Abstract
Background
Insomnia is the most prevalent sleep disorder globally. Nuciferine (NF), a bioactive constituent extracted from Nelumbo nucifera leaves, is recognized for its diverse pharmacological activities. However, its sleep-regulating effects have not been investigated. This study aimed to delineate the therapeutic effects and underlying mechanisms of NF in mitigating insomnia.
Methods
The sedative-hypnotic effects of NF were assessed employing locomotor activity test, pentobarbital-induced sleep test, and electroencephalography-based sleep profiling. Insomnia symptoms in rodents were induced by serotonin (5-HT) depletion and environmental stress. The potential mechanisms of NF’s action through the regulation of central serotonin system were also explored.
Results
Nuciferine attenuated locomotor activity and extended pentobarbital-induced sleep duration in a dose-dependent manner. It also significantly augmented total and non–rapid eye movement (NREM) sleep time and enhanced delta power at frequencies of 0.5 and 1 Hz in normal rats. Sleep analysis revealed that NF effectively reversed the reduction in total and NREM sleep time caused by environmental stress from cage changing. NF treatment also proved effective against insomnia induced by 5-HT depletion, as evidenced by increased sleep duration and reduced sleep latency. Further investigation revealed a synergetic effect of NF and 5-hydroxytryptophan, alone with increased 5-HT and 5-HT1A receptor levels in the hypothalamus of insomniac mice following NF administration.
Conclusions
The results demonstrate NF’s hypnotic effects and its ability to alleviate insomnia, providing preclinical evidence for its potential as a naturally derived treatment for insomnia.
Keywords: nuciferine, insomnia, sedative-hypnotic, EEG
Significance statement.
Insomnia affects up to 30% of the global population and is associated with an increased risk of mood disorders, cardiovascular diseases, and obesity. However, the side effects and potential for dependency associated with hypnotic drugs limit their application in treating insomnia. Nuciferine (NF), a bioactive compound from Nelumbo nucifera leaves, is known for its pharmacological properties, but its sleep-regulating effects remain unexplored. This study assessed NF’s sedative-hypnotic effects using rodent behavior and electroencephalography (EEG)-based sleep analysis. The results indicate that NF effectively enhanced sleep in rodents suffering from insomnia. Specifically, NF enhanced non–rapid eye movement (NREM) sleep and increased the EEG delta power spectrum. Our research also found that the sleep-enhancing effects of NF were associated with the modulation of 5-HT and 5-HT1A receptor in the hypothalamus. This study may provide preliminary evidence supporting the potential of NF as a novel treatment for insomnia.
INTRODUCTION
Insomnia is the most prevalent sleep disorder, affecting an estimated 10%-30% of the global population.1 Chronic insomnia disrupts daily life and is associated with a range of comorbidities, including anxiety and depression. Additionally, it is associated with an elevated risk of neurodegenerative diseases such as Parkinson’s, Alzheimer’s, and epilepsy.2
Lotus (Nelumbo nucifera) leaves are extensively used both as a dietary component and a medicinal resource. In Traditional Chinese Medicine, N. nucifera leaves are valued for their antipyretic and anti-inflammatory properties, addressing conditions such as heatstroke, diarrhea, and fever. Ancient literature also documents their use in “calming emotions” and modern research suggests that these effects may be linked to their rich alkaloids content.3
Nuciferine (NF), an aromatic ring-containing alkaloid, is a major component of the alkaloid fraction extracted from N. nucifera leaves. NF is reported to exhibit a diverse range of pharmacological activities, encompassing anticancer, anti-inflammatory, antioxidant, and vasorelaxant effects.4,5 It has been shown to induce specific behavioral effects in rats, such as catalepsy, enhancement of morphine-induced analgesia, and anticonvulsant activity.5 Despite these findings, the role of NF in the treatment of insomnia remains unexplored.
Rodent models of insomnia, developed based on various pathophysiological mechanisms, are crucial for the evaluation of pharmacological treatments. The “first-night effect” a well-documented phenomenon, where unfamiliar sleep environments disrupt sleep duration and quality, is commonly observed in humans, such as long-distance travelers.6 The immediate onset of insomnia symptoms in rats can be triggered by a change in their rearing environment, specifically through the alteration of housing cages. This method is referred to as cage change-induced insomnia.7 Additionally, p-chlorophenylalanine (PCPA), which inhibits tryptophan hydroxylase (a key enzyme in serotonin biosynthesis), reduces serotonin levels, disrupts the sleep/wake cycle, and induces insomnia.8 The PCPA-induced insomnia model is widely recognized for establishing animal models in pathophysiological research and pharmacological evaluation.4,9 Given the complexity of insomnia features, employing a multiple-model approach is crucial for a comprehensively assessment of drug efficacy and understanding underlying mechanisms.
Electroencephalography (EEG) and electromyography (EMG) are pivotal tools in sleep studies and are recognized as the gold standard for sleep assessment. Advancements in wireless physiological signal telemetry technology have optimized the method for collecting EEG and EMG signals. This method minimizes the restrictions on the subjects, thus facilitating the preservation of authentic sleep patterns and supports the long-term, stable monitoring of signals.10,11
In the current investigation, we sequentially evaluated the sedative and hypnotic effects of NF using established behavioral paradigms, including the locomotor activity test and the pentobarbital-induced sleep test (PIST). Subsequently, wireless physiological EEG telemetry was employed to investigate the effects of NF on sleep duration, sleep architecture, and power density spectrum in both normal rats and those subjected to cage change-induced insomnia. Additionally, we utilized the PCPA-induced insomnia model to further elucidate the therapeutic effects and underlying mechanisms of NF in treating insomnia. In summary, our study investigates the sedative and hypnotic effects of NF using various rodent models and EEG-based sleep analysis, demonstrating its potential as a therapeutic agent for insomnia and highlighting its distinctive impact on sleep architecture compared with traditional treatments.
METHODS
Animals
Adult male ICR mice (22-25 g) and Sprague-Dawley rats (220-240 g) were purchased from SPF Biotechnology (Beijing, China). Animals were housed in a suitable environment maintained at an ambient temperature of 24 ± 1 °C, 55 ± 5% humidity, and a 12 h/12 h light-dark cycle (light period, 08:00-20:00). Food and water were provided ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee (ethics approval number: IACUC-DWZX-2022-781) and performed following the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering.
