Abstract
Human immunodeficiency virus (HIV) infection produces neurological comorbidities including HIV-associated neurocognitive disorder (HAND) and chronic pain. HIV also increases the risk of developing an alcohol use disorder (AUD). With the rising prevalence of AUD in women and people with HIV (PWH), understanding the neurobiological impact of alcohol in these populations is important. We examined proteomic alterations in the hippocampus and anterior cingulate cortex (ACC), brain regions critical for cognition and affective pain, in a female rhesus macaque model of chronic binge alcohol administration and SIV infection. Adult female rhesus macaques received either chronic binge alcohol (CBA, 13–14 g/kg/week of alcohol) or water (VEH) via gastric catheter. All animals were inoculated with simian immunodeficiency virus (SIVmac251) and treated with antiretroviral therapy (ART). Brain samples were processed for proteomic analysis, and quantitative discovery-based proteomics identified differentially expressed proteins in both brain regions comparing CBA treatment to VEH. Ingenuity Pathway Analysis (IPA) was also used to predict pathway activation. CBA significantly altered 147 proteins in the hippocampus and 176 proteins in the ACC. IPA revealed alterations in 39 canonical pathways in the hippocampus and 62 canonical pathways in the ACC. Fourteen common canonical pathways were enriched in both regions, including synaptogenesis and protein kinase A (PKA) signaling. These discoveries expand our understanding of how alcohol alters proteins of critical signaling pathways in vulnerable brain regions in the context of SIV/HIV infection and may lead to the development of new pharmacological treatment avenues for neurological dysfunction in women with HIV who use alcohol.
Keywords: Alcohol, Anterior cingulate cortex, Cognition, Hippocampus, Simian immunodeficiency virus, Proteomics
Introduction
Human immunodeficiency virus (HIV) infection leads to profound pathophysiological consequences at both peripheral and central nervous system levels, including increased neuroinflammation, impaired neurogenesis, and neurodegeneration (Kaul et al. 2005; Maxi et al. 2016). These neurological changes can manifest behaviorally in the forms of cognitive impairment, termed HIV-associated neurocognitive disorder (HAND), and enhanced pain symptoms, termed HIV-associated neuropathy (HIV-N) (Singer et al. 2010; Ghosh et al. 2012; Jazebi et al. 2021; Simon et al. 2021a). Furthermore, there is a high prevalence of psychiatric comorbidities in people with HIV (PWH), including alcohol use disorder (AUD), generalized anxiety disorder (GAD), major depressive disorder (MDD), and posttraumatic stress disorder (PTSD), also potentially driven by underlying neuroadaptations (Galvan et al. 2002; Neigh et al. 2016; Crane et al. 2017; Camara et al. 2020).
PWH have a greater likelihood of alcohol misuse compared to the general population, and approximately 30% of PWH meet criteria for an AUD, compared to 10% of the general population (Galvan et al. 2002; Park et al. 2016; Koch et al. 2019; Duko et al. 2019; Yen et al. 2022; SAMHSA 2022). Furthermore, AUD in PWH can result in aggravated HIV-related biomedical and psychological consequences and can exacerbate comorbidities including HAND and HIV-N (Molina et al. 2014; Fama et al. 2016). Indeed, alcohol consumption can exacerbate systemic and neuroinflammation and result in immune dysfunction, contributing to the severity of HIV-associated neuropsychiatric comorbidities, including HIV-associated neurocognitive disorder (Pandrea et al. 2010; Monnig 2017).
With antiretroviral therapy (ART) advances extending the longevity of PWH and the rising prevalence of AUD in women (Grant et al. 2017), understanding the impact of HIV and alcohol on the female nervous system is increasingly important.
Sex is a significant factor in the HIV/AIDS epidemic. In 2023, an estimated 20.5 million women were living with HIV globally, accounting for more than half of adult PWH (World Health Organization 2024). Women with HIV endure more mental health challenges than men with HIV, including a higher motivation to consume alcohol in response to negative emotions and pain (Cook et al. 2016). Higher alcohol consumption may exacerbate both cognitive decline and neuropathic pain (Pahng et al. 2017), putting women with comorbid HIV and AUD at a heightened risk for future declines in mental health. Importantly, women are more susceptible to brain damage due to chronic alcohol use, and women are at heightened risk for developing age-related cognitive decline and chronic pain in the context of AUD (Hommer 2003; Mann et al. 2005; Maki et al. 2009; Levine et al. 2021; Fitzpatrick-Schmidt and Edwards 2023). This elevated risk, coupled with the rising prevalence of AUD in women and longer life expectancy in PWH due to advances in ART, necessitates further investigation to better understand the mechanisms of cognitive decline and other brain-related disorders in this vulnerable patient population.
Dysregulation of hippocampal and anterior cingulate cortex (ACC) signaling may play a role in neuroplastic changes seen in both HIV and AUD. The hippocampus is a key component of the limbic system, plays a prominent role in learning and memory, and can undergo atrophy in the context of HAND due to impaired neural progenitor cell proliferation and differentiation (Venkatesan et al. 2007; Lee et al. 2013; Yonelinas 2013). The anterior cingulate cortex (ACC) is a frontal cortical region that integrates bidirectional connections with the hippocampus and other limbic system structures and has prominent roles in cognition, emotional regulation, and the affective processing of pain (Stevens et al. 2011; Pahng et al. 2017). Importantly, the hippocampus and ACC are particularly vulnerable to the effects of alcohol and may play a key role in the development of AUD due to their central role at the intersection of emotion and motivation (Edwards and Koob 2010; Egli et al. 2012; Kutlu and Gould 2016; Pahng et al. 2017; Mira et al. 2019). The two regions also functionally interact to regulate cognitive-affective processing (Wang et al. 2021). One recent study discovered that functional connectivity between the hippocampus and ACC is reduced in individuals suffering from both AUD and HIV infection, and that this deficit is correlated with lifetime alcohol consumption (Honnorat et al. 2022).
Our previous work revealed that alcohol promotes simian immunodeficiency virus (SIV)-induced cognitive deficits in male rhesus macaques (Winsauer et al. 2002). From a neurobiological perspective, we also discovered that chronic binge alcohol (CBA) administration in simian immunodeficiency virus (SIV)-infected male rhesus macaques upregulates hippocampal genes involved in inflammation and immune function and dysregulates genes involved in neurogenesis (Maxi et al. 2016). Additional work indicates that CBA in adolescent male rhesus macaques leads to a long-lasting impairment in hippocampal proliferation and neurogenesis and corresponding neurodegeneration (Taffe et al. 2010). Furthermore, a macaque model of binge alcohol self-administration has revealed a bidirectional relationship with heavy alcohol consumption and deficits in cognitive and behavioral flexibility (Shnitko et al. 2019, 2020). Unfortunately, few studies have examined the impact of both alcohol and HIV on the brain proteome, and only one study has utilized proteomic profiling in HIV + drinkers, although this was done on plasma exosomes (Kodidela et al. 2020).
To better understand the neurobiological impact of comorbid HIV and CBA use in women, the current study sought to investigate proteomic neuroadaptations in the hippocampus and ACC of SIV-infected female rhesus macaques exposed to CBA administration. The overarching goal of this experimental design is to determine how HIV and comorbid AUD risk (in this case, modeled using SIV infection in the context of longitudinal CBA administration) may lead to future cognitive and neuropsychiatric comorbidities by identifying proteins and pathways altered within this context. Our model approximates the definition of “extreme binge” drinking from the National Institute on Alcohol Abuse and Alcoholism (NIAAA), and such blood alcohol levels are commonly achieved in individuals either at-risk for or suffering from AUD. Based on our previous studies, we hypothesized that proteins associated with inflammation, immune signaling, and neurodevelopment would be altered by chronic binge alcohol in the context of SIV. The revelation of neuroadaptations and signaling networks within these key brain areas in highly valid preclinical models of comorbid HIV and AUD is critical for understanding neurological deficits and promoting novel therapeutic avenues to improve quality of life in women with HIV.
Materials and Methods
Animal Study Design
Experiments were approved by the Institutional Animal Care and Use Committee at Louisiana State University Health Sciences Center in New Orleans, LA (IACUC #5128, LSUHSC-NO Comprehensive Alcohol-HIV/AIDS Research Center, Expiration Date: June 20, 2025). The study adhered to the National Institute of Health (NIH) guidelines for the care and use of experimental animals. The experimental design has been described in detail in previous publications in both male (Molina et al. 2014; Maxi et al. 2016) and female (Poret et al. 2021; Simon et al. 2021b) rhesus macaques, and an overview of the timeline is shown in Fig. 1. In brief, adult female rhesus macaques (Macaca mulatta) ages 6–9 years were randomly assigned to a group receiving either chronic binge alcohol (CBA) or isovolumetric water (VEH) administration. Gastric catheter implantation surgeries were performed to administer 13–14 g/kg/week of alcohol (30% ethanol in water, w/v). A single 30-min infusion of 30% w/v alcohol was delivered via intragastric catheter in the mornings 5 days a week. Blood alcohol levels peaked at 50–60 mM (~ 230 mg/dL) at approximately two hours after initiation of alcohol infusion. The alcohol dosing regimen models extreme binge alcohol administration that places humans at risk for developing an AUD and has been historically used in our alcohol center and non-human primate model of alcohol-induced organ pathophysiology and cognitive impairments (Winsauer et al. 2002). CBA-treated animals do not lose consciousness and are intoxicated for no more than 2–3 h. In order to determine the effects of alcohol on SIV disease progression in a controlled setting, the same dose of alcohol and SIV virus were provided to all animals. Following three months of CBA or VEH administration, all animals were inoculated intravaginally with SIVmac251. After 2.5 months, animals achieved viral set-point and were started on daily subcutaneous injections of antiretroviral therapy (ART) consisting of 30 mg/kg of emtricitabine (FTC) and 20 mg/kg of Tenofovir [9-(2-phosphonomethoxypropyl) adenine, PMPA] (Gilead Sciences Inc., Foster City, CA). In total, two treatment groups were assigned randomly: VEH (n = 6) and CBA (n = 6). Animals received CBA or VEH for a total of 14.5 months, were infected with SIV for 11.5 months, and were on ART for 9 months. At the end of the treatment regimens, all animals were euthanized according to the American Veterinary Medical Associations guidelines. Data for SIV viral loads, T-cell counts, and markers of immune activation for these animals have been published previously (McTernan et al. 2022; Gallegos et al. 2024).
Fig. 1.
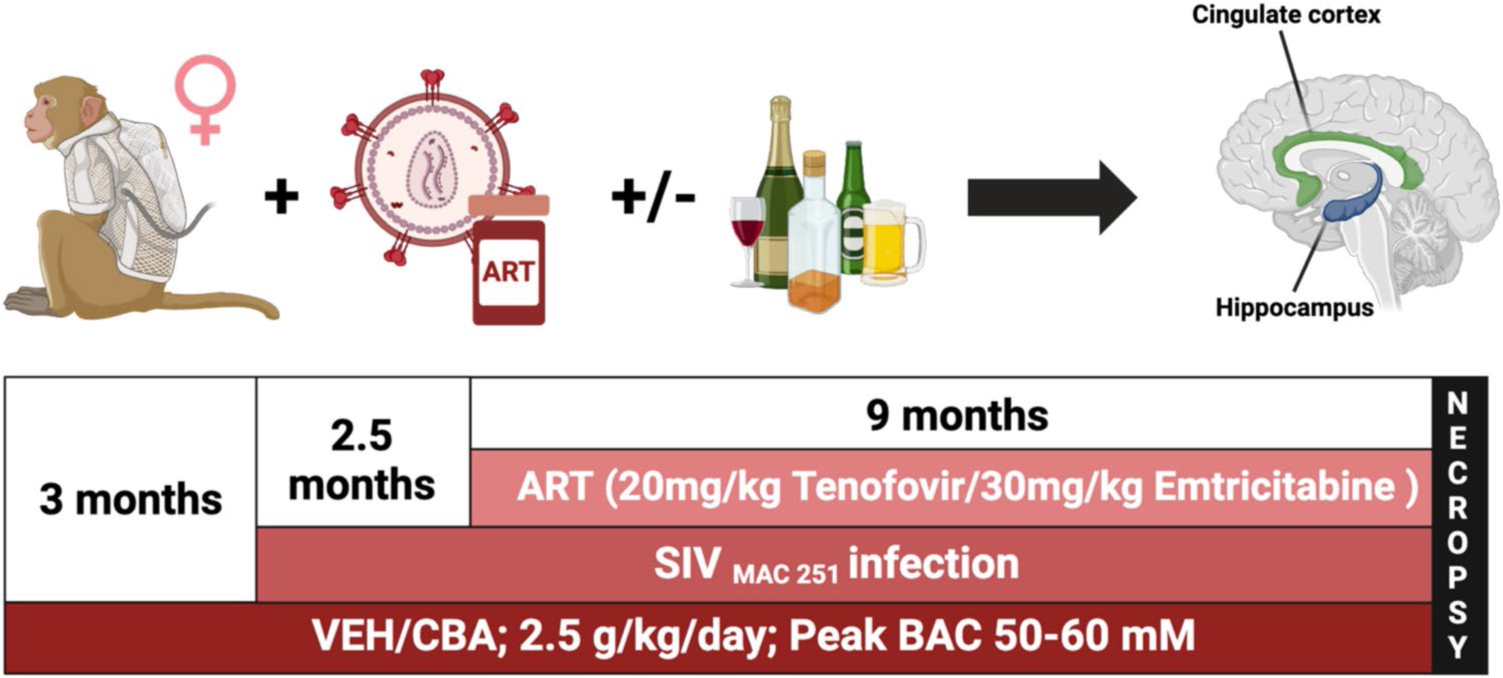
A schematic showing the timeline for the nonhuman primate study. Adult female rhesus macaques were randomly assigned to chronic binge alcohol (CBA) or isovolumetric water (VEH) groups. Alcohol was administered at 13–14 g/kg/week (30% ethanol in water, wt/vol; infusions 30 min/day, 5d/wk). peaked at 50–60 mM (230 mg%) at approximately two hours after initiation of alcohol infusion. Following three months of CBA or VEH administration, all animals were inoculated intravaginally with SIVmac251. After 2.5 months, animals achieved viral set-point and were started on daily subcutaneous injections of antiretroviral therapy consisting of 30 mg/kg of emtricitabine (FTC) and 20 mg/kg of Tenofovir [9-(2-phosphonomethoxypropyl) adenine, PMPA] (Gilead Sciences Inc., Foster City, CA). Finally, all animals were euthanized humanely at the study end-point, brains were snap-frozen, and the anterior cingulate cortex and hippocampus were isolated and prepared for proteomic analysis. Animals received CBA or VEH for a total of 14.5 months, were infected with SIV for 11.5 months, and were on ART for 9 months
Tissue Preparation and Protein Assay
Whole brains were hemisected and snap frozen in −30 °C isopentane at necropsy and the right hemisphere was stored at −80°C until dissection. Due to the nature of this longitudinal study (2015–2021), samples were stored at −80 for a period of years. Cohorts of animals were balanced in terms of treatment groups to mitigate effects related to differential time of tissue storage. Regional brain dissections were taken from 6 mm coronal brain slices and guided by the Paxinos Rhesus Macaque Brain Atlas, Second Edition (Paxinos et al. 2009). Regions collected for this analysis include the anterior cingulate cortex (Brodmann area 24, + 12.0 mm to + 6.0 mm from Bregma) and whole hippocampus (−10 mm to −16 mm from Bregma) from the right hemisphere only.
