic toxicity cannot be assessed without in vivo models.
Fig. 7.
Cytotoxicity and genotoxicity studies. A: The impact of silver(I) complexes 1–5 on the viability of SK-MEL-28 malignant melanoma cells. Data represent the mean ± SEM from three independent experiments. B: a Flow cytometry analysis of Annexin V-FITC/PI dual staining in SK-MEL-28 cells treated with OHBT. b The graph illustrates the percentages of apoptotic and necrotic cells in OHBT-treated samples compared to the control. Data are presented as mean ± SEM from three independent experiments. *p < 0.05 indicates a statistically significant difference compared to the negative control. C DNA damage observed following treatment with OHBT. b DNA damage was observed following treatment with BrOHMBT. Cells exposed to 30 mM H2O2 for 1 h were the positive control. Data are presented as mean ± SEM from three independent experiments. *p < 0.05 indicates a statistically significant difference compared to the negative control.
Reproduced from ref [87]. Copyright 2023, Elsevier
Silver(I) complexes exhibit intriguing structural chemistry and hold significant promise as potential anticancer agents. However, despite their potential, the literature on silver(I) complexes in the context of anticancer activity remains limited. In particular, systematic studies focusing on the interaction of diphosphines with silver and their resultant biological applications are sparse [18, 88]. More strikingly, research on silver(I) monophosphine complexes is even less explored, with only a handful of studies systematically addressing their coordination chemistry and bioactivity. A thorough and ongoing review of the existing scientific literature has confirmed this gap, underscoring the need for in-depth investigations into the coordination behaviour of silver(I) monophosphine complexes and their potential as therapeutic agents [18, 88]. Given these complexes'structural diversity and promising chemical properties, there is a compelling scientific motivation to explore their mechanism of cytotoxicity and how they induce cell death in cancerous cells. Addressing this gap, Meijboom et al. [89] have patented a new class of silver(I) monophosphine complexes designed explicitly for anticancer applications. The general formula represents these novel complexes: [89]
where:
Ag represents silver in the + 1-oxidation state.
X is a coordinating or non-coordinating anion selected from Cl⁻, Br⁻, I⁻, F⁻, CN⁻, SCN⁻, PO₄3⁻, NO₃⁻, NO₂⁻, ClO₄⁻, BF₄⁻, PF₆⁻, SO₄2⁻, CO₃2⁻, CH₃CO₂⁻, CF₃CO₂⁻, and CF₃SO₃⁻.
L is a monodentate phosphine ligand of the general formula PR₃.
m is an integer ranging from 1 to 4, inclusive.
n is an integer from 1 to ∞ inclusive.
These novel silver(I) monophosphine complexes provide a new avenue for exploring metal-based therapeutics with potential applications in targeted anticancer therapies. Their structural versatility and coordination chemistry are anticipated to contribute significantly to developing new metal-based pharmaceuticals [89]. The patenting of these silver(I) monophosphine complexes marks a significant advancement in medicinal inorganic chemistry. By systematically investigating their coordination chemistry and biological activity, this invention aims to provide a deeper understanding of their mode of action at the molecular level. The potential for these complexes to serve as effective anticancer agents lies in their unique electronic properties, ligand stabilisation effects, and the ability to modulate their interactions with biological targets. Furthermore, these findings open up new opportunities for designing and optimising silver(I)-based drugs, contributing to the broader effort of developing metal-based chemotherapeutic agents with enhanced efficacy and selectivity.
Conclusions
Silver(I) phosphine complexes represent a promising class of metal-based anticancer agents with potent cytotoxicity, distinct mechanisms of action, and favourable selectivity over conventional platinum drugs. Their ability to induce apoptosis via mitochondrial disruption and ROS generation provides a strategic advantage in overcoming resistance, particularly in cisplatin-refractory cancers.
This review highlights key advances in the synthesis, structure, and biological activity of silver(I) phosphine complexes, drawing comparisons with other non-platinum metals. Despite encouraging in vitro data, challenges such as poor aqueous solubility, off-target effects in some analogues, and a lack of in vivo validation remain barriers to clinical translation.
To aid compound prioritization, Table 2 summarizes IC₅₀ values across malignant and non-malignant cell lines. Selectivity indices emphasize that not all silver(I) complexes are equally safe—underscoring the importance of ligand optimization, metal-to-ligand ratios, and anion selection in improving efficacy while minimizing toxicity.
Table 2.
