Abstract
While bulk RNA sequencing and single-cell RNA sequencing have shed light on cellular heterogeneity and potential molecular mechanisms in the musculoskeletal system in both physiological and various pathological states, the spatial localization of cells and molecules and intercellular interactions within the tissue context require further elucidation. Spatial transcriptomics has revolutionized biological research by simultaneously capturing gene expression profiles and in situ spatial information of tissues, gradually finding applications in musculoskeletal research. This review provides a summary of recent advances in spatial transcriptomics and its application to the musculoskeletal system. The classification and characteristics of data acquisition techniques in spatial transcriptomics are briefly outlined, with an emphasis on widely-adopted representative technologies and the latest technological breakthroughs, accompanied by a concise workflow for incorporating spatial transcriptomics into musculoskeletal system research. The role of spatial transcriptomics in revealing physiological mechanisms of the musculoskeletal system, particularly during developmental processes, is thoroughly summarized. Furthermore, recent discoveries and achievements of this emerging omics tool in addressing inflammatory, traumatic, degenerative, and tumorous diseases of the musculoskeletal system are compiled. Finally, challenges and potential future directions for spatial transcriptomics, both as a field and in its applications in the musculoskeletal system, are discussed.
Subject terms: Bone, Pathogenesis
Introduction
Since the advent of next-generation sequencing in 2005 (ref. 1), transcriptomics research has made substantial advances. Bulk RNA sequencing (RNA-seq) provides information on gene expression, RNA structure, and protein translation, and is capable of identifying new genes and deciphering signal networks related to physiological states and pathological processes.2,3 However, RNA-seq only detects the average gene expression levels of mixed cells, masking phenotypic details of individual cells and the differences in transcriptomes between cells, leading to the potential dilution and oversight of important transcripts in specific cell types.4 To overcome this limitation, single-cell RNA sequencing (scRNA-seq) was introduced by Tang et al.5 in 2009, achieving the quantification of transcriptomes within individual cells for the first time. It provides a powerful tool for studying cellular heterogeneity, characterizing new cell types and states, and elucidating regulatory networks between cell clusters.6 However, scRNA-seq requires the harvest of single live cells from tissues without inducing cell stress or death. During cell separation, the disruption of intercellular connections and changes in the external microenvironment can lead to alterations in the internal transcriptome.4 Additionally, the hardness of bone and cartilage tissues and the specific cellular morphology of muscle cells present challenges in preparing single-cell suspensions from these tissues.7
Despite the unique data provided by RNA-seq and scRNA-seq for exploring tissue cellular heterogeneity, transcriptomic analysis by either of these techniques results in loss of the spatial context of cells,8 which is closely related to biological function.9 For example, from the enthesis with higher calcification to the tendon mid-body, and then to the myotendinous junction, the spatial organization of cells within the tendon is crucial for their ability to bear and transmit tensile forces.10 Similarly, the transcriptional heterogeneity of subpopulations of skeletal stem and progenitor cells (SSPCs) may depend on their diverse spatial positioning and ecological niches within the bone marrow. Even subtle changes in localization or intercellular crosstalk of SSPCs in the bone marrow microenvironment can profoundly impact their functional state or cell fate.11 Furthermore, although researchers can theoretically infer potential mechanisms based on receptor–ligand interactions from RNA-seq and scRNA-seq data, the biological feasibility of such mechanisms still requires further validation, specifically whether the interacting cells are in close spatial proximity and express the necessary genes in that spatial context.12 All these points emphasize the significant role of spatial location information in biomedical research.
The concept of spatial transcriptomics (ST) was first introduced in 2016 by the Lundeberg research group.13 Unlike traditional sequencing methods, it allows for the quantification and localization of the transcriptome while preserving the original spatial context. This approach provides data on associated cellular gene expression levels and spatial location information, creating a visual spatial transcriptome map that integrates with tissue morphological characteristics. For example, ST has been used to construct the first spatiotemporal transcriptomic atlas of human embryonic limb development.14 Moreover, ST helps to decipher the true gene expression of cells in situ within tissues, avoiding biases in detection caused by the loss of certain cell types or changes in cell transcriptomes during the single-cell dissociation process. For example, despite the important role adipocytes play in the bone marrow microenvironment, their fragility and large size make them difficult to analyze by scRNA-seq.15 Similarly, the multinucleated nature of mature osteoclasts16 and muscle fibers,17 along with the fragile nature and large cell volume of true hypertrophic chondrocytes,18 pose significant challenges for single-cell isolation and analysis. The spatial location information provided is advantageous for elucidating physiological and pathological mechanisms. At the subcellular level, the spatial localization of messenger RNA (mRNA) plays a crucial role in precisely controlling protein synthesis and function, helping to elucidate the spatial regulation of gene activity. At the cellular level, clarifying the spatial positions of cells within tissues aids in identifying cell types, defining cell functions, and deciphering the spatial organization of cells and their intercellular interaction networks.19,20 For example, ST has been utilized to analyze the cellular composition, spatial organizational structure, and intercellular crosstalk within the microenvironment of the SSPC niche in the bone marrow.12 As ST introduces a spatial dimension to gene expression studies, it offers a novel perspective and methodology for biomedical research, earning it the title of “Method of the Year 2020” by Nature Methods.21
ST technologies can be divided into two main categories based on RNA detection strategies: imaging-based ST technologies and sequencing-based ST technologies.9 With the continuous development of microscopic imaging and processing strategies and sequencing technologies, improvements in sequencing cost-effectiveness, and ongoing enhancements in computational strategies, the capabilities for data acquisition and analysis in ST have rapidly advanced. Currently, ST technologies have achieved unbiased whole-transcriptome analysis,13 nanoscale spatial resolution,22 and 3D construction of transcriptomic landscapes,23,24 among other multidimensional functionalities. In terms of applications, ST has been widely used in various fields including embryonic development,25–29 oncology,30,31 immunology,32,33 and neuroscience.34,35 However, the application of ST to the musculoskeletal system is still in the developmental stage. While Feng et al. provided a valuable overview of single-cell and spatial omics in musculoskeletal disorder research,36 a comprehensive review specifically focused on the advances and challenges of ST in this field remains lacking.
This article provides a brief overview of the development of ST technologies, focusing on the latest technological advances and widely-utilized representative technologies, along with a brief workflow for integrating ST into musculoskeletal system research. We summarize research progress in revealing the physiological mechanisms of the musculoskeletal system, particularly during developmental processes, as well as new findings and achievements in the study of inflammatory, traumatic, degenerative, and tumorous diseases of the musculoskeletal system. Finally, we discuss the current challenges and future developments, including challenges and prospects for the application of ST to the musculoskeletal system, the 3D landscape of the transcriptome, spatial multi-omics and spatiotemporal omics, and the application of artificial intelligence (AI) in ST.
Developments and classification of ST
Previous reviews have already provided a high-quality summary of ST data acquisition technologies.9,20,37–41 Therefore, this section provides only a brief overview of these technologies, focusing on the latest technological innovations and widely-adopted representative methods.
Imaging-based ST technologies
Imaging-based ST technologies (Table 1) include in situ hybridization (ISH) techniques, which utilize labeled probes containing complementary sequences to detect target RNA, and in situ sequencing (ISS) techniques that directly sequence RNA in its native tissue context.
Table 1.
Imaging-Based ST Technologies
| Method | Inventor | Year established | Sample type | Advantages | Limitations | Gene detection efficiency | Targeted/ transcriptome-wide | Spatial resolution | Commercial platform (vendor) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| ISH-based | ||||||||||
| smFISH | Femino et al. | 1998 | FF/FFPE | High sensitivity | Low throughput | Nearly 100% | Targeted | Subcellular | RNAscope (Bio-Techne) | 44,45 |
| seqFISH | Lubeck et al. | 2014 | FF/FFPE | Multiplexing | High cost, high error, optical crowding, limited view | 84% | Targeted | Subcellular | Molecular Cartography (Resolve Biosciences) | 47,225 |
| MERFISH | Chen et al. | 2015 | FF/FFPE | Multiplexing, improved detection robustness | High cost, limited view | 80%–95% | Targeted | Subcellular | MERSCOPE (Vizgen) | 49,226 |
| seqFISH+ | Eng et al. | 2019 | FF | Higher level of multiplexing, each image displays part of the transcripts, reducing optical crowding | High cost, time-consuming, limited view: analysis of small tissue profiles, only 1 mm2 | 49% | Targeted | Subcellular | NA | 48,50 |
| EEL FISH | Borm et al. | 2022 | FF | Multiplexing, high throughput, low cost, gapless RNA capture, eliminates tissue background impact | Low detection sensitivity | 2.6%–13.2% | Targeted | Subcellular | Rebus Esper (Rebus Biosystems) | 50 |
| CosMx SMI | Nanostring | 2022 | FF/FFPE | Automated equipment, targeted detection of RNA and proteins at the spatial level, high signal-to-noise ratio | Low throughput | One or two copies per cell | Targeted | Subcellular | CosMx SMI (Nanostring) | 51 |
| ISS-based | ||||||||||
| FISSEQ | Lee et al. | 2014 | FF/FFPE | Whole transcriptome analysis | Limited read length, extremely low detection efficiency, high proportion of rRNA reads, low throughput, expensive, optical crowding | < 0.005% | Transcriptome-wide | Subcellular | RC2 (ReadCoor) | 56,227 |
| STARmap | Wang et al. | 2018 | FF | High signal-to-noise ratio, high sensitivity, high precision, capable of resolving thick tissue sections | Low throughput, limited view | Slightly better than scRNA-seq | Targeted | Subcellular | Plexa In Situ Analyzer (Stellaromics) | 54 |
| Exseq | Alon et al. | 2021 | FF/FFPE | Optional targeted and non-targeted, nanoscale subcellular spatial resolution | Long imaging time | ~43.4% (Targeted) | Targeted or transcriptome-wide | Subcellular | NA | 22 |
| Xenium | 10x Genomics | 2023 | FF/FFPE | High detection sensitivity for low-level expressed genes, high specificity, fast data output capability | Platform capable of combined proteomic analysis pending release | 1.4 times higher than scFFPE-seq | Targeted | Subcellular | Xenium (10x Genomics) | 53 |
| Electro-seq | Li et al. | 2023 | Live cells | Paired detection of 3D transcriptional states and electrophysiological states | The causal relationship between gene expression and electrophysiology still needs further exploration | NA | Targeted | Cellular | NA | 55 |
ISH-based ST technologies
ISH techniques have evolved from early radioactive methods42,43 to fluorescence-labeled approaches, which have been continuously refined over the past decades.44,45 RNAscope, as one of the earliest commercially-available ST platforms, detects a limited number of genes with high sensitivity at subcellular resolution.46 To enhance RNA detection throughput, researchers have employed multiplexing encoding strategies, using unique fluorescent sequences or binary codes corresponding to individual RNAs. SeqFISH,47 seqFISH+,48 MERFISH,49 EEL FISH50 and NanoString’s Spatial Molecular Imaging (SMI) technology51 have significantly expanded the scale of target detection. MERFISH employs a unique binary encoding strategy integrated with error correction schemes, significantly enhancing the robustness of transcript recognition.49 EEL FISH uses electrophoresis to move RNA to a capture plane with minimal lateral dispersion, reducing tissue background interference while decreasing the time required for thick tissue z-axis imaging, albeit at the cost of losing axial resolution.50 SMI utilizes detection panels covering up to 18 000 genes and is the first imaging-based ST technology claiming near-whole transcriptome coverage in human or mouse samples. Additionally, it enables targeted multi-omics detection of RNA and proteins.51 Overall, the notable advantage of ISH-based ST technologies lies in their ability to detect low-abundance transcripts with high sensitivity while directly capturing their precise spatial information.
ISS-based ST technologies
Targeted ISS techniques utilize padlock probes and rolling-circle amplification to achieve in situ sequencing of target RNA.52 Xenium can detect low-abundance genes with high sensitivity and specificity and has rapid data output capabilities.53 The newly-introduced Xenium Prime 5 K assays can simultaneously detect up to 5 000 genes in human or mouse samples. Additionally, researchers can design fully customizable gene panels containing up to 480 genes to meet specific research needs. The commercialized version of STARmap, Plexa In Situ Analyzer, enables the mapping of spatial gene expression patterns in thick tissue samples with intact structural integrity, providing high-resolution 3D multi-omics perspectives.54 Electro-seq, a yet-to-be-commercialized technology, integrates chronic electrophysiological recordings with the construction of 3D transcriptome landscapes, providing a promising tool for characterizing cell states and developmental trajectories in electrogenic tissues, such as skeletal muscles.55
The introduction of non-targeted ISS techniques has facilitated a shift from targeting only known sequences to exploring unknown genes. FISSEQ56 and ExSeq22 are capable of unbiased whole-transcriptome analysis, albeit at the cost of low gene detection efficiency. Compared to ISH techniques, ISS-based ST technologies enable the detection of both targeted and non-targeted transcripts, offer a higher signal-to-noise ratio, and possess the capability to detect single-nucleotide variations.57 However, due to inefficient reverse transcription steps and the low ligation efficiency of padlock probes, ISS-based ST technologies exhibit lower detection sensitivity, particularly when employing multiplexing strategies.57,58
Advantages and limitations of imaging-based ST technologies
Imaging-based ST technologies share several common advantages. First, high sensitivity and subcellular resolution are two key strengths. However, gains in sensitivity and spatial resolution come at the cost of detecting fewer transcripts.59 Second, these technologies are broadly applicable to formalin-fixed and paraffin-embedded (FFPE) samples, which is particularly valuable in musculoskeletal research, especially when working with hard tissues such as bone. This will be elaborated upon in detail later in the text. Like their advantages, the limitations of imaging-based ST technologies are also pronounced. First, the targeted strategy restricts new discoveries of genes and their spatial positions that are not included in the prespecified target panel. Second, imaging-based ST technologies often entail high temporal and financial costs, requiring expensive and complex microscopes or staining apparatus. Third, the maximum imaging area of currently available technologies, provided by Xenium at 4.72 cm², limits their applicability to long tissue sections, such as the longitudinal sections of tendons. Fourth, the imaging data from a single sample can reach several hundred gigabytes, posing significant challenges for data storage, processing, and sharing.
Sequencing-based ST technologies
Sequencing-based ST technologies (Table 2) record spatial positions by selecting regions of interest (ROIs) or using spatial barcodes. Spatial barcodes can be organized in an array on the capture surface, printed onto tissues via microfluidic channels, or directly tagged to individual cells or nuclei.
Table 2.
