Abstract
Determination of metanephrines in saliva has been reported as a pain- and hazard-free alternative to plasma-based tests with a potential to reduce false-positive results during diagnostic work-up for neuroendocrine tumours. No validated method has, however, been made available in the published literature so far. The aim of this work was to develop an LC-MS/MS method for the measurement of metanephrine, normetanephrine and 3-methoxytyramine in human saliva. As hyposalivation represents a real-life challenge in situations with catecholamine excess, special effort was made to minimise specimen volume and to make the assay suitable also for paediatric and geriatric populations. The method validation was performed according to relevant guidelines and included recovery from three different collection swabs (Salivette, SalivaBio and Salimetrics). Selectivity, interferences, matrix effects, limits of quantification, linearity of calibration, sample volume, trueness, within-run and total analytical repeatability, robustness, carry-over and stability were evaluated. A sample volume as low as 20 μL was acceptable for all analytes, but 50 μL sample volume gave lower LLOQ, higher accuracy and better precision. Total analytical variation was <15% and the minimum turnaround time was 3 h. Among the sample collection devices tested, highest recovery was achieved with SalivaBio (93–104%). Metanephrines were stable in saliva when stored at ambient temperature for 6 h, and tolerated at least 1 week of freezing at −20°C. The non-invasiveness, ease of specimen collection, better stability of analytes compared to plasma and analytical performance make the method relevant for both research and diagnostics of states with catecholamine excess.
Plain language summary
A new method to measure breakdown products of adrenalin, noradrenalin and dopamine in saliva is proposed. The main advantage of saliva test compared to traditional blood-based assays is that specimen can be collected without stress and pain. The method can improve diagnostics of hormone-producing tumours by reducing likelihood of hormone elevations due to stress and pain (false positive results) and avoiding hazards associated with blood collection, particularly in children.
Keywords: saliva, metanephrines, catecholamines, liquid chromatography–mass spectrometry, adrenal medulla
Introduction
Stress and anxiety are typically associated with a hormonal response that involves release of catecholamines such as epinephrine, norepinephrine and small amounts of dopamine from chromaffin cells of the nervous system, including the adrenal medulla (1, 2, 3). Measurement of plasma-free metanephrines is a standard approach in screening and diagnostics of neuroendocrine tumours, such as pheochromocytoma and paraganglioma, producing catecholamines (4). Yet, it is clinically challenging and economically demanding to differentiate between organic disorders and the rather prevalent physiological elevations of metanephrines caused by stress.
Saliva as a matrix for measurement of metanephrines, especially in the setting of stress-free sampling at home, has the potential to reduce false positive results and the need for repetitive samplings in the diagnostic workup of neuroendocrine tumours (5). It provides several other advantages over traditional plasma-based assays, such as simple pre-analytical processing and non-invasiveness, which makes it the preferred alternative in paediatric populations, injecting drug users, patients with anaemia or anxiety and frail elderly prone to syncope.
Eijkelenkamp et al. reported diagnostic sensitivity of 89% and specificity of 87% for supine salivary metanephrines determined with LC-MS/MS in pheochromocytoma and paraganglioma (6). Although the diagnostic accuracy was slightly inferior to plasma-based assays, this pioneering work has proven that saliva tests could be a clinically useful option. Performance of infant-sized swabs and minimal volume of saliva needed were, however, not reported in this or other publications from the same group (6, 7). We have therefore aimed to develop and validate an LC-MS/MS assay to determine clinically relevant catecholamine metabolites metanephrine, normetanephrine and 3-methoxytyramine in small volumes of saliva. By this, we attempted to make the assay suitable also for paediatric and geriatric populations.
To our knowledge, this is the first published method validated for metanephrines in salivary samples suitable for new-borns and patients with hyposalivation. This extends the possibility for valid salivary sampling in many patient groups.
Materials and methods
Chemicals and reagents
Stock solutions of metanephrine, normetanephrine and 3-methoxytyramine and deuterated internal standards were purchased from Cerilliant (USA). Mucin, sodium chloride (NaCl), sodium phosphate monobasic dehydrate (NaH2PO4), sodium hydroxide (NaOH), potassium chloride (KCl), potassium thiocyanate (KSCN), urea and uric acid were purchased from Merck Life Science AS (Norway). Ammonium acetate, formic acid (FA, 98–100% for LC-MS) and water (LC-MS grade HiPerSolv®) were obtained from VWR (Oslo, Norway). Methanol (MeOH) was LC grade or better, and solvents from Merck Life Sciences, Sigma-Aldrich (USA) and VWR were used. Ephedrine, pseudoephedrine, 3,4-methyl-dioxy-amphetamine (MDA), 3,4-methylenedioxymethamphetamine (MDMA), amoxyline and sulphasalazine were obtained from Sigma-Aldrich. 3-O-methyldopa was purchased from LGC (UK); D,L-4-hydroxy-3-methoxymethamphetamine hydrochloride (HMMA) was obtained from Ipomed (France); and levodopa was purchased from European Pharmacopeia (France).