Comparative IC₅₀ values (µM) of representative metal-based complexes against cancerous and non-cancerous cell lines
| Complex | Cancer cell line | IC₅₀ (µM, cancer cells) | Non-malignant cell line | IC₅₀ (µM, non-malignant cells) | Selectivity Index (SI) |
|---|---|---|---|---|---|
| AgSCN{P(4-MeOC6H4)3}2 | SNO (esophageal) | 3.5 | HDF-a, HEK-293 | > 10 | High (≥ 3) |
| Ag-diphenyl-2-pyridylphosphine (Complex 1) | HT-29 (colorectal) | 7.49 | HEK-293, MRHF | > 50 | ~ 6.7 |
| Ag-cyclohexyldiphenylphosphine (1:2, 1:3 ratios) | SNO, MCF-7, A375, A549 | < 10 (approx.) | HEK-293 | ~ 10–15 | ~ 1–2 |
| Ag-cyanide-phosphine complex | SNO (esophageal) | ~ 2.5 | Not specified | < 2.5 (high toxicity) | < 1 (low selectivity) |
| Ag-thiocyanate-phosphine (1:1 to 1:3 ratios) | SNO (esophageal) | < 10 | Not specified | Not available | Likely high |
| Ag-HT-29 Complex 1 | HT-29 (colorectal) | 7.49 | HEK-293, MRHF | > 50 | ~ 6.7 |
| BrOHMBT (thiosemicarbazone derivative) | SK-MEL-28 (melanoma) | 0.64 | Not specified | Not available | Not reported |
| Cisplatin | Multiple | 17–22 | HEK-293 | 2.0 (SI ≈ 2) | ~ 2.0 |
The structure–activity relationship (SAR) analysis across the reviewed silver(I), ruthenium(II), osmium(II), iridium(III), and bismuth(III) complexes reveals key trends in how ligand modifications and coordination environments influence anticancer potency and selectivity. Notably, the following observations can be made:
Halogenation (e.g., Cl, Br substituents) on aromatic rings often enhances cytotoxicity, likely due to increased lipophilicity and membrane permeability. For instance, complexes with bromo-substituted ligands showed lower IC₅₀ values in A549 and HT-29 cells.
Methoxy substitutions (–OCH₃) tend to improve selectivity by modulating electron density and hydrophilicity, as seen in AgSCN{P(4-MeOC₆H₄)₃}₂, which showed high selectivity indices toward esophageal cancer cells over non-malignant lines.
Metal-to-ligand ratios affect complex geometry and bioactivity. For silver(I) phosphines, 1:2 and 1:3 ratios generally enhanced stability and activity compared to 1:1 analogue. However, excessive ligand bulk can hinder cell permeability.
Anion variation (e.g., chloride, nitrate, thiocyanate) significantly influences hydrolytic stability and cellular uptake. Nitrate-coordinated bismuth(III) and thiocyanate-coordinated silver(I) complexes exhibited higher activity and apoptotic potential.
Chelation and geometry also correlate with activity. Six-coordinate Bi(III) complexes and half-sandwich Ir(III) compounds with C,N or C,O bidentate ligands displayed higher apoptotic effects and ROS induction than lower-coordination analogues.
Hence, the most promising anticancer complexes demonstrated optimized ligand electronic properties, stable coordination geometries, favourable metal-to-ligand ratios, and strategic use of anions to enhance bioactivity and selectivity. These SAR trends provide valuable guidance for future ligand design and metal complex optimization in metallodrug development.
Altogether, these findings offer a rational basis for designing next-generation silver-based metallodrugs. Continued progress will depend on multidisciplinary efforts to optimize pharmacokinetics, incorporate tumour-targeting strategies, and validate efficacy through in vivo models. With these developments, silver(I) phosphine complexes could evolve into a vital component of the future anticancer arsenal.
Prospects and directions
Exploring silver(I) phosphine complexes as anticancer agents continues to reveal promising opportunities for scientific advancement and clinical application. While current studies demonstrate their cytotoxic potency, selective targeting capabilities, and unique mechanisms of action, challenges remain in optimising bioavailability, enhancing selectivity, minimising systemic toxicity, and evaluating long-term safety. Addressing these limitations is critical for the successful translation of these compounds into viable cancer therapies.
One fundamental approach to improving the therapeutic potential of silver(I) phosphine complexes involves optimizing ligand design. The coordination environment of these complexes significantly influences their solubility, stability, and biological activity. Future studies should focus on refining the steric and electronic properties of phosphine ligands to enhance their coordination chemistry and maximize anticancer efficacy. Additionally, functionalizing these complexes with tumour-targeting biomolecules such as folic acid, peptides, or monoclonal antibodies could improve specificity and reduce off-target cytotoxicity. Investigating mixed-ligand systems, including phosphine-thiosemicarbazone or phosphine-carboxylate hybrids, may further exploit synergistic effects that improve anticancer activity.
Another critical area of research involves addressing the limited aqueous solubility and rapid clearance of silver(I) phosphine complexes. Emerging drug delivery strategies, such as nanoparticle-based carriers, liposomes, polymeric nanoparticles, and dendrimers, could significantly improve the stability and systemic circulation of these compounds. Furthermore, silver(I) complex-loaded micelles or hydrogels could facilitate controlled and sustained drug release at tumour sites, minimising systemic toxicity and enhancing therapeutic efficacy. Encapsulation in biodegradable carriers also presents an opportunity to ensure targeted intracellular delivery while reducing unintended accumulation in healthy tissues.