Protein Extraction and Digestion
To the weighted tissue (20 mg minimum) from regional brain samples, the same volume (in μL) of ABC Buffer (100 mM Ammonium Bicarbonate, pH 8) and 8 volumes of 100% methanol (MetOH) were added (i.e. for 20 mg tissue, add 20 μL buffer and 160 μL MetOH). Samples were then homogenized using Bead Ruptor 96 (OMNI International, USA) and 6 large beads. Suspensions (180 μL) were transferred into a new Eppendorf tube, centrifuged at 14,000 × g for 5 min and the supernatant was discarded. Pellets were washed twice with cold acetone and dried in a SpeedVac concentrator. For digestion, protein pellets were solubilized in 20 μL of Denaturation Buffer (25 mM ammonium bicarbonate, pH8.0; 10 mM TCEP; 5% SDC (sodium deoxycholate)), incubated for 10 min at 60°C, and alkylated using 5 μL Alkylation Buffer (100 mM Iodoacetamide in water, prepared fresh). The incubation continued for an additional 60 min at room temperature in the dark. Following alkylation, samples were diluted with 175 μL of dilution buffer (25 mM ammonium bicarbonate, pH8.0), and 2 μL of Trypsin solution (1 μg/μL) were added. Samples were subsequently incubated overnight at 37°C. To stop the reaction and remove the SDC, 10 μL of 10% trifluoroacetic acid (TFA) was added, and the mixture was incubated for 30 min at room temperature and centrifuged at 15,000 × g (SDC precipitates at low pH). Supernatants were then transferred into the new tubes and directly used for liquid chromatographymass spectrometry (LC–MS) analysis.
Liquid Chromatography-Mass Spectrometry (LC–MS) Analysis
All analyses were carried out using a Bruker nanoElute2 System coupled to a timsTOF fleX 2 mass spectrometer. The mobile phases were composed of Solvent A (0.1% formic acid in 3% ACN) and Solvent B (0.1% formic acid in ACN). The injection volume was 2 μL. Following injection, the peptides were separated on C-18 reversed phase PepSep column (0.150 × 250 mm; 1.5 nm particle; Bruker) coupled with the CaptiveSpray ionization source of the mass spectrometer. The flow rate and temperature of the column chamber were set to 800 nL/min and 50 °C. Separation of peptides was achieved at the following gradient: T = 0 min: 0% B; T = 40 min: 26% B; T = 40.5 min: 95% B; T = 41.5 min: 95% B; T = 42 min: 0% B; T = 45 min: 0% B (column re-equilibration). Mass spectrometry data was collected in positive, Data Dependent Acquisition (DDA) – PASEF mode under the following conditions: a capillary voltage of 1,500 V; source temperature of 180 °C; dry gas flow at 3 L/min; and an acquisition range of 100 – 1,700 m/z. The time settings were as follows: 1/K0 Start: 0.60 Vs/cm2; 1/K0 End: 1.60 Vs/cm2; Ramp Time: 100 ms; Accumulation Time: 100 ms; Duty Cycle: 100%; and Ramp Rate: 9.42 Hz.
Proteomic Data Analysis
Proteomic data were initially processed using MaxQuant, leveraging the UniProt Macaca mulatta database to identify proteins with the label-free quantification (LFQ) method. LFQ intensities were processed and prepared using an in-house R script. Proteins detected in at least five out of six replicates of samples in any given group were classified as detected proteins for that group. The median gap-filling method addressed potential gaps in protein intensity measurements. Statistical analyses were conducted using moderated Student’s t-test with Empirical Bayes statistics of the R limma package (limma_3.58.1) to identify differentially expressed proteins, with the significance level alpha set at 0.05 and absolute log2-transformed fold change (|log2FC|) > 0.322 (corresponding to a linear FC of 1.25) (Smyth et al. 2005). Using the R stat package (stat_4.4.1), principal component analysis (PCA) was performed on centered protein expression levels to visualize the variation among groups for each region separately. For further analysis, Ingenuity Pathway Analysis (IPA) and GraphPad Prism 9 for macOS (San Diego, CA, USA) were employed to elucidate pathway interactions and perform statistical analyses.
Results
Proteomic analysis of the hippocampus and anterior cingulate cortex (ACC) detected a total of 3,756 proteins in the hippocampus and 3,805 proteins in the ACC (Fig. 2). Of the proteins detected in the hippocampus, 3,534 (94.1%) were detected in both the CBA and VEH groups, while 127 proteins (3.4%) were detected only in the CBA group and 95 proteins (2.5%) were detected only in the VEH group (Fig. 2a). In the ACC, 3,594 proteins (94.5%) were detected in both groups, while 92 proteins (2.4%) were detected only in the CBA group and 119 proteins (3.1%) were detected only in the VEH group (Fig. 2b). Pairwise analysis revealed CBA significantly altered levels of 147 proteins in the hippocampus (Fig. 3a) and 176 proteins in the ACC (Fig. 3b). In the hippocampus, 79 proteins were upregulated, and 68 proteins were downregulated, while in the ACC 96 proteins were upregulated and 80 proteins were downregulated.
Fig. 2.

Venn diagram depicting proteins detected in the CBA, VEH, or both groups in the hippocampus (a) and anterior cingulate cortex (ACC) (b). a. 3,756 proteins were detected in the hippocampus, with 3,534 of these proteins identified in both groups. b. 3,805 proteins were detected in the ACC, with 3,594 of these proteins identified in both groups
Fig. 3.

Volcano plot depicting proteins significantly altered by chronic binge alcohol (CBA) in the hippocampus and anterior cingulate cortex (ACC). a. In the hippocampus, CBA resulted in upregulation of 79 proteins and downregulation of 68 proteins. b. In the ACC, CBA resulted in upregulation of 96 proteins and downregulation of 80 proteins
Of the significantly regulated proteins, 14 were detected in both the ACC and hippocampus (Fig. 4). Five proteins were upregulated in both brain regions, including adhesion G protein-coupled receptor B2 (ADGRB2), neuron-specific calcium-binding protein hippocalcin (HPCA), synemin (SYNM), thiol methyltransferase 1A (TMT1A), and transthyretin (TTR). Seven proteins were downregulated in both regions, including aldehyde dehydrogenase 3 family member B1 (ALDH3B1), Rac/Cdc42 guanine nucleotide exchange factor 6 (ARHGEF6), carboxylic ester hydrolase (CES1), citramalyl Co-A lyase (CLYBL), 5-oxoprolinase (ATP-hydrolysing; OPLAH), synapsin III (SYN3), and zinc finger CCCH domain-containing protein 15 (ZC3H15). The remaining two proteins were differentially regulated with both proteins upregulated in the hippocampus and downregulated in the ACC (alanyl-tRNA synthetase domain containing 1 (AARSD1) and glycogen debranching enzyme (AGL)). Table 1 shows these 14 proteins and their log2-fold change (log2FC) for each brain region.
Fig. 4.

Venn diagram (A) and heat map (B) showing proteins altered by CBA in the hippocampus and anterior cingulate cortex (ACC). 14 proteins were altered by CBA in both the hippocampus and ACC
Table 1.
Chronic binge alcohol significantly altered 14 proteins in both the hippocampus and anterior cingulate cortex of SIV-infected female macaques
| Hippocampus |
Anterior cingulate cortex |
|||||||
|---|---|---|---|---|---|---|---|---|
| UniProt | Gene name | Description | log2FC | P-Value | Expression | log2FC | P-Value | Expression |
|
| ||||||||
| F6PQH0 | AARSD1 | Alanyl-tRNA synthetase domain containing 1 | 0.54063771 | 0.03173684 | Up-regulated | −0.386603 | 0.0172036 | Down-regulated |
| A0A5F8A4G7 | ADGRB2 | Adhesion G protein-coupled receptor B2 | 0.56426081 | 0.01017682 | Up-regulated | 0.45588502 | 0.01384375 | Up-regulated |
| F7EAR8 | AGL | Glycogen debranching enzyme | 0.33182155 | 0.0366304 | Up-regulated | −0.4206269 | 0.01371138 | Down-regulated |
| A0A1D5QTX0 | ALDH3B1 | Aldehyde dehydrogenase 3 Family Member B1 | −0.4680561 | 0.02047928 | Down-regulated | −0.575973 | 0.0263026 | Down-regulated |
| F6Q0Q9 | ARHGEF6 | Rac/Cdc42 guanine nucleotide exchange factor 6 | −0.4172266 | 0.00474548 | Down-regulated | −0.4440599 | 0.01484991 | Down-regulated |
| F7AI42 | CES1 | Carboxylic ester hydrolase | −0.4499579 | 0.01313481 | Down-regulated | −0.5073875 | 0.0248774 | Down-regulated |
| F7ETT5 | CLYBL | Citramalyl-CoA lyase | −1.0751604 | 0.00519463 | Down-regulated | −14.486856 | 1.82E-11 | Down-regulated |
| F7HSC9 | HPCA | Neuron-specific calcium-binding protein hippocalcin | 0.64673274 | 0.00967482 | Up-regulated | 0.47923652 | 0.00684953 | Up-regulated |
| A0A5F8ARZ5 | OPLAH | 5-oxoprolinase, ATP-hydrolysing | −0.4194829 | 0.04640646 | Down-regulated | −0.4572641 | 0.00826011 | Down-regulated |
| F7HFD1 | SYN3 | Synapsin III | −0.4407369 | 0.01491037 | Down-regulated | −0.4689005 | 0.00429813 | Down-regulated |
| F7C3M5 | SYNM | Synemin | 0.69988633 | 1.78E-05 | Up-regulated | 0.60734439 | 0.0199949 | Up-regulated |
| A0A5F8AU93 | TMT1A | Thiol methyltrans-ferase 1A | 0.64149998 | 0.00479771 | Up-regulated | 0.46004815 | 0.02060176 | Up-regulated |
| A0A1D5QXZ0 | TTR | Transthyretin | 1.25076596 | 0.04387351 | Up-regulated | 1.19095385 | 0.01180823 | Up-regulated |
| F7H9Q2 | ZC3H15 | Zinc finger CCCH domain-containing protein 15 | −0.5009104 | 0.01454721 | Down-regulated | −14.202414 | 1.57E-21 | Down-regulated |
Several proteins were detected in only one group in both brain regions. In the hippocampus, 20 proteins were detected only in VEH animals, while 17 proteins were detected only in CBA animals. Table 2 shows these hippocampal proteins and their respective p-values for each group. In the ACC, 16 proteins were detected only in the VEH animals and 11 proteins were detected only in the CBA animals. Table 3 shows these ACC proteins and their respective p-values for each group.
