Abstract
Context
Prior research has explored the effect of synbiotics, the combination of probiotics and prebiotics, on the gut microbiota in clinical populations. However, evidence related to the effect of synbiotics on the gut microbiota in healthy adults has not been reviewed to date.
Objective
A systematic review and meta-analysis was conducted to comprehensively investigate the effect of synbiotics on the gut microbiota and inflammatory markers in populations of healthy adults.
Data Sources
Scopus, PubMed, Web of Science, ScienceDirect, MEDLINE, CINAHL, and The Cochrane Library were systematically searched to retrieve randomized controlled trials examining the primary outcome of gut microbiota or intestinal permeability changes after synbiotic consumption in healthy adults. Secondary outcomes of interest were short-chain fatty acids, inflammatory biomarkers, and gut microbiota diversity.
Data Extraction
Weighted (WMD) or standardized mean difference (SMD) outcome data were pooled in restricted maximum likelihood models using random effects. Twenty-seven articles reporting on 26 studies met the eligibility criteria (n = 1319).
Data Analysis
Meta-analyses of 16 studies showed synbiotics resulted in a significant increase in Lactobacillus cell count (SMD, 0.74; 95% confidence interval [CI], 0.15, 1.33; P = 0.01) and propionate concentration (SMD, 0.22; 95% CI, 0.02, 0.43; P = 0.03) compared with controls. A trend for an increase in Bifidobacterium relative abundance (WMD, 0.97; 95% CI, 0.42, 2.52; P = 0.10) and cell count (SMD, 0.82; 95% CI, 0.13, 1.88; P = 0.06) was seen. No significant differences in α-diversity, acetate, butyrate, zonulin, IL-6, CRP, or endotoxins were observed.
Conclusion
This review demonstrates that synbiotics modulate the gut microbiota by increasing Lactobacillus and propionate across various healthy adult populations, and may result in increased Bifidobacterium. Significant variations in synbiotic type, dose, and duration should be considered as limitations when applying findings to clinical practice.
Systematic Review Registration
PROSPERO no. CRD42021284033.
Keywords: gut microbiome, gut microbiota, healthy adult, inflammation, prebiotic, probiotic, symbiotic
INTRODUCTION
There is a growing global consumer interest in gut microbiota therapies and their related health benefits. These therapies may include probiotics, prebiotics, or synbiotics, each having different effects on human health. A recent survey of 16 000 participants from 16 countries reported that 48% of respondents consumed probiotics daily, or almost daily, with interests driven by the functional benefits to the gut microbiome and immune system.1
Probiotics are live bacteria that have demonstrated benefits to human health when consumed in adequate amounts.2 Probiotics have been shown to confer strain-specific clinical benefits for multiple conditions, including gastrointestinal symptoms,3 irritable bowel syndrome,4 Helicobacter pylori eradication,5 infection,6,7 type 2 diabetes mellitus,8 and chronic kidney disease.9 Despite their clinical benefits, there is limited evidence to demonstrate that probiotics modulate the gut microbiota. A systematic review by Kristensen et al10 found no significant effect on any microbiota measurements after probiotic supplementation in 7 randomized controlled trials with healthy adults. Similarly, another review of healthy adults by McFarland et al11 found a limited capacity for probiotics to alter the gut microbiota in only 4 of 29 studies.
Prebiotics are substrates that are selectively utilized by the host gut bacteria to provide a health benefit.12 The most widely researched prebiotics are inulin, galacto-oligosaccharide (GOS), and fructo-oligosaccharide (FOS), and these have demonstrated the ability to modulate the human gut microbiota in healthy adult populations by increasing the abundance of beneficial bacteria.13,14
The term “synbiotic” refers to the combination of probiotic and prebiotic therapies.15 Given the lack of evidence demonstrating significant microbiota modulation from probiotic supplementation alone, and the recent evidence of the effect of prebiotics on bacterial populations, synbiotics have emerged as a potential microbiota-modulating and therapeutic option.15 To date, the clinical benefits of synbiotics have been explored in many chronic and acute disease populations, including infections,16 irritable bowel syndrome,17 critical illness,18 overweight/obesity,19 and diabetes.8 However, the evidence related to the effect of synbiotics on the gut microbiota has rarely been investigated. Systematic reviews to date have included a limited number of synbiotic intervention trials alongside probiotic and prebiotic interventions, examining the effects on the gut microbiota in adults with chronic conditions.20–22 In chronic kidney disease, 2 synbiotic interventions showed a significant increase in Bifidobacterium.21 In inflammatory bowel disease, a further 2 synbiotic interventions each found contrasting results.22 One included study reported an increase in Bifidobacterium after supplementation with Bifidobacterium longum probiotic and an inulin/oligofructose prebiotic23; yet, the second study reported no significant difference to Bifidobacterium or Lactobacillus after supplementation with probiotic Bifidobacterium breve and a GOS prebiotic,24 A systematic review of the effect of pro-, pre- and synbiotics on the gut microbiome in major depressive disorder did not include any synbiotic intervention trials that reported on gut microbiota outcomes. 20 Thus, there is a lack of systematic reviews synthesizing the evidence for the effect of synbiotics on the human gut microbiota and, to our knowledge, the effect of synbiotics on the gut microbiota of healthy adult populations has not been systematically reviewed.
Given the global consumer interest in gut microbiota health and supplements, particularly from health-conscious populations,1,25 it is essential to ascertain to what extent microbiota-modulating therapies are of benefit to healthy adults. A 2020 Australian survey (n = 1265) found that 58.9% of participants were probiotic consumers. These respondents were mostly female, more educated, and reported healthier lifestyle behaviors related to fruit intake, physical activity, and alcohol intake risk compared with respondents who did not take probiotics.25 No data were collected on the respondents’ gut microbiota and the survey only focused on probiotics, and not pre- or synbiotic therapies. An investigation of the effect of microbiota-modulating therapies on the gut microbiota and other associated health benefits in healthy adult populations is timely. Moreover, to the authors’ knowledge, the evidence for the effect of synbiotic therapies on inflammatory biomarkers has not been reviewed, despite the well-known role of the gut microbiota and short-chain fatty acids (SCFAs) on regulation of the immune system.26
Thus, this review aimed to examine the effects of oral synbiotic supplementation on the modulation of the gut microbiota and inflammatory biomarkers in healthy adult populations. The specific research question was as follows: “In healthy adults, what is the impact of synbiotic consumption on the gut microbiota and inflammatory markers?”
METHODS
Study protocol
This was a systematic review and meta-analysis of randomized trials examining the effects of oral synbiotic supplementation on measurements of gut microbiota and clinical biomarkers in healthy adult populations. The systematic review was conducted according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions,27 and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.28 The review was first registered in the International Prospective Register of Systematic Reviews on 18 November 2021 (PROSPERO CRD42021284033) with amendments made on 6 December 2022. The PICO (Population, Intervention or exposure, Outcomes) model was used to define the research question (Table 1).
Table 1.
PICO criteria for inclusion and exclusion of studies
| Parameter | Criterion |
|---|---|
| Population | Healthy adults aged 18–65 y |
| Intervention or exposure | Synbiotic consumption (probiotic + prebiotic), in supplement or food fortified form |
| Comparison | Placebo or standard diet |
| Outcomes | Changes in gut microbiota composition or intestinal permeability (primary outcome). Additionally, α-diversity, SCFAs, and inflammatory biomarkers (secondary outcomes) |
Abbreviation: SCFA, short-chain fatty acid.
The systematic search was conducted across Scopus, PubMed, Web of Science Core Collection, ScienceDirect, MEDLINE (via EBSCO), CINAHL (via EBSCO), and The Cochrane Library on 24 October 2021 and updated on 30 May 2022. The search strategy used Medical Subject Heading (MeSH) terms, where available, and keywords identified from relevant literature. The full search strategy is presented in Appendix S1 (see the Supporting Information online). No limitations were applied to article language or date of publication. Reference lists of eligible articles were hand-searched for potential studies meeting the eligibility criterion.
Study eligibility
The criteria for eligibility were as follows: (1) randomized or quasi-randomized controlled trials (including parallel and crossover designs), (2) studies conducted with healthy adults aged 18 years or older, (3) oral synbiotic consumption in supplement or fortified food form, (4) placebo as comparator, (5) studies where the effect of synbiotics could be isolated from other interventions including medication or dietary changes, and (6) studies assessing modulation to the gut microbiome composition or intestinal permeability, considered as the primary outcome for this review. Secondary outcome measures included in this review were SCFAs, inflammatory biomarkers, and measures of gut microbiota diversity. Healthy adults were defined as those without a significant clinical disease that impacts the gut microbiota and/or inflammation, including inflammatory bowel disease, viral or bacterial infective states, cancer, gastrointestinal surgery, autoimmune conditions, enteral nutrition, critical illness, neurodegenerative diseases, liver disease, or chronic kidney disease.
The following exclusion criteria were applied: (1) animal or in vitro studies; (2) non-intervention studies including observational and case-control studies; (3) protocols, abstracts, expert opinions, letters, seminars, books, or book chapters; and (4) study populations of children or populations with clinical diseases outlined above. If studies had more than 2 arms, only the comparison of synbiotics to placebo/control arm was considered.
Study selection
After removal of duplicates, articles were imported to Covidence Systematic review software (Veritas Health Innovation)29 for title and abstract screening by 3 independent reviewers (D.J.C., K.C., Y.P.), with any disagreement resolved via consensus. Full-text screening was then performed by 1 reviewer (D.J.C.), with articles of concern discussed among the research team (K.C., Y.P.). Where multiple articles reported results from a single study, the associated articles were linked, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions.27 Reference lists of included articles were manually searched to identify additional relevant articles.
Data extraction
Data extraction was performed by 1 reviewer (D.J.C.) in consultation with the research team (K.L., Y.P., K.C.). Data were extracted from each study for the following: citation, country, study design, population description, sample size, study duration, synbiotic type, dose of synbiotic, and details of control and baseline demographic data (shown in Table 230–56). Study findings data were extracted and categorized as microbiota outcomes, SCFAs, diversity and richness, and inflammatory biomarkers (shown in Table 330–56). All data are reported as continuous variables. The mean final values or mean change values with respective standard deviations (SDs) (or standard error/95% confidence interval [CI]) were collated for each outcome, where possible. When not available, the mean final values and respective SDs were converted from provided data, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions.27 Where necessary, median and interquartile range values were converted to mean and SD using the formula developed by Wan et al.57 Where the published article did not provide adequate information, authors were contacted by email for additional details. Data from intention-to-treat analyses were extracted for use in this meta-analysis, where possible. Where this was not available, data from per-protocol analyses were used and the impact considered in the risk-of-bias assessment.
Table 2.
