Abstract
Objective
This study aimed to investigate whether endometrial thickness (EmT) change prognosticates the ongoing pregnancy rate (OPR) in hormonally prepared frozen-thawed embryo transfers (FETs) in women with treated intrauterine adhesion (IUA).
Methods
We prospectively examined 261 FET cycles in women with IUA. Ultimately, 156 patients were included in the final analysis. The primary outcome was OPR. The association between the EmT change ratio and OPR, as well as the relationship between the EmT change and serum hormone concentration, was analyzed.
Results
The intraclass correlation coefficient for repeated EmT measurements was 0.944 (95% CI: 0.933–0.954, P < 0.001). Subdividing by the expansion cutoff from 5% to 15%, the 10% expansion group had the highest OPR with optimal sensitivity and specificity. Regarding the baseline characteristics, there were no statistically significant differences between the two groups. Nevertheless, the OPR increased significantly in cycles with endometrial expansion ≥ 10% compared to those with no expansion (55.3% vs 26.3%, P=0.001). The difference was still significant after adjustment between the two groups (adjusted OR, 3.74; 95% CI 1.68–8.34, P=0.001). No correlation was found between the EmT change and serum hormone concentrations.
Conclusion
Endometrial expansion was significantly correlated with higher OPR in women with treated IUA in the hormonal protocol for FET.
Keywords: endometrial thickness, frozen embryo transfer, intrauterine adhesion, pregnancy outcomes, progesterone
Introduction
The human endometrium experiences dramatic histological changes from the proliferative through the secretory phase under hormonal control of the ovary to prepare itself for successful embryo implantation, which involves visible intraluminal secretion, stromal edema, and prominence of spiral arterioles in the mid-secretory stage.1 This limited span is termed the “window of implantation” (WOI), which refers to the transient timeframe when an endometrium becomes receptive to blastocysts.2 At present, displacement of the WOI can be detected using genetic testing tools,3,4 but transvaginal ultrasound is still a staple of endometrial receptivity assessment in infertility care.
Intrauterine adhesion (IUA) is characterized by fibrous strips across the inner wall of the uterus, which affects approximately 6% of women undergoing assisted reproductive technology.5,6 This pathology can be asymptomatic and remains undiscovered, but frequently leads to menstrual disturbances and fertility disorders. A relatively thin endometrium is a key clinical manifestation of dysfunctional endometria in most patients.
The live birth rate declined significantly following a decrease in endometrial thickness (EmT) below 7 mm in frozen-thawed embryo transfers (FETs).7 In clinical practice, we noted that the EmT in some patients with IUA may decrease dramatically on the day of embryo transfer (ET), which raises the concern of proceeding forward with ETs. Studies investigating the effect of changes in EmT after progesterone supplementation on reproductive outcomes are heterogeneous.8–10 In hormone replacement therapy (HRT)-FET cycles, endometrial compaction (thinness from the endometrial transformation day to the day of ET) was associated with an increased ongoing pregnancy rate,8 but the same association was not detected in another study.9 A study of 3091 single frozen-thawed blastocyst transfer cycles showed that an expansion in EmT after progesterone administration was correlated with a higher pregnancy rate.10 The aforementioned studies only included women without IUA. To date, no study has explored the effect of the EmT response to progesterone treatment in patients with IUA.
The objective of our study was to assess the influence of EmT changes in response to progesterone on reproductive outcomes in postoperative patients with IUA undergoing hormonally prepared frozen-thawed blastocyst transfer cycles.
Materials and Methods
Study Design and Population
A single-center, prospective cohort study of 261 patients with postoperative IUA undergoing their first FET cycle was conducted from December 2020 to August 2023 at the Reproductive Medicine Center of Xiangya Hospital. Ultimately, a total of 156 patients were included in the final analysis. This study was observational with no intervention and was approved by the Ethics Committee of the Reproductive Medicine Center of Xiangya Hospital (reference number 2020010), which complies with the Declaration of Helsinki. The informed consents were obtained before patients’ participation.
The inclusion criteria were as follows: (1) women with an IUA history and IUA were identified and treated by hysteroscopy; (2) the AFS score was less than or equal to 4 before embryo transfer; (3) all included women were invited to receive a hormone replacement protocol for their first frozen-thawed blastocyst transfer cycles after adhesiolysis; and (4) EmT ≥ 6 mm or a trilaminar endometrial pattern (EmP) presented on ultrasound, estradiol concentration ≥ 100 pg/mL, and progesterone concentration < 1 ng/mL on the day of progesterone administration. No age or body mass index (BMI) limit was applied when enrolling the patients.
Women were excluded from this study if they had untreated hydrosalpinx, endometrial polyps, endometritis, stage III–IV endometriosis, or uterine fibroids protruding into the endometrial cavity. Patients who did not undergo ET were excluded from the study. In cases where the EmT was not accurately measured, the cycle was excluded from further analysis.
Endometrial Preparation and EmT Assessment
The patient received a hormonal protocol for FET. For the hormonal protocol, patients were administered 6 mg/day estradiol valerate (Progynova, Delpharm Lille) from the third day of menstruation, which may increase after six days to 8 mg/day for an additional six days based on the patients’ EmT. Estradiol valerate was administered for at least 12 days. When the endometrium reached at least 6 mm or presented with a triple-layer pattern, luteal phase support was initiated. Vaginal progesterone capsules (Utrogestan, Besins Healthcare, France) at a dose of 200 mg three times daily and oral progesterone capsules at a dose of 200 mg daily were administered to patients until the day of serum hCG testing. Blastocysts were transferred on day 6 of progesterone administration, and hormone levels were retested. The uterus was displayed in a sagittal plane with the cervical canal visible on ultrasound when assessing the EmT. The EmT was measured using an electronic caliper, and the images were stored. The EmT evaluation was repeated thrice, with the probe reinserted into the vagina for every new scan. The mean of three consecutive measurements was calculated for the data analysis. All EmT assessments were performed transvaginally by Dr. Y. L. (with over 20 years of two-dimensional ultrasound experience) using an 8-mHZ transducer (GE Voluson S6, USA) under standardized operation guidelines. EmT was defined as the maximum value between the two opposing walls of the endometrium-myometrium interfaces.
