Abstract
Introduction
Opioid-induced constipation (OIC) is a common side effect of chronic opioid therapy that significantly impacts quality of life and healthcare costs. Naldemedine, a peripherally acting mu-opioid receptor antagonist, has shown efficacy in treating OIC. However, real-world evidence on naldemedine use in the United States is limited, particularly in older adults. We aimed to describe naldemedine use in real-world settings in the US, focusing on clinical characteristics, comorbidity profiles, co-prescribed medications, and healthcare resource utilization (HCRU), with a specific emphasis on older adults.
Methods
This retrospective study analyzed data from the 2017–2022 Merative™ MarketScan® Commercial and Medicare Databases. We identified 2110 naldemedine users aged ≥ 30 years on chronic opioid therapy. Demographic and clinical characteristics, co-prescribed medications, and HCRU were evaluated. Subgroup analysis focused on patients aged ≥ 65 years.
Results
The study cohort (66% women, median age 56 years, 14% aged ≥ 65 years) presented a significant comorbidity burden, with 57% having hypertension, 36% diabetes, and 25% chronic pulmonary disease with a Charlson Comorbidity Index ≥ 2 in 38% of subjects. Polypharmacy (i.e., use of five or more distinct drugs, excluding naldemedine) was very common (76%, 82% in ≥ 65 years). The most frequent indications for naldemedine were chronic back pain and radiculopathy. Oxycodone, hydrocodone, and morphine were the most commonly prescribed opioids. After initiating naldemedine, 30% of patients showed a reduction in hospitalizations per patient per year, with a more pronounced effect in older adults (37%). Potential drug–drug interactions with CYP3A4 inducers or inhibitors were infrequent and did not appear to impact HCRU.
Conclusions
This real-world study demonstrates that naldemedine is predominantly used in middle-aged adults with comorbidities and polypharmacy. Naldemedine use was associated with reduced HCRU, particularly in older adults, suggesting potential benefits in managing OIC. The findings support the safety and effectiveness of naldemedine in real-world settings, including in older patients with multiple comorbidities.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40122-025-00720-y.
Keywords: Naldemedine, Opioid-induced constipation, Real-world
Key Summary Points
| Why carry out this study? | |
| Opioid-induced constipation (OIC) is a common side effect of chronic opioid therapy that significantly impacts quality of life and healthcare costs. | |
| After its approval for OIC treatment, there is still limited real-world evidence on naldemedine use in the United States, particularly in older adults and patients with multiple comorbidities. | |
| What was learned from the study? | |
| In this real-world study of 2110 naldemedine users, the medication was predominantly prescribed to middle-aged adults with significant comorbidity burden (38% with Charlson Comorbidity Index ≥ 2) and high rates of polypharmacy (76%). | |
| Naldemedine use was associated with reduced healthcare resource utilization, particularly in older adults (37% reduction in hospitalizations), and showed an acceptable safety profile even in the context of potential CYP3A4 drug interactions, supporting its effectiveness in real-world settings. |
Introduction
Opioids are a class of drugs used to manage severe chronic pain in adults. A study by the Centers for Disease Control and Prevention (CDC) using the 2019 National Health Interview Survey (NHIS) estimated that 22% of adults with chronic pain were prescribed opioids in 2019 [1]. Routinely used opioids include morphine, codeine, oxycodone, hydrocodone, and fentanyl. Common side effects include gastrointestinal symptoms such as constipation, nausea, vomiting, and neurologic symptoms such as drowsiness [2]. During chronic opioid use, opioid-induced bowel dysfunction is a potentially debilitating side effect [3]. This condition is a collection of various gastrointestinal motility disorders, of which opioid-induced constipation (OIC) is the most frequent [3]. OIC is a common and troublesome side effect that has negative effects on patient well-being as well as the healthcare system. Studies have found that OIC affects from 10 to 70% of patients taking chronic opioid therapy leading to higher total healthcare costs, decreased work productivity, and activity impairment [4–6].
There are multiple options for the clinical management of OIC. First-line treatment typically consists of non-pharmacologic measures such as increased fluid intake, physical activity, and increased dietary fiber intake. However, these lifestyle modifications often prove inadequate in the treatment of OIC. A common treatment regimen for OIC is a gastrointestinal stimulant (e.g., senna/bisacodyl) with or without a stool softener (e.g., docusate), or daily administration of an osmotic laxative (e.g., polyethylene glycol) [7]. For refractory cases, treatment options include peripherally acting mu-opioid receptor antagonists (PAMORAs), such as naldemedine and naloxegol [7]. Registration trials and subsequent pairwise and network meta-analysis found that naldemedine is effective and well tolerated in the treatment of OIC [8–12]. In addition, naldemedine was also shown to be able to efficiently prevent OIC [13, 14]. However, data from clinical trials or cohort studies are generally conducted in controlled settings with selected patient populations. While providing reliable estimates of safety and efficacy, they cannot give insights into the actual prescribing practice, long-term safety and effectiveness in routine clinical practice, or its impact on healthcare resource utilization (HCRU). To this purpose, real-world data complements this gap in knowledge by offering snapshots into medication use in broader populations, also including older adults with multiple comorbidities and receiving polypharmacy.
