Abstract
Background
Peak expiratory flow (PEF) serves as a direct indicator of the functional status of the respiratory system. Higher body fat content, especially abdominal obesity, may relate to a deterioration in long-term respiratory function. The "A Body Shape Index" (ABSI) better assesses abdominal obesity, but its association with PEF is poorly understood.
Methods
The analysis demonstrated data from 14,386 middle-aged and older adults from the 2015 China Health and Retirement Longitudinal Study (CHARLS). ABSI, a sex-specific metric integrating waist circumference, weight, and height via allometric modeling derived from Chinese anthropometrics, was analyzed against PEF/PEF prediction using multivariable linear and spline regressions to characterize nonlinear associations. Threshold effects, subgroup, and sensitivity analyses ensured robustness.
Results
This research showed a negative relationship between ABSI and both PEF and PEF predictions. An inverted L-shaped curve in the spline analysis characterized the association between ABSI and PEF/PEF prediction across the sexes. The ABSI threshold was 0.0782 and 0.0691 in males and females, respectively.
Conclusions
Abdominal obesity negatively affects respiratory function, with ABSI thresholds varying by sex. Therefore, weight management should focus on a healthy ABSI to reduce abdominal obesity and safeguard respiratory health.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-025-02599-2.
Keywords: A body shape index, Peak expiratory flow, Respiratory function tests, Abdominal obesity
Introduction
Respiratory function is crucial for human health, among middle-aged and older individuals, which progressively deteriorates with advancing age. Age-related decline in respiratory function contributes to health issues such as chronic obstructive pulmonary disease (COPD), pneumonia, and other respiratory conditions [1, 2]. Research indicates that reduced respiratory function is strongly associated with a heightened risk of cardiovascular disease, highlighting the importance of understanding changes in respiratory function and their impact [2]. Peak expiratory flow (PEF) serves as a crucial and affordable metric for assessing respiratory health that reflects airway openness and respiratory muscle strength [3]. In addition to diagnosing and monitoring asthma and COPD, it can also indicate non-respiratory issues such as dementia and sarcopenia [4–6]. Therefore, studying PEF in middle-aged and older individuals is vital for public health.
Obesity significantly affects respiratory function and has become a serious public health issue, particularly given the increasing obesity rates among the population of middle-aged and older people in China. Estimates show that the rate of overweight and obesity among Chinese adults could rise to 65.3% by 2030, with the estimated number of affected individuals potentially reaching 789.95 million [7]. A cohort study conducted in the rural regions of Henan, China, showed that the prevalence of abdominal obesity among individuals aged 40 and older was as high as 53.2% [8]. A population-based study of 121,965 participants found that the risk of impaired respiratory function was nearly twice as high in individuals with abdominal obesity [9]. Cohort studies have pointed to an association between abdominal obesity and compromised breathing ability [10, 11]. Therefore, a reliable indicator for evaluating abdominal obesity could potentially be associated with pulmonary function. But previous research has predominantly focused on waist circumference (WC) or the waist-to-hip ratio, neglecting the integration of WC and body mass index (BMI) for a comprehensive assessment and ignoring variations in how fat is distributed based on sex. The introduction of "A Body Shape Index" (ABSI) has addressed these limitations. Among the numerous indicators used to assess obesity, ABSI offers distinct advantages, particularly in evaluating abdominal obesity. Established by Krakauer et al. in 2012, the ABSI integrates height, BMI, and WC for comprehensive assessment beyond individual metrics. A high ABSI reflects a waist measurement that surpasses what is expected for a given height and weight, underscoring the accumulation of volume in the central body [12–15]. As a conventional metric, BMI has limited ability to predict diseases because it does not distinguish between fat and lean mass, nor does it account for the distribution of adipose tissue [13]. In addition, due to differences in hormones, different sexes exhibit different patterns of fat distribution. Males are more likely to have an "apple-shaped" body, while females tend to have a "pear-shaped" body [16, 17]. Due to differences in fat distribution caused by sex rely solely on indicators such as BMI, WC, or waist-to-hip ratio to assess abdominal obesity is inaccurate. Compared with conventional BMI, ABSI has shown enhanced predictive capability regarding the risk and mortality associated with various chronic conditions, such as cardiovascular diseases, metabolic disorders, and chronic kidney disease [13–15].
Although there is an established negative association between abdominal obesity and compromised respiratory function [9–11], research concurrently examining BMI, WC, and sex-specific fat distribution is limited. Consequently, it is vital to explore the relationship involving ABSI, an index incorporating BMI, WC, sex-specific fat distribution, and PEF, a key indicator of respiratory function. However, there is a paucity of research on this topic, particularly studies investigating the association between ABSI values calculated using formulas specific to the Chinese population and PEF within the same demographic group. Consequently, this research utilized data from the 2015 China Health and Retirement Longitudinal Study (CHARLS) to assess the relationship between ABSI and PEF in middle-aged and older Chinese adults. This research sought to expand the knowledge of the effects of obesity on respiratory function. These insights are essential to inform future clinical practices and develop public health strategies.
Methods
Study population and design
CHARLS is an ongoing, nationally representative longitudinal survey in China, established in 2011. CHARLS collects high-quality data through structured questionnaire interviews from a nationally representative sample of Chinese people aged 45 and older, using multistage stratified sampling proportional to size. It included participants from urban and rural areas, ensuring coverage of the substantial rural–urban differences in lifestyle, socioeconomic status, and health outcomes that characterize the Chinese population. Participants completed a standardized questionnaire to gather sociodemographic, lifestyle, and health-related data. The participants underwent biennial follow-ups after the baseline survey. The survey sample was nationally representative and included individually weighted variables. The CHARLS study design has been described previously [18].
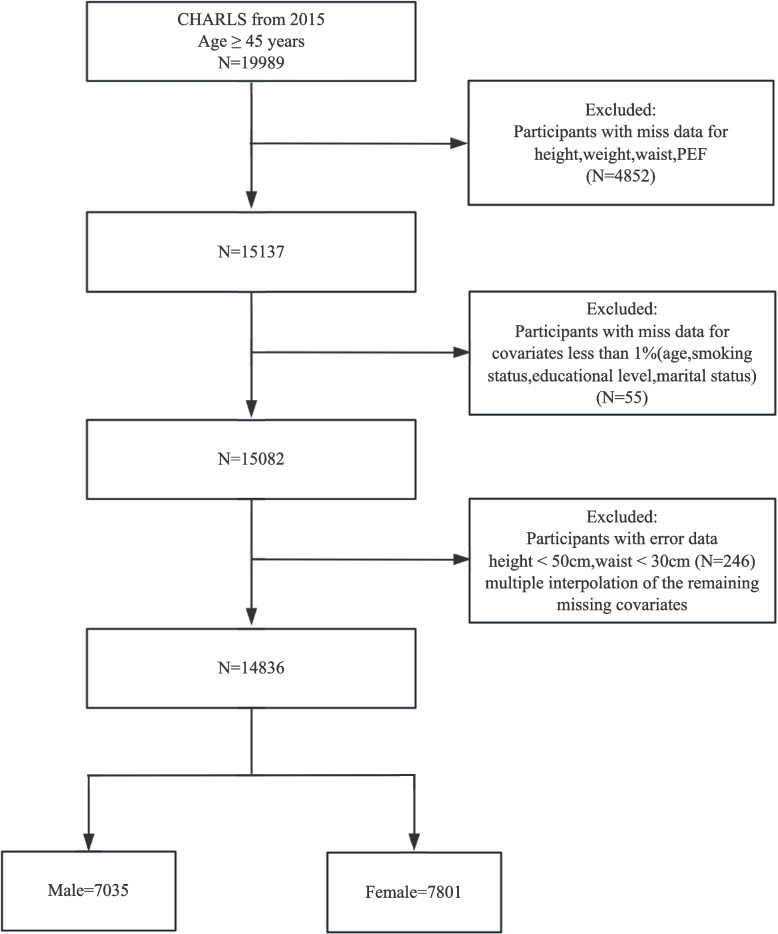
This research conducted a retrospective analysis of data from 19,989 individuals aged 45 and older, using the CHARLS 2015 dataset. Out of these, 5,153 were omitted due to the following exclusion criteria: (1) no information about height, weight, WC, or PEF (N = 4,852); (2) lack of information on marital status, smoking, educational status, and age (N = 55); and (3) height < 50 cm and WC < 30 cm erroneous data (N = 246). Multiple imputations were used to address the missing data in the covariates. Subsequently, 14,836 participants were categorized into five groups based on ABSI quintiles, as illustrated in Fig. 1.
Fig. 1.
Participant inclusion flowchart (China health and retirement longitudinal study [CHARLS])
The research complied with the applicable ethical guidelines set forth by national or institutional research committees, as well as the principles outlined in the Declaration of Helsinki established in 1964 and its subsequent amendments. It was also conducted in compliance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Reporting Guidelines for observational studies [19].