Silver (I) phosphine complexes hold significant potential for combination therapies because they can induce mitochondria-mediated apoptosis. Their integration with existing chemotherapeutic agents, such as cisplatin, doxorubicin, or 5-fluorouracil, could enhance treatment efficacy and mitigate resistance. Additionally, co-administration with immune checkpoint inhibitors, including anti-PD-1 and anti-CTLA-4 antibodies, may boost immune-mediated tumour suppression. Incorporating redox-modulating agents, such as thioredoxin reductase inhibitors, could further amplify oxidative stress-induced apoptosis, offering a novel therapeutic strategy for resistant cancer types.
While the anticancer potential of silver(I) phosphine complexes has been established in vitro, their precise molecular interactions remain insufficiently characterised. Future research should focus on proteomic and transcriptomic analyses to elucidate the cellular pathways affected by these complexes. Advanced intracellular tracking methods, including fluorescence or Raman spectroscopy, could provide insights into their subcellular distribution and accumulation. Computational modelling techniques, such as molecular docking and dynamics simulations, would further help predict interactions with key biomolecular targets, including DNA, mitochondrial proteins, and cell surface receptors.
Preclinical evaluations and clinical trials are essential to translate these findings into clinical applications. Rigorous, long-term toxicity studies must be conducted to assess systemic accumulation and potential adverse effects. Pharmacokinetic and biodistribution analyses should determine the metabolic pathways, clearance rates, and optimal dosing regimens. Comparative efficacy trials in patient-derived xenograft models should also be undertaken to evaluate how silver(I) phosphine complexes perform relative to standard-of-care drugs such as cisplatin, oxaliplatin, and auranofin.
Another important consideration is the environmental impact of silver(I)-based pharmaceuticals. As these compounds become integrated into medical applications, concerns arise regarding their accumulation in wastewater systems and potential long-term ecological effects. Therefore, researchers must prioritise green synthesis methodologies to minimise environmental burdens. Developing effective silver recovery and recycling strategies within pharmaceutical waste streams will also be crucial for sustainability. Additionally, thorough ecological impact assessments should be performed to ensure that large-scale production of these compounds does not compromise environmental integrity.
Author contributions
RM: Conceptualized the review and drafted sections related to the structural diversity and chemical properties of silver(I) phosphine complexes. Reviewed and edited the manuscript for scientific rigour and supervised the overall project. AOCI: Co-conceptualized the review, conducted the comprehensive literature analysis, and drafted the initial manuscript. Managed the compilation and comparative evaluation of data from various studies, contributed to the discussion on the comparative analysis of silver(I) phosphine complexes with other metal-based drugs, and prepared the manuscript for submission. SSR: Contributed to the conceptual framing of the review in the context of nanostructured materials and their biomedical applications. Critically reviewed and edited the manuscript for scientific content and clarity and provided expert input on nanotechnology-based drug delivery strategies relevant to silver(I) complexes. Participated in refining the prospects and future directions section and approved the final version of the manuscript.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Informed consent
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Reinout Meijboom, Email: rmeijboom@uj.ac.za.
Austine Ofondu Chinomso Iroegbu, Email: aoiroegbu@gmail.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Cytlak UM, Dyer DP, Honeychurch J, Williams KJ, Travis MA, Illidge TM. Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat Rev Immunol. 2022;22(2):124–38. 10.1038/s41577-021-00568-1. [DOI] [PubMed] [Google Scholar]