Artificial saliva, calibrators and internal quality controls
Artificial saliva was used as a matrix for calibrators and internal quality controls (QCs) and prepared as described by Denys (8) and De Nys (9), but without addition of α-amylase. Briefly, 1L artificial saliva contained KCl (896 mg), NaH2PO4 (888 mg), NaCl (298 mg), KSCN (200 mg), urea (200 mg), mucin (50 mg), 1 M NaOH (1.8 mL) and uric acid (15 mg, added as stock solution).
Calibration solutions at four levels, all within the method’s linear range, were prepared from certified reference material stock solutions (Cerilliant) by adequate dilutions with artificial saliva. The concentration of calibrators is shown in Table 1. In-house calibration solutions were compared to calibration solutions (6PLUS1, Chromsystems, Germany) used in our validated routine method for plasma-free metanephrines (10).
Table 1.
Calibrator concentrations, lower (LLOQ) and upper (ULOQ) limit of quantification.
| Metanephrine (nmol/L) | Normetanephrine (nmol/L) | 3-methoxytyramine (nmol/L) | |
|---|---|---|---|
| Calibrator 1 | 0.82 | 0.87 | 0.43 |
| Calibrator 2 | 1.9 | 2.0 | 0.72 |
| Calibrator 3 | 5.1 | 5.5 | 1.2 |
| Calibrator 4 | 21 | 23 | 16 |
| LLOQ | 0.14 | 0.15 | 0.20 |
| ULOQ | 100 | 100 | 30 |
QCs at three concentrations levels (Table 2) were prepared with artificial saliva as matrix. Stock solutions were from the same certified reference material, however, using different pipettes and on different days.
Table 2.
Concentrations of internal QCs observed bias, within-run repeatability and total analytical repeatability.
| QC concentration level | Metanephrine | Normetanephrine | 3-methoxytyramine | ||||||
|---|---|---|---|---|---|---|---|---|---|
| QC 1 | QC 2 | QC 3 | QC 1 | QC 2 | QC 3 | QC 1 | QC 2 | QC 3 | |
| QC concentration, nmol/L | 0.27 | 0.60 | 2.7 | 0.29 | 0.64 | 2.9 | 0.40 | 0.75 | 1.4 |
| Bias, mean % from target | −12 | 8.3 | 9.5 | −6.2 | 6.8 | 10 | −13 | −0.3 | 8.9 |
| Within-run repeatability, CV% | 8.4 | 3.1 | 3.5 | 14 | 5.1 | 4.1 | 13 | 3.7 | 3.0 |
| Total analytical repeatability, CV% | 14 | 11 | 7.0 | 26 | 11 | 12 | 20 | 11 | 12 |
The internal standard working solution was prepared from stock solutions (Cerilliant) and had a metanephrine-d3 concentration of 1.0 nmol/L; the normetanephrine-d3 and 3-methoxytyramine-d4 concentrations were 2.0 nmol/L.
Sample collection and pre-analytical processing
Saliva samples for method development and validation were obtained from pooled anonymous left-overs from clinical routine, volunteers connected to the study team and research projects; personal information was not recorded. The Data Protection Officer at the Oslo University Hospital approved the use of anonymous biological material (reference 21/10703). Samples were collected using three different swabs: SalivaBio infant swab (Salimetrics, USA), Micro·SALTM for Children (Oasis Diagnostics® Corporation, USA) and Salivette® with synthetic swab (Sarstedt, Germany).
Saliva samples were stored on-swab in storage tubes (Salimetrics or Sarstedt), placed at −20°C within 10–90 min after collection and at −80°C upon arrival of the samples at the laboratory. Before analysis, SalivaBio and Salivette swabs were thawed (room temperature, 30–60 min) and centrifuged at 1,500 g for 15 min to release saliva from these swabs. Saliva obtained with MicroSAL was pressed through a narrow bore tube connected to a 2 mL microcentrifuge tube upon collection, and placed at −20°C within 10–90 min followed by transfer to −80°C for long-term storage.