ABSI assessment
The ABSI, developed by Krakauer et al., is a comprehensive index derived from American population data used to evaluate body shape and abnormal obesity by integrating WC, height, and BMI [12]. However, the standardized coefficients of BMI and height vary across populations with different body type patterns and racial backgrounds [20]. To ensure applicability to the Chinese population, the ABSI formulated by Wang et al. in 2020, based explicitly on the Chinese population, was used [21]. This formula was developed utilizing three anthropometric measurements specific to the Chinese population: weight, height, and WC. Utilizing the allometric scaling approach, we conducted linear regression using log-transformed WC measurements as the response parameter, with logarithmically converted values for body mass and stature serving as predictive factors. Given the variations in body morphology between males and females, sex-specific ABSI formulae were derived. Height was assessed with a vertical altimeter, while weight was determined using a scale during the physical examination. Anthropometric assessments were conducted with a flexible measuring tape to record the horizontal plane at the umbilical level, capturing abdominal perimeter dimensions while subjects maintained an upright standing posture.
Male:
Female:
Respiratory function assessment
In the CHARLS, respiratory function was evaluated under the supervision of certified healthcare professionals and trained volunteers. Subjects were trained to stand straight, inhale deeply, and position their lips securely on a disposable mouthpiece. They then forcefully exhaled at maximum velocity. Three trials were conducted, with the best reading from these respiratory efforts selected for analysis. The predicted peak expiratory flow (PEF prediction) calculation for males was performed using the formula: 75.6 + 20.4 × age − 0.41 × age2 + 0.002 × age3 + 1.19 × height (cm) [22]. The formula for females is as follows: 282.0 + 1.79 × age – 0.046 × age2 + 0.68 × height (cm). Airflow limitation (AL) is characterized by a PEF/PEF prediction ratio of < 80%, whereas severe airflow limitation (SAL) is identified when this ratio is < 60% [22, 23].
Covariates
Covariates included categorical variables (self-reported via questionnaire: sex, age, race, residence, marital status, smoking status, alcohol consumption, educational level; diagnosed via self-report and physician diagnoses [medical records]: hypertension, diabetes, stroke, heart disease, dyslipidemia, kidney disease) and continuous variables (self-reported age, sleep duration; measured via blood assays: plasma glucose, low-density lipoprotein [LDL], total cholesterol [TC], creatinine, triglycerides [TG], Cystatin C). Detailed protocols are available at: https://charls.charlsdata.com/pages/Data/2015-charls-wave4/zh-cn.html.
Statistical analysis
Spline regression and nonlinear modeling
All the participants were separated into quintile categories based on their ABSI levels. Continuous variables are presented as mean (standard deviation; SD) or median (interquartile range; IQR), and categorical variables as frequencies or percentages (n, %). Baseline characteristics were analyzed using one-way analysis of variance (ANOVA) and the Kruskal–Wallis test for normally and non-normally distributed continuous variables, respectively. The chi-square test was used for categorical variables. Multiple imputations (five iterations) were used for covariates with > 1% missing values. Supplemental Table 1 displays the variables and extent of missingness. Linear regression models were applied to the overall, male, and female populations to calculate the coefficients (β) and 95% confidence interval (95% CI) for the association between ABSI and both PEF and PEF/PEF prediction. When utilizing linear regression models, it was ensured that all underlying assumptions, including posterior predictive checks, linearity, homogeneity of variance, influential observations, collinearity, and normality of residuals, were thoroughly validated. All models met these criteria. Logistic regression models assessed the association between ABSI and AL or SAL with odds ratios (OR) and 95% CIs. To prevent large ABSI regression coefficients, the ABSI values were scaled by a factor of 100. Model 1 remained unadjusted, whereas Model 2 was adjusted for demographic variables, including age, race, residence, educational level, marital status, and sleep patterns. Model 3 incorporated smoking and drinking status and chronic diseases, including diabetes, hypertension, stroke, heart disease, kidney disease, and dyslipidemia. Model 4 enhanced the analysis by factoring serum biomarkers, including creatinine, glucose, TG, LDL, TC, and Cystatin C. The selection of covariates was based on demographic variables, the presence of chronic diseases, changes in effect estimates exceeding 10%, and indicators previously reported in the literature as potentially affecting PEF [23]. A multiple regression analysis ensured the robustness of the results after multiple imputations. A smoothing spline curve was utilized to investigate the possible nonlinear relationship between ABSI and PEF/PEF prediction, with adjustments made for the variables in Model 4.
Threshold determination using two-piece linear regression
A smoothed two-piece linear regression model was used to examine the association threshold between ABSI and PEF/PEF prediction, with adjustments for the variables in Model 4.
Subgroup and sensitivity analyses
Interaction and subgroup analyses were performed according to age, sex, race, drinking status, and smoking status using linear regression models, adjusting for the variables in Model 4. These subgroups were selected based on the fundamental characteristics and lifestyle factors study of the population that significantly influence respiratory function. Four sensitivity analyses were conducted to ensure the robustness of the results. The first repeated analyses were performed according to the ABSI quartiles. Secondly, we performed a sensitivity analysis of the association between the three ABSI components and PEF. As the third sensitivity analysis, missingness indicators (dummy variables) were integrated into regression models to evaluate potential bias from incomplete data. Fourth, to mitigate the influence of self-reported and physician-diagnosed chronic pulmonary diseases (namely chronic bronchitis, emphysema, and pulmonary heart disease) on the findings, these participants were excluded from the study.
Software and significance threshold
Statistical analyses were conducted using R Statistical Software (v4.2.2; R Foundation for Statistical Computing; http://www.R-project.org) and a free statistical analysis platform (v2.0; Beijing Free Clinical Medical Technology Co., Ltd.). Descriptive statistics were calculated for all participants, and statistical significance was assessed using a two-tailed test with a P value threshold of < 0.05.
Results
Characteristics of the population by ABSI
This study included 14,836 participants with an average age of 60.5 ± 9.8 years. Of these, 7,035 (47.4%) were male, and the mean ABSI was 0.0729 ± 0.0062. Table 1 presents the characteristics of the participants across the ABSI quintiles. Higher ABSI scores were typically associated with older age, male sex, unmarried status, alcohol consumption, smoking status, and higher educational level. Additionally, higher ABSI was associated with diabetes, hypertension, stroke, kidney diseases, heart diseases, dyslipidemias, and higher levels of glucose, creatinine, TC, LDL, Cystatin C, and TG.
Table 1.