- 3.Banfill K, Giuliani M, Aznar M, Franks K, McWilliam A, Schmitt M, Sun F, Vozenin MC, Faivre Finn C. Cardiac toxicity of thoracic radiotherapy: existing evidence and future directions. J Thorac Oncol. 2021;16(2):216–27. 10.1016/j.jtho.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chrysostomou D, Roberts LA, Marchesi JR, Kinross JM. Gut microbiota modulation of efficacy and toxicity of cancer chemotherapy and immunotherapy. Gastroenterology. 2023;164(2):198–213. 10.1053/j.gastro.2022.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Paprocka R, Wiese-Szadkowska M, Janciauskiene S, Kosmalski T, Kulik M, Helmin-Basa A. Latest developments in metal complexes as anticancer agents. Coord Chem Rev. 2022;452: 214307. 10.1016/j.ccr.2021.214307. [Google Scholar]
- 6.Banerjee S, Banerjee S. Metal-based complexes as potential anti-cancer agents. Anticancer Agents Med Chem. 2022;22(15):2684–707. 10.2174/1871520622666220331085144. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh S. Cisplatin: the first metal based anticancer drug. Bioorg Chem. 2019;88: 102925. 10.1016/j.bioorg.2019.102925. [DOI] [PubMed] [Google Scholar]
- 8.Raju SK, Praveen SK, Maruthamuthu M, Mohanpriya K. Silver complexes as anticancer agents: a perspective review. German J Pharmaceut Biomater. 2022;1(1):06–28. 10.5530/gjpb.2022.1.3. [Google Scholar]
- 9.Katanić Stanković JS, Selaković D, Rosić G. Oxidative damage as a fundament of systemic toxicities induced by cisplatin—the crucial limitation or potential therapeutic target? Int J Mol Sci. 2023;24(19):14574. 10.3390/ijms241914574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perše M, Večerić-Haler Ž. Cisplatin-induced rodent model of kidney injury: characteristics and challenges. Biomed Res Int. 2018;2018:1–29. 10.1155/2018/1462802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gudikandula K, Charya Maringanti S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J Exp Nanosci. 2016;11(9):714–21. 10.1080/17458080.2016.1139196. [Google Scholar]
- 12.Clement JL, Jarrett PS. Antibacterial silver. Met Based Drugs. 1994;1(5–6):467–82. 10.1155/MBD.1994.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell AD, Hugo WB. 7 Antimicrobial activity and action of silver. Amsterdam: Elsevier; 1994. p. 351–70. 10.1016/S0079-6468(08)70024-9. [DOI] [PubMed] [Google Scholar]
- 14.Kuchar J, Rust J, Lehmann CW, Mohr F. Silver(I) complexes with camphorsulfonato and phosphine ligands: structural diversity and antibacterial activity. Inorg Chem. 2020;59(15):10557–68. 10.1021/acs.inorgchem.0c00982. [DOI] [PubMed] [Google Scholar]
- 15.Medici S, Peana M, Nurchi VM, Zoroddu MA. Medical uses of silver: history, myths, and scientific evidence. J Med Chem. 2019;62(13):5923–43. 10.1021/acs.jmedchem.8b01439. [DOI] [PubMed] [Google Scholar]
- 16.Scalambra F, Lorenzo-Luis P, de los Ríos I, Romerosa A. New findings in metal complexes with antiproliferative activity containing 1,3,5-triaza-7-phosphaadamantane (PTA) and derivative ligands. Eur J Inorg Chem. 2019. 10.1002/ejic.201801426. [Google Scholar]
- 17.Medici S, Peana M, Crisponi G, Nurchi VM, Lachowicz JI, Remelli M, Zoroddu MA. Silver coordination compounds: a new horizon in medicine. Coord Chem Rev. 2016;327–328:349–59. 10.1016/j.ccr.2016.05.015. [Google Scholar]
- 18.Meijboom R, Bowen RJ, Berners-Price SJ. Coordination complexes of silver(I) with tertiary phosphine and related ligands. Coord Chem Rev. 2009;253(3–4):325–42. 10.1016/j.ccr.2008.03.001. [Google Scholar]
- 19.Omondi B, Meijboom R. Concomitant polymorphic behavior of Di-μ-thiocyanato-κ2N: S;κ 2S: N-Bis[Bis(Tri-p-fluorophenylphosphine-κ P)silver(I)]. Acta Crystallogr B. 2010;66(1):69–75. 10.1107/S0108768109048769. [DOI] [PubMed] [Google Scholar]
- 20.Chainok K, Neville SM, Forsyth CM, Gee WJ, Murray KS, Batten SR. Supramolecular architecture of silver(I) coordination polymers containing polydentate N-donor ligands. CrystEngComm. 2012;14(10):3717. 10.1039/c2ce25225b. [Google Scholar]
- 21.Schultheiss N, Powell DR, Bosch E. Silver(I) coordination chemistry of 2,6-diarylpyrazines. π-stacking, anion coordination, and steric control. Inorg Chem. 2003;42(17):5304–10. 10.1021/ic034277m. [DOI] [PubMed] [Google Scholar]
- 22.Şahin-Bölükbaşı S, Cantürk-Kılıçkaya P, Kılıçkaya O. Silver(I)-N-heterocyclic carbene complexes challenge cancer; evaluation of their anticancer properties and in silico studies. Drug Dev Res. 2021;82(7):907–26. 10.1002/ddr.21822. [DOI] [PubMed] [Google Scholar]
- 23.Marichev KO, Patil SA, Patil SA, Heras Martinez HM, Bugarin A. N-heterocyclic carbene metal complexes as therapeutic agents: a patent review. Expert Opin Ther Pat. 2022;32(1):47–61. 10.1080/13543776.2021.1965992. [DOI] [PubMed] [Google Scholar]