Reagents
NF (S3821) and 5-hydroxytryptophan (5-HTP) (S2374) were purchased from Selleck Chemicals (Houston, TX, USA). Pentobarbital (No.11715) and PCPA (C6506) were purchased from Sigma-Aldrich. Diazepam (DZ) (U1023403, Sinopharm Chemical Reagent Co., Ltd.), antibodies for 5-HT1A (ab85615, Abcam), and GAPDH (D16H11, Cell Signaling Technology) were purchased from respective manufacturers, as were the 5-HT enzymatic assay kit (H104-1-1, Nanjing Jiancheng Bioengineering Institute), polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA), radioimmunoprecipitation assay (RIPA) lysis buffer (Servicebio Biotechnology, Wuhan, China), RNA pure Tissue&Cell Kit (DNase I) (CW0560S, CWBIO), PrimeScript RT Master Mix R (RR036A, TaKaRa), MagicSYBR Mixture (CW3008M, CWBIO), 5-HT1A, and GAPDH primers (Sangon Biotech).
Locomotor Activity Test
The effect of NF on locomotor activity in mice was assessed in a 50 × 50 × 50 cm test chamber using the DigBehv video tracking system (Jiliang Software Technology Co., Ltd., Shanghai, China). Sixty mice were divided into 6 groups: Control (20% DMSO, 10 mL/kg), DZ (3 mg/kg, 10 mL/kg), and NF (3, 10, 20, and 30 mg/kg, 10 mL/kg). Vehicle, DZ, or NF were administered intraperitoneally 20 min prior to testing. Movement recording and analysis was conducted according to literature.9
Pentobarbital-Induced Sleep Test
The hypnotic effects of NF were assessed according to published methods.12 The threshold and subthreshold doses of pentobarbital sodium were determined based on the loss of the righting reflex in preliminary trials. Mice were randomly assigned to 6 groups (n = 10): control (20% DMSO, 10 mL/kg, i.p.), DZ (3 mg/kg, 10 mL/kg, i.p.), and NF groups (3, 10, 20, and 30 mg/kg, 10 mL/kg, i.p.), and 20 min after treatment, pentobarbital was administered at either a subthreshold or threshold dose (30 and 48 mg/kg). Animals that fell asleep within 15 min of pentobarbital administration were included in the analysis.13 Sleep latency was defined as the interval from pentobarbital injection to the loss of the righting reflex, and sleep duration as the interval from the loss to the recovery of the righting reflex. In the subthreshold test, drug administration and dosages adhered to the same protocol, and the number of sleeping animals within 15 min was counted. All experiments were conducted in a quiet environment.
Transmitter Implantation
Rats were anesthetized using isoflurane. The electrode placement coordinates were adjusted according to the rat stereotaxic atlas. Epidural EEG electrodes were placed above the medial prefrontal cortex (3 mm anterior to the bregma, close to the midline) and parietal cortex overlaying both the left and right hippocampi (2.8 mm posterior to the bregma, 3.3 mm lateral to the midline). Wireless radiotelemetry transmitters (HD-S02, DSI, United States) were placed in a subcutaneous pocket on the back of the rats, and the electrodes were fixed using dental cement. EMG electrodes were inserted into the cervical trapezius. All animals were allowed to recover for 7 days before experiments.
Physiological Signal Recording and Analysis
EEG, EMG, body temperature, and activity signals from individually housed rats were collected using PRC-1 receiver pads (DSI) and connected to a data exchange matrix (MX2, DSI). EEG (0.5-30 Hz) and EMG (16-128 Hz) signals were filtered, digitized at 128 Hz using Ponemah software (v6.5.1, DSI), and imported into NeuroScore software (v3.3.1, DSI) for vigilance staging. Vigilance states (active wake, wake, NREM sleep, and REM sleep) were scored in 10 s epochs using a standard algorithm. The duration and number of bouts for each stage were calculated. Power density spectrum analysis was conducted following established methods using MATLAB R2019a software.14 EEG spectral power was classified into the following frequency bands: delta (0.5-4 Hz), theta (4-8 Hz), alpha (8-12 Hz), sigma (12-16 Hz), low beta (16-20 Hz), and high beta (20-30 Hz).15
Sleep Analysis in Normal Rats
After recovery from implantation surgery, the rats were housed individually. Subsequently, 3 crossover and repeated measurements were conducted, each separated by a 1-week washout period.16 Rats (n = 6) received intraperitoneal injections of either vehicle, DZ (3 mg/kg), or NF (30 mg/kg, 10 mL/kg) at 08:30. Recording session was initiated at 09:00.
Cage Change-Induced Insomnia Model
The cage change-induced insomnia model is an established rodent model of acute environmental stress intended to induce mild stress and disrupt sleep-wake states in rats.17 In this experiment, insomnia was induced by transferring the rats into unfamiliar housing cages, previously occupied by other male rats for 5-7 days, 3 times over a 6 h period. The effects of NF on insomnia were assessed using wireless EEG telemetry. Rats were divided into 4 groups (n = 5 or 6): control (20% DMSO, 10 mL/kg, i.p.), cage change (CA, 20% DMSO, 10 mL/kg, i.p.), DZ (3 mg/kg, 10 mL/kg, i.p.), and NF groups (30 mg/kg, 10 mL/kg, i.p.). Following a 7-day recovery period postimplant surgery, baseline data were collected. On the eighth day at 08:30, rats were injected with DZ, NF, or vehicle. At 09:00, they were transferred to unfamiliar cages, and simultaneous EEG recording began. Subsequent cage changes occurred at 11:00 and 13:00. Data collection concluded at 15:00. Vigilance states, including REM sleep, NREM sleep, Wake, and Active Wake, were analyzed as described in section 2.6.
PCPA-Induced Insomnia Model
Parachlorophenylalanine (PCPA), a tryptophan hydroxylase inhibitor, can induce insomnia in rodents when administered intraperitoneally by blocking 5-HT synthesis.18 In this study, mice were randomly assigned to 6 groups (n = 10): control (20% DMSO, 10 mL/kg, i.p.), PCPA (20% DMSO, 10 mL/kg, i.p.), and NF groups (10, 20, and 30 mg/kg, 10 mL/kg, i.p.). Except for the control group, all mice received PCPA (400 mg/kg, 10 mL/kg, i.p.) for 3 consecutive days, and 24 h post–treatment, locomotor activity, and PIST were conducted.