Baseline characteristics of participants stratified by a body shape index quintile
| Variables | Total (N = 14,836) | Q1 (N = 2967) | Q2 (N = 2967) | Q3 (N = 2967) | Q4 (N = 2967) | Q5 (N = 2968) | P value |
|---|---|---|---|---|---|---|---|
| ≤ 6.86 | 6.86–7.18 | 7.18–7.46 | 7.46–7.76 | ≥ 7.76 | |||
| Demographic | |||||||
| Sex, n (%) | < 0.001 | ||||||
| Female | 7801 (52.6) | 2696 (90.9) | 2308 (77.8) | 1493 (50.3) | 782 (26.4) | 522 (17.6) | |
| Male | 7035 (47.4) | 271 (9.1) | 659 (22.2) | 1474 (49.7) | 2185 (73.6) | 2446 (82.4) | |
| Age, Mean ± SD (y) | 60.5 ± 9.8 | 56.5 ± 8.5 | 59.0 ± 8.8 | 60.5 ± 9.2 | 61.5 ± 9.8 | 65.1 ± 10.5 | < 0.001 |
| Race, n (%) | < 0.001 | ||||||
| Non-Han | 970 (6.5) | 222 (7.5) | 199 (6.7) | 181 (6.1) | 167 (5.6) | 201 (6.8) | |
| Han | 12,388 (83.5) | 2516 (84.8) | 2514 (84.7) | 2510 (84.6) | 2488 (83.9) | 2360 (79.5) | |
| Unknown | 1478 (10.0) | 229 (7.7) | 254 (8.6) | 276 (9.3) | 312 (10.5) | 407 (13.7) | |
| Residence, n (%) | 0.067 | ||||||
| Urban community | 5583 (37.6) | 1142 (38.5) | 1069 (36.0) | 1093 (36.8) | 1111 (37.4) | 1168 (39.4) | |
| Rural village | 9253 (62.4) | 1825 (61.5) | 1898 (64.0) | 1874 (63.2) | 1856 (62.6) | 1800 (60.6) | |
| Educational Level, n (%) | < 0.001 | ||||||
| Illiterate | 6362 (42.9) | 1374 (46.3) | 1461 (49.2) | 1314 (44.3) | 1099 (37) | 1114 (37.5) | |
| Primary school | 4149 (28.0) | 791 (26.7) | 771 (26.0) | 826 (27.8) | 867 (29.2) | 894 (30.1) | |
| Junior high school | 2842 (19.2) | 529 (17.8) | 500 (16.9) | 568 (19.1) | 648 (21.8) | 597 (20.1) | |
| High school or above | 1483 (10.0) | 273 (9.2) | 235 (7.9) | 259 (8.7) | 353 (11.9) | 363 (12.2) | |
| Marital status, n (%) | < 0.001 | ||||||
| No | 1920 (12.9) | 298 (10.0) | 393 (13.2) | 404 (13.6) | 387 (13.0) | 438 (14.8) | |
| Yes | 12,916 (87.1) | 2669 (90.0) | 2574 (86.8) | 2563 (86.4) | 2580 (87.0) | 2530 (85.2) | |
| Health status and function | |||||||
| Height, Mean ± SD (m) | 1.6 ± 0.1 | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | < 0.001 |
| Weight, Mean ± SD (kg) | 60.1 ± 11.8 | 60.0 ± 13.0 | 58.2 ± 10.2 | 59.8 ± 11.1 | 62.1 ± 11.9 | 60.4 ± 12.4 | < 0.001 |
| WC, Mean ± SD (cm) | 86.5 ± 10.7 | 81.5 ± 11.5 | 85.0 ± 10.2 | 86.5 ± 10.1 | 88.5 ± 9.5 | 90.7 ± 9.9 | < 0.001 |
| ABSI**, Mean ± SD | 7.29 ± 0.62 | 6.47 ± 0.62 | 7.02 ± 0.09 | 7.32 ± 0.01 | 7.60 ± 0.08 | 8.05 ± 0.31 | < 0.001 |
| PEF, Mean ± SD (L/min) | 318.3 ± 125.9 | 294.6 ± 92.3 | 299.7 ± 108.7 | 325.1 ± 129.6 | 349.1 ± 137.7 | 322.8 ± 145.8 | < 0.001 |
| PEF/PEF prediction, Mean ± SD (%) | 83.7 ± 27.2 | 84.6 ± 23.8 | 84.1 ± 26.0 | 84.0 ± 26.5 | 84.8 ± 27.9 | 81.0 ± 30.9 | < 0.001 |
| Drinking status, n (%) | < 0.001 | ||||||
| No | 7972 (53.7) | 2193 (73.9) | 1994 (67.2) | 1560 (52.6) | 1187 (40.0) | 1038 (35.0) | |
| Yes | 6864 (46.3) | 774 (26.1) | 973 (32.8) | 1407 (47.4) | 1780 (60.0) | 1930 (65.0) | |
| Smoking status, n (%) | < 0.001 | ||||||
| Nevera | 8398 (56.6) | 2583 (87.1) | 2264 (76.3) | 1633 (55.0) | 1077 (36.3) | 841 (28.3) | |
| Currentb | 4175 (28.1) | 233 (7.9) | 479 (16.1) | 927 (31.2) | 1229 (41.4) | 1307 (44.0) | |
| Pastc | 2263 (15.3) | 151 (5.1) | 224 (7.5) | 407 (13.7) | 661 (22.3) | 820 (27.6) | |
| Sleep duration, Mean ± SD (h) | 6.4 ± 1.9 | 6.4 ± 1.9 | 6.3 ± 2.0 | 6.4 ± 2.0 | 6.4 ± 1.9 | 6.5 ± 1.9 | 0.016 |
| Hypertension, n (%) | < 0.001 | ||||||
| No | 6136 (41.4) | 1265 (42.6) | 1357 (45.7) | 1256 (42.3) | 1182 (39.8) | 1076 (36.3) | |
| Yes | 7318 (49.3) | 1321 (44.5) | 1327 (44.7) | 1440 (48.5) | 1542 (52.0) | 1688 (56.9) | |
| Unknown | 1382 (9.3) | 381 (12.8) | 283 (9.5) | 271 (9.1) | 243 (8.2) | 204 (6.9) | |
| Diabetes, n (%) | < 0.001 | ||||||
| No | 10,502 (70.8) | 2060 (69.4) | 2129 (71.8) | 2121 (71.5) | 2082 (70.2) | 2110 (71.1) | |
| Yes | 2418 (16.3) | 420 (14.2) | 471 (15.9) | 446 (15.0) | 525 (17.7) | 556 (18.7) | |
| Unknown | 1916 (12.9) | 487 (16.4) | 367 (12.4) | 400 (13.5) | 360 (12.1) | 302 (10.2) | |
| Heart Diseases, n (%) | < 0.001 | ||||||
| No | 10,406 (70.1) | 1978 (66.7) | 2066 (69.6) | 2113 (71.2) | 2113 (71.2) | 2136 (72.0) | |
| Yes | 2324 (15.7) | 457 (15.4) | 510 (17.2) | 434 (14.6) | 450 (15.2) | 473 (15.9) | |
| Unknown | 2106 (14.2) | 532 (17.9) | 391 (13.2) | 420 (14.2) | 404 (13.6) | 359 (12.1) | |
| Stroke, n (%) | < 0.001 | ||||||
| No | 12,350 (83.2) | 2384 (80.4) | 2525 (85.1) | 2473 (83.4) | 2460 (82.9) | 2508 (84.5) | |
| Yes | 448 (3.0) | 71 (2.4) | 62 (2.1) | 85 (2.9) | 114 (3.8) | 116 (3.9) | |
| Unknown | 2038 (13.7) | 512 (17.3) | 380 (12.8) | 409 (13.8) | 393 (13.2) | 344 (11.6) | |
| Dyslipidemias, n (%) | < 0.001 | ||||||
| No | 10,095 (68.0) | 1930 (65.0) | 2046 (69.0) | 2037 (68.7) | 2024 (68.2) | 2058 (69.3) | |
| Yes | 2346 (15.8) | 460 (15.5) | 478 (16.1) | 460 (15.5) | 467 (15.7) | 481 (16.2) | |
| Unknown | 2395 (16.1) | 577 (19.4) | 443 (14.9) | 470 (15.8) | 476 (16.0) | 429 (14.5) | |
| Kidney Diseases, n (%) | < 0.001 | ||||||
| No | 11,458 (77.2) | 2222 (74.9) | 2317 (78.1) | 2310 (77.9) | 2294 (77.3) | 2315 (78.0) | |
| Yes | 1276 (8.6) | 220 (7.4) | 248 (8.4) | 240 (8.1) | 273 (9.2) | 295 (9.9) | |
| Unknown | 2102 (14.2) | 525 (17.7) | 402 (13.5) | 417 (14.1) | 400 (13.5) | 358 (12.1) | |
| Serum biomarkers | |||||||
| Glucose, Mean ± SD (mg/dL) | 103.5 ± 35.2 | 99.8 ± 29.0 | 101.3 ± 30.2 | 102.6 ± 32.8 | 106.0 ± 39.5 | 108.2 ± 42.8 | < 0.001 |
| Creatinine, Mean ± SD (mg/dL) | 0.8 ± 0.3 | 0.7 ± 0.2 | 0.8 ± 0.3 | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.9 ± 0.4 | < 0.001 |
| TC, Mean ± SD (mg/dL) | 184.2 ± 36.3 | 187.0 ± 35.5 | 186.5 ± 36.3 | 184.0 ± 36.8 | 183.0 ± 36.1 | 180.3 ± 36.4 | < 0.001 |
| LDL, Mean ± SD (mg/dL) | 102.4 ± 28.8 | 103.8 ± 28.6 | 103.4 ± 28.7 | 102.4 ± 28.9 | 102.4 ± 29.0 | 99.9 ± 28.8 | < 0.001 |
| Cystatin C, Mean ± SD (mg/dL) | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.9 ± 0.3 | < 0.001 |
| TG, Median (IQR) (mg/dL) | 115.9 (83.2, 170.8) | 112.4 (82.3, 166.4) | 116.8 (85.0, 169.0) | 112.4 (81.4, 168.6) | 116.8 (83.2, 176.1) | 117.7 (84.1, 174.3) | 0.021 |
ABSI a body shape index, PEF peak expiratory flow, TC total cholesterol, TG triglycerides, LDL low-density lipoprotein, WC waist circumference, SD standard deviation, IQR interquartile range
**Continuous variable: ABSI was scaled by a factor of 100
aDefined as individuals with no history of smoking, including cigarette smoking, pipe use, or chewing tobacco, where smoking is classified as having consumed more than 100 cigarettes or an equivalent amount of tobacco in their lifetime
bDefined as individuals with a history of smoking cigarettes, smoking a pipe, or chewing tobacco, either currently or in the past, with smoking classified as having consumed more than 100 cigarettes in their lifetime
cDefined as an individual with a history of smoking cigarettes, using a pipe, or chewing tobacco, with smoking defined as having consumed more than 100 cigarettes or equivalent, but who has now quit
Association between ABSI and PEF , PEF/PEF prediction, AL and SAL
Following multivariate adjustments, ABSI was significantly negatively associated with PEF and PEF/PEF prediction. In men, a 0.01 unit increase in ABSI resulted in a 9.83 L/min reduction in PEF (β = −9.83, 95% CI: −17.26 to −2.4, P = 0.010) and a 3.30 unit decrease in PEF/PEF prediction (β = −3.30, 95% CI: −5.05 to −1.55, P < 0.001). Stratification by ABSI quintile showed that the highest ABSI subgroup (Q5, 0.0793 to 0.114) experienced a 13.32 L/min decrease in PEF and 3.86 unit decrease in PEF/PEF prediction compared with the lowest ABSI subgroup (Q1, 0.0231 to 0.0729). These associations were consistent in separate analyses of the general population and females (Tables 2 and 3). As shown in Table 4, in the overall study population, an increase in ABSI is significantly associated with elevated levels of AL (OR 1.17, 95% CI 1.08–1.28, P < 0.001) and SAL (OR 1.30, 95% CI 1.19–1.41, P < 0.001). These associations are consistent across both male and female participants.