Characteristics of the included studies according to population subtypes
| Reference | Study design | Participants | Sample size, n (n = synbiotic group) | Duration | Probiotic/day | Prebiotic/day | Control/day | Microbiota or intestinal permeability analysis | Inflammatory markers |
|---|---|---|---|---|---|---|---|---|---|
| Healthy adults | |||||||||
| Casiraghi et al,55 Italy | Parallel RCT | Healthy adults; 22-47 y | 26 (13) | 4 wk | 109 CFU Lactobacillus acidophilus 74-2 5× and 109 CFU Bifidobacterium lactis 420 | 9.25 g Inulin | 500 mL milk | Culture | — |
| Palaria et al,30 USA | Crossover RCT | Healthy adults; mean age 30 y | 52 | 3 wk | 109–1010 CFU of B. animalis subsp. lactis Bb-12 + yogurt starter blend containing Streptococcus thermophilus and Lactobacillus bulgaricus | 1 g Inulin | 94 g Milk | qPCR | — |
| van Zanten et al,31 Denmark | Crossover RCT | Healthy adults; 18-50 y | 18 | 3 wk | 109 CFU L. acidophilus NCFM | 5 g Cellobiose | 5 g Maltodextrin | 16s rRNA V3-4; qPCR | — |
| Roberts et al,32 UK | Parallel RCT | Recreational athletes; mean age 35 y | 20 (10) | 3 mo | 1010 CFU L. acidophilus CUL-60, 1010 CFU L. acidophilus CUL-21, 9.5 × 109 CFU Bifidobacterium bifidum CUL-20, 0.5 × 109 CFU Bifidobacterium animalis subsp. lactis CUL-34 | 5.6 g FOS | 2 g Corn flour | Urinary lactulose and mannitol recovery | Plasma endotoxin |
| Rajkumar et al,33 India | Parallel RCT | Healthy adults; 20-25 y; BMI 18.5–24.9 kg/m2 | 30 (15) | 6 wk | 2 × 109 CFU Lactobacillus salivarius | 10 g FOS | Gelatin capsule | Culture | hs-CRP, TNF-α, IL-6, and IL-1β |
| Valle et al,34 Brazil | Parallel RCT | Healthy adults; 18-22 y; enrolled at Brazil Army Cadet school | 80 (40) | 1 mo | 10.3-log CFU L. acidophilus LA-5, 11.0-log CFU B. animalis BB-12 | 2.3 g Inulin | 60 g Ice cream | 16s rRNA V3-4 | — |
| Childs et al,35 UK | Crossover RCT | Healthy adults; 25-65 y; BMI 20-30 kg/m2 | 44 | 3 wk | 109 CFU B. animalis subsp. lactis Bi-07 | 8 g XOS | 8 g Maltodextrin | qPCR; FISH with 16s rRNA; flow cytometry | Fecal IgA, salivary IgA, IL-6, IL-10, TNF-α, TNF-β |
| Constipation | |||||||||
| Kim et al,36 Korea | Parallel RCT | Adults with Rome III constipation | 30 (20) | 1 mo | Lacticaseibacillus casei 3451, Lactobacillus plantarum 3501, Lactobacillus rhamnosus 3201, Bifidobacterium lactis 4301, B. breve 4401, Lactococcus lactis 2301 (dose unspecified) | XOS, oat fiber, psyllium husk fiber (dose unspecified) | Glucose anhydrocrystalline, maltodextrin, and corn starch | 16s rRNA V3-4 | — |
| Bazzocchi et al,37 Italy | Parallel RCT | 18-65 y; Rome III constipation | 31 (19) | 2 mo | L. plantarum, L. acidophilus, L. rhamnosus, Bifidobacteriumlongum, and Bifidobacteriumbreve (dose unspecified) | 5 g Psyllium | Maltodextrin | 16s rDNA; denaturing gradient gel electrophoresis; qPCR | — |
| Bogovič Matijašić et al,38 Slovenia | Parallel RCT | 16-65 y; Rome III constipation–predominant IBS | 76 (33) | 1 mo | 6.48 × 109 CFU L. acidophilus La-5 and 9 × 109 CFU B. animalis subsp. lactis BB-12 | 7.2 g Mixed dietary fiber (inulin and FOS) | Heat-treated fermented milk | Random amplified polymorphic DNA RAPD profiling; PCR; 16s rRNA V4 | — |
| Anzawa et al,39 Japan | Crossover RCT | 20-64 y; constipation experienced 3-5 days/wk | 60 | 2 wk | 1010 CFU of B. animalis subsp. lactis GCL2505 | 2 g Inulin | Fermented milk | qPCR | — |
| Neyrinck et al,40 Belgium | Parallel RCT | 50-70 y; Rome III constipation | 27 (13) | 1 mo | 5 × 109 CFU B. animalis lactis Vesalius 002/bag (2 bags for first 5 d, then 1 bag for remaining 25 d) | 4.95 g FOS/bag (2 bags for first 5 d, then 1 bag for remaining 25 d) | 5 g Maltodextrin | 16s rDNA V1-3; plasma intestinal fatty-acid binding protein (iFABP) | IL-6, IL-8, IL-1β, IL-10, IL-17a, TNF-α, IFN-γ, Monocyte chemotactic protein (MCP)-1 |
| Elderly adults | |||||||||
| Shioiri et al,41 Japan | Crossover RCT | Healthy adults; mean age 74 y | 20 | 2 wk | 3 × 1010 CFU L. casei Shirota | 2.5 g GOS | 80 mL Fermented milk | Culture | — |
| Lee et al,56 Korea | Parallel RCT | Healthy females; ≥65 y | 51 (35) | 1 wk or 4 wk | 10 × 109 CFU B. animalis spp. Lactis HY8002, 5 × 109 CFU L. casei HY2782, and 5 × 109 CFU L. plantarum HY7712 | 8 g Mixed dietary fiber (polydextrose, chicory dietary fiber, lactulose, and wheat, XOS) | Placebo drink (unspecified) | 16s rRNA V3-4; zonulin | — |
| Ouwehand et al,42 Finland | Parallel RCT | Healthy adults; ≥65 y; regularly take NSAIDs | 51 (24 completed) | 2 wk | 2 × 109 CFU/g L. acidophilus NCFM | 5 g Lactilol | 5 g Sucrose | qPCR | TNF-α and fecal IgA |
| Macfarlane et al,43 UK | Crossover RCT | Healthy adults; 65-90 y; BMI 18.5–30.0 kg/m2 | 47 | 1 mo | 4 × 1011 B. longum | 12 g Synergy 1 (inulin + FOS) | 12 g Maltodextrin | FISH with 16s rRNA | CRP, IgA g/L; IgG g/L; IL-10 + IL-8 + IL-6 + TNF-α |
| Lehtinen et al,44 UK | Crossover RCT | Healthy adults; >60 y |
|
3 wk | 1 × 109 CFU B. animalis subsp. Lactis Bi-07 | 8 g GOS | 8 g Maltodextrin | FISH with 16s rRNA; qPCR | IFN-γ, IL-1β, IL-6, IL-8, IL-10, TNF-α, and IgA |
| Costabile et al,45 UK | Crossover RCT | Healthy adults; 60-80 y | 40 | 3 wk | Synbiotic 1: 1 × 1010 CFU L. rhamnosus GG-PB12 | 6 g Soluble corn fiber | Maltodextrin | 16s rRNA V3-4; qPCR | hs-CRP, IL-6, and IL-8 |
| Synbiotic 2: 1 × 1010 CFU L. rhamnosus GG | |||||||||
| Type 2 diabetes | |||||||||
| Kassaian et al,46 Iran | Parallel RCT | Adults with prediabetes | 80 (40) | 6 mo | 1.5 × 109 CFU each L. acidophilus, B. lactis, B. bifidum, and B. longum | 6 g Inulin | 6 g Maltodextrin | qPCR | — |
| Horvath et al,47 Austria | Parallel RCT | >18 y; diagnosed T2DM; BMI 30-40 kg/m2 | 41 (21) | 6 mo | 1.5 × 1010 CFU blend of B. bifidum W23, B. lactis W51, B. lactis W52, L. acidophilus W37, L. casei W56, L. brevis W63, L. salivarius W24, Lactococcuslactis W58, and Lc. lactis W19 | 10 g Omnilogic Plus (OMNi-BIOTIC®) (GOS + FOS, 8 g active prebiotic) | 6 g Starch | 16s rDNA V1-2; zonulin; diamine | — |
| Kanazawa et al,48 Japan | Parallel RCT | 30-80 y; T2DM treated with diet and exercise alone | 88 (44) | 6 mo | 3 × 108 Lc. paracasei YIT 9029 and 3 × 108 B. breve YIT 12272 | 7.5 g GOS | Not stated | qPCR; 16s rRNA V1-2 | hs-CRP and IL-6 |
| Overweight adults | |||||||||
| Krumbeck et al,49 USA | Parallel RCT | 18-65 y; BMI 30-40 kg/m2 | 58 (39) | 3 wk | Synbiotic 1: 1 × 109 CFU Bifidobacterium adolescentis IVS-1 | 5 g GOS | 7 g Lactose | qPCR; 16s rRNA V5-6; urinary lactulose and mannitol | Serum endotoxin |
| Synbiotic 2: 1 × 109 CFU B. animalis subsp. lactis BB-12 | |||||||||
| Hibberd et al,50 USA | Parallel RCT | 18-65 y; BMI 28.0–34.9 kg/m2 | 113 (56) | 6 mo | 1010 CFU B. animalis subsp. lactis 420 | 12 g Litesse® Ultra polydextrose (IFF) | 12 g Micro-crystalline cellulose | 16s rRNA V4; zonulin | hs-CRP |
| Stenman et al,51 Finland | Parallel RCT | 18-65 y; BMI 28.0–34.9 kg/m2 | 113 (56) | 6 mo | 1010 CFU B. animalis subsp. lactis 420 | 12 g Litesse® Ultra polydextrose (IFF) | 12 g Micro-crystalline cellulose | Zonulin | hs-CRP, Lipopolysaccharide, Endotoxin, and IL-6 |
| Angelino et al,52 Italy | Parallel RCT | 30-65 y; BMI 25-35 kg/m2; healthy sedentary adults | 46 (23) | 3 mo | Bacillus coagulans BC30 1 × 107 CFU/g pasta (dose unspecified) | Whole-wheat pasta supplemented with barley β-glucan (dose unspecified) | Whole-wheat pasta | qPCR | hs-CRP, IL-6, IL-10, and TNF-α |
| Asthma and migraines | |||||||||
| McLoughlin et al,53 Australia | Crossover RCT | Adults with doctor-diagnosed, stable asthma | 17 | 1 wk | 7.5 × 109 CFU L. acidophilus LA-5, 8.75 × 109 CFU L. rhamnosus GG and 8.75 × 109 CFU B. animalis subsp. lactis BB-12 | 12 g Inulin | 12 g Maltodextrin | qPCR; 16s rRNA V4 | — |
| Ghavami et al,54 Iran | Parallel RCT | 20-50 y; adult women with migraines diagnosed according to the ICDH-3 criteria. | 80 (40) | 3 mo | 2 × 109 CFU L. casei, L. acidophilus, L. rhamnosus, L. helveticus, L. bulgaricus, L. plantarum, L. gasseri, B. breve, B. longum, B. lactis, B. bifidum, and S. thermophilus | FOS (dose unspecified) | Starch | Zonulin | hs-CRP |
Abbreviations: BMI, body mass index; CFU, colony-forming units; CRP, C-reactive protein; FISH, fluorescence in situ hybridization; FOS, fructo-oligosaccharide; GOS, galacto-oligosaccharide; hs-CRP, high-sensitivity C-reactive protein; IFN-γ, interferon-γ; IgA, immunoglobulin A; IgG, immunoglobulin G; IL, interleukin; NSAID, nonsteroidal anti-inflammatory drug; PCR, polymerase chain reaction; qPCR, quantitative polymerase chain reaction; RCT, randomized controlled trial; T2DM, type 2 diabetes mellitus; TNF-α, tumor necrosis factor α; XOS, xylooligosaccharide.
Table 3.