Synergetic Effect of 5-HTP and NF in Pentobarbital-Induced Sleep Test
To investigate the relationship between NF’s sedative-hypnotic effects and the serotonin system, the impact of combined NF and 5-HTP administration on sleep was examined.19 Groups (n = 10) including the control (20% DMSO, 10 mL/kg, i.p.), 5-HTP (2.5 mg/kg, 10 mL/kg, i.p.), and NF groups (3, 10, and 20 mg/kg, 10 mL/kg, i.p.) were administered vehicle or NF. This was done 20 mins after 5-HTP administration, followed by a 15 mins interval before pentobarbital injection. The PIST was conducted in accordance with the method described in section 2.4.
Sample Collection and 5-HT Level Analysis
The level of 5-HT in the hypothalamus of mice in the PCPA-induced insomnia experiment was detected by enzyme-linked immunosorbent assay (ELISA), as previously reported but with slight modification.8 Mice in each group were sacrificed via cervical dislocation, the skull was removed, and the brain tissue was exposed and completely removed on ice. The brain tissue was then thoroughly homogenized and centrifuged at 3000 rpm for 20 mins, and the supernatant was collected. The 5-HT content was measured according to the manufacturer’s instruction, with absorbance read at 450 nm using a microplate reader.
5-HT1A Receptor Analysis via Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction
The expression levels of 5-HT1A mRNA in the hypothalamus of mice involved in the PCPA insomnia experiment were measured via real-time quantitative reverse transcription-polymerase chain reaction (RT-qPCR).20,21 Primers are designed via the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/) and PrimerBank (https://pga.mgh.harvard.edu/primerbank/) databases. 5-HT1A receptor primer sense sequences: GACAGGCGGCAACGATACT, antisense sequences: CCAAGGAGCCGATGAGATAGTT, GADPH primer sense sequences: AGGTCGGTGTGAACGGATTTG, and antisense sequences: TGTAGACCATGTAGTTGAGGTCA.
5-HT1A Receptor Analysis via Western Blotting
The expression levels of 5-HT1A in the hypothalamus of mice from the PCPA insomnia experiment were measured using western blotting.20 Hypothalamic tissue proteins were extracted using RIPA lysis buffer with protease inhibitors. Protein samples were loaded onto 10% sodium dodecyl sulfate-polyacrylamide gels to separate the target proteins and transferred to PVDF membranes. Post–incubation of primary and secondary antibodies, protein bands on membranes were visualized using an electrochemiluminescence system. The gray values of the protein bands were quantified using the ImageJ software and normalized to the internal reference protein (GAPDH).
Statistical Analysis
Statistical data are expressed as mean ± standard error of the mean (SEM). Statistical analyses were performed using GraphPad Prism 9 software (RRID: SCR_002798 (http://www.graphpad.com/), San Diego, CA, USA). Statistical significance was determined using one-way or two-way ANOVA. P-values <.05 were considered statistically significant.
RESULTS
Sedative Effect of NF in Normal Mice
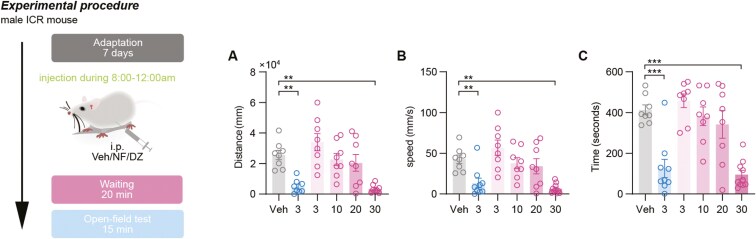
The sedative activity of NF (3-30 mg) was examined by measuring spontaneous activity in normal mice after its administration compared with the classic sedative drug diazepam (DZ). As shown in Figure 1A-C, the total distance (P <.01), average speed (P <.01), and duration of activity (P <.001) were significantly reduced compared with the control group after DZ (3 mg/kg) and NF (30 mg/kg) administration. The effects of NF exhibited a dose-dependent enhancement (3, 10, 20, and 30 mg/kg), with a significant difference observed at 30 mg/kg. NF at 30 mg/kg exerted a central inhibitory effect on normal mice, comparable to that of DZ at 3 mg/kg.
Figure 1.
The effects of NF on normal mice in locomotor activity test (A-C). (A) Total distance of activity, (B) average speed of spontaneous activity, and (C) time of spontaneous activity. Data are presented as the mean ± SEM (n = 8). Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparisons test (A, B, and C). **P <.01, ***P <.001, and ns means no significance. The gray represents the control group, the blue represents diazepam group (3 mg/kg), and the red represents NF group (3, 10, 20, and 30 mg/kg).
Hypnotic Effect of NF in Normal Mice
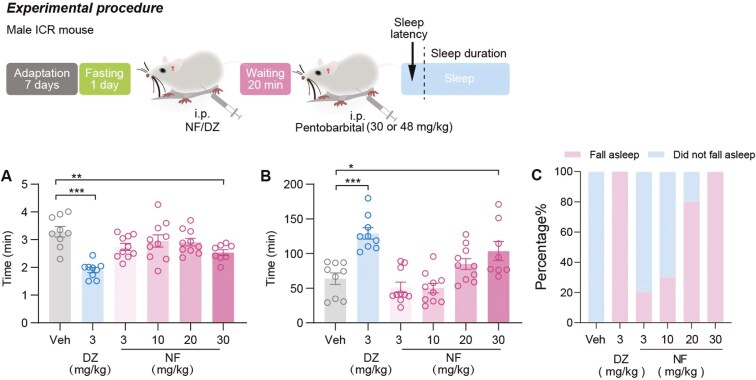
The hypnotic effects of NF were further examined using the PIST. As shown in Figure 2A-B, the dose of 30 mg/kg NF significantly reduced sleep latency (P <.01) and extended sleep duration compared with the control group (P <.05). To eliminate the potential drug interactions between NF and pentobarbital that might affect pentobarbital-induced sleep, a subthreshold dose of PIST was conducted. At the dose of 30 mg/kg, mice administered with NF achieved a 100% sleep onset rate (Figure 2C). In Figure 2B-C, the increase in sleep duration and sleep rate also demonstrated a positive relationship with the dose of NF within the range of 3-30 mg/kg.