Table 2.
In the hippocampus, 20 proteins were detected only in the vehicle animals, while 17 proteins were only detected in the chronic binge alcohol animals
| UniProt | Gene name | Description | P-value | |
|---|---|---|---|---|
|
| ||||
| Only detected in VEH | ||||
| F6QST9 | CACNG2 | Voltage-dependent calcium channel gamma-2 subunit | 1.28E-17 | |
| F6R9U7 | SLC38A3 | Sodium-coupled neutral amino acid transporter 3 | 1.34E-20 | |
| F7DKJ0 | CLVS1 | Clavesin-1 | 1.56E-25 | |
| G7NFS5 | SLC27A4 | long-chain-fatty-acid–CoA ligase | 9.16E-19 | |
| A0A5F8AVF2 | MFSD6 | Major facilitator superfamily domain containing 6 | 1.44E-22 | |
| F6ZS77 | CHD4 | DNA helicase | 4.87E-18 | |
| F7EQ66 | PDS5B | PDS5 cohesin associated factor B | 4.88E-25 | |
| G7NCH2 | PPP2R5B | Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit | 3.16E-20 | |
| F6S0I2 | XKR4 | XK-related protein | 2.65E-19 | |
| F6WRA1 | SPECC1L | Cytospin-A | 3.04E-23 | |
| F7CB80 | SESTD1 | SEC14 domain and spectrin repeat-containing protein 1 | 1.26E-24 | |
| F6V197 | KCNJ10 | ATP-sensitive inward rectifier potassium channel 10 | 7.11E-16 | |
| Q5TM62 | MRPS18B | Small ribosomal subunit protein mS40 | 5.57E-19 | |
| F6S7U8 | LTN1 | E3 ubiquitin-protein ligase listerin | 8.25E-23 | |
| F6PQ65 | HSPB6 | Heat shock protein beta-6 | 2.97E-17 | |
| F7G2I9 | ERMP1 | Endoplasmic reticulum metallopeptidase 1 | 4.03E-19 | |
| H9H3P6 | CFH | Complement factor H | 2.40E-17 | |
| F7GDT2 | SNX30 | Sorting nexin-30 | 3.37E-13 | |
| F7GYQ8 | RPAP1 | RNA polymerase II associated protein 1 | 6.54E-19 | |
| F7AG75 | RGS6 | Regulator of G protein signaling 6 | 2.37E-14 | |
| Only detected in CBA | ||||
| F7B8J7 | NSMF | NMDA receptor synaptonuclear signaling and neuronal migration factor | 1.10E-22 | |
| A0A5F8AHI7 | GSTA2 | Glutathione S-transferase | 4.67E-15 | |
| G7MYN0 | MESD | LRP chaperone MESD | 1.12E-24 | |
| A0A1D5QYD1 | STRADA | STE20-related kinase adapter protein alpha | 3.16E-16 | |
| A0A1D5QYW7 | FXN | Frataxin, mitochondrial | 2.38E-22 | |
| I0FM31 | FGFR1OP2 | FGFR1 onco partner 2 | 6.37E-23 | |
| H9Z9T3 | RWDD1 | RWD domain containing 1 | 1.32E-23 | |
| F7DUH0 | CPSF7 | Cleavage and polyadenylation specific factor 7 | 1.01E-21 | |
| F7HNU4 | SHROOM2 | Shroom family member 2 | 4.21E-21 | |
| I2CTJ0 | RHOT2 | Mitochondrial Rho GTPase | 6.18E-23 | |
| F7GJI8 | DPY30 | Protein dpy-30 homolog | 2.84E-22 | |
| F6QI72 | KCNIP2 | Kv channel-interacting protein 2 isoform 2q | 1.81E-21 | |
| F6ZWH1 | PTDSS1 | Phosphatidylserine synthase | 9.96E-24 | |
| A0A5F7ZTS4 | RGS10 | Regulator of G protein signaling 10 | 3.08E-23 | |
| F7F4F6 | UFL1 | E3 UFM1-protein ligase 1 | 2.14E-20 | |
| A0A5F8A8L3 | F13A1 | Coagulation factor XIII A chain | 5.13E-21 | |
| F7A6I9 | ZNF638 | Zinc finger protein 638 | 1.68E-24 | |
Table 3.
In the anterior cingulate cortex, 16 proteins were detected only in the vehicle animals, while 12 proteins were only detected in the chronic binge alcohol animals
| UniProt | Gene name | Description | P-value |
|---|---|---|---|
|
| |||
| Onlv detected in VEH | |||
| A0A5F7ZB67 | PKLR | Pyruvate kinase | 1.05E-11 |
| G7NGN4 | LOC706710 | Large ribosomal subunit protein mL62 | 6.10E-21 |
| F7GFS1 | GGACT | Gamma-glutamylaminecyclotransferase | 8.53E-23 |
| A0A5F8A0J9 | COQ3 | Ubiquinone biosynthesis O-methyltransferase, mitochondrial | 1.01E-14 |
| F7AHG4 | CCDC47 | PAT complex subunit CCDC47 | 2.04E-22 |
| A0A5F7ZPN9 | SNRPE | Small nuclear ribonucleoprotein E | 6.42E-19 |
| A0A1D5Q9D5 | SKIC2 | SKI2 subunit of superkiller complex | 1.47E-21 |
| F7HS52 | GAB1 | GRB2 associated binding protein 1 | 8.99E-19 |
| F7AVV4 | RNF141 | RING finger protein 141 | 5.10E-16 |
| F7G9W1 | NRP2 | Neurolipin | 2.91E-13 |
| F6T885 | ADHFE1 | Hydroxyacid-oxoacid transhydrogenase, mitochondrial | 9.59E-13 |
| F6YW03 | STARD10 | PCTP-like protein | 8.87E-13 |
| F7BX36 | VARS2 | Valine-tRNA ligase, mitochondrial | 2.08E-21 |
| F6QI47 | POLR2B | DNA-directed RNA polymerase subunit beta | 1.31E-21 |
| F7ETT5 | CLYBL | Citramalyl-CoA lyase | 1.82E-11 |
| F7H9Q2 | ZC3H15 | Zinc finger CCCH domain-containing protein 15 | 1.57E-21 |
| Only detected in CBA | |||
| F6Q743 | CNN2 | Calponin | 1.69E-18 |
| A0A1D5QQF1 | FTL | Ferritin light chain | 3.81E-18 |
| A0A5F8A311 | BZW1 | Basic leucine zipper and W2 domains 1 | 4.34E-23 |
| I0FLZ7 | NHP2 | H/ACA ribonucleoprotein complex subunit 2 | 2.36E-20 |
| F7B3S2 | MRPL14 | Large ribosomal subunit protein uL14m | 1.22E-18 |
| F6UV16 | TEX2 | Testis expressed 2 | 1.70E-23 |
| F7GMS1 | MTMR7 | Phosphatidylinositol-3-phosphatase | 2.54E-17 |
| F7BAI3 | RBKS | Ribokinase | 3.41E-18 |
| Q5PXZ9 | TIMP3 | Metalloproteinase inhibitor 3 | 5.27E-12 |
| H9EQ69 | RMND5A | RING-type E3 ubiquitin transferase | 2.19E-18 |
| A0A1D5QYR9 | SNAP23 | Synaptosomal-associated protein | 2.67E-16 |
| F7GUL5 | MMAA | Metabolism of cobalamin associated A | 8.66E-14 |
To better understand how CBA alters the hippocampal and ACC proteomes, we utilized principal component analysis (PCA) to compare the proteomic profiles from the two treatment groups in each brain region. PCA analysis revealed that the first two principal components (PCs) account for about 90% of the variability in our data in each region (Fig. 5, Supplementary Fig. 1). In the hippocampus, PC1 accounted for 89.0% of the variability, while PC2 accounted for 3.04%. In the ACC, PC1 accounted for 82.9%, while PC2 accounted for 6.5%. The percentage of variances explained by individual components for Hippocampus and ACC regions are provided in the supplementary bar plot Supplementary Fig. 1. Supplementary Table 1 and 2 contain the loading values for the top 50 proteins in the hippocampus and the ACC, respectively.
Fig. 5.

Principal component analysis (PCA) score plot of proteomics data for the hippocampus (A) and anterior cingulate cortex (B)
Ingenuity pathway analyses (IPA) was performed to identify canonical pathways altered by CBA in the hippocampus and ACC of SIV-infected female macaques. Canonical pathways are defined as well-characterized, essential metabolic and cell signaling pathways that are generally conserved across species. In the hippocampus, 39 canonical pathways were significantly enriched by CBA, including SNARE signaling, glutaminergic receptor signaling, and opioid signaling (Table 4). In the ACC, 62 canonical pathways were significantly enriched by CBA, including actin cytoskeleton signaling, extracellular matrix organization, and post-translational protein phosphorylation (Table 5).
Table 4.
Ingenuity pathway analysis revealed 39 hippocampal pathways were significantly enriched by chronic binge alcohol administration (p < 0.05)
| Ingenuity canonical pathway | Molecules | Z-score | P-value |
|---|---|---|---|
|
| |||
| Acetylcholine Receptor Signaling Pathway | ADCY5, CACNA2D2, CACNG2, CAMK2D, CAMK2G, GUCY1A1 | −2.449 | 0.001 |
| Adrenergic Receptor Signaling Pathway (Enhanced) | ADCY5, CACNA2D2, CACNG2, GUCY1A1 | −2 | 0.028 |
| Autism Signaling Pathway | ALDH3B1, CACNA2D2, CACNG2, CAMK2D, CAMK2G | −0.447 | 0.032 |
| Calcium Signaling | CACNA2D2, CACNG2, CAMK2D, CAMK2G, MYL1 | −2 | 0.009 |
| cAMP-mediated signaling | ADCY5, AKAP12, CAMK2D, CAMK2G, GUCY1A1, RGS10 | −2 | 0.002 |
| Cardiac conduction | CACNA2D2, CAMK2D, CAMK2G, KCNIP2, SCN3B | 0 | 0.001 |
| Corticotropin Releasing Hormone Signaling | ADCY5, CACNA2D2, CACNG2, GUCY1A1 | 0 | 0.011 |
| CREB Signaling in Neurons | ADCY5, ADGRB2, CACNA2D2, CACNG2, CAMK2D, CAMK2G, GRIK5, GUCY1A1 | −1.134 | 0.024 |
| Dilated Cardiomyopathy Signaling Pathway | ADCY5, CACNA2D2, CACNG2, CAMK2D, CAMK2G, GUCY1A1, MYL1 | −2 | 0.000 |
| GABAergic Receptor Signaling Pathway (Enhanced) | ADCY5, CACNA2D2, CACNG2, GUCY1A1, SLC38A3 | 1.342 | 0.001 |
| Glutaminergic Receptor Signaling Pathway (Enhanced) | ADCY5, CACNA2D2, CACNG2, CAMK2D, CAMK2G, GRIK5, GUCY1A1, SCN3B, SLC38A3 | −1.667 | 0.000 |
| Gustation Pathway | ADCY5, CACNA2D2, CACNG2, GUCY1A1, SCN3B | −1 | 0.006 |
| ILK Signaling | ARHGEF6, MYL1, PPP2R5B, RHOT2 | 2 | 0.029 |
| Mitochondrial Dysfunction | CACNA2D2, CACNG2, CAMK2D, CAMK2G, RHOT2 | −0.447 | 0.048 |
| Necroptosis Signaling Pathway | CAMK2D, CAMK2G, CYLD, PYGL | −2 | 0.012 |
| Netrin Signaling | CACNA2D2, CACNG2, CAMK2D, CAMK2G, DCC, ENAH | −0.816 | 0.000 |
| Neurovascular Coupling Signaling Pathway | CACNA2D2, CACNG2, GUCY1A1, KCNJ10 | 0 | 0.045 |
| Neutrophil degranulation | AGL, ALDH3B1, ANXA2, ATP8A1, PYGL, TMEM30A, TMT1A, TTR | 0 | 0.006 |
| Opioid Signaling Pathway | ADCY5, CACNA2D2, CACNG2, CAMK2D, CAMK2G, GUCY1A1, RGS10, RGS6 | −1.134 | 0.000 |
| Orexin Signaling Pathway | ADCY5, CACNA2D2, CACNG2, GUCY1A1 | −2 | 0.048 |
| Parkinson’s Signaling Pathway | ALDH3B1, CACNA2D2, CACNG2, CAMK2D, CAMK2G | −2.236 | 0.032 |
| Protein Kinase A Signaling | ADCY5, AKAP12, CAMK2D, CAMK2G, DCC, GUCY1A1, MYL1, PYGL | −0.447 | 0.003 |
| RAF/MAP kinase cascade | CAMK2D, CAMK2G, KIT, KSR1, PPP2R5B | −0.447 | 0.018 |
| RAR Activation | ADCY5, CAMK2D, CAMK2G, GUCY1A1, KIT, PRMT2, RHOT2 | −1.134 | 0.012 |
| Response to elevated platelet cytosolic Ca2 + | ENDOD1, F13A1, HABP4, SCG3 | 1 | 0.007 |
| RHO GTPase cycle | ABL2, AKAP12, ARHGEF6, BRK1, CIT, CPSF7, ELMO2 | 1.134 | 0.015 |
| Role of NFAT in Cardiac Hypertrophy | ADCY5, CACNA2D2, CACNG2, CAMK2D, CAMK2G, GUCY1A1 | −2.236 | 0.002 |
| Serotonin Receptor Signaling | ADCY5, CACNA2D2, CACNG2, CAMK2D, CAMK2G, F13A1, GUCY1A1, RHOT2 | −1.414 | 0.006 |
| Signaling by Rho Family GTPases | ARHGEF6, CIT, MYL1, RHOT2, SEPTIN6 | 0.447 | 0.019 |
| Signaling by ROBO receptors | ABL2, DCC, ENAH, GPC1, NRP1 | 2.236 | 0.001 |
| Signaling by VEGF | BRK1, ELMO1, ELMO2, NRP1 | 1 | 0.003 |
| Sleep NREM Signaling Pathway | ADCY5, CAMK2D, CAMK2G, GUCY1A1 | 0 | 0.004 |
| SNARE Signaling Pathway | ADCY5, CACNA2D2, CAMK2D, CAMK2G, GUCY1A1, MYL1, SYN3 | −1.134 | 0.000 |
| Synaptic Long Term Depression | CACNA2D2, CACNG2, GUCY1A1, PPP2R5B | −1 | 0.028 |
| Synaptogenesis Signaling Pathway | ADCY5, CACNA2D2, CAMK2D, CAMK2G, GUCY1A1, SYN3 | −2 | 0.010 |
| Thrombin Signaling | ADCY5, ARHGEF6, CAMK2D, CAMK2G, GUCY1A1, MYL1, RHOT2 | 0.447 | 0.000 |
| White Adipose Tissue Browning Pathway | ADCY5, CACNA2D2, CACNG2, GUCY1A1 | −2 | 0.008 |
| WNT/SHH Axonal Guidance Signaling Pathway | ADCY5, CAMK2D, CAMK2G, GPC1, GUCY1A1 | 0.447 | 0.002 |
| Xenobiotic Metabolism PXR Signaling Pathway | ALDH3B1, CAMK2D, CAMK2G, GSTA3 | 1 | 0.026 |
Table 5.