Sequencing-Based ST Technologies
| Method | Inventor | Year established | Sample type | Advantages | Limitations | Gene detection efficiency | Targeted/ transcriptome-wide | Spatial resolution | Commercial platform (vendor) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| ROI-selection-based | ||||||||||
| LCM | Emmert-Buck et al. | 1996 | FF/FFPE | Highly suitable for FFPE, whole transcriptome analysis, high spatial resolution | Time-consuming, low throughput | NA | Targeted or transcriptome-wide | Cellular | LCM(Arcturus, PALM, Leica) | 60 |
| Geo-seq | Chen et al. | 2017 | FF | Robust, more sensitive than LCM | Low throughput, difficult to achieve single-cell spatial resolution | NA | transcriptome-wide | Multicellular | NA | 24 |
| GeoMx DSP | NanoString | 2019 | FF/FFPE | Multiplexing, suitable for FFPE samples, capable of combining multi-omics detection | Limited sensitivity | NA | transcriptome-widea | Variable, minimum up to 10 μm | GeoMx DSP (NanoString) | 61 |
| Spatial barcode-based | ||||||||||
| Visium | 10× Genomics | 2019 | FF/FFPE | Unbiased whole transcriptome analysis, adaptable to FFPE tissues | Limited RNA capture efficiency, limited spatial resolution | >6.9% | transcriptome-widea | 55 μm | Visium (10× Genomics) | 13 |
| Slide-Seq | Rodriques et al. | 2019 | FF | Unbiased whole transcriptome analysis, high spatial resolution | Not suitable for highly heterogeneous samples, low capture efficiency, in situ sequencing is time-consuming | 0.3% | transcriptome-wide | 10 μm | NA | 66 |
| DBiT-seq | Liu et al. | 2020 | FF/FFPE | Unbiased whole transcriptome analysis, variable spatial resolution, suitable for FFPE tissues, detection of RNA and proteins in a spatial context | Limited spatial resolution | ~15.5% | transcriptome-wide | Variable (10 μm, 25 μm, 50 μm) | NA | 68 |
| Slide-seqV2 | Stickels et al. | 2021 | FF | Unbiased whole transcriptome analysis, high spatial resolution, improved capture efficiency | Capture efficiency remains low | Approximately 10 times higher than Slide-seq | transcriptome-wide | 10 μm | Curio Seeker (Curio Biosciences) | 67 |
| Stereo-seq | BGI Genomics | 2021 | FF/FFPE | Unbiased whole transcriptome analysis, very high spatial resolution, large detection area | Capture efficiency remains low | Comparable to Visium | transcriptome-wide | 220 nm | STOmics Stereo-seq, Stereo-seq OMNI (BGI Genomics) | 28,38,228 |
| XYZeq | Lee et al. | 2021 | FF | Unbiased whole transcriptome analysis, true single-cell resolution | Limited spatial resolution | NA | transcriptome-wide | 500 μm (spot-to-spot center distance) | NA | 70 |
| sci-Space | Srivatsan et al. | 2021 | FF | Unbiased whole transcriptome analysis, true single-cell resolution | Limited spatial resolution | NA | transcriptome-wide | ~222 μm (spot-to-spot center distance) | NA | 71 |
| xDBiT | Wirth et al. | 2023 | FF/FFPE | Unbiased whole transcriptome analysis, improved gene detection efficiency and throughput compared to DBiT-seq | Limited spatial resolution | Higher than DBiT-seq | transcriptome-wide | 50 μm | NA | 69 |
| Visium HD | 10×Genomics | 2024 | FFPE | Using about 18 000 probes for near-whole transcriptome level analysis, no gaps in capture area | Only compatible with human and mouse FFPE tissues | NA | transcriptome-widea | 2 μm | Visium HD (10× Genomics) | 229 |
aNear-complete transcriptome coverage can only be achieved in human/mouse samples or evolutionarily similar species
ROI-selection-based ST technologies
Separation of ROIs through physical dissection or the application of optical or molecular markers represents a straightforward and effective strategy for obtaining spatial location information. Laser capture microdissection (LCM)60 is one of the representative techniques for physical dissection, utilizing ultra violet (UV) lasers to cut tissues or infrared radiation (IR) lasers to fuse tissues with a membrane to isolate ROIs. It is highly compatible with FFPE samples and extensively used in ST research. Geo-seq, which combines LCM with scRNA-seq, enables the exploration of cellular heterogeneity within ROIs.24 Using optical or molecular markers to designate ROIs avoids the impacts of physical dissection on the transcriptome. GeoMx Digital Spatial Profiler (GeoMx DSP)61 enables the quantitative analysis of RNA and proteins within ROIs in FFPE and FF samples by utilizing cleavable oligonucleotides and antibodies. In summary, the advantage of such technologies lies in the ability for researchers to select ROIs based on functional units of the tissue. However, the selection of ROIs is labor-intensive, requires prior knowledge, and carries the risk of potential selection bias. Moreover, these technologies typically do not offer single-cell spatial resolution.
Spatial barcode-based ST technologies
Since 2016, researchers have utilized spatial barcodes and unique molecular identifiers for the localization and quantification of RNA. Spatial Transcriptomics (ST)13 pioneered this approach, capturing transcripts at array sites containing spatial barcodes. Visium, an upgraded version of ST commercialized by 10x Genomics, has improved detection efficiency and spatial resolution. Visium is now compatible with FFPE samples62 and supports Nanopore long-read sequencing.63 Two improved versions of Visium, RNA-rescue ST (RRST)64 and spatial total RNA-sequencing (STRS),65 enable effective analysis of RNA from low-quality FF tissues and detection of a full spectrum of RNA, including non-coding RNA, respectively. The introduction of Slide-Seq66 enabled spatial resolution to reach near single-cell accuracy for the first time in such technologies. Slide-seqV2 (ref. 67) has undergone improvements in bead synthesis, array indexing, and library preparation, enhancing detection efficiency. Termed as the “ultra-wide-angle ten billion-pixel camera of life”, Stereo-seq28 uses DNA Nanoballs for unbiased whole-transcriptome detection at nanoscale resolution and high sensitivity in large fields of view. Notably, the cDNA generated by Stereo-seq requires sequencing on a specialized sequencer. Visium HD, recently released by 10x Genomics, achieves continuous tissue coverage without capture gaps, obtaining high-quality data at the micrometer scale.
Strategies that print spatial barcodes onto tissues via microfluidic channels effectively circumvent issues of RNA lateral diffusion during capture. DBiT-seq,68 as a representative technology of this strategy, enables spatial quantification of mRNA and proteins. xDBiT,69 an upgraded version of DBiT-seq, improves gene detection efficiency and throughput. Compared to capture array-based approaches, microfluidics-based ST technologies are more cost-effective.58
Due to the complexity of cell contours, a single capture area may capture transcripts from multiple cells, and transcripts within a single cell may contribute to multiple capture points. Therefore, performing actual single-cell level transcriptomic analysis is highly challenging. In XYZeq70 and sci-Space,71 spatial barcodes are used to mark individual cells or nuclei, rather than capture points, achieving true single-cell resolution at the expense of lower spatial resolution.
Advantages and limitations of sequencing-based ST technologies
Compared to image-based strategies, these techniques significantly improve throughput and whole transcriptome unbiased analysis capabilities. In addition, sequencing-based ST technologies can identify gene expression patterns over larger areas, with Stereo-seq offering a maximum area of up to 13.2 cm × 13.2 cm.28 Furthermore, most of these technologies require only standard sequencing equipment rather than expensive specialized instruments, facilitating their widespread application in ST research. Moreover, sequencing is considerably less time-consuming compared to imaging processes. To date, these technologies have achieved a range of spatial resolutions, spanning from tens of microns to submicron levels. However, their relatively limited sensitivity remains a common limitation. In addition, poly-A-based capture strategies tend to favor the detection of highly-expressed genes and are not compatible with FFPE samples.
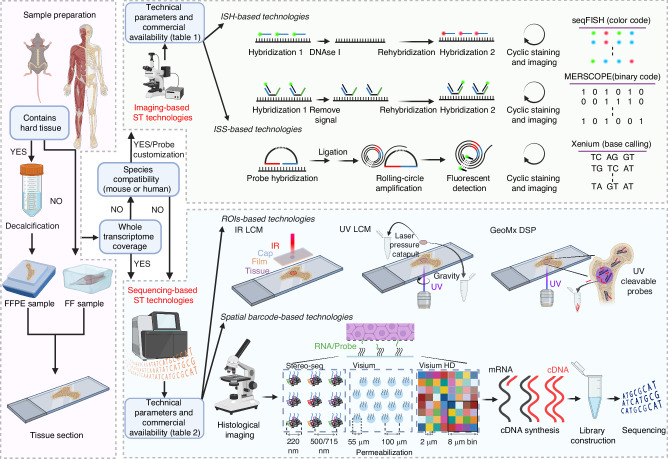
A concise workflow for integrating ST into musculoskeletal system research (Fig. 1)
Fig. 1.
A Concise Workflow for Integrating ST into Musculoskeletal System Research and Overview of the Main ST Technologies. The figure highlights key considerations for conducting ST research in the musculoskeletal system, such as sample types, transcriptome coverage, and species compatibility, in order to select between imaging-based or sequencing-based technologies. It also illustrates the main ST technologies, which researchers can choose based on different technical parameters and commercial availability. However, it is important to acknowledge that the choice of technology is a complex process, and this workflow may not be universally applicable to all biological questions. Researchers should carefully weigh their options when making a technological choice. Furthermore, it should be noted that some probe-based sequencing platforms, such as GeoMx DSP, Visium V2, and Visium HD, are limited to near-whole transcriptome detection in humans and mice only. cDNA complementary DNA. Created in BioRender. Wang, H. (2025) https://BioRender.com/e71m592
In the context of musculoskeletal system research, sample compatibility plays a crucial role in the selection of ST platforms. When working with hard tissues such as bone, FFPE samples are often preferred due to the need for decalcification. The compatible sample types for various ST technologies are summarized in Tables 1 and 2. Additionally, previous studies have provided a focused summary of ST platforms specifically adapted for FFPE samples.72 The biological questions to be addressed are a prerequisite for selecting an ST platform. Most sequencing-based ST technologies offer the ability to achieve unbiased whole-transcriptome coverage, making them well-suited for hypothesis-generating exploratory studies. In contrast, imaging-based ST technologies provide high-sensitivity detection of target genes, making them more appropriate for hypothesis testing and clinical research.73 Furthermore, species compatibility is a crucial consideration in the decision-making process. Imaging-based commercial platforms and probe-based sequencing platforms, such as GeoMx DSP, Visium V2, and Visium HD, are restricted to transcriptome detection in mouse, human, or evolutionarily similar species. Although customization of probe panels is feasible, it substantially increases both the complexity and cost of experiments. After addressing fundamental considerations such as sample compatibility, research objectives, and species adaptability, specific technical parameters, such as sensitivity and spatial resolution, along with the advantages and limitations of each technique, become critical factors for further evaluation. As we have discussed above, trade-offs often exist between various technical parameters within a single technology or platform.74 In recent years, advances in ST technologies and the emergence of new commercial platforms have progressed in parallel, both entering a phase of rapid development. Compared to technologies confined to laboratory settings, commercial platforms are more robust and mature, offering essential technical support and user-friendly analytical interfaces, making them a more accessible yet higher-cost option.74 Finally, given the complementary nature of imaging-based and sequencing-based ST technologies, employing two complementary methods within the same study, such as the “wide-angle” Visium combined with the “focused” RNAscope,75 can significantly enhance our ability to explore the spatial transcriptome landscape, offering a more comprehensive and detailed perspective.
Applications of ST in the musculoskeletal system
Although RNA-seq and scRNA-seq have facilitated our understanding of the physiological and pathological mechanisms within the musculoskeletal system, further clarification is needed regarding the spatial heterogeneity of gene expression, spatial relationships between cells, and their interactions based on spatial context. Currently, the application of ST to the musculoskeletal system is still in its developmental stages. This section aims to introduce the advances in ST concerning the physiological mechanisms of the musculoskeletal system, particularly during developmental processes (Table 3), as well as new findings and breakthroughs enabled by ST in the study of various diseases of the musculoskeletal system (Table 4).
Table 3.
Advances in the Application of ST to Physiological Mechanisms of the Musculoskeletal System
| Author | ST Technologies | Resource | Tissues/ Structures | Sample type | Key research achievements | References |
|---|---|---|---|---|---|---|
| Xiao et al. | Visium/RNAscope | mice | femurs | FFPE | Confirmed the feasibility of applying spatial barcode-based ST technologies to fully mineralized mature long bone tissue. Analyzed the cellular composition and interaction networks of the SSPCs’ microenvironment. | 12 |
| Zhang et al. | Visium/RNA-ISH | human | embryonic limb | FF | Constructed the first single-cell spatiotemporal transcriptome landscape of human embryonic limb development. | 14 |
| Mirzazadeh et al. | RRST | mice | growth plate | FF | Identified significantly-upregulated soluble factors in the SOC and SOC-adjacent areas of mice, including Ccl9, Basp1, and Apln in the SOC area and Msmp in the SOC-adjacent area which may influence spatially-proximate chondrocytes. | 64 |
| Piña et al. | Visium/RNAscope | mice | secondary palate | FFPE | Accurately pinpointed the onset of palatal ossification between E14.5 and E15.5, and identified the spatiotemporal localization of marker genes (Deup1, Lrrc23, Dynlrb2) during palatal fusion. | 75 |
| Gribaudo et al. | tomo-seq/RNAscope | organoid models | - | FF | Confirmed that human trunk self-organization organoid model can replicate multi-tissue concomitant morphogenesis of the spinal cord and vertebral column similar to in vivo conditions. | 76 |
| Chen et al. | Visium/ISS | mice | intervertebral disc | FF | Constructed the first spatial transcriptome atlas of the intervertebral disc. | 77 |
| Zhang et al. | stereo-seq | mice | shoulder region | not mentioned | Intricately described the complexity and spatial heterogeneity of fibrocartilage attachment site cells in the shoulder region of postnatal mice. Revealed the molecular dynamics during fibrocartilage differentiation. | 78 |
| Lui et al. | LCM/RNA-ISH | mice | tibial articular cartilage | FF/FFPE | Identified new signaling pathways with spatial regulation during the growth of mouse articular cartilage. Revealed similarities in gene spatial expression patterns from the superficial to the deep regions of joint cartilage and from hypertrophic to resting zones of growth plate cartilage. | 79 |
| Chau et al. | manual microdissection/RNA-ISH | rat | proximal tibial epiphyses | FF/FFPE | Revealed similarities in gene spatial expression patterns from the superficial to the deep regions of joint cartilage and from hypertrophic to resting zones of growth plate cartilage. | 80 |
| Bian et al. | Visium/FISH | mice | Hindlimbs | FFPE | Uncovered the critical role of the G protein-coupled receptor ADGRG6 in regulating chondrocyte proliferation and differentiation, as well as maintaining growth plate homeostasis by Indian Hedgehog signaling. | 81 |
| Tong et al. | LCM | mice | knee joints | FF | Provided critical insights into the molecular and spatial mechanisms driving SOC development. Indicated that mesenchymal progenitors in the periarticular region around epiphyseal cartilage play a critical role in initiating SOC development and forming subchondral bone. | 82 |
| Tower et al. | Visium | mice | calvaria | FF | Discovered that the presence of sensory innervation maintains the undifferentiated state of mesenchymal cells in cranial sutures to keep cranial sutures patent, while the absence of sensory innervation leads to dysregulation of BMP/TGF-β signaling, manifesting as premature closure of cranial sutures. | 83 |
| Baccin et al. | LCM | mice | femurs | FF | Established the first spatial atlas of the bone marrow microenvironment. | 84 |
| D’Ercole et al. | Visium/LCM | mice | tibialis anterior, associated extensor digitorum longus | FF | Examined the distinct morphofunctional regions in muscle and their reaction to reversible nerve injury, emphasizing the polyamine pathway as a possible contributor to muscle atrophy. | 85 |
| Karlsen et al. | Visium | human | myotendinous junction | FF | Analyzed the different myofibre domains in the human myotendinous junction at the single-nucleus spatial level. | 86 |
| Steffen et al. | Visium | rat | patellar tendons | FF | Presented the first spatial gene expression landscape of healthy tendon, and clarified the spatial expression patterns of tendon-associated genes. | 87 |
Table 4.
