Abstract
Skin autofluorescence (SAF), reflecting advanced glycation endproducts’ accumulation in tissue, has been proposed as a noninvasive aging biomarker. Yet, SAF has not been compared with well-established blood-based aging biomarkers such as MetaboHealth in association with frailty. Furthermore, no previous study determined the longitudinal association of SAF with frailty.
We used 2 382 Doetinchem Cohort Study participants’ (aged 46.0–85.4) cross-sectional data, of whom 1 654 had longitudinal SAF measurements. SAF was measured using the AGE Reader. MetaboHealth was calculated on 1H-NMR-metabolomics. Linear regressions were used for the associations of SAF and MetaboHealth on the 36-deficit frailty index and logistic regressions for being pre-frail or frail as determined by the frailty phenotype. Longitudinal associations were determined by an interaction term between age and SAF in a linear mixed model.
SAF and MetaboHealth were associated with higher odds of pre-frailty (odd ratios per standard deviation SAF: 1.21 [1.10–1.32], MetaboHealth: 1.35 [1.24–1.49]) and frailty (SAF: 1.70 [1.41–2.06], MetaboHealth: 1.90 [1.57–2.32]). When mutually adjusted, both aging biomarkers remained associated with pre-frailty (SAF: 1.16 [1.05–1.27], MetaboHealth 1.33 [1.21–1.46]) and frailty (SAF: 1.52 [1.25–1.85], MetaboHealth: 1.75 [1.43–2.14]). Additionally, SAF and MetaboHealth were associated with higher frailty index scores (percentage increase per standard deviation SAF: 1.35 [1.00–1.70], MetaboHealth: 1.87 [1.54–2.20]), also after mutual adjustment (SAF: 1.02 [0.68–1.37], MetaboHealth: 1.69 [1.35–2.02]). SAF was also longitudinally associated with the frailty index (percentage per unit/year increase: 0.12 [0.07–0.16]).
The mutual independence of SAF and MetaboHealth implies they capture distinct aspects of the aging process. Altogether, these findings emphasize SAF’s clinical potential as an age-related decline biomarker, which could be further enhanced when combined with MetaboHealth.
Keywords: Biomarkers, Epidemiology, Human aging, MetaboHealth, Molecular
Background
Frailty, a hallmark of aging, is characterized by age-associated declined physiological functioning and increased vulnerability to age-related diseases (1). However, the onset and level of frailty vary between individuals and cannot be explained by chronological age alone (2). Frailty is associated with higher risks of mortality, hospitalization, falls, and long-term care admissions, thereby affecting the quality of life (1,3,4). The predominantly used frailty definitions are the frailty phenotype (5), a measure of merely physical frailty, and the frailty index (6), a measure of accumulated age-related deficits.
Several invasive blood-based molecular biomarkers of biological age, such as MetaboHealth (7)—a metabolomics-based aging biomarker—have been proposed to quantify and predict an individual’s age-related decline (8,9). In recent studies, skin autofluorescence (SAF) has emerged as a promising biomarker for assessing biological age (10,11). SAF can be assessed noninvasively by placing the forearm on the AGE Reader device, reflecting the long-term accumulation of advanced glycation end products (AGEs) in tissue (12,13). AGEs are compounds formed through nonenzymatic condensation between the carbonyl groups of reducing sugars and the free amino groups in proteins, lipids, or nucleic acids, followed by subsequent rearrangements into stable, irreversible end-products (14,15). Glycation has effects through three main pathways: (i) AGE tissue accumulation, (ii) in situ glycation, which leads to tissue structure damage, and (iii) receptor (RAGE) activation, which activates pro-inflammatory and pro-oxidative signaling pathways (15–17). Together, they lead to chronic inflammation/oxidative stress, fibrosis, and tissue stiffness (15–17).
Previous studies have demonstrated cross-sectional associations between SAF and MetaboHealth with both the frailty phenotype and frailty index and their components (8,10,18,19). However, the association of SAF with the frailty index and frailty phenotype has not been validated in another cohort, nor have any longitudinal studies examined the relationship between SAF and frailty. Moreover, the associations of SAF and MetaboHealth with frailty have not been compared, while for the use of biomarkers in public health or clinical practice, a noninvasive marker would be preferred over an invasive marker if it conveyed the same information as the invasive marker.
Therefore, in the current study, we determine, cross-sectionally and longitudinally, the associations of SAF with the frailty phenotype and the frailty index and compare the strength of the cross-sectional association with that of MetaboHealth.
Method
Study Population
The study population consisted of participants from the Doetinchem Cohort Study (DCS) (20), a study of adult residents of Doetinchem, the Netherlands. The study is ongoing and now includes seven examinations spaced at 5-year intervals.
In the current study, we included 2 382 participants who participated in the sixth examination (2013–2017) and had available information on SAF, MetaboHealth, the frailty index, and the frailty phenotype. Among these participants, 1 325 also participated in the seventh examination (2018–2022) and had available information on SAF, the frailty index, and the frailty phenotype. The external Medical Ethics Committees of the Netherlands Organization for Applied Scientific Research and of the University of Utrecht approved the study (NL19158.041.07 and NL63779.041.17), and all participants provided informed consent.
Frailty Phenotype
Following Fried’s criteria (5), participants were considered pre-frail if they exhibited 1 or 2 of the frailty phenotype components and frail when ≥3 components were present. The components included weakness, slowness, low physical activity, exhaustion, and unintentional weight loss.
[1] Weakness was defined as being in the lowest quintile of handgrip strength stratified by sex and body mass index (BMI).
[2] Slowness was in the original frailty phenotype measured by gait speed, which was unavailable in the sixth Doetinchem Cohort Study examination round. Therefore, we defined slowness as the inability to stand up from a chair without using one’s arms or taking >14.15 seconds for women and >14.67 for men to perform the Five Times Sit to Stand Test (FTSTST), following previously established cutoffs (21). We chose the FTSTST based on prior research indicating a strong correlation between low gait speed and a high FTSTST score (21–24). The FTSTST was exclusively conducted in participants aged ≥60, given the a priori expectation that issues on this test don’t typically arise before this age, implying that those under 60 were considered not slow.
[3] Low physical activity was determined based on the Dutch version of the validated European Prospective Investigation into Cancer and Nutrition physical activity questionnaire (25). When participants were in the lowest sex-stratified quintile, we defined them as low physically active.
[4] Exhaustion was identified through two statements in the Center for Epidemiological Studies Depression (CES-D) scale (26): (a) “I felt that everything I did was an effort”; (b) “I could not get going.” Individuals were categorized as exhausted if they answered frequently or mostly on ≥1 of these statements.