Microbiota and inflammatory outcomes after synbiotic consumption
| Study ID | Microbiota outcomes | SCFA | Richness and diversity | Inflammatory markers |
|---|---|---|---|---|
| Healthy adults | ||||
| Casiraghi et al,55 Italy | ↑ Bifidobacterium and Lactobacillus | — | — | — |
| Palaria et al,30 USA |
|
— | — | — |
| van Zanten et al,31 Denmark |
|
|
↔ PCoA weighted UniFrac distance | — |
| Roberts et al,32 UK |
|
— | — |
|
| Rajkumar et al,33 India |
|
— | — | ↓ hs-CRP, TNF-α, IL-6, and IL-1β |
| Valle et al,34 Brazil |
|
|
|
— |
| Childs et al,35 UK |
|
|
— |
|
| Constipation | ||||
| Kim et al,36 Korea |
|
— |
|
— |
| Bazzocchi et al,37 Italy |
|
— | — | — |
| Bogovič Matijašić et al,38 Slovenia |
|
— |
|
— |
| Anzawa et al,39 Japan |
|
— | — | — |
| Neyrinck et al,40 Belgium |
|
— |
|
|
| Elderly adults | ||||
| Shioiri et al,41 Japan |
|
— | — | — |
| Lee et al,56 Korea |
|
— | — | — |
| Ouwehand et al,42 Finland | ↑ Bifidobacterium and L. acidophilus | — | — | ↔ TNF-α and IgA |
| Macfarlane et al,43 UK |
|
|
— |
|
| Lehtinen et al,44 UK | ↔ All microbes assessed |
|
— |
|
| Costabile et al,45 UK |
|
— |
 PCoA of Bray-Curtis dissimilarity showed microbial shifts towards higher Parabacteroides and Ruminococcaceae abundance in both synbiotic groups. PCoA of Bray-Curtis dissimilarity showed microbial shifts towards higher Parabacteroides and Ruminococcaceae abundance in both synbiotic groups. |
|
|
|
|||
| Type 2 diabetes | ||||
| Kassaian et al,46 Iran | ↔ All microbes assessed | — | — | — |
| Horvath et al,47 Austria |
|
— |
|
— |
| Kanazawa et al,48 Japan |
|
|
↓ Phylogenetic Diversity, OTU and Shannon Index at 12wks (no changes at 24wks) | ↔ hs-CRP and IL-6 |
| Overweight adults | ||||
| Krumbeck et al,49 USA |
|
— | ↔ α-Diversity and β-diversity (measures unspecified) | — |
|
— | — | — | |
| Hibberd et al,50 USA |
|
|
|
↓ hs-CRP |
| Stenman et al,51 Finland | ↔ Zonulin | — | — |
|
| Angelino et al,52 Italy |
|
— | — |
|
| Asthma and migraines | ||||
| McLoughlin et al,53 Australia |
|
|
|
— |
| Ghavami et al,54 Iran | ↓ Zonulin | — | — | ↓ hs-CRP |
Abbreviations: F/B, Firmicutes/Bacteroidetes; hs-CRP, high-sensitivity C-reactive protein; iFABP, intestinal fatty-acid binding protein; IFN-γ, interferon-γ; IgA, immunoglobulin A; IgG, immunoglobulin G; IL, interleukin; LPS, lipopolysaccharide; OTU, operational taxonomic unit; PCoA, principal coordinates analysis; SCFA, short-chain fatty acid; TNF, tumor necrosis factor.
Statistical analysis
Meta-analyses were performed where 3 or more studies reported on an outcome and where medians/means with SDs could be obtained or calculated. Stata version 17 software (release 17; StataCorp LLC) was used to perform random-effects meta-analyses using the meta esize command for weighted Hedge’s g standardized mean difference (SMD) and meta set command for weighted mean difference (WMD). The random-effects model was chosen to address statistical heterogeneity due to the assumption of real differences in the treatment effect related to the differences in study populations, synbiotic intervention, and duration.58 Restricted maximum likelihood to estimate heterogeneity variance was used, as recommended by Langan et al.59
The WMD (with 95% CI) was computed from mean change or final values, where possible, and Hedge’s g weighted SMD (with 95% CI) where measurement methods hindered a weighted mean difference calculation. Crossover trials were initially analyzed the same way as parallel trials, using a paired analysis. This approach has been noted as likely to be conservative according to the Cochrane Handbook,27 and was required due to a lack of available data on the results of the first period of crossover trials. Where parallel trials included 2 separate synbiotic interventions, these interventions were included as separate studies and labeled “A” and “B.”49 To minimize unit-of-analysis error, the control group population was split between the 2 intervention arms. Paired analyses of crossover studies were performed with correlation coefficients of 0.25, 0.5, and 0.75, as a sensitivity analysis to determine if the paired analyses underweighted the crossover studies.27 In addition, “leave-one-out” sensitivity analyses were conducted to explore the effect of removing each individual study from the overall meta-analysis estimates. For calculating the standard error of the difference for sensitivity analyses, where a crossover trial had a different number of participants who completed the intervention and control due to dropout, the highest population number was considered. The I2 test statistic estimated the proportion of total variation that was attributable to between-study heterogeneity, with a score of 30%–60% taken to indicate moderate heterogeneity, 50%–90% substantial heterogeneity, and 75%–100% considerable heterogeneity.27
Quality assessment
The Cochrane Collaboration Risk of Bias Tool 2.060 was used to determine the risk of bias in the randomized controlled trials, with the effect of assignment to intervention considered. Five domains were assessed for risk of bias, including (1) randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of outcome, and (5) selection of reported results. An additional domain—period and carryover effects—was assessed in crossover trials. The risk-of-bias assessment was appraised by 1 reviewer (D.J.C.).
The quality of the body of evidence was determined by 3 reviewers independently (D.J.C., K.C., K.L.) using Grading of Recommendations Assessment, Development and Evaluation (GRADE).61 (GRADEpro GDT: GRADEpro Guideline Development Tool [software]; McMaster University and Evidence Prime, 2022. Available from gradepro.org).
RESULTS
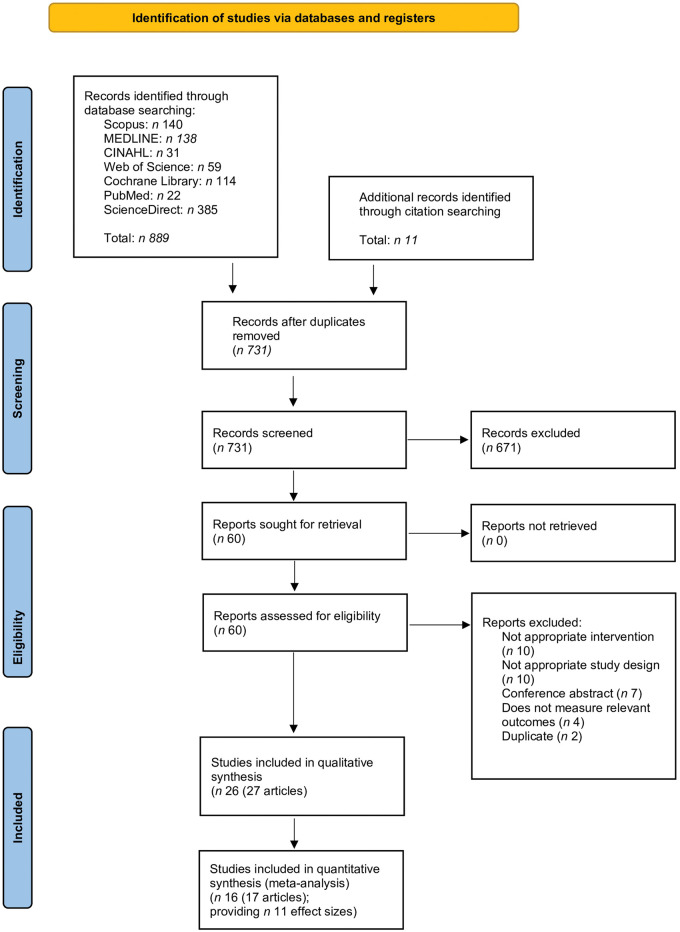
A total of 782 articles were identified from the systematic search strategy, reference lists, and scoping search. After removal of duplicates, 731 articles were screened and 60 full-text articles were reviewed for eligibility. Thirty-two of these articles were excluded, with the most common reasons being that they did not investigate an oral synbiotic in supplement or food form, were not conducted in a healthy adult population, did not include a randomized or quasi-randomized controlled trial, or that the article was a study protocol. This resulted in 27 articles reporting on 26 studies being included in this review (n = 1319) (Fig. 1).62 From 17 of these articles reporting on 16 studies, 11 effect sizes were available for inclusion in the meta-analysis.
Figure 1.
Flow diagram of the literature search process. Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study characteristics
Characteristics of the included studies are outlined in detail in Table 2. Eighteen studies had a parallel design,32–34,36–38,40,42,46–52,54,55 and 9 studies had a crossover design.30,31,35,39,41,43–45,53 All included studies were randomized controlled trials. The duration of included studies ranged from 1 week53 to 6 months.46–48,50,51 Five studies30,32,34,38,50 collected additional outcome data 5 days34 to 1 month50 postintervention. Studies were conducted in Australia,53 Italy,37,52,55 the United States,30,50 Korea,36,56 Denmark,31 the United Kingdom,32,35,43–45 India,33 Brazil,34 Slovenia,38 Japan,39,41,48 Belgium,40 Finland,42,51 Iran,46,54 and Austria.47 Studies included participants with constipation,36–40 healthy adults,30–35,55 older adults,41–45,56 as well as those who were overweight or obese,42,49,50,52 or had type 2 diabetes or prediabetes,46–48 asthma,53 or chronic migraines.54
The synbiotic interventions were either supplemented as a capsule,31–33,35–37,39,42–47,49–51,53,54 yogurt,30 ice cream,34 milk beverage,38,41,55,56 powder,40 or pasta.52 All probiotic interventions contained Lactobacillus and/or Bifidobacterium, either a single strain31,33,35,39–45,49–52 or multi-strain.32,34,36–38,46,48,53–56 The most common species in single- and multi-strain probiotics were Lactobacillus acidophilus,31,32,34,37,38,46,47,53–55 Lactobacillus rhamnosus,36,37,42,45,53,54 and Bifidobacterium animalis.30,32,34–36,38–40,44,46,47,49–51,53–56 Four studies included strains not from the Bifidobacterium or Lactobacillus genera in the multi-strain probiotic. These were Lactococcus lactis36,47 and Streptococcus thermophilis.30,54 One study included Bacillus coagulans BC30 as the single probiotic strain.52 The most common prebiotic interventions were inulin,30,34,38,39,43,46,53,55 GOS,41,44,47–49 FOS,32,33,38,40,43,47,54,56 and xylo-oligosaccharide.35,36,56
Microbiota analysis techniques included bacterial cell counts (n = 3),33,41,55 qPCR cell counts (n = 5),30,39,42,46,52 16S rRNA gene sequencing (n = 3),34,36 intestinal permeability reported as urinary lactulose-to-mannitol ratio (n = 1),32 or zonulin (n = 2),32,51,54 whereas the majority of studies utilized a combination of methodologies (n = 14).31,35,37,38,40,43–45,47–50,53,56 This variation in testing resulted in a combination of quantitative bacterial counts with various measurement units, relative abundance data, and qualitative descriptions of microbiome changes reported in study outcomes. The substantial heterogeneity in outcome data hindered the ability to perform meta-analyses on microbiota outcomes, except for Lactobacillus, Bifidobacterium, zonulin, α-diversity, and SCFAs.