Ingenuity pathway analysis revealed 62 anterior cingulate cortex pathways were significantly enriched by chronic binge alcohol administration (p < 0.05)
| Ingenuity canonical pathway | Molecules | Z-score | P-value |
|---|---|---|---|
|
| |||
| ABRA Signaling Pathway | ACTA2, ACTB, ACTC1, CALD1, GSN, MYH11, MYL9, TAGLN, TPM1, TPM2, VCL | 2.111 | 0.000 |
| Actin Cytoskeleton Signaling | ACTA2, ACTB, ACTC1, ACTN4, APC, ARHGEF6, FGF12, FLNA, FN1, GSN, MYH11, MYL12B, MYL9, RRAS, TLN1, VCL | 2.138 | 0.000 |
| Acute Phase Response Signaling | ALB, APOA1, APOA2, C1QB, CRABP1, FN1, FTL, RRAS, SERPINA3, TTR, VWF | 0.816 | 0.000 |
| Agrin Interactions at Neuromuscular Junction | ACTA2, ACTB, ACTC1, ARHGEF6, PKLR, RRAS | 1.342 | 0.000 |
| Amyloid fiber formation | APOA1, GSN, MFGE8, TTR | 2 | 0.002 |
| Atherosclerosis Signaling | ALB, APOA1, APOA2, APOD, COL18A1 | 2.236 | 0.002 |
| Autism Signaling Pathway | ALDH18A1, ALDH3B1, ALDH4A1, APC, FMR1, RRAS | −0.816 | 0.019 |
| Binding and Uptake of Ligands by Scavenger Receptors | ALB, APOA1, COL4A1, COL4A2, FTL, HBB | 2.449 | 0.000 |
| Caveolar-mediated Endocytosis Signaling | ACTA2, ACTB, ACTC1, ALB, CAVIN1, FLNA | 2 | 0.000 |
| Cell junction organization | ACTB, ARHGEF6, FLNA, NECTIN3, RSU1 | 1.342 | 0.000 |
| Deubiquitination | ACTB, APC, PSME1, PSME2, RNF123, WDR20 | 0 | 0.009 |
| DHCR24 Signaling Pathway | ALB, APOA1, APOA2, APOD, RRAS, TTR | 2.449 | 0.000 |
| Dilated Cardiomyopathy Signaling Pathway | ACTA2, ACTB, ACTC1, CAMK1, CAMK4, CNN1, DES, GAB1, MYH11, MYL9, TPM1 | −1.667 | 0.000 |
| EPH-Ephrin signaling | ACTB, MYH11, MYL12B, MYL9 | 2 | 0.004 |
| Epithelial Adherens Junction Signaling | MYL12B, NECTIN3, RRAS, VCL | 1 | 0.022 |
| Extracellular matrix organization | BGN, COL18A1, COL4A1, COL4A2, FN1, LUM, TTR | 2.646 | 0.000 |
| Fcγ Receptor-mediated Phagocytosis in Macrophages and Monocytes | ACTA2, ACTB, ACTC1, TLN1 | 2 | 0.004 |
| Gap Junction Signaling | ACTA2, ACTB, ACTC1, CSNK1D, PLCD3, RRAS | 0.816 | 0.023 |
| Glutaminergic Receptor Signaling Pathway (Enhanced) | CAMK4, DGKE, FMR1, PLCD3, SCN4B, VAMP2 | −0.816 | 0.023 |
| Glycerophospholipid biosynthesis | CHKB, GPCPD1, SLC44A1, STARD10 | −2 | 0.005 |
| GP6 Signaling Pathway | COL18A1, COL4A1, COL4A2, TLN1 | 2 | 0.011 |
| Hepatic Fibrosis Signaling Pathway | ACTA2, APC, CSNK1D, FTL, MYL12B, MYL9, RRAS | 2.236 | 0.023 |
| ILK Signaling | ACTA2, ACTB, ACTC1, ACTN4, ARHGEF6, FLNA, FN1, MYH11, MYL9, RSU1, TGFB1I1, VCL | 1.155 | 0.000 |
| Integrin cell surface interactions | COL18A1, COL4A1, COL4A2, FBN1, FN1, LUM, VWF | 2.646 | 0.000 |
| Integrin Signaling | ACTA2, ACTB, ACTC1, ACTN4, GSN, MYL12B, MYL9, RRAS, TLN1, VCL | 3 | 0.000 |
| L1CAM interactions | ACTB, ANK3, LYPLA2, NRP2, SCN4B, SPTBN4 | −1.633 | 0.000 |
| Leukocyte Extravasation Signaling | ACTA2, ACTB, ACTC1, ACTN4, TIMP3, VCL | 1.342 | 0.002 |
| LXR/RXR Activation | ALB, APOA1, APOA2, APOD, TTR | 2.236 | 0.002 |
| Neutrophil degranulation | AGL, ALDH3B1, CNN2, FTL, GSN, HBB, S100A11, SER-PINA3, SNAP23, TMT1A, TTR, VCL |
2.309 | 0.000 |
| Nuclear Cytoskeleton Signaling Pathway | ACTA2, ACTB, ACTC1, DES, FN1 | 2.236 | 0.017 |
| PAK Signaling | ARHGEF6, MYL12B, MYL9, RRAS | 1 | 0.008 |
| Parkinson’s Signaling Pathway | ACTA2, ACTB, ACTC1, ALDH18A1, ALDH3B1, ALDH4A1 | −2.449 | 0.018 |
| Paxillin Signaling | ACTA2, ACTB, ACTC1, ACTN4, ARHGEF6, RRAS, TLN1, VCL | 2.121 | 0.000 |
| Post-translational protein phosphorylation | ALB, APOA1, APOA2, FBN1, FN1, MFGE8, VWA1 | 2.646 | 0.000 |
| Production of Nitric Oxide and Reactive Oxygen Species in Macrophages | ALB, APOA1, APOA2, APOD | 2 | 0.041 |
| Protein Kinase A Signaling | FLNA, MTMR7, MYL12B, MYL9, PDE4B, PLCD3, PYGM | −1 | 0.022 |
| Pulmonary Fibrosis Idiopathic Signaling Pathway | ACTA2, ACTB, ACTC1, COL18A1, COL4A1, COL4A2, FN1, RRAS | 2.449 | 0.002 |
| RAF/MAP kinase cascade | ACTB, FN1, NEFL, PSME1, PSME2, SPTBN4, TLN1, VCL, VWF | 1.667 | 0.000 |
| Regulation of Actin-based Motility by Rho | ACTA2, ACTB, ACTC1, GSN, MYL12B, MYL9 | 2.236 | 0.000 |
| Regulation of Insulin-like Growth Factor (IGF) transport and uptake by IGFBPs | ALB, APOA1, APOA2, FBN1, FN1, MFGE8, VWA1 | 2.646 | 0.000 |
| Response to elevated platelet cytosolic Ca2 + | ACTN4, ALB, APOA1, FLNA, FN1, SERPINA3, TIMP3, TLN1, VCL, VWF | 3.162 | 0.000 |
| RHO GTPase cycle | ACTB, ACTC1, ARHGAP39, ARHGEF6, CAVIN1, CDC42SE2, CHN1, SNAP23, TEX2, WIPF3 |
0.632 | 0.001 |
| RHO GTPases activate PAKs | FLNA, MYH11, MYL12B, MYL9 | 2 | 0.000 |
| RHOA Signaling | ACTA2, ACTB, ACTC1, MYL12B, MYL9, NRP2 | 2.236 | 0.000 |
| RHOGDI Signaling | ACTA2, ACTB, ACTC1, ARHGEF6, MYH11, MYL12B, MYL9 | −1.633 | 0.001 |
| RNA Polymerase II Transcription | POLR2B, RNMT, SNRPE, SRSF6 | −2 | 0.019 |
| Role of Osteoclasts in Rheumatoid Arthritis Signaling Pathway | CAMK4, COL18A1, COL4A1, COL4A2, GSN, RRAS | 1.633 | 0.019 |
| Semaphorin interactions | MYH11, MYL12B, MYL9, RRAS, TLN1 | 2.236 | 0.000 |
| Semaphorin Neuronal Repulsive Signaling Pathway | MYL12B, MYL9, NRP2, PDE4B, RRAS | −0.447 | 0.004 |
| Sertoli Cell-Germ Cell Junction Signaling Pathway (Enhanced) | ACTA2, ACTB, ACTC1, NECTIN3, RRAS | −1.342 | 0.022 |
| Sertoli Cell-Sertoli Cell Junction Signaling | ACTA2, ACTB, ACTC1, ACTN4, NECTIN3, RRAS, VCL | 2.646 | 0.001 |
| Signaling by Rho Family GTPases | ACTA2, ACTB, ACTC1, ARHGEF6, DES, MYL12B, MYL9 | 1.89 | 0.002 |
| Smooth Muscle Contraction | ACTA2, CALD1, MYH11, MYL12B, MYL9, TLN1, TPM1, TPM2, VCL | 3 | 0.000 |
| SNARE Signaling Pathway | MYH11, MYL9, SNAP23, SYN3, VAMP2 | 2.236 | 0.002 |
| Striated Muscle Contraction | ACTC1, DES, TPM1, TPM2 | 2 | 0.000 |
| Synaptogenesis Signaling Pathway | CHN1, NECTIN3, RRAS, SYN3, TLN1, VAMP2 | 0.816 | 0.020 |
| Thrombin Signaling | ARHGEF6, CAMK1, CAMK4, MYL12B, MYL9, PLCD3, RRAS | −0.447 | 0.001 |
| VEGF Signaling | ACTA2, ACTB, ACTC1, ACTN4, RRAS, VCL | 1.633 | 0.000 |
| Virus Entry via Endocytic Pathways | ACTA2, ACTB, ACTC1, FLNA, RRAS | 2.236 | 0.001 |
| Wound Healing Signaling Pathway | ACTA2, COL18A1, COL4A1, COL4A2, FN1, RRAS | 2.449 | 0.007 |
| Xenobiotic Metabolism AHR Signaling Pathway | ALDH18A1, ALDH3B1, ALDH4A1, NQO2 | −2 | 0.003 |
| Xenobiotic Metabolism CAR Signaling Pathway | ALDH18A1, ALDH3B1, ALDH4A1, SOD3 | −1 | 0.043 |
IPA analysis provides z-score values representing potential pathway activation (positive z-score) or potential pathway inhibition (negative z-score). In the hippocampus, CBA inhibited 29 pathways and activated 10 pathways (Fig. 6). The hippocampal pathways with the lowest z-scores (i.e., greatest inhibition) included acetylcholine receptor signaling, Parkinson’s signaling, and synaptogenesis signaling. Among the most activated hippocampal pathways (highest z-scores) were signaling by ROBO receptors and ILK signaling. In the ACC, CBA activated 49 canonical pathways and inhibited 13 pathways (Fig. 7). The most activated pathways in the ACC included response to elevated platelet cytosolic Ca2+, integrin signaling, smooth muscle contraction, post-translational protein phosphorylation, and extracellular matrix organization, while the most inhibited pathways included Parkinson’s signaling, xenobiotic metabolism AHR signaling, glycerophospholipid biosynthesis, and RNA polymerase II transcription.