[5] Unintentional weight loss was defined as losing >5% of body weight compared with the previous visit.
Frailty Index
The frailty index (6), previously used in DCS (27), is composed of 36 age-associated deficits incorporating information on chronic conditions (n = 22) and cognitive (n = 3), physical (n = 7), and psychological (n = 3) functioning. For a full description of all deficits, see Supplementary Table 1. Health deficits were categorized into binary or ternary states: 0 representing complete absence, 0.5 indicating partial presence, and 1 denoting full presence of the deficit. Based on prior research (28), we only included participants with available information on >30 deficits. Next, the frailty index was calculated by dividing the total sum of deficits by the number of deficits considered. As diabetes, BMI, and renal functioning were part of the frailty index and were included in the statistical models, we also calculated the frailty index without these deficits for the purpose of sensitivity analyses. Furthermore, we also calculated domain-specific scores, all using the same procedure as the frailty indices. These indices, excluding specified deficits, were only calculated when the same ratio of non-missing deficits as in the full frailty index, that is, ≥5/6, were available.
Skin Autofluorescence
The AGE Reader SU (DiagnOptics Technologies B.V., Groningen, The Netherlands) was initially used in the DCS starting May 2013 to measure SAF. A second AGE Reader SU was introduced in June 2016. In May 2021, these devices were replaced by two AGE Readers MU (DiagnOptics Technologies B.V.). Measurements on all devices followed similar procedures: forearm skin was cleaned with alcohol to remove lotions, and approximately 4 cm2 of forearm skin was exposed to an excitation light with a peak wavelength of ~378 nm. The AGE Reader utilizes the fluorescence properties of AGEs, analyzing emission and reflection spectra to estimate skin AGEs and generating numerical values in arbitrary units (AU), where higher scores indicate higher tissue AGEs levels. The automated software of the AGE Reader integrates skin reflectance values falling within the 6%–10% range (Fitzpatrick type V skin color) into SAF values, excluding participants with skin reflections below 6% (Fitzpatrick type VI) (29). None of our participants had skin reflections below this threshold. We Z-scaled SAF values per examination, considering measurements >3 standard deviations from the mean as outliers. This led to the exclusion of 28 measurements from the sixth and 18 measurements from the seventh examination.
MetaboHealth
MetaboHealth is a score based on 14 metabolites measured in ethylenediaminetetraacetic acid plasma via high‐throughput nuclear magnetic resonance using the Nightingale platform 2020 assay. This score is based on a previous study in 44 168 European participants from 12 European cohorts and includes metabolite measures of lipid metabolism, fatty acid metabolism, glycolysis, fluid balance, and inflammation (7). We calculated MetaboHealth using the MiMIR R-package (30). A higher score represents a higher metabolomics-based biological age.
Selection and Assessment of Covariates
We selected covariates when previous research indicated they were associated with SAF, or MetaboHealth, and frailty (7,31–36). Age was determined as the chronological age at SAF measurement. Sex was based on self-reported sex. Socioeconomic status was based on the highest attained education following UNESCO classification (37). Smoking status was assessed through questionnaires and, in our analyses, categorized into never, former, and current smokers, with never smokers as the reference category. Weight and height were measured at the research center, from which BMI was calculated. Diabetes status was determined based on self-report or random glucose measurement >=11.1 mmol/l. Renal function was estimated from plasma creatinine using the 2021 CKD-EPI formula (38). Considering the documented seasonal effects of SAF (11) and metabolomics (39) measurements, we determined the season of measurement using meteorological seasons. The device used was included in the longitudinal analyses as a categorical variable, with the first AGE-reader SU as the reference category, as we observed a confounding effect of the used device on our association.
Statistical Analysis
We determined differences in baseline characteristics between the participants with and without information on SAF and frailty during the seventh examination. For this, we used a Student’s t-test for normally distributed continuous variables, a Mann–Whitney test for non-normal distributed continuous variables, and χ2 test for binary and categorical variables.
The Mann–Whitney tests were used to determine whether there were differences in SAF and MetaboHealth values between participants for each component of the frailty phenotype separately. Logistic regression analyses were used to determine the cross-sectional associations between raw and Z-score normalized SAF and MetaboHealth with the frailty phenotype and its components. Linear regression analyses were used to determine the cross-sectional associations between raw and Z-score normalized aging biomarkers with the frailty index and its domains. We include the raw analyses for future meta-analyses but chose to scale the aging biomarkers to be able to compare their results. In the first model, we adjusted analyses for age, sex, socioeconomic status, and season of measurement (11,39). In the second model, we included the other aging biomarker to determine whether the effects of SAF and MetaboHealth on frailty were independent of each other. Next, we performed sensitivity analyses on both models, adjusting for diabetes status, BMI, renal function, and smoking status, given the established influence of these factors on SAF and MetaboHealth (7,31–34). We determined the interaction terms between SAF and MetaboHealth with sex to determine whether sex was an effect modifier in the associations of these aging biomarkers and frailty.
Next, we examined the associations of SAF with changes in frailty with aging. To assess the dynamics of SAF over time, we determined the Spearman’s correlation coefficient between SAF measures from the two visits. We determined the longitudinal association of SAF and changes in the frailty phenotype and its components using logistic mixed model analyses with the Nelder-Mead optimizer. Additionally, linear mixed model analyses were used for the longitudinal associations of SAF with the frailty index and its domains. In all analyses, we included an interaction term between chronological age and SAF to determine the change in frailty with aging as measured by SAF. Furthermore, we added the device as a covariate to adjust for differences that could be attributed to different devices used between examinations. Additionally, we performed a sensitivity analysis including only participants with measurements on the first AGE Reader SU.
All analyses were performed in R 4.3.2. Language refinement was done using ChatGPT and Grammarly. Subsequentially, the authors reviewed and edited the text as needed and take full responsibility for the content of the publication.
Results
Participants Characteristics
We included 2 382 DCS participants with cross-sectional information on SAF, MetaboHealth, frailty phenotype, and frailty index from the sixth examination. Of them, for 1 654 participants, also information was available on SAF and both frailty measures in the seventh examination. MetaboHealth was not available for the seventh examination. Participants who attended both examinations were, on average, younger, with lower BMI, MetaboHealth, SAF, and frailty index scores and higher renal function during the sixth examination. Additionally, participants with longitudinal SAF information available were also more likely to be highly educated and less likely to have a diabetes diagnosis or be frail at the sixth examination (Table 1).