Deuterated internal standards (ISTD, Cerilliant, 100 μL) prepared in 0.1% FA (v/v) were added to 50 μL of each blank, sample, calibrator or control sample and allowed to equilibrate for at least 10 min. The pH was adjusted to 7 with 50 mM ammonium acetate (600 μL) before weak cation-exchange (WCX) solid phase extraction (SPE, Evolute Express 30 mg, Biotage, Sweden). After application of the samples, the WCX plate was washed with 1 mL H2O, followed by 1 mL 50/50 MeOH/AcN (50/50 v/v). Samples were eluted from the WCX plate using 500 μL 5% FA in 50/50 MeOH/AcN, pH 3. The solvent was removed under a gentle stream of nitrogen (55°C) and the samples were reconstituted in 75 μL H2O.
LC-MS/MS method
LC-MS/MS analysis was performed using a UHPLC system (AB Sciex LLC, USA) coupled to a 6500+ QTrap mass spectrometer (Sciex). Twenty microlitres of the extracted sample were injected onto a 2.1 × 50 mm, 1.8 μm high-strength silica-C18 (HSS-T3) column (Waters, USA). A linear gradient at a flow rate of 0.3 mL/min was used to separate and elute analytes using 0.1% FA in water (A) and 0.1% FA in MeOH (B) as mobile phase. The linear gradient started at 2% B, which was held for 0.5 min, followed by 2–20% B (0.5–1.2 min), 20% B (1.2–3.0 min) and a 1-minute wash (100% B). The chromatographic and global MS-settings are given in Supplementary Table 1 (see section on Supplementary materials given at the end of the article).
Detection was performed with positive electrospray ionization and multiple reaction monitoring (MRM) using scheduled MRM. The ion transitions monitored for quantification are given in Supplementary Table 2 and are specific (e.g. without loss of water). In addition, one (ISTD) or two (analytes) qualifier ions were monitored (Supplementary Table 2).
LC-MS/MS method validation
Validation was performed according to relevant current guidelines (11, 12, 13) and internal procedures and included assessment of selectivity (specificity), interferences, matrix effects, lower and upper limit of quantification (LLOQ and ULOQ), linearity of calibration, sample volume, trueness (recovery and bias), within-run repeatability, total analytical repeatability, robustness, carry-over, stability and recovery from sample collection swab. All experiments included in method validation were based on sample volume of 50 μL, unless specified otherwise.
Specificity was defined as the ability to separate the isobaric compounds normetanephrine and adrenaline and other isobaric interferences previously described (14, 15, 16, 17, 18). Ion ratios for quantifier and qualifier ions were monitored. Spectral interference was tested by injection of standard solutions containing either analytes or internal standards and visual inspection of chromatograms.
Interferences by phospholipids were tested by injecting saliva samples from adults (n = 6) and premature infants (n = 3) and scanning for precursor ions of the product m/z 184 in the range of m/z 200–1,000. Blank artificial saliva with added ISTD (zero calibrator) and blank water with or without ISTD were injected directly or following WXC extraction. Blank matrix and blank water applied to saliva collection swabs and followed by WCX extraction were analysed in several batches.
Matrix effects were tested in different ways: i) by comparing two sets of QC solutions: blank saliva matrix with analytes spiked after sample preparation and solvent spiked with analytes without sample preparation (n = 6 for both sets at two QC levels). Absence of matrix effects was defined as 100 ± 20%; ii) by injecting anonymous saliva samples (n = 3 from premature infants and n = 6 from adults) and recording the total ion current (TIC) as MS scan; and iii) by comparing the mean ISTD peak areas in blank water (n = 15) and blank saliva (n = 14) with mean ISTD peak areas in matrix from project samples (n = 214).
LLOQ was defined as the lowest concentration giving a within-run CV% below 20% and a signal-to-noise (S/N) ratio of >10. ULOQ was defined as the highest calibrator concentration where the calculated value was within ±15% from the theoretical value.
Acceptable linearity was defined as R2 > 0.995 for a 4-point calibration curve. Placement of calibrators (n = 3) in the first and the last wells of a 96-well plate was compared. All calculated concentrations of the calibrators should be within ±10% of their respective theoretical values.
The acceptability of a sample volume lower than 50 μL was tested using internal QC samples. Calibrators had a volume of 50 μL, and QCs were prepared with a lower volume. A sample volume below 50 μL was permitted if the QCs bias with lower sample volume did not exceed ±10% compared to a volume of 50 μL.
Trueness was defined as the lack of significant bias of the internal QC samples from their target value. Acceptable bias was ±15% (FDA (11), EMA (12) and internal experience).
Recovery from the different saliva collection swabs was assessed using calibrator solutions. A detailed description of the experiment is given in the Supplementary materials. Recovery was considered acceptable within 100 ± 20%.