Advances in the application of ST to various diseases of the musculoskeletal system
| Author | ST Technologies | Resource | Tissues/ Structures | Sample type | Pathologies/Disorders | References |
|---|---|---|---|---|---|---|
| Inflammatory Diseases | ||||||
| Vickovic et al. | ST | human | synovial tissue | FF | rheumatoid arthritis | 92 |
| Meng et al. | Visium | human | synovial tissue | FF | rheumatoid arthritis | 93 |
| Smith et al. | Visium | human | synovial tissue | FF | rheumatoid arthritis | 94 |
| Rauber et al. | Visium | human | synovial tissue | FF | psoriatic arthritis/rheumatoid arthritis | 95 |
| Zheng et al. | Visium | human | synovial tissue | FF | osteoarthritis/rheumatoid arthritis | 96 |
| Carlberg et al. | ST | human | synovial tissue | FF | rheumatoid arthritis/spondyloarthritis | 97 |
| Hardt et al. | Visium | human | synovial tissue | FF | rheumatoid arthritis | 98 |
| MacDonald et al. | CosMx SMI | human | synovial tissue | FFPE | rheumatoid arthritis | 99 |
| Kenney et al. | Visium | mice | popliteal lymph nodes | FF | rheumatoid arthritis | 100 |
| Traumatic Diseases | ||||||
| McKellar et al. | STRS | mice | tibialis anterior muscles | FF | muscle injuries | 65 |
| McKellar et al. | Visium | mice | tibialis anterior muscles | FF | muscle injuries | 109 |
| Young et al. | Visium/smFISH | mice | gastrocnemius muscles/tibialis anterior muscles | FF | muscular dystrophy/muscle injuries | 110 |
| Larouche et al. | Visium | mice | tibialis anterior muscles | FF | volumetric muscle loss | 111 |
| Ackerman et al. | Visium | mice | hind paws | FF | tendon injuries | 112 |
| Cherief et al. | Visium | mice | Achilles tendon | FF | tendon injuries | 113 |
| Kang et al. | Visium | mice | Achilles tendon | FFPE | traumatic heterotopic ossification | 114 |
| Tower et al. | Visium | mice | digit | FF | limb defects | 117 |
| Wan et al. | Visium | mice | calvarium | FF | bone defects | 118 |
| Jiang et al. | Visium | mice | femurs | FFPE | normal and pathological fractures | 119 |
| Rios et al. | Visium | mice/human | pseudarthrosis | FFPE | fracture in neurofibromatosis type 1 | 120 |
| Mathavan et al. | Visium | mice | femurs | FFPE | fractures | 121 |
| Foster et al. | Visium | mice | dorsal skin | FF | skin injuries | 123 |
| Yang et al. | Visium | mice | dorsal skin | FF | skin injuries | 124 |
| Chen et al. | Visium | human | umbilical cord | FF | skin injuries | 125 |
| Degenerative Diseases | ||||||
| Fan et al. | Geo-seq | human | knee articular cartilage | FF | osteoarthritis | 134 |
| Yang et al. | Visium | human | anterior cruciate ligaments | not mentioned | osteoarthritis | 137 |
| Perez et al. | GeoMx DSP | human | vastus lateralis | FFPE | sarcopenia | 138 |
| Akbar et al. | Visium | human | hamstring tendon/supraspinatus tendon | not mentioned | tendinopathy | 139 |
| Fu et al. | Visium | human | supraspinatus tendon | not mentioned | tendinopathy | 140 |
| Tumorous Diseases | ||||||
| Zhang et al. | Visium | human | tumor specimens | FF | chordoma | 148 |
| Wrenn et al. | GeoMx DSP | mice | tumor specimens | FFPE | Ewing sarcoma | 150 |
| Li et al.a | ST | human | tumor specimens | FF | pan-cancer with M1 macrophage infiltration | 152 |
| Ihle et al. | GeoMx DSP | human | tumor specimens | FFPE | prostate cancer bone metastasis | 156 |
aLi et al. did not perform ST experiments and their ST data were sourced from SpatialDB.230
Advances in the application of ST to physiological mechanisms of the musculoskeletal system
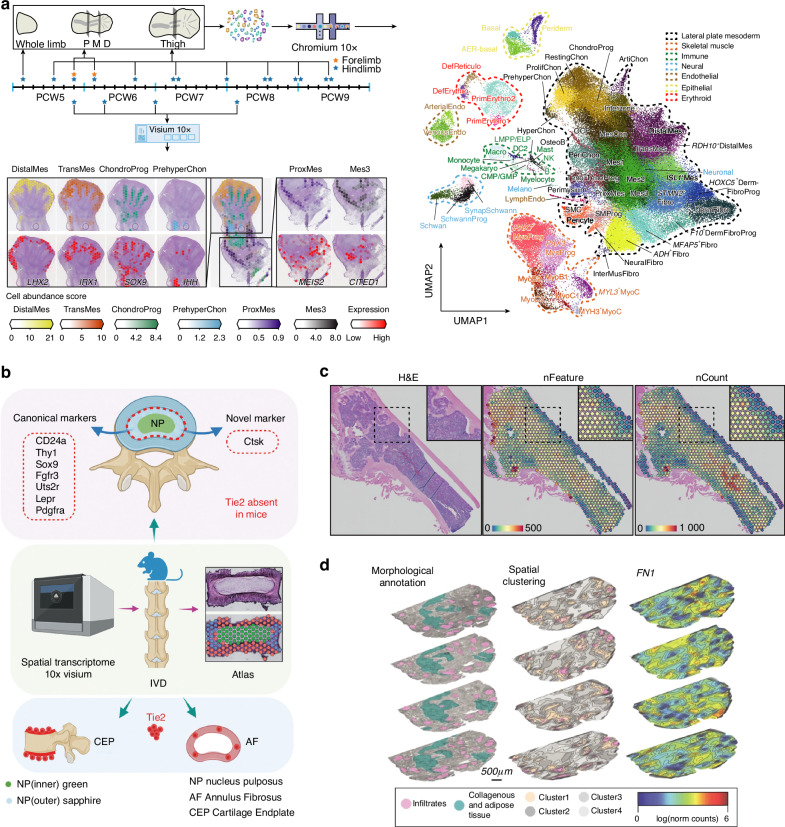
ST provides new insights into the physiological mechanisms of the musculoskeletal system at various developmental stages including the embryonic, juvenile, and mature phases. The embryonic development stage of the musculoskeletal system involves a complex and finely-coordinated evolution of cells and changes in gene spatial expression patterns. Although studies on model organisms have elucidated the fundamental mechanisms of limb development in vertebrates, the spatiotemporal characteristics of this process in humans remain incompletely understood. Zhang et al.14 conducted an analysis of multiple time points during human embryonic limb development using scRNA-seq and Visium. Utilizing Uniform Manifold Approximation and Projection (UMAP), they visualized 125 955 human embryonic limb cells identified through scRNA-seq. The spatial data revealed the spatial distribution of specific cell types and the spatial expression patterns of their corresponding marker genes (Fig. 2a). Consequently Zhang et al.14 successfully provided the first intricate depiction of the single-cell spatiotemporal transcriptome landscape of human embryonic limb development. The study further deciphered the cellular evolutionary pathways and cell spatial positioning determination processes from early limb formation to complete morphogenesis. It revealed two phases of human skeletal muscle development characterized by different cellular states and identified temporally-regulated gene expression patterns crucial for limb formation. The detailed portrayal of human limb development by Zhang et al.14 is valuable for gaining a deeper understanding of the mechanisms behind congenital limb syndromes and improving their diagnosis and treatment strategies. Moreover, this study also revealed substantial homology between mice and humans, demonstrating high similarity in the spatial expression patterns of genes controlling forelimb/hindlimb and proximal–distal identity, confirming mice as a reliable model for studying human physiological and pathological mechanisms.14 In addition, ST also contributed to the spatial analysis of the palatal fusion process75 and to the development and validation of complex higher-order organoid models related to embryonic development.76
Fig. 2.
Application of ST in Physiological Mechanisms and Inflammatory Diseases of the Musculoskeletal System. a At different time-points of human embryonic limb development, scRNA-seq and ST have been used to construct a spatiotemporal transcriptomic map. UMAP visualization of 125 955 human embryonic limb cells. In human posterior limb tissue slices at PCW6.2, spatial heatmaps of specific cell types and corresponding marker genes.14 Copyright © 2023, The Author(s). b Multiple canonical markers detected in the murine NP region, but Tie2 only in the CEP or AF region.77 © 2024 The Authors. Advanced Science published by Wiley-VCH GmbH. c H&E-stained sections of adult murine femur and heatmaps of the number of unique genes (nFeature) or unique transcripts (nCount) detected at each capture site.12 Copyright © 2023, The Author(s). d Morphological annotation, spatial clustering, and spatial expression pattern of FN1 of a serum-positive RA patient sample.92 Copyright © 2022, The Author(s). P proximal, M middle, D distal, PCW post-conception week, infiltrates leukocyte infiltration sites
The juvenile phase of the musculoskeletal system represents a dynamic period of growth and differentiation, marked by significant cellular turnover and the establishment of mature tissue structures. ST enables precise parsing of gene spatial expression patterns during this critical developmental window, providing unique insights into cellular hierarchy and lineage specification through the combination of lineage tracing. Nucleus pulposus progenitor cells (NPPCs) play a crucial role in maintaining cellular refreshment and supporting the development of nucleus pulposus (NP) tissue.77 However, the spatial differentiation trajectory of NP cells remains to be further explored. Chen et al.77 constructed the first spatial transcriptome atlas of the intervertebral disc (IVD) of juvenile mice using Visium, combined with lineage tracing to identify cells located at the periphery of the NP and expressing Cathepsin K (Ctsk) as NPPCs, which generate the entire NP adult tissue. Meanwhile, Tie2, long suggested as a marker for NPPCs, was not observed in the juvenile mouse NP but was found in the cartilage endplate (CEP) and annulus fibrosus (AF), as confirmed through ISS (Fig. 2b). Spatial analysis of the IVD suggests that previous reports regarding Tie2+ cells in NP tissue may have been influenced by potential contamination from Tie2+ cells in adjacent tissues such as the CEP or AF. Moreover, the existence of Tie2+ NPPCs in humans and other species remains to be further investigated. Beyond the IVD, ST has also been applied to the analysis of developing fibrocartilage attachment sites,78 articular cartilage,79 growth plate cartilage,80,81 the secondary ossification center (SOC),64,82 and the cranium.83
Analyzing the mature stage of the musculoskeletal system through ST helps improve understanding of its physiological characteristics under steady-state conditions and provides a foundation for exploring various diseases. The homeostatic microenvironment of bone marrow provides essential support for the physiological self-renewal and differentiation of SSPCs.12 Baccin et al.,84 using scRNA-seq and LCM-seq, established the first spatial atlas of the bone marrow microenvironment, revealing different perivascular niches contributed by the spatial heterogeneity of Cxcl12-abundant reticular cell subpopulations. Xiao et al.12 confirmed the feasibility of applying spatial barcode-based ST technologies to fully mineralized adult long bones. Specifically, the study utilized the Visium platform with a probe panel covering approximately 20 000 genes to analyze decalcified FFPE sections of mature mouse femur, successfully demonstrating a high transcript recovery efficiency (Fig. 2c). Xiao et al.12 further analyzed the cellular composition and interaction networks within the microenvironment of the SSPCs, providing a comprehensive understanding of both local and global regulatory SSPC networks. Additionally, ST has been applied to the spatial characterization of mature skeletal muscle,85 myotendinous junctions,86 and tendons.87
Applications of ST in inflammatory diseases of the musculoskeletal system
Rheumatoid arthritis (RA) is a common chronic systemic autoimmune disease affecting between 0.25% and 1% of the global population.88,89 Despite the continuous development and application of disease-modifying antirheumatic drugs, which control disease activity in most patients with RA, a proportion of patients still experience limited treatment benefits.90 The pathological mechanisms behind the clinical manifestations and treatment responses of RA remain to be explored further.91 ST offers a new means to reach an in-depth understanding of the complex pathological mechanisms of musculoskeletal inflammatory diseases such as RA. Vickovic et al.92 analyzed the differences in cellular composition and spatial organization and interactions of cells in the synovial sites of patients with seropositive and seronegative RA, constructing an exploratory multi-dimensional view of RA by combining tissue morphology with spatial transcriptome landscapes. To further explore the pathological mechanisms of RA and discover new therapeutic target clues, researchers have conducted detailed studies on fibroblast-like synoviocytes (FLSs),93–96 immune cells,92,97–99 and synovial draining lymph nodes100 using ST.
FLSs are considered tissue-resident cells involved in the initiation, maintenance, and resolution of chronic inflammation in arthritis, and their activation is a key step in the occurrence and development of arthritis.101,102 Smith et al.94 by analyzing the chromatin accessibility and gene expression profiles of FLSs, constructed a spatial atlas of FLS cell states influenced by local exposure to TNF, IFN-γ, and IL-1β during active RA. It is particularly noteworthy that inhibition of IL-1 may improve the activated state of the lining FLSs and prevent further joint damage.94 The role of FLSs during the remission and relapse of inflammation remains a topic for further investigation. Rauber et al.95 found that during the remission process of arthritis, FLSs transitioned from an MMP3 + /IL6+ phenotype to a CD200 + DKK3+ phenotype. Their ST analysis of synovium from RA and psoriatic arthritis patients showed that MMP3 + /IL6+ FLSs were spatially proximate to inflammatory immune cells in areas of active inflammation, whereas CD200 + DKK3+ FLSs co-localized with type 2 innate lymphoid cells in areas of inflammation resolution, further indicating that CD200+ FLSs formed an inflammatory resolution-promoting microenvironment in arthritis.95 Meng et al.93 integrated single-cell and spatial transcriptomics data to construct the spatial transcriptome landscape of the synovium in patients with RA undergoing sustained remission and relapse, identifying co-localization in the lining layer of recurrent RA patients among macrophages considered precursors to osteoclasts that express CTSK+, pro-inflammatory M1-type macrophages, and CD55+ lining FLSs, which significantly increased in proportion in the synovium during relapse. The study by Meng et al. also revealed the crucial role of the fibroblast growth factor (FGF) signaling pathway in the recurrence of RA, with a notable increase in expression levels in the lining FLSs of recurrent RA patients.93 Further research indicated that knocking out FGF10 or inhibiting FGF receptor 1 (FGFR1) effectively regulated the activity of the FGF pathway, reducing bone erosion, and offering new hope for the remission of recurrent RA.93
Immune cells play an undeniable role in the occurrence and progression of arthritis. In chronic inflammatory sites of non-lymphoid tissues, organized structures formed by the aggregation of immune cells are referred to as tertiary lymphoid organs (TLOs).103 Although scRNA-seq provides unique data on the cellular heterogeneity of RA synovial tissue,104,105 the limited understanding of the spatial organization of TLOs within RA synovium hinders our deeper insights into the pathogenesis of RA and its therapeutic responses. Vickovic et al.92 conducted serial sectioning of synovial tissue from RA patients, identifying leukocyte infiltration sites through morphological annotation, and delineating key spatial regions characterized by distinct gene expression patterns using unsupervised clustering approaches. The study also demonstrated the spatial expression pattern of Fibronectin 1 (FN1). The expression level of FN1 is positively correlated with transforming growth factor beta (TGF-β) activity, and TGF-β, due to its pivotal role in joint destruction, is considered a crucial target for monitoring disease progression and developing therapeutic strategies.106 In summary, Vickovic et al. constructed a fused morphological 3D spatial transcriptome landscape of synovial tissue from RA patients (Fig. 2d). The study also identified differences in the spatial gene expression patterns between seropositive and seronegative RA patients within or around TLOs. By integrating scRNA-seq data, it further revealed the specific localization patterns of different cell types.92 Additionally, other ST studies have contributed to uncovering the key roles of T cells,97 B cells,98 and dendritic cells99 in RA synovium.
Kenney et al.100 were the first to use combined single-cell transcriptomics and ST to analyze the pathological changes in draining lymph nodes during the progression of RA. The study revealed that in the draining lymph nodes of late-stage arthritis, close crosstalk between ALCAM+ macrophages and CD6 + T cells promoted B cell differentiation into plasma cells and IgG2b+ class switching.100 The aggregation of IgG2b+ plasma cells near the MARCO+ sinusoids in the draining lymph nodes was associated with the exacerbation of late-stage arthritis.100 The findings of Kenney et al. further deepen our understanding of the relationship between arthritis progression and lymphatic dysfunction. In future, the therapeutic potential of targeted inhibition of CD6 in RA still requires further exploration.