Fig. 6.

Chronic binge alcohol significantly altered 39 canonical pathways in the hippocampus
Fig. 7.

Chronic binge alcohol significantly altered 62 canonical pathways in the anterior cingulate cortex
Several pathways were altered by CBA in both the hippocampus and ACC (Fig. 8). Five canonical pathways were inhibited in both brain regions, including Parkinson’s signaling, dilated cardiomyopathy signaling, protein kinase A signaling, autism signaling, and glutaminergic receptor signaling pathways. Four canonical pathways were activated in both regions, including RHO GTPase cycle, ILK signaling, signaling by RHO Family GTPases, and response to elevated platelet cytosolic Ca2+. Interestingly, five pathways were differentially altered in the two brain regions, with one pathway (thrombin signaling) activated in the hippocampus and inhibited in the ACC and three pathways (synaptogenesis signaling, RAF/MAP kinase cascade, and SNARE signaling) inhibited in the hippocampus and activated in the ACC. One pathway (neutrophil degranulation) was activated in the ACC but had a z-score of 0 in the hippocampus, indicating that, although individual proteins in the pathway were significantly altered, overall pathway activity was not altered.
Fig. 8.

Chronic binge alcohol significantly enriched 14 canonical pathways in both the hippocampus and anterior cingulate cortex
Discussion
Extending our previous work describing the pathophysiological effects of alcohol in a preclinical rhesus macaque animal model of chronic SIV infection and ART treatment (Molina et al. 2015), the current study aimed to examine the impact of longitudinal chronic binge alcohol (CBA) exposure on the hippocampal and ACC proteome in female macaques. Our quantitative proteomic approach discovered that CBA results in differential protein expression profiles in the hippocampus and ACC of female macaques. Our analyses also identified numerous proteins and several pathways significantly enriched by CBA across the two brain regions. This study builds on previous work from our group, which investigated CBA-induced alterations in the hippocampal and frontal cortex transcriptome in SIV-infected male macaques. Interestingly, and in contrast to this previous study and our initial hypothesis, we did not observe CBA-induced enrichment of proteins involved in inflammatory and immune signaling in female macaques. Other recent CNS proteomic analyses have focused on the impact of HIV or alcohol on the brain proteome (Doke et al. 2021; Teng et al. 2023; Enculescu et al. 2019; Grantham and Farris 2019); but to our knowledge, this is the first study to examine the dual neuroproteomic impact of chronic binge alcohol in the context of SIV infection. Below, we summarize our overall findings for each region, highlight the most significantly differentially expressed proteins, and also discuss select systems related to HIV, AUD, and cognition that could serve as viable targets for pharmacotherapy and future mechanistic investigation.
Effects of CBA in the Hippocampus
In the hippocampus, 147 proteins were dysregulated by CBA, with about half of these proteins up-regulated and half down-regulated (Fig. 3A). Of hippocampal proteins expressed in both CBA and VEH animals, the largest expression increase observed was transthyretin (Table 6). Transthyretin (TTR) is a carrier protein responsible for transportation of thyroxine and retinol (Sousa et al. 2007; Ueda 2022). Importantly, TTR can also bind to amyloid beta peptide, a peptide whose accumulation is a key pathological feature of Alzheimer’s disease (AD; Sousa et al. 2007). Amyloid beta has also been shown to accumulate in the frontal cortex of HIV-positive individuals and act synergistically with Tat, one of the HIV viral proteins, to synergistically increase neurotoxicity (Achim et al. 2009; Hategan et al. 2017). TTR levels have been shown to be altered both in patients with AD and in rodent models of AD (Riisøen 1988; Serot et al. 1997; Li et al. 2011). One clinical study found that participants with higher blood TTR levels were at heightened risk of mild cognitive impairment (Nakamura et al. 2023). Another study showed serum TTR concentrations are elevated in association with higher daily alcohol consumption (Jono et al. 2016); however, to our knowledge, no studies have investigated TTR in the context of CBA exposure or AUD. Future studies should explore neuronal TTR as a potential mechanism of alcohol-related cognitive decline. In addition, neuronal pentraxin-2 (NPTX2) was also upregulated in the hippocampus of CBA animals. NPTX2 is a protein involved in excitatory synapse formation involved in clustering AMPA glutamate receptors at synapses (Chapman et al. 2019). Previous studies have shown that NPTX2 may contribute to brain atrophy and memory decline in AD (Swanson et al. 2016). Furthermore, there is evidence that NPTX2 is also upregulated in the context of Parkinson’s disease and epilepsy (Chapman et al. 2019). Previous work using an alcohol self-administration model in cynomolgus macaques found chronic ethanol altered ionotropic glutamate receptor complexes in the prefrontal cortex, and a proteomic study performed in post-mortem human brain tissue revealed changes in the glutamate transporter GLAST/EAAT1 (Acosta et al. 2010; Kashem et al. 2021). Thus, NPTX2 and glutamatergic signaling may also play a role in SIV- and CBA-induced neurological deficits.
Table 6.
Literature review of top differentially expressed proteins in the"hippocampus
| UniProt ID | Gene name | Description | Relevant literature |
|---|---|---|---|
|
| |||
| Top Upregulated Proteins (Expressed in Both Groups) | |||
| A0A1D5QXZ0 | TTR | Transthyretin |
Preclinical Mice who consumed alcohol for 30 days and were treated with naltrexone had increased transthyretin in the prefrontal cortex (Yu et al. 2011) Clinical Daily alcohol consumption is associated with increased serum transthyretin (Jono et al. 2016) People with higher levels of transthyretin are at increased risk of mild cognitive impairment (Nakamura et al. 2023) Women with HIV had lower serum transthyretin than seronegative women (Kiefer et al. 2018) |
| F7FQM7 | APOA1 | Apolipoprotein A1 |
Clinical People with HIV have increased anti-apolipoprotein A1 auto-antibodies (Frias et al. 2024) |
| A0A5F8AMA1 | CA1 | Carbonic anhydrase 1 |
Preclinical Alcohol inhibits erythrocyte carbonic anhydrase in rats (Coban et al. 2008) Review that discusses potential of carbonic anhydrases as targets to protect against neurovascular dysfunction in Alzheimer’s disease (Lemon et al. 2021) |
| Top Downregulated Proteins (Expressed in Both Groups) | |||
| A0A5F8A6B8 | MPZ | Myelin protein P0 |
Preclinical Chronic alcohol exposure in mice inhibits myelinogenesis in the prefrontal cortex and corpus callosum (Guo et al. 2021) Clinical Two patients with HIV encephalopathy had increased cerebrospinal fluid myelin basic protein, a marker for central nervous system demyelination (Pfister et al. 1989) MPZ mutations are associated with peripheral neuropathies, including Charcot-Marie-Tooth disease (Bienfait et al. 2006; Raasakka et al. 2019) |
| A0A1D5RDW1 | PMP2 | Myelin P2 protein |
Preclinical PMP2 plays a role in myelin thickening and ATP production during remyelination following nerve crush injury (Hong et al. 2024) |
| F6XQH6 | PRX | Periaxin | Periaxin mutations are associated with peripheral neuropathies including Charcot-Marie Tooth and Dejerine-Sottas neuropathy (Datta et al. 2019; Boerkoel et al. 2001; Peddareddygari 2012) |
The most downregulated hippocampal protein detected in both CBA and VEH animals was myelin protein zero (MPZ; Table 6). MPZ is the main structural component of myelin sheath in the peripheral nervous system, and disruption of MPZ is associated with the development of peripheral neuropathies (Raasakka et al. 2019). Chronic alcohol consumption can lead to the development of alcoholic neuropathy, a polyneuropathy characterized by damage to sensory, motor, and autonomic nerve fibers (Dudek et al. 2020). Although the exact mechanism of alcoholic neuropathy is not well characterized, it is thought that disruption of myelin in the peripheral nerves contributes, as in other peripheral neuropathies. The downregulation of MPZ thus provides evidence of myelin disruption in a macaque model of CBA, which has been shown in previous transcriptomic studies examining the prefrontal cortex in mice and rhesus macaques exposed to chronic alcohol (Bogenpohl et al. 2019). Several proteins were only detected in the hippocampus of CBA animals, including the mitochondrial proteins glutathione S-transferase and frataxin and the ER protein phosphatidylserine synthase. Indeed, previous work has reported alterations in nucleus accumbens glutathione signaling pathways in alcohol-drinking macaques (Womersley et al. 2024). Alteration of these mitochondrial proteins, including glutathione S-transferase, suggests disruptions due to CBA are impacting the mitochondria of hippocampal neurons, which could suggest dysregulation of hippocampal bioenergetics that would be expected to be critical for cognitive function.
Ingenuity pathway analyses revealed that CBA significantly altered 39 hippocampal pathways, including inhibition of acetylcholine receptor signaling, orexin signaling, and serotonin receptor signaling, each representing potential pharmacotherapeutic targets. Acetylcholine is a neurotransmitter critically involved in memory, attention, and motivation and normally supports cognition and anti-inflammatory processes (Hasselmo 2006; Han et al. 2017). Reductions in acetylcholine signaling may reflect cognitive deficits and heightened inflammation in the hippocampus of CBA animals. Importantly, hippocampal acetylcholine deficiency is one of the hallmarks of AD, a progressive neurodegenerative disorder and the most common cause of dementia, and cholinesterase inhibitors such as donepezil and rivastigmine are prescribed for mild and moderate AD to improve cognitive and behavioral symptoms (Chen et al. 2022). Interestingly, acetylcholine receptor antagonists may impair cognition in monkeys with a history of chronic heavy drinking, while acetylcholine receptor agonists improved cognitive deficits in socially subordinate, but not socially dominant, monkeys (Galbo-Thomma et al. 2024).
Deficits in hippocampal serotonin signaling have been shown in several monkey models involving early life stress and alcohol consumption. Early maternal separation in male rhesus macaques results in lower serotonin transporter binding potential in several brain regions, including the hippocampus and anterior cingulate gyrus (Ichise et al. 2006). Another study similarly showed early maternal separation reduces serotonin metabolites and serotonin turnover in the hippocampus and increases ethanol consumption in male rhesus macaques (Huggins et al. 2012). Furthermore, low baseline serotonin levels in the cerebrospinal fluid were associated with greater alcohol intake in male and female nonhuman primates (Wood et al. 2022). These studies, together with the present study, provide evidence that there may be a link between serotonergic dysfunction and alcohol dependence. To further explore the potential contribution of these and other neurotransmitter signaling pathways in cognition and negative affect, recently developed behavioral paradigms can be applied to functionally investigate future cohorts of SIV-infected and CBA-treated macaques (Shnitko et al. 2017).
A previous study conducted a proteomic analysis of multiple brain regions, including the hippocampus and prefrontal cortex, on the brains of adult males with and without AUD (Teng et al. 2023). Interestingly, only two hippocampal pathways were associated with differentially expressed in the AUD brains compared to control, and these pathways did not overlap with the pathways we observed in the present analysis. The majority of the AUD-altered pathways they observed were in the amygdala, including the enrichment of several pathways we saw altered by CBA in the hippocampus (adrenergic, opioid, and GABA receptor signaling).
Effects of CBA in the Anterior Cingulate Cortex
In the anterior cingulate cortex (ACC), 176 proteins were significantly altered by CBA (Fig. 3B). Of ACC proteins expressed in both CBA and VEH animals, the largest increases observed were actin alpha-2 and calponin-1, both of which are involved in smooth muscle contraction (Yuan 2015; Liu and Jin 2016; Table 7). The most inhibited ACC protein detected in both groups was voltage-gated sodium channel subunit β4, a protein that promotes excitability by slowing sodium channel inactivation and may be involved in alcohol consumption based on evidence from rodent models and humans with alcohol use disorder (Blednov et al. 2019; Table 7). Other significantly upregulated proteins in the ACC included apolipoproteins A1, A2, and D. Apolipoproteins are proteins that bind to lipids to form lipoproteins, or soluble complexes that transport lipids (Elliott et al. 2010). While apolipoprotein E is largely recognized as a major genetic risk factor for AD, recent work suggests other apolipoproteins, including apolipoprotein A1 and apolipoprotein D, may also play a role in neurodegenerative and neuropsychiatric disorders, including AD, Parkinson’s disease, and schizophrenia (Elliott et al. 2010; Qiang et al. 2013; Dassati et al. 2014; Tong et al. 2022). Furthermore, apolipoprotein A2 was found to be elevated in the serum in a nonhuman primate model of ethanol self-administration, suggesting its potential as a peripheral biomarker of ethanol consumption (Freeman et al. 2006). Interestingly, a proteomic study examining exosomal proteins derived from plasma of HIV-infected alcohol drinkers have also reported a downregulation of apolipoprotein A2 (Kodidela et al. 2020).