Within-run repeatability was assessed by analysing internal QC samples at three concentration levels (n = 10 replicates) and averaging the CV% obtained on 3–4 days (Table 1). The total analytical repeatability (within-laboratory reproducibility) was calculated as long-term CV% (at least ten different runs on separate days) of internal QCs at three levels. Acceptable coefficients of variation were defined as 15% (FDA (11), EMA (12) and internal experience).
Stability was assessed by spiking internal QC 2 and 3 (artificial saliva) to four different pools of human saliva. On-swab stability was tested for 3 and 6 h at ambient temperature, and for 7 days at −20°C compared to snap-freezing and storage at −80°C. Individual samples from voluntary adults were used to assess the stability of metanephrine and normetanephrine at ambient temperature for 7 days.
Robustness was tested by making minor changes in column temperature or composition of mobile phase.
Carry-over in the LC-system was tested with blank injections after injections of a sample with high analyte concentration. Method carry-over was tested by extracting a sample with low analyte concentration before and after a sample with high analyte concentration.
Diurnal variation
Anonymous adult volunteers (n = 9) were recruited. The participants were asked to collect saliva specimens according to standard procedure recommended by Sarstedt (2 min sample collection time) performed at the following settings: i) supine position right after awakening; ii) 20 min of supine rest following sample 1; iii) 20 min of sitting following sample 2; iv) 20 min of supine rest following coffee-free and low-catecholamine breakfast (avoiding nuts and fruits such as bananas, pineapple fruit juice, tomatoes, potatoes and beans); v) following 15 min of otherwise regular physical exercise or any physical effort; vi) 20 min of supine rest following sample 5; vii) 20 min of supine rest before evening teeth brushing; and viii) 20 min supine rest following evening teeth brushing. Swabs were stored at room temperature for a maximum of 24 h, and placed at −80°C before analysis. Mixed effects models were built in the GraphPad Prism 10.1.2 (GraphPad Software LLC, USA), with settings as fixed effects, saliva collection kit number (=individual participants) as a random effect and assumed sphericity. Test for linear trend between group means followed morning-to-afternoon group order.
Results
Method validation
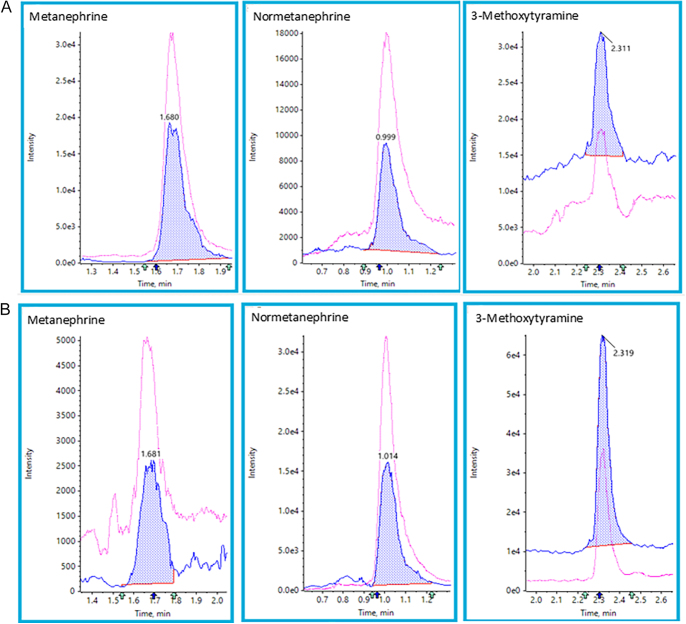
Baseline separation was achieved for normetanephrine and adrenaline or 3-O-methyldopa and 3-methoxytyramine. No interference from ephedrine, pseudoephedrine, MDA, MDMA, HMMA, amoxyline or sulphasalazine was observed. Spectral interference was not observed for any analyte/internal standard. Separation of metanephrine and 3-methoxytyramine did prevent spectral interference between these analytes. Two different qualifier ion-ratios were used in the evaluation of analytes, while one qualifier ion ratio was used in the evaluation of internal standards in samples. The method is specific for metanephrine, normetanephrine and 3-methoxytyramine. An example chromatogram at the lowest calibration level and from a representative sample is shown in Fig. 1A and B.
Figure 1.
(A) Example chromatograms of CAL 1 level. (B) Example chromatogram of a sample; calculated concentrations were 0.14 nmol/L (metanephrine), 3.6 nmol/L (normetanephrine) and 1.1 nmol/L (3-methoxytyramine).