Applications of ST in traumatic diseases of the musculoskeletal system
Globally, trauma accounts for over one in ten deaths, with non-fatal injuries potentially leading to impaired motor function and reduced quality of life.107 Clinicians and researchers have long pursued functional regeneration and the avoidance of non-functional fibrotic repair in tissues such as skin, muscle, and tendon following trauma, as well as deciphering the spatial molecular mechanisms linking local mechanical environments to cellular responses during fracture healing to promote optimal bone repair. However, limited understanding of the cellular and molecular spatiotemporal mechanisms during healing has prevented the consensus on effective improvements in healing outcomes. ST offers new hope for studying regenerative mechanisms and identifying intervention targets in skeletal muscle, tendon and bone, as well as skin.
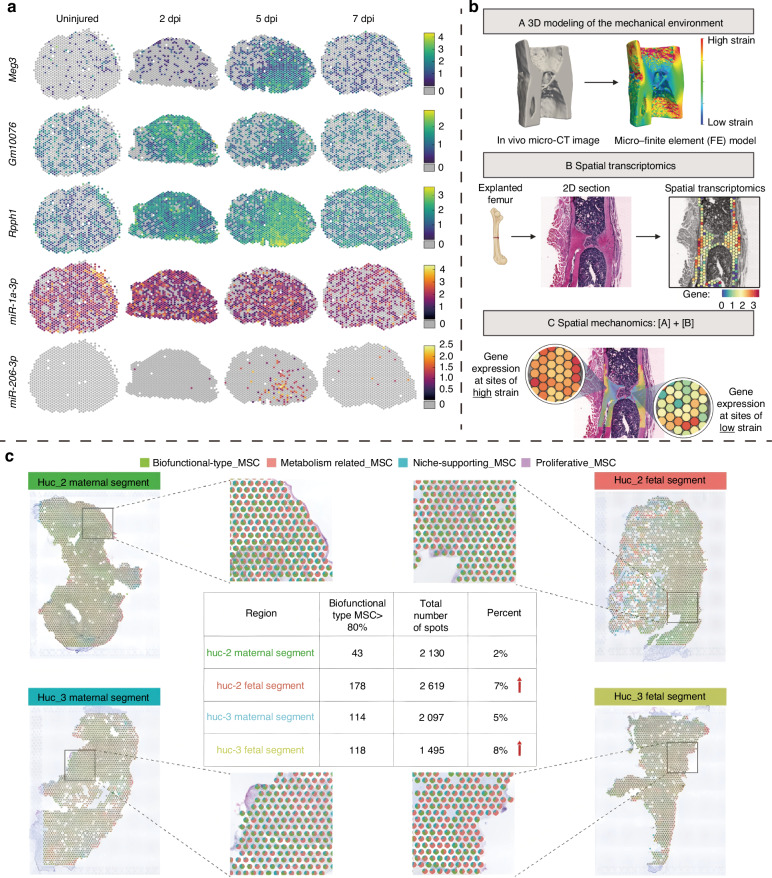
Muscle and tendon injury repair is a coordinated process involving multiple cell types and complex gene regulatory networks.65,108 ST has been used to analyze the spatial characteristics of cellular and gene expression patterns in skeletal muscle regeneration,65,109–111 tendon healing,112,113 and traumatic heterotopic ossification.114 As an example, McKellar et al.65 applied STRS to the skeletal muscle regeneration process, conducting ST analysis on uninjured tibialis anterior muscles and those at days 2, 5 and 7 post-injury in mice. They robustly identified previously undetected or poorly-detected transcripts, including Meg 3, GM 10076, Rpph1, as well as highly-abundant mature miRNAs, such as miR − 206 − 3p and miR − 1a − 3p, and demonstrated their corresponding spatiotemporal expression patterns (Fig. 3a). While the pivotal role of miRNAs in skeletal muscle regeneration has been extensively studied,115,116 the standard Poly-A-based Visium workflow has limited their detection in a spatial context. McKellar et al. made a pioneering contribution to the spatial transcriptome landscape of skeletal muscle regeneration by including non-coding RNA components.65 As another instance of ST being applied to muscle regeneration, Larouche et al.111 attempted to elucidate the cellular and molecular spatiotemporal mechanisms behind failed muscle tissue regeneration and fibrotic scar formation following volumetric muscle loss, mediated by immune and stem cell dysregulation. In tissue sections, the volumetric muscle loss area can be divided into a defect zone with complete muscle loss, an intact zone with fully preserved muscle, and a transition zone in between.111 Analyses showed that at day 7 post-injury, scar-associated macrophages colocalized with mesenchymal-derived cells in the defect zone, characterized by high expression of inflammation-and collagen deposition-related genes, while muscle stem cells were primarily found in the transition zone, enriched with developmental myogenic genes, and were almost absent from the defect zone.111 Over time, inflammation in the volumetric muscle loss defect and transition zones subsided, but fibrotic remodeling intensified.111 Further analysis indicated that interactions between scar-associated macrophages and mesenchymal-derived cells promoted fibrosis progression, which was unfavorable for MuSC-mediated regeneration, and that disrupting this crosstalk via TGF-β inhibition created a microenvironment conducive to MuSC-mediated muscle regeneration which also impeded fibrotic remodeling in the defect zone.111
Fig. 3.
Application of ST in Traumatic Diseases of the Musculoskeletal System. a Spatiotemporal expression pattern of Meg3, Gm10076, Rpph1, miR − 206 − 3p and miR − 1a − 3p at different time-points before and after injury during the regeneration process of murine anterior tibial muscle.65 Copyright © 2022, The Author(s). b In vivo micro-CT imaging is used for micro-finite element analysis to create 3D landscapes of the mechanical environment at the tissue scale. ST analysis of explanted femurs was performed to construct 2D spatial transcriptome landscapes. Finally, gene spatial expression patterns under different mechanical strains were constructed through visual alignment.121 Copyright © 2025, The American Association for the Advancement of Science. c Deconvolution of Visium capture spots from different regions of the umbilical cord was performed to visualize cell type proportions. The color coding represents different cell types, while pie charts illustrate the proportion of each cell type at each spot. The proportion of spots in the fetal segment of the umbilical cord, where functional MSCs comprise over 80% of the cells, was higher compared to the maternal segment.125© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH. dpi day post-injury, Huc human umbilical cord
Up to now, ST has been applied in studies related to digit regeneration,117 cranial defect repair mediated by bone repair materials,118 and both normal and pathological fracture healing.119–121 Given the important role of mechanoregulation in skeletal regeneration and reconstruction, Mathavan et al.121 developed an ST-based mechanomics platform to explore the transcriptomic features of cells in different local mechanical environments during fracture healing within the spatial framework. Specifically, the researchers first performed in vivo micro-CT imaging on a mouse femoral defect model weekly and further used micro-finite element analysis to construct a 3D model of the mechanical environment. Three weeks after the fracture, the mice were divided into two groups: the Loaded group, which received cyclic mechanical loading, and the Control group, which received sham loading. Subsequently, the researchers conducted ST analysis on longitudinal sections of the femurs from both groups at five weeks post-fracture to construct a spatial transcriptomic landscape. Finally, by integrating spatial multimodal data, including bone morphology, mechanical environment, and gene spatial expression patterns, the study provided a clearer understanding of the molecular mechanisms of fracture healing under local mechanical regulation (Fig. 3b). The study indicates that gene expression features in high-strain regions are associated with osteogenic responses, characterized by upregulation of genes such as Spp1 and Col1a2. In contrast, gene expression features in low-strain regions show dominance of bone resorption, with upregulation of genes like S100a8 and Ncf1 (ref. 121). In addition, spatial analysis of differentially-expressed genes at the fracture site confirmed a significantly enhanced osteogenic response in the Loaded groups compared to the Control group, as evidenced by the upregulation of osteogenic differentiation and activity markers (e.g., Sp7, Bglap), mineralizing osteocyte markers (e.g., Phex, Dmp1), and mature osteocyte markers (e.g., Mepe, Sost).121 In the future, utilizing ST technologies with higher resolution and constructing 3D transcriptomic landscapes to build a 3D mechanomics platform will offer finer and more intuitive insights in this field, promoting the discovery of mechano-responsive targets to facilitate fracture healing.
The wound healing process involves a complex and finely-coordinated cellular and molecular mechanism, and research in this area has been ongoing for over a century.122 ST has provided valuable spatial insights into both the normal process and the involvement of biomaterials during wound healing.123,124 Furthermore, ST has contributed to advances in cell-based therapies related to wound repair.125 Mesenchymal stem cells (MSCs), for which Wharton’s jelly MSCs (WJ-MSCs) make an excellent representative model, have broad clinical application potential for promoting tissue repair and regeneration126 and regulating immunity.127 However, their high heterogeneity may be one of the key reasons for their inconsistent clinical efficacy.128 Chen et al.125 conducted a systematic analysis of WJ-MSCs by combining scRNA-seq and ST. scRNA-seq identified four subtypes of WJ-MSCs, with the S100A9 + CD29 + CD142+ functional MSC subtype, which promotes wound healing, showing potential as a therapeutic agent for wound healing. ST analysis of maternal and fetal segments from two human umbilical cords (UCs) revealed the spatial heterogeneity of molecular and functional characteristics across different regions of the UC. Finally, the researchers used SPOTlight129 to integrate scRNA-seq and ST data for deconvolution of Visium capture spots. By calculating the proportion of capture spots in which functional MSCs constituted more than 80% of the cells, they found that functional MSCs were relatively enriched in the fetal segment of the UC compared to the maternal segment125 (Fig. 3c). This suggests that the fetal segment of the UC is an ideal source of this MSC subtype.
Applications of ST in degenerative diseases of the musculoskeletal system
Osteoarthritis (OA) is a common degenerative disease primarily affecting the knee and hip joints, characterized by joint pain and functional impairment.130 In South Korea, the prevalence of knee OA among individuals over 50 years old is as high as 35.1% (ref. 131), and the prevalence of OA continues to rise globally.132 Differences in subtype among OA patients and the spatial heterogeneity of chondrocytes present challenges for the precise diagnosis and treatment of OA.133 Fan et al.134 integrated Geo-seq and scRNA-seq data to create a single-cell and regional spatially-resolved transcriptome landscape of human knee cartilage, both with and without OA, identifying 11 chondrocyte clusters and their specific marker genes, including newly-identified pre-inflammatory and inflammatory chondrocyte clusters. As a benefit of the acquisition of spatial information, Fan et al.134 discovered that in OA, most chondrocyte clusters are situated in transcriptionally-quiescent middle and deep zones, whereas prehypertrophic chondrocytes and prefibrocartilage chondrocytes are primarily concentrated in areas of the joint surface and superficial regions that are transcriptionally active and enriched with OA-associated differentially-expressed genes. Further studies indicated that the inflammatory chondrocyte group has the potential to activate MIF-CD74-mediated cartilage degradation in the knee joints of OA patients, that the prehypertrophic chondrocytes and prefibrocartilage chondrocytes groups are crucial for distinguishing individuals with or without OA and for OA subtyping respectively, and that the hypertrophic chondrocyte group is a key cluster associated with susceptibility to OA in European and East Asian populations, providing important clues for precise diagnosis and interventions for OA.134
The anterior cruciate ligament is a crucial ligament of the knee joint, and its degeneration can lead to knee instability and chronic pain, potentially promoting the development and progression of OA.135,136 Yang et al.137 combined single-cell transcriptomics and ST to map the single-cell spatial transcriptome landscape of healthy and degenerated anterior cruciate ligaments, identifying and localizing various cell subtypes. By analyzing their interactions, Yang et al.137 found that FGF and TGF-β signaling pathways might mediate extracellular matrix remodeling in anterior cruciate ligament degeneration, and targeting FGF7–FGFR1 and TGFB1–TGFBR2 could be effective therapeutic strategies. The proximity of fibroblasts to immune and endothelial cells, revealed by ST, further supports the analysis of cell interactions.137
ST has also been applied to the study of aging skeletal muscle138 and tendon.139,140 Tendinopathy, also a common degenerative disease of the musculoskeletal system, is accompanied by pain and causes impaired motor function, severely affecting quality of life. The incidence of rotator cuff tendinopathy is as high as 5.5% (refs. 141,142). The unclear molecular and cellular mechanisms underlying tendinopathy have led to the lack of a unified and effective treatment method. Through combined single-cell and spatial transcriptomics analysis of healthy tendons and tendon samples from patients with tendinopathy, Akbar et al.139 revealed dysregulation of immune homeostasis in tendons with tendinopathy initiated by endothelial and matrix cells. Fu et al.140 identified and located tendon cell subgroups, further proposing that targeting spatially-proximate endothelial cell subgroups and macrophages, which alter the niche of tendon stem/progenitor cells, might be effective therapeutic strategies. They also discovered the developmental characteristics of tendinopathy from inflammatory infiltration through chondrogenesis to chondral ossification, suggesting that the essence of tendinopathy is heterotopic ossification of the tendon.140 Future research that distinguishes the severity of tendinopathy rather than simply categorizing samples as healthy or diseased may provide a more nuanced perspective.
Applications of ST in tumorous diseases of the musculoskeletal system
The tumor microenvironment (TME) is composed of dynamically-changing populations of various cell types and extracellular matrix, and analyzing its complex cellular heterogeneity and intercellular interaction networks in a spatial context provides new insights into the mechanisms of tumor occurrence and progression, prognosis assessment, and the development of new tumor markers and therapeutic targets.143 Cancer-associated fibroblasts (CAFs) are one of the predominant stromal cell types in the TME and play a pivotal role.144,145 Chordomas, originating from embryonic remnants of the notochord, are rare mesenchymal tumors most commonly found in the clival region and sacrococcygeal area.146,147 Zhang et al.148 used ST to confirm the presence of a novel CAF cluster, termed endoplasmic reticulum stress-CAFs (ERS-CAFs), in the chordoma TME, originally identified via scRNA-seq. The spatial data indicated that ERS-CAFs are located close to tumor cells, potentially promoting tumor progression through direct crosstalk, and the proximity of ERS-CAFs to tumor cells correlates with the severity of malignancy and patient prognosis.148 Ewing’s sarcoma is a poorly-differentiated malignancy of small round cells, occurring predominantly during adolescence, and has a poor prognosis.149 Wrenn et al.150 combined ST and single-cell proteomics to identify spatial heterogeneity among Ewing’s sarcoma cell subpopulations. Their study, augmented by multiplex immunofluorescence staining, found CD73+ Ewing’s sarcoma cells exhibited similarities with CAFs in upregulating extracellular matrix protein expression and deposition, and they, therefore, categorized this subgroup as CAF-like tumor cells.150 Their findings underscore the significant role of CAF-like tumor cells in remodeling the TME to promote tumor initiation and progression.150
Beyond CAFs and tumor cells, immune cells in the TME also play a crucial role in either promoting or inhibiting tumor initiation and development, drawing significant research interest.151 Integrating data from bulk, single-cell, and spatial transcriptomics, validated through immunostaining, established proteasome activator complex subunit 2 (PSME2) as a pan-cancer biomarker in cancers infiltrated by M1 macrophages.152 Osteosarcoma is a highly-aggressive malignant bone tumor primarily affecting children with a low survival rate.153 Researchers found that overexpression of PSME2 in osteosarcoma cells significantly inhibits their proliferation, invasion, and migration, and consequently they screened the PSME2 agonist Irinotecan, which in combination with paclitaxel, was found to synergistically promote apoptosis in osteosarcoma cells.152
Metastatic prostate cancer has a high mortality rate, with bones being a primary metastatic site.154 Bone metastases from prostate cancer exhibit three pathological subtypes: lytic, blastic, and mixed.155 Ihle et al.156 used GeoMx DSP and immunohistochemical staining to reveal differences in immune cell-enriched biological pathways between blastic and lytic lesions in the TME. The former displayed high levels of pSTAT3 and components of the JAK-STAT pathway, while the latter showed enrichment of pAKT activity and PI3K-AKT pathway components. Compared to lytic lesions, blastic lesions have multiple enriched immune checkpoints, including IDO-1, OX40L, B7-H4, and PD-L1, highlighting potential targets of immune therapy in bone metastases of blastic prostate cancer.156
ST holds substantial potential in analyzing the TME of musculoskeletal systems, providing deep insights into tumor pathology and offering new perspectives for disease diagnosis, treatment, and prognostic prediction. Furthermore, ST aids in validating regulatory networks constructed based on bioinformatics analysis from RNA-seq data. For instance, Huang et al.157 developed a specific regulatory network based on prognostic stemness-related signatures for infiltrative breast cancer bone metastases, supported by ST and other multi-omics data.