- 24.Valencia-Lazcano AA, Hassan D, Pourmadadi M, Shamsabadipour A, Behzadmehr R, Rahdar A, Medina DI, Díez-Pascual AM. 5-Fluorouracil nano-delivery systems as a cutting-edge for cancer therapy. Eur J Med Chem. 2023;246: 114995. 10.1016/j.ejmech.2022.114995. [DOI] [PubMed] [Google Scholar]
- 25.Alzahrani S, Al Doghaither H, Al-ghafari A, Pushparaj P. 5-Fluorouracil and capecitabine therapies for the treatment of colorectal cancer (review). Oncol Rep. 2023;50(4):175. 10.3892/or.2023.8612. [DOI] [PubMed] [Google Scholar]
- 26.Vasile Scaeteanu G, Badea M, Olar R. Coordinative compounds based on unsaturated carboxylate with versatile biological applications. Molecules. 2024;29(10):2321. 10.3390/molecules29102321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogner AN, Stiers KM, Tanner JJ. Structure, biochemistry, and gene expression patterns of the proline biosynthetic enzyme pyrroline-5-carboxylate reductase (PYCR), an emerging cancer therapy target. Amino Acids. 2021;53(12):1817–34. 10.1007/s00726-021-02999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang M, Su X, Zhong X, Lan Y, Yang F, Qin Y, Jiang C. Recent development of schiff-base metal complexes as therapeutic agents for lung cancer. J Mol Struct. 2024;1318: 139403. 10.1016/j.molstruc.2024.139403. [Google Scholar]
- 29.Presenjit, Chaturvedi S, Singh A, Gautam D, Singh K, Mishra AK. An insight into the effect of Schiff base and their d and f block metal complexes on various cancer cell lines as anticancer agents: a review. Anticancer Agents Med Chem 2024;24(7): 488–503. 10.2174/0118715206280314231201111358. [DOI] [PubMed]
- 30.Ji P, Wang P, Chen H, Xu Y, Ge J, Tian Z, Yan Z. Potential of copper and copper compounds for anticancer applications. Pharmaceuticals. 2023;16(2):234. 10.3390/ph16020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno-Alcántar G, Picchetti P, Casini A. Gold complexes in anticancer therapy: from new design principles to particle-based delivery systems. Angewandte Chemie Int Edn. 2023. 10.1002/anie.202218000. [DOI] [PubMed] [Google Scholar]
- 32.Lee SY, Kim CY, Nam T-G. Ruthenium complexes as anticancer agents: a brief history and perspectives. Drug Des Devel Ther. 2020;14:5375–92. 10.2147/DDDT.S275007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Štarha P, Křikavová R. Platinum(IV) and platinum(II) anticancer complexes with biologically active releasable ligands. Coord Chem Rev. 2024;501: 215578. 10.1016/j.ccr.2023.215578. [Google Scholar]
- 34.Suárez-Moreno GV, Hernández-Romero D, García-Barradas Ó, Vázquez-Vera Ó, Rosete-Luna S, Cruz-Cruz CA, López-Monteon A, Carrillo-Ahumada J, Morales-Morales D, Colorado-Peralta R. Second and third-row transition metal compounds containing benzimidazole ligands: an overview of their anticancer and antitumour activity. Coord Chem Rev. 2022;472: 214790. 10.1016/j.ccr.2022.214790. [Google Scholar]
- 35.Sahoo D, Deb P, Basu T, Bardhan S, Patra S, Sukul PK. Advancements in platinum-based anticancer drug development: a comprehensive review of strategies, discoveries, and future perspectives. Bioorg Med Chem. 2024;112: 117894. 10.1016/j.bmc.2024.117894. [DOI] [PubMed] [Google Scholar]
- 36.Ranasinghe R, Mathai ML, Zulli A. Cisplatin for cancer therapy and overcoming chemoresistance. Heliyon. 2022;8(9): e10608. 10.1016/j.heliyon.2022.e10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ota A, Tajima M, Mori K, Sugiyama E, Sato VH, Sato H. The selective cytotoxicity of silver thiosulfate, a silver complex, on MCF-7 breast cancer cells through ROS-induced cell death. Pharmacol Rep. 2021;73(3):847–57. 10.1007/s43440-021-00260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chrysouli MP, Banti CN, Kourkoumelis N, Panayiotou N, Markopoulos GS, Tasiopoulos AJ, Hadjikakou SK. Chloro(Triphenylphosphine)Gold(I) a forefront reagent in gold chemistry as apoptotic agent for cancer cells. J Inorg Biochem. 2018;179:107–20. 10.1016/j.jinorgbio.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Berners-Price SJ, Mirabelli CK, Johnson RK, Mattern MR, McCabe FL, Faucette LF, Sung CM, Mong SM, Sadler PJ, Crooke ST. In vivo antitumor activity and in vitro cytotoxic properties of Bis[1,2-bis(diphenylphosphino)ethane]gold(I) chloride. Cancer Res. 1986;46(11):5486–93. [PubMed] [Google Scholar]
- 40.Kriel FH, Coates J. Synthesis and antitumour activity of gold(I) and silver(I) complexes of hydrazine-bridged diphosphine ligands. S Afr J Chem. 2012;46:271–9. [Google Scholar]