Blastocysts were assessed by an embryologist, based on the evaluation system proposed by Gardner.11 The blastocysts were categorized into three quality groups based on published literature: good (3–6AA/AB/BA), fair (3–6BB), and poor (3–6BC/CB).12
We calculated the difference between the EmT on the day of ET and on the day of progesterone addition. The change ratio of EmT was computed as the difference between EmT on the ET day and progesterone addition day divided by the EmT on the progesterone addition day. Patients were stratified into an expansion ≥ 10% group (those whose EmT change ratio was calculated to increase ≥ 10%) and a no-expansion group (those whose EmT change ratio was calculated to increase < 10%) according to the optimal cutoff calculated using receiver operating characteristic (ROC) curve analysis.
Definitions of Reproductive Outcomes
The primary outcome was the ongoing pregnancy rate, which was defined as viable pregnancy at 12 weeks of gestation. Secondary outcomes included positive β-hCG (defined as a serum concentration of beta-hCG exceeding 10 mIU/mL), clinical pregnancy (visualization of a gestational sac or heartbeats on ultrasound at 6 weeks of gestation), implantation rate (the percentage of visible gestational sacs among transferred embryos at the clinical pregnancy stage), and early spontaneous abortion (spontaneous loss of intrauterine gestation before 12 weeks of gestation).
Statistical Analysis
Data were tested for normality using the Shapiro–‒Wilk test before statistical inference. Continuous variables were expressed as mean ± standard deviation or median (interquartile range, IQR) according to normality or non-normality of the data and were compared using Student’s t-test or Mann–Whitney U-test. Categorical data were expressed as frequencies (percentages) and compared using the chi-square test.
The reliability of repeated EmT measurements was evaluated using the intraclass correlation coefficient (ICC) with two-way random effects and absolute agreement. The ICC was reported based on a single measurement. The agreement between repeated EmT measurements (the first and second measurements) was assessed using Bland–Altman plots with limits of agreement (LoA).
The sensitivity and specificity for patients whose endometrial thickness did not expand or expanded by 5%, 10%, or 15% were obtained via ROC curve analysis. Univariate logistic regression analysis was performed to assess the effects of various confounders on the reproductive outcomes. In univariate logistic analysis, hormone levels were categorized into two groups based on their median concentrations. Covariates with a significance level of P ≤ 0.2 in the univariate logistic analysis, as well as those regarded as potential confounding factors based on published studies and clinical experience, were included in the multiple adjustment model. The EmT change, age, BMI, number of adhesiolyses, estradiol and progesterone level on the day of starting progesterone, and estradiol/progesterone ratio on the day of starting progesterone and ET were included as adjusted variables. The EmT change was inputted into the models as a grouped variable (expanded ≥ 10% vs expanded < 10%).
The relationship between hormone concentration and EmT changes (differences and ratios) was explored using Spearman’s rank-order correlation coefficients.
All statistical analyses were conducted using the IBM Statistical Package for the Social Sciences (version 26.0; IBM Corp., USA). Differences were considered statistically significant at a two-sided P < 0.05.
Results
Measurement Reliability and Agreement
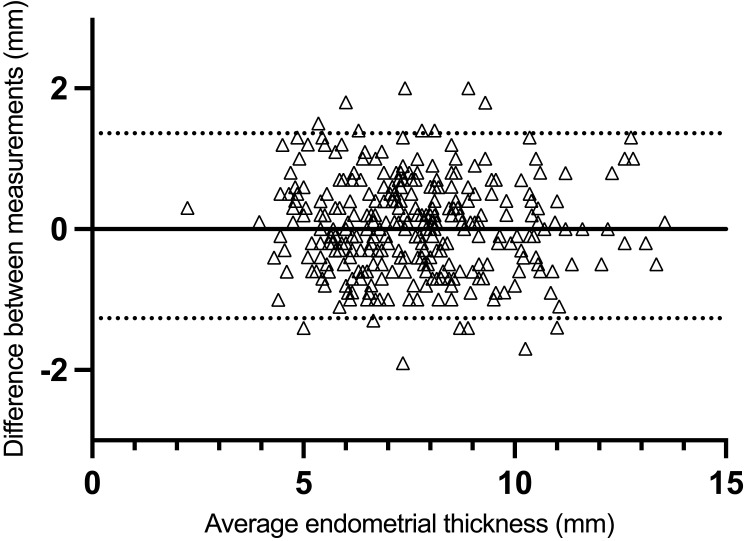
The ICC for repeated EmT measurements was 0.944 (95% CI: 0.933–0.954, P < 0.001), indicating good reliability among the three EmT measurements performed by a single rater. As previously reported, ICC values should be ≥ 0.9 when applying this method in clinical practice.13 In addition, 95% LoA was −1.27 to 1.36 (Figure 1).
Figure 1.
Bland-Altman plots for intraobserver agreement of measurement of endometrial thickness by ultrasound. Horizontal lines show estimates of lower and upper limits of agreement.