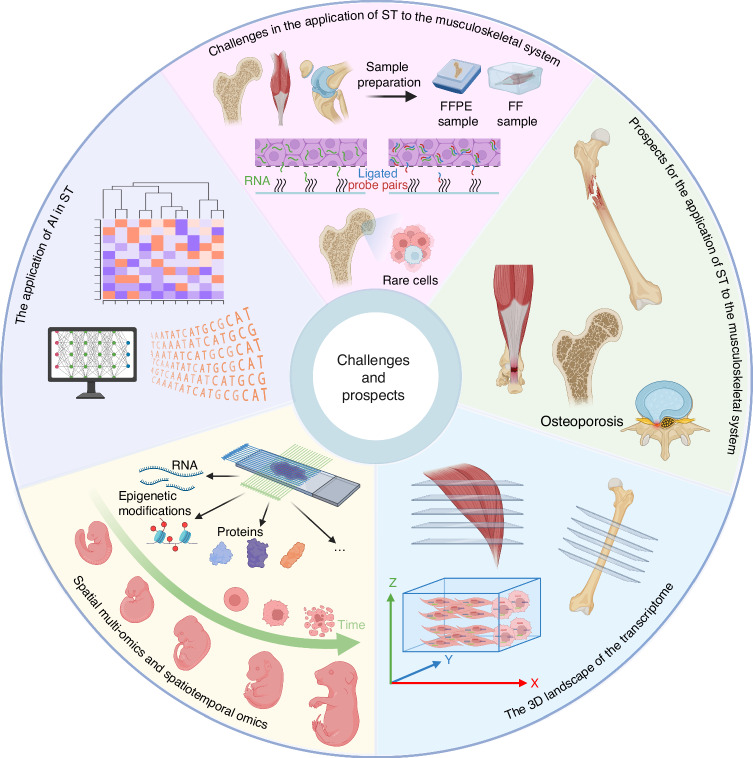
Challenges and prospects (Fig. 4)
Fig. 4.
Challenges and Prospects. Challenges and prospects faced by spatial transcriptomics itself and its application to the musculoskeletal system. AI Artificial intelligence, FFPE Formalin fixed and paraffin embedded, FF Fresh frozen. Created in BioRender. Wang, H. (2025) https://BioRender.com/e71m592
Challenges in the application of ST to the musculoskeletal system
In current single-cell and spatial multi-omics research, most high-quality data originate from soft tissue samples. Related tissues in the musculoskeletal system, such as bone, cartilage, and tendon, contain tougher, denser, and even mineralized extracellular matrices, with relatively lower cell content,158 presenting challenges for conducting single-cell and spatial multi-omics studies. This section discusses the challenges and potential solutions in sample preparation for ST, the permeabilization process, and effective identification of rare cell types in the bone marrow, with the aim of promoting wider application of ST to the musculoskeletal system.
Due to the dense and hardened nature of bone and sclerotic bone lesions, an additional decalcification step is required in sample processing compared to soft tissues.159 However, most decalcification agents contain strong acids that degrade nucleic acids, leading to RNA degradation.160 In contrast, using EDTA for decalcifying bone specimens significantly improves nucleic acid recovery rates, and ultrasonic vibration can reduce decalcification time.161 In addition, performing daily imaging assessments during the decalcification process allows for determination of the minimum decalcification time required for specific samples, thereby mitigating the negative impact of this process on RNA quality.120 Standardized, nucleic acid-friendly mild decalcification protocols still need further development and improvement. The extracellular matrix of cartilage tissue is primarily composed of proteoglycans and collagen, with cellular content making up only about 5%–10% of the total volume.162 The low cellularity of cartilage means that the total RNA content is likely to be lower than that of tissues with higher cell densities, highlighting the importance of maintaining adequate quantities and quality of RNA when conducting transcriptomic analyses on cartilage tissues. During the preparation of cartilage samples, rapidly processing samples at low temperatures to inactivate RNAses, avoiding the use of chemical fixatives, choosing fresh samples and minimizing storage time, and quickly thawing frozen samples are widely agreed-upon methods to effectively prevent RNA degradation.158 Notably, it has been reported that RNA degradation levels are significantly increased in cartilage samples from arthritis patients.163 Special attention should be given to RNA preservation when studying such samples. For tendon tissue, due to its dense and parallel arrangement of collagen fibers and relatively thin structure, obtaining high-quality longitudinal sections of tendons also presents certain challenges.87 Notably, a comprehensive protocol for preparing ST samples from FFPE tissues of the murine musculoskeletal system, including bone and muscle, has recently been established. This development is poised to facilitate the broader application of ST in musculoskeletal system research.164
FFPE is a widely-used biological sample fixation method in clinical practice, offering significant advantages, including long-term preservation of tissue samples at room temperature while maintaining structural and morphological integrity.165 Importantly, FFPE tissues adhere better to slides than FF samples, reducing the likelihood of tissue detachment. However, the FFPE protocol also has limitations; excessive fixation can cause extensive RNA cross-linking, reducing RNA quality.165 A de-crosslinking step can improve RNA accessibility and integrity and thus enhance analytical quality. However, researchers must find a fine balance between adequate de-crosslinking and preventing RNA degradation during this process. Xiao et al.12 and Ihle et al.156 have confirmed the feasibility of applying ST to FFPE samples of normal bone and of prostate cancer bone metastases, respectively. Currently, many ST technologies have been adapted for FFPE samples, providing a platform for ST analysis of highly-mineralized tissues and making ST more accessible for clinical research. Since FF tissues avoid RNA cross-linking and degradation due to long-term storage, their gene detection efficiency is superior to that of FFPE tissues.62,68 To obtain high-quality ST data, analyzing FF tissues remains an indispensable choice. The technique of RRST, which has the capability to analyze FF samples with low-quality RNA, offers three improvements over the commercially-available Visium FFPE solution: it uses a shorter formalin fixation step and omits the de-crosslinking step to prevent RNA cross-linking and degradation, and it includes a baking step to enhance tissue section adhesion and thus address the propensity of FF tissues to detach.64 RRST has been successfully applied to FF samples of mouse bone and cartilage tissues.64 In the future, combining this improved strategy with higher spatial resolution, spatial barcode-based ST technologies will enable researchers to gain more refined analytical perspectives from decalcified hard tissues and other FF samples with low RNA quality.
Currently, two main permeabilization strategies are generally applied. The first is the PolyA capture-based strategy used in Visium V1, where mRNA in the tissue is released and captured by oligonucleotide sequences containing Poly dT on the capture panel. The second is the probe-based strategy employed in Visium V2 and Visium HD, which uses paired probes targeting the protein-coding regions of RNA based on a gene panel.64 Subsequently, ligated probe pairs, rather than RNA, are released from the tissue and captured by the capture panel. The preferred tissue samples for the PolyA capture-based strategy are FF samples, as they preserve polyadenylated transcripts well. The duration of permeabilization for the PolyA capture-based strategy needs to be experimentally determined to achieve the strongest fluorescence signal and minimal lateral diffusion of RNA. Optimizing permeabilization time is a delicate balancing act since it is necessary to sufficiently capture RNA while avoiding prolonged permeabilization that could cause lateral RNA diffusion. However, the optimal permeabilization time may vary between different tissues, and when a section contains different tissues such as bone, cartilage, and soft tissues like muscle, researchers need to select the optimal permeabilization time for the specific tissue area to be focused on, or consider the whole section to balance the suboptimal permeabilization time suitable for all tissues. There are no reported strategies that allow different degrees of permeabilization on the same section for different tissues, limiting the use of the PolyA capture-based strategy on sections containing multiple tissue types. Due to the degradation and fragmentation of RNA molecules in FFPE samples,166 the capture efficiency of the PolyA capture-based strategy significantly decreases. Therefore, for FFPE samples, researchers need to choose a probe-based strategy which uses a uniform permeabilization scheme for different tissues without the need to experiment with permeabilization times. However, it is important to note that, as previously mentioned, probe-based strategies are limited to humans, mice, or evolutionarily similar species and are not suitable for other models, such as axolotls.167 RRST, compared to the standard Visium probe-based strategy, significantly increases the number of unique genes and unique molecular identifiers detected in cartilage tissue, with a more uniform distribution, producing high-quality bone and cartilage ST data for analysis.64 The dense and extensively cross-linked extracellular matrix of cartilage and tendon may be a potential obstacle for transcript or probe permeabilization. However, there have been no reports demonstrating enhanced permeabilization effects by enzymatic degradation of the extracellular matrix during cartilage and tendon permeabilization, and its effectiveness and potential impacts on transcriptional profile and spatial accuracy require further consideration.
Currently, ST studies related to cartilage tissue and its sample preparation are relatively limited. Cartilage is an essential component of the intervertebral disc, and our previous research77 has demonstrated the feasibility of using the PolyA capture-based Visium technique on 3-week-old mouse IVD FF samples, providing a preliminary reference for ST studies in cartilage tissues.
In bone marrow, the abundance of different cell types varies by orders of magnitude,168 with non-hematopoietic cells, which include mesenchymal-origin stromal cells, accounting for less than 0.5% of the total cell count in adult mouse bone marrow.169 This makes the effective identification of rare cell types in the bone marrow particularly challenging. ST technology based on spatial barcodes typically lacks actual single-cell resolution, necessitating the use of deconvolution to determine the cell types and their proportions within each spot and to identify potentially present rare cell types.170 This step is often achieved by integrating scRNA-seq data or combining high-resolution H&E images, or through reference-free methods. Given that scRNA-seq is a powerful tool for identifying rare cell populations, and considering the abundance of bone marrow scRNA-seq datasets currently available,15,84 we emphasize the use of scRNA-seq to effectively identify rare cell types and further map them to the bone marrow niche, thus enhancing the analytical power of ST data. When identifying rare cell types in bone marrow using scRNA-seq, the critical roles of sample preparation, enrichment of rare cells, and the choice of library preparation platforms and marker genes must be fully considered.171 First, preparing scRNA-seq samples by enzymatic digestion of bone marrow tissues rather than by bone marrow aspiration or bone flushing enables the capture and identification of rare cells closely adhering to the bone surface, such as fibro-mesenchymal stromal cells, avoiding the detection bias of the mesenchymal stromal cell component towards adipocytes.15,84 Second, using prior knowledge of cell surface markers and anatomical locations to utilize flow cytometry or laser microscopy can significantly enhance the detectability of rare cells through cytometric or anatomic enrichment.171 For example, Hoxb5+ long-term hematopoietic stem cells constitute only about 0.001% of the nucleated cells in mouse bone marrow and are difficult to detect without enrichment.172 Third, using a plate-based full-length sequencing platform instead of a droplet-based short-read sequencing platform increases the scope and depth of RNA capture,173 improving the likelihood of detecting rare cell types and states in bone marrow. Fourth, methods such as CellSIUS174 and scPNMF175 have been developed to optimize the selection of marker genes, facilitating the identification of rare cell types in bone marrow. Additionally, integrating multiple bone marrow scRNA-seq datasets also assists in identifying rare stromal cell subpopulations.176 Furthermore, by integrating single-cell multi-omics data to enhance the comprehensive understanding of cellular heterogeneity, deep learning models such as MarsGT177 can more effectively infer and identify rare cells within the bone marrow.
Different deconvolution packages have their pros and cons. To improve the reliability and accuracy of data analysis, researchers have developed a joint predictive pipeline utilizing Seurat,178 CellTrek,179 and Cell2Location,180 successfully pinpointing the rare cell types, Cxcl12-abundant reticular cells in the bone marrow and Pdgfra+Sca1+ SSPCs in the periosteum.12 It is worth noting that deconvolution strategies based on scRNA-seq data depend on the quality and completeness of reference data and the compatibility between single-cell and spatial data. While we now have access to a wealth of bone marrow scRNA-seq datasets, we cannot guarantee that these datasets encompass all cell types and states. Packages that offer reference-free deconvolution capabilities such as CARD181 and STdeconvolve182 might help discover as-yet unidentified rare cell types. Conversely, for rare cell types with known marker genes, targeted ST technologies such as Xenium and GeoMx DSP can be used for detection.183 Additionally, studies with Seq-Scope184 and HDST185 have confirmed the capability of high-resolution spatial barcode-based ST technology to accurately identify and precisely locate rare cell types, suggesting the potential value of applying higher resolution technologies to bone marrow ST research.
Prospects for the application of ST to the musculoskeletal system
ST has already contributed to substantial progress in the study of the musculoskeletal system, and we believe there is still significant potential for further advances in this field. Fracture is a common and frequent disorder of the musculoskeletal system, with approximately 5% to 10% of fracture patients experiencing delayed healing or non-union.186 The study by Rios et al.120 was the first to apply ST to human bone. However, existing ST studies on fractures, including this one, remain limited to analyses at only one or two specific time points.119–121 Comprehensive single-cell spatiotemporal transcriptomic maps covering the entire healing process of fractures at different anatomical sites are still lacking. Conducting combined scRNA-seq and ST analysis at different fracture sites and at different stages of the fracture-healing process could help us deepen our understanding of the mechanisms behind fracture healing and aid in the development of strategies to promote healing. Osteoporosis increases the risk of fractures and is associated with many skeletal diseases.187 The 3D gene expression atlas of trabecular and cortical bone under the influence of osteoporosis remain to be drawn. Lumbar disc herniation, often caused by intervertebral disc degeneration, is a common cause of lower back pain and chronic disability in the elderly, but its pathological mechanisms are not yet fully understood.188 Recently, Chen et al.77 constructed a spatial transcriptome atlas of the IVD without degeneration, while the spatial expression patterns of genes in the NP, AF, and CEP under intervertebral disc degeneration conditions still need further mapping. Additionally, athletes and fitness enthusiasts are often plagued by overuse injuries.189 In the future, using ST to reveal the response mechanisms underlying the spatial expression patterns of genes in tissues such as tendons and ligaments under different mechanical loads could help in formulating scientific training strategies and preventing injuries.