Analytes or ISTDs did not show significant peaks in blank matrix or blank water. This indicates that the method, including sample preparation, is free from interferences. Precursor ion scans did not indicate any significant interferences from phospholipids. Matrix effects were in the range of 98–111%. TIC of the MS scan did not show peaks that might give electrospray ionization (ESI) signal suppression. ISTD peak areas were similar in water, artificial saliva and human saliva (Supplemental Table 3); this indicates no matrix effects and justifies the use of artificial saliva for calibration and internal QC. In summary, these observations show that the method is not prone to matrix effects or other interferences.
The LLOQ and ULOQ were based on sample volumes of 50 μL (Table 1). All analytes had an acceptable linearity over a relevant concentration range. The linearity remained equally good when the calibration solutions were placed at the end of a 96-well plate, indicating that the method’s working range was 96 samples. Provided high enough concentration (>LLOQ), a smaller sample volume down to 10 μL for metanephrine and normetanephrine and 20 μL for 3-methoxytyramine could be used without loss of accuracy. An analyte concentration was also calculated and reported for sample volumes below 50 μL when ion ratios were acceptable, and the signal-to-noise ratio was above 10.
QC samples’ bias from their target value, within-run and total analytical repeatability are shown in Table 2. A bias below ±15% was observed for all three analytes, notably also at concentrations around LLOQ (QC1).
All analytes showed an acceptable repeatability (mean below the predefined target of 15%) at all concentration levels. Total analytical repeatability was within the pre-defined limits (15%) at any concentration level for metanephrine, and at middle and high concentration level for normetanephrine and 3-methoxytyramine (n = 25–32 for all). Higher total analytical variation (15–20%) was expected for QC1 according to the definition of LLOQ.
Small changes in mobile phase additive, column temperature or mobile phase flowrate did not change peak shape or selectivity, showing that the method is robust.
Carry-over was not observed in the LC-system or in the method including sample preparation.
Test of saliva collection swabs
We tested recovery from three saliva collection swabs (Table 3); two were suitable for neonatal population (MicroSal and SalivaBio) and one (Salivette) for adults only. Recovery for calibrator levels 2–4 from the sample collection swab ranged from 93 to 104% for the SalivaBio swab; 90–113% for the MicroSal swab; and 85–107% for the Salivette swab. SalivaBio and MicroSal showed good recoveries, even within ±15% for all analytes with an exception for the lowest level of methoxytyramine (149% at calibrator level 1 for Salimetrics). Lowest recovery (<90%) from the Salivette swab was observed at one concentration level for metanephrine and 3-methoxytyramine. Recoveries from the Salivette swab were in general around ±90% at all concentration levels for normetanephrine and at two concentration levels for metanephrine and 3-methoxytyramine. The other two swabs showed recoveries of 95–100%. However, a small interference for 3-methoxytyramine from the WCX plate (Supplemental Fig. 1) may give higher recoveries at the lowest concentration level.
Table 3.
Recovery data given as the mean % of three replicates.
| Analyte | SalivaBio | Salivette | MicroSal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAL1 | CAL2 | CAL3 | CAL4 | CAL1 | CAL2 | CAL3 | CAL4 | CAL1 | CAL2 | CAL3 | CAL4 | |
| Metanephrine | 97 | 94 | 99 | 101 | 105 | 88 | 91 | 97 | 97 | 107 | 104 | 105 |
| Normetanephrine | 104 | 93 | 97 | 94 | 92 | 93 | 90 | 94 | 93 | 101 | 102 | 97 |
| 3-methoxytyramine | (149)* | 95 | 94 | 104 | 95 | 107 | 90 | 85 | (113)* | 101 | 101 | 90 |
Interference from WXC plate in the recovery experiment setup.
Sample stability
On-swab stability for SalivaBio was tested at room temperature and at −20°C (Table 4) using spiked saliva pools or non-pooled individual samples obtained from voluntary adults. Metanephrine and normetanephrine showed reduced on-swab stability when stored at ambient temperature for 7 days, but all analytes were stable at room temperature for 6 h. Because of this, all experimental samples used in this study were placed at −20°C within 90 min from specimen collection time. Stored at −20°C, metanephrines were stable for 1 week. Samples were transferred to −80°C immediately upon arrival to the laboratory.
Table 4.
Stability of metanephrines in saliva at room temperature and in saliva at −20°C, given as the mean bias from day 0.
| Analyte | Room temperature | −20C | ||
|---|---|---|---|---|
| 3 h | 6 h | 1 week | 1 week | |
| Metanephrine | 2%, n = 8 | −2%, n = 8 | 39%, n = 26 | 0%, n = 8 |
| Normetanephrine | −5%, n = 8 | −5%, n = 8 | 46%, n = 25 | −5%, n = 8 |
| 3-methoxytyramine | −5%, n = 8 | −7%, n = 8 | -* | −7%, n = 8 |
Methoxytyramine 7 days not tested, samples from healthy adults did not contain 3-methoxytyramine.