Characteristics of the Population
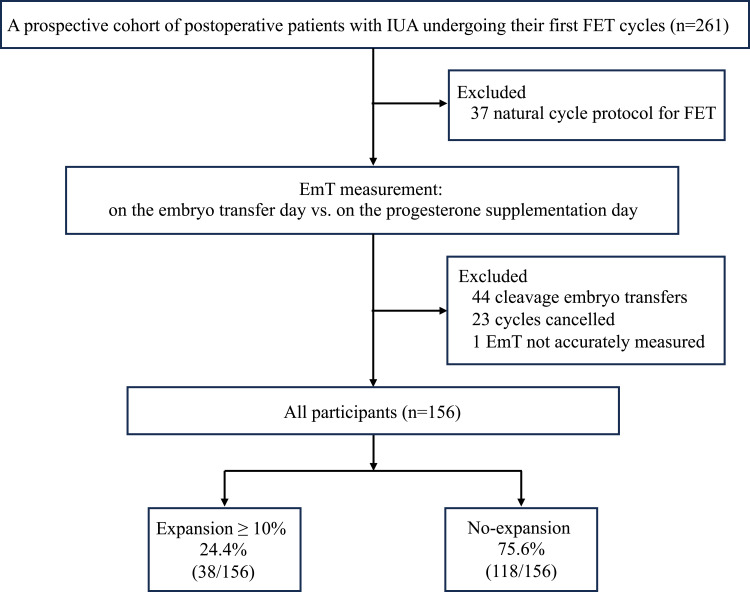
We prospectively enrolled 261 postoperative patients with IUA who underwent their first FET cycle after hysteroscopic adhesiolysis (Figure 2). A total of 105 patients were excluded from this study; 37 used a natural cycle protocol for endometrial preparation, 44 underwent cleavage embryo transfers, and 23 had their cycles cancelled because of poor EmT and EmP. Another patient was excluded because of multiple areas of visible uterine fluid on ultrasound, which prevented accurate recording of the endometrial lining, leaving 156 patients included in the final study.
Figure 2.
Flowchart of patients.
Abbreviations: EmT, endometrial thickness; FET, frozen-thawed embryo transfer; IUA, intrauterine adhesion.
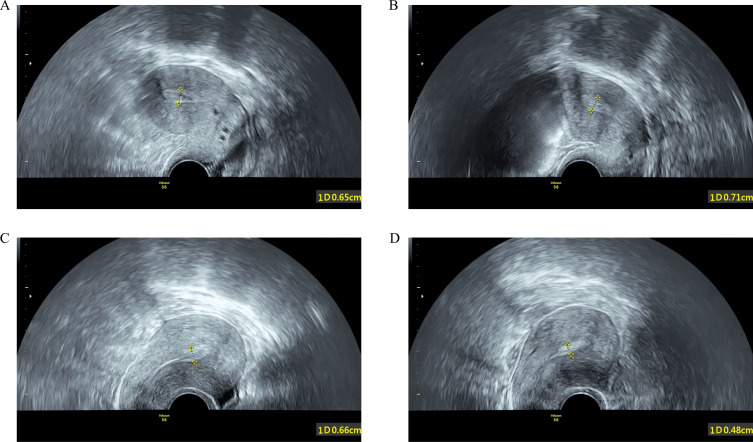
Among all FET cycles, 24.4% (38/156) of the women had an EmT change ratio ≥ 10% (categorized as the expansion ≥ 10% group) (Figure 3A and B), and 75.6% (118/156) of the patients had an EmT change ratio < 10% (categorized as the no-expansion group) (Figure 3C and D). Furthermore, 36.5% (57/156) and 64.7% (101/156) of the cycles had EmTs less than 7 mm and 8 mm, respectively, in the estrogen-only phase. A total of 40.4% (63/156) and 62.2% (97/156) of the cycles had EmTs less than 7 mm and 8 mm on the ET day, respectively. Additionally, 89.1% (139/156) of patients were categorized as having moderate-to-severe IUA based on the AFS score on the initial surgery. Among the 156 cycles, 45.5% (71/156) achieved clinical pregnancy and 33.3% (52/156) achieved ongoing pregnancy.
Figure 3.
Ultrasound images of the endometrium. Endometrial expansion measured on the progesterone administration day (A) and on the embryo transfer day (B) from the same cycle in one patient. Endometrial compaction measured on the progesterone administration day (C) and on the embryo transfer day (D) from the same cycle in another patient. All images were taken by transvaginal grayscale ultrasound on a sagittal plane.
Relationship Between EmT Change and Ongoing Pregnancy Rate
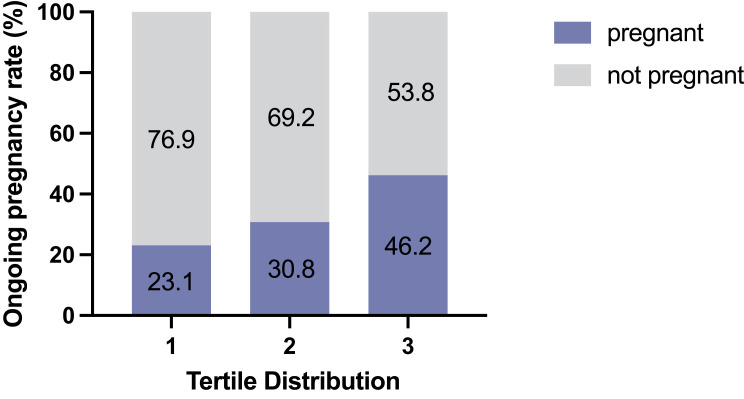
The EmT change ratios were used to distribute expansion changes into tertiles. The ongoing pregnancy rate increased with the increasing tertile of EmT change (increased percentage of expansion) (Figure 4).