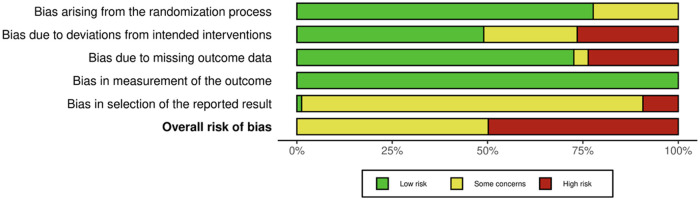
Risk of bias
The risk-of-bias assessment is shown in Fig. 5 and Figs. S5 and S6 (see the Supporting Information online). No studies were considered at low risk of bias. The risk of bias was considered high in 13 studies and as some concerns noted in 14 studies. The high risk of bias was mostly related to concerns with deviations from the intended intervention due to failure to analyze data according to intention-to-treat (domain 2) and missing outcome data (domain 3).
Figure 5.
Risk of bias as a weighted proportion of total studies.
Effect of synbiotics on study outcomes
The number of studies and effect sizes, and the results of each meta-analysis, are shown in Table 4 and Figs. 2–4. Summary data for each study are available in Table S1 (see the Supporting Information online). In line with the Cochrane Handbook, due to the small number of studies included in the meta-analyses, subgroup analyses were not performed.27
Table 4.
Changes in outcomes following synbiotic consumption, compared with control
| Outcome | No. of studies and references | Effect sizes, n | Participants, n | Results |
Inconsistency |
|||
|---|---|---|---|---|---|---|---|---|
| Effect size (WMD or SMD) | 95% CI | P | I2 (%) | P | ||||
| Bifidobacterium relative abundance (%) | 334,49,53 | 4 | 146 | 0.97 WMD | −0.20, 2.14 | 0.10 | 93.0 | <0.001 |
| Bifidobacterium cell count | 831,37,41–43,48,52,55 | 8 | 378 | 0.82 SMD | −0.03, 1.67 | 0.06 | 93.1 | <0.001 |
| Lactobacillus cell count | 431,37,48,55 | 4 | 169 | 0.74 SMD | 0.15, 1.33 | 0.01 | 69.4 | 0.02 |
| Zonulin (ng/mL) | 347,50,54 | 3 | 168 | −0.24 WMD | −4.34, 3.86 | 0.91 | 90.5 | <0.001 |
| Shannon Index | 334,48,50 | 3 | 218 | 0.02 WMD | −0.21, 0.26 | 0.84 | 86.3 | <0.001 |
| Acetate concentration | 634,41–43,51,53 | 6 | 372 | 0.27 SMD | −0.02, 0.57 | 0.07 | 49.1 | 0.07 |
| Propionate concentration | 634,41–43,51,53 | 6 | 372 | 0.22 SMD | 0.02, 0.43 | 0.03 | 0.0 | 0.79 |
| Butyrate concentration | 634,41–43,51,53 | 6 | 372 | 0.17 SMD | −0.03, 0.37 | 0.10 | 0.0 | 0.62 |
| IL-6 (pg/mL) | 543,45,48,51,52 | 5 | 390 | −0.76 WMD | −2.21, 0.70 | 0.31 | 77.6 | 0.06 |
| CRP (mg/L) | 543,50–52,54 | 5 | 378 | −0.12 WMD | −0.51, 0.26 | 0.54 | 44.6 | 0.54 |
| Endotoxin (EU/mL) | 332,47,50 | 3 | 129 | −0.00 WMD | −0.03, 0.02 | 0.79 | 52.3 | 0.05 |
Abbreviations: CI, confidence interval; CRP, C-reactive protein; IL, interleukin; SMD, standardized mean difference; WMD, weighted mean difference.
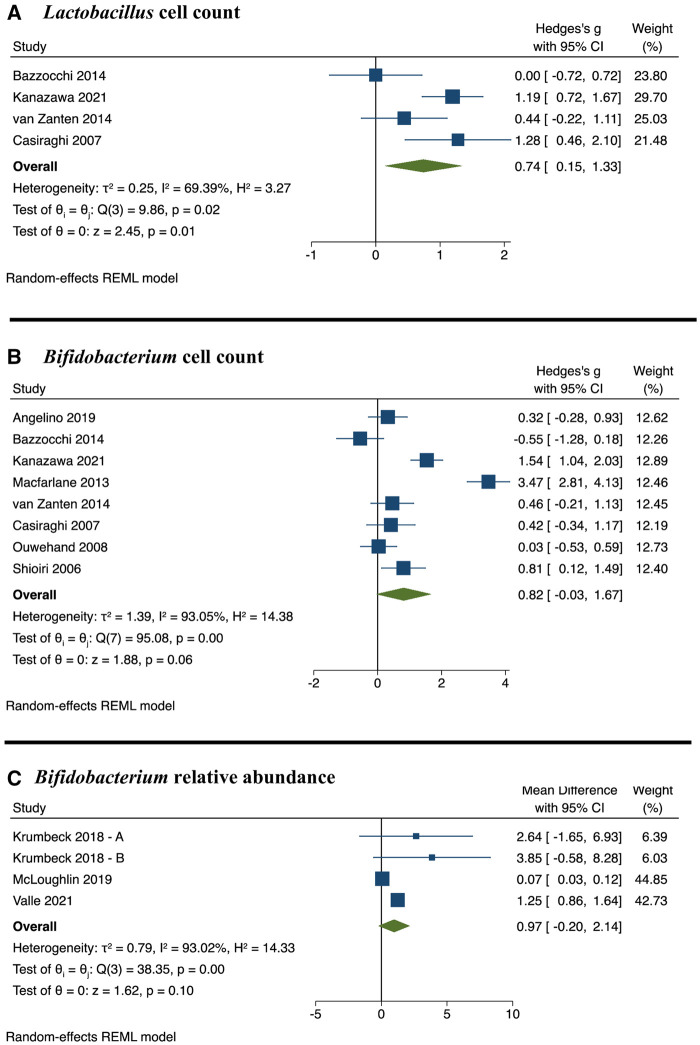
Figure 2.
Forest plot of weighted standardized mean differences or standardized mean difference and 95% CIs for synbiotics compared with control. (A) Lactobacillus cell count; (B) Bifidobacterium cell count; and (C) Bifidobacterium relative abundance. Abbreviations: CI, confidence interval; REML, restricted maximum likelihood.
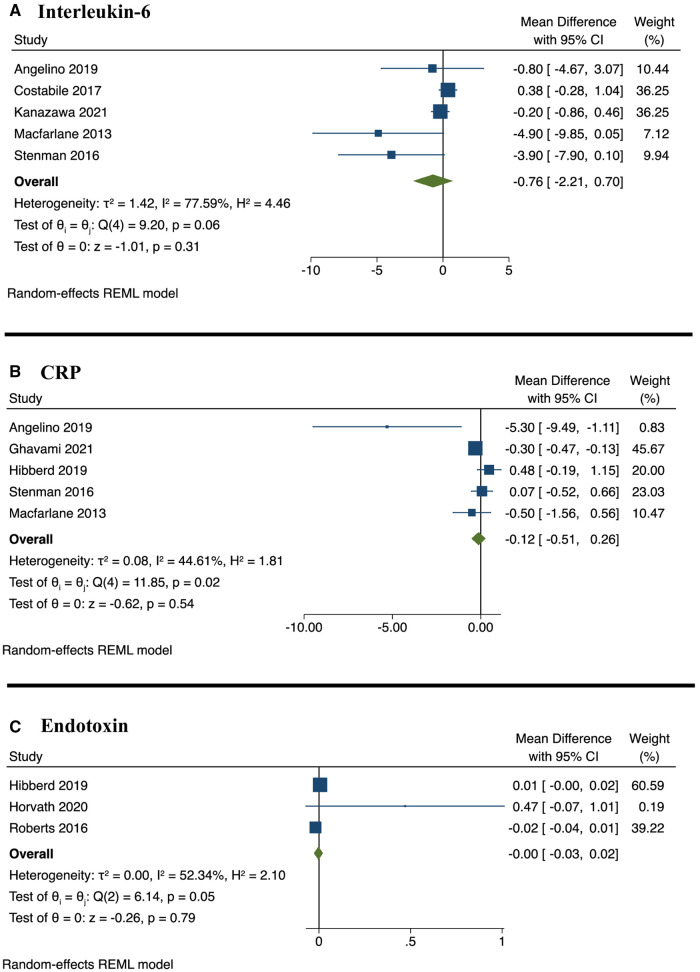
Figure 4.
Forest plot of weighted mean difference and 95% CIs for synbiotics compared with control. (A) Interleukin-6; (B) CRP; and (C) endotoxin. Abbreviations: CI, confidence interval; CRP, C-reactive protein; REML, restricted maximum likelihood.
Changes in gut microbiota composition
A narrative synthesis of all studies reveals that the only consistent change to the gut microbiota after a synbiotic intervention, seen across all population subtypes, was an increase in Lactobacillus and/or Bifidobacterium relative abundance or cell count measures. A few changes to bacterial phylum relative abundance were reported, with an increase in Firmicutes seen in 1 study after supplementation with Bifidobacterium for 1 month,43 but a decrease was seen by 2 other studies after supplementation with a probiotic containing members of the Firmicutes phylum, specifically Lactobacillus or Lacticaseibacillus for 1 week or 6 months, respectively.48,56 These 3 studies displayed some concerns of risk of bias. An increase in Actinobacteria phylum was reported by 3 studies after supplementation with a probiotic containing Bifidobacterium for 3 weeks,49 1 month,43 or 6 months48; Proteobacteria43 and Bacteroidetes48,51,56 abundance decreased after synbiotic intervention in 2 studies. A reduction in bacterial genus or family from the Clostridiales order was seen in 2 studies among a healthy adult population after a 3-week intervention,30,31 yet Childs et al35 reported no change to this bacterial order among healthy adults after the same duration of intervention. Two of these studies displayed some concerns of risk of bias31,35 and 1 study displayed a high risk of bias.30
Lactobacillus
A total of 4 studies exploring the effect of synbiotic consumption on Lactobacillus cell count were included in the meta-analysis.31,37,48,55 Each study included a Lactobacillus strain as the probiotic, with the prebiotic and duration of intervention being inulin for 4 weeks,55 cellobiose for 3 weeks,31 psyllium for 2 months,37 or GOS for 6 months.48 Synbiotic consumption resulted in a statistically significant increase in the Lactobacillus cell count, with an SMD of 0.74 (95% CI: 0.15, 1.33; P = 0.01) (Table 4, Fig. 2). The effect estimate was similar after using different correlation coefficients (0.25, 0.5, and 0.75) (see Table S3 in the Supporting Information online). Sensitivity analyses revealed that excluding Kanazawa et al48 and Casiraghi et al55 led to a nonsignificant effect size of an SMD of 0.55 (95% CI: -0.15, 1.25; P = 0.13) and an SMD of 0.59 (95% CI: -0.12, 1.29; P = 0.10), respectively (see Fig. S1 in the Supporting Information online).