Despite the high prevalence of OIC and its various adverse impacts on patients and the healthcare system, there is no known current published real-world evidence on the use of naldemedine in the United States (US).
In the present study we aimed to describe naldemedine use in the real-world setting in the US, by reporting the clinical characteristics of naldemedine users along with their comorbidity profile, co-prescribed drugs, and risk of drug–drug interaction. A specific focus in older subjects aged 65 years or more is included, and the potential impact on health-care resources use is reported before and after naldemedine initiation.
Methods
Data Source
We used the 2017–2022 Merative™ MarketScan® Commercial and Medicare Databases to identify the eligible study subjects. MarketScan is one of the largest proprietary US claims databases used for healthcare research with de-identified longitudinal, patient-level claims and specialty data for > 273 million unique patients. The Commercial database contains health insurance claims across the continuum of healthcare as well as enrolment data from large employers and health plans across the United States which provide private healthcare coverage for employees, their spouses, and dependents. The Medicare database includes Medicare-eligible retirees with employer-sponsored Medicare Supplemental plans and Medicare Advantage plans. These databases include healthcare claims with diagnosis and procedure codes for medical encounters in both inpatient and outpatient setting, dispensing of outpatient prescription medications, and days of supply (https://www.merative.com/documents/brief/marketscan-explainer-general).
Ethical Approval
This study is conducted only using an existing anonymous, commercially available secondary healthcare claims database that meets the US Health Insurance Portability and Accountability Act (HIPAA) requirement. Therefore, the data do not meet the definition of human subject research.
Sample Selection
We selected naldemedine users aged 30 years or older who received chronic opioid therapy (as defined by cumulative opioid exposure for > 30 days within the 180 days prior to or on the date of initiating naldemedine). Of note, according to the U.S. label indication, naldemedine is indicated for the treatment of OIC in adult patients with chronic non-cancer pain, including patients with chronic pain related to prior cancer or its treatment who do not require frequent (e.g., weekly) opioid dosage escalation. The date of the first prescription or dispensing of the newly prescribed naldemedine was defined as the study index date. All patients must have had a minimum 6-month continuous insurance enrolment in the database prior to the index date. Out of 3139 individuals who had received at least one prescription of naldemedine between January 1, 2017 and December 31, 2022, 2340 were on chronic opioid therapy and 2141 had a minimum of 6 months continuous enrolment in the database prior to the index date. Of these, 2110 subjects were aged 30 years or more on the index date, and were included in the present analysis (Supplementary Fig. 1).
Study Measures
The study variables included gender, employee status, Charlson comorbidity index (CCI) [15], Frailty index based on the algorithm from Kim et al., [16] and polypharmacy defined as the concurrent use of five or more distinct generically named drugs, excluding naldemedine. Naldemedine treatment duration was calculated from the index date to the date of the last dispensing plus its days of supply and 30 additional days as a grace period. The frequency of naldemedine co-prescription with CYP3A4 inducers or inhibitors was reported considering the reported potential significant interactions [17]. The impact on HRCU was assessed by considering the number of hospitalizations and of overnight stays. Interactions with primary care and other facilities of the healthcare system could not be considered because these events are not captured by the database. Number of hospitalizations per patient per year (PPPY) was calculated using number of hospitalizations during the follow-up period divided by follow-up time in years before and after the naldemedine index date. Number of overnight stays per patient per year was calculated in the same way. Change in the number of hospitalizations per patient per year is the difference between events PPPY after starting naldemedine and before starting naldemedine.
Statistical Analyses
Naldemedine users were grouped into < 65 and ≥ 65 years old by their age at index date. Continuous variables were described with median and interquartile range (first to third quartile (Q1–Q3), while categorical ones with absolute numbers and percentages. The analyses were conducted using the statistical software SAS 9.4 (Cary, NC, USA).
Results
General Characteristics of the Study Cohort Taking Naldemedine
Out of 2110 individuals included in the analysis, 1401 (66%) were women, and 298 (14%) were people aged 65 years or more (Table 1). The majority were full-time employed (65%), and around two-thirds of individuals presented at least one chronic condition, most commonly arterial hypertension (57%), dyslipidemia (30%), diabetes mellitus (36%), chronic pulmonary disease (25%), rheumatic disease (11%), chronic liver disease (10%) or malignancy (10%). As expected, older people had a higher burden of comorbidities with more than half of older participants having a CCI ≥ 3, with an increased prevalence of arterial hypertension (73% vs. 55%), dyslipidemia (41% vs. 28%), diabetes mellitus (54% vs. 33%), congestive heart failure (17% vs. 4%), peripheral vascular disease (25% vs. 7%), cerebrovascular disease (15% vs. 6%), malignancy (19% vs. 10%), pulmonary (34% vs. 24%) and renal (20% vs. 6%) disease compared to younger subjects. Overall, 10% of patients were frail according to the Frailty Index, and over 75% of patients used five or more drugs concurrently within 30 days of starting naldemedine. Polypharmacy was more frequent in older patients (82% vs. 75% in younger ones).