Table 1.
Characteristics of the Study Population
| All | Only 6th Examination | Both Examinations | p-Value | |
|---|---|---|---|---|
| N | 2 382 | 728 | 1 654 | |
| Women (%) | 1 247 (52.4) | 382 (52.5) | 865 (52.%) | .97 |
| Age (years) at 6th examination (SD) | 63.7 (8.9) | 67.0 (9.6) | 62.2 (8.2) | <.01 |
| Age (years) at 7th examination (SD) | 67.8 (8.2) | 67.8 (8.2) | ||
| Highest attained education | <.01 | |||
| Primary education (%) | 64 (2.7) | 35 (4.8) | 29 (1.8) | |
| Lower vocational or intermediate general education (%) | 823 (34.6) | 327 (44.9) | 496 (30.0) | |
| Intermediate vocational or secondary education (%) | 825 (34.6) | 195 (26.8) | 630 (38.1) | |
| Higher vocational education (%) | 670 (28.1) | 171 (23.5) | 499 (30.2) | |
| BMI (kg/m2) at 6th examination (SD) | 26.7 (4.2) | 27.1 (4.3) | 26.5 (4.1) | <.01 |
| BMI (kg/m2) at 7th examination (SD) | 26.6 (4.4) | 26.6 (4.4) | ||
| Smoking status at 6th examinationa | .06 | |||
| Current smoker (%) | 282 (11.8) | 109 (15.0) | 173 (10.5) | |
| Former smoker (%) | 1 178 (49.5) | 369 (50.7) | 809 (48.9) | |
| Never smoker (%) | 897 (37.7) | 242 (33.2) | 655 (39.6) | |
| Smoking status at 7th examinationa | ||||
| Current smoker (%) | 115 (7.0) | 115 (7.0) | ||
| Former smoker (%) | 857 (51.8) | 857 (51.8) | ||
| Never smoker (%) | 658 (39.8) | 658 (39.8) | ||
| Diabetes at 6th examination (%) | 150 (6.3) | 61 (8.4) | 89 (5.4) | .01 |
| Diabetes at 7th examination (%) | 111 (6.7) | 111 (6.7) | ||
| Renal function (eGFR) at 6th examination [IQR] | 77.8 [68.4, 87.3] | 76.2 [65.7, 85.7] | 78.5 [69.4, 88.1] | <.01 |
| Renal function (eGFR) at 7th examination [IQR] | 72.7 [64.4, 82.7] | 72.7 [64.4, 82.7] | ||
| Skin autofluorescence (AU) at 6th examination [IQR] | 2.3 [2.1, 2.7] | 2.5 [2.2, 2.8] | 2.3 [2.0, 2.6] | <.01 |
| Skin autofluorescence (AU) at 7th examination [IQR] | 2.4 [2.1, 2.8] | 2.4 [2.1, 2.8] | ||
| MetaboHealth (AU) [IQR] | −0.07 [−0.32, 0.22] | 0.07 [−0.21, 0.37] | −0.12 [−0.35, 0.16] | <.01 |
| Frailty phenotype at 6th examination | ||||
| Frail (%) | 161 (6.8) | 94 (12.9) | 67 (4.1) | <.01 |
| Pre-frail (%) | 1 141 (47.9) | 382 (52.5) | 759 (45.9) | |
| Not frail (%) | 1 080 (45.3) | 252 (34.6) | 828 (50.1) | |
| Frailty index (0–1) at 6th examination [IQR] | 0.07 [0.03, 0.13] | 0.09 [0.04, 0.17] | 0.06 [0.03, 0.11] | <.01 |
| Frailty phenotype at 7th examination | ||||
| Frail (%) | 114 (6.9) | 114 (6.9) | ||
| Pre-frail (%) | 829 (50.1) | 829 (50.1) | ||
| Not frail (%) | 711 (43.0) | 711 (43.0) | ||
| Frailty index (0–1) at 7th examination [IQR] | 0.08 [0.03, 0.14] | 0.08 [0.03, 0.14] |
Notes: AU = arbitrary units; BMI = body mass index; eGFR = estimated glomerular filtration rate; IQR = interquartile range; SD = standard deviation. Values represent number of participants and percentages for categorical variables, mean and standard deviation for age and BMI and median and interquartile range for renal function, skin autofluorescence, MetaboHealth, and the frailty index. The p-value indicates differences in baseline characteristics between the participants with and without a measurement during the seventh examination using a Student’s t-test for normally distributed continuous variables, a Mann–Whitney test for non-normal distributed continuous variables, and χ2 test for binary and categorical variables.
aSmoking status information was missing for 25 participants (of whom 17 had two SAF measurements) at the sixth examination and for 20 participants at the seventh examination.
Cross-Sectional Analyses: Frailty Phenotype
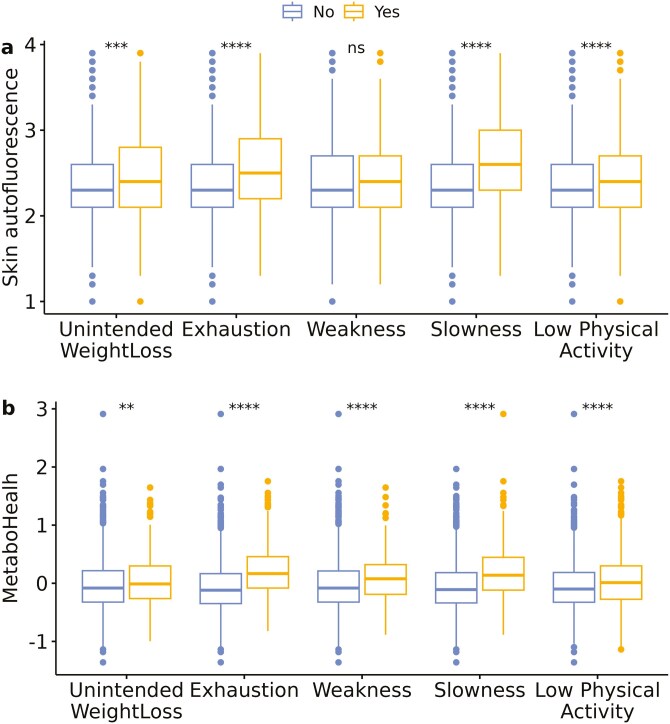
As illustrated in Figure 1, higher median SAF values and MetaboHealth scores were observed in individuals with unintended weight loss, exhaustion, slowness, and low physical activity compared with those without unintended weight loss, no exhaustion, no slowness, and normal physical activity, respectively. Higher median MetaboHealth scores were also observed with weakness compared with non-weak participants.