The 3D landscape of the transcriptome
The spatial heterogeneity of bones, bone junctions, and skeletal muscles is not limited to two-dimensional planes but is also manifested in complex three-dimensional structures such as the network of trabecular bones, the varying cell density and collagen fiber arrangement from the superficial to deep layers of joint cartilage, and the intricate 3D patterns of blood vessels and nerve distributions within muscles. While current ST methods primarily focus on acquiring data in two-dimensional planes, accurately mapping and analyzing 3D structures are more complex and challenging. Tomo-seq processes RNA-seq for slices taken along the X, Y, and Z axes of three identical samples to reconstruct the 3D spatial transcriptome landscape by integrating data across these axes.23 Combining Tomo-seq and LCM, Geo-seq also has the capability of transcriptomic 3D reconstruction.24 Using innovative physical micro-sectioning techniques and algorithms similar to CT reconstruction, STRP-seq successfully reconstructs 3D gene expression patterns.190 STARmap performs high-resolution in situ imaging of target RNA in thick tissue slices, enabling 3D gene expression analysis of tissue samples.54 STARmap PLUS extends this capability to map transcripts and proteins in 3D.191 Electro-seq offers a potentially-powerful tool for characterizing cell states and developmental trajectories in electrogenic tissues such as skeletal muscles, incorporating chronic electrophysiological recordings while constructing 3D transcriptome landscapes.55 Open-ST can reconstruct virtual tissue blocks up to 350 μm thick, providing a 3D view of gene expression, cell types, and local signal transmission.192
Building 3D transcriptome landscapes from merely two-dimensional slice data involves overcoming challenges in data alignment and integration. Yet, the implementation of such strategies allows the construction of volumetric gene expression atlases to no longer be confined to specific ST methods. Zeira et al.193 introduced PASTE, which considers both transcriptional similarity and spatial proximity, analyzing consecutive 2D slice data to resolve the transcriptomic state of entire tissues in 3D space. Jones et al.194 proposed GPSA, which aligns spatial coordinates of sample data across tissue slices and data modalities using a common coordinate system, utilizing sparse 2D slices to construct a 3D atlas.
Accurate parsing and robust reconstruction of 3D structures will further enhance our understanding of internal interactions and functional dynamics within complex biological systems, especially the spatial relationships at the cellular and molecular levels and their implications for disease progression and tissue regeneration.
Spatial multi-omics and spatiotemporal omics
Spatial multi-omics is a cutting-edge frontier that integrates various omics layers, including but not limited to epigenomics, transcriptomics, proteomics, and metabolomics, within a spatial context to provide multidimensional data, comprehensively uncovering the intricate micro-mechanisms of life processes.195 The development of spatial single-omics has propelled the continuous progress and innovation of spatial multi-omics. Typically, spatial multi-omics landscapes are constructed by using different spatial single-omics methods on adjacent slices. However, even adjacent slices can exhibit heterogeneity in spatial structure and cellular composition, and spatial resolutions may vary across different single-omics methods.195 DBiT-seq68 was the first technology to enable spatial multi-omics sequencing on the same slice,196 using a microfluidic deterministic barcoding strategy to detect the entire transcriptome and dozens of targeted proteins. Similarly, spatial-CITE-seq197 also maps both transcriptomes and proteins but targets up to hundreds of proteins. As previously mentioned, STARmap PLUS191 has the ability to perform 3D mapping of both transcripts and proteins. Combining CITE-seq with spatial barcode-based ST technologies, SM-Omics198 and SPOTS199 further realize spatial multi-omics, although with limited spatial resolution. Spatial ATAC–RNA-seq200 and Spatial CUT&Tag–RNA-seq200 analyze chromatin accessibility or histone modifications alongside the transcriptome, allowing for comprehensive studies on the complex processes of gene expression regulation. Dedicated frameworks like SpatialData201 have been developed for spatial multi-omics data. These frameworks greatly facilitate access to, sharing, and analysis of large-scale multidimensional data, providing powerful tools to overcome the limitations and challenges of traditional spatial omics data processing.
The journal “Nature” listed spatial multi-omics technologies as one of the top seven technologies to watch in 2022.202 Spatial multi-omics has begun to provide multi-omics landscapes with tissue context for projects like the Human Cell Atlas, aiming to build a detailed reference map of human cell types and states, significantly improving our understanding of the structure and function of human cells.203 However, spatial multi-omics is still in its developmental stage. For genomics and transcriptomics, the prevalent use of short-read sequencing technologies limits the detection of genetic variations and spatial alternative splicing.204 In proteomics, strategies that simultaneously offer high throughput and high spatial resolution still need further development. For metabolomics, constrained as it is by specific sample preparation methods, it is challenging to integrate with other spatial omics data on the same slice.195 Spatial epigenomics provides valuable data on gene regulation and personalized medicine, yet the simultaneous detection of DNA methylation, chromatin accessibility, and histone modifications on the same slice to finely and comprehensively delineate epigenetic features remains to be realized.200 Furthermore, the integration and analysis of multi-omics data present unprecedented challenges to existing bioinformatics tools. Lastly, high spatial resolution and true single-cell resolution, coupled with low cost and high throughput, remain the goals we strive for.
Currently, research on ST, especially applied to bone tissue in the musculoskeletal system, is indeed very limited. The decalcification process in bones typically leads to RNA degradation,12 which is one of the main limitations for conducting ST research in bone tissues; however, this process has little impact on the antigenicity of proteins in the sample, thus not hindering subsequent antibody-based spatial proteomics analyses.205 There exists a nonlinear complex relationship between mRNA and the proteins translated from it, often leading to discrepancies in their levels of expression.206 It is noteworthy that, compared to proteomic data, transcriptomic data tend to present a more complex landscape, whereas the proteome appears more stable and straightforward.207 Therefore, spatial proteomics analysis provides us with more direct and authentic insights into cellular functions and states.208 A recent study15 utilized scRNA-seq and co-detection by indexing, a spatial proteomics technique based on multiple antibodies, to analyze the cellular composition and spatial landscape of the human bone marrow niche, focusing on the enrichment and analysis of low-abundance non-hematopoietic cells. By integrating single-cell transcriptomic data with spatial proteomic data, intercellular signaling and spatial proximity within the bone marrow were revealed. Additionally, the co-detection by indexing maps helped uncover the expansion of mesenchymal stromal cells and their spatial proximity and co-enrichment with leukemic blasts in acute myeloid leukemia.15 To our knowledge, this study is the first to construct a spatial multi-omics map of the human bone marrow through the integration of multi-omics data across different dimensions, providing a key multi-omics perspective for comprehensively analyzing the human bone marrow niche. Based on this, we emphasize the significant potential and value of constructing spatial multi-omics landscapes through the combination of spatial single-omics and other omics without spatial resolution, rather than relying on the premise that all omics data have spatial resolution. Although this article mainly focuses on ST, it is undeniable that spatial proteomics may offer a more practical option for spatial multi-omics research in bone tissues.
Spatiotemporal omics not only focuses on the distribution and function of cells and molecules in space but also considers how these characteristics change over time. Currently, ST can only capture the transient states of continuous processes, limiting our deep understanding of the dynamic evolution of physiological and pathological processes. Methods to construct a spatiotemporal transcriptome atlas rely on sampling multiple specimens at different time-points within the same physiological or pathological process. Continuous molecular analysis of live cells to decipher cell dynamics has been a focus area for researchers. Live-seq,209 for the first time, enabled continuous observation and analysis of single-cell transcriptomic spectra and downstream molecules in the same cell without causing significant cell perturbations. Live-seq provided temporal resolution to scRNA-seq. Future enhancements to Live-seq that add tissue spatial context, such as combining it with ROI-selection-based ST technologies, will greatly advance the development of spatiotemporal transcriptomics. Additionally, continuous advances in mass spectrometry will significantly enhance our understanding and exploration of the proteome, peptidome, and metabolome in spatiotemporal multi-omics studies.210
The application of AI in ST
The multidimensional data from ST, which include complex gene expression matrices, 2D and even 3D spatial information, and histological images, demand high capabilities in data processing and analysis.211 As ST technologies continue to evolve, moving toward higher-dimensional spatial multi-omics and spatiotemporal omics, the volume and complexity of data are set to increase. This progression poses new challenges for data handling and analysis, but it also creates significant opportunities for AI, particularly for deep learning (DL) strategies within machine learning. Currently, DL has been extensively applied to various aspects of ST analysis. For instance, models like SpaGCN212 and stLearn213 can effectively cluster data based on gene transcription features and spatial distributions. SpaGCN212 and STAGATE214 can efficiently identify spatially-variable genes. Tools such as stPlus215 and Tangram216 integrate single-cell and spatial transcriptomics data to impute missing genes. stLearn213 and GCNG217 leverage DL methods to identify critical ligand–receptor pairs, thereby inferring intercellular communication networks. For sequencing-based ST technologies, methods like DSTG218 and Tangram216 can predict the proportions of different cell types at mixed cell loci, achieving deconvolution of cell types. For imaging-based ST technologies, tools such as Baysor219 and JSTA220 effectively segment cells. Deep learning models such as SAUCIE,221 DESC,222 and CarDEC223 have been developed to overcome batch effects in scRNA-seq. Although methods like SEDR224 and STAGATE214 have limitations in handling batch effects in ST, particularly in not considering histological images, they represent early attempts and provide preliminary solutions to alleviate batch effects in this field. We believe that the continuous innovations in AI, especially DL, coupled with the further opening and sharing of ST data, is crucial for fully unlocking the potential of these data. This will provide unprecedented insights into the complex molecular mechanisms of health and disease.
Conclusion
This article outlines the development of ST data acquisition technologies, highlighting new technologies, advances, and representative methods, providing a concise workflow for incorporating ST into musculoskeletal system research, summarizing the applications of ST in the musculoskeletal system, and discussing the challenges and prospects for ST itself and its application in the musculoskeletal system. When conducting ST studies, the sample preparation and permeation processes of the musculoskeletal system, especially bone, cartilage, and tendon tissues, as well as the effective identification of rare cell types in the bone marrow, remain challenging. As capabilities in sample processing, tissue permeabilization, and rare cell identification continue to advance, along with the deep application of AI in ST, the field of ST is constantly evolving towards spatial multi-omics and spatiotemporal omics, continuously advancing and innovating. This progress is also reflected in the transition from 2D transcriptomic data to 3D transcriptomic landscapes, providing us with a more comprehensive and in-depth new perspective on understanding gene expression patterns within organisms. In the future, further application of ST to problems such as fractures and lumbar disc herniation will push research on the musculoskeletal system into a new stage. We believe that the data acquisition and analysis technologies of ST will continue to develop rapidly, bringing continuous breakthroughs to research into the musculoskeletal system.
Acknowledgements
This work was supported by The National Natural Science Youth Foundation of China (Grant No. 82102584). The Figs. 1 and 4 were Created with BioRender.com.
Author contributions
W.H.Y. and C.P. collected the related papers and wrote the manuscript. W.H.Y. and C.P. investigated and prepared the figures and the tables. C.W. and X.R. helped to revise the manuscript. W.J., L.H.Z. and H.Z.Y. made important suggestions for the paper. H.J. made outstanding contributions to the revision of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by The National Natural Science Youth Foundation of China (Grant No. 82102584).
Competing interests
The authors declare no competing interest.
Footnotes
These authors contributed equally: Haoyu Wang, Peng Cheng
Contributor Information
Ren Xu, Email: xuren526@xmu.edu.cn.
Wei Chen, Email: surgeonchenwei@126.com.
References
- 1.Margulies, M. et al. Genome sequencing in open microfabricated high density picoliter reactors. Nature437, 376–380 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang, Z., Gerstein, M. & Snyder, M. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet.10, 57–63 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stark, R., Grzelak, M. & Hadfield, J. RNA sequencing: the teenage years. Nat. Rev. Genet.20, 631–656 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Li, X. & Wang, C.-Y. From bulk, single-cell to spatial RNA sequencing. Int. J. Oral. Sci.13, 36 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang, F. et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods6, 377–382 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Saliba, A. E., Westermann, A. J., Gorski, S. A. & Vogel, J. Single-cell RNA-seq: advances and future challenges. Nucleic Acids Res.42, 8845–8860 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang, T. et al. Single-cell RNA sequencing in orthopedic research. Bone Res.11, 10 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang, B., Lee, J. H. & Bang, D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp. Mol. Med50, 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao, A., Barkley, D., Franca, G. S. & Yanai, I. Exploring tissue architecture using spatial transcriptomics. Nature596, 211–220 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villasenor, S. & Grinstein, M. Spatial high-resolution analysis of gene expression levels in tendons. J. Vis. Exp.10.3791/65852 (2024). [DOI] [PubMed] [Google Scholar]
- 11.Kurenkova, A. D., Medvedeva, E. V., Newton, P. T. & Chagin, A. S. Niches for skeletal stem cells of mesenchymal origin. Front. Cell Dev. Biol.8, 592 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao, X. et al. Spatial transcriptomic interrogation of the murine bone marrow signaling landscape. Bone Res.11, 59 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ståhl, P. L. et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science353, 78–82 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Zhang, B. et al. A human embryonic limb cell atlas resolved in space and time. Nature635, 668–678 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandyopadhyay, S. et al. Mapping the cellular biogeography of human bone marrow niches using single-cell transcriptomics and proteomic imaging. Cell187, 3120–3140 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenblatt, M. B. et al. The unmixing problem: a guide to applying single-cell RNA sequencing to bone. J. Bone Miner. Res.34, 1207–1219 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams, K., Yokomori, K. & Mortazavi, A. Heterogeneous skeletal muscle cell and nucleus populations identified by single-cell and single-nucleus resolution transcriptome assays. Front. Genet.13, 835099 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hata, K. et al. Serinc5 regulates sequential chondrocyte differentiation by inhibiting Sox9 function in pre-hypertrophic chondrocytes. J. Cell. Physiol.240, e31490 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holt, C. E. & Bullock, S. L. Subcellular mRNA localization in animal cells and why it matters. Science326, 1212–1216 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao, J. et al. Uncovering an organ’s molecular architecture at single-cell resolution by spatially resolved transcriptomics. Trends Biotechnol.39, 43–58 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Marx, V. Method of the year: spatially resolved transcriptomics. Nat. Methods18, 9–14 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Alon, S. et al. Expansion sequencing: spatially precise in situ transcriptomics in intact biological systems. Science371, eaax2656 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Junker, J. P. et al. Genome-wide RNA tomography in the zebrafish embryo. Cell159, 662–675 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Chen, J. et al. Spatial transcriptomic analysis of cryosectioned tissue samples with Geo-seq. Nat. Protoc.12, 566–580 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Asp, M. et al. A spatiotemporal organ-wide gene expression and cell atlas of the developing human heart. Cell179, 1647–1660.e1619 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Fawkner-Corbett, D. et al. Spatiotemporal analysis of human intestinal development at single-cell resolution. Cell184, 810–826.e823 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calvanese, V. et al. Mapping human haematopoietic stem cells from haemogenic endothelium to birth. Nature604, 534–540 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen, A. et al. Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball-patterned arrays. Cell185, 1777–1792.e1721 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Farah, E. N. et al. Spatially organized cellular communities form the developing human heart. Nature627, 854–864 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galeano Niño, J. L. et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature611, 810–817 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arora, R. et al. Spatial transcriptomics reveals distinct and conserved tumor core and edge architectures that predict survival and targeted therapy response. Nat. Commun.14, 5029 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, S. et al. Spatial maps of T cell receptors and transcriptomes reveal distinct immune niches and interactions in the adaptive immune response. Immunity55, 1940–1952.e1945 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krausgruber, T. et al. Single-cell and spatial transcriptomics reveal aberrant lymphoid developmental programs driving granuloma formation. Immunity56, 289–306.e287 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen, W.-T. et al. Spatial transcriptomics and in situ sequencing to study Alzheimer’s disease. Cell182, 976–991.e919 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Zheng, P. et al. Integrated spatial transcriptome and metabolism study reveals metabolic heterogeneity in human injured brain. Cell Rep. Med.4, 101057 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng, S. et al. Application of single-cell and spatial omics in musculoskeletal disorder research. Int. J. Mol. Sci.24, 2271 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asp, M., Bergenstråhle, J. & Lundeberg, J. Spatially resolved transcriptomes—next generation tools for tissue exploration. BioEssays42, e1900221 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Moses, L. & Pachter, L. Museum of spatial transcriptomics. Nat. Methods19, 534–546 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Williams, C. G. et al. An introduction to spatial transcriptomics for biomedical research. Genome Med.14, 68 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robles-Remacho, A., Sanchez-Martin, R. M. & Diaz-Mochon, J. J. Spatial transcriptomics: emerging technologies in tissue gene expression profiling. Anal. Chem.95, 15450–15460 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yue, L. et al. A guidebook of spatial transcriptomic technologies, data resources and analysis approaches. Comput. Struct. Biotechnol. J.21, 940–955 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.GALL, J. G. & PARDUE, M. L. Formation and detection of RNA-DNA hybrid molecules in cytological preparations. Proc. Natl. Acad. Sci. USA63, 378–383 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.John, H. A., Birnstiel, M. L. & Jones, K. W. RNA-DNA hybrids at the cytological level. Nature223, 582–587 (1969). [DOI] [PubMed] [Google Scholar]
- 44.Femino, A. M., Fay, F. S., Fogarty, K. & Singer, R. H. Visualization of single RNA transcripts in Situ. Science280, 585–590 (1998). [DOI] [PubMed] [Google Scholar]
- 45.Raj, A. et al. Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods5, 877–879 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, F. et al. RNAscope. J. Mol. Diagn.14, 22–29 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lubeck, E. et al. Single-cell in situ RNA profiling by sequential hybridization. Nat. Methods11, 360–361 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eng, C.-H. L. et al. Transcriptome-scale super-resolved imaging in tissues by RNA seqFISH+. Nature568, 235–239 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen, K. H. et al. RNA imaging Spatially resolved, highly multiplexed RNA profiling in single cells. Science348, aaa6090 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borm, L. E. et al. Scalable in situ single-cell profiling by electrophoretic capture of mRNA using EEL FISH. Nat. Biotechnol.41, 222–231 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He, S. et al. High-plex imaging of RNA and proteins at subcellular resolution in fixed tissue by spatial molecular imaging. Nat. Biotechnol.40, 1794–1806 (2022). [DOI] [PubMed] [Google Scholar]
- 52.Ke, R. et al. In situ sequencing for RNA analysis in preserved tissue and cells. Nat. Methods10, 857–860 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Janesick, A. et al. High resolution mapping of the tumor microenvironment using integrated single-cell, spatial and in situ analysis. Nat. Commun.14, 8353 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, X. et al. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science361, eaat5691 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li, Q. et al. Multimodal charting of molecular and functional cell states via in situ electro-sequencing. Cell186, 2002–2017.e2021 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee, J. H. et al. Fluorescent in situ sequencing (FISSEQ) of RNA for gene expression profiling in intact cells and tissues. Nat. Protoc.10, 442–458 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larsson, C., Grundberg, I., Söderberg, O. & Nilsson, M. In situ detection and genotyping of individual mRNA molecules. Nat. Methods7, 395–397 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Jain, S. & Eadon, M. T. Spatial transcriptomics in health and disease. Nat. Rev. Nephrol.20, 659–671 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Megas, S. et al. Spatial transcriptomics of the respiratory system. Annu. Rev. Physiol.87, 447–470 (2024). [DOI] [PubMed] [Google Scholar]
- 60.Emmert-Buck, M. R. et al. Laser capture microdissection. Science274, 998–1001 (1996). [DOI] [PubMed] [Google Scholar]
- 61.Merritt, C. R. et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat. Biotechnol.38, 586–599 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Gracia Villacampa, E. et al. Genome-wide spatial expression profiling in formalin-fixed tissues. Cell Genom.1, 100065 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lebrigand, K. et al. The spatial landscape of gene expression isoforms in tissue sections. Nucleic Acids Res.51, e47 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mirzazadeh, R. et al. Spatially resolved transcriptomic profiling of degraded and challenging fresh frozen samples. Nat. Commun.14, 509 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McKellar, D. W. et al. Spatial mapping of the total transcriptome by in situ polyadenylation. Nat. Biotechnol.41, 513–520 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodriques, S. G. et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science363, 1463–1467 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stickels, R. R. et al. Highly sensitive spatial transcriptomics at near-cellular resolution with Slide-seqV2. Nat. Biotechnol.39, 313–319 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu, Y. et al. High-spatial-resolution multi-omics sequencing via deterministic barcoding in tissue. Cell183, 1665–1681.e1618 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wirth, J. et al. Spatial transcriptomics using multiplexed deterministic barcoding in tissue. Nat. Commun.14, 1523 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee, Y. et al. XYZeq: Spatially resolved single-cell RNA sequencing reveals expression heterogeneity in the tumor microenvironment. Sci. Adv.7, eabg4755 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Srivatsan, S. R. et al. Embryo-scale, single-cell spatial transcriptomics. Science373, 111–117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pang, J. B. et al. Spatial transcriptomics and the anatomical pathologist: Molecular meets morphology. Histopathology84, 577–586 (2024). [DOI] [PubMed] [Google Scholar]
- 73.Pentimalli, T. M., Karaiskos, N. & Rajewsky, N. Challenges and opportunities in the clinical translation of high-resolution spatial transcriptomics. Annu. Rev. Pathol.20, 405–432 (2024). [DOI] [PubMed] [Google Scholar]
- 74.Valihrach, L., Zucha, D., Abaffy, P. & Kubista, M. A practical guide to spatial transcriptomics. Mol. Asp. Med.97, 101276 (2024). [DOI] [PubMed] [Google Scholar]
- 75.Piña, J. O. et al. Multimodal spatiotemporal transcriptomic resolution of embryonic palate osteogenesis. Nat. Commun.14, 5687 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gribaudo, S. et al. Self-organizing models of human trunk organogenesis recapitulate spinal cord and spine co-morphogenesis. Nat. Biotechnol.42, 1243–1253 (2023). [DOI] [PubMed] [Google Scholar]
- 77.Chen, Y. et al. Characterization of the nucleus pulposus progenitor cells via spatial transcriptomics. Adv. Sci.11, e2303752 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang, T. et al. Single-cell RNA sequencing reveals cellular and molecular heterogeneity in fibrocartilaginous enthesis formation. eLife12, e85873 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lui, J. C. et al. Spatial regulation of gene expression during growth of articular cartilage in juvenile mice. Pediatr. Res.77, 406–415 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marcu, K. B. et al. Gene expression profiling reveals similarities between the spatial architectures of postnatal articular and growth plate cartilage. PLoS One9, e103061 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bian, F. et al. The G protein-coupled receptor ADGRG6 maintains mouse growth plate homeostasis through IHH signaling. J. Bone Miner. Res.39, 1644–1658 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tong, W. et al. Periarticular mesenchymal progenitors initiate and contribute to secondary ossification center formation during mouse long bone development. Stem Cells37, 677–689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tower, R. J. et al. Spatial transcriptomics reveals a role for sensory nerves in preserving cranial suture patency through modulation of BMP/TGF-β signaling. Proc. Natl. Acad. Sci. USA118, e2103087118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baccin, C. et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat. Cell Biol.22, 38–48 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.D’Ercole, C. et al. Spatially resolved transcriptomics reveals innervation-responsive functional clusters in skeletal muscle. Cell Rep.41, 111861 (2022). [DOI] [PubMed] [Google Scholar]
- 86.Karlsen, A. et al. Distinct myofibre domains of the human myotendinous junction revealed by single nucleus RNA-seq. J. Cell Sci.136, jcs260913 (2023). [DOI] [PubMed] [Google Scholar]
- 87.Steffen, D., Mienaltowski, M. & Baar, K. Spatial gene expression in the adult rat patellar tendon. Matrix Biol.10.1016/j.mbplus.2023.100138 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Finckh, A. et al. Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol.18, 591–602 (2022). [DOI] [PubMed] [Google Scholar]
- 89.Di Matteo, A., Bathon, J. M. & Emery, P. Rheumatoid arthritis. Lancet402, 2019–2033 (2023). [DOI] [PubMed] [Google Scholar]
- 90.Huang, J. et al. Promising therapeutic targets for treatment of rheumatoid arthritis. Front. Immunol.12, 686155 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gravallese, E. M., Longo, D. L. & Firestein, G. S. Rheumatoid arthritis—common origins, divergent mechanisms. N. Engl. J. Med.388, 529–542 (2023). [DOI] [PubMed] [Google Scholar]
- 92.Vickovic, S. et al. Three-dimensional spatial transcriptomics uncovers cell type localizations in the human rheumatoid arthritis synovium. Commun. Biol.5, 129 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meng, X. et al. Role and therapeutic potential for targeting fibroblast growth factor 10/FGFR1 in relapsed rheumatoid arthritis. Arthritis Rheumatol.76, 32–47 (2023). [DOI] [PubMed] [Google Scholar]
- 94.Smith, M. H. et al. Drivers of heterogeneity in synovial fibroblasts in rheumatoid arthritis. Nat. Immunol.24, 1200–1210 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rauber, S. et al. CD200+ fibroblasts form a pro-resolving mesenchymal network in arthritis. Nat. Immunol.25, 682–692 (2024). [DOI] [PubMed] [Google Scholar]
- 96.Zheng, L. et al. ITGA5+ synovial fibroblasts orchestrate proinflammatory niche formation by remodelling the local immune microenvironment in rheumatoid arthritis. Ann. Rheum. Dis.84, 232–252 (2024). [DOI] [PubMed] [Google Scholar]
- 97.Carlberg, K. et al. Exploring inflammatory signatures in arthritic joint biopsies with Spatial Transcriptomics. Sci. Rep.9, 18975 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hardt, U. et al. Integrated single cell and spatial transcriptomics reveal autoreactive differentiated B cells in joints of early rheumatoid arthritis. Sci. Rep.12, 11876 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.MacDonald, L. et al. Synovial tissue myeloid dendritic cell subsets exhibit distinct tissue-niche localization and function in health and rheumatoid arthritis. Immunity57, 2843–2862 (2024). [DOI] [PubMed] [Google Scholar]
- 100.Kenney, H. M. et al. Multi-omics analysis identifies IgG2b class-switching with ALCAM-CD6 co-stimulation in joint-draining lymph nodes during advanced inflammatory-erosive arthritis. Front. Immunol.14, 1237498 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davidson, S. et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol.21, 704–717 (2021). [DOI] [PubMed] [Google Scholar]
- 102.Friščić, J. et al. The complement system drives local inflammatory tissue priming by metabolic reprogramming of synovial fibroblasts. Immunity54, 1002–1021.e1010 (2021). [DOI] [PubMed] [Google Scholar]
- 103.Bery, A. I. et al. Role of tertiary lymphoid organs in the regulation of immune responses in the periphery. Cell. Mol. Life Sci.79, 359 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stephenson, W. et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic instrumentation. Nat. Commun.9, 791 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol.20, 928–942 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med.365, 2205–2219 (2011). [DOI] [PubMed] [Google Scholar]
- 107.Messerer, D. A. C. et al. Immunopathophysiology of trauma-related acute kidney injury. Nat. Rev. Nephrol.17, 91–111 (2020). [DOI] [PubMed] [Google Scholar]
- 108.Nichols, A. E. C., Best, K. T. & Loiselle, A. E. The cellular basis of fibrotic tendon healing: challenges and opportunities. Transl. Res.209, 156–168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McKellar, D. W. et al. Large-scale integration of single-cell transcriptomic data captures transitional progenitor states in mouse skeletal muscle regeneration. Commun. Biol.4, 1280 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Young, L. V. et al. Muscle injury induces a transient senescence‐like state that is required for myofiber growth during muscle regeneration. FASEB J.36, e22587 (2022). [DOI] [PubMed] [Google Scholar]
- 111.Larouche, J. A. et al. Spatiotemporal mapping of immune and stem cell dysregulation after volumetric muscle loss. JCI Insight8, e162835 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ackerman, J. E. et al. Defining the spatial-molecular map of fibrotic tendon healing and the drivers of Scleraxis-lineage cell fate and function. Cell Rep.41, 111706 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cherief, M. et al. TrkA- mediated sensory innervation of injured mouse tendon supports tendon sheath progenitor cell expansion and tendon repair. Sci. Transl. Med.15, eade4619 (2023). [DOI] [PubMed] [Google Scholar]
- 114.Kang, H. et al. The HIF-1alpha/PLOD2 axis integrates extracellular matrix organization and cell metabolism leading to aberrant musculoskeletal repair. Bone Res. 12, 17 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mok, G. F., Lozano-Velasco, E. & Munsterberg, A. microRNAs in skeletal muscle development. Semin. Cell Dev. Biol.72, 67–76 (2017). [DOI] [PubMed] [Google Scholar]
- 116.Giagnorio, E. et al. MyomiRs and their multifaceted regulatory roles in muscle homeostasis and amyotrophic lateral sclerosis. J. Cell Sci.134, jcs258349 (2021). [DOI] [PubMed] [Google Scholar]
- 117.Tower, R. J. et al. Spatial transcriptomics reveals metabolic changes underly age-dependent declines in digit regeneration. eLife11, e71542 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wan, Z. et al. Mgp high‐expressing MSCs orchestrate the osteoimmune microenvironment of collagen/nanohydroxyapatite‐mediated bone regeneration. Adv. Sci.11, e2308986 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang, W., Caruana, D. L., Back, J. & Lee, F. Y. Unique spatial transcriptomic profiling of the murine femoral fracture callus: a preliminary report. Cells13, 522 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rios, J. J. et al. Spatial transcriptomics implicates impaired BMP signaling in NF1 fracture pseudarthrosis in murine and patient tissues. JCI Insight9, e176802 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mathavan, N. et al. Spatial transcriptomics in bone mechanomics: Exploring the mechanoregulation of fracture healing in the era of spatial omics. Sci. Adv.11, eadp8496 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pena, O. A. & Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol.25, 599–616 (2024). [DOI] [PubMed] [Google Scholar]
- 123.Foster, D. S. et al. Integrated spatial multiomics reveals fibroblast fate during tissue repair. Proc. Natl. Acad. Sci. USA118, e2110025118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang, Y. et al. Tracing immune cells around biomaterials with spatial anchors during large-scale wound regeneration. Nat. Commun.14, 5995, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen, P. et al. Single‐cell and spatial transcriptomics decodes Wharton’s jelly‐derived mesenchymal stem cells heterogeneity and a subpopulation with wound repair signatures. Adv. Sci.10, e2204786 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xia, X. et al. Mesenchymal stem cells promote healing of nonsteroidal anti-inflammatory drug-related peptic ulcer through paracrine actions in pigs. Sci. Transl. Med.11, eaat7455 (2019). [DOI] [PubMed] [Google Scholar]
- 127.Levy, O. et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv.6, eaba6884 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou, T. et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol.14, 24 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elosua-Bayes, M. et al. SPOTlight: seeded NMF regression to deconvolute spatial transcriptomics spots with single-cell transcriptomes. Nucleic Acids Res.49, e50 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Glyn-Jones, S. et al. Osteoarthritis. Lancet386, 376–387 (2015). [DOI] [PubMed] [Google Scholar]