Figure 2.
The hypnotic effect of NF on normal mice in a pentobarbital-induced sleep test. (A) Sleep latency, (B) sleep duration, and (C) fall asleep rate. Data are presented as the mean ± SEM (n = 8-10). Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparisons test. *P <.05, **P <.01, ***P <.001, and ns means no significance. The gray represents the control group, the blue represents diazepam group (3 mg/kg), and the red represents NF group (3, 10, 20, and 30 mg/kg).
Sleep-Regulatory Effects of NF in Free-Moving Rats
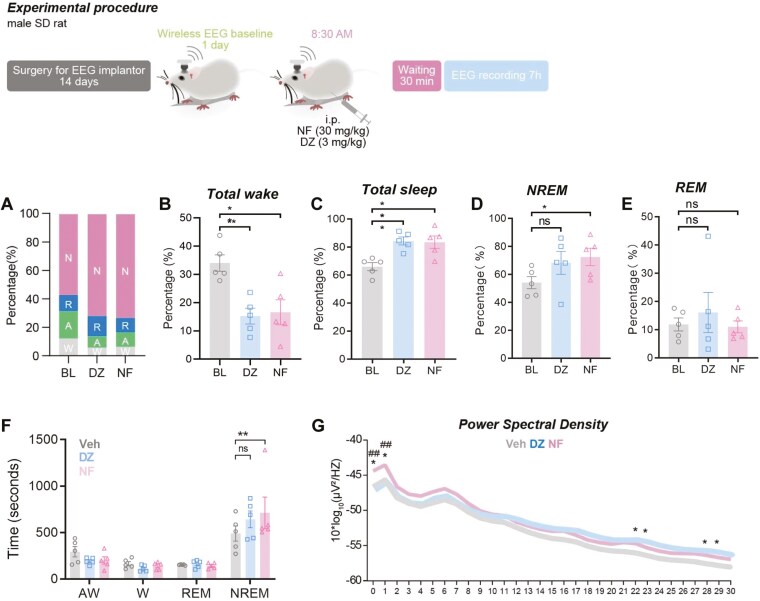
To further examine the effects of NF on sleep architecture, wireless EEG and EMG recordings and subsequent analyses were performed. It was observed that after NF and DZ administration, the total wake time decreased significantly (P <.01 and P <.01, respectively; Figure 3B) and the duration of total sleep time increased significantly compared with baseline (P <.01 and P <.01, respectively; Figure 3C). NF increased the cumulative time (P <.05, Figure 3D) and mean bouts duration of NREM sleep compare with baseline (P <.01, Figure 3F). In contrast, DZ exhibited no significant effect on the regulation of NREM sleep.
Figure 3.
The effect of NF on sleep architecture in free-moving rats. The time proportion of NREM, REM, Active wake, and Wake over the recording period (A). Percentage of total wake (wake and active wake) (B), total sleep (C), NREM sleep (D), and REM sleep (E) of each group. Mean bouts duration of NREM, REM, Active wake, and Wake (F). The effect of NF on power density spectral in sleep. EEG power density curves and quantitative changes in power of 0.5-30 Hz frequency bands (G). The gray represents the baseline, the blue represents diazepam group (3 mg/kg), and the red represents NF group in the G (30 mg/kg). Data are presented as the mean ± SEM (n = 6). Statistical analysis was performed using repeated one-way or two-way ANOVA followed by Tukey’s multiple comparisons test. *P <.05, **P <.01 vs baseline, ##P <.01 vs diazepam.
Typical examples of the compressed spectral array, EEG, and EMG polygraphic recordings were obtained from rats administered with vehicle, DZ, or NF. The EEG power density spectrum in the 0.5-30 Hz frequency range was plotted over a 7 h recording period. As shown in Figure 3G, NF increased the power density in the range of 0.5-4 Hz (delta power), with significant differences observed at 0.5 Hz and 1 Hz compared with baseline and DZ (P <.05 and P <.01, respectively). DZ mainly increased power density in the higher frequency range, with significant differences observed at 22, 23, 28, and 29 Hz compared with baseline (P <.05). In summary, the enhancement of delta power density in the sleep of NF-administered mice suggests an increased depth of NREM sleep, whereas DZ did not exhibit such an effect.
Sleep-Improving Effects of NF in Cage Change-Induced Insomnia
The therapeutic effects of NF were further evaluated using cage-change induced insomnia model. Based on the results of sleep analysis, we found that the CA group showed a significant increase in total wake compared with baseline (P <.05, Figure 4B), while total sleep (P <.01, Figure 4C) and NREM sleep (P <.05, Figure 4D) time were significantly decreased. After the administration of NF and DZ, both compounds were found to decrease total wake time (Figure 4B) and to prolong total sleep (P <.05, Figure 4C) and NREM sleep time (P <.01 and P <.05, respectively, Figure 4D).
Figure 4.
The sleep improving effects of NF in rats with cage change-induce insomnia. The time proportion of NREM, REM, Active wake, and Wake over the recording period (A). Percentage of total wake (wake and active wake) (B), total sleep (C), NREM sleep(D), and REM sleep (E) of each group were analyzed. Hourly distribution of total sleep, REM sleep, and NREM sleep time (F-H). The light gray represents the baseline, the dark gray represents the cage change group, the blue represents diazepam group (3 mg/kg), and the red represents NF group in the F (30 mg/kg). Data are presented as the mean ± SEM (n = 5 or 6). Statistical analysis was performed using repeated one-way ANOVA followed by Tukey’s multiple comparisons test (B-D), and two-way ANOVA followed by Dunnett’s multiple comparisons test (F-H). *P <.05, **P <.01 vs the baseline, #P <.05, ##P <.01 vs the CA group, and ns means no significance.
To understand the dynamic effects of NF on sleep, we analyzed the hourly distribution of sleep time for each group throughout the entire recording period. Overall, the CA group showed a decrease in total sleep and NREM sleep duration throughout the period, with a significant difference observed at 11:00, as shown in Figure 4F. The enhancement in total sleep time attributed to NF administration was significant at 09:00 and 11:00, coinciding with the timing of the first 2 cage changes (P <.05 and P <.05 respectively, Figure 4F). Regarding NREM sleep, both NF and DZ exhibited significant enhancement at specific time points: NF at 09:00 and 12:00 (P <.05 and P <.05 respectively, Figure 4H) and DZ at 09:00 (P <.01, Figure 4H). NF effectively improved the insomnia symptoms induced by cage change, as evidenced by increased total sleep and NREM sleep time.