Table 1.
Baseline demographic and clinical characteristics of naldemedine users
| Total (N = 2110) | Age groups | ||
|---|---|---|---|
| < 65 years (N = 1812) | ≥ 65 years (N = 298) | ||
| Demographic and clinical information | |||
| Age (years), median (Q1–Q3) | 56 (49–62) | 54 (48–59) | 71 (67–79) |
| Female, n (%) | 1401 (66.4%) | 1240 (68.4%) | 161 (54.0%) |
| Employment status, n (%) | |||
| Active full-time | 1367 (64.8%) | 1312 (72.4%) | 55 (18.5%) |
| Active part-time or seasonal | 19 (0.9%) | 18 (1.0%) | 1 (0.3%) |
| Early retiree | 153 (7.3%) | 136 (7.5%) | 17 (5.7%) |
| Medicare eligible retiree | 207 (9.8%) | 32 (1.8%) | 175 (58.7%) |
| Chronic conditions, n (%) | |||
| Arterial hypertension | 1204 (57.1%) | 987 (54.5%) | 217 (72.8%) |
| Dyslipidemia | 631 (29.9%) | 509 (28.1%) | 122 (40.9%) |
| Myocardial infarction | 71 (3.4%) | 48 (2.6%) | 23 (7.7%) |
| Congestive heart failure | 126 (6.0%) | 75 (4.1%) | 51 (17.1%) |
| Peripheral vascular disease | 204 (9.7%) | 129 (7.1%) | 75 (25.2%) |
| Cerebrovascular disease | 156 (7.4%) | 110 (6.1%) | 46 (15.4%) |
| Dementia | 18 (0.9%) | 8 (0.4%) | 10 (3.4%) |
| Chronic pulmonary disease | 530 (25.1%) | 429 (23.7%) | 101 (33.9%) |
| Rheumatic disease | 234 (11.1%) | 202 (11.1%) | 32 (10.7%) |
| Peptic ulcer disease | 61 (2.9%) | 52 (2.9%) | 9 (3.0%) |
| Mild liver disease | 210 (10.0%) | 178 (9.8%) | 32 (10.7%) |
| Moderate or severe liver disease | 14 (0.7%) | 9 (0.5%) | 5 (1.7%) |
| Diabetes without chronic complication | 531 (25.2%) | 430 (23.7%) | 101 (33.9%) |
| Diabetes with chronic complication | 238 (11.3%) | 176 (9.7%) | 62 (20.8%) |
| Hemiplegia or paraplegia | 49 (2.3%) | 41 (2.3%) | 8 (2.7%) |
| Renal disease | 170 (8.1%) | 111 (6.1%) | 59 (19.8%) |
| Malignancy | 227 (10.8%) | 172 (9.5%) | 55 (18.5%) |
| Metastatic solid tumor | 80 (3.8%) | 68 (3.8%) | 12 (4.0%) |
| AIDS/HIV | 10 (0.5%) | 9 (0.5%) | 1 (0.3%) |
| Charlson Comorbidity Index, median (Q1, Q3) | 1 (0, 3) | 1 (0, 2) | 3 (1, 5) |
| 0 | 733 (34.7%) | 683 (37.7%) | 50 (16.8%) |
| 1 | 521 (24.7%) | 472 (26.0%) | 49 (16.4%) |
| 2 | 278 (13.2%) | 230 (12.7%) | 48 (16.1%) |
| ≥ 3 | 578 (27.4%) | 427 (23.6%) | 151 (50.7%) |
| Frailty Index1 | |||
| Median (Q1–Q3) | 0.18 (0.15, 0.21) | 0.17 (0.15, 0.20) | 0.20 (0.16, 0.23) |
| Frail (≥ 0.25) | 216 (10.2%) | 156 (8.6%) | 60 (20.1%) |
| Number of drugs used per patient within 30 days of starting naldemedine, median (Q1–Q3) | 8 (6, 12) | 8 (0, 12) | 9 (6, 12) |
| Polypharmacy2 within 30 days of starting naldemedine | 1597 (75.7%) | 1363 (75.2%) | 245 (82.2%) |
| Most common pain syndromes at naldemedine start | |||
| Low back pain (M545) | 1149 (54.5%) | 990 (54.6%) | 159 (53.4%) |
| Chronic pain syndrome (G894) | 1060 (50.2%) | 933 (51.5%) | 127 (42.6%) |
| Radiculopathy-lumbar region (M5416) | 868 (41.1%) | 739 (40.8%) | 129 (43.3%) |
| Other chronic pain (G8929) | 839 (39.8%) | 718 (39.6%) | 121 (40.6%) |
| Cervicalgia (M542) | 677 (32.1%) | 612 (33.8%) | 65 (21.8%) |