Table 2.
Association of ABSI and PEF (Multiple imputation)
| Categories | Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| β(95%CI) | P value | β(95%CI) | P value | β(95%CI) | P value | β(95%CI) | P value | ||
| All | ABSI** | 15.38 (12.13 ~ 18.63) | < 0.001 | −6.28 (−9.56 ~ −3.00) | < 0.001 | −6.22 (−9.87 ~ −2.57) | 0.001 | −6.15 (−10.13 ~ −2.17) | 0.002 |
| ABSI** Quintile | |||||||||
| Q1 (1.70 ~ 6.86) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | |
| Q2 (6.86 ~ 7.18) | 5.11 (−1.22 ~ 11.44) | 0.114 | 3.48 (−1.98 ~ 8.94) | 0.211 | 3.09 (−2.95 ~ 9.12) | 0.317 | 3.81 (−2.71 ~ 10.33) | 0.252 | |
| Q3 (7.18 ~ 7.46) | 30.50 (24.17 ~ 36.83) | < 0.001 | 3.20 (−2.57 ~ 8.96) | 0.277 | 3.76 (−2.60 ~ 10.12) | 0.246 | 3.67 (−3.22 ~ 10.57) | 0.296 | |
| Q4 (7.46 ~ 7.76) | 54.48 (48.15 ~ 60.81) | < 0.001 | 3.81 (−2.40 ~ 10.03) | 0.229 | 3.90 (−2.94 ~ 10.75) | 0.264 | 0.97 (−6.53 ~ 8.47) | 0.799 | |
| Q5 (7.76 ~ 11.40) | 28.22 (21.89 ~ 34.55) | < 0.001 | −13.19 (−19.83 ~ −6.55) | < 0.001 | −13.36 (−20.68 ~ −6.03) | < 0.001 | −13.81 (−21.91 ~ −5.71) | 0.001 | |
| P for trend | < 0.001 | 0.001 | 0.003 | 0.003 | |||||
| Male | |||||||||
| ABSI** | −35.17 (−41.35 ~ −28.99) | < 0.001 | −10.62 (−16.6 ~ −4.64) | 0.001 | −10.03 (−16.73 ~ −3.33) | 0.003 | −9.83 (−17.26 ~ −2.40) | 0.010 | |
| ABSI** Quintile | |||||||||
| Q1 (2.31 ~ 7.29) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | |
| Q2 (7.29 ~ 7.51) | 8.58 (−1.34 ~ 18.50) | 0.090 | 7.39 (−1.93 ~ 16.71) | 0.120 | 6.87 (−3.68 ~ 17.42) | 0.202 | 5.43 (−5.96 ~ 16.83) | 0.350 | |
| Q3 (7.51 ~ 7.71) | −0.52 (−10.44 ~ 9.40) | 0.919 | 5.87 (−3.44 ~ 15.18) | 0.216 | 4.71 (−5.81 ~ 15.24) | 0.380 | −0.21 (−11.76 ~ 11.34) | 0.972 | |
| Q4 (7.71 ~ 7.93) | −11.56 (−21.48 ~ −1.64) | 0.022 | 4.64 (−4.76 ~ 14.04) | 0.334 | 1.30 (−9.34 ~ 11.95) | 0.810 | −1.45 (−13.17 ~ 10.27) | 0.809 | |
| Q5 (7.93 ~ 11.40) | −52.89 (−62.81 ~ −42.97) | < 0.001 | −12.84 (−22.51 ~ −3.17) | 0.009 | −10.71 (−21.45 ~ 0.03) | 0.051 | −13.32 (−25.32 ~ −1.31) | 0.030 | |
| P for trend | < 0.001 | 0.017 | 0.032 | 0.018 | |||||
| Female | |||||||||
| ABSI** | −28.89 (−32.18 ~ −25.60) | < 0.001 | −7.19 (−10.59 ~ −3.78) | < 0.001 | −6.98 (−10.76 ~ −3.19) | < 0.001 | −6.80 (−10.89 ~ −2.71) | 0.001 | |
| ABSI** Quintile | |||||||||
| Q1 (1.70 ~ 6.67) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | |
| Q2 (6.67 ~ 6.92) | 2.77 (−3.11 ~ 8.66) | 0.356 | 2.77 (−3.11 ~ 8.66) | 0.356 | 1.76 (−4.8 ~ 8.33) | 0.598 | 0.60 (−6.44 ~ 7.65) | 0.867 | |
| Q3 (6.92 ~ 7.13) | −0.61 (−6.51 ~ 5.29) | 0.840 | −0.61 (−6.51 ~ 5.29) | 0.840 | 0.36 (−6.18 ~ 6.89) | 0.914 | 1.09 (−5.97 ~ 8.15) | 0.762 | |
| Q4 (7.13 ~ 7.40) | −5.53 (−11.56 ~ 0.50) | 0.072 | −5.53 (−11.56 ~ 0.50) | 0.072 | −2.90 (−9.53 ~ 3.72) | 0.390 | −3.44 (−10.64 ~ 3.76) | 0.349 | |
| Q5 (7.40 ~ 10.00) | −16.48 (−22.89 ~ −10.07) | < 0.001 | −16.48 (−22.89 ~ −10.07) | < 0.001 | −13.75 (−20.75 ~ −6.75) | < 0.001 | −13.99 (−21.64 ~ −6.35) | < 0.001 | |
| P for trend | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||
ABSI a body shape index, PEF peak expiratory flow, TC total cholesterol, TG triglycerides, LDL low-density lipoprotein, β coefficient, CI confidence interval
Model 1: not adjusted
Model 2: adjusted for age, race, residence, educational status, marital status, sleep duration(total population adjusted for sex)
Model 3: adjusted for model 2, additionally adjusted for smoking status, drinking status, hypertension, diabetes, heart diseases, stroke, dyslipidemias, kidney diseases
Model 4: adjusted for model 3, additionally adjusted for creatinine, glucose, TC, TG, LDL, Cystatin C
**Continuous variable: ABSI was scaled by a factor of 100
Table 3.