- 131.Hong, J. W., Noh, J. H. & Kim, D. J. The prevalence of and demographic factors associated with radiographic knee osteoarthritis in Korean adults aged ≥ 50 years: the 2010-2013 Korea National Health and Nutrition Examination Survey. PLoS One15, e0230613 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hunter, D. J. & Bierma-Zeinstra, S. Osteoarthritis. Lancet393, 1745–1759 (2019). [DOI] [PubMed] [Google Scholar]
- 133.Ji, Q. et al. Single-cell RNA-seq analysis reveals the progression of human osteoarthritis. Ann. Rheum. Dis.78, 100–110 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fan, Y. et al. Unveiling inflammatory and prehypertrophic cell populations as key contributors to knee cartilage degeneration in osteoarthritis using multi- omics data integration. Ann. Rheum. Dis.83, 926–944 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Loeser, R. F. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin. Geriatr. Med.26, 371–386 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thompson, S. et al. Twenty-year outcomes of a longitudinal prospective evaluation of isolated endoscopic anterior cruciate ligament reconstruction with patellar tendon autografts. Am. J. Sports Med.43, 2164–2174 (2015). [DOI] [PubMed] [Google Scholar]
- 137.Yang, R. et al. A single-cell atlas depicting the cellular and molecular features in human anterior cruciate ligamental degeneration: A single cell combined spatial transcriptomics study. eLife12, e85700 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Perez, K. et al. Single nuclei profiling identifies cell specific markers of skeletal muscle aging, frailty, and senescence. Aging14, 9393–9422 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Akbar, M. et al. Single cell and spatial transcriptomics in human tendon disease indicate dysregulated immune homeostasis. Ann. Rheum. Dis.80, 1494–1497 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fu, W., Yang, R. & Li, J. Single-cell and spatial transcriptomics reveal changes in cell heterogeneity during progression of human tendinopathy. BMC Biol.21, 132 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maffulli, N., Longo, U. G., Kadakia, A. & Spiezia, F. Achilles tendinopathy. Foot Ankle Surg.26, 240–249 (2020). [DOI] [PubMed] [Google Scholar]
- 142.Millar, N. L. et al. Tendinopathy. Nat. Rev. Dis. Prim.7, 1 (2021). [DOI] [PubMed] [Google Scholar]
- 143.Walsh, L. A. & Quail, D. F. Decoding the tumor microenvironment with spatial technologies. Nat. Immunol.24, 1982–1993 (2023). [DOI] [PubMed] [Google Scholar]
- 144.Liu, T. et al. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J. Hematol. Oncol.12, 86 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer20, 174–186 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gill, C. M., Fowkes, M. & Shrivastava, R. K. Emerging therapeutic targets in chordomas: a review of the literature in the genomic era. Neurosurgery86, E118–E123 (2020). [DOI] [PubMed] [Google Scholar]
- 147.Al Shihabi, A. et al. Personalized chordoma organoids for drug discovery studies. Sci. Adv.8, eabl3674 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang, T.-L. et al. Integrating single-cell and spatial transcriptomics reveals endoplasmic reticulum stress-related CAF subpopulations associated with chordoma progression. Neuro Oncol.26, 295–308 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Riggi, N., Longo, D. L., Suvà, M. L. & Stamenkovic, I. Ewing’s Sarcoma. N. Engl. J. Med.384, 154–164 (2021). [DOI] [PubMed] [Google Scholar]
- 150.Wrenn, E. D. et al. Cancer-associated fibroblast-like tumor cells remodel the Ewing sarcoma tumor microenvironment. Clin. Cancer Res.29, 5140–5154 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lei, X. et al. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett.470, 126–133 (2020). [DOI] [PubMed] [Google Scholar]
- 152.Li, R. et al. PSME2 offers value as a biomarker of M1 macrophage infiltration in pan-cancer and inhibits osteosarcoma malignant phenotypes. Int. J. Biol. Sci.20, 1452–1470 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pingping, B. et al. Incidence and mortality of sarcomas in Shanghai, China, during 2002–2014. Front. Oncol.9, 662 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin.69, 7–34 (2019). [DOI] [PubMed] [Google Scholar]
- 155.Weilbaecher, K. N., Guise, T. A. & McCauley, L. K. Cancer to bone: a fatal attraction. Nat. Rev. Cancer11, 411–425 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ihle, C. L. et al. Distinct tumor microenvironments of lytic and blastic bone metastases in prostate cancer patients. J. Immunother. Cancer7, 293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang, R. et al. Construction of bone metastasis-specific regulation network based on prognostic stemness-related signatures in breast invasive carcinoma. Front. Oncol.10, 613333 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pagani, S. et al. RNA extraction from cartilage: issues, methods, tips. Int. J. Mol. Sci.24, 2120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sailer, V. et al. Bone biopsy protocol for advanced prostate cancer in the era of precision medicine. Cancer124, 1008–1015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Singh, V. M. et al. Analysis of the effect of various decalcification agents on the quantity and quality of nucleic acid (DNA and RNA) recovered from bone biopsies. Ann. Diagn. Pathol.17, 322–326 (2013). [DOI] [PubMed] [Google Scholar]
- 161.Zheng, G. et al. Clinical mutational profiling of bone metastases of lung and colon carcinoma and malignant melanoma using next-generation sequencing. Cancer Cytopathol.124, 744–753 (2016). [DOI] [PubMed] [Google Scholar]
- 162.Vincent, T. L., McClurg, O. & Troeberg, L. The extracellular matrix of articular cartilage controls the bioavailability of pericellular matrix-bound growth factors to drive tissue homeostasis and repair. Int. J. Mol. Sci.23, 6003 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ruettger, A., Neumann, S., Wiederanders, B. & Huber, R. Comparison of different methods for preparation and characterization of total RNA from cartilage samples to uncover osteoarthritis in vivo. BMC Res. Notes3, 7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wehrle, E. et al. Protocol for preparing formalin-fixed paraffin-embedded musculoskeletal tissue samples from mice for spatial transcriptomics. STAR Protoc.5, 102986 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gao, X. H. et al. Comparison of fresh frozen tissue with formalin-fixed paraffin-embedded tissue for mutation analysis using a multi-gene panel in patients with colorectal cancer. Front. Oncol.10, 310 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lin, X. et al. A comparative analysis of RNA sequencing methods with ribosome RNA depletion for degraded and low-input total RNA from formalin-fixed and paraffin-embedded samples. BMC Genomics20, 831 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhong, J. et al. Multi-species atlas resolves an axolotl limb development and regeneration paradox. Nat. Commun.14, 6346 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gomariz, A. et al. Quantitative spatial analysis of haematopoiesis-regulating stromal cells in the bone marrow microenvironment by 3D microscopy. Nat. Commun.9, 2532 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Boulais, P. E. et al. The majority of CD45(-) Ter119(-) CD31(-) bone marrow cell fraction is of hematopoietic origin and contains erythroid and lymphoid progenitors. Immunity49, 627–639 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li, H. et al. A comprehensive benchmarking with practical guidelines for cellular deconvolution of spatial transcriptomics. Nat. Commun.14, 1548 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gulati, G. S. et al. Profiling cell identity and tissue architecture with single-cell and spatial transcriptomics. Nat. Rev. Mol. Cell Biol.26, 11–31 (2024). [DOI] [PubMed] [Google Scholar]
- 172.Chen, J. Y. et al. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature530, 223–227 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ziegenhain, C. et al. Comparative analysis of single-cell RNA sequencing methods. Mol. Cell65, 631–643 (2017). [DOI] [PubMed] [Google Scholar]
- 174.Wegmann, R. et al. CellSIUS provides sensitive and specific detection of rare cell populations from complex single-cell RNA-seq data. Genome Biol.20, 142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Song, D. et al. scPNMF: sparse gene encoding of single cells to facilitate gene selection for targeted gene profiling. Bioinformatics37, i358–i366 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cheng, C., Chen, W., Jin, H. & Chen, X. A review of single-cell RNA-seq annotation, integration, and cell–cell communication. Cells12, 1970 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang, X. et al. MarsGT: Multi-omics analysis for rare population inference using single-cell graph transformer. Nat. Commun.10.1038/s41467-023-44570-8 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell184, 3573–3587 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wei, R. et al. Spatial charting of single-cell transcriptomes in tissues. Nat. Biotechnol.40, 1190–1199 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kleshchevnikov, V. et al. Cell2location maps fine-grained cell types in spatial transcriptomics. Nat. Biotechnol.40, 661–671 (2022). [DOI] [PubMed] [Google Scholar]
- 181.Ma, Y. & Zhou, X. Spatially informed cell type deconvolution for spatial transcriptomics. Nat. Biotechnol.40, 1349–1359 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Miller, B. F. et al. Reference-free cell type deconvolution of multi-cellular pixel-resolution spatially resolved transcriptomics data. Nat. Commun.13, 2339 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tilburg, J. et al. Spatial transcriptomics of murine bone marrow megakaryocytes at single-cell resolution. Res Pr. Thromb. Haemost.7, 100158 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cho, C.-S. et al. Microscopic examination of spatial transcriptome using Seq-Scope. Cell184, 3559–3572.e3522 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vickovic, S. et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat. Methods16, 987–990 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Toosi, S., Behravan, N. & Behravan, J. Nonunion fractures, mesenchymal stem cells and bone tissue engineering. J. Biomed. Mater. Res. A106, 2552–2562 (2018). [DOI] [PubMed] [Google Scholar]
- 187.Compston, J. E., McClung, M. R. & Leslie, W. D. Osteoporosis. Lancet393, 364–376 (2019). [DOI] [PubMed] [Google Scholar]
- 188.Francisco, V. et al. A new immunometabolic perspective of intervertebral disc degeneration. Nat. Rev. Rheumatol.18, 47–60 (2022). [DOI] [PubMed] [Google Scholar]
- 189.Aicale, R., Tarantino, D. & Maffulli, N. Overuse injuries in sport: a comprehensive overview. J. Orthop. Surg. Res.13, 309 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schede, H. H. et al. Spatial tissue profiling by imaging-free molecular tomography. Nat. Biotechnol.39, 968–977 (2021). [DOI] [PubMed] [Google Scholar]
- 191.Zeng, H. et al. Integrative in situ mapping of single-cell transcriptional states and tissue histopathology in a mouse model of Alzheimer’s disease. Nat. Neurosci.26, 430–446 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schott, M. et al. Open-ST: High-resolution spatial transcriptomics in 3D. Cell187, 3953–3972.e26 (2024). [DOI] [PubMed] [Google Scholar]
- 193.Zeira, R., Land, M., Strzalkowski, A. & Raphael, B. J. Alignment and integration of spatial transcriptomics data. Nat. Methods19, 567–575 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jones, A., Townes, F. W., Li, D. & Engelhardt, B. E. Alignment of spatial genomics data using deep Gaussian processes. Nat. Methods20, 1379–1387 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vandereyken, K., Sifrim, A., Thienpont, B. & Voet, T. Methods and applications for single-cell and spatial multi-omics. Nat. Rev. Genet.24, 494–515 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Baysoy, A., Bai, Z., Satija, R. & Fan, R. The technological landscape and applications of single-cell multi-omics. Nat. Rev. Mol. Cell Biol.24, 695–713 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu, Y. et al. High-plex protein and whole transcriptome co-mapping at cellular resolution with spatial CITE-seq. Nat. Biotechnol.41, 1405–1409 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vickovic, S. et al. SM-Omics is an automated platform for high-throughput spatial multi-omics. Nat. Commun.13, 795 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ben-Chetrit, N. et al. Integration of whole transcriptome spatial profiling with protein markers. Nat. Biotechnol.41, 788–793 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang, D. et al. Spatial epigenome-transcriptome co-profiling of mammalian tissues. Nature616, 113–122 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Marconato, L. et al. SpatialData: an open and universal data framework for spatial omics. Nat. Methods22, 58–62 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Eisenstein, M. Seven technologies to watch in 2022. Nature601, 658–661 (2022). [DOI] [PubMed] [Google Scholar]
- 203.Madissoon, E. et al. A spatially resolved atlas of the human lung characterizes a gland-associated immune niche. Nat. Genet.55, 66–77 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sakamoto, Y., Sereewattanawoot, S. & Suzuki, A. A new era of long-read sequencing for cancer genomics. J. Hum. Genet.65, 3–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Charnwichai, P. et al. Rapid decalcification of articular cartilage and subchondral bone using an ultrasonic cleaner with EDTA. Acta Histochem.125, 152009 (2023). [DOI] [PubMed] [Google Scholar]
- 206.Bosia, C. et al. RNAs competing for microRNAs mutually influence their fluctuations in a highly non-linear microRNA-dependent manner in single cells. Genome Biol.18, 37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cai, L., Friedman, N. & Xie, X. S. Stochastic protein expression in individual cells at the single molecule level. Nature440, 358–362 (2006). [DOI] [PubMed] [Google Scholar]
- 208.Chu, L. X. et al. Spatiotemporal multi-omics: exploring molecular landscapes in aging and regenerative medicine. Mil. Med. Res.11, 31 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen, W. et al. Live-seq enables temporal transcriptomic recording of single cells. Nature608, 733–740 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pade, L. R. et al. Biological mass spectrometry enables spatiotemporal ‘omics: From tissues to cells to organelles. Mass Spectrom. Rev.43, 106–138 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zahedi, R. et al. Deep learning in spatially resolved transcriptfomics: a comprehensive technical view. Brief. Bioinform.25, bbae082 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hu, J. et al. SpaGCN: integrating gene expression, spatial location and histology to identify spatial domains and spatially variable genes by graph convolutional network. Nat. Methods18, 1342–1351 (2021). [DOI] [PubMed] [Google Scholar]
- 213.Pham, D. et al. Robust mapping of spatiotemporal trajectories and cell-cell interactions in healthy and diseased tissues. Nat. Commun.14, 7739 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dong, K. & Zhang, S. Deciphering spatial domains from spatially resolved transcriptomics with an adaptive graph attention auto-encoder. Nat. Commun.13, 1739 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Shengquan, C. et al. stPlus: a reference-based method for the accurate enhancement of spatial transcriptomics. Bioinformatics37, i299–i307 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Biancalani, T. et al. Deep learning and alignment of spatially resolved single-cell transcriptomes with Tangram. Nat. Methods18, 1352–1362 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yuan, Y. & Bar-Joseph, Z. GCNG: graph convolutional networks for inferring gene interaction from spatial transcriptomics data. Genome Biol.21, 300 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Song, Q. & Su, J. DSTG: deconvoluting spatial transcriptomics data through graph-based artificial intelligence. Brief. Bioinform.22, bbaa414 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Petukhov, V. et al. Cell segmentation in imaging-based spatial transcriptomics. Nat. Biotechnol.40, 345–354 (2022). [DOI] [PubMed] [Google Scholar]
- 220.Littman, R. et al. Joint cell segmentation and cell type annotation for spatial transcriptomics. Mol. Syst. Biol.17, e10108 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Amodio, M. et al. Exploring single-cell data with deep multitasking neural networks. Nat. Methods16, 1139–1145 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li, X. et al. Deep learning enables accurate clustering with batch effect removal in single-cell RNA-seq analysis. Nat. Commun.11, 2338, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lakkis, J. et al. A joint deep learning model enables simultaneous batch effect correction, denoising, and clustering in single-cell transcriptomics. Genome Res. 31, 1753–1766 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Xu, H. et al. Unsupervised spatially embedded deep representation of spatial transcriptomics. Genome Med16, 12 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Shah, S. et al. Single-molecule RNA detection at depth by hybridization chain reaction and tissue hydrogel embedding and clearing. Development143, 2862–2867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Xia, C. et al. Spatial transcriptome profiling by MERFISH reveals subcellular RNA compartmentalization and cell cycle-dependent gene expression. Proc. Natl. Acad. Sci. USA116, 19490–19499 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lee, J. H. Quantitative approaches for investigating the spatial context of gene expression. WIREs Syst. Biol. Med.9, e1369 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ferri-Borgogno, S. et al. Molecular, metabolic, and subcellular mapping of the tumor immune microenvironment via 3D targeted and non-targeted multiplex multi-omics analyses. Cancers16, 846 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yin, Y. et al. Unraveling spatial complexity of the tumor microenvironment: a whole transcriptomic perspective with Visium HD. Cancer Res.84, abstr. 3645 (2024). [Google Scholar]
- 230.Fan, Z., Chen, R. & Chen, X. SpatialDB: a database for spatially resolved transcriptomes. Nucleic Acids Res.48, D233–D237 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]