Therapeutic Effects of NF on PCPA-Induced Insomnia
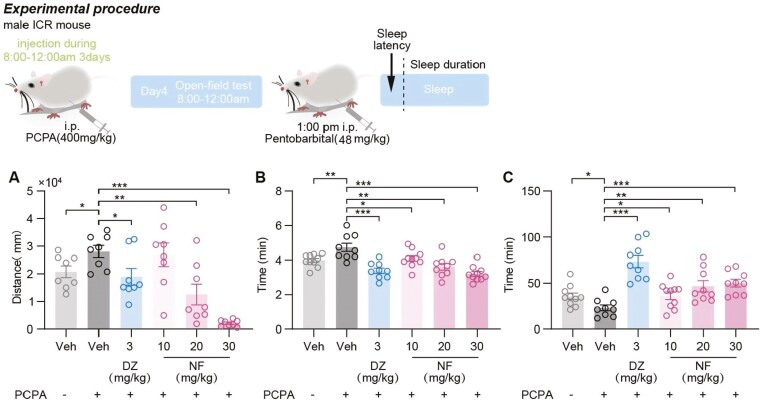
Administration of PCPA led to behavioral and physiological changes in mice, including weight loss, decreased food intake, and increased startle response. The spontaneous activity test, conducted 24 h postfinal PCPA administration as depicted in Figure 5A, showed a significant increase in distance traveled by model mice compared with controls (P <.05). Administration of DZ (3 mg/kg) and NF (20 and 30 mg/kg) significantly reduced the spontaneous activity in insomniac mice (P <.05, .01, and <.001, respectively), with NF (30 mg/kg) exhibiting a stronger effect than DZ (3 mg/kg).
Figure 5.
The sedative-hypnotic effects of NF in PCPA-induced insomnia mice. (A) Distance of spontaneous activity were measured. (B) Sleep latency and (C) sleep duration were measured in the PIST. Data are presented as the mean ± SEM (n = 8-10). Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparisons test. *P <.05, **P <.01, and ***P <.001.
The therapeutic effects of NF on PCPA-induced insomnia in animals were further assessed using PIST. The model mice exhibited significantly prolonged sleep latency and shortened sleep duration compared with the control group (P <.01 and <.05, respectively, Figure 5B-C), confirming the successful establishment of the insomnia model. DZ (3 mg/kg) and NF (10, 20, and 30 mg/kg) significantly reduced sleep latency in insomniac mice compared with the model group (P <.001, .05, .01, and .001, respectively) and increased sleep duration (P <.001, .05, .01, and .001, respectively).
NF’s Modulatory Effect on the Serotonin System in PCPA-Induced Insomniac Mice
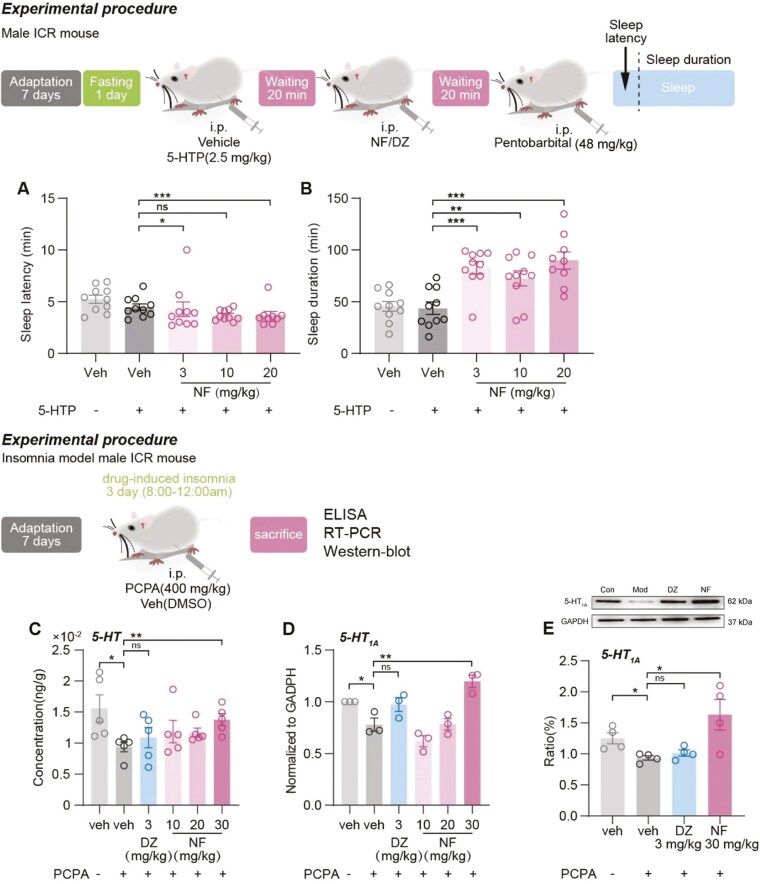
As illustrated in Figure 6A-B, 5-HTP (2.5 mg/kg) significantly enhanced the hypnotic effect of NF. Co-administration with 5-HTP led to significant reductions in sleep latency at NF doses of 3 and 20 mg/kg (P <.05 and <.001, respectively) and extended sleep duration compared with the control group (P <.001, .01 and .001, respectively).
Figure 6.
The synergic effect of 5-HTP and NF in pentobarbital-induced sleep test. (A) Sleep latency and (B) sleep duration. Data are presented as the mean ± SEM (n = 8-10). Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparisons test. *P <.05, **P <.01, ***P <.001, and ns means no significance. NF’s modulation of the 5-HT System. (C) The concentration of 5-HT in the hypothalamus was detected by ELISA. (E) The level of 5-HT1A receptor mRNA in the hypothalamus was tested by RT-qPCR. (D and E) The expression level of 5-HT1A receptor in the hypothalamus was detected by western blotting. Data are presented as the mean ± SEM (n = 3-6). Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparisons test. *P <.05, **P <.01, and ns means no significance.