Figure 1.
Boxplots of SAF and MetaboHealth by the presence of different frailty phenotype components. The figure illustrates boxplots per frailty phenotype component of (A) skin autofluorescence, (B) MetaboHealth. Asterix indicates the significance of the Mann–Whitney test of participants in whom a certain component was absent (left/blue) or present (yellow/right), with ns indicating p > .05; *p < .05 and p > .01; **p < .01 and p > .001; ***p < .001 and p > .0001; and ****p < .0001.
An increase of one unit in both SAF and MetaboHealth was associated with higher odds of prevalent pre-frailty. Specifically, the odds ratio (OR) per unit increase in SAF was 1.53 (95% confidence interval 1.24; 1.89), and for MetaboHealth, it was 2.06 (1.65–2.57). Similarly, for prevalent frailty, the OR per unit increase in SAF was 3.35 (2.18–5.21) and 4.45 (2.85–7.05) for MetaboHealth. These associations were observed in a multivariable logistic regression model adjusting for age, sex, season, and socioeconomic status (Supplementary Table 2).
To facilitate the comparison of the results between SAF and MetaboHealth, we Z-transformed both aging biomarkers. We observed higher odds ratios for MetaboHealth than SAF on both prevalent pre-frailty (SAF OR per standard deviation [SD] increase: 1.21 [1.10–1.32], MetaboHealth: 1.35 [1.24–1.49]) and frailty (OR per SD SAF: 1.70 [1.41–2.06], MetaboHealth: 1.90 [1.57–2.32]). These results indicate that per SD increase in SAF, the odds of being pre-frail were 21% higher, the odds of being frail 70% higher, and respectively 35% and 90% higher per SD increase in MetaboHealth. Furthermore, both were associated with higher odds of having any of the components of the frailty phenotype, although the association between SAF and exhaustion was not statistically significant (Table 2).
Table 2.
Cross-Sectional Analyses of Skin Autofluorescence and MetaboHealth With Frailty
| Odds on being/having | Ncases/N (%) | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SAF | MetaboHealth | SAF | MetaboHealth | ||||||
| OR(CI) | pFDR | OR(CI) | pFDR | OR(CI) | pFDR | OR(CI) | pFDR | ||
| Pre-frail (reference non-frail) | 1 141/2 221 (51%) | 1.21 (1.10–1.32) | <.001 | 1.35 (1.24–1.49) | <0.001 | 1.16 (1.05–1.27) | 4.22 × 10−3 | 1.33 (1.21–1.46) | <.001 |
| Frail (reference non-frail) | 161/1 241 (13%) | 1.70 (1.41–2.06) | <.001 | 1.90 (1.57–2.32) | <0.001 | 1.52 (1.25–1.85) | <0.001 | 1.75 (1.43–2.14) | <.001 |
| Unintended weight loss (no/yes) | 322/2 382 (14%) | 1.27 (1.12–1.44) | <.001 | 1.14 (1.01–1.28) | 0.04 | 1.25 (1.10–1.42) | 1.16 × 10−3 | 1.09 (0.96–1.23) | .20 |
| Weakness (no/yes) | 431/2 382 (18%) | 1.16 (1.03–1.30) | .01 | 1.56 (1.39–1.75) | <0.001 | 1.06 (0.94–1.19) | 0.40 | 1.54 (1.37–1.73) | <.001 |
| Exhaustion (no/yes) | 186/2 382 (8%) | 1.10 (0.94–1.29) | .24 | 1.30 (1.12–1.51) | 1.14 × 10−3 | 1.05 (0.89–1.23) | 0.62 | 1.29 (1.11–1.50) | 1.97 × 10−3 |
| Slowness (no/yes) | 355/2 382 (15%) | 1.34 (1.18–1.51) | <.001 | 1.30 (1.15–1.47) | <.001 | 1.28 (1.13–1.45) | <0.001 | 1.23 (1.09–1.40) | 2.20 × 10−3 |
| Low physical activity (no/yes) | 720/2 382 (30%) | 1.21 (1.10–1.33) | <.001 | 1.23 (1.12–1.35) | <.001 | 1.17 (1.06–1.29) | 2.85 × 10−3 | 1.20 (1.09–1.32) | <.001 |
| Outcome | N | Model 1 | Model 2 | ||||||
| SAF | MetaboHealth | SAF | MetaboHealth | ||||||
| %(CI | pFDR | %(CI | pFDR | %(CI | pFDR | %(CI | pFDR | ||
| Frailty index (0–100%) | 2 382 | 1.35 (1.00–1.70) | <.001 | 1.87 (1.54–2.20) | <.001 | 1.02 (0.68–1.37) | <.001 | 1.69 (1.35–2.02) | <.001 |
| Frailty index without BMI, renal function, diabetes (0–100%) | 2 379 | 1.26 (0.90–1.61) | <.001 | 1.58 (1.24–1.92) | <.001 | 0.99 (0.63–1.35) | <.001 | 1.40 (1.06–1.74) | <.001 |
| Chronic conditions (0–100%) | 2 381 | 1.06 (0.71–1.40) | <.001 | 1.80 (1.47–2.13) | <.001 | 0.73 (0.39–1.08) | <.001 | 1.67 (1.33–2.00) | <.001 |
| Cognitive domain (0–100%) | 2 067 | 2.13 (1.24–3.02) | <.001 | 1.87 (1.00–2.75) | <.001 | 1.84 (0.94–2.74) | <.001 | 1.55 (0.66–2.43) | <.001 |
| Physical domain (0–100%) | 2 380 | 1.66 (1.08–2.23) | <.001 | 2.41 (1.86–2.96) | <.001 | 1.23 (0.65–1.81) | <.001 | 2.19 (1.63–2.75) | <.001 |
| Psychological domain (0–100%) | 2 321 | 1.68 (0.89–2.48) | <.001 | 1.10 (0.33–1.87) | 5.31 × 10−3 | 1.52 (0.71–2.33) | <.001 | 0.83 (0.04–1.61) | .04 |
Notes: Model 1 adjusts for age, sex, and socioeconomic status. Model 2 includes additional adjustments for the other biomarker. % = percentage increase; BMI = body mass index; CI = indicates 95%-confidence interval; N = number of participants; Ncases = number of cases; OR = odds ratio; pFDR = p-value after false discovery rate correction; SAF = skin autofluorescence.