Figure 4.
The endometrial thickness changes were divided into tertiles. Tertile 3 represents the most endometrial expansion and tertile 1 represents the most endometrial compaction.
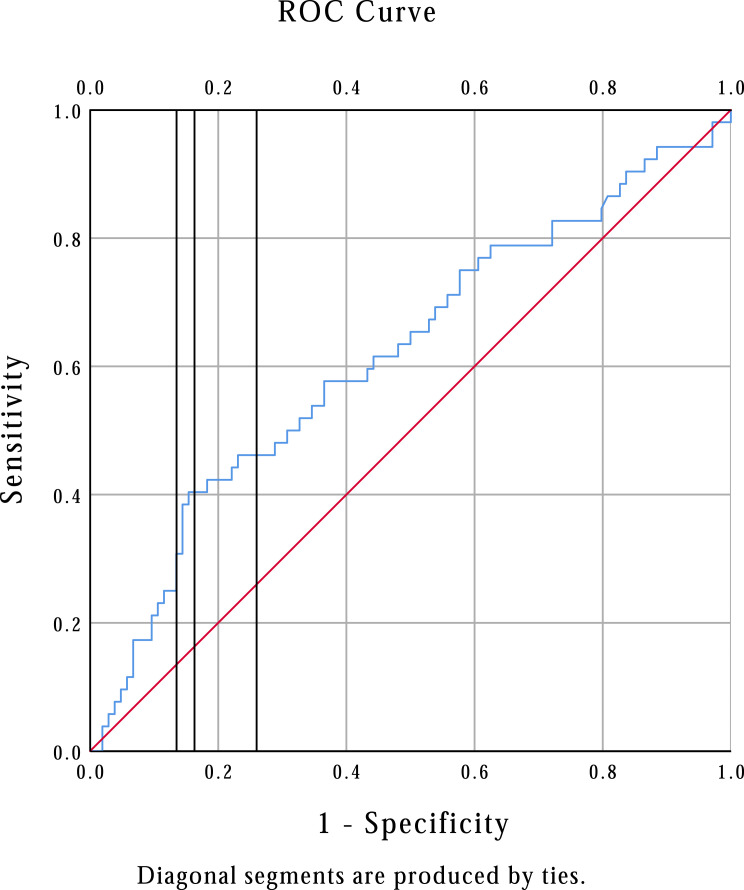
When defining our groups at expansion cutoffs from 5% to 15%, the ongoing pregnancy rate was significantly higher at all expansion cutoffs (Table 1). Sensitivity and specificity for each level of endometrial expansion calculated from the ROC curve are shown in Figure 5. Given a sensitivity of 40.4% and specificity of 83.7%, the 10% expansion level was chosen as the cut-off for subsequent analysis.
Table 1.
Ongoing Pregnancy Rate at Different Degrees of Expansion From 5% to 15%
| Expansion | Ongoing Pregnancy | P | Sensitivity | Specificity |
|---|---|---|---|---|
| ≥5% (n=51) | 24 (47.1%) | 0.011 | 0.462 | 0.740 |
| <5% (n=105) | 28 (26.7%) | |||
| ≥10% (n=38) | 21 (55.3%) | 0.001 | 0.404 | 0.837 |
| <10% (n=118) | 31 (26.3%) | |||
| ≥15% (n=29) | 15 (51.7%) | 0.020 | 0.288 | 0.865 |
| <15% (n=127) | 37 (29.1%) |
Figure 5.
Receiver operating characteristic (ROC) curve for endometrial expansion and ongoing pregnancy rate. Vertical lines from left to right represent 15%, 10%, and 5% expansion. The area under the curve is 0.618, 95% confidence interval 0.522–0.714; P=0.016.
Univariate logistic analysis was employed to examine the effect of each parameter on ongoing pregnancy rate (Supplemental Table 1). Briefly, the serum estradiol/progesterone ratio on the FET day was negatively correlated with the ongoing pregnancy rate (odds ratio [OR] 0.440, 95% CI 0.218–0.888; P = 0.022). Nevertheless, EmT on the ET day was positively correlated with ongoing pregnancy rate (OR 1.211, 95% CI 1.028–1.425; P = 0.022). Endometrial expansion, defined as an increase of ≥ 10% in the EmT change ratio, significantly increased the ongoing pregnancy rate (OR 3.467, 95% CI 1.622–7.410; P = 0.001).
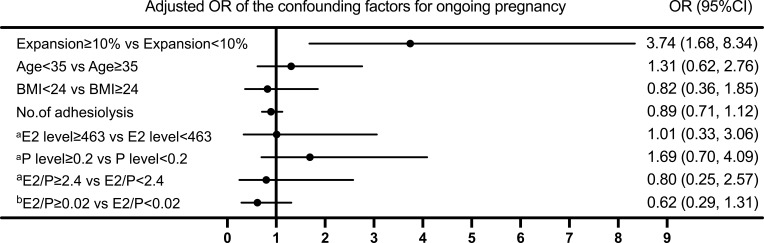
A multivariate logistic regression model was used to investigate the correlation between the EmT change ratio and the ongoing pregnancy rate (Figure 6). When the EmT change ratio was stratified into two groups and taking the <10% expansion group as a reference, the ≥10% expansion group had a positive correlation with the ongoing pregnancy rate in the adjusted model (OR 3.74, 95% CI 1.68–8.34; P = 0.001).
Figure 6.
Risk factors for ongoing pregnancy of IUA patients in hormonally prepared frozen-thawed embryo transfer cycles. aon the progesterone initiation day. bon the embryo transfer day.