To investigate the effect of NF on the serotonergic system, hypothalamic 5-HT levels were measured using ELISA. As shown in Figure 6C, 5-HT levels significantly decreased (P <.05) in PCPA-induced insomnia mice, confirming successful model establishment. NF increased hypothalamic 5-HT concentrations in a dose-dependent manner, with a 30- mg/kg dose reversing the reduced 5-HT levels (P <.01), whereas diazepam did not produce this effect.
The 5-HT1A mRNA expression level was detected by RT-qPCR. The results showed the 5-HT1A mRNA level in the hypothalamus decreased significantly (P <.05, Figure 6D) in model group. Following administration of NF, 5-HT1A receptor mRNA level showed an upward trend, with a significant increase observed at a 30 mg/kg dose (P <.01), whereas DZ had no effect. Similar results were observed with 5-HT1A protein expression. In Figure 6E, 30 mg/kg NF also effectively elevated 5-HT1A receptor expression compared with mice treated with PCPA alone.
DISCUSSION
Although literature has documented the sedative and hypnotic effects of the total alkaloid fraction extract from N. nucifera leaves.22 The effects of the monomer compound NF on sleep are still unclear. Research on the impact of NF on the central nervous system is limited. Ye et al. reported that NF exhibits psychoactive effects at doses ranging from 10 to 50 mg/kg.23 Additionally, Chen and colleagues found that doses of 10, 20, and 40 mg/kg of NF improved outcomes in acute ischemic stroke.24 In this study, we aimed to investigate the sedative and hypnotic effects of NF and to elucidate its possible mechanisms at doses ranging from 3 to 30 mg/kg. To address this research gap and explore the potential of NF in sleep regulation, a suite of behavioral assays was designed and executed.
In this study, the locomotor activity test was employed to assess changes in spontaneous activity levels of rodents.25 A reduction in spontaneous activity among mice typically indicates the sedative effects of a substance, suggesting decreased excitability within the central nervous system.9 The observed suppression of spontaneous activity in the current study indicates that NF exerts a dose-dependent sedative effect on the central nervous system. Notably, a dosage of 30 mg/kg demonstrated sedative efficacy equivalent to that of DZ (3 mg/kg).
In pharmacological research, evaluating hypnotic effects often involves assessing interactions with barbiturates, focusing on metrics such as sleep latency and total sleep duration in rodents.9,25 Subthreshold doses of pentobarbital are frequently used, with sleep onset rate as an important indicator. Pentobarbital’s hepatic metabolism is primarily mediated by cytochrome P450 enzymes, and drugs that inhibit these enzymes may enhance its hypnotic effects by slowing its metabolism. Literature indicates that substances without intrinsic hypnotic activity, such as chloramine, the alkaloid fraction of Helietta apiculata, and SKF525-A, can prolong pentobarbital-induced sleep duration by inhibiting cytochrome P450.26-28 NF is also an inhibitor of cytochrome P450 enzymes.29,30 To exclude the potential influence of its inhibitory effects on the synergistic hypnotic interaction with pentobarbital, we employed the subthreshold dose PIST. A dose of 30 mg/kg pentobarbital was administered, which is insufficient to induce falling asleep in mice when used alone. This approach was designed to minimize the confounding effects of enzyme inhibitors and isolate the sedative properties of NF. The results demonstrated that NF still exhibited sedative-hypnotic effects after ruling out its inhibitory action on cytochrome P450 enzymes. Therefore, the inhibitory effect of nuciferine on cytochrome P450 enzymes does not significantly contribute to its hypnotic synergy with pentobarbital.
Our results demonstrated that NF enhances pentobarbital’s hypnotic action, as evidenced by reduced sleep latency and prolonged sleep duration at a dose of 30 mg/kg. Neither 2.5 mg/kg of 5-HTP nor NF at 3 or 10 mg/kg individually exhibited significant hypnotic effects. However, the combination of 2.5 mg/kg 5-HTP and 3 mg/kg NF produced significant hypnosis in mice, whereas NF alone required a minimum effective dose of 30 mg/kg to show significant effects. These findings suggest that NF’s hypnotic effects are likely mediated through the serotonergic pathway.
Based on the results of classical pharmacological assessments, we further investigated the hypnotic effects of NF using EEG-based sleep analysis. This approach allowed us to observe sleep patterns without interference from additional agents. The results showed that both NF and DZ significantly affected sleep parameters in freely moving rats within 7 h of administration, increasing total sleep duration and reducing active wake time. Notably, NF increased NREM sleep duration by prolonging the duration of NREM bouts and significantly increasing the number of NREM bouts within the 960-1920 s range, suggesting enhanced consolidation of NREM sleep. The depth of NREM sleep was also improved by NF treatment, as evidenced by an increase in the delta power in the power density spectrum analysis. These effects were not observed with DZ, a typical benzodiazepine sedative-hypnotic drug. DZ’s pharmacological effects are primarily mediated by enhancing the binding affinity of the GABA transmitter to the GABAA receptor.31 Although DZ showed a stronger synergistic effect with pentobarbital sodium compared with NF in PIST studies, it did not significantly affect the duration or average bout length of NREM sleep, nor delta power density. Clinical studies have similarly reported that DZ reduces deep sleep (N3 stage of NREM) and REM sleep without improving NREM consolidation.32 In the treatment of cage change-induced insomnia, NF significantly increased both total sleep time and NREM sleep duration, highlighting its distinctive efficacy in improving NREM sleep and overall sleep quality.
To further investigate NF’s effects on alleviating insomnia, we established a 5-HT-depleted insomnia model in mice using PCPA. NF effectively reversed insomnia symptoms in these model mice, including prolonged sleep latency and shortened sleep duration in the PIST. These therapeutic effects prompted us to examine NF’s regulation of neurotransmitters in the central nervous system. The improvement of specific insomnia symptoms induced by 5-HT depletion suggests that NF may act on the 5-HT system. Indeed, literature indicates that NF’s pharmacological actions are closely related to the serotonin system and involve direct interactions with a series of serotonin receptors.33 Nuciferine exerts diverse pharmacological effects through interactions with multiple 5-HT receptor subtypes, including: antagonism at 5-HT2A, 5-HT2C, and 5-HT2B receptors, inverse agonism at the 5-HT7 receptor, partial agonism at the 5-HT6 receptor, and agonism at the 5-HT1A receptor.3 Farrell et al. also identified 5-HT1A as a potential target of NF and validated the interaction between NF and the serotonin system through various behavioral paradigms.34
We explored the potential targets of NF by utilizing a subthreshold dose of PIST (also to exclude the cytochrome P450 inhibition effect of NF), as well as employing 5-HT receptor inhibitors. The results indicate that compared with antagonists of 5-HT2, 5-HT6, and 5-HT7 receptors, the 5-HT1A receptor antagonist WAY100635 effectively inhibits the synergistic induction of sleep onset in mice by NF and pentobarbital (refer to Supplementary Materials). Based on these results, we further assessed hypothalamic 5-HT content and the transcription and translation levels of its 1A-type receptors, founding that NF significantly elevated these markers. These findings suggest that NF’s sleep-regulating effects may involve the serotonin system and 5-HT1A receptors.