| Post-laminectomy syndrome-not elsewhere classified (M961) | 470 (22.3%) | 399 (22.0%) | 71 (23.8%) |
| Radiculopathy-cervical region (M5412) | 438 (20.8%) | 393 (21.7%) | 45 (15.1%) |
Data expressed as absolute numbers (percentage) for categorical variables, or as median (first-third quartile, Q1–Q3) or mean (standard deviation) for continuous variables as appropriate
1The Frailty Index was calculated according to the algorithm developed by Kim-Dae Hyun; Gautam-Nileesa-2020-"SAS Programs—Claims-Based Frailty Index"-https://doi.org/10.80/DVN/HM8DOI
2Polypharmacy defined as concurrent use of five or more distinct generical-named drugs, excluding naldemedine
Co-Prescribed Opioid and Non-Opioid Therapy and Risk of Drug–Drug Interactions
All patients were on opioid therapy for chronic non-cancer pain at naldemedine prescription and 87% of patients continued opioid therapy also after naldemedine start (Table 2). The most common pain syndromes for opioid prescription were low back pain (55%), lumbar radiculopathy (41%), cervicalgia (32%) and cervical radiculopathy (21%), without significant differences across age groups (Table 1). The most commonly prescribed opioids were oxycodone (76% pre- and 59% post-naldemedine initiation), hydrocodone (47% pre- and 34% post-naldemedine initiation), morphine (28% pre- and 21% post-naldemedine initiation), fentanyl (26% pre- and 18% post-naldemedine initiation), hydromorphone (22% pre- and 16% post-naldemedine initiation) and buprenorphine (18% pre- and 14% post-naldemedine initiation). In the first 6 months after naldemedine initiation, only 42% and 23% of patients had to change or reduce their opioid treatment, respectively, compared to 78% and 37% in the 6 months before naldemedine (Supplementary Table 1). This observation was generally consistent across the studied age groups. Moreover, older subjects presented comparable opioid continuation rate after naldemedine start (85% vs. 89%) compared to younger individuals (Table 2).
Table 2.
Opioid use by 5% of naldemedine users
| Within 12 months before starting naldemedine | After starting naldemedine* | |||||
|---|---|---|---|---|---|---|
| Total | < 65 years | ≥ 65 years | Total | < 65 years | ≥ 65 years | |
| Opioid | 2110 (100.0%) | 1812 (100.0%) | 298 (100.0%) | 1870 (88.63%) | 1616 (89.18%) | 254 (85.23%) |
| Codeine | ||||||
| Acetaminophen/codeine phosphate | 106 (5.02%) | 91 (5.02%) | 15 (5.03%) | 65 (3.08%) | 58 (3.20%) | 7 (2.35%) |
| Buprenorphine | ||||||
| Buprenorphine | 263 (12.46%) | 238 (13.13%) | 25 (8.39%) | 206 (9.76%) | 184 (10.15%) | 22 (7.38%) |
| Buprenorphine hydrochloride | 29 (1.37%) | 28 (1.55%) | 1 (0.34%) | 25 (1.18%) | 24 (1.32%) | 1 (0.34%) |
| Buprenorphine/naloxone | 85 (4.03%) | 77 (4.25%) | 8 (2.68%) | 75 (3.55%) | 70 (3.86%) | 5 (1.68%) |
| Fentanyl | ||||||
| Fentanyl | 539 (25.55%) | 476 (26.27%) | 63 (21.14%) | 390 (18.48%) | 347 (19.15%) | 43 (14.43%) |
| Fentanyl citrate | 1 (0.05%) | 1 (0.06%) | - | 4 (0.19%) | 2 (0.11%) | 2 (0.67%) |
| Hydrocodone | ||||||