Table 7.
Literature review of top differentially expressed proteins in the"anterior cingulate cortex
| UniProt ID | Gene name | Description | Relevant literature |
|---|---|---|---|
|
| |||
| Top Upregulated Proteins (Expressed in Both Groups) | |||
| F7HJJ8 | ACTA2 | Alpha-2 actin |
Preclinical Chronic alcohol consumption in mice alters hippocampal actin cytoskeleton (Gao et al. 2024) A review that discusses the role of the actin cortex in the initiation of HIV infection (Spear et al. 2012) |
| A0A1D5QTD2 | CNN1 | Calponin 1 |
Preclinical CNN1 expression reduced with alcohol consumption in oligodendrocytes of mice (Bazzi et al. 2023) |
| A0A1D5QLJ5 | MFGE8 | Milk fat globule epidermal growth factor 8 |
Preclinical MFGE8 improves reduces apoptosis and attenuates organ injury in alcohol intoxicated rats (Wu et al. 2010; Chaung et al. 2019) Pre-treatment with MFGE8 reduces apoptosis and inflammation after surgical brain injury and traumatic brain injury in rats (Xiao et al. 2018; Gao et al. 2018) |
| Top Downregulated Proteins (Expressed in Both Groups) | |||
| A0A5F8A459 | SCN4B | Sodium voltage-gated channel beta subunit 4 |
Preclinical Scn4b knockout in mice alters hypnotic effects of alcohol but does not alter alcohol consumption (Blednov et al. 2019) Scn4b gene expression altered in alcohol-preferring mice and rats (Mulligan et al. 2006; Tabakoff et al. 2008) Clinical Scn4b gene expression altered in humans with alcohol use disorder (Farris et al. 2015) |
| F7DWC1 | MAGI3 | Membrane associated guanylyl kinase, WW, and PDZ domain containing 3 | — |
| F7ATN1 | MOXD1 | Monooxygenase DBH-like 1 |
Preclinical MOXD1 is predictive of alcoholic liver disease in a mouse model (Pan et al. 2024) |
Several ACC proteins were detected only in CBA animals, including ferritin light chain and metabolism of cobalamin associated A. Ferritin is an intracellular protein that stores iron and is comprised of a light chain and a heavy chain (Kotla et al. 2022). Elevated ferritin is associated with iron overload and heavy alcohol consumption and can result in ferroptosis, or cell death due to iron accumulation and lipid peroxidation (Kotla et al. 2022). Previous work has shown chronic intermittent alcohol increases iron-positive cells in the hippocampus and prefrontal cortex and is associated with anxiety and depression (Xu et al. 2022). Furthermore, inhibiting ferroptosis prevents alcohol-induced neuronal damage (Xu et al. 2022). Interestingly, higher ferritin heavy chain in the cerebrospinal fluid may be neuroprotective in PWH (Kaur et al. 2021). Future work with our nonhuman primate model will follow up on this finding with ex vivo assessments (e.g., Seahorse-based assays) to better understand the role of ferroptosis in CBA-induced deficits. Metabolism of cobalamin associated A is a protein involved in translocation of cobalamin (vitamin B12) into the mitochondria. Importantly, cobalamin is one of the vitamins that can become deficient in patients with chronic AUD, resulting in megaloblastic anemia and demyelination of peripheral nerves (Green et al. 2017; Madebo et al. 2022). Thus, it is interesting that the metabolism and translocation of this vitamin is upregulated in our CBA animals. This may be due to a compensatory upregulation of cobalamin-associated enzymes due to a CBA-induced vitamin deficiency.
Canonical pathways that were most significantly activated by CBA in the ACC included Parkinson’s signaling, xenobiotic metabolism aryl hydrocarbon receptor (AhR) signaling, and glycerophospholipid biosynthesis. CBA also inhibited several canonical pathways in the ACC, including response to elevated platelet cytosolic Ca2+, post-translational protein phosphorylation, and extracellular matrix organization.
Commonalities Between the Hippocampus and Anterior Cingulate Cortex
Overall, 14 common proteins (Fig. 4, Table 1) and 14 common canonical pathways (Fig. 8) were altered by CBA in both the hippocampus and ACC, suggesting some similarities in the effects of alcohol on distinct brain regions. As described above, the negative effects of lifetime alcohol consumption on executive function were found to be mediated by functional connectivity between the hippocampus and ACC in humans (Honnorat et al. 2022), suggesting that circuit-based mechanisms linking these two crucial regions may be particularly vulnerable to excessive alcohol exposure. Many of the individual protein alterations were in the same direction (i.e., upregulation or downregulation in both brain regions). For example, transthyretin was upregulated in both regions, while citramaloyl-CoA lyase was downregulated in both regions. Synapsin III was also downregulated in both regions, potentially indicating reductions in neurogenesis and neuronal plasticity based on its role in these processes (Porton et al. 2011). Indeed, previous work has shown CBA in nonhuman primates impairs hippocampal neurogenesis (Taffe et al. 2010).
Several canonical pathways were altered in both brain regions. Interestingly, synaptogenesis and SNARE signaling were significantly enriched by CBA in each brain region. In the hippocampus, synaptogenesis and SNARE signaling were inhibited, suggesting chronic alcohol reduces the formation of new synapses and neurotransmitter release in the hippocampus. In contrast, synaptogenesis and SNARE signaling pathways were activated in the ACC, suggesting CBA increases synapse formation and neurotransmitter release in this region. Thus, chronic alcohol may differentially alter synaptic signaling across these two regions, which may be due to functionally distinct roles for the two areas. For example, the hippocampus is heavily involved in spatial learning and long-term memory, while the ACC plays a more specific role in working memory and affective pain processing. These unique roles may explain the differential effects of CBA on regional physiology and how alcohol impacts these two sets of functional memory domains.
Other pathways affected by CBA were predicted to be altered in the same direction (i.e., both activated or both inhibited). Parkinson’s signaling was inhibited in both regions, which may indicate altered dopaminergic signaling in response to CBA in these dopaminoceptive regions, while integrin-linked kinase (ILK) signaling was activated in both regions, which may play influence hippocampal neurogenesis (Xu et al. 2015). Protein kinase A (PKA) signaling was also inhibited across both regions, suggesting a decrease in PKA-driven phosphorylation events and intracellular signaling cascades, a function that regulates diverse physiological processes including synaptic plasticity, memory, and metabolism that may be dysregulated across several neurological and psychiatric disorders (Glebov-McCloud et al. 2024). Interestingly, alcohol acutely activates PKA signaling, and PKA signaling may be critically involved in regulation of voluntary alcohol consumption (Thiele et al. 2000; Ron and Messing 2013). Particularly notable is the previously described role of reduced PKA activity in the nucleus accumbens in alcohol drinking (Misra and Pandey 2006) and reduced PKA signaling in the central amygdala in alcohol withdrawal-related anxiety (Pandey et al. 2005). Chronic intermittent alcohol exposure also dysregulates PKA-regulated genes in the medial prefrontal cortex, nucleus accumbens, and amygdala (Repunte-Canonigo et al. 2007). These deficits may extend to other areas including the hippocampus and ACC to mediate additional deleterious alcohol-related behaviors. Numerous studies have described interventions targeting an inhibition of cAMP phosphodiesterases (PDEs) to boost cAMP/PKA activity and reduce alcohol drinking (Logrip et al. 2014; Logrip 2015), and a recent NIH study recommended that PET imaging of PDE4B in individuals with alcohol use disorder (AUD) should be considered in conjunction with ongoing trials of PDE4 inhibitors to treat alcohol withdrawal and reduce alcohol consumption (Tang et al. 2024). A previous study examining proteomic alterations in postmortem brains from patients with AUD found metabolic changes in proteins associated with glycolysis (phosphofructokinase, glyceraldehyde-3-phosphate) and oxidative phosphorylation (NADH dehydrogenase iron-sulfur protein 8, cytoplasmic aspartate aminotransferase) in the prefrontal cortex (Enculescu et al. 2019); however, we did not see similar alterations in the ACC.
Limitations and Future Directions
While the analysis of female macaque brain represents a major strength of the current study, there are some limitations of bioinformatics analyses on rhesus macaque systems, as the monkey proteomic and IPA databases are less well characterized compared to those for humans and rodents. Another limitation is the exclusion of male animals in the present cohort, although ongoing studies are currently being conducted in male macaques. Future studies are necessary to extend these findings at the individual protein level and functional studies will be vital to confirm the role of specific pathways in corresponding behavioral tests. Another limitation of this study is the lack of SIV-negative control animals. Female macaques were particularly dimcult to source during the study period to include this control group. Ongoing macaque cohorts incorporate this factor, and we recognize that the results from the present analyses must be limited to interpretation of alterations in the context of SIV. Additionally, all animals in this experiment were treated with ART, which can itself have significant neurotoxic effects (Robertson et al. 2012). Thus, we are unable to tease out the effects of CBA without exposure to SIV and ART, and future studies should examine this to better understand how ART modulates the relationship between SIV infection and CBA exposure. Furthermore, the ART regimen we use here was the current standard at the time when the experiment was conducted (2015–2021); current cohorts of animals have an updated ART regimen that includes bictegravir. Finally, in comparison to our fixed alcohol administration model, Grant and colleagues have developed a model of alcohol self-administration in monkeys that allows for the study of the impact of voluntary chronic alcohol consumption and its comorbidities (Baker et al. 2023), including effects on cognitive behaviors (Shnitko et al. 2020; Grant et al. 2021). Nevertheless, while there are many advantages of the voluntary self-administration model, the ability to control the amount of alcohol consumed in our animal model is a strength of our present study.
Summary
Together, the results from this study suggest that CBA administration in SIV-infected female macaques produces widespread alterations in the hippocampal and ACC proteomes. Our discoveries indicate that certain proteins and pathways are similarly altered across both brain regions, including upregulation of transthyretin and inhibition of Parkinson’s and protein kinase A signaling pathways. Importantly, CBA may also differentially impact these regions, suggesting CBA-induced deficits may differ by brain region and may be specific to the functional role of that region. Ongoing studies seek to expand this work to males to better understand the role of sex on the impact of CBA on brain regional proteomics, especially the differential impact on specific brain areas including the hippocampus and ACC. Overall, the hippocampus and ACC represent important areas at the intersection of alcohol- and HIV-induced cognitive decline and for the discovery of potential therapeutic targets for treatment of brain-related disorders in women with HIV.
Supplementary Material
Acknowledgements
This work was supported by the LSU Health-New Orleans Comprehensive Alcohol Research Center (P60AA009803 (PEM)), as well as training and research grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA): F30AA030941 (TFS), T35AA021097 (PEM), T32AA007577 (PEM), and R01AA025996 (SE). Data Availability Statement: All primary data will be made available upon reasonable request and will be uploaded to the NIAAA Data Archive within six months of publication.
Footnotes
Competing Interests The authors declare no competing interests.
Declarations
None to report.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11481-025-10179-5.
Data Availability
No datasets were generated or analysed during the current study.