In conclusion, our investigation substantiated the sedative-hypnotic and sleep-enhancing properties of NF and highlighted its advantages over diazepam, particularly in regulating NREM sleep and delta power density. Our research into the mechanism by which NF ameliorates PCPA-induced insomnia revealed the involvement of the serotonergic system. These findings enhance the understanding of the psychoactive properties of alkaloid extracts from N. nucifera leaves, positioning NF as a promising candidate for therapeutic intervention in insomnia. Nonetheless, further comprehensive studies are warranted to delineate the safety and efficacy profiles of NF, ensuring its suitability for clinical applications. Future research should focus on elucidating the precise pharmacological functions and underlying mechanisms through which NF exerts its ameliorative effects on insomnia.
Supplementary Material
Acknowledgments
None.
Contributor Information
Luo-Xuan Wang, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
Yu-Meng Liu, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
Yong-Fang Gu, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
Lu Li, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
Ren-Hong Qiu, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
Yan-Kai Wang, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
Jin Yang, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
Ji Wang, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
Yang Zhang, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
Shuo Li, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
Qiong-Yin Fan, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
Rui Xue, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
Jing-Cao Li, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
You-Zhi Zhang, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China.
Author contributions
Luo-Xuan Wang (Formal analysis, Methodology, Visualization, Writing—original draft, Writing—review & editing), Yu-Meng Liu (Data curation, Investigation, Software, Writing—original draft, Writing—review & editing), Yong-Fang Gu (Data curation), Lu Li (Data curation), Ren-Hong Qiu (Data curation), Yan-Kai Wang (Data curation), Jin Yang (Data curation), Ji Wang (Data curation), Yang Zhang (Writing—review & editing), Shuo Li (Writing—review & editing), Qiong-Yin Fan (Writing—review & editing), Rui Xue (Writing—review & editing), Jing-Cao Li (Conceptualization, Validation, Writing—review & editing), and youzhi zhang (Funding acquisition, Project administration, Supervision, Writing—review & editing)
Funding
None.
Conflict of interest
The authors declare no conflicts of interest.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Morin CM, Jarrin DC.. Epidemiology of insomnia: prevalence, course, risk factors, and public health burden. Sleep Med Clin. 2022;17:173–191. https://doi.org/ 10.1016/j.jsmc.2022.03.003 [DOI] [PubMed] [Google Scholar]
- 2. Bollu PC, Kaur H.. Sleep medicine: insomnia and sleep. Mo Med. 2019;116:68–75. [PMC free article] [PubMed] [Google Scholar]
- 3. Farrell MS, McCorvy JD, Huang XP, et al. In vitro and in vivo characterization of the Alkaloid Nuciferine. Zhou H, ed. PLoS One. 2016;11:e0150602. https://doi.org/ 10.1371/journal.pone.0150602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li D, Liu B, Fan Y, et al. Nuciferine protects against folic acid-induced acute kidney injury by inhibiting ferroptosis. Br J Pharmacol. 2021;178:1182–1199. https://doi.org/ 10.1111/bph.15364 [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Yao W, Li B, et al. Nuciferine modulates the gut microbiota and prevents obesity in high-fat diet-fed rats. Exp Mol Med. 2020;52:1959–1975. https://doi.org/ 10.1038/s12276-020-00534-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agnew HW, Webb WB, Williams RL.. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263–266. https://doi.org/ 10.1111/j.1469-8986.1966.tb02650.x [DOI] [PubMed] [Google Scholar]
- 7. Michaud JC, Muyard JP, Capdevielle G, et al. Mild insomnia induced by environmental perturbations in the rat: a study of this new model and of its possible applications in pharmacological research. Arch Int Pharmacodyn Ther. 1982;259:93–105. [PubMed] [Google Scholar]
- 8. Dong YJ, Jiang NH, Zhan LH, et al. Soporific effect of modified Suanzaoren Decoction on mice models of insomnia by regulating Orexin-A and HPA axis homeostasis. Biomed Pharmacother. 2021;143:112141. https://doi.org/ 10.1016/j.biopha.2021.112141 [DOI] [PubMed] [Google Scholar]
- 9. Zhong Y, Zheng Q, Hu P, et al. Sedative and hypnotic effects of Perilla frutescens essential oil through GABAergic system pathway. J Ethnopharmacol. 2021;279:113627. https://doi.org/ 10.1016/j.jep.2020.113627 [DOI] [PubMed] [Google Scholar]
- 10. Hubbard J, Gent TC, Hoekstra MMB, et al. Rapid fast-delta decay following prolonged wakefulness marks a phase of wake-inertia in NREM sleep. Nat Commun. 2020;11:3130. https://doi.org/ 10.1038/s41467-020-16915-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim JJ, Gharpure A, Teng J, et al. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature. 2020;585:303–308. https://doi.org/ 10.1038/s41586-020-2654-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song Y, Yang CJ, Wang ZB, et al. Chemical constituents of Eleutherococcus sessiliflorus extract and its sedative-hypnotic effect. Nat Prod Res. 2017;31:1995–2000. https://doi.org/ 10.1080/14786419.2016.1272106 [DOI] [PubMed] [Google Scholar]