| Acetaminophen/hydrocodone bitartrate | 909 (43.08%) | 789 (43.54%) | 120 (40.27%) | 666 (31.56%) | 585 (32.28%) | 81 (27.18%) |
| Hydrocodone bitartrate | 76 (3.60%) | 70 (3.86%) | 6 (2.01%) | 52 (2.46%) | 49 (2.70%) | 3 (1.01%) |
| Hydrocodone bitartrate/ibuprofen | 8 (0.38%) | 6 (0.33%) | 2 (0.67%) | 2 (0.09%) | 1 (0.06%) | 1 (0.34%) |
| Hydromorphone | ||||||
| Hydromorphone | 244 (11.56%) | 217 (11.98%) | 27 (9.06%) | 173 (8.20%) | 156 (8.61%) | 17 (5.70%) |
| Hydromorphone hydrochloride | 211 (10.00%) | 192 (10.60%) | 19 (6.38%) | 171 (8.10%) | 156 (8.61%) | 15 (5.03%) |
| Methadone | ||||||
| Methadone | 72 (3.41%) | 62 (3.42%) | 10 (3.36%) | 73 (3.46%) | 63 (3.48%) | 10 (3.36%) |
| Methadone hydrochloride | 71 (3.36%) | 61 (3.37%) | 10 (3.36%) | 71 (3.36%) | 61 (3.37%) | 10 (3.36%) |
| Morphine | ||||||
| Morphine | 213 (10.09%) | 194 (10.71%) | 19 (6.38%) | 129 (6.11%) | 115 (6.35%) | 14 (4.70%) |
| Morphine sulfate | 372 (17.63%) | 316 (17.44%) | 56 (18.79%) | 300 (14.22%) | 250 (13.80%) | 50 (16.78%) |
| Morphine sulfate/naltrexone hydrochloride | 19 (0.90%) | 17 (0.94%) | 2 (0.67%) | 12 (0.57%) | 10 (0.55%) | 2 (0.67%) |
| Oxycodone | ||||||
| Oxycodone | 113 (5.36%) | 104 (5.74%) | 9 (3.02%) | 98 (4.64%) | 90 (4.97%) | 8 (2.68%) |
| Oxycodone hydrochloride | 690 (32.70%) | 581 (32.06%) | 109 (36.58%) | 531 (25.17%) | 449 (24.78%) | 82 (27.52%) |
| Acetaminophen/oxycodone hydrochloride | 806 (38.20%) | 690 (38.08%) | 116 (38.93%) | 607 (28.77%) | 528 (29.14%) | 79 (26.51%) |
| Tapentadol | ||||||
| Tapentadol hydrochloride | 136 (6.45%) | 124 (6.84%) | 12 (4.03%) | 85 (4.03%) | 77 (4.25%) | 8 (2.68%) |
*After starting naldemedine and up to 30 days after the last day of naldemedine exposure
Regarding the risk of interaction involving the CYP3A4 enzyme, only 16 (1%) patients took naldemedine concomitantly with a CYP3A4 inducer (such as carbamazepine and haloperidol [Supplementary Table 2]), while 333 (16%) presented a concomitant use of a CYP3A4 inhibitor, most frequently with carvedilol and omeprazole (Supplementary Table 2). There were no identifiable reports of drug–drug interactions involving naldemedine in this cohort. This is consistent with the known pharmacological profile of naldemedine [17].
Impact on Healthcare Resource Utilization (HCRU)
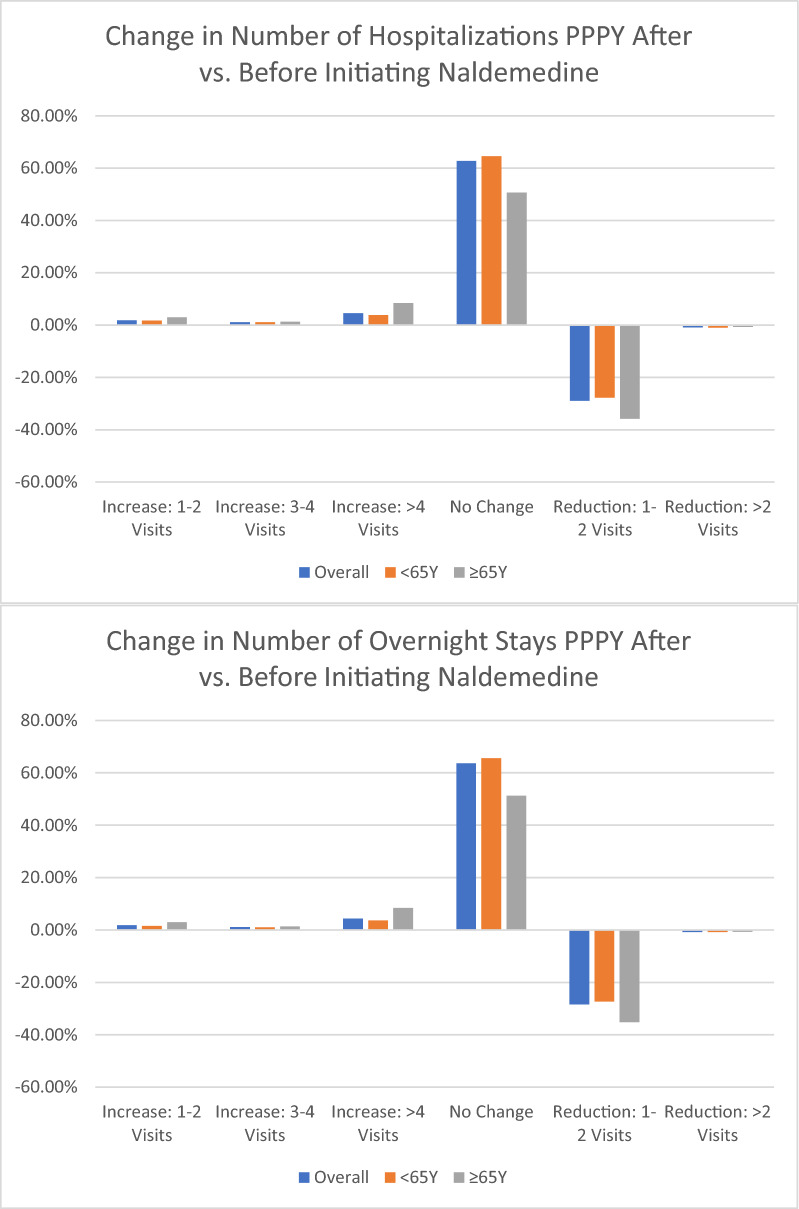
Overall, 435 (21%) and 418 (20%) subjects had at least one hospitalization or at least one hospital overnight stay in the 12 months before starting naldemedine (Table 3). Median follow-up after starting naldemedine was 17 months (Q1–Q3 7–31) with a median naldemedine intake duration of 88 days (Q1–Q3 60–169). Overall, 278 (13.2%) subjects had at least one hospitalization in the 6 months before starting naldemedine, while 196 (9.3%) subjects had hospitalization in the 6 months after naldemedine. Similarly, the number of subjects with an overnight stay was 265 (12.6%) in the 6 months before naldemedine and 187 (8.9%) in the 6 months after starting naldemedine. When comparing