When both scaled SAF and scaled MetaboHealth were included in the same model, both remained independently associated with being pre-frail (OR per SD SAF: 1.16 [1.05–1.27], MetaboHealth 1.33 [1.21–1.46]) and frail (OR per SD SAF: 1.52 [1.25–1.85], MetaboHealth 1.75 [1.43–2.14]). Furthermore, we only observed a small attenuation of the effect sizes for the different components of the frailty phenotype when both SAF and MetaboHealth were mutually adjusted (Table 2).
Cross-Sectional Analyses: Frailty Index
Furthermore, one unit increase in both SAF and MetaboHealth was associated with higher frailty index scores (percentage increase in frailty index (%) per unit increase SAF: 2.97 [2.21–3.73], MetaboHealth: 4.31 [3.55–5.07]) (Supplementary Table 2). After we standardized the aging biomarkers to enhance comparability, we observed higher effect estimates for MetaboHealth compared with SAF (% per SD 1.35 [1.00–1.70], MetaboHealth: 1.87 [1.54–2.20]). These associations remained statistically significant in a sensitivity analysis using the frailty index without the deficits of BMI, diabetes, and renal function (% per SD SAF: 1.26 [0.90–1.61], MetaboHealth: 1.58 [1.24–1.92]). These associations were observed across all domains of the frailty index (Table 2).
Moreover, the association of scaled SAF and scaled MetaboHealth with the frailty index was mutually independent (% per SD SAF: 1.02 [0.68–1.37], MetaboHealth: 1.69 [1.35–2.02]) and all domains (Table 2). This mutual independence remained in a sensitivity analysis using the frailty index without deficits of BMI, diabetes, and renal function (% per SD SAF: 0.99 [0.63–1.35], MetaboHealth: 1.40 [1.06–1.74]).
Sensitivity analyses additionally adjusting for BMI, smoking status, renal function, and diabetes did not notably change any of the previously mentioned results (Supplementary Table 3). There was no significant interaction term between SAF or MetaboHealth and sex (p > .1) for either the frailty phenotype, its components, the frailty index, or the different domains of the frailty index.
Longitudinal Analyses
SAF had a Spearman’s rank correlation coefficient of 0.58 between examinations. The interaction term between age (in years) and SAF (per AU) suggested longitudinal associations with the frailty phenotype, showing marginally increased odds of being pre-frail (OR per AU/year 1.02 [1.00–1.04]) with a similar effect size when only including participants who were measured two times with the same device (OR per AU/year 1.02 [1.00–1.05]) (Table 3, Supplementary Table 4). As shown in Table 3, we observed a relatively large effect estimate for the association of SAF with being frail (OR per AU/year 1.39 [0.93–2.08]), yet this result was not statistically significant. However, this association attenuated when adjusting for smoking status, diabetes, renal function, and BMI (OR per AU/year 1.02 [0.97–1.08]) (Supplementary Table 4), suggesting that these factors confounded the longitudinal association between SAF and being frail.
Table 3.
Longitudinal Associations of Skin Autofluorescence
| Odds on being/having | N | Nobs | Odd ratio (95% CI) | pFDR |
|---|---|---|---|---|
| Pre-frail (reference non-frail) | 2 260 | 3 761 | 1.02 (1.00–1.04) | .03 |
| Frail (reference non-frail) | 1 534 | 2 066 | 1.39 (0.93–2.08) | .16 |
| Unintended weight loss (no/yes) | 2 382 | 4 036 | 1.01 (0.99–1.03) | .62 |
| Weakness (no/yes) | 2 382 | 4 036 | 1.10 (1.02–1.17) | .02 |
| Exhaustion (no/yes) | 2 382 | 4 036 | 1.03 (0.96–1.12) | .47 |
| Slowness (no/yes) | 2 382 | 4 036 | 0.97 (0.94–1.01) | .22 |
| Low physical activity (no/yes) | 2 382 | 4 036 | 1.02 (1.00–1.05) | .15 |
| Outcome | N | Nobs | Beta (CI) | pFDR |
| Frailty index (0–100%) | 2 382 | 4 036 | 0.12 (0.07–0.16) | <.001 |
| Frailty index without BMI, renal function, diabetes (0–100%) | 2 380 | 4 031 | 0.12 (0.07–0.17) | <.001 |
| Chronic conditions (0–100%) | 2 382 | 4 031 | 0.06 (0.01–0.11) | .05 |
| Cognitive domain (0–100%) | 2 226 | 3 616 | 0.47 (0.32–0.62) | <.001 |
| Physical domain (0–100%) | 2 380 | 3 456 | 0.19 (0.10–0.29) | <.001 |
| Psychological domain (0–100%) | 2 347 | 3 952 | 0.05 (−0.08–0.18) | .49 |
Notes: Effect estimates indicate the interaction term between age and skin autofluorescence per arbitrary unit per year. Analyses are adjusted for sex, season of measurement, AGE-reader used, and socioeconomic status. BMI = body mass index; CI = 95%-confidence interval; N = number of participants; Nobs = number of observations; pFDR = p-value after false discovery rate correction.
Moreover, we observed longitudinal associations of SAF with the frailty index (% per AU/year 0.12 [0.07–0.16]) with similar effect estimates after excluding BMI, diabetes, and renal function from the frailty index (% per AU/year 0.12 [0.07–0.17]). These results indicate that for each year increase in age, one AU unit of SAF is associated with a 0.12% higher frailty index. We observed stronger effect sizes when restricting to participants with the same device for both measurements for the normal frailty index (% per AU/year 0.20 [0.13–0.27]) and the frailty index without the three before-mentioned deficits (% per AU/year 0.20 [0.12–0.27]), as shown in Supplementary Table 4. Domain-specific analyses suggested that the cognitive domain (% per AU/year 0.47 [0.32–0.62]), the physical domain (% per AU/year 0.19 [0.10–0.29]), and the prevalence of chronic diseases (% per AU/year 0.06 [0.01–0.11]) drove these longitudinal associations. Sensitivity analyses, including adjustments for BMI, diabetes, and renal function, did not notably alter the results (Supplementary Table 4).
Interaction terms between SAF and sex did not reach statistical significance (p > .1) in associations with the frailty phenotype, its individual components, the frailty index, or its domains.
Discussion
In this first study with longitudinal measurements of SAF in a population-based cohort, we validated the cross-sectional associations between the noninvasive, easy-to-measure SAF and the frailty phenotype and frailty index. Furthermore, we compared the associations of SAF and frailty with those between the more invasive MetaboHealth and frailty. Our findings showed similar effect sizes between the two biomarkers, with generally higher effect sizes for MetaboHealth. Moreover, we demonstrated that higher SAF was associated with frailty index increases over time. We did not observe sex-specific associations.