- 13. Wang S, Wang C, Peng D, et al. Agarwood essential oil displays sedative-hypnotic effects through the GABAergic system. Molecules (Basel, Switzerland). 2017;22:2190. https://doi.org/ 10.3390/molecules22122190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buysse DJ, Germain A, Hall ML, et al. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008;31:1673–1682. https://doi.org/ 10.1093/sleep/31.12.1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu YM, Pietrone R, Cashmere JD, et al. EEG power during waking and NREM sleep in primary insomnia. J Clin Sleep Med. 2013;9:1031–1037. https://doi.org/ 10.5664/jcsm.3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cano G, Mochizuki T, Saper CB.. Neural circuitry of stress-induced insomnia in rats. J. Neurosci. 2008;28:10167–10184. https://doi.org/ 10.1523/JNEUROSCI.1809-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McKenna JT, Gamble MC, Anderson-Chernishof MB, et al. A rodent cage change insomnia model disrupts memory consolidation. J Sleep Res. 2019;28:e12792. https://doi.org/ 10.1111/jsr.12792 [DOI] [PubMed] [Google Scholar]
- 18. Hao YF, Luo T, Lu ZY, Shen CY, Jiang JG.. Targets and underlying mechanisms related to the sedative and hypnotic activities of saponins from Rhodiola rosea L. (crassulaceae). Food Funct. 2021;12:10589–10601. https://doi.org/ 10.1039/d1fo01178b [DOI] [PubMed] [Google Scholar]
- 19. Wang LE, Bai YJ, Shi XR, et al. Spinosin, a C-glycoside flavonoid from semen Zizhiphi Spinozae, potentiated pentobarbital-induced sleep via the serotonergic system. Pharmacol Biochem Behav. 2008;90:399–403. https://doi.org/ 10.1016/j.pbb.2008.03.022 [DOI] [PubMed] [Google Scholar]
- 20. Limón-Morales O, Soria-Fregozo C, Arteaga-Silva M, Vázquez-Palacios G, Bonilla-Jaime H.. Altered expression of 5-HT1A receptors in adult rats induced by neonatal treatment with clomipramine. Physiol Behav. 2014;124:37–44. https://doi.org/ 10.1016/j.physbeh.2013.10.026 [DOI] [PubMed] [Google Scholar]
- 21. Nonogaki K, Kaji T.. Liraglutide, a GLP-1 receptor agonist, which decreases hypothalamic 5-HT2A receptor expression, reduces appetite and body weight independently of serotonin synthesis in mice. J Diabetes Res. 2018;2018:6482958. https://doi.org/ 10.1155/2018/6482958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yan MZ, Chang Q, Zhong Y, et al. Lotus leaf alkaloid extract displays sedative-hypnotic and anxiolytic effects through GABAA receptor. J Agric Food Chem. 2015;63:9277–9285. https://doi.org/ 10.1021/acs.jafc.5b04141 [DOI] [PubMed] [Google Scholar]
- 23. Ye LH, He XX, You C, et al. Pharmacokinetics of Nuciferine and N-Nornuciferine, two major alkaloids from Nelumbo nucifera leaves, in rat plasma and the brain. Front Pharmacol. 2018;9:902. https://doi.org/ 10.3389/fphar.2018.00902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen C, Duan F, Xie Y, et al. Nuciferine attenuates acute ischemic stroke in a rat model: a metabolomic approach for the mechanistic study. Mol Omics. 2022;18:765–778. https://doi.org/ 10.1039/d2mo00158f [DOI] [PubMed] [Google Scholar]
- 25. Deng L, Shi AM, Wang Q.. Sedative-hypnotic and anxiolytic effects and the mechanism of action of aqueous extracts of peanut stems and leaves in mice. J Sci Food Agric. 2018;98:4885–4894. https://doi.org/ 10.1002/jsfa.9020 [DOI] [PubMed] [Google Scholar]
- 26. Goloubkova TD, Heckler E, Rates SM, Henriques JA, Henriques AT.. Inhibition of cytochrome P450-dependent monooxygenases by an alkaloid fraction from Helietta apiculata markedly potentiate the hypnotic action of pentobarbital. J Ethnopharmacol. 1998;60:141–148. https://doi.org/ 10.1016/s0378-8741(97)00139-6 [DOI] [PubMed] [Google Scholar]
- 27. Ishaq S, Rasheed MA, Ashraf M, et al. Effect of calcium hypochlorite and chloramine on blood biochemistry and sodium pentobarbital induced sleeping time in mice. Pak J Pharm Sci. 2016;29:1625–1632. [PubMed] [Google Scholar]
- 28. Salort G, Álvaro-Bartolomé M, García-Sevilla JA.. Pentobarbital and other anesthetic agents induce opposite regulations of MAP kinases p-MEK and p-ERK, and upregulate p-FADD/FADD neuroplastic index in brain during hypnotic states in mice. Neurochem Int. 2019;122:59–72. https://doi.org/ 10.1016/j.neuint.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 29. Hu L, Xu W, Zhang X, et al. In-vitro and in-vivo evaluations of cytochrome P450 1A2 interactions with nuciferine. J Pharm Pharmacol. 2010;62:658–662. https://doi.org/ 10.1211/jpp.62.05.0015 [DOI] [PubMed] [Google Scholar]
- 30. Ye LH, He XX, Kong LT, et al. Identification and characterization of potent CYP2D6 inhibitors in lotus leaves. J Ethnopharmacol. 2014;153:190–196. https://doi.org/ 10.1016/j.jep.2014.02.014 [DOI] [PubMed] [Google Scholar]
- 31. Li P, Eaton MM, Steinbach JH, Akk G.. The benzodiazepine diazepam potentiates responses of α1β2γ2L γ-aminobutyric acid type A receptors activated by either γ-aminobutyric acid or allosteric agonists. Anesthesiology. 2013;118:1417–1425. https://doi.org/ 10.1097/ALN.0b013e318289bcd3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Atkin T, Comai S, Gobbi G.. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70:197–245. https://doi.org/ 10.1124/pr.117.014381 [DOI] [PubMed] [Google Scholar]
- 33. Zhao T, Zhu Y, Zhao R, et al. Structure-activity relationship, bioactivities, molecular mechanisms, and clinical application of nuciferine on inflammation-related diseases. Pharmacol Res. 2023;193:106820. https://doi.org/ 10.1016/j.phrs.2023.106820 [DOI] [PubMed] [Google Scholar]
- 34. Farrell MS, McCorvy JD, Huang XP, et al. In vitro and in vivo characterization of the Alkaloid Nuciferine. PLoS One. 2016;11:e0150602. https://doi.org/ 10.1371/journal.pone.0150602 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.