References
- Achim CL, Adame A, Dumaop W et al. (2009) Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol 4:190–199. 10.1007/s11481-009-9152-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta G, Hasenkamp W, Daunais JB et al. (2010) Ethanol self-administration modulation of NMDA receptor subunit and related synaptic protein mRNA expression in prefrontal cortical fields in cynomolgus monkeys. Brain Res 1318:144–154. 10.1016/j.brainres.2009.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EJ, Moore S, Gonzales SW, Grant KA (2023) Long-term drinking stability in the open-access self-administration monkey model. Alcohol 113:41–48. 10.1016/j.alcohol.2023.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzi SA, Maguire C, Mayfield RD, Melamed E (2023) Alcohol induces concentration-dependent transcriptomic changes in oligodendrocytes. bioRxiv 2023.09.22.559075. 10.1101/2023.09.22.559075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienfait HME, Faber CG, Baas F et al. (2006) Late onset axonal Charcot-Marie-Tooth phenotype caused by a novel myelin protein zero mutation. J Neurol Neurosurg Psychiatry 77:534–537. 10.1136/jnnp.2005.073437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Bajo M, Roberts AJ et al. (2019) Scn4b regulates the hypnotic effects of ethanol and other sedative drugs. Genes Brain Behav 18:e12562. 10.1111/gbb.12562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerkoel CF, Takashima H, Stankiewicz P et al. (2001) Periaxin mutations cause recessive Dejerine-Sottas neuropathy. Am J Hum Genet 68:325–333. 10.1086/318208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenpohl JW, Smith ML, Farris SP et al. (2019) Cross-species co-analysis of prefrontal cortex chronic ethanol transcriptome responses in mice and monkeys. Front Mol Neurosci 12:197. 10.3389/fnmol.2019.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara A, Sow MS, Touré A et al. (2020) Anxiety and depression among HIV patients of the infectious disease department of Conakry University Hospital in 2018. Epidemiol Infect 148:e8. 10.1017/S095026881900222X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman G, Shanmugalingam U, Smith PD (2019) The role of neuronal pentraxin 2 (NP2) in regulating glutamatergic signaling and neuropathology. Front Cell Neurosci 13:575. 10.3389/fncel.2019.00575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaung WW, Brenner M, Yen H-T et al. (2019) Recombinant human milk fat globule-EGF factor VIII (rhMFG-E8) as a therapy for sepsis after acute exposure to alcohol. Mol Med 25:52. 10.1186/s10020-019-0118-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z-R, Huang J-B, Yang S-L, Hong F-F (2022) Role of cholinergic signaling in Alzheimer’s disease. Molecules 27:1816. 10.3390/molecules27061816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban TA, Beydemir S, Gülçin I, Ekinci D (2008) The effect of ethanol on erythrocyte carbonic anhydrase isoenzymes activity: an in vitro and in vivo study. J Enzyme Inhib Med Chem 23:266–270. 10.1080/14756360701474780 [DOI] [PubMed] [Google Scholar]
- Cook RL, Cook CL, Karki M et al. (2016) Perceived benefits and negative consequences of alcohol consumption in women living with HIV: a qualitative study. BMC Public Health 16:263. 10.1186/s12889-016-2928-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane HM, McCaul ME, Chander G et al. (2017) Prevalence and factors associated with hazardous alcohol use among persons living with hiv across the US in the current era of antiretroviral treatment. AIDS Behav 21:1914–1925. 10.1007/s10461-017-1740-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassati S, Waldner A, Schweigreiter R (2014) Apolipoprotein D takes center stage in the stress response of the aging and degenerative brain. Neurobiol Aging 35:1632–1642. 10.1016/j.neurobiolaging.2014.01.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Kataria S, Govindarajan R (2019) A case report on Charcot-Marie-Tooth disease with a novel periaxin gene mutation. Cureus 11:e5111. 10.7759/cureus.5111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doke M, Ramasamy T, Sundar V et al. (2021) Proteomics profiling with SWATH-MS quantitative analysis of changes in the human brain with HIV infection reveals a differential impact on the frontal and temporal lobes. Brain Sci 11:1438. 10.3390/brainsci11111438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek I, Hajduga D, Sieńko C et al. (2020) Alcohol-Induced neuropathy in chronic alcoholism: causes, pathophysiology, diagnosis, and treatment options. Curr Pathobiol Rep 8:87–97. 10.1007/s40139-020-00214-w [DOI] [Google Scholar]
- Duko B, Ayalew M, Ayano G (2019) The prevalence of alcohol use disorders among people living with HIV/AIDS: a systematic review and meta-analysis. Subst Abuse Treat Prev Policy 14:52. 10.1186/s13011-019-0240-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Koob GF (2010) Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol 5:393–401. 10.2217/fnl.10.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S (2012) Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev 36:2179–2192. 10.1016/j.neubiorev.2012.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DA, Weickert CS, Garner B (2010) Apolipoproteins in the brain: implications for neurological and psychiatric disorders. Clin Lipidol 51:555–573. 10.2217/CLP.10.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enculescu C, Kerr ED, Yeo KYB et al. (2019) Proteomics reveals profound metabolic changes in the alcohol use disorder brain. ACS Chem Neurosci 10:2364–2373. 10.1021/acschemneuro.8b00660 [DOI] [PubMed] [Google Scholar]
- Fama R, Sullivan EV, Sassoon SA et al. (2016) Impairments in component processes of executive function and episodic memory in alcoholism, HIV infection, and HIV infection with alcoholism comorbidity. Alcohol Clin Exp Res 40:2656–2666. 10.1111/acer.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SP, Arasappan D, Hunicke-Smith S et al. (2015) Transcriptome organization for chronic alcohol abuse in human brain. Mol Psychiatry 20:1438–1447. 10.1038/mp.2014.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick-Schmidt T, Edwards S (2023) Within and beyond the binary: sex and gender differences in pain and alcohol use disorder. Curr Addict Rep. 10.1007/s40429-023-00534-y [DOI] [Google Scholar]
- Freeman WM, Gooch RS, Lull ME et al. (2006) Apo-aii is an elevated biomarker of chronic non-human primate ethanol self-administration. Alcohol Alcohol 41:300–305. 10.1093/alcalc/agl021 [DOI] [PubMed] [Google Scholar]
- Frias MA, Pagano S, Bararpour N et al. (2024) People living with HIV display increased anti-apolipoprotein A1 auto-antibodies, inflammation, and kynurenine metabolites: a case-control study. Front Cardiovasc Med 11:1343361. 10.3389/fcvm.2024.1343361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbo-Thomma LK, Epperly PM, Blough BE et al. (2024) Cognitive-enhancing effects of acetylcholine receptor agonists in group-housed cynomolgus monkeys who drink ethanol. J Pharmacol Exp Ther 389:258–267. 10.1124/jpet.123.001854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos EM, Simon L, Molina PE (2024) Chronic binge alcohol mediated hepatic metabolic adaptations in SIV-infected female rhesus macaques. Alcohol and Alcoholism 59:agae060. 10.1093/alcalc/agae060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA et al. (2002) The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol 63:179–186. 10.15288/jsa.2002.63.179 [DOI] [PubMed] [Google Scholar]
- Gao Y-Y, Zhang Z-H, Zhuang Z et al. (2018) Recombinant milk fat globule-EGF factor-8 reduces apoptosis via integrin β3/FAK/PI3K/AKT signaling pathway in rats after traumatic brain injury. Cell Death Dis 9:845. 10.1038/s41419-018-0939-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D-P, Wang L-W, Xie D-L et al. (2024) Chronic alcohol exposure alters the levels and assembly of the actin cytoskeleton and microtubules in the adult mouse hippocampus. J Integr Neurosci 23:118. 10.31083/j.jin2306118 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Chandran A, Jansen JP (2012) Epidemiology of HIV-related neuropathy: a systematic literature review. AIDS Res Hum Retroviruses 28:36–48. 10.1089/AID.2011.0116 [DOI] [PubMed] [Google Scholar]
- Glebov-McCloud AGP, Saide WS, Gaine ME, Strack S (2024) Protein Kinase A in neurological disorders. J Neurodevelop Disord 16:9. 10.1186/s11689-024-09525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD et al. (2017) Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiat 74:911–923. 10.1001/jamapsychiatry.2017.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Newman N, Gonzales S, Shnitko TA (2021) Replicability in measures of attentional set-shifting task performance predicting chronic heavy drinking in rhesus monkeys. Alcohol 96:93–98. 10.1016/j.alcohol.2021.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham EK, Farris SP (2019) Bioinformatic and biological avenues for understanding alcohol use disorder. Alcohol 74:65–71. 10.1016/j.alcohol.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Allen LH, Bjørke-Monsen A-L et al. (2017) Vitamin B12 deficiency. Nat Rev Dis Primers 3:17040. 10.1038/nrdp.2017.40 [DOI] [PubMed] [Google Scholar]
- Guo F, Zhang Y-F, Liu K et al. (2021) Chronic exposure to alcohol inhibits new myelin generation in adult mouse brain. Front Cell Neurosci 15:732602. 10.3389/fncel.2021.732602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Li X, Hao J (2017) The cholinergic anti-inflammatory pathway: an innovative treatment strategy for neurological diseases. Neurosci Biobehav Rev 77:358–368. 10.1016/j.neubiorev.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Hasselmo ME (2006) The role of acetylcholine in learning and memory. Curr Opin Neurobiol 16:710–715. 10.1016/j.conb.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hategan A, Bianchet MA, Steiner J et al. (2017) HIV Tat protein and amyloid-p peptide form multifibrillar structures that cause neurotoxicity. Nat Struct Mol Biol 24:379–386. 10.1038/nsmb.3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer DW (2003) Male and female sensitivity to alcohol-induced brain damage. Alcohol Res Health 27:181–185 [PMC free article] [PubMed] [Google Scholar]
- Hong J, Garfolo R, Kabre S et al. (2024) PMP2 regulates myelin thickening and ATP production during remyelination. Glia 72:885–898. 10.1002/glia.24508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honnorat N, Fama R, Müller-Oehring EM et al. (2022) Alcohol use disorder and its comorbidity with HIV infection disrupts anterior cingulate cortex functional connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging 7:1127–1136. 10.1016/j.bpsc.2020.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins KN, Mathews TA, Locke JL et al. (2012) Effects of early life stress on drinking and serotonin system activity in rhesus macaques: 5-hydroxyindoleacetic acid in cerebrospinal fluid predicts brain tissue levels. Alcohol 46:371–376. 10.1016/j.alcohol.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise M, Vines DC, Gura T et al. (2006) Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. J Neurosci 26:4638–4643. 10.1523/JNEUROSCI.5199-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazebi N, Evans C, Kadaru HS et al. (2021) HIV-related neuropathy: pathophysiology, treatment and challenges. J Neurol Exp Neurosci 7:15–24. 10.17756/jnen.2021-082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jono H, Su Y, Obayashi K et al. (2016) Sources of variation of transthyretin in healthy subjects in East and Southeast Asia: Clinical and experimental evidence for the effect of alcohol on transthyretin metabolism. Clin Chim Acta 458:5–11. 10.1016/j.cca.2016.04.011 [DOI] [PubMed] [Google Scholar]
- Kashem MA, Šerý O, Pow DV et al. (2021) Actions of alcohol in brain: genetics, metabolomics, GABA receptors, proteomics and glutamate transporter GLAST/EAAT1. Curr Mol Pharmacol 14:138–149. 10.2174/1874467213666200424155244 [DOI] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S et al. (2005) HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ 12:878–892. 10.1038/sj.cdd.4401623 [DOI] [PubMed] [Google Scholar]
- Kaur H, Bush WS, Letendre SL et al. (2021) Higher CSF ferritin heavy-chain (Fth1) and transferrin predict better neurocognitive performance in people with HIV. Mol Neurobiol 58:4842–4855. 10.1007/s12035-021-02433-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer EM, Hoover DR, Shi Q et al. (2018) Longitudinal evaluation of markers of inflammation in HIV-positive and HIV-negative Rwandan women. HIV Med 19:734–744. 10.1111/hiv.12665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Fitzpatrick AL, Rapp SR et al. (2019) Alcohol Consumption and risk of dementia and cognitive decline among older adults with or without mild cognitive impairment. JAMA Netw Open 2:e1910319. 10.1001/jamanetworkopen.2019.10319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodidela S, Wang Y, Patters BJ et al. (2020) Proteomic Profiling of exosomes derived from plasma of HIV-infected alcohol drinkers and cigarette smokers. J Neuroimmune Pharmacol 15:501–519. 10.1007/s11481-019-09853-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotla NK, Dutta P, Parimi S, Das NK (2022) The role of ferritin in health and disease: recent advances and understandings. Metabolites 12:609. 10.3390/metabo12070609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Gould TJ (2016) Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: contributions to development and maintenance of addiction. Learn Mem 23:515–533. 10.1101/lm.042192.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-H, Amin ND, Venkatesan A et al. (2013) Impaired neurogenesis and neurite outgrowth in an HIV-gp120 transgenic model is reversed by exercise via BDNF production and Cdk5 regulation. J Neurovirol 19:418–431. 10.1007/s13365-013-0194-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon N, Canepa E, Ilies MA, Fossati S (2021) Carbonic anhydrases as potential targets against neurovascular unit dysfunction in Alzheimer’s disease and stroke. Front Aging Neurosci 13:772278. 10.3389/fnagi.2021.772278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DA, Gross AL, Briceño EM et al. (2021) Sex differences in cognitive decline among US adults. JAMA Netw Open 4:e210169. 10.1001/jamanetworkopen.2021.0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Masliah E, Reixach N, Buxbaum JN (2011) Neuronal production of transthyretin in human and murine Alzheimer’s disease: is it protective? J Neurosci 31:12483–12490. 10.1523/JNEUROSCI.2417-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Jin J-P (2016) Calponin isoforms CNN1, CNN2 and CNN3: Regulators for actin cytoskeleton functions in smooth muscle and non-muscle cells. Gene 585:143–153. 10.1016/j.gene.2016.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML (2015) Phosphodiesterase regulation of alcohol drinking in rodents. Alcohol 49:795–802. 10.1016/j.alcohol.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Vendruscolo LF, Schlosburg JE et al. (2014) Phosphodiesterase 10A regulates alcohol and saccharin self-administration in rats. Neuropsychopharmacology 39:1722–1731. 10.1038/npp.2014.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madebo T, Bemanian M, Vold JH et al. (2022) Vitamin B12 levels, substance use patterns and clinical characteristics among people with severe substance use disorders: a cohort study from western norway. Nutrients 14:1941. 10.3390/nu14091941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K et al. (2009) Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology 72:1661–1668. 10.1212/WNL.0b013e3181a55f65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B et al. (2005) Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clin Exp Res 29:896–901. 10.1097/01.alc.0000164376.69978.6b [DOI] [PubMed] [Google Scholar]