- 41.Lupidi G, Avenali L, Bramucci M, Quassinti L, Pettinari R, Khalife HK, Gali-Muhtasib H, Marchetti F, Pettinari C. Synthesis, properties, and antitumor effects of a new mixed phosphine gold(I) compound in human colon cancer cells. J Inorg Biochem. 2013;124:78–87. 10.1016/j.jinorgbio.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Kim JH, Reeder E, Parkin S, Awuah SG. Gold(I/III)-phosphine complexes as potent antiproliferative agents. Sci Rep. 2019;9(1):12335. 10.1038/s41598-019-48584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caruso F, Villa R, Rossi M, Pettinari C, Paduano F, Pennati M, Daidone MG, Zaffaroni N. Mitochondria are primary targets in apoptosis induced by the mixed phosphine gold species chlorotriphenylphosphine-1,3-Bis(diphenylphosphino)propanegold(I) in melanoma cell lines. Biochem Pharmacol. 2007;73(6):773–81. 10.1016/j.bcp.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Lee JH, Berger JM. Cell cycle-dependent control and roles of DNA topoisomerase II. Genes (Basel). 2019;10(11):859. 10.3390/genes10110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martín-Encinas E, Conejo-Rodríguez V, Miguel JA, Martínez-Ilarduya JM, Rubiales G, Knudsen BR, Palacios F, Alonso C. Novel phosphine sulphide gold(i) complexes: topoisomerase i inhibitors and antiproliferative agents. Dalton Trans. 2020;49(23):7852–61. 10.1039/D0DT01467B. [DOI] [PubMed] [Google Scholar]
- 46.Dandash F, Léger DY, Fidanzi-Dugas C, Nasri S, Brégier F, Granet R, Karam W, Diab-Assaf M, Sol V, Liagre B. In vitro anticancer activity of new gold(III) porphyrin complexes in colon cancer cells. J Inorg Biochem. 2017;177:27–38. 10.1016/j.jinorgbio.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 47.Ott I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord Chem Rev. 2009;253(11–12):1670–81. 10.1016/j.ccr.2009.02.019. [Google Scholar]
- 48.Dragutan I, Dragutan V, Demonceau A. Editorial of special issue ruthenium complex: the expanding chemistry of the ruthenium complexes. Molecules. 2015;20(9):17244–74. 10.3390/molecules200917244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levina A, Mitra A, Lay PA. Recent developments in ruthenium anticancer drugs. Metallomics. 2009;1(6):458. 10.1039/b904071d. [DOI] [PubMed] [Google Scholar]
- 50.Dwyer FP, Gyarfas EC, Rogers WP, Koch JH. Biological activity of complex ions. Nature. 1952;170(4318):190–1. 10.1038/170190a0. [DOI] [PubMed] [Google Scholar]
- 51.Sadique S, Baqer AA, Salman AW, Iqbal MA, Kadim MM, Jamil F, Majeed A, Manahil S, Altaf A. Ruthenium complexes for breast cancer therapy. Rev Inorg Chem. 2024;44(2):191–208. 10.1515/revic-2023-0010. [Google Scholar]
- 52.Motswainyana WM, Ajibade PA. Anticancer activities of mononuclear ruthenium(II) coordination complexes. Adv Chem. 2015;2015:1–21. 10.1155/2015/859730. [Google Scholar]
- 53.Mendoza-Ferri MG, Hartinger CG, Mendoza MA, Groessl M, Egger AE, Eichinger RE, Mangrum JB, Farrell NP, Maruszak M, Bednarski PJ, Klein F, Jakupec MA, Nazarov AA, Severin K, Keppler BK. Transferring the concept of multinuclearity to ruthenium complexes for improvement of anticancer activity. J Med Chem. 2009;52(4):916–25. 10.1021/jm8013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang L, Wu X, Shi C, Wen H, Wu S, Chen J, Huang C, Wang Y, Liu Y. Synthesis and characterization of polypyridine ruthenium(II) complexes and anticancer efficacy studies in vivo and in vitro. J Inorg Biochem. 2022;236: 111963. 10.1016/j.jinorgbio.2022.111963. [DOI] [PubMed] [Google Scholar]
- 55.Engelbrecht Z, Roberts KE, Hussan A, Amenuvor G, Cronjé MJ, Darkwa J, Makhubela BCE, Sitole L. Synthesis and anti-cancer activity of bis-amino-phosphine ligand and its ruthenium(II) complexes. Bioorg Med Chem Lett. 2020;30(20): 127492. 10.1016/j.bmcl.2020.127492. [DOI] [PubMed] [Google Scholar]
- 56.Čanović P, Simović AR, Radisavljević S, Bratsos I, Demitri N, Mitrović M, Zelen I, Bugarčić ŽD. Impact of aromaticity on anticancer activity of polypyridyl ruthenium(II) complexes: synthesis, structure, DNA/protein binding, lipophilicity and anticancer activity. J Biol Inorg Chem. 2017;22(7):1007–28. 10.1007/s00775-017-1479-7. [DOI] [PubMed] [Google Scholar]
- 57.Conti L, Macedi E, Giorgi C, Valtancoli B, Fusi V. Combination of light and Ru(II) polypyridyl complexes: recent advances in the development of new anticancer drugs. Coord Chem Rev. 2022;469: 214656. 10.1016/j.ccr.2022.214656. [Google Scholar]
- 58.Kanaoujiya R, Meenakshi, Srivastava S, Singh R, Mustafa G. Recent advances and application of ruthenium complexes in tumor malignancy. Mater Today Proc. 2023;72:2822–2827. 10.1016/j.matpr.2022.07.098.