the number of hospitalization per-person-per-year before and after starting naldemedine, 630 (30%) patients presented a reduction of at least one hospitalization, and 616 (29%) patients had a reduction of at least one overnight stay (Fig. 1). Older participants presented a higher rate of hospitalization or of overnight stay (27% and 27%) before starting naldemedine along with a greater reduction of hospitalization after starting naldemedine (Fig. 1). Similarly, about 37% of older individuals would have a reduction of hospitalization per patient per year, and 36% would have a reduction of overnight stays per patient per year, compared to 29% and 28% in younger subjects, respectively.
Table 3.
Healthcare resource use in naldemedine users
| Total | Age groups | ||
|---|---|---|---|
| < 65 years | ≥ 65 years | ||
| At least 1 hospitalization within 12 months before starting naldemedine | 435 (20.6%) | 355 (19.6%) | 80 (26.8%) |
| N Change: N of hospitalizations PPPY after vs. before initiating naldemedine | |||
| Increase: 1–2 visits | 39 (1.8%) | 30 (1.7%) | 9 (3.0%) |
| Increase: 3–4 visits | 24 (1.1%) | 20 (1.1%) | 4 (1.3%) |
| Increase: > 4 visits | 95 (4.5%) | 70 (3.9%) | 25 (8.4%) |
| No Change | 1322 (62.7%) | 1171 (64.6%) | 151 (50.7%) |
| Reduction: 1–2 visits | 610 (28.9%) | 503 (27.8%) | 107 (35.9%) |
| Reduction: > 2 visits | 20 (0.9%) | 18 (1.0%) | 18 (1.0%) |
| At least 1 overnight stay within 12 months before starting naldemedine | 418 (19.8%) | 339 (18.7%) | 79 (26.5%) |
| N Change: N of overnight stays PPPY after vs. before initiating naldemedine | |||
| Increase: 1–2 visits | 37 (1.8%) | 28 (1.5%) | 9 (3.0%) |
| Increase: 3–4 visits | 23 (1.1%) | 19 (1.0%) | 4 (1.3%) |
| Increase: > 4 visits | 92 (4.4%) | 67 (3.7%) | 25 (8.4%) |
| No change | 1342 (63.6%) | 1189 (65.6%) | 153 (51.3%) |
| Reduction: 1–2 visits | 599 (28.4%) | 494 (27.3%) | 105 (35.2%) |
| Reduction: > 2 visits | 17 (0.8%) | 15 (0.8%) | 2 (0.7%) |
PPPY, per patient per year
Fig. 1.
Change in number of hospitalizations (A) or of overnight stays (B) after starting naldemedine. PPPY, per person per year
Of note, subjects with a co-prescription of a CYP3A4 inducer or inhibitor predominantly reported a reduction of hospitalizations (39% vs. 28%) or overnight stays (38% vs. 27%) compared to subjects without co-prescriptions.
Discussion
In the present study, we found that the majority of naldemedine users in the US were middle-aged adults with a significant burden of comorbidities, polypharmacy, and HCRU. The principal indications for naldemedine prescription were chronic back pain and radiculopathy, and the most frequent co-prescribed opioids were oxycodone, hydrocodone and morphine. Older people represented a relatively low proportion (~ 16%) of the study sample but were characterized by a more severe comorbidity profile. After starting naldemedine, a reduction in HCRU (such as hospitalization or overnight stay) was found in around ~ 40% of older subjects compared to ~ 30% in younger ones.