The cross-sectional relationship between SAF and MetaboHealth with frailty phenotype and index aligns with previous research in the Rotterdam Study (8,10). In the Rotterdam Study participants with cross-sectional frailty index measures, each unit increase in SAF corresponded to a 2.6% increase in frailty index, consistent with our cohort’s 3.0% increase. Yet, in contrast with our findings, SAF was not significantly associated with unintended weight loss in the Rotterdam Study, although it had a point estimate of 1.17. Therefore, more well-powered studies are needed to give insight into the association of SAF with unintentional weight loss. MetaboHealth was in the Rotterdam Study association with the scaled frailty phenotype and scaled frailty index (8), precluding direct comparison with our results. Furthermore, in both the current study and the Rotterdam Study, SAF and MetaboHealth remained associated with the frailty phenotype and frailty index even after adjustment for traditional risk factors (8,10). This consistency strengthens the evidence that these biomarkers capture frailty risk beyond conventional risk factors and emphasizes their ability as holistic biomarkers. Contrary, in a smaller study including 423 French participants aged ≥75, of whom 35% had chronic kidney disease, compared with 11% in the Rotterdam Study and 10% in the current cohort, no association of SAF with prevalent frailty as measured by the frailty phenotype was observed (19). Given the association between renal function and both AGEs (33) and frailty (40,41), the comparability of our results with this French study is constrained. However, this discrepancy underscores the necessity for additional research involving participants with reduced renal function to gain deeper insights into the clinical applicability within this subgroup.
Furthermore, our results showed similar and independent relationships between SAF and MetaboHealth with frailty, indicating an added value in measuring both. No previous study has compared SAF to MetaboHealth or any other omics-based aging biomarker. However, in a previous study, we compared MetaboHealth to epigenetic biomarkers, where we also observed independent associations of MetaboHealth and DNAm GrimAge with different frailty measures (8). Therefore, we urge future studies to compare SAF to other invasive aging biomarkers, aiming to gain further insights into the distinct contributions of different omics layers to the aging process.
The moderate correlation of SAF across visits suggests that SAF is a dynamic measure of aging. This variability over time might be explained by responsiveness to lifestyle changes (42). Yet, this moderate correlation might also be the result of measurement errors. Therefore, additional longitudinal studies are necessary to ascertain SAF’s reliability.
Moreover, in our relatively healthy cohort, we observed that increases in SAF were longitudinally associated with increases of 0.12% per AU/year in the frailty index independent from age, sex, socioeconomic status, device used, and season of measurement. A cross-sectional study of healthy individuals demonstrated that SAF increased by about 0.023 AU annually up to age 70 (10,43). Furthermore, in individuals over 70 with early-stage dementia, increases up to 0.4 AU/year were reported (10,44). Therefore, although an increase of 0.12% per AU/year might not seem much, added to the expected increase in SAF and frailty with age (6,43) and given the healthy nature of our cohort, SAF might have serious potential as an aging biomarker for high-risk individuals when tracked longitudinally. This finding aligns with prior research on a single SAF measurement and longitudinal outcomes, indicating associations with disability, mortality, and severe postsurgical complications the year post-surgery in older cardiac patients (18).
We did not observe a robust longitudinal association of SAF with physical frailty. This finding is in line with a previous study on a single SAF measurement with incident frailty as measured by the frailty phenotype after four years in a subset of the earlier discussed older French community-dwelling cohort (19). The absence of observed longitudinal associations with the frailty phenotype may potentially stem from the categorization of physical frailty, which could result in reduced sensitivity to detect changes compared with continuous metrics. This hypothesis is further supported by the attenuation of the association with frailty in the current study, which included relatively healthy participants after additional adjustments for smoking status, diabetes, renal function, and BMI. Unlike the frailty phenotype, the continuous frailty index remained robust, suggesting that the continuous frailty index may capture more subtle and earlier signs of aging.
Cross-sectionally and longitudinally, we did not observe effect modification of sex in the associations of SAF and MetaboHealth with neither the frailty phenotype nor the frailty index. These findings are in line with the cross-sectional results from the Rotterdam Study, where no statistically significant interaction terms were reported in the association of SAF with the frailty phenotype (10) and MetaboHealth with five different frailty measures (8). Unfortunately, we could not validate the longitudinal results due to the absence of other studies providing longitudinal information on SAF and frailty. Consequently, future research with longitudinal SAF and frailty data should be used to validate the absence of sex-specific effects before asserting their nonexistence.
This study’s strengths lie in the availability of both SAF and metabolomics, enabling a direct comparison between SAF and the established blood-based aging biomarker MetaboHealth. Furthermore, our longitudinal assessment of SAF and frailty provides valuable insights into their interrelationship. However, the absence of longitudinal MetaboHealth data at corresponding time points limits longitudinal comparison. Additionally, we had information on the two most commonly used frailty metrics. However, previous research indicated that different frailty metrics consider different participants frail (8,43), leading to different association patterns of aging biomarkers with different frailty metrics. The results can thus not be generalized to other frailty metrics. Therefore, future research on the association with other frailty metrics is needed. Moreover, the lack of another cohort with longitudinal SAF and frailty data hinders external validation. Additionally, there is evident selection bias towards healthier individuals attending the seventh examination, making the results less generalizable to a frailer population. Furthermore, this selection bias has likely led to an underestimation of the association between SAF and frailty, as participants who were already frail or probably had the strongest increase in frailty over time were underrepresented. Given our data’s restriction to a maximum of two measurements per participant, we were not able to detect potential nonlinear SAF-frailty associations. Future investigations should prioritize longitudinal data collection for MetaboHealth, SAF, and frailty at multiple time points to validate our results, conduct longitudinal comparisons, and explore nonlinear trends. Lastly, clinical studies are encouraged to evaluate the clinical applicability of SAF and MetaboHealth as aging biomarkers.
In summary, we demonstrate cross-sectional and longitudinal associations of SAF measurement with frailty. Cross-sectionally, SAF and MetaboHealth displayed similar associations with both physical frailty and the frailty index, indicating that the noninvasiveness of SAF measurement does not hinder its performance as a holistic aging biomarker. Moreover, the associations between both aging biomarkers with frailty were independent of each other, suggesting that each captures a different part of the aging process, with possible advantages of measuring both. Altogether, these insights suggest the potential of SAF as a measure of age-related decline, with the prospect of enhanced applicability when combined with MetaboHealth.