Association of ABSI and PEF/PEF prediction (Multiple imputation)
| Categories | Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| β(95%CI) | P value | β(95%CI) | P value | β(95%CI) | P value | β(95%CI) | P value | ||
| All | ABSI** | −2.08 (−2.78 ~ −1.38) | < 0.001 | −3.12 (−3.98 ~ −2.27) | < 0.001 | −3.16 (−4.13 ~ −2.20) | < 0.001 | −3.16 (−4.21 ~ −2.10) | < 0.001 |
| ABSI** Quintile | |||||||||
| Q1 (1.70 ~ 6.86) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | ||
| Q2 (6.86 ~ 7.18) | −0.49 (−1.87 ~ 0.89) | 0.486 | −0.61 (−2.03 ~ 0.82) | 0.403 | −0.50 (−2.10 ~ 1.09) | 0.537 | −0.30 (−2.02 ~ 1.43) | 0.734 | |
| Q3 (7.18 ~ 7.46) | −0.63 (−2.01 ~ 0.75) | 0.368 | −2.33 (−3.83 ~ −0.83) | 0.002 | −1.83 (−3.51 ~ −0.15) | 0.033 | −1.70 (−3.52 ~ 0.12) | 0.068 | |
| Q4 (7.46 ~ 7.76) | 0.23 (−1.15 ~ 1.61) | 0.739 | −2.64 (−4.26 ~ −1.02) | 0.001 | −2.36 (−4.17 ~ −0.54) | 0.011 | −2.90 (−4.88 ~ −0.91) | 0.004 | |
| Q5 (7.76 ~ 11.40) | −3.63 (−5.01 ~ −2.25) | < 0.001 | −6.06 (−7.79 ~ −4.33) | < 0.001 | −5.84 (−7.78 ~ −3.90) | < 0.001 | −5.81 (−7.95 ~ −3.67) | < 0.001 | |
| P for trend | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||
| Male | |||||||||
| ABSI** | −4.01 (−5.30 ~ −2.71) | < 0.001 | −3.35 (−4.73 ~ −1.96) | < 0.001 | −3.25 (−4.83 ~ −1.67) | < 0.001 | −3.30 (−5.05 ~ −1.55) | < 0.001 | |
| ABSI** Quintile | |||||||||
| Q1 (2.31 ~ 7.29) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | ||
| Q2 (7.29 ~ 7.51) | 1.77 (−0.32 ~ 3.85) | 0.097 | 1.46 (−0.70 ~ 3.63) | 0.186 | 1.35 (−1.14 ~ 3.84) | 0.287 | 1.07 (−1.62 ~ 3.76) | 0.436 | |
| Q3 (7.51 ~ 7.71) | 1.03 (−1.06 ~ 3.12) | 0.333 | 0.94 (−1.23 ~ 3.10) | 0.396 | 0.74 (−1.74 ~ 3.22) | 0.561 | −0.32 (−3.04 ~ 2.41) | 0.819 | |
| Q4 (7.71 ~ 7.93) | 0.71 (−1.38 ~ 2.79) | 0.506 | 0.38 (−1.80 ~ 2.56) | 0.733 | −0.34 (−2.85 ~ 2.17) | 0.789 | −0.98 (−3.75 ~ 1.78) | 0.486 | |
| Q5 (7.93 ~ 11.40) | −4.50 (−6.59 ~ −2.41) | < 0.001 | −3.72 (−5.96 ~ −1.47) | 0.001 | −3.10 (−5.63 ~ −0.57) | 0.016 | −3.86 (−6.69 ~ −1.03) | 0.008 | |
| P for trend | < 0.001 | 0.002 | 0.008 | 0.003 | |||||
| Female | |||||||||
| ABSI** | −3.84 (−4.82 ~ −2.86) | < 0.001 | −3.01 (−4.08 ~ −1.93) | < 0.001 | −3.10 (−4.31 ~ −1.89) | < 0.001 | −3.12 (−4.42 ~ −1.82) | < 0.001 | |
| ABSI** Quintile | |||||||||
| Q1 (1.70 ~ 6.67) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | 0(Ref) | ||
| Q2 (6.67 ~ 6.92) | 0.45 (−1.37 ~ 2.26) | 0.630 | 0.56 (−1.30 ~ 2.41) | 0.556 | 0.29 (−1.80 ~ 2.39) | 0.783 | −0.09 (−2.34 ~ 2.15) | 0.937 | |
| Q3 (6.92 ~ 7.13) | −1.39 (−3.20 ~ 0.43) | 0.134 | −0.74 (−2.60 ~ 1.12) | 0.435 | −0.44 (−2.53 ~ 1.64) | 0.678 | −0.24 (−2.49 ~ 2.01) | 0.837 | |
| Q4 (7.13 ~ 7.40) | −2.42 (−4.24 ~ −0.61) | 0.009 | −2.47 (−4.37 ~ −0.57) | 0.011 | −1.72 (−3.83 ~ 0.40) | 0.112 | −1.98 (−4.28 ~ 0.31) | 0.091 | |
| Q5 (7.40 ~ 10.0) | −7.03 (−8.85 ~ −5.22) | < 0.001 | −6.14 (−8.15 ~ −4.12) | < 0.001 | −5.43 (−7.66 ~ −3.19) | < 0.001 | −5.53 (−7.97 ~ −3.09) | < 0.001 | |
| P for trend | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||
ABSI a body shape index, PEF peak expiratory flow, PEF/PEF prediction ratio of peak expiratory flow to predicted peak expiratory flow, TC total cholesterol, TG triglycerides, LDL low-density lipoprotein, β coefficient, CI confidence interval
Model 1: not adjusted
Model 2: adjusted for age, race, residence, educational status, marital status, sleep duration(total population adjusted for sex)
Model 3: adjusted for model 2, additionally adjusted for smoking status, drinking status, hypertension, diabetes, heart diseases, stroke, dyslipidemias, kidney diseases
Model 4: adjusted for model 3, additionally adjusted for creatinine, glucose, TC, TG, LDL, Cystatin C
**Continuous variable: ABSI was scaled by a factor of 100
Table 4.
Association of continuous ABSI** with AL and SAL risk (Multiple imputation)
| Categories | Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | ||
| All | AL | 1.15 (1.09–1.21) | < 0.001 | 1.17 (1.09–1.25) | < 0.001 | 1.17 (1.09–1.27) | < 0.001 | 1.17 (1.08–1.28) | < 0.001 |
| SAL | 1.36 (1.27–1.46) | < 0.001 | 1.30 (1.19–1.41) | < 0.001 | 1.29 (1.18–1.40) | < 0.001 | 1.30 (1.19–1.41) | < 0.001 | |
| Male | AL | 1.26 (1.15–1.39) | < 0.001 | 1.17 (1.05–1.31) | 0.004 | 1.18 (1.05 ~ 1.33) | 0.007 | 1.18 (1.03–1.34) | 0.017 |
| SAL | 1.58 (1.38–1.80) | < 0.001 | 1.37 (1.20–1.56) | < 0.001 | 1.36 (1.19 ~ 1.56) | < 0.001 | 1.38 (1.20–1.57) | < 0.001 | |
| Female | AL | 1.32 (1.21–1.43) | < 0.001 | 1.16 (1.06–1.27) | 0.001 | 1.16 (1.05–1.29) | 0.003 | 1.17 (1.05–1.30) | 0.006 |
| SAL | 1.52 (1.37–1.70) | < 0.001 | 1.27 (1.14–1.42) | < 0.001 | 1.27 (1.13–1.42) | < 0.001 | 1.27 (1.14–1.42) | < 0.001 | |
ABSI a body shape index, TC total cholesterol, TG triglycerides, LDL low-density lipoprotein, SAL severe airflow limitation, AL airflow limitation, OR odds ratio, CI confidence interval
Model 1: not adjusted
Model 2: adjusted for age, sex, race, residence, educational status, marital status, sleep duration
Model 3: adjusted for model 2, additionally adjusted for smoking status, drinking status, hypertension, diabetes, heart diseases, stroke, dyslipidemias, kidney diseases
Model 4: adjusted for model 3, additionally adjusted for creatinine, glucose, TC, TG, LDL, Cystatin C
**Continuous variable: ABSI was scaled by a factor of 100
Smoothing spline curve regression model
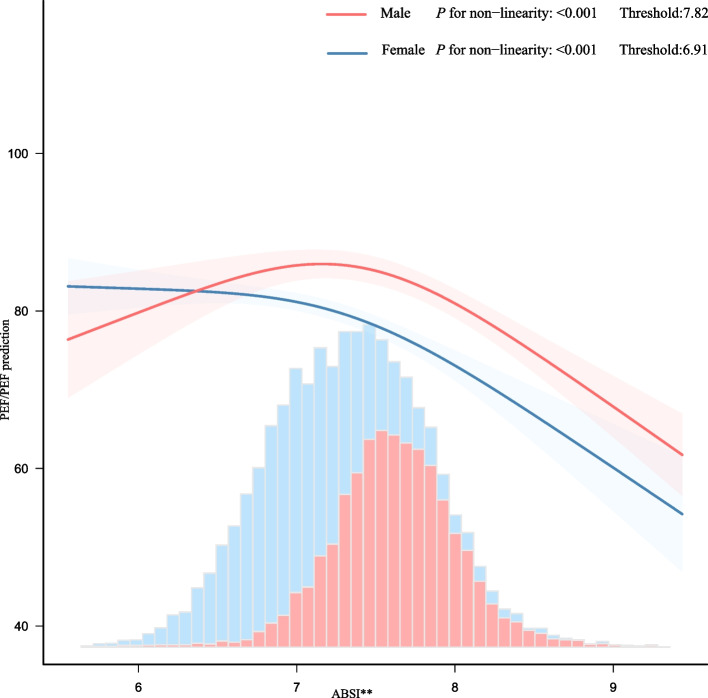
The association between ABSI and PEF/PEF prediction across sexes was characterized by an inverted L-shaped curve in the spline analysis (Fig. 2). A threshold value of 0.0782 was identified in the threshold analysis of men. Each 0.01 unit rise in ABSI beyond the threshold decreased PEF/PEF prediction by 13.047 units (95% CI: −18.285 to −7.809, P < 0.001). Conversely, when the ABSI was < 0.0782, no significant association was observed between ABSI and PEF/PEF prediction. Similarly, a threshold of 0.0691 was established for the threshold analysis of females. Beyond this threshold, each 0.01 unit increase in ABSI corresponded to a reduction in PEF/PEF prediction by 8.138 units (95% CI: −10.549 to −5.726, P < 0.001). When the ABSI was < 0.0691, no significant association was found between the ABSI and PEF/PEF prediction (Table 5).