Bifidobacterium
A total of 11 studies exploring the effect of synbiotic consumption on Bifidobacterium relative abundance or cell count were included in the meta-analysis.31,34,37,41–43,48,49,52,53,55 Seven of these studies supplemented with Bifidobacterium with or without Lactobacillus.34,37,43,48,49,53,55 The prebiotic and duration of interventions were as follows: inulin for 1 week53 or 4 weeks34,43,55; cellobiose for 3 weeks31; psyllium for 2 months37; GOS for 2 weeks,41 3 weeks,49 or 6 months48; lactilol for 2 weeks42; FOS for 4 weeks43; or β-glucan for 3 months.52 The meta-analysis effect estimates showed that synbiotic consumption did not result in significant differences in Bifidobacterium relative abundance or cell count (Table 4, Fig. 2). However, the effect estimate for Bifidobacterium cell count became significant after using correlation coefficients 0.25, 0.5, and 0.75 (see Table S2 in the Supporting Information online). Sensitivity analyses showed substantial study effects when omitting McLoughlin et al53 from Bifidobacterium relative abundance (WMD: 1.47; 95% CI: 0.42 to 2.52; P = 0.006) and omitting Bazzocchi et al37 from Bifidobacterium cell count (SMD: 1.01; 95% CI: 0.13, 1.88; P = 0.024) (see Figs. S2 and S3 in the Supporting Information online).
Change in intestinal permeability
Among the 4 randomized controlled trials assessing zonulin as a marker of intestinal permeability, 3 reported a significant reduction after a 3-month54 or 6-month intervention47,50 and 1 reported no change after a 3-month intervention.51 Of the studies reporting a significant reduction, 2 displayed a high risk of bias47,54 and the other reported some concerns of risk of bias.50 The study reporting no change displayed some concerns of risk of bias.51 The meta-analysis effect estimate showed that synbiotic consumption did not result in significant changes in zonulin (Table 4, Fig. 3).
Figure 3.
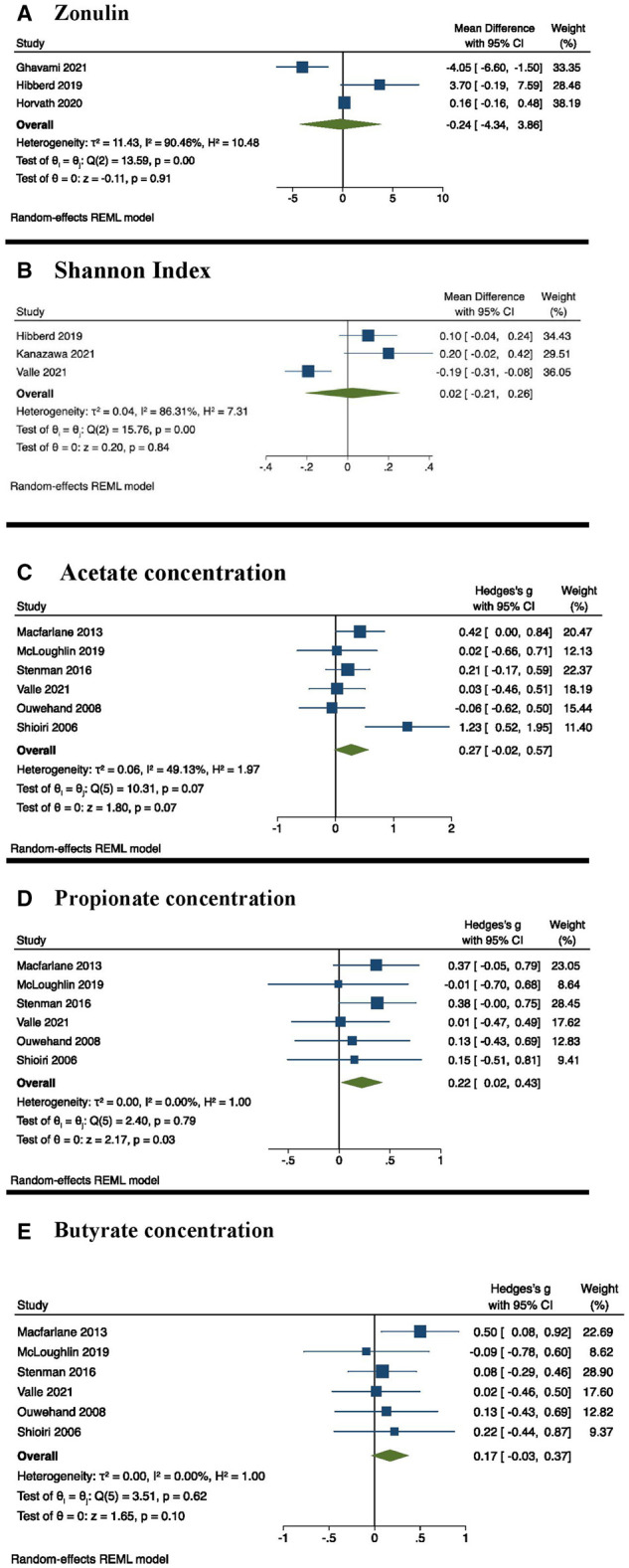
Forest plot of weighted mean difference or standardized mean difference and 95% CIs for synbiotics compared with control. (A) Zonulin; (B) Shannon Index; (C) acetate concentration: (D) propionate concentration; and (E) butyrate concentration. Abbreviations: CI, confidence interval; REML, restricted maximum likelihood.
Change in α-diversity
An increase in α-diversity, measured as the Shannon Index and Phylogenetic Diversity, was reported by Hibberd et al50 after a 6-month intervention. In contrast, 2 studies found a decrease in various α-diversity measurements after a 1-month36 or 6-month48 intervention; however, for Kanazawa et al48 this reduction was only present at 12 weeks and not 24 weeks. Two of these studies displayed some concerns of risk of bias,36,50 and 1 displayed a high risk of bias.48
The meta-analysis effect estimate showed that synbiotic consumption did not result in significant changes in the Shannon Index (Table 4, Fig. 3). Sensitivity analysis showed substantial changes when omitting Valle et al34 from Shannon Index assessments (WMD: 0.13; 95% CI: 0.01, 0.25; P = 0.031) (see Fig. S5 in the Supporting Information online).
Changes in SCFAs
The meta-analysis effect estimate showed that synbiotic consumption did not result in significant differences in the SCFAs acetate and butyrate (Table 4, Fig. 3). Inconsistencies in the reported SCFA outcomes were seen across all included studies. Five studies reported no significant changes to measured SCFAs after synbiotic supplementation for 1 week,53 3 weeks,31,35,44 or 6 months.50 Three studies reported significant increases in SCFA production after supplementation for 1 month34,43 or 6 months.48 Of the studies reporting no significant change in SCFAs, 4 displayed some concerns of risk of bias31,35,50,53 and 1 displayed a high risk of bias.44 Of the 3 studies reporting a significant increase in SCFAs, 2 displayed a high risk of bias34,48 and 1 displayed some concerns of risk of bias.43
Propionate
A total of 6 studies exploring the effect of synbiotic consumption on propionate concentration were included in the meta-analysis.34,41–43,51,53 The duration of interventions ranged from 1 week53 to 6 months.51 Synbiotic consumption resulted in a statistically significant increase in the propionate concentration, with an SMD of 0.22 (95% CI: 0.02, 0.43; P = 0.03) (Table 4, Fig. 3). The effect estimate was no longer significant after using different correlation coefficients (0.25, 0.5, and 0.75) (see Table S3 in the Supporting Information online). Sensitivity analyses revealed that excluding Macfarlane et al43 and Stenman et al51 led to a nonsignificant effect size of an SMD of 0.18 (95% CI: -0.05, 0.41; P = 0.12) and an SMD of 0.16 (95% CI: -0.08, 0.40; P = 0.18), respectively (see Fig. S7 in the Supporting Information online).
Changes in inflammatory markers
There were significant reductions in proinflammatory cytokines and inflammatory biomarkers reported by 8 studies across 6 population subtypes.32,33,40,43,45,50,52,54 The duration of interventions ranged from 3 weeks45 to 6 months.50 Four of these studies displayed a high risk of bias32,33,52,54 and 4 displayed some concerns of risk of bias.40,43,45,50 The meta-analysis estimates showed that synbiotic consumption did not result in a significant difference in interleukin (IL)-6, C-reactive protein (CRP), or endotoxin (Table 4, Fig. 4). However, the effect estimate for IL-6 was significant when using the correlation coefficient of 0.5 (see Table S3 in the Supporting Information online). Sensitivity analyses showed substantial changes when omitting Hibberd et al50 from CRP estimates (WMD: −0.28; 95% CI: −0.45, −0.12; P = 0.001) (see Fig. S4 in the Supporting Information online).
Quality of the body of evidence
The quality of the body of evidence was determined using GRADE61 (see Table S4 in the Supporting Information online). The quality of the body of evidence was “very low” for Bifidobacterium relative abundance, Lactobacillus and zonulin due to downgrades for risk of bias, inconsistency, and imprecision. The quality of the body of evidence was “low” for Bifidobacterium cell count, CRP, and acetate due to downgrades for both risk of bias and inconsistency. The quality of the body of evidence was “moderate” for the Shannon Index, propionate, and butyrate after being downgraded only for risk of bias.
DISCUSSION
To the authors’ knowledge, this systematic review and meta-analysis is the first to investigate the impact of synbiotic intake on the gut microbiota and inflammatory biomarkers in populations of adults with no significant clinical disease impacting the gut microbiota and/or inflammation. The results of the analysis of randomized controlled trials suggest that synbiotic interventions may increase the abundance of Lactobacillus cell count and propionate concentration but likely have no significant effect on zonulin, α-diversity measured as Shannon Index, acetate, butyrate, IL-6, CRP, or endotoxin concentration. When interpreting these findings, it should be noted that approximately half of the included studies displayed a high risk of bias, while some concerns regarding the risk of bias were noted in the remaining studies. The narrative synthesis of all included randomized trials suggests that synbiotics may also increase the abundance of Bifidobacterium measures. The nonsignificant meta-analysis effect estimate for Bifidobacterium cell count should be interpreted with caution, due to a significant effect seen when using correlation coefficients of 0.25, 0.5, and 0.75 for crossover studies, as well as sensitivity analyses revealing a substantial study effect by Bazzocchi et al.37 This study was the only included study with a negative Hedge’s g SMD value (-0.55; 95% CI: -1.28, 0.18), likely explaining why its exclusion resulted in a significant Bifidobacterium cell count estimate of an SMD of 1.01 (95% CI: 0.13, 1.88; P = 0.02).
Given the fact that all probiotic supplements contained single or multiple strains from Lactobacillus and Bifidobacterium genera, it is not surprising to see a consistent increase in these 2 genera. Yet, these changes are not evident when supplementing with probiotics alone. A systematic review examining alterations in fecal microbiota composition among healthy adults found no observed changes to fecal microbiota bacterial profile or measures of α-diversity after probiotic supplementation.10 The results from this review therefore suggest that synbiotics can modulate the gut microbiota in healthy adult populations, in contrast to the cumulative evidence for probiotic interventions alone. Given that only 5 studies collected data during a follow-up period, the results of this systematic review are unable to identify whether synbiotics offer a long-lasting or only a transient effect on the human gut microbiome. Three of these follow-up studies found that none of the significant differences in the measured gut microbiome outcomes had remained during the follow-up period.30,34,38 One month after a 6-month intervention period, Hibberd et al50 reported an increased abundance of Lactobacillus in the intervention group. Roberts et al32 reported that 6 days after finishing a 3-month synbiotic intervention, participants continued to display a significantly reduced plasma endotoxin level; however, during this time, participants also completed an endurance triathlon event. Probiotic intervention trials have similarly shown inconsistencies regarding the duration of lasting effects on the gut microbiome.63,64 It is uncertain from findings of this review whether synbiotics offer any advantage over probiotics in this regard.