Abbreviations: BMI, body mass index; E2, estradiol; P, progesterone; OR, odds ratio.
Comparison Between Groups
The baseline characteristics and reproductive outcomes between the expansion ≥ 10% group and no-expansion group are presented in Table 2. The two groups were comparable in baseline characteristics, such as age, BMI, etiology of fertility treatment, infertility type and duration, ovarian function tests, IUA etiology, number of miscarriages and adhesiolysis, maximum IUA score, EmT and EmP on the endometrial transformation day, hormone levels on the endometrial transformation and ET days, number of embryos transferred, and rate of good-quality embryos transferred. Nevertheless, the EmT on the ET day was greater in the expansion ≥ 10% group than in the no-expansion group (9.3 mm vs 7.0 mm, P < 0.001). Additionally, the difference in the EmT was 1.8 (1.2, 2.5) mm in the expansion ≥ 10% group and −0.50 (−1.2, 0.1) mm in the no-expansion group (P < 0.001). Regarding pregnancy outcomes, the expansion ≥ 10% group had a higher ongoing pregnancy rate (55.3% vs 26.3%, P = 0.001), positive β-hCG rate (71.1% vs 50.8%, P = 0.029), clinical pregnancy rate (63.2% vs 39.8%, P = 0.012), and implantation rate (61.9% vs 35.8%, P = 0.003) than the no-expansion group. There was no significant difference in the rate of early spontaneous abortion between the two groups.
Table 2.
Comparison of Baseline Characteristics and Reproductive Outcomes Between the Two Groups
| Parameter | Expansion ≥10% (n=38) | No expansion (n=118) | P |
|---|---|---|---|
| Age (y) | 34.1±5.2 | 34.3±3.7 | 0.75 |
| BMI (kg/m2) | 21.6±2.9 | 22.2±2.7 | 0.28 |
| ART etiology | 0.27 | ||
| Tubal | 20 (52.6) | 70 (59.3) | |
| Diminished ovarian reserve | 9 (23.7) | 13 (11.0) | |
| Preimplantation genetic testing | 4 (10.5) | 17 (14.4) | |
| Others | 5 (13.2) | 18 (15.3) | |
| Infertility type | 0.64 | ||
| Primary | 7 (18.4) | 26 (22.0) | |
| Secondary | 31 (81.6) | 92 (78.0) | |
| Infertility duration (y) | 4.0 (2.0, 6.3) | 4.0 (2.0, 7.0) | 0.78 |
| Basal serum FSH (mIU/mL) | 6.9 (5.7, 8.2) | 6.5 (5.6, 8.0) | 0.54 |
| Basal serum estradiol (pg/mL) | 49.9 (33.3, 63.8) | 42.0 (32.3, 56.6) | 0.26 |
| Basal serum LH (mIU/mL) | 5.2 (3.8, 9.5) | 5.1 (4.1, 6.7) | 0.93 |
| Anti-Müllerian hormone (ng/mL) | 2.3 (0.9, 3.7) | 2.5 (1.5, 4.2) | 0.21 |
| Antral follicle count (n) | 13.0 (7.0, 20.0) | 14.0 (8.0, 21.0) | 0.30 |
| IUA etiology | 0.59 | ||
| Intrauterine operation* | 30 (78.9) | 88 (74.6) | |
| Unknown | 8 (21.1) | 30 (25.4) | |
| No. of miscarriages | 1.0 (0.8, 2.0) | 1.0 (0.8, 2.0) | 0.84 |
| Maximum IUA score† | 8.0 (6.0, 9.3) | 8.0 (7.0, 10.0) | 0.47 |
| No. of adhesiolysis | 2.0 (1.0, 3.0) | 2.0 (2.0, 4.0) | 0.33 |
| EmT before P administration (mm) | 7.7 (6.6, 8.8) | 7.5 (6.6, 8.3) | 0.79 |
| EmP before P administration | 1.00 | ||
| A/B | 34 (89.5) | 107 (90.7) | |
| C | 4 (10.5) | 11 (9.3) | |
| Serum estradiol level (pg/mL)‡ | 462.8 (207.2, 693.0) | 460.4 (249.2, 846.4) | 0.33 |
| Serum progesterone level (ng/mL)‡ | 0.2 (0.1, 0.3) | 0.2 (0.1, 0.3) | 0.42 |
| Serum estradiol/progesterone ratio‡ | 2.4 (1.0, 5.1) | 2.4 (1.1, 5.4) | 0.85 |
| EmT on the ET day (mm) | 9.3 (8.3, 11.1) | 7.0 (5.6, 8.0) | <0.001 |
| EmP on the ET day | 0.51 | ||
| B | 5 (13.2) | 21 (17.8) | |
| C | 33 (86.8) | 97 (82.2) | |
| Serum estradiol level (pg/mL)§ | 241.1 (178.3, 380.6) | 232.0 (157.4, 436.3) | 0.91 |
| Serum progesterone level (ng/mL)§ | 15.9 (8.5, 19.7) | 14.3 (10.1, 19.5) | 0.85 |
| Serum estradiol/progesterone ratio§ | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.03) | 0.83 |
| ΔEmT¶ | 1.8 (1.2, 2.5) | −0.50 (−1.2, 0.1) | <0.001 |
| No. of embryos transferred | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | 0.40 |
| Quality of embryos transferred | 0.30 | ||
| Good | 4 (10.5) | 11 (9.3) | |
| Fair | 9 (23.7) | 44 (37.3) | |
| Poor | 25 (65.8) | 63 (53.4) | |
| Ongoing pregnancy rate | 21 (55.3) | 31 (26.3) | 0.001 |
| Positive β-hCG rate | 27 (71.1) | 60 (50.8) | 0.029 |
| Clinical pregnancy rate | 24 (63.2) | 47 (39.8) | 0.012 |
| Implantation rate n/N (%) | 26/42 (61.9) | 49/137 (35.8) | 0.003 |
| Early spontaneous abortion rate n/N (%) | 3/24 (12.5) | 16/47 (34.0) | 0.052 |
Note: Data are presented as mean ± SD or median (interquartile range), or n (%), unless otherwise indicated. * Intrauterine operation included curettage, induced labor, and transcervical resection of the septum. † Based on the AFS scoring system (1988). ‡ On the day of progesterone administration. § On the day of embryo transfer. ¶ΔEmT, difference in EmT between the embryo transfer day and starting progesterone day.