Supplementary Material
Acknowledgments
We want to gratefully acknowledge the participants and staff of the Doetinchem Cohort Study.
Contributor Information
Lieke M Kuiper, Center for Prevention, Lifestyle and Health, National Institute for Public Health and Environment (RIVM), Bilthoven, the Netherlands; Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, the Netherlands.
H Susan J Picavet, Center for Prevention, Lifestyle and Health, National Institute for Public Health and Environment (RIVM), Bilthoven, the Netherlands.
M Liset Rietman, Center for Prevention, Lifestyle and Health, National Institute for Public Health and Environment (RIVM), Bilthoven, the Netherlands.
Martijn E T Dollé, Center for Health Protection, National Institute for Public Health and Environment (RIVM), Bilthoven, the Netherlands.
W M Monique Verschuren, Center for Prevention, Lifestyle and Health, National Institute for Public Health and Environment (RIVM), Bilthoven, the Netherlands; Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands.
Gustavo Duque, (Biological Sciences Section).
Funding
This work was supported by the Netherlands Organization for Health Research and Development (ZonMW) (research grant 457001001 for the VOILA project). The Doetinchem Cohort Study was supported by the Ministry of Health, Welfare and Sport of the Netherlands and the National Institute for Public Health and the Environment.
Conflict of Interest
None.
Author Contributions
L.M.K. and M.E.T.D. conceptualized the study. L.M.K., M.E.T.D., and W.M.M.V. designed the methodology. L.M.K. performed the formal analysis, created visualization and wrote the original draft. H.S.J.P., M.L.R., and W.M.M.V. curated the data. W.M.M.V. and M.E.T.D. were responsible for funding acquisition and supervised the project. None of the funders had any role in the design and conduct of the study, the collection, management, analyses, and interpretation of the data, or the preparation of the manuscript. All authors revised and agreed on the final version of the manuscript.
References
- 1. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–1375. https://doi.org/ 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
- 2. Lowsky DJ, Olshansky SJ, Bhattacharya J, Goldman DP. Heterogeneity in healthy aging. J Gerontol A Biol Sci Med Sci. 2014;69(6):640–649. https://doi.org/ 10.1093/gerona/glt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Lepeleire J, Iliffe S, Mann E, Degryse JM. Frailty: An emerging concept for general practice. Br J Gen Pract. 2009;59(562):e177–e182. https://doi.org/ 10.3399/bjgp09x420653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. https://doi.org/ 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fried LP, Tangen CM, Walston J, et al. Frailty in Older Adults: Evidence for a Phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):146–156. https://doi.org/ 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 6. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–336. https://doi.org/ 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deelen J, Kettunen J, Fischer K, et al. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat Commun. 2019;10(1):1–8. https://doi.org/ 10.1038/s41467-019-11311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuiper LM, Polinder-Bos HA, Bizzarri D, et al. Epigenetic and metabolomic biomarkers for biological age: a comparative analysis of mortality and frailty risk. J Gerontol A Biol Sci Med Sci. 2023;78(10):1753–1762. https://doi.org/ 10.1093/gerona/glad137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zonneveld MH, Kuhaili NA, Mooijaart SP, et al. Increased 1H-NMR metabolomics-based health score associates with declined cognitive performance and functional independence in older adults at risk of cardiovascular disease. GeroScience. 2024. https://doi.org/ 10.1007/s11357-024-01391-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waqas K, Chen J, Rivadeneira F, Uitterlinden AG, Voortman T, Carola Zillikens M. Skin autofluorescence, a noninvasive biomarker of advanced glycation end-products, is associated with frailty: the Rotterdam Study. J Gerontol A Biol Sci Med Sci. 2022;77(10):2032–2039. https://doi.org/ 10.1093/gerona/glac025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramsauer B, Graaff R, Sikole A, et al. Skin Autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, decreases during the summer in dialysis patients. Artif Organs. 2019;43(2):173–180. https://doi.org/ 10.1111/aor.13320 [DOI] [PubMed] [Google Scholar]
- 12. Meerwaldt R, Graaf R, Oomen PHN, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47(7):1324–1330. https://doi.org/ 10.1007/s00125-004-1451-2 [DOI] [PubMed] [Google Scholar]
- 13. Atzeni IM, van de Zande SC, Westra J, Zwerver J, Smit AJ, Mulder DJ. The AGE Reader: A non-invasive method to assess long-term tissue damage. Methods. 2022;203:533–541. https://doi.org/ 10.1016/j.ymeth.2021.02.016 [DOI] [PubMed] [Google Scholar]
- 14. Twarda‐clapa A, Olczak A, Białkowska AM, Koziołkiewicz M. Advanced Glycation End‐Products (AGEs): formation, chemistry, classification, receptors, and diseases related to AGEs. Cells. 2022;11(8):1312. https://doi.org/ 10.3390/cells11081312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drenth H, Zuidema S, Bautmans I, Hobbelen H. The role of inflammaging and advanced glycation end products on paratonia in patients with dementia. Exp Gerontol. 2020;142:111125. https://doi.org/ 10.1016/j.exger.2020.111125 [DOI] [PubMed] [Google Scholar]
- 16. Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci. 2010;65A(9):963–975. https://doi.org/ 10.1093/gerona/glq074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frimat M, Daroux M, Litke R, Nevière R, Tessier FJ, Boulanger E. Kidney, heart and brain: Three organs targeted by ageing and glycation. Clin Sci. 2017;131(11):1069–1092. https://doi.org/ 10.1042/CS20160823 [DOI] [PubMed] [Google Scholar]
- 18. Smoor RM, van Dongen EPA, Verwijmeren L, et al. Advanced glycation end products for preoperative frailty screening in older cardiac surgery patients. J Am Geriatr Soc. 2023;71(8):2520–2529. https://doi.org/ 10.1111/jgs.18361 [DOI] [PubMed] [Google Scholar]
- 19. Pilleron S, Rajaobelina K, Teguo MT, et al. Accumulation of advanced glycation end products evaluated by skin autofluorescence and incident frailty in older adults from the Bordeaux Three-City cohort. PLoS One. 2017;12(10):e0186087. https://doi.org/ 10.1371/journal.pone.0186087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verschuren WMM, Blokstra A, Picavet HSJ, Smit HA. Cohort profile: The Doetinchem Cohort Study. Int J Epidemiol. 2008;37(6):1236–1241. https://doi.org/ 10.1093/ije/dym292 [DOI] [PubMed] [Google Scholar]