Standardisation of specimen collection: time of day, posture, physical activity, intake of food, coffee and teeth brushing
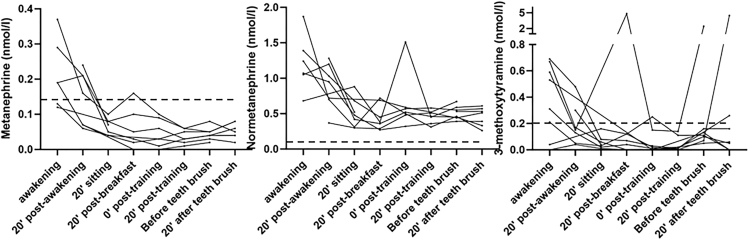
Salivary concentrations of metanephrine and normetanephrine were considerably higher in the morning hours, falling below the level of their corresponding reference intervals in plasma by midday (P for linear trend <0.001 for both metabolites). A similar pattern, although not statistically significant, was seen for 3-methoxytyramine. Most of the spikes were observed midday following physical activity and intake of food, but no consistent differences were noted in relationship to posture, physical activity, eating or teeth brush. Lowest average levels of metanephrine and normetanephrine in combination with least variation were observed in the late afternoon following 20 min of supine rest before teeth brushing (Fig. 2).
Figure 2.
Diurnal variation of salivary metanephrines in healthy adults. The dotted lines indicate LLOQs.
Discussion
Here, we describe a thoroughly validated LC-MS/MS method to determine metanephrine, normetanephrine and 3-methoxytyramine in saliva with a minimum turnaround time of 3–4 h. Traditionally, metanephrines were analysed in plasma using liquid chromatography with electrochemical detection (‘HLPC’). However, LC-MS methods are more specific and have shorter turnaround times compared to HPLC (17).
Among the many advantages of using saliva as the sample material is its ease of collection, hassle- and hazard-free nature, simple pre-analytical processing and possibility to monitor rapid hormonal changes. It is worth mentioning that urine samples also share many of the abovementioned advantages; however, contrary to saliva samples, it can detect only significant catecholamine excess that has been accumulated over a period of time.
The main focus of method development has been the downscaling of sample volume to suit neonatal and geriatric population while retaining the same analytical quality of plasma metanephrines that we have experienced in our laboratory over the past 5–6 years.
With the present method, we were able to perform quantification in saliva volumes as low as 20 μL.
Osinga et al. reported the use of an established plasma LC-MS/MS method for salivary metanephrines (7). However, the plasma method was based on a large sample volume (500 μL, (19)). The reference intervals for salivary metanephrine and normetanephrine in a study by Eijkelenkamp were reported to be 0.04–0.25 and 0.20–1.46 nmol/L, respectively (6). Our LLOQ for metanephrine is 0.14 nmol/L and for normetanephrine is 0.15 nmol/L, which both are below the upper reference value (40% lower for metanephrine and 90% lower for normetanephrine). Since the clinical need is to diagnose the states of catecholamine excess, our method is suitable to distinguish between individuals with normal and elevated values.
We characterized the performance of three different collection swabs. There were some minor differences in recovery of metanephrines, but in general, all three swabs may be used to obtain saliva for this test. Surprisingly, the Salivette swab, which performed well for more hydrophobic steroid hormones (20, 21), showed slightly lower recoveries for the hydrophilic metanephrines compared to the other two swabs. If Salivette is used, sample pre-treatment should include application of calibration and internal control solutions to the swab, which eventually will increase the cost of the method. SalivaBio gave best overall performance for both panels of steroids and metanephrines tested in our lab (20). The observed interference at the low level for 3-methoxytyramine originated from the WCX plates. However, it was not present to the same extent in the subsequent runs as in the recovery experiment. Careful examination of chromatograms and peak integration for samples with low concentrations of 3-methoxytyramine is mandatory to avoid overestimation.
Our results show that swabs need to be frozen within 6 h. On-swab stability at −20°C for more than 1 week has not been investigated in the present study, and we therefore suggest to limit the time for domestic storage combined with transport/shipment up to 7 days and to place the expressed sample in a new cryovial at −80°C immediately upon arrival at the laboratory. Relatively short on-swab stability at room temperature is one of the disadvantages of the present method. However, plasma-free metanephrines require special sampling as well (10): blood specimen has to be drawn by certified professionals in equipped facilities, centrifuged within 30 min, frozen and sent frozen to the laboratory, while 20 min supine rest before specimen collection is simply not achievable in out-patient settings at all.