The efficacy and safety of naldemedine in treating OIC was demonstrated in several phase 3 randomized placebo-controlled trials, both in patients without cancer (COMPOSE I-II-III)[9, 10] and with cancer (COMPOSE IV-V) [11]. These findings were supported by a network meta-analysis [8], and a positive impact on subjective measures of patient-reported outcomes was also shown [12]. However, since its first approval by regulatory agencies in 2017, only two retrospective studies have analyzed the characteristics of patients prescribed naldemedine in the real-world setting. Yamada et al. [18] retrospectively described a group of 51 patients with cancer prescribed with naldemedine in a single center in Japan. More recently, Kessoku et al. [19] reported data on 2361 naldemedine users with chronic cancer pain extracted from a Japanese administrative database.
By analyzing a large claims database, the present study provides a comprehensive analysis of naldemedine use in a real-world setting in the USA, primarily focusing on the clinical characteristics, comorbidity profiles, co-prescribed medications, and HCRU. In contrast to Yamada et al. [18], all subjects in the study were patients without cancer, which is consistent with the current indication for naldemedine prescription in the US. Consequently, patients in this study were relatively younger with a higher proportion of women, but also a minor (but not negligible) proportion of older subjects(> 65 years)was included. Oxycodone was the most co-prescribed opioid drug in around two-thirds of patients, while hydromorphone was relatively more prescribed in Kessoku et al. [19].
The potential interactions with CYP3A4 inducers were limited as only 1% of naldemedine users were co-prescripted with one of known inducers, while a significantly higher proportion of patients were co-prescribed a CYP3A4 inhibitor (16%), which -of note- was higher than previously reported by Yamada et al. [18] (6%). Of note, we could not rely upon a formal screening for adverse drug reactions (or of interaction-related medical problems), but the absence of an increase in HRCU between the 17% people at risk of interaction and the remaining 83% suggests that naldemedine was well tolerated also in the context of polypharmacy. As expected, older age was associated with multimorbidity and polypharmacy, but indirect indexes of safety and efficacy of naldemedine (such as the variation of HCRU) showed an even larger potential benefit in older compared to younger people. This suggests the tolerability profile of naldemedine is not a major cause for concern in routine clinical use. Notably, this study is the first to report on naldemedine use in a real-world cohort of patients without cancer, with a specific focus on subjects aged 65 years or older. This is particularly significant as older patients are especially vulnerable to both the side effects of opioids and the complications of OIC. The observed reduction in hospitalizations and overnight stays after naldemedine initiation is a promising finding, suggesting that effective management of OIC could lead to substantial improvements in overall health outcomes and resource utilization, especially in older adults. The reduction in opioid regimen changes and dosage adjustments is a further finding in this direction: by alleviating OIC symptoms, naldemedine likely contributed to improved opioid tolerance, reducing the need for modifications in opioid therapy and enabling a more stable pain management approach. This stability is particularly relevant in patients with chronic pain, as frequent opioid adjustments can complicate treatment adherence and efficacy.
The main strength of the present study resides in the use of a large, comprehensive claims database, which allowed for a robust analysis of naldemedine use in a real-world setting across a diverse population. This enhances the generalizability of the findings to the broader population of patients without cancer prescribed with naldemedine. Additionally, the focus on older adults provides valuable insights into a particularly vulnerable subset of the population. However, there are also several limitations to consider. The retrospective nature of the study introduces potential biases related to the accuracy and completeness of the recorded data. The reliance on claims data may not capture all relevant clinical details, such as the severity of comorbidities, the effect on constipation or the precise reasons for medication/opioid changes. Furthermore, the study could not include a control group of patients not treated with naldemedine, given that this was not a clinical trial randomizing patient to conventional laxatives or naldemedine. Indeed, in real life, the use of naldemedien is usually driven by failure of conventional laxatives. Accordingly, a formal comparison of naldemedine users and users of conventional laxatives would be unable to validly compare the HCRU between these two groups. Moreover, we can hypothesize, but not prove, a causal association between naldemedine use and reduction in HCRU, as also other unexplored factors might have a contributory role. Lastly, the profile of safety with regard to potential interactions of naldemedine with CYP3A4 was indirectly assessed, in the absence of normal reporting of adverse drug reactions. MarketScan represents patients covered by commercial insurances, rather than a random sample from the U.S. population, and therefore are not representative of the U.S. population, especially elderly populations. The results should be interpreted with caution regarding their applicability to other populations.
In conclusion, the present study reports the real-world application of naldemedine among patients without cancer in the USA, emphasizing the clinical complexity of this population, the challenges posed by multimorbidity and polypharmacy, and the positive impact of effective OIC treatment on aspects of HCRU, particularly in older and frail patients. Further research is warranted to continue monitoring the long-term safety and efficacy of naldemedine in diverse patient populations and to optimize treatment strategies for those with the greatest clinical needs.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
Antonio De Vincentis, Bin Cai, Marco Moscarda, Peter Barnes, and Raffaele Antonelli Incalzi contributed to the drafting and critical revision of the manuscript for important intellectual concepts. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study and the Rapid Service Fee were funded by Shionogi BV.