Abbreviations: ART, assisted reproductive technology; BMI, body mass index; EmT, endometrial thickness; EmP, endometrial pattern; ET, embryo transfer; FSH, follicle-stimulating hormone; IUA, intrauterine adhesion; LH, luteinizing hormone; P, progesterone.
Relationship between EmT Change and Hormone Concentrations
Associations between EmT changes (differences and ratios) and hormonal concentrations were analyzed using Spearman’s rank-order correlation coefficients (Table 3). There was no significant correlation between the EmT changes and serum hormone concentrations.
Table 3.
Spearman Rank-Order Correlations Between Hormone Concentrations and EmT Change
| Characteristics | EmT Change (mm) | EmT Change Ratio |
|---|---|---|
| Serum estradiol concentration (pg/mL)* | −0.040 | −0.055 |
| Serum progesterone concentration (ng/mL)* | −0.098 | −0.099 |
| Serum estradiol/ progesterone ratio* | 0.018 | 0.010 |
| Serum estradiol concentration (pg/mL)† | 0.079 | 0.063 |
| Serum progesterone concentration (ng/mL)† | −0.074 | 0.072 |
| Serum estradiol/ progesterone ratio† | 0.002 | −0.010 |
Notes: * On the day of progesterone administration. † on the day of embryo transfer. P > 0.05 in any of the correlations.
Discussion
To our knowledge, this is the first study to evaluate EmT changes after progesterone administration in women with treated IUA during the hormonal cycle of frozen-thawed blastocyst transfer. Women whose endometrium expanded by ≥ 10% after progesterone initiation had a dramatically higher ongoing pregnancy rate than those whose endometrium expanded by < 10%.
The relationship between EmT changes after progesterone initiation and pregnancy rates in fresh ET, specifically FET cycles in non-thin endometrial patients, has been investigated in the past few years with contradictory results.8,10,14–18 Bu et al and Jin et al from the same center investigated the relationship between EmT changes and reproductive outcomes in patients who underwent naturally prepared single frozen-thawed blastocyst transfers.10,15 Similar to our findings, they reported that an increase in the EmT after progesterone treatment resulted in better clinical outcomes. However, two previous studies revealed that the ongoing pregnancy rate significantly increased as the percentage of compaction increased.8,16 Their results were based on a second transabdominal ultrasound examination as opposed to the first transvaginal ultrasound examination. Interestingly, the results did not show the same correlation in the subjects with an initial lining (EmT before P administration) of 7–8 mm. Women with an EmT of < 7 mm were excluded from the analysis in previous studies. In the present study, only women with identified and treated IUA were recruited. In this population, we expected thin endometrial linings, but the median EmT on progesterone initiation day was greater than 7 mm in this cohort, which was surprising. There are four possible reasons for this phenomenon: First, if the endometrium was too thin, the cycle was cancelled and excluded from the analysis. Second, not all patients with IUA had thin endometria. Some patients, even those with severe IUA, may present with normal EmT after standard treatment via hysteroscopic adhesiolysis. Third, the average EmT on progesterone initiation day was approximately 10 mm in non-IUA patients as previously reported,19 which was far thicker than that in our study. Finally, a key factor may lie in the study’s selection criteria, which included only patients with a postoperative AFS score of less than or equal to 4, indicating mild IUA. This means that the selected patients already had a better prognosis for endometrial recovery compared to the broader postoperative IUA population, potentially giving them an advantage in EmT development.
One important reason for such contradictory studies lies in the retrospective nature of previous investigations. The reliability and agreement of the EmT measurements were not evaluated. Additionally, we speculate that the discrepancy between our study and others is due to the inclusion of a distinct population. The participants in our study were more likely to have an inadequate response to both estrogen and progesterone. A deficiency in sex hormone receptors has been confirmed in the endometrium of women with IUA.20,21 These patients usually have endometrium that is unresponsive or poorly responsive to hormone therapy. Single-cell transcriptome analysis of the thin endometrium revealed an impaired capacity of endometrial epithelial cells in the proliferative phase and dysfunctional endometrial mesenchymal cells in the secretory phase.22 Another possible explanation for this difference may be that the type, route, and dose of progesterone administered were heterogeneous among studies. It has been postulated that secretory histological endometrial development is driven by the duration of progesterone exposure rather than serum concentration.23 However, two other studies showed that the formulations and dosages of progesterone for luteal support affected the pharmacokinetic profiles of progesterone, inducing different frequencies of adequate secretory transformation of the endometrium.24,25 Moreover, a low progesterone concentration may lead to abnormal endometrial development, which is characterized by the differential expression of functional proteins.23 Intriguingly, researchers observed that endometrial thickness was thinner in the abnormally low progesterone group than in the normal progesterone group, although the difference was not statistically significant because of the sample size limitation. Although the serum progesterone concentrations were comparable between the expansion ≥ 10% group and the no-expansion group in our study, it is reasonable to question whether the progesterone concentrations in the endometrium were equal between the two groups. Given that the route of progesterone administration influences endometrial concentration,26 tissue progesterone levels may vary among studies. Labarta et al reported that a higher uterine concentration of progesterone was associated with a receptive endometrium, as determined by the ERA test.27
The EmT changes were divided into tertiles rather than quartiles because the median EmT change ratio was negative, indicating endometrial compaction, resulting in no obvious increase in ongoing pregnancy at the 50th percentile. In addition, we failed to detect an association between EmT changes and serum hormone concentrations, which is consistent with a previously published study,17 suggesting that there is currently no serum hormonal basis for EmT changes.