Within-subject variation of metanephrines was lowest by the end of day. We suggest a standardized procedure for specimen collection in the late afternoon following 20 min of supine rest, if the test’s scope is diagnostics of neuroendocrine tumours. Small bleedings from microinjuries in oral mucosa, for example, elicited by teeth brushing, have been previously suggested as a possible source of falsely elevated hormone concentrations in saliva (22). We observed no effect of teeth brushing in this study. This probably reflects comparable metanephrine levels in saliva and plasma and implicates no need for specimen collection standardisation other than the time of day and supine rest. Our experiments, however, were performed on healthy adults, and thus validity of our conclusions for patients with bleeding gum disorders cannot be affirmed in this study. Although Eijkelenkamp et al. reported good sensitivity and specificity (6), more studies are needed to confirm that their findings are applicable to the method developed and reported by us.
It is recommended to avoid consumption of coffee and caffeine-containing beverages before sampling of plasma-free metanephrines (5). We performed an experiment to examine whether the intake of coffee may influence levels of salivary metanephrines; however, only a small number of subjects participated in this experiment, and thus no clear trend was observed (data not shown). Normetanephrine and possibly 3-methoxytyramine may be affected, and these finding necessitate further exploration. Relevant analytical interferences for LC-MS/MS as reported by Davison et al. (14) were tested and ruled out ephedrine, pseudoephedrine, MDA, MDMA, HMMA, amoxyline, sulphasalazine, adrenaline and 3-O-methyldopa. Pharmacological interferences (14) such as coffee, nicotine, food, amphetamines, cocaine and others were not investigated within the frame of this work but should be undertaken as a diagnostic utility study with relevant patient categories.
In conclusion, the present LC-MS/MS method to determine metanephrines is validated for saliva volumes as low as 20–50 μL. This makes it a non-invasive alternative to plasma-based assays. In future, diagnostic accuracy study should be undertaken to assess the utility of this method in the screening and diagnostics of neuroendocrine tumours, adrenal function and stress, particularly in the paediatric setting.
Supplementary materials
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the work reported.
Funding
This work was supported by the Foundation Dam under grant number 389 455. In collaboration with the Norwegian Prematurity Organization, a user representative has taken part at all stages of the present work, including planning, data analysis and manuscript preparation.
Author contribution statement
SRD helped in the development of methodology, formal analysis, writing of the original draft, visualization, writing of the review and editing. PMT helped with resources, writing of the review and editing and project administration. TS helped in the development of methodology, validation, formal analysis, investigation and data curation. THL helped with investigation and data curation. SNZ performed conceptualization, designing of methodology, investigation, formal analysis, writing of the original draft, visualization, project administration, funding acquisition, writing of the review and editing.
Acknowledgments
The authors thank study participants and colleagues who have contributed saliva. We thank Sheba Maria Lothe for reviewing the language.
References
- 1.Carmichael SW. The adrenal medulla. In Principles of Medical Biology. Volume 10A: Molecular and Cellular Endocrinology, pp 207–225. Eds Bittar EE & Bittar N. London, UK: JAI Press. [Google Scholar]
- 2.Peitzsch M, Novos T, Kaden D, et al. Harmonization of LC-MS/MS measurements of plasma free normetanephrine, metanephrine, and 3-methoxytyramine. Clin Chem 2021. 67 1098–1112. ( 10.1093/clinchem/hvab060) [DOI] [PubMed] [Google Scholar]
- 3.Wong DL, Tai TC, Wong-Faull DC, et al. Epinephrine: a short- and long-term regulator of stress and development of illness: a potential new role for epinephrine in stress. Cell Mol Neurobiol 2012. 32 737–748. ( 10.1007/s10571-011-9768-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenhofer G, Kopin IJ & Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 2004. 56 331–349. ( 10.1124/pr.56.3.1) [DOI] [PubMed] [Google Scholar]