Fig. 2.
association between ABSI and PEF/PEF prediction. Curves indicate the association between ABSI and PEF/PEF predictions, adjusted for confounders. P < 0.001 for non-linearity in both sexes. Histograms show ABSI distribution. The width of each bar represents the bin range (group interval), and the height indicates the frequency of data within that bin range. TC: total cholesterol; TG: triglycerides; LDL: low-density lipoprotein. Adjusted for age, race, residence, educational status, marital status, sleep duration, smoking status, drinking status, hypertension, diabetes, heart diseases, stroke, dyslipidemias, kidney diseases, creatinine, glucose. TC, TG, LDL, Cystatin C. Only 0.91% ~ 99.86% of the data is displayed. ** Continuous variable: ABSI was scaled by a factor of 100
Table 5.
Threshold effect analysis of the ABSI and PEF/PEF prediction
| ABSI** | Adjusted model | ||
|---|---|---|---|
| β (95% CI) | P value | ||
| Male | |||
| < 7.82 | −0.103(−2.767 ~ 2.561) | 0.939 | |
| ≥ 7.82 | −13.047(−18.285 ~ −7.809) | < 0.001 | |
| Likelihood Ratio test | < 0.001 | ||
| Female | |||
| < 6.91 | 1.382(−1.938 ~ 4.702) | 0.415 | |
| ≥ 6.91 | −8.138(−10.549 ~ −5.726) | < 0.001 | |
| Likelihood Ratio test | < 0.001 | ||
TC total cholesterol, TG triglycerides, LDL low-density lipoprotein, β coefficient, CI confidence interval. Adjusted for age, race, residence, educational status, marital status, sleep duration, smoking status, drinking status, hypertension, diabetes, heart diseases, stroke, dyslipidemias, kidney diseases, creatinine, glucose, TC, TG, LDL, Cystatin C. Only 0.91% ~ 99.86% of the data is displayed
**Continuous variable: ABSI was scaled by a factor of 100
Subgroup analysis
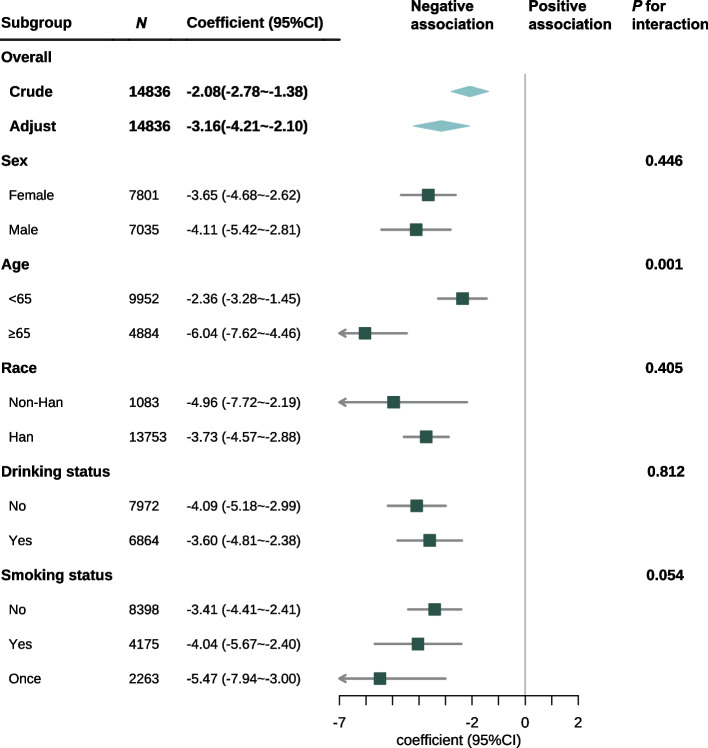
Subgroup analysis was performed across various subgroups to assess potential differences in the association between ABSI and PEF/PEF prediction. No significant interactions were found in subgroups categorized according to sex, race, alcohol consumption, or smoking status. In the age-stratified subgroup, an interaction existed between ABSI and PEF/PEF prediction, as indicated by the likelihood ratio test P value of 0.001) (Fig. 3).
Fig. 3.
Subgroup analysis of the association between ABSI and PEF/PEF prediction in the entire population. TC: total cholesterol; TG: triglycerides; LDL: low-density lipoprotein; CI: confidence interval. Adjusted for race, residence, educational status, marital status, sleep duration, hypertension, diabetes, heart diseases, stroke, dyslipidemias, kidney diseases, creatinine, glucose, TC, TG, LDL, Cystatin C. ABSI was scaled by a factor of 100
Sensitivity analysis
First, the findings remained unchanged according to the ABSI quartiles (Supplemental Table 2). Second, a sensitivity analysis was performed to examine the association between the three ABSI components and PEF. Supplemental Table 3 demonstrates that after adjusting for all variables, height, weight, and WC were found to be independently associated with PEF. Third, Sensitivity analyses with missing indicators showed consistent negative associations between ABSI and PEF/PEF prediction across methods, with aligned trend directions (Supplementary Table 4). Fourth, after excluding patients with chronic pulmonary disease, the results remained robust (Supplementary Table 5).
Discussion
This study investigated the association between the ABSI and respiratory function in middle-aged and elderly Chinese individuals. The main findings were as follows: (1) There was a negative association between ABSI and both PEF and PEF/PEF prediction in these age groups, as determined by regression analysis. A higher ABSI was proportionally related to a higher risk of AL and SAL, even after controlling confounders related to this research. (2) There was a significant threshold effect between ABSI and PEF/PEF prediction, with different thresholds for different sexes.
The decline in respiratory function among middle-aged and older individuals is significantly influenced by obesity, particularly abdominal obesity, as confirmed by multiple cross-sectional [9, 24] and cohort studies [10, 11, 25]. BMI, a common measure for assessing weight-related health, has limitations not only in distinguishing between fat and lean mass but also in accounting for sex differences or adipose tissue distribution [13]. This is particularly important because different body fat distributions in men and women can have distinct effects on respiratory health. In contrast, the ABSI, introduced in 2012, combines WC, height, and weight to assess abdominal obesity independently, without relying on BMI. Research shows that ABSI is more strongly associated with early mortality and health risks, including diabetes, metabolic syndrome, and cardiovascular disease, than BMI or WC alone [13–15]. The study innovatively applied the ABSI formula derived from a Chinese population to assess its association with PEF and demonstrated the stronger correlation of ABSI with respiratory function. Furthermore, the identified sex-specific thresholds for ABSI in relation to PEF highlight the importance of considering sex differences in body fat distribution and respiratory health, which is consistent with findings from other populations [26]. This approach provides a more nuanced understanding of the effect of abdominal obesity on respiratory function, particularly in the context of sex-specific differences.
PEF indicates airway patency, large airway function, and respiratory muscle strength [22, 27]. Research suggests that individuals with metabolic syndrome (MetS) demonstrate a reduction in their creatinine-to-cystatin C ratio (CCR) [28]. The CCR is a marker of reactive muscle mass that exerts protective effects on respiratory function [29]. A reduction in CCR is associated with a deterioration in pulmonary function, particularly abdominal obesity, a predominant characteristic of MetS. Consequently, an increase in the ABSI score, which signifies abdominal obesity, was associated with a decline in PEF and PEF/PEF prediction. Furthermore, numerous studies have directly corroborated that abdominal obesity contributes to a reduction in muscle mass [30–32], subsequently demonstrating increased ABSI and decreased PEF. There are several other mechanisms by which obesity affects respiratory function. Studies have shown that obese patients have significant differences in ventilatory dynamics in the resting state and during exercise compared with normal-weight individuals [33]. Obesity leads to reduced vital capacity, increased respiratory rate, and increased physiological dead space, which may lead to dyspnea and decreased exercise endurance [34, 35]. Obesity also affects inflammatory responses in the respiratory tract. The airways of obese patients are usually accompanied by higher levels of inflammation [36, 37]. Furthermore, obesity disrupts the balance of the autonomic nervous system, marked by heightened sympathetic activity and reduced parasympathetic function. This dysregulation leads to an increase in bronchial smooth muscle tone, thereby elevating airway resistance during forced expiration and further impairing PEF [38, 39]. In addition, obesity exacerbates sleep-disordered breathing conditions, such as obstructive sleep apnea, wherein recurrent cycles of hypoxia and reoxygenation induce chronic airway inflammation and diaphragm fatigue. These interactions establish a vicious cycle that perpetuates declines in PEF, even during daytime respiration [40, 41]. Additionally, it is well known that respiratory function declines with age [1], and the subgroup analysis revealed an interaction effect with age. Muscle mass and function gradually decline with increasing age, and the respiratory muscles are no exception [42]. Aging of the respiratory muscles is a major reason for a decline in respiratory function [43].