The microbiota-modulating effects demonstrated by synbiotics in this review have similarly been shown by prebiotic interventions. The prebiotics most supplemented in this review—inulin, GOS, and FOS—have consistently demonstrated the ability to increase Bifidobacterium and Lactobacillus bacteria.65–67 GOS and FOS, at doses of 5–10 g and 2.5–10 g, respectively, have increased Bifidobacterium populations in healthy adult populations, after as few as 7 days of supplementation.65–67 Additionally, the systematic review and meta-analysis by So et al68 examining the effect of dietary fiber interventions on the gut microbiota in healthy adults reported a significant increase in Lactobacillus and Bifidobacterium compared with placebo or low-fiber comparators. Subgroup analyses identified that prebiotic dietary fibers resulted in a significantly greater increase in Bifidobacterium compared with comparators. The systematic review by Ferro et al69 examined the effect of prebiotics, probiotics, and synbiotics on the gut microbiota and health outcomes in infants, and reported an increase of fecal Bifidobacterium measures after prebiotic and synbiotic supplementation, but not after probiotics. These results further suggest that prebiotics, and therefore synbiotics, have a greater capacity to alter the microbiota than probiotic supplementation. Given that prebiotics have demonstrated the ability to modulate the gut microbiota of healthy adults in a similar manner to the synbiotic interventions included in this review,65–68 it is difficult to ascertain if the microbiota-modulating effects reported in this study are due to the unique synbiotic combination or due to the prebiotics alone.
The human gut microbiota is a stable and resilient ecosystem.70 It is therefore not surprising that introducing a bacterial species alone, in the form of probiotics in a transient manner, does not substantially alter the microbiota. Additionally, despite dietary interventions having demonstrated the ability to rapidly alter the human gut microbiota, these alterations revert to the original structure after ceasing dietary interventions.71 Although probiotics introduce new species, prebiotics when supplemented alone provide a substrate for pre-existing microbial species. Given that all humans possess Bifidobacterium and Lactobacillus genera in their gut microbiota, providing substrates that feed these endogenous bacteria may be a more effective strategy than introducing exogenous bacterial species into a resilient ecosystem. It is widely recognized that Bifidobacterium and Lactobacillus are healthy gut bacteria, having demonstrated key roles in the production of SCFAs, immune system function, maintaining stability and diversity of the gut microbiota, and providing various clinical benefits.72–74 Interventions to increase these bacterial genera are widely favored, particularly Bifidobacterium due to the demonstrated decrease in this genus throughout adulthood compared with infancy.75 Thus, the proliferation of these beneficial bacteria demonstrated in this review is a positive health-enhancing result.
The lack of change in α-diversity seen in this meta-analysis, a measure of the richness and evenness of bacterial species, is not unexpected given the results of previous studies. The reviews by So et al68 and Kristensen et al10 similarly did not find a significant change in α-diversity after prebiotic and probiotic supplementation in healthy adults, respectively. Additional reviews reporting on changes in α-diversity after microbiota-modulating therapies further demonstrate inconsistencies. A recent systematic review20 found no significant change in α-diversity measures after probiotic or synbiotic supplementation in adults with major depressive disorder. In contrast, a meta-analysis reported a significant increase in α-diversity after weight-loss interventions, yet this significant increase was not present when pooling the effect of food-based interventions alone.76 Similarly, another systematic review found minimal changes in α-diversity after food-based weight-loss interventions.77 These findings align with the discussion by So et al68 that current literature suggests that short-term dietary or microbiota interventions are unable to alter microbial diversity,71,78,79 yet observational studies assessing habitual dietary intake show positive correlations between dietary diversity and microbiota diversity.80,81 Thus, perhaps assessing changes in α-diversity from short-term studies is not a useful indicator of the overall health of the human gut microbiota, given the stability and resilience of the microbial ecosystem.82 Coyte et al83 provide a topical discussion on the interactions between diversity, microbial competition, and microbial stability, demonstrating that microbial diversity does not always correlate with a stable and therefore healthy microbiome. In light of this ecological concept, human gut microbiota research may benefit from taking greater care to consider measures of microbial diversity as indicators of microbiome health.84
This review reported no significant reductions to any inflammatory markers after synbiotic supplementation, which is somewhat in contrast to previous literature. Overweight, obesity, prediabetes, and diabetes, collectively referred to as the metabolic syndrome, are associated with a low-grade inflammatory response due to metabolic endotoxemia.85 This response is closely related to alterations in the intestinal microbiota.86 One meta-analysis found a significant reduction in CRP by an SMD of -0.38 (P = 0.00) after synbiotic or probiotic supplementation in adults with diabetes87; however, another meta-analysis found no significant change in any inflammatory markers after probiotic supplements in adults with diabetes.88 Anti-inflammatory effects after probiotic, prebiotic, and synbiotic supplementation have additionally been demonstrated in adults who are overweight or obese.89,90 This review synthesized the data from 7 studies of adults with overweight, obesity, or diabetes,46–52 with the remaining 20 studies including participants not known to have raised inflammatory markers at baseline. Subgroup analyses were not able to be performed in these 7 studies due to the small number of studies included in each meta-analysis effect estimate. It is likely that changes to inflammatory markers after synbiotic supplementation were not see in this review due to the majority of included studies examining adults not known to have raised inflammatory biomarkers and a relatively small sample size of only 5 studies assessing CRP43,50–52,54 and/or IL-643,45,48,51,52 included in the meta-analysis.
Short-chain fatty acids, particularly butyrate, have demonstrated anti-inflammatory effects as butyrate is the main substrate for colonic epithelium barrier cells, thereby preserving the integrity of this barrier.26 In vitro studies have demonstrated that SCFAs can modulate the expression of inflammatory cytokines by colonic epithelial cells.26 Additionally, SCFAs may have a direct impact on immune cells by modulating cell signaling and metabolism, which have effects on the innate and adaptive immune system.26 This review demonstrated a significant increase in propionate SCFAs after synbiotic supplementation, but not acetate or butyrate, and the data were not available to pool total SCFA production. The varied findings of the effect of synbiotics on SCFAs in healthy adults in this review are in line with previous literature. The recent systematic review by Vinelli et al91 investigating the effect of dietary fiber interventions in healthy adults found that 7 studies reported a significant increase in total SCFAs, while 5 studies reported no changes in total SCFAs. Authors of that review concluded that dietary fibers, including prebiotics, do not produce unequivocal increases in SCFAs in healthy adults.91
Strengths
All studies included in the meta-analysis were randomized controlled trials and therefore provide the highest degree of evidence. This review also followed a robust methodology including prospective registration of the review protocol and duplicate initial screening of the included articles. Additionally, a comprehensive search was conducted at 2 time points to maximize the likelihood that all eligible trials were included. Considering the heterogeneity in intervention type and population, data were analyzed using a random-effects model to provide a more conservative average effect of the intervention.
Limitations
Various limitations in this review warrant discussion and consideration. Despite all included studies assessing synbiotic interventions, variation in the type of prebiotics and probiotics, doses, and duration resulted in heterogeneity. Studies ranged from 1 week to 6 months in duration, and due to the lack of subgroup analyses, it was not possible to identify an optimal duration for supplementation. Although all included study participants were considered healthy adults who had no significant clinical disease impacting the gut microbiota and/or inflammatory conditions, population characteristics still varied considerably and 6 population subtypes were identified. Moreover, heterogeneity regarding the microbiota measurements and reporting existed among the included studies. Studies with next-generation sequencing, cell culturing, and non-sequencing molecular-based methods were included in this review, resulting in differences in the bacterial identification and specificity.92 This substantial heterogeneity, particularly due to synbiotic type and population characteristics, limits the translational and clinical application of the evidence. This systematic review is, therefore, unable to provide evidence for certain synbiotic combinations that offer greater effects on the gut microbiota or inflammation in healthy adult populations.
Additional limitations include the high risk of bias present in 13 included studies, particularly bias related to deviations from the intended intervention and missing outcome data, which may have affected the overall results. Further, due to the small sample sizes, funnel plots to test for publication bias were not conducted. The quality of the body of evidence was downgraded in all assessed outcomes (evaluated using GRADE61) due to concerns with risk of bias, inconsistency, and imprecision. This further confirms the inappropriateness of the current body of evidence presented in this review for translation into clinical practice.
Recommendations
To reduce the heterogeneity and improve the specificity of results, further reviews pooling data only from next-generation sequencing methods are needed. Additionally, further high-quality randomized controlled trials exploring different doses and durations of synbiotics among homogenous healthy adult populations are needed to identify dose–response relationships and the most effective synbiotic regimens. Further well-designed studies that examine differing lengths of supplementation for the same dose and combination of synbiotics are needed to inform evidence-based clinical recommendations. Consideration also needs to be given to the desired microbiota outcome. Given the complexities of human gut microbiota research involving methodological differences and interdisciplinary research teams, standardized reporting guidelines are necessary to promote consistency and reproducibility. The STORMS (Strengthening The Organisation and Reporting of Microbiome Studies) tool, published in 2021, is a 17-item checklist providing guidance for concise and complete reporting of human microbiome studies.93 Widespread adoption of this reporting guideline may reduce between-study heterogeneity and facilitate future comparative analysis of microbiome research. As outlined in the STORMS tool, it is essential for authors to publish or make available all measured quantitative data from human microbiome clinical trials to ensure that results from eligible studies can be pooled in meta-analyses. To reduce the risk of bias in future clinical trials, intention-to-treat analysis is required, with statistical analytical methods being prespecified in clinical trial registrations or protocols, and the reporting of missing outcome data to improve transparency.
CONCLUSION
This systematic review and meta-analysis of the effects of synbiotics on the gut microbiota and inflammatory biomarkers in healthy adults found evidence for a favorable effect on Lactobacillus cell count and propionate concentration, suggesting modulation of the gut microbiota. However, the nonsignificant differences in Bifidobacterium, α-diversity, zonulin, acetate, and butyrate suggest a limited effect on the gut microbiota composition, structure, and function. The nonsignificant effect estimate for Bifidobacterium measures should be interpreted with caution due to the significant effect size seen with differing correlation coefficients and when excluding 2 studies. Additionally, the nonsignificant differences in CRP, IL-6, and endotoxin concentrations suggest a lack of consistent evidence for the effects of synbiotics on inflammation in healthy adult populations. This is the first review to date to provide evidence for the microbiota-modulating effect of various synbiotic doses and combinations among healthy adult populations, and highlights the need for continued exploration of synbiotics as microbiota-modulating therapies.
Supplementary Material
Acknowledgments
Author contributions. D.J.C., Y.P., and K.C. designed the study and conducted study screening and application of eligibility criteria. D.J.C. extracted the data. D.J.C. and E.P.N. performed statistical analyses. D.J.C., K.L., E.P.N., Y.P., and K.C. assisted with data interpretation and analysis. D.J.C. drafted the manuscript. K.C., Y.P., K.L., and E.P.N. provided critical review of the manuscript. All authors have read and approved the final manuscript.
Funding. No external funding was received to support this work.
Declaration of interest. D.J.C. is supported by an Australian Government Research Training Program scholarship. All other authors have no relevant interests to declare.