- 59.Lei J, Liu Y, Yin M, Li S, Wang Z, Chen Y. Coordination environment dependence of anticancer activity in cyclometalated bismuth(III) complexes with C, O-chelating ligands. J Inorg Biochem. 2024;256: 112571. 10.1016/j.jinorgbio.2024.112571. [DOI] [PubMed] [Google Scholar]
- 60.Getreuer P, Marretta L, Toyoglu E, Dömötör O, Hejl M, Prado-Roller A, Cseh K, Legin AA, Jakupec MA, Barone G, Terenzi A, Keppler BK, Kandioller W. Investigating the anticancer potential of 4-phenylthiazole derived Ru(ii) and Os(ii) metalacycles. Dalton Trans. 2024;53(12):5567–79. 10.1039/D4DT00245H. [DOI] [PubMed] [Google Scholar]
- 61.Lv A, Li G, Zhang P, Tao R, Li X, Ren X, Li P, Liu X, Yuan X-A, Liu Z. Design and anticancer behaviour of cationic/neutral half-sandwich iridium(III) imidazole-phenanthroline/phenanthrene complexes. J Inorg Biochem. 2024;257: 112612. 10.1016/j.jinorgbio.2024.112612. [DOI] [PubMed] [Google Scholar]
- 62.Von Hofmann AW. XXIV. Contributions to the history of the phosphorus-bases. Philos Trans R Soc Lond. 1860;150:497–533. 10.1098/rstl.1860.0025. [Google Scholar]
- 63.AW VonHofmann. XXII. Contributions to the History of the Phosphorus-Bases. Philos Trans R Soc Lond1860, 150, 409–448. 10.1098/rstl.1860.0023.
- 64.Hofmann AW. XXV—Contributions to the history of the phosphorus-bases. Q J Chem Soc. 1861;13(4):289–325. 10.1039/QJ8611300289. [Google Scholar]
- 65.Von Hofmann AW. XXIII. Contributions to the history of the phosphorus-bases. Philos Trans R Soc Lond. 1860;150:449–96. 10.1098/rstl.1860.0024. [Google Scholar]
- 66.Cahours AAT. Recherches Sur Les Radicaux Organométalliques; Impremerie De Mallet-Bachelier: Rue du Jardinet, 75006 Paris, France, 1860.
- 67.Mann FG, Wells AF, Purdie D. 386. The Constitution of Complex Metallic Salts. Part VI. The constitution of the phosphine and arsine derivatives of silver and aurous halides. The Configuration of the Co-Ordinated Argentous and Aurous Complex. J Chem Soc (Resumed). 1937. 10.1039/jr9370001828. [Google Scholar]
- 68.Ahrland S, Chatt J, Davies NR, Williams AA. The relative affinities of co-ordinating atoms for silver ion. Part II. Nitrogen, phosphorus, and arsenic. J Chem Soc Resumed. 1958. 10.1039/jr9580000276. [Google Scholar]
- 69.Ahrland S, Chatt J, Davies NR. The relative affinities of ligand atoms for acceptor molecules and ions. Q Rev Chem Soc. 1958;12(3):265. 10.1039/qr9581200265. [Google Scholar]
- 70.Booth G. Complexes of the transition metals with phosphines, arsines, and stibines. In: Advances in inorganic chemistry and radiochemistry. Amsterdam: Elsevier. 1964; vol. 6, pp 1–69.
- 71.Altaf M, Stoeckli-Evans H. Silver(I) tertiary phosphine complexes: influence of the anion on the structural and spectroscopic properties. Polyhedron. 2010;29(2):701–8. 10.1016/j.poly.2009.10.008. [Google Scholar]
- 72.Bachman RE, Andretta DF. Metal−ligand bonding in coinage metal−phosphine complexes: the synthesis and structure of some low-coordinate silver(I)−phosphine complexes. Inorg Chem. 1998;37(21):5657–63. 10.1021/ic980726k. [DOI] [PubMed] [Google Scholar]
- 73.Baranov AYu, Rakhmanova MI, Samsonenko DG, Malysheva SF, Belogorlova NA, Bagryanskaya IYu, Fedin VP, Artemev AV. Silver(I) and gold(I) complexes with Tris[2-(2-Pyridyl)Ethyl]phosphine. Inorganica Chim Acta. 2019;494:78–83. 10.1016/j.ica.2019.05.015. [Google Scholar]
- 74.Qian T-T, Xie Y-F, Shi H-T, Jia A-Q, Zhang Q-F. New adducts of silver(I) Halides, AgX (X = Cl, Br), with bidentate phosphine ligands: syntheses and molecular structures of [Ag3(Μ3-Cl)2(μ-Dppm)3][PF6], [Ag3(Μ3-Br)2(μ-Dppm)3][AgBr 2], {[Et4N][Ag2 (μ-Br)3(μ-Dppe)]}n, [Et4N]2[(AgCl2)2(μ-Dppe)], and [(AgCl)2(μ-Dppp)2]. Zeitschrift für Naturforschung B. 2017;72(5):327–34. 10.1515/znb-2016-0193. [Google Scholar]
- 75.Abdul Halim SNA, Nordin FJ, Mohd Abd Razak MR, Mohd Sofyan NRF, Abdul Halim SN, Rajab NF, Sarip R. Synthesis, characterization, and evaluation of silver(I) complexes with mixed-ligands of thiosemicarbazones and diphenyl(p-Tolyl)phosphine as biological agents. J Coord Chem. 2019;72(5–7):879–93. 10.1080/00958972.2019.1577400. [Google Scholar]