The association between ABSI and PEF exhibited sex differences, aligning with the findings of Zeng et al. [26] that the impact of general obesity on respiratory function is more pronounced in women. In contrast, the link between central obesity and respiratory function is more evident in men. This can be attributed to variations in body fat distribution: women typically have a"pear-shaped"body morphology with more fat in the lower body, and men have an"apple-shaped"form with higher abdominal fat concentration [16, 17]. Hormonal differences likely play a key role; elevated estrogen in women promotes adipose tissue storage, especially in the lower extremities, whereas testosterone in men enhances muscle hypertrophy and reduces visceral fat [44, 45]. These factors contribute to the sex-specific thresholds observed in the study, with women having a lower threshold than men due to their lower baseline ABSI associated with a"pear-shaped"body and men having a higher baseline ABSI associated with an"apple-shaped"body.
Interestingly, some studies have found that increased BMI protects against COPD [46], especially in male patients. Additionally, obesity is negatively correlated with mortality in patients with acute respiratory distress syndrome [47, 48]. Similarly, in the study, the associations were nonlinear and below the ABSI threshold, and an increased ABSI did not negatively impact respiratory function. It is speculated that a lower ABSI value not only reflects lower abdominal fat but also indicates low body weight or malnutrition. At this stage, an increase in ABSI may lead to not only the accumulation of abdominal fat but also an increase in skeletal muscle. As skeletal muscle is positively associated with respiratory function [49], an increase in ABSI below the threshold does not temporarily decrease PEF. In addition, increased chest wall elastance in obese patients may provide protective benefits by partially absorbing transpulmonary pressure, thereby reducing ventilator-induced lung injury [50]. These results were consistent with the findings.
The study involved a substantial cross-sectional analysis of a Chinese population. It employed the ABSI formula to evaluate the association between abdominal obesity and PEF, thereby providing a robust assessment of respiratory function. Through the objective measurement of various anthropometric indices, the independent impact of ABSI on PEF was assessed, with the reliability of the findings supported by a sensitivity analysis. The findings indicate that ABSI thresholds can be effectively integrated into clinical guidelines for managing obesity and assessing respiratory risk, with adjustments for sex differences. This allows for more personalized and targeted interventions. Additionally, the study provides valuable insights into public health policies to reduce abdominal obesity among middle-aged and older adults, crucial for preserving respiratory function and enhancing health outcomes. The cost-effectiveness of PEF as a screening tool was also highlighted in the study, emphasizing its potential as a practical approach for the early detection and management of respiratory issues associated with obesity, especially in resource-limited settings.
This study had some limitations. First, while adjusting for multiple covariates in the statistical model, residual confounding variables might still affect the observed relationship linking ABSI to respiratory function, including unmeasured parameters like nutritional intake patterns and exercise frequency metrics. In addition, due to differences in baseline data between the included and excluded participants, it was not possible to exclude the effect of non-random missing data on the results. Second, while the primary focus is on the relationship between abdominal obesity and pulmonary function, it should be acknowledged that impaired respiratory capacity could theoretically exacerbate adiposity due to limitations in physical activity. However, Due to the cross-sectional nature of this study data, causal inferences cannot be made. Therefore, longitudinal studies are necessary to understand the temporal dynamics. Future research may benefit from employing Mendelian randomization or mediation analyses to investigate bidirectional pathways, particularly within populations exhibiting early-stage respiratory dysfunction. Third, the CHARLS cohort study was limited by the absence of detailed data on medication usage, such as bronchodilators and beta-blockers, which could potentially impact respiratory function. Although adjustments were made for hypertension and cardiovascular diseases—conditions frequently managed with these medications—there remains a possibility of unaccounted drug-specific effects. Furthermore, PEF measurements were not standardized to a specific time of day (e.g., morning versus afternoon), which may have introduced variability due to diurnal fluctuations in respiratory function. The lack of additional spirometry indicators, such as forced expiratory volume in one second (FEV1) and forced vital capacity (FVC), further compounds these limitations. Consequently, the findings should be interpreted with caution due to potential pharmacological confounding, temporal variability, and limited respiratory function metrics. Future research should aim to include comprehensive pharmacotherapy records and standardize measurement protocols, including both timing and spirometry panels, to enhance the robustness of the results. Fourth, data from the 2015 CHARLS database were used. Future studies should include larger sample sizes, more diverse ethnic groups, and extended multicenter cohorts to confirm and replicate these findings.
Conclusions
Sex-specific ABSI thresholds enhance the screening process for abdominal obesity among Chinese middle-aged and older adults, thereby aiding in the early identification of respiratory risks. The incorporation of these thresholds into clinical practice promotes personalized interventions and cost-effective monitoring. Future research should aim to validate the causal relationships and generalize these thresholds across diverse populations to optimize the management of obesity-related respiratory conditions.
Supplementary Information
Supplementary Material 1: Supplemental Table 1. displays the variables and extent of missingness. Supplemental Table 2. Association of ABSI quartiles with PEF/PEF prediction in the total population (Multiple imputation). Supplemental Table 3. Association of three components (Height, Weight, Waist) of ABSI and PEF. Supplementary Table 4. Association of ABSI and PEF/PEF prediction (dummy variables). Supplemental Table 5. Association of ABSI quintile with PEF/PEF prediction in the total population (Exclude patients with chronic pulmonary disease).
Acknowledgements
The authors thank the CHARLS staff, investigators, and participants. Thanks to the Free Statistics team for providing technical assistance and valuable tools for data analysis and visualization. We thank Dr. Liu jie (People’s Liberation Army of China General Hospital, Beijing, China) for helping in this revision.
Abbreviations
- PEF
Peak expiratory flow
- ABSI
A Body Shape Index
- CHARLS
China Health and Retirement Longitudinal Study
- COPD
Chronic obstructive pulmonary disease
- BMI
Body mass index
- STROBE
STrengthening the Reporting of OBservational studies in Epidemiology
- AL
Airflow limitation
- SAL
Severe airflow limitation
- LDL
Low-density lipoprotein
- TC
Total cholesterol
- TG
Triglycerides
- OR
Odds ratios
- CI
Confidence interval
- SD
Standard deviation
- IQR
Interquartile range
- WC
Waist circumference
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
Authors’ contributions
GSJ: data collection, data analysis, manuscript writing. LCF: data collection, XXQ, JW: data analysis, manuscript editing. YL: project development, manuscript editing. All authors have read and approved this manuscript.
Funding
No funding was received for conducting this study.
Data availability
The data that support the findings of this study are available in China Health and Retirement Longitudinal Study. (https://charls.pku.edu.cn/).
Declarations
Ethical approval and consent to participate
Ethical approval was obtained from the Institutional Review Boards of Peking University (No. IRB00001052-11015) for the CHARLS, and all participants gave informed consent.
Consent for publication
All authors have reviewed the final version of the manuscript and approve its submission for publication.
Competing interests
The authors declare no competing interests.
Footnotes
"The original online version of this article was revised: the authors reported a mistake with the authorship. Guosong Jiang is not a corresponding author."
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/1/2025
"The original online version of this article was revised: the authors reported a mistake with the authorship. Guosong Jiang is not a corresponding author."