Data availability statement. Access to data are available on request (djb983@uowmail.edu.au).
Contributor Information
Denelle J Cosier, School of Medicine, Indigenous and Health Sciences, University of Wollongong, Wollongong, New South Wales, Australia.
Kelly Lambert, School of Medicine, Indigenous and Health Sciences, University of Wollongong, Wollongong, New South Wales, Australia.
Elizabeth P Neale, School of Medicine, Indigenous and Health Sciences, University of Wollongong, Wollongong, New South Wales, Australia.
Yasmine Probst, School of Medicine, Indigenous and Health Sciences, University of Wollongong, Wollongong, New South Wales, Australia.
Karen Charlton, School of Medicine, Indigenous and Health Sciences, University of Wollongong, Wollongong, New South Wales, Australia.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Appendix S1 Full search strategy
Table S1 Summary data for meta-analyses
Table S2 Sensitivity analyses using varying correlation coefficients for cross-over studies
Table S3 Justification for risk of bias judgments
Table S4 GRADE assessment of the quality of the body of evidence
Table S5 PRISMA Checklist
Figure S1 Effect for estimate of Lactobacillus cell count if one study is omitted
Figure S2 Effect for estimate of Bifidobacterium relative abundance (%) if one study is omitted
Figure S3 Effect for estimate of Bifidobacterium cell count if one study is omitted
Figure S4 Effect for estimate of zonulin (ng/mL) if one study is omitted
Figure S5 Effect for estimate of Shannon Index if one study is omitted
Figure S6 Effect for estimate of acetate concentration if one study is omitted
Figure S7 Effect for estimate of propionate concentration if one study is omitted
Figure S8 Effect for estimate of butyrate concentration if one study is omitted
Figure S9 Effect for estimate of interleukin-6 (pg/mL) if one study is omitted
Figure S10 Effect for estimate of CRP (mg/L) if one study is omitted
Figure S11 Effect for estimate of endotoxin (EU/mL) if one study is omitted
Figure S12 Risk of bias assessment summary
REFERENCES
- 1.Chr. Hansen Holding A/S. New Study of Consumer Understanding of Probiotics Points to Significant Opportunities for the Food Industry. Denmark: Chr. Hansen Holding A/S; 2022. [Google Scholar]
- 2.Hill C, Guarner F, Reid G, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. [DOI] [PubMed] [Google Scholar]
- 3.Hungin A, Mulligan C, Pot B, et al. ; European Society for Primary Care Gastroenterology. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice–an evidence‐based international guide. Aliment Pharmacol Ther. 2013;38:864–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Didari T, Mozaffari S, Nikfar S, et al. Effectiveness of probiotics in irritable bowel syndrome: updated systematic review with meta-analysis. World J Gastroenterol. 2015;21:3072–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McFarland LV, Huang Y, Wang L, et al. Systematic review and meta-analysis: multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United European Gastroenterol J. 2016;4:546–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McFarland LV. Probiotics for the primary and secondary prevention of C. difficile infections: a meta-analysis and systematic review. Antibiotics (Basel). 2015;4:160–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vouloumanou EK, Makris GC, Karageorgopoulos DE, et al. Probiotics for the prevention of respiratory tract infections: a systematic review. Int J Antimicrob Agents. 2009;34:197.e191–197.e110. [DOI] [PubMed] [Google Scholar]
- 8.Bock PM, Telo GH, Ramalho R, et al. The effect of probiotics, prebiotics or synbiotics on metabolic outcomes in individuals with diabetes: a systematic review and meta-analysis. Diabetologia. 2021;64:26–41. [DOI] [PubMed] [Google Scholar]
- 9.Thongprayoon C, Kaewput W, Hatch ST, et al. Effects of probiotics on inflammation and uremic toxins among patients on dialysis: a systematic review and meta-analysis. Dig Dis Sci. 2019;64:469–479. [DOI] [PubMed] [Google Scholar]
- 10.Kristensen NB, Bryrup T, Allin KH, et al. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8:52. doi: 10.1186/s13073-016-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFarland LV. Use of probiotics to correct dysbiosis of normal microbiota following disease or disruptive events: a systematic review. BMJ Open. 2014;4:e005047. doi: 10.1136/bmjopen-2014-005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 13.Finegold SM, Li Z, Summanen PH, et al. Xylooligosaccharide increases bifidobacteria but not lactobacilli in human gut microbiota. Food Funct. 2014;5:436–445. [DOI] [PubMed] [Google Scholar]
- 14.Le Bastard Q, Chapelet G, Javaudin F, et al. The effects of inulin on gut microbial composition: a systematic review of evidence from human studies. Eur J Clin Microbiol Infect Dis. 2020;39:403–413. doi: 10.1007/s10096-019-03721-w. [DOI] [PubMed] [Google Scholar]
- 15.Swanson KS, Gibson GR, Hutkins R, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17:687–701. doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan CKY, Tao J, Chan OS, et al. Preventing respiratory tract infections by synbiotic interventions: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;11:979–988. doi: 10.1093/advances/nmaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford AC, Harris LA, Lacy BE, et al. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044–1060. doi: 10.1111/apt.15001. [DOI] [PubMed] [Google Scholar]
- 18.Manzanares W, Lemieux M, Langlois PL, et al. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care. 2016;19:262. doi: 10.1186/s13054-016-1434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzumura EA, Bersch-Ferreira ÂC, Torreglosa CR, et al. Effects of oral supplementation with probiotics or synbiotics in overweight and obese adults: a systematic review and meta-analyses of randomized trials. Nutr Rev. 2019;77:430–450. doi: 10.1093/nutrit/nuz001. [DOI] [PubMed] [Google Scholar]
- 20.Alli SR, Gorbovskaya I, Liu JCW, et al. The gut microbiome in depression and potential benefit of prebiotics, probiotics and synbiotics: a systematic review of clinical trials and observational studies. Int J Mol Sci. 2022;23:5–15. doi: 10.3390/ijms23094494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McFarlane C, Ramos CI, Johnson DW, et al. Prebiotic, probiotic, and synbiotic supplementation in chronic kidney disease: a systematic review and meta-analysis. J Ren Nutr. 2019;29:209–220. doi: 10.1053/j.jrn.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Zhang XF, Guan XX, Tang YJ, et al. Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: a systematic review and meta-analysis. Eur J Nutr. 2021;60:2855–2875. doi: 10.1007/s00394-021-02503-5. [DOI] [PubMed] [Google Scholar]
- 23.Steed H, Macfarlane GT, Blackett KL, et al. Clinical trial: the microbiological and immunological effects of synbiotic consumption—a randomized double-blind placebo-controlled study in active Crohn's disease. Aliment Pharmacol Ther. 2010;32:872–883. doi: 10.1111/j.1365-2036.2010.04417.x. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa H, Matsumoto S, Ohashi Y, et al. Beneficial effects of probiotic bifidobacterium and galacto-oligosaccharide in patients with ulcerative colitis: a randomized controlled study. Digestion. 2011;84:128–133. doi: 10.1159/000322977. [DOI] [PubMed] [Google Scholar]
- 25.Khalesi PS, Vandelanotte PC, Thwaite BT, et al. Awareness and attitudes of gut health, probiotics and prebiotics in Australian adults. J Diet Suppl. 2021;18:418–432. doi: 10.1080/19390211.2020.1783420. [DOI] [PubMed] [Google Scholar]
- 26.Gill PA, van Zelm MC, Muir JG, et al. Review article: short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther. 2018;48:15–34. doi: 10.1111/apt.14689. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3 (updated February 2022). Cochrane Collaboration; 2022.
- 28.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ. 2009;6:e1000097. [PMC free article] [PubMed] [Google Scholar]
- 29.Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia; 2022. Available at: www.covidence.org.
- 30.Palaria A, Johnson-Kanda I, O'Sullivan DJ.. Effect of a synbiotic yogurt on levels of fecal bifidobacteria, clostridia, and enterobacteria. Appl Environ Microbiol. 2012;78:933–940. doi: 10.1128/aem.05848-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Zanten GC, Krych L, Röytiö H, et al. Synbiotic Lactobacillus acidophilus NCFM and cellobiose does not affect human gut bacterial diversity but increases abundance of lactobacilli, bifidobacteria and branched-chain fatty acids: a randomized, double-blinded cross-over trial. FEMS Microbiol Ecol. 2014;90:225–236. doi: 10.1111/1574-6941.12397. [DOI] [PubMed] [Google Scholar]
- 32.Roberts JD, Suckling CA, Peedle GY, et al. An exploratory investigation of endotoxin levels in novice long distance triathletes, and the effects of a multi-strain probiotic/prebiotic, antioxidant intervention. Nutrients. 2016;8:733. doi: 10.3390/nu8110733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajkumar H, Kumar M, Das N, et al. Effect of probiotic Lactobacillus salivarius UBL S22 and prebiotic fructo-oligosaccharide on serum lipids, inflammatory markers, insulin sensitivity, and gut bacteria in healthy young volunteers: a randomized controlled single-blind pilot study. J Cardiovasc Pharmacol Ther. 2015;20:289–298. doi: 10.1177/1074248414555004. [DOI] [PubMed] [Google Scholar]
- 34.Valle M, Vieira IA, Fino LC, et al. Immune status, well-being and gut microbiota in military supplemented with synbiotic ice cream and submitted to field training: a randomised clinical trial. Br J Nutr. 2020;126:1794–1808. doi: 10.1017/S0007114521000568. [DOI] [PubMed] [Google Scholar]
- 35.Childs CE, Röytiö H, Alhoniemi E, et al. Xylo-oligosaccharides alone or in synbiotic combination with Bifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: a double-blind, placebo-controlled, randomised, factorial cross-over study. Br J Nutr. 2014;111:1945–1956. [DOI] [PubMed] [Google Scholar]
- 36.Kim MC, Lee S, Park JK, et al. Effects of ID-HWS1000 on the perception of bowel activity and microbiome in subjects with functional constipation: a randomized, double-blind placebo-controlled study. J Med Food. 2021;24:883–893. doi: 10.1089/jmf.2020.4746. [DOI] [PubMed] [Google Scholar]
- 37.Bazzocchi G, Giovannini T, Giussani C, et al. Effect of a new synbiotic supplement on symptoms, stool consistency, intestinal transit time and gut microbiota in patients with severe functional constipation: a pilot randomized double-blind, controlled trial. Tech Coloproctol. 2014;18:945–953. doi: 10.1007/s10151-014-1201-5. [DOI] [PubMed] [Google Scholar]
- 38.Bogovič Matijašić B, Obermajer T, Lipoglavšek L, et al. Effects of synbiotic fermented milk containing Lactobacillus acidophilus La-5 and Bifidobacterium animalis ssp. lactis BB-12 on the fecal microbiota of adults with irritable bowel syndrome: a randomized double-blind, placebo-controlled trial. J Dairy Sci. 2016;99:5008–5021. doi: 10.3168/jds.2015-10743. [DOI] [PubMed] [Google Scholar]