- 76.MohdSofyan NRF, Nordin FJ, MohdAbdRazak MR, Abdul Halim SNA, MohdKhir NAF, Muhammad A, Rajab NF, Sarip R. New silver complexes with mixed thiazolidine and phosphine ligands as highly potent antimalarial and anticancer agents. J Chem. 2018. 10.1155/2018/8395374. [Google Scholar]
- 77.Banti CN, Kyros L, Geromichalos GD, Kourkoumelis N, Kubicki M, Hadjikakou SK. A novel silver iodide metalo-drug: experimental and computational modelling assessment of its interaction with intracellular DNA, lipoxygenase and glutathione. Eur J Med Chem. 2014;77:388–99. 10.1016/j.ejmech.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 78.Engelbrecht Z, Meijboom R, Cronjé MJ. The ability of silver(I) thiocyanate 4-methoxyphenyl phosphine to induce apoptotic cell death in esophageal cancer cells is correlated to mitochondrial perturbations. Biometals. 2018;31(2):189–202. 10.1007/s10534-017-0051-9. [DOI] [PubMed] [Google Scholar]
- 79.Human Z, Munyaneza A, Omondi B, Sanabria NM, Meijboom R, Cronjé MJ. The induction of cell death by phosphine silver(I) thiocyanate complexes in SNO-esophageal cancer cells. Biometals. 2015;28(1):219–28. 10.1007/s10534-014-9817-5. [DOI] [PubMed] [Google Scholar]
- 80.Potgieter K, Engelbrecht Z, Naganagowda G, Cronjé MJ, Meijboom R. Anticancer activity of silver(I) cyclohexyldiphenylphosphine complexes toward SNO cancer cells. J Coord Chem. 2017;70(15):2644–58. 10.1080/00958972.2017.1366466. [Google Scholar]
- 81.Human-Engelbrecht Z, Meijboom R, Cronjé MJ. Apoptosis-inducing ability of silver(I) cyanide-phosphines useful for anti-cancer studies. Cytotechnology. 2017;69(4):591–600. 10.1007/s10616-017-0070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaur N, Popli P, Tiwary N, Swami R. Small molecules as cancer targeting ligands: shifting the paradigm. J Control Release. 2023;355:417–33. 10.1016/j.jconrel.2023.01.032. [DOI] [PubMed] [Google Scholar]
- 83.Helmy LA, Abdel-Halim M, Hassan R, Sebak A, Farghali HAM, Mansour S, Tammam SN. The other side to the use of active targeting ligands; the case of folic acid in the targeting of breast cancer. Colloids Surf B Biointerfaces. 2022;211: 112289. 10.1016/j.colsurfb.2021.112289. [DOI] [PubMed] [Google Scholar]
- 84.Naganagowda G, Engelbrecht Z, Potgieter K, Malan FP, Ncube P, Cronjé MJ, Meijboom R. Synthesis, crystal structure and spectral studies of silver(I) cyclohexyldiphenylphosphine complexes: towards the biological evaluation on malignant and non-malignant cells. J Coord Chem. 2023;76(1):45–60. 10.1080/00958972.2023.2164854. [Google Scholar]
- 85.Roberts KE, Engelbrecht Z, Potgieter K, Meijboom R, Cronjé MJ. Silver(I) bromide phosphines induce mitochondrial-mediated apoptosis in malignant human colorectal cells. Biomedicines. 2023;11(10):2794. 10.3390/biomedicines11102794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roberts KE, Cronjé M, Engelbrecht Z. Insights into silver(I) phosphine complexes in targeting cell death and metastatic mechanisms in malignant cell lines, University of the Witwatersrand, Johannesburg, 2023. https://wiredspace.wits.ac.za/items/3a67a569-5818-40ea-983a-e882dd884f61. Accessed 18 Feb 2025.
- 87.Ooi TC, Nordin FJ, Rahmat NS, Abdul Halim SNA, Sarip R, Chan KM, Rajab NF. Genotoxicity and apoptotic effect of silver(I) complexes with mixed-ligands of thiosemicarbazones and diphenyl(p-Tolyl)phosphine on malignant melanoma cells, SK-MEL-28. Mutation Res Genetic Toxicol Environ Mutagenesis. 2023;886: 503581. 10.1016/j.mrgentox.2022.503581. [DOI] [PubMed] [Google Scholar]
- 88.Zartilas S, Hadjikakou SK, Hadjiliadis N, Kourkoumelis N, Kyros L, Kubicki M, Baril M, Butler IS, Karkabounas S, Balzarini J. Tetrameric 1:1 and monomeric 1:3 complexes of silver(I) halides with tri(p-Tolyl)-phosphine: a structural and biological study. Inorganica Chim Acta. 2009;362(3):1003–10. 10.1016/j.ica.2007.07.034. [Google Scholar]
- 89.Meijboom, R.; Cronjé, M. J. Use of silver (i) complexes as anticancer agents. 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.