Change history
8/27/2025
A Correction to this paper has been published: 10.1186/s12944-025-02678-4
References
- 1.Vijg J, Suh Y. Genome instability and aging. Annu Rev Physiol. 2013;75:645–68. [DOI] [PubMed] [Google Scholar]
- 2.Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med. 2019;7(4):358–64. [DOI] [PubMed] [Google Scholar]
- 3.Donahue PT, Balasubramanian A, Davoudi A, Wanigatunga AA, Schrack JA, Carlson MC. Population reference equations for handheld peak expiratory flow in older U.S. adults. Respir Med. 2024;234:107811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grande G, Li Y, Trevisan C, et al. Lung function in relation to brain aging and cognitive transitions in older adults: a population-based cohort study. Alzheimers Dement. 2024;20(8):5662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He YY, Jin ML, Chang J, Wang XJ. Associations of sarcopenia with peak expiratory flow among community-dwelling elderly population: based on the China Health and Retirement Longitudinal Study (CHARLS). Eur Geriatr Med. 2024;15(1):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halpin DMG, Meltzer EO, Pisternick-Ruf W, et al. Peak expiratory flow as an endpoint for clinical trials in asthma: a comparison with FEV1. Respir Res. 2019;20(1):159. Published 2019 Jul 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Zhao L, Gao L, Pan A, Xue H. Health policy and public health implications of obesity in China. Lancet Diabetes Endocrinol. 2021;9(7):446–61. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Wu W, Mao Z, et al. Prevalence and influencing factors of overweight and obesity in a Chinese rural population: the Henan Rural Cohort Study. Sci Rep. 2018;8(1):13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leone N, Courbon D, Thomas F, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179(6):509–16. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Zhu L, Wei Y, et al. Association between adiposity measures and COPD risk in Chinese adults. Eur Respir J. 2020;55(4):1901899. Published 2020 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park Y, Kim J, Kim YS, et al. Longitudinal association between adiposity changes and lung function deterioration. Respir Res. 2023;24(1):44. Published 2023 Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7(7):e39504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji M, Zhang S, An R. Effectiveness of A Body Shape Index (ABSI) in predicting chronic diseases and mortality: a systematic review and meta-analysis. Obes Rev. 2018;19(5):737–59. [DOI] [PubMed] [Google Scholar]
- 14.Yang N, Zhuo J, Xie S, et al. A body shape index and its changes in relation to all-cause mortality among the chinese elderly: a retrospective cohort study. Nutrients. 2023;15(13):2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim B, Kim G, Kim E, et al. The a body shape index might be a stronger predictor of chronic kidney disease than BMI in a senior population. Int J Environ Res Public Health. 2021;18(24):12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piché ME, Vasan SK, Hodson L, Karpe F. Relevance of human fat distribution on lipid and lipoprotein metabolism and cardiovascular disease risk. Curr Opin Lipidol. 2018;29(4):285–92. [DOI] [PubMed] [Google Scholar]
- 17.Piché ME, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: an update. Prog Cardiovasc Dis. 2018;61(2):103–13. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. [DOI] [PubMed] [Google Scholar]
- 20.Cheung YB. “A Body Shape Index” in middle-age and older Indonesian population: scaling exponents and association with incident hypertension. PLoS One. 2014;9(1):e85421. Published 2014 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Han Y, Chen T, Han Y. A body shape index constructed and its association with blood pressure among Chinese adults. Chin J Public Health. 2020;36(04):588–91. [Google Scholar]
- 22.Pulmonary function professional group of respiratory branch of Chinese medical association. Guideline of pulmonary function testing—peak expiratory flow and its variability. Chin J Tubere Respir Dis. 2017;40(6):426–30. [Google Scholar]
- 23.Li J, Sun Q, Zhang H, et al. Serum-Creatinine-to-Cystatin C-to-Waist-Circumference ratios as an indicator of severe airflow limitation in older adults. J Clin Med. 2023;12(22):7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu YE, Chen SC, Geng JH, Wu DW, Wu PY, Huang JC. Obesity-related indices are associated with longitudinal changes in lung function: a large taiwanese population follow-up study. Nutrients. 2021;13(11):4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park S, Jung SY, Kwon JW. Sex differences in the association between asthma incidence and modifiable risk factors in Korean middle-aged and older adults: NHIS-HEALS 10-year cohort. BMC Pulm Med. 2019;19(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng X, Liu D, An Z, Li H, Song J, Wu W. Obesity parameters in relation to lung function levels in a large Chinese rural adult population. Epidemiol Health. 2021;43:e2021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y, Wu X, Zhao Y, et al. Relationships between muscle strength, lung function, and cognitive function in Chinese middle-aged and older adults: A study based on the China health and retirement longitudinal study (CHARLS). J Formos Med Assoc. Published online April 8, 2024. [DOI] [PubMed]
- 28.Chen JH, Chen JY, Chen YC, Li WC. Sex difference in the association between creatinine-to-cystatin C ratio and metabolic syndrome among Chinese adults [published correction appears in Front Endocrinol (Lausanne). 2024 Oct 17;15:1502904. Front Endocrinol (Lausanne). 2024;15:1389295. [DOI] [PMC free article] [PubMed]
- 29.Wang K, Jia S, Zhao W, Ge M, Dong B. The creatinine-to-cystatin C ratio (a surrogate marker of muscle mass) as a predictor of lung function decline in older adults: a nationwide longitudinal study in China. Respir Med. 2023;211:107197. [DOI] [PubMed] [Google Scholar]
- 30.van de Bool C, Rutten EP, Franssen FM, Wouters EF, Schols AM. Antagonistic implications of sarcopenia and abdominal obesity on physical performance in COPD. Eur Respir J. 2015;46(2):336–45. [DOI] [PubMed] [Google Scholar]
- 31.Lynch GM, Murphy CH, Castro EM, Roche HM. Inflammation and metabolism: the role of adiposity in sarcopenic obesity. Proc Nutr Soc. Published online July 16, 2020. [DOI] [PubMed]
- 32.Cava E, Yeat NC, Mittendorfer B. Preserving healthy muscle during weight loss. Adv Nutr. 2017;8(3):511–9. Published 2017 May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMurray RG, Ondrak KS. Effects of being overweight on ventilatory dynamics of youth at rest and during exercise. Eur J Appl Physiol. 2011;111(2):285–92. [DOI] [PubMed] [Google Scholar]
- 34.O’Donnell DE, O’Donnell CD, Webb KA, Guenette JA. Respiratory consequences of mild-to-moderate obesity: impact on exercise performance in health and in chronic obstructive pulmonary disease. Pulm Med. 2012;2012:818925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ora J, Laveneziana P, Wadell K, Preston M, Webb KA, O’Donnell DE. Effect of obesity on respiratory mechanics during rest and exercise in COPD. J Appl Physiol (1985). 2011;111(1):10–9. [DOI] [PubMed] [Google Scholar]
- 36.Yücel ÜÖ, Çalış AG. The relationship between general and abdominal obesity, nutrition and respiratory functions in adult asthmatics. J Asthma. 2023;60(6):1183–90. [DOI] [PubMed] [Google Scholar]
- 37.Dixon AE, Poynter ME. Mechanisms of asthma in obesity. Pleiotropic aspects of obesity produce distinct asthma phenotypes. Am J Respir Cell Mol Biol. 2016;54(5):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lambert GW, Schlaich MP, Eikelis N, Lambert EA. Sympathetic activity in obesity: a brief review of methods and supportive data. Ann N Y Acad Sci. 2019;1454(1):56–67. [DOI] [PubMed] [Google Scholar]
- 39.Camoretti-Mercado B, Lockey RF. Airway smooth muscle pathophysiology in asthma. J Allergy Clin Immunol. 2021;147(6):1983–95. [DOI] [PubMed] [Google Scholar]
- 40.Isono S. Obesity and obstructive sleep apnoea: mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology. 2012;17(1):32–42. [DOI] [PubMed] [Google Scholar]
- 41.Kimura H, Ota H, Kimura Y, Takasawa S. Effects of Intermittent Hypoxia on Pulmonary Vascular and Systemic Diseases. Int J Environ Res Public Health. 2019;16(17):3101. Published 2019 Aug 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsson L, Degens H, Li M, et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. 2019;99(1):427–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gea J, Ausín P, Martínez-Llorens JM, Barreiro E. Respiratory muscle senescence in ageing and chronic lung diseases. Eur Respir Rev. 2020;29(157):200087. Published 2020 Sep 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su SY, Lin TH, Liu YH, et al. Sex difference in the associations among obesity-related indices with hyperuricemia in a large taiwanese population study. Nutrients. 2023;15(15):3419. Published 2023 Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato Y, Fujimoto S, Konta T, et al. Body shape index: sex-specific differences in predictive power for all-cause mortality in the Japanese population. PLoS One. 2017;12(5):e0177779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–17. [DOI] [PubMed] [Google Scholar]
- 47.Tea K, Zu Y, Chung CH, et al. The relationship between metabolic syndrome and mortality among patients with acute respiratory distress syndrome in acute respiratory distress syndrome network and prevention and early treatment of acute lung injury network trials. Crit Care Med. 2024;52(3):407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudym D, Pham T, Rackley CR, et al. Mortality in patients with obesity and acute respiratory distress syndrome receiving extracorporeal membrane oxygenation: the multicenter ECMObesity study. Am J Respir Crit Care Med. 2023;208(6):685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park CH, Yi Y, Do JG, Lee YT, Yoon KJ. Relationship between skeletal muscle mass and lung function in Korean adults without clinically apparent lung disease. Medicine (Baltimore). 2018;97(37):e12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ball L, Serpa Neto A, Pelosi P. Obesity and survival in critically ill patients with acute respiratory distress syndrome: a paradox within the paradox. Crit Care. 2017;21(1):114. Published 2017 May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplemental Table 1. displays the variables and extent of missingness. Supplemental Table 2. Association of ABSI quartiles with PEF/PEF prediction in the total population (Multiple imputation). Supplemental Table 3. Association of three components (Height, Weight, Waist) of ABSI and PEF. Supplementary Table 4. Association of ABSI and PEF/PEF prediction (dummy variables). Supplemental Table 5. Association of ABSI quintile with PEF/PEF prediction in the total population (Exclude patients with chronic pulmonary disease).
Data Availability Statement
The data that support the findings of this study are available in China Health and Retirement Longitudinal Study. (https://charls.pku.edu.cn/).