- 39.Anzawa D, Mawatari T, Tanaka Y, et al. Effects of synbiotics containing Bifidobacterium animalis subsp. lactis GCL2505 and inulin on intestinal bifidobacteria: a randomized, placebo-controlled, crossover study. Food Sci Nutr. 2019;7:1828–1837. doi: 10.1002/fsn3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neyrinck AM, Rodriguez J, Taminiau B, et al. Improvement of gastrointestinal discomfort and inflammatory status by a synbiotic in middle-aged adults: a double-blind randomized placebo-controlled trial. Sci Rep. 2021;11:2627. doi: 10.1038/s41598-020-80947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shioiri T, Yahagi K, Nakayama S, et al. The effects of a synbiotic fermented milk beverage containing Lactobacillus casei strain shirota and transgalactosylated oligosaccharides on defecation frequency, intestinal microflora, organic acid concentrations, and putrefactive metabolites of sub-optimal health state volunteers: a randomized placebo-controlled cross-over study. Bioscience Microflora. 2006;25:137–146. doi: 10.12938/bifidus.25.137. [DOI] [Google Scholar]
- 42.Ouwehand AC, Tiihonen K, Saarinen M, et al. Influence of a combination of Lactobacillus acidophilus NCFM and lactitol on healthy elderly: intestinal and immune parameters. Br J Nutr. 2009;101:367–375. doi: 10.1017/s0007114508003097. [DOI] [PubMed] [Google Scholar]
- 43.Macfarlane S, Cleary S, Bahrami B, et al. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: a randomised, double-blind, placebo-controlled crossover study. Aliment Pharmacol Ther. 2013;38:804–816. doi: 10.1111/apt.12453. [DOI] [PubMed] [Google Scholar]
- 44.Lehtinen MJ, Maneraat S, Childs CE, et al. Consumption of Bifidobacterium animalis subsp. Lactis Bi-07 in a clinical trial enhances ex vivo phagocytic activity in healthy elderly adults. Immunology. 2012;137:730. doi: 10.1111/imm.12002. [DOI] [Google Scholar]
- 45.Costabile A, Bergillos-Meca T, Rasinkangas P, et al. Effects of soluble corn fiber alone or in synbiotic combination with Lactobacillus rhamnosus GG and the pilus-deficient derivative GG-PB12 on fecal microbiota, metabolism, and markers of immune function: a randomized, double-blind, placebo-controlled, crossover study in healthy elderly (saimes study). Front Immunol. 2017;8:1443. doi: 10.3389/fimmu.2017.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kassaian N, Feizi A, Rostami S, et al. The effects of 6 mo of supplementation with probiotics and synbiotics on gut microbiota in the adults with prediabetes: a double blind randomized clinical trial. Nutrition. 2020;79-80:110854. doi: 10.1016/j.nut.2020.110854. [DOI] [PubMed] [Google Scholar]
- 47.Horvath A, Leber B, Feldbacher N, et al. Effects of a multispecies synbiotic on glucose metabolism, lipid marker, gut microbiome composition, gut permeability, and quality of life in diabesity: a randomized, double-blind, placebo-controlled pilot study. Eur J Nutr. 2020;59:2969–2983. doi: 10.1007/s00394-019-02135-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanazawa A, Aida M, Yoshida Y, et al. Effects of synbiotic supplementation on chronic inflammation and the gut microbiota in obese patients with type 2 diabetes mellitus: a randomized controlled study. Nutrients. 2021;13:1–19. doi: 10.3390/nu13020558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krumbeck JA, Rasmussen HE, Hutkins RW, et al. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome. 2018;6:121. doi: 10.1186/s40168-018-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hibberd AA, Yde CC, Ziegler ML, et al. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef Microbes. 2019;10:121–135. doi: 10.3920/BM2018.0028. [DOI] [PubMed] [Google Scholar]
- 51.Stenman LK, Lehtinen MJ, Meland N, et al. Probiotic with or without fiber controls body fat mass, associated with serum zonulin, in overweight and obese adults—randomized controlled trial. EBioMedicine. 2016;13:190–200. doi: 10.1016/j.ebiom.2016.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angelino D, Martina A, Rosi A, et al. Glucose- and lipid-related biomarkers are affected in healthy obese or hyperglycemic adults consuming a whole-grain pasta enriched in prebiotics and probiotics: a 12-week randomized controlled trial. J Nutr. 2019;149:1714–1723. doi: 10.1093/jn/nxz071. [DOI] [PubMed] [Google Scholar]
- 53.McLoughlin R, Berthon BS, Rogers GB, et al. Soluble fibre supplementation with and without a probiotic in adults with asthma: a 7-day randomised, double blind, three way cross-over trial. EBioMedicine. 2019;46:473–485. doi: 10.1016/j.ebiom.2019.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghavami A, Khorvash F, Heidari Z, et al. Effect of synbiotic supplementation on migraine characteristics and inflammatory biomarkers in women with migraine: results of a randomized controlled trial. Pharmacol Res. 2021;169:105668. doi: 10.1016/j.phrs.2021.105668. [DOI] [PubMed] [Google Scholar]
- 55.Casiraghi MC, Canzi E, Zanchi R, et al. Effects of a synbiotic milk product on human intestinal ecosystem. J Appl Microbiol. 2007;103:499–506. doi: 10.1111/j.1365-2672.2006.03273.x. [DOI] [PubMed] [Google Scholar]
- 56.Lee S, You H, Lee Y, et al. Intake of MPRO3 over 4 weeks reduces glucose levels and improves gastrointestinal health and metabolism. Microorganisms. 2021;10:88. doi: 10.3390/microorganisms10010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riley RD, Higgins JPT, Deeks JJ.. Interpretation of random effects meta-analyses. BMJ. 2011;342: D 549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 59.Langan D, Higgins JPT, Jackson D, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10:83–98. doi: 10.1002/jrsm.1316. [DOI] [PubMed] [Google Scholar]
- 60.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 61.Schünemann HJ, Oxman AD, Brozek J, et al.; GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–1110. doi: 10.1136/bmj.39500.677199.AE. Erratum in: BMJ. 2008;336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bjerg A, Sørensen M, Krych L, et al. The effect of Lactobacillus paracasei subsp. paracasei L. casei W8® on blood levels of triacylglycerol is independent of colonisation. Benef Microbes. 2015;6:263–269. [DOI] [PubMed] [Google Scholar]
- 64.Lahti L, Salonen A, Kekkonen RA, et al. Associations between the human intestinal microbiota, Lactobacillus rhamnosus GG and serum lipids indicated by integrated analysis of high-throughput profiling data. PeerJ 2013;1:e32. doi: 10.7717/peerj.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bouhnik Y, Raskine L, Simoneau G, et al. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am J Clin Nutr. 2004;80:1658–1664. doi: 10.1093/ajcn/80.6.1658. [DOI] [PubMed] [Google Scholar]
- 66.Davis LMG, Martínez I, Walter J, et al. A dose dependent impact of prebiotic galactooligosaccharides on the intestinal microbiota of healthy adults. Int J Food Microbiol. 2010;144:285–292. doi: 10.1016/j.ijfoodmicro.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 67.Bouhnik Y, Raskine L, Simoneau G, et al. The capacity of short-chain fructo-oligosaccharides to stimulate faecal bifidobacteria: a dose-response relationship study in healthy humans. Nutr J. 2006;5:8–30. doi: 10.1186/1475-2891-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.So D, Whelan K, Rossi M, et al. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;107:965–983. doi: 10.1093/ajcn/nqy041. [DOI] [PubMed] [Google Scholar]
- 69.Ferro LE, Crowley LN, Bittinger K, et al. Effects of prebiotics, probiotics, and synbiotics on the infant gut microbiota and other health outcomes: a systematic review. Crit Rev Food Sci Nutr. 2023;63:5620–5642. doi: 10.1080/10408398.2021.2022595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Picard C, Fioramonti J, Francois A, et al. Review article: Bifidobacteria as probiotic agents—physiological effects and clinical benefits. Aliment Pharmacol Ther. 2005;22:495–512. doi: 10.1111/j.1365-2036.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 73.Berstad A, Raa J, Midtvedt T, et al. Probiotic lactic acid bacteria—the fledgling cuckoos of the gut? Microb Ecol Health Dis. 2016;27:31557–31529. doi: 10.3402/mehd.v27.31557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu H, Chen X, Zhang S, et al. Gut microbiota, the potential biological medicine for prevention, intervention and drug sensitization to fight diseases. Nutrients. 2022;14:10–28. doi: 10.3390/nu14204220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arboleya S, Watkins C, Stanton C, et al. Gut bifidobacteria populations in human health and aging. Front Microbiol. 2016;7:1204. doi: 10.3389/fmicb.2016.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koutoukidis DA, Jebb SA, Zimmerman M, et al. The association of weight loss with changes in the gut microbiota diversity, composition, and intestinal permeability: a systematic review and meta-analysis. Gut Microbes. 2022;14:2020068. doi: 10.1080/19490976.2021.2020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bliesner A, Eccles-Smith J, Bates C, et al. Impact of food-based weight loss interventions on gut microbiome in individuals with obesity: a systematic review. Nutrients. 2022;14:14. doi: 10.3390/nu14091953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walker AW, Ince J, Duncan SH, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 81.McDonald D, Hyde E, Debelius JW, et al. ; American Gut Consortium. American gut: an open platform for citizen science microbiome research. mSystems. 2018;3:e00031-00018. doi: Doi: 10.1128/mSystems.00031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fassarella M, Blaak EE, Penders J, et al. Gut microbiome stability and resilience: elucidating the response to perturbations in order to modulate gut health. Gut. 2021;70:595–605. doi: 10.1136/gutjnl-2020-321747. [DOI] [PubMed] [Google Scholar]
- 83.Coyte KZ, Schluter J, Foster KR.. The ecology of the microbiome: networks, competition, and stability. Science. 2015;350:663–666. [DOI] [PubMed] [Google Scholar]
- 84.Johnson KVA, Burnet PWJ.. Microbiome: should we diversify from diversity? Gut Microbes. 2016;7:455–458. doi: 10.1080/19490976.2016.1241933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wellen KE, Hotamisligil GS.. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/jci25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kallus SJ, Brandt LJ.. The intestinal microbiota and obesity. J Clin Gastroenterol. 2012;46:16–24. [DOI] [PubMed] [Google Scholar]
- 87.Zheng HJ, Guo J, Jia Q, et al. The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2019;142:303–313. doi: 10.1016/j.phrs.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 88.Samah S, Ramasamy K, Lim SM, et al. Probiotics for the management of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2016;118:172–182. doi: 10.1016/j.diabres.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 89.Mazidi M, Rezaie P, Ferns GA, et al. Impact of probiotic administration on serum C-reactive protein concentrations: systematic review and meta-analysis of randomized control trials. Nutrients. 2017;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fernandes R, do Rosario VA, Mocellin MC, et al. Effects of inulin-type fructans, galacto-oligosaccharides and related synbiotics on inflammatory markers in adult patients with overweight or obesity: a systematic review. Clin Nutr. 2017;36:1197–1206. doi: 10.1016/j.clnu.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 91.Vinelli V, Biscotti P, Martini D, et al. Effects of dietary fibers on short-chain fatty acids and gut microbiota composition in healthy adults: a systematic review. Nutrients. 2022;14:14. doi: 10.3390/nu14132559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sarangi AN, Goel A, Aggarwal R.. Methods for studying gut microbiota: a primer for physicians. J Clin Exp Hepatol. 2019;9(1):62–73. doi: 10.1016/j.jceh.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mirzayi C, Renson A, Zohra F, et al. ; Massive Analysis and Quality Control Society. Reporting guidelines for human microbiome research: the STORMS checklist. Nat Med. 2021;27:1885–1892. doi: 10.1038/s41591-021-01552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.