Data Availability
The datasets generated and analyzed during the current study are not publicly available due to restrictions related to the Merative™ MarketScan® Commercial and Medicare Databases.
Declarations
Conflict of Interest
Antonio De Vincentis and Raffaele Antonelli Incalzi have no conflicts of interest to declare with the present study. Bin Cai, Marco Moscarda, and Peter Barnes are employees of Shionogi at the time of writing.
Ethical Approval
This study is conducted only using an existing anonymous, commercially available secondary healthcare claims database that meets the US Health Insurance Portability and Accountability Act (HIPAA) requirement. Therefore, the data do not meet the definition of human subject research.
References
- 1.Dahlhamer JM, Connor EM, Bose J, Lucas JL, Zelaya CE. Prescription opioid use among adults with chronic pain: United States. Natl Health Stat Report. 2019;2021:1–9. [PubMed] [Google Scholar]
- 2.Opioids [Internet]. 2023 [cited 2024 Apr 26]. Available from: https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/opioids.
- 3.Ketwaroo GA, Cheng V, Lembo A. Opioid-induced bowel dysfunction. Curr Gastroenterol Rep. 2013;15:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Candrilli SD, Davis KL, Iyer S. Impact of constipation on opioid use patterns, health care resource utilization, and costs in cancer patients on opioid therapy. J Pain Palliat Care Pharmacother. 2009;23:231–41. [DOI] [PubMed] [Google Scholar]
- 5.Bell T, Annunziata K, Leslie JB. Opioid-induced constipation negatively impacts pain management, productivity, and health-related quality of life: findings from the National Health and Wellness Survey. J Opioid Manag. 2009;5:137–44. [DOI] [PubMed] [Google Scholar]
- 6.Varrassi G, Banerji V, Gianni W, Marinangeli F, Pinto C. Impact and consequences of opioid-induced constipation: a survey of patients. Pain Ther. 2021;10:1139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sizar O, Genova R, Gupta M. Opioid-induced constipation. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 Apr 26]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK493184/. [PubMed]
- 8.Ouyang R, Li Z, Huang S, Liu J, Huang J. Efficacy and safety of peripherally acting mu-opioid receptor antagonists for the treatment of opioid-induced constipation: a Bayesian network meta-analysis. Pain Med. 2020;21:3224–32. [DOI] [PubMed] [Google Scholar]
- 9.Hale M, Wild J, Reddy J, Yamada T, Ferreira JCA. Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): two multicentre, phase 3, double-blind, randomised, parallel-group trials. Lancet Gastroenterol Hepatol. 2017;2:555–64. [DOI] [PubMed] [Google Scholar]
- 10.Webster LR, Nalamachu S, Morlion B, Reddy J, Baba Y, Yamada T, et al. Long-term use of naldemedine in the treatment of opioid-induced constipation in patients with chronic noncancer pain: a randomized, double-blind, placebo-controlled phase 3 study. Pain. 2018;159:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katakami N, Harada T, Murata T, Shinozaki K, Tsutsumi M, Yokota T, et al. Randomized phase III and extension studies of naldemedine in patients with opioid-induced constipation and cancer. J Clin Oncol. 2017;35:3859–66. [DOI] [PubMed] [Google Scholar]
- 12.Katakami N, Harada T, Murata T, Shinozaki K, Tsutsumi M, Yokota T, et al. Randomized phase III and extension studies: efficacy and impacts on quality of life of naldemedine in subjects with opioid-induced constipation and cancer. Ann Oncol. 2018;29:1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamano J, Higashibata T, Kessoku T, Kajiura S, Hirakawa M, Oyamada S, et al. Naldemedine for opioid-induced constipation in patients with cancer: a multicenter, double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2024;42:4206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozaki A, Kessoku T, Tanaka K, Yamamoto A, Takahashi K, Takeda Y, et al. Effectiveness of naldemedine compared with magnesium oxide in preventing opioid-induced constipation: a randomized controlled trial. Cancers (Basel). 2022;14:2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 16.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in medicare data: development and validation of a claims-based frailty index. J Gerontol Ser A. 2018;73:980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naldemedine_SmPC.pdf [Internet]. [cited 2024 Aug 16]. Available from: https://ec.europa.eu/health/documents/community-register/2019/20190218143647/anx_143647_en.pdf.
- 18.Yamada M, Jimaru Y, Torii S, Mitsuba N, Takahashi K. A Retrospective observational study of factors affecting the efficacy of concurrent prescription of naldemedine for opioid-induced constipation caused by oxycodone tablets. Biol Pharm Bull. 2023;46:1826–31. [DOI] [PubMed] [Google Scholar]
- 19.Kessoku T, Higashibata T, Morioka Y, Naya N, Koretaka Y, Ichikawa Y, et al. Naldemedine and magnesium oxide as first-line medications for opioid-induced constipation: a comparative database study in Japanese patients with cancer pain. Cureus. 2024;16: e55925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to restrictions related to the Merative™ MarketScan® Commercial and Medicare Databases.