- Maxi JK, Dean M, Zabaleta J et al. (2016) Chronic binge alcohol administration dysregulates hippocampal genes involved in immunity and neurogenesis in simian immunodeficiency virus-infected macaques. Biomolecules 6:43. 10.3390/biom6040043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTernan PM, Siggins RW, Catinis A et al. (2022) Chronic binge alcohol and ovarian hormone loss dysregulate circulating immune cell SIV co-receptor expression and mitochondrial homeostasis in SIV-infected rhesus macaques. Biomolecules 12:946. 10.3390/biom12070946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira RG, Lira M, Tapia-Rojas C et al. (2019) Effect of alcohol on hippocampal-dependent plasticity and behavior: role of glutamatergic synaptic transmission. Front Behav Neurosci 13:288. 10.3389/fnbeh.2019.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra K, Pandey SC (2006) The decreased cyclic-AMP dependent-protein kinase A function in the nucleus accumbens: a role in alcohol drinking but not in anxiety-like behaviors in rats. Neuropsychopharmacology 31:1406–1419. 10.1038/sj.npp.1300900 [DOI] [PubMed] [Google Scholar]
- Molina PE, Bagby GJ, Nelson S (2014) Biomedical consequences of alcohol use disorders in the HIV-infected host. Curr HIV Res 12:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, Amedee AM, Winsauer P et al. (2015) Behavioral, metabolic, and immune consequences of chronic alcohol or cannabinoids on HIV/AIDs: studies in the non-human primate SIV model. J Neuroimmune Pharmacol 10:217–232. 10.1007/s11481-015-9599-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA (2017) Immune activation and neuroinflammation in alcohol use and HIV infection: evidence for shared mechanisms. Am J Drug Alcohol Abuse 43:7–23. 10.1080/00952990.2016.1211667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ et al. (2006) Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci U S A 103:6368–6373. 10.1073/pnas.0510188103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Imaoka M, Tazaki F et al. (2023) Serum transthyretin level and its relationship with cognitive function in community-dwelling older people: cross sectional and longitudinal study. Arch Gerontol Geriatr 115:105226. 10.1016/j.archger.2023.105226 [DOI] [PubMed] [Google Scholar]
- Neigh GN, Rhodes ST, Valdez A, Jovanovic T (2016) PTSD co-morbid with HIV: Separate but equal, or two parts of a whole? Neurobiol Dis 92:116–123. 10.1016/j.nbd.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahng AR, McGinn MA, Paulsen RI, Edwards S (2017) The prefrontal cortex as a critical gate of negative affect and motivation in alcohol use disorder. Curr Opin Behav Sci 13:139–143. 10.1016/j.cobeha.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T, Zhao Z, Lu J et al. (2024) Fenofibrate inhibits MOXD1 and PDZK1IP1 expression and improves lipid deposition and inflammation in mice with alcoholic fatty liver. Life Sci 336:122321. 10.1016/j.lfs.2023.122321 [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T (2005) Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest 115:2762–2773. 10.1172/JCI24381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Happel KI, Amedee AM et al. (2010) Alcohol’s role in HIV transmission and disease progression. Alcohol Res Health 33:203–218 [PMC free article] [PubMed] [Google Scholar]
- Park LS, Hernández-Ramírez RU, Silverberg MJ et al. (2016) Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis. AIDS 30:273–291. 10.1097/QAD.0000000000000922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Petrides M, Toga AW (2009) The Rhesus Monkey Brain in Stereotaxic Coordinates, 2nd edn. Academic Press [Google Scholar]
- Peddareddygari (2012) Analysis of association of deletion in the repeat region of the periaxin gene with late onset motor neuropathy. J Neurol Res. 10.4021/jnr154w [DOI] [Google Scholar]
- Pfister HW, Einhäupl KM, Wick M et al. (1989) Myelin basic protein in the cerebrospinal fluid of patients infected with HIV. J Neurol 236:288–291. 10.1007/BF00314458 [DOI] [PubMed] [Google Scholar]
- Poret JM, Guidry JJ, Simon L, Molina PE (2021) Chronic binge alcohol and ovariectomy dysregulate omental adipose tissue metaboproteome in simian immunodeficiency virus-infected female macaques. Physiol Genomics 53:358–371. 10.1152/physiolgenomics.00001.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porton B, Wetsel WC, Kao H-T (2011) Synapsin III: role in neuronal plasticity and disease. Semin Cell Dev Biol 22:416–424. 10.1016/j.semcdb.2011.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang JK, Wong YC, Siderowf A et al. (2013) Plasma apolipoprotein A1 as a biomarker for Parkinson disease. Ann Neurol 74:119–127. 10.1002/ana.23872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raasakka A, Ruskamo S, Kowal J et al. (2019) Molecular structure and function of myelin protein P0 in membrane stacking. Sci Rep 9:642. 10.1038/s41598-018-37009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Lutjens R, van der Stap LD, Sanna PP (2007) Increased expression of protein kinase A inhibitor alpha (PKI-alpha) and decreased PKA-regulated genes in chronic intermittent alcohol exposure. Brain Res 1138:48–56. 10.1016/j.brainres.2006.09.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riisøen H (1988) Reduced prealbumin (transthyretin) in CSF of severely demented patients with Alzheimer’s disease. Acta Neurol Scand 78:455–459. 10.1111/j.1600-0404.1988.tb03687.x [DOI] [PubMed] [Google Scholar]
- Robertson K, Liner J, Meeker RB (2012) Antiretroviral neurotoxicity. J Neurovirol 18:388–399. 10.1007/s13365-012-0120-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Messing RO (2013) Signaling pathways mediating alcohol effects. Curr Top Behav Neurosci 13:87–126. 10.1007/7854_2011_161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA, Center for Behavioral Health Statistics and Quality (2022) National Survey on Drug Use and Health. Table 5.9A—Alcohol use disorder in past year: among people aged 12 or older; by age group and demographic characteristics, numbers in thousands, 2022 and 2023. Available from: https://www.samhsa.gov/data/report/2023-nsduh-detailed-tables. Accessed 2 Aug 2024
- Serot J-M, Christmann D, Dubost T, Couturier M (1997) Cerebrospinal fluid transthyretin: aging and late onset Alzheimer’s disease. J Neurol Neurosurg Psychiatry 63:506–508. 10.1136/jnnp.63.4.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitko TA, Allen DC, Gonzales SW et al. (2017) Ranking cognitive flexibility in a group setting of rhesus monkeys with a set-shifting procedure. Front Behav Neurosci 11:55. 10.3389/fnbeh.2017.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitko TA, Gonzales SW, Grant KA (2019) Low cognitive flexibility as a risk for heavy alcohol drinking in non-human primates. Alcohol 74:95–104. 10.1016/j.alcohol.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitko TA, Gonzales SW, Newman N, Grant KA (2020) Behavioral flexibility in alcohol-drinking monkeys: the morning after. Alcohol Clin Exp Res 44:729–737. 10.1111/acer.14289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Edwards S, Molina PE (2021a) Pathophysiological consequences of at-risk alcohol use; implications for comorbidity risk in persons living with human immunodeficiency virus. Front Physiol 12:758230. 10.3389/fphys.2021.758230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Torres D, Saravia A et al. (2021b) Chronic binge alcohol and ovariectomy-mediated impaired insulin responsiveness in SIV-infected female rhesus macaques. Am J Physiol Regul Integr Comp Physiol 321:R699–R711. 10.1152/ajpregu.00159.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer EJ, Valdes-Sueiras M, Commins D, Levine A (2010) Neurologic presentations of AIDS. Neurol Clin 28:253–275. 10.1016/j.ncl.2009.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK (2005) limma: Linear Models for Microarray Data. In: Gentleman R, Carey VJ, Huber W et al. (eds) Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer-Verlag, New York, pp 397–420 [Google Scholar]
- Sousa JC, Cardoso I, Marques F et al. (2007) Transthyretin and Alzheimer’s disease: Where in the brain? Neurobiol Aging 28:713–718. 10.1016/j.neurobiolaging.2006.03.015 [DOI] [PubMed] [Google Scholar]
- Spear M, Guo J, Wu Y (2012) The trinity of the cortical actin in the initiation of HIV-1 infection. Retrovirology 9:45. 10.1186/1742-4690-9-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens FL, Hurley RA, Taber KH (2011) Anterior cingulate cortex: unique role in cognition and emotion. J Neuropsychiatry Clin Neurosci 23:121–125. 10.1176/jnp.23.2.jnp121 [DOI] [PubMed] [Google Scholar]
- Swanson A, Willette AA, Alzheimer’s Disease Neuroimaging Initiative (2016) Neuronal Pentraxin 2 predicts medial temporal atrophy and memory decline across the Alzheimer’s disease spectrum. Brain Behav Immun 58:201–208. 10.1016/j.bbi.2016.07.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Kechris K et al. (2008) The genomic determinants of alcohol preference in mice. Mamm Genome 19:352–365. 10.1007/s00335-008-9115-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD et al. (2010) Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc Natl Acad Sci U S A 107:11104–11109. 10.1073/pnas.0912810107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Kim SW, Olsen-Dufour A et al. (2024) PET imaging in rat brain shows opposite effects of acute and chronic alcohol exposure on phosphodiesterase-4B, an indirect biomarker of cAMP activity. Neuropsychopharmacology. 10.1038/s41386-024-01988-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng P-N, Barakat W, Tran SM et al. (2023) Brain proteomic atlas of alcohol use disorder in adult males. Transl Psychiatry 13:318. 10.1038/s41398-023-02605-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J et al. (2000) High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci 20:RC75. 10.1523/JNEUROSCI.20-10-j0003.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Gong S, Zhang Y et al. (2022) Association of circulating apolipoprotein AI levels in patients with Alzheimer’s disease: a systematic review and meta-analysis. Front Aging Neurosci 14:899175. 10.3389/fnagi.2022.899175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M (2022) Transthyretin: Its function and amyloid formation. Neurochem Int 155:105313. 10.1016/j.neuint.2022.105313 [DOI] [PubMed] [Google Scholar]
- Venkatesan A, Nath A, Ming G, l, Song H, (2007) Adult hippocampal neurogenesis: regulation by HIV and drugs of abuse. Cell Mol Life Sci 64:2120–2132. 10.1007/s00018-007-7063-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, John Y, Barbas H (2021) Pathways for contextual memory: the primate hippocampal pathway to anterior cingulate cortex. Cereb Cortex 31:1807–1826. 10.1093/cercor/bhaa333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, Moerschbaecher JM, Brauner IN et al. (2002) Alcohol unmasks simian immunodeficiency virus-induced cognitive impairments in rhesus monkeys. Alcohol Clin Exp Res 26:1846–1857. 10.1097/01.ALC.0000042171.80435.F1 [DOI] [PubMed] [Google Scholar]
- Womersley JS, Obellianne C, Padula AE et al. (2024) Adaptations in glutathione-based redox protein signaling pathways and alcohol drinking across species. Biomed Pharmacother 180:117514. 10.1016/j.biopha.2024.117514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood EK, Lemmon DP, Schwandt ML et al. (2022) Central nervous system monoamine metabolite response to alcohol exposure is associated with future alcohol intake in a nonhuman primate model (Macaca mulatta). Addict Biol 27:e13142. 10.1111/adb.13142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2024) HIV data and statistics. WHO. https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics. Accessed 12 Dec 2024 [Google Scholar]
- Wu R, Chaung WW, Zhou M et al. (2010) Milk fat globule EGF factor 8 attenuates sepsis-induced apoptosis and organ injury in alcohol-intoxicated rats. Alcohol Clin Exp Res 34:1625–1633. 10.1111/j.1530-0277.2010.01248.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Li G, Chen Y et al. (2018) Milk Fat Globule-Epidermal Growth Factor-8 Pretreatment Attenuates Apoptosis and Inflammation via the Integrin-β3 Pathway after Surgical Brain Injury in Rats. Front Neurol 9:96. 10.3389/fneur.2018.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X-F, Li T, Wang D-D et al. (2015) Integrin-linked kinase is essential for environmental enrichment enhanced hippocampal neurogenesis and memory. Sci Rep 5:11456. 10.1038/srep11456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Xiong Q, Tian X et al. (2022) Alcohol exposure induces depressive and anxiety-like behaviors via activating ferroptosis in mice. Int J Mol Sci 23:13828. 10.3390/ijms232213828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen F-S, Wang S-I, Lin S-Y et al. (2022) The impact of heavy alcohol consumption on cognitive impairment in young old and middle old persons. J Transl Med 20:155. 10.1186/s12967-022-03353-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP (2013) The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behav Brain Res 254:34–44. 10.1016/j.bbr.2013.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Halder D, Baek MN et al. (2011) Changes in the expression of transthyretin and protein kinase Cγ genes in the prefrontal cortex in response to naltrexone. Neurosci Lett 488:288–293. 10.1016/j.neulet.2010.11.049 [DOI] [PubMed] [Google Scholar]
- Yuan S-M (2015) α-Smooth Muscle Actin and ACTA2 Gene Expressions in Vasculopathies. Braz J Cardiovasc Surg 30:644–649. 10.5935/1678-9741.20150081 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.