As for EmT on the ET day, it is a mediator variable rather than a confounding factor in this study. The EmT on the ET day is a component of the calculated difference of EmT change, and there is a mathematical dependency between the two variables, which does not meet the independence requirement for a confounding factor. Additionally, a previous study confirmed that the EmT on the ET day seemed to be not important for the success rate.28
The strengths of this study include its prospective nature, the homogeneous population of patients undergoing HRT cycles for frozen-thawed blastocyst transfers, the fact that all women were diagnosed with the postoperative state of IUA, the relatively more confounding factors that we collected, especially the hormone levels tested at the two separate timepoints, the highly significant differences in pregnancy outcomes, the endometrial measurements all performed by means of vaginal ultrasound, and the reliability and agreement of the three EmT measurements.
Limitations
Our study had some limitations. The limited sample size of this patient population was an intrinsic disadvantage of this study. Only those who underwent ET were included; consequently, the pregnancy rates might have been overestimated in this population. In addition, all included women had identified and treated for IUA, a specific group that is likely to have impaired endometrial function. Therefore, these findings should not be generalized to other patients undergoing FET cycles. Another limitation was that embryonic factors could not be excluded because not all embryos transferred were tested for aneuploidy by preimplantation genetic testing. However, the number of embryos transferred and embryo quality were similar between the two groups.
Conclusions
In summary, the expansion of the EmT before hormonal cycle frozen-thawed blastocyst transfers in women with postoperative IUA resulted in a marked increase in the ongoing pregnancy rate compared to women whose endometrial lining did not expand. A second ultrasound examination of the endometrium is necessary in these patients. Cycles should be considered for cancellations, if the endometrium does not expand.
Acknowledgments
We are grateful to the American Journal Experts for their professional manuscript services.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant number 82371682), the National Key Research and Development Program of China (grant no. 2021YFC2700404), and the National Natural Science Foundation of China (grant no. 81801537).
Data Sharing Statement
The data for this study are available from the corresponding author upon reasonable request.
Ethical Approval and Informed Consent
This study was observational with no intervention and was approved by the Ethics Committee of the Reproductive Medicine Center of Xiangya Hospital (reference number 2020010). Written informed consent was obtained before patients’ participation.
Author Contributions
B W and Y L are the corresponding authors. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1(1):3–25. doi: 10.1016/S0015-0282(16)30062-0 [DOI] [PubMed] [Google Scholar]
- 2.Beier-Hellwig K, Sterzik K, Bonn B, Beier HM. Contribution to the physiology and pathology of endometrial receptivity: the determination of protein patterns in human uterine secretions. Hum Reprod. 1989;4(suppl 1):115–120. doi: 10.1093/humrep/4.suppl_1.115 [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100(3):818–824. doi: 10.1016/j.fertnstert.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 4.He A, Zou Y, Wan C, et al. The role of transcriptomic biomarkers of endometrial receptivity in personalized embryo transfer for patients with repeated implantation failure. J Transl Med. 2021;19(1):176. doi: 10.1186/s12967-021-02837-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santamaria X, Mas A, Cervelló I, Taylor H, Simon C. Uterine stem cells: from basic research to advanced cell therapies. Hum Reprod Update. 2018;24(6):673–693. doi: 10.1093/humupd/dmy028 [DOI] [PubMed] [Google Scholar]
- 6.Magalhaes RS, Williams JK, Yoo KW, Yoo JJ, Atala A. A tissue-engineered uterus supports live births in rabbits. Nat Biotechnol. 2020;38(11):1280–1287. doi: 10.1038/s41587-020-0547-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu KE, Hartman M, Hartman A, Luo ZC, Mahutte N. The impact of a thin endometrial lining on fresh and frozen–thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod. 2018;33(10):1883–1888. doi: 10.1093/humrep/dey281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zilberberg E, Smith R, Nayot D, et al. Endometrial compaction before frozen euploid embryo transfer improves ongoing pregnancy rates. Fertil Steril. 2020;113(5):990–995. doi: 10.1016/j.fertnstert.2019.12.030 [DOI] [PubMed] [Google Scholar]
- 9.Jin Z, Shi H, Lu M, Bu Z, Huo M, Zhang Y. Endometrial thickness changes after progesterone administration do not affect the pregnancy outcomes of frozen-thawed euploid blastocyst transfer: a retrospective cohort study. Fertil Steril. 2021;116(6):1502–1512. doi: 10.1016/j.fertnstert.2021.08.008 [DOI] [PubMed] [Google Scholar]