- 21. de Abreu DCC, Porto JM, Tofani PS, Braghin RMB, Freire Junior RC. Prediction of reduced gait speed using 5-time sit-to-stand test in healthy older adults. J Am Med Dir Assoc. 2022;23(5):889–892. https://doi.org/ 10.1016/j.jamda.2021.11.002 [DOI] [PubMed] [Google Scholar]
- 22. Bernabeu-Mora R, Medina-Mirapeix F, Llamazares-Herrán E, De Oliveira-Sousa SL, Sánchez-Martinez P, Escolar-Reina P. The accuracy with which the 5 times sit-to-stand test, versus gait speed, can identify poor exercise tolerance in patients with COPD A cross-sectional study. Medicine. 2016;95(35):e4740. https://doi.org/ 10.1097/MD.0000000000004740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yee XS, Ng YS, Allen JC, et al. Performance on sit-to-stand tests in relation to measures of functional fitness and sarcopenia diagnosis in community-dwelling older adults. Eur Rev Aging Phys Act. 2021;18(1):1. https://doi.org/ 10.1186/s11556-020-00255-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Correia M de A, Cucato GG, Lanza FC, et al. Relationship between gait speed and physical function in patients with symptomatic peripheral artery disease. Clinics. 2019;74:e1254. https://doi.org/ 10.6061/clinics/2019/e1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pols MA, Peeters PH, Ocké MC, Slimani N, Bueno-de-Mesquita BH, Collete HJA. Estimation of reproducibility and relative validity of the questions included in the EPIC physical activity questionnaire. Int J Epidemiol. 1997;26(1):S181–S189. https://doi.org/ 10.1093/ije/26.suppl_1.s181 [DOI] [PubMed] [Google Scholar]
- 26. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. https://doi.org/ 10.1177/014662167700100306 [DOI] [Google Scholar]
- 27. Samson LD, Boots AMH, Verschuren WMM, Picavet HSJ, Engelfriet P, Buisman AM. Frailty is associated with elevated CRP trajectories and higher numbers of neutrophils and monocytes. Exp Gerontol. 2019;125:110674. https://doi.org/ 10.1016/j.exger.2019.110674 [DOI] [PubMed] [Google Scholar]
- 28. Schoufour JD, Erler NS, Kiefte-de Jong JC, et al. Design of a frailty index among community living middle-aged and older people: the Rotterdam study. Maturitas. 2017;97:14–20. https://doi.org/ 10.1016/j.maturitas.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 29. Koetsier M, Nur E, Chunmao H, et al. Skin color independent assessment of aging using skin autofluorescence. Opt Express. 2010;18(14):14416–14429. https://doi.org/ 10.1364/OE.18.014416 [DOI] [PubMed] [Google Scholar]
- 30. Bizzarri D, Reinders MJT, Beekman M, Slagboom PE, van den Akker EB. Van Den Akker EB. MiMIR: R-shiny application to infer risk factors and endpoints from Nightingale Health’s 1H-NMR metabolomics data. Bioinformatics. 2022;38(15):3847–3849. https://doi.org/ 10.1093/bioinformatics/btac388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hosseini M, Razavi Z, Ehsani AH, Firooz A, Afazeli S. Clinical significance of non-invasive skin autofluorescence measurement in patients with diabetes: a systematic review and meta-analysis. E Clin Med. 2021;42:101194. https://doi.org/ 10.1016/j.eclinm.2021.101194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cerami C, Founds H, Nicholl I, et al. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci U S A. 1997;94(25):13915–13920. https://doi.org/ 10.1073/pnas.94.25.13915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hörner DV, Taal MW. Skin autofluorescence: an emerging biomarker in persons with kidney disease. Curr Opin Nephrol Hypertens. 2019;28(6):507–512. https://doi.org/ 10.1097/MNH.0000000000000549 [DOI] [PubMed] [Google Scholar]
- 34. Smit AP, Herber G-CM, Kuiper LM, Loef B, Picavet HSJ, Verschuren WMM. Past or present; which exposures predict metabolomic aging better? The doetinchem cohort study. J Gerontol A. 2023;79(2):glad202. https://doi.org/ 10.1093/gerona/glad202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuiper LM, Smit AP, Bizzarri D, et al. Lifestyle factors and metabolomic aging biomarkers: meta-analysis of cross-sectional and longitudinal associations in three prospective cohorts. Mech Ageing Dev. 2024;220:111958. https://doi.org/ 10.1016/j.mad.2024.111958 [DOI] [PubMed] [Google Scholar]
- 36. Feng Z, Lugtenberg M, Franse C, et al. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: a systematic review of longitudinal studies. PLoS One. 2017;12(6):e0178383. https://doi.org/ 10.1371/journal.pone.0178383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rietveld CA, Medland SE, Derringer J, et al. GWAS of 126,559 individuals identifies genetic variants associated with educational attainment. Science. 2013;340(6139):1467–1471. https://doi.org/ 10.1126/science.1235488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin c–based equations to estimate GFR without Race. N Engl J Med. 2021;385(19):1737–1749. https://doi.org/ 10.1056/nejmoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bar N, Korem T, Weissbrod O, et al. A reference map of potential determinants for the human serum metabolome. Nature. 2020;588(7836):135–140. https://doi.org/ 10.1038/s41586-020-2896-2 [DOI] [PubMed] [Google Scholar]
- 40. Reese PP, Cappola AR, Shults J, et al. Physical performance and frailty in chronic kidney disease. Am J Nephrol. 2013;38(4):307–315. https://doi.org/ 10.1159/000355568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chowdhury R, Peel NM, Krosch M, Hubbard RE. Frailty and chronic kidney disease: A systematic review. Arch Gerontol Geriatr. 2017;68:135–142. https://doi.org/ 10.1016/j.archger.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 42. Moqri M, Herzog C, Poganik JR, et al. Biomarkers of aging for the identification and evaluation of longevity interventions. Cell. 2023;186(18):3758–3775. https://doi.org/ 10.1016/j.cell.2023.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koetsier M, Lutgers HL, De Jonge C, Links TP, Smit AJ, Graaff R. Reference values of skin autofluorescence. Diabetes Technol Ther. 2010;12(5):399–403. https://doi.org/ 10.1089/dia.2009.0113 [DOI] [PubMed] [Google Scholar]
- 44. Drenth H, Zuidema SU, Krijnen WP, Bautmans I, van der Schans C, Hobbelen H. Advanced glycation end-products are associated with the presence and severity of paratonia in early stage alzheimer disease. J Am Med Dir Assoc. 2017;18(7):636.e7–636.e12. https://doi.org/ 10.1016/j.jamda.2017.04.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.