- 5.Därr R, Pamporaki C, Peitzsch M, et al. Biochemical diagnosis of phaeochromocytoma using plasma-free normetanephrine, metanephrine and methoxytyramine: importance of supine sampling under fasting conditions. Clin Endocrinol 2014. 80 478–486. ( 10.1111/cen.12327) [DOI] [PubMed] [Google Scholar]
- 6.Eijkelenkamp K, Osinga TE, van Faassen M, et al. Diagnostic accuracy of salivary metanephrines in pheochromocytomas and paragangliomas. Clin Chem 2021. 67 1090–1097. ( 10.1093/clinchem/hvab064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osinga TE, van der Horst-Schrivers AN, van Faassen M, et al. Mass spectrometric quantification of salivary metanephrines-A study in healthy subjects. Clin Biochem 2016. 49 983–988. ( 10.1016/j.clinbiochem.2016.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denys S, Tack K, Caboche J, et al. Bioaccessibility, solid phase distribution, and speciation of Sb in soils and in digestive fluids. Chemosphere 2009. 74 711–716. ( 10.1016/j.chemosphere.2008.09.088) [DOI] [PubMed] [Google Scholar]
- 9.De Nys S, Putzeys E, Vervliet P, et al. A novel high sensitivity UPLC-MS/MS method for the evaluation of bisphenol A leaching from dental materials. Sci Rep 2018. 8 6981. ( 10.1038/s41598-018-24815-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hormone Laboratory, Oslo University Hospital . Analyseoversikt. Oslo, Norway: Oslo University Hospital. (https://metodebok.no/index.php?action=chapter&item=9yRN2DFz) [Google Scholar]
- 11.U.S. Food and Drug Administration (FDA) . Bioanalytical method validation guidance for industry. Silver Spring, MD, USA: FDA. (https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf) [Google Scholar]
- 12.European Medicines Agency . Guideline on bioanalytical method validation. EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2**. London, UK: European Medicines Agency. (https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf) [Google Scholar]
- 13.Clinical and Laboratory Standards Institute (CLSI) . Liquid chromatography-mass spectrometry methods. In CLSI Guideline C62, 2nd edn. Wayne, PA, USA: CLSI. (https://clsi.org/shop/standards/c62/) [Google Scholar]
- 14.Davison AS, Jones DM, Ruthven S, et al. Clinical evaluation and treatment of phaeochromocytoma. Ann Clin Biochem 2018. 55 34–48. ( 10.1177/0004563217739931) [DOI] [PubMed] [Google Scholar]
- 15.Eisenhofer G, Brown S, Peitzsch M, et al. Levodopa therapy in Parkinson’s disease: influence on liquid chromatographic tandem mass spectrometric-based measurements of plasma and urinary normetanephrine, metanephrine and methoxytyramine. Ann Clin Biochem 2014. 51 38–46. ( 10.1177/0004563213487894) [DOI] [PubMed] [Google Scholar]
- 16.Peitzsch M, Adaway JE & Eisenhofer G. Interference from 3-O-methyldopa with ultra-high performance LC-MS/MS measurements of plasma metanephrines: chromatographic separation remains important. Clin Chem 2015. 61 993–996. ( 10.1373/clinchem.2015.239962) [DOI] [PubMed] [Google Scholar]
- 17.Petteys BJ, Graham KS, Parnás ML, et al. Performance characteristics of an LC–MS/MS method for the determination of plasma metanephrines. Clin Chim Acta 2012. 413 1459–1465. ( 10.1016/j.cca.2012.06.006) [DOI] [PubMed] [Google Scholar]
- 18.Twentyman JM, Cradic KW, Singh RJ, et al. Ionic cross talk can lead to overestimation of 3-methoxytyramine during quantification of metanephrines by mass spectrometry. Clin Chem 2012. 58 1156–1158. ( 10.1373/clinchem.2012.186601) [DOI] [PubMed] [Google Scholar]
- 19.de Jong WH, Graham KS, van der Molen JC, et al. Plasma free metanephrine measurement using automated online solid-phase extraction HPLC–tandem mass spectrometry. Clin Chem 2007. 53 1684–1693. ( 10.1373/clinchem.2007.087114) [DOI] [PubMed] [Google Scholar]
- 20.Dahl SR, Bakke LH, Thorsby PM, et al. An LC-MS/MS assay for simultaneous determination of 13 steroid hormones and two synthetic steroids in saliva: potential utility for paediatric population and beyond. Scand J Clin Lab Invest 2024. 84 527–534. ( 10.1080/00365513.2024.2437620) [DOI] [PubMed] [Google Scholar]
- 21.Büttler RM, Bagci E, Brand HS, et al. Testosterone, androstenedione, cortisol and cortisone levels in human unstimulated, stimulated and parotid saliva. Steroids 2018. 138 26–34. ( 10.1016/j.steroids.2018.05.013) [DOI] [PubMed] [Google Scholar]
- 22.Kivlighan KT, Granger DA, Schwartz EB, et al. Quantifying blood leakage into the oral mucosa and its effects on the measurement of cortisol, dehydroepiandrosterone, and testosterone in saliva. Horm Behav 2004. 46 39–46. ( 10.1016/j.yhbeh.2004.01.006) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a