- 10.Bu Z, Yang X, Song L, Kang B, Sun Y. The impact of endometrial thickness change after progesterone administration on pregnancy outcome in patients transferred with single frozen-thawed blastocyst. Reprod Biol Endocrinol. 2019;17(1):99. doi: 10.1186/s12958-019-0545-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner DK, Schoolcraft WB. In vitro culture of human blastocyst. In: Jansen R, Mortimer D, editors. Toward Reproductive Certainty: Infertility and Genetics Beyond 1999. Carnforth, United Kingdom: Parthenon Press; 1999:378–388. [Google Scholar]
- 12.Hou Z, He A, Zhang Q, et al. Endometrial fluid aspiration immediately prior to embryo transfer does not affect IVF/vitrified-warmed embryo transfer outcomes – a prospective matched cohort study. Reprod BioMedicine Online. 2022;44(3):486–493. doi: 10.1016/j.rbmo.2021.11.004 [DOI] [PubMed] [Google Scholar]
- 13.Coelho Neto MA, Roncato P, Nastri CO, Martins WP. True Reproducibility of UltraSound Techniques (TRUST): systematic review of reliability studies in obstetrics and gynecology. Ultrasound Obstet Gyne. 2015;46(1):14–20. doi: 10.1002/uog.14654 [DOI] [PubMed] [Google Scholar]
- 14.Ye J, Zhang J, Gao H, et al. Effect of endometrial thickness change in response to progesterone administration on pregnancy outcomes in frozen-Thawed embryo transfer: analysis of 4465 cycles. Front Endocrinol. 2020;11:546232. doi: 10.3389/fendo.2020.546232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin Z, Li J, Yang E, et al. Effect of endometrial thickness changes on clinical pregnancy rates after progesterone administration in a single frozen-thawed euploid blastocyst transfer cycle using natural cycles with luteal support for PGT-SR- and PGT-M-assisted reproduction: a retrospective cohort study. Reprod Biol Endocrinol. 2021;19(1):154. doi: 10.1186/s12958-021-00841-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas J, Smith R, Zilberberg E, et al. Endometrial compaction (decreased thickness) in response to progesterone results in optimal pregnancy outcome in frozen-thawed embryo transfers. Fertil Steril. 2019;112(3):503–509.e1. doi: 10.1016/j.fertnstert.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 17.Olgan S, Dirican EK, Sakinci M, et al. Endometrial compaction does not predict the reproductive outcome after vitrified–warmed embryo transfer: a prospective cohort study. Reprod BioMedicine Online. 2022;45(1):81–87. doi: 10.1016/j.rbmo.2022.02.025 [DOI] [PubMed] [Google Scholar]
- 18.Ju W, Wei C, Lu X, et al. Endometrial compaction is associated with the outcome of artificial frozen-thawed embryo transfer cycles: a retrospective cohort study. J Assist Reprod Genet. 2023;40(7):1649–1660. doi: 10.1007/s10815-023-02809-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youngster M, Mor M, Kedem A, et al. Endometrial compaction is associated with increased clinical and ongoing pregnancy rates in unstimulated natural cycle frozen embryo transfers: a prospective cohort study. J Assist Reprod Genet. 2022;39(8):1909–1916. doi: 10.1007/s10815-022-02544-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao Y, Sun H, Zhu H, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a Phase I clinical trial. Stem Cell Res Ther. 2018;9(1):192. doi: 10.1186/s13287-018-0904-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Shi L, Lin X, et al. Unresponsive thin endometrium caused by Asherman syndrome treated with umbilical cord mesenchymal stem cells on collagen scaffolds: a pilot study. Stem Cell Res Ther. 2021;12(1):420. doi: 10.1186/s13287-021-02499-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Li Y, Chen X, et al. Single‐cell transcriptome analysis uncovers the molecular and cellular characteristics of thin endometrium. FASEB J. 2022;36(3). [DOI] [PubMed] [Google Scholar]
- 23.Usadi RS, Groll JM, Lessey BA, et al. Endometrial development and function in experimentally induced luteal phase deficiency. J Clin Endocrinol Metab. 2008;93(10):4058–4064. doi: 10.1210/jc.2008-0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miles RA, Paulson RJ, Lobo RA, Press MF, Dahmoush L, Sauer MV. Pharmacokinetics and endometrial tissue levels of progesterone after administration by intramuscular and vaginal routes: a comparative study. Fertil Steril. 1994;62(3):485–490. doi: 10.1016/S0015-0282(16)56935-0 [DOI] [PubMed] [Google Scholar]
- 25.Blake EJ, Norris PM, Dorfman SF, Longstreth J, Yankov VI. Single and multidose pharmacokinetic study of a vaginal micronized progesterone insert (Endometrin) compared with vaginal gel in healthy reproductive-aged female subjects. Fertil Steril. 2010;94(4):1296–1301. doi: 10.1016/j.fertnstert.2009.06.014 [DOI] [PubMed] [Google Scholar]
- 26.Duijkers IJM, Klingmann I, Prinz R, et al. Effect on endometrial histology and pharmacokinetics of different dose regimens of progesterone vaginal pessaries, in comparison with progesterone vaginal gel and placebo. Hum Reprod. 2018;33(11):2131–2140. doi: 10.1093/humrep/dey288 [DOI] [PubMed] [Google Scholar]
- 27.Labarta E, Sebastian-Leon P, Devesa-Peiro A, et al. Analysis of serum and endometrial progesterone in determining endometrial receptivity. Hum Reprod. 2021;36(11):2861–2870. doi: 10.1093/humrep/deab184 [DOI] [PubMed] [Google Scholar]
- 28.Griesinger G, Trevisan S, Cometti B. Endometrial thickness on the day of embryo transfer is a poor predictor of IVF treatment outcome. Human Reprod Open. 2018;2018(1). doi: 10.1093/hropen/hox031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this study are available from the corresponding author upon reasonable request.