Abstract
Background
Congenital cytomegalovirus (cCMV) infection imposes a substantial economic burden on both families and society in China. There is currently a paucity of dynamic models to study cytomegalovirus (CMV) vaccination strategies for China’s high-seroprevalence population (over 95%). Recent clinical trials demonstrated that the messenger RNA (mRNA) vaccine candidate, mRNA-1647, exhibited potential efficacy in both preinfection and postinfection contexts. This study aims to assess the cost-effectiveness of various CMV vaccination strategies for Chinese young women.
Method
An age-structured dynamic model was adopted, using Mathematica software, to simulate three strategies: (1) no vaccination (status quo); (2) pre-marriage vaccination (age 20–28 years); (3) reproductive-age vaccination (age 20–40 years). The vaccine was assumed to have 50% coverage and 50% efficacy for the first 5 years, with efficacy gradually decreasing over the next 15 years, costing US$300 per treatment course. This study period covers 2025–2050. Health outcomes included reductions in cCMV infection incidence, morbidity and mortality. We conducted cost-effectiveness, scenario and sensitivity analyses, discounting costs and disability-adjusted life years (DALYs) at 3% annually. The strategy would be considered cost-effective if the incremental cost-effectiveness ratio (ICER) was below China’s 2023 per capita gross domestic product (US$12 675).
Results
By 2050, pre-marriage and reproductive-age vaccination strategies could prevent cCMV infection incidence by 38.8% (95% uncertainty interval [UI], 33.7% to 43.5%) and 43.3% (38.3% to 47.1%), respectively, with ICERs of US$4751 (4124 to 5378) and US$10 814 (10 290 to 11 338) per DALY averted compared with the status quo. However, the reproductive-age strategy is not cost-effective, with an ICER of US$25 553 (12 566 to 36 126) versus the pre-marriage strategy.
Conclusions
Prioritising pre-marriage vaccination could control cCMV infection in China. Our findings would inform public health policies and guide future research on optimising CMV vaccination strategies.
Keywords: Epidemiologic Factors, Age Factors, Public Health Practice, Communicable Disease Control, economics
WHAT IS ALREADY KNOWN ON THIS TOPIC
Previous studies have explored cytomegalovirus (CMV) vaccination strategies but lacked dynamic modelling assessments tailored to China’s high-seroprevalence population (over 95%).
The messenger RNA-1647 vaccine shows potential with estimated 50% efficacy against CMV acquisition in seronegative and seropositive individuals.
WHAT THIS STUDY ADDS
This study reveals that both pre-marriage and reproductive-age vaccination strategies are effective, reducing congenital CMV (cCMV) incidence by 38.8% and 43.3%, respectively.
Notably, the pre-marriage strategy is the most cost-effective, with an ICER of US$4751 per DALY averted, well below China’s willingness-to-pay threshold.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These findings highlight the importance of prioritising pre-marriage vaccination to control cCMV, informing public health policies and guiding future research on optimising CMV vaccination strategies.
Introduction
Cytomegalovirus (CMV) infection constitutes one of the most prevalent congenital infections during the neonatal period, carrying substantial public health ramifications.1 Globally, congenital CMV (cCMV) infection poses a significant health challenge, profoundly affecting the health and developmental outcomes of countless newborns.2 Research has demonstrated a correlation between the likelihood of cCMV infection and the seroprevalence of CMV within a given population. The vast majority of newborns with cCMV infection are born to seropositive young women.3 4 Seroprevalence rates worldwide vary between 50% and 98%, whereas the seroprevalence in the Chinese population surpasses 95%.5 6 cCMV infection is a predominant aetiological factor in permanent neonatal hearing loss, contributing to 15%–20% of all non-genetic hearing loss cases globally.7 8 In China, this proportion escalates from 25% to 35%.9 Furthermore, neonates suffering from cCMV infection face a heightened risk of developing additional severe neurodevelopmental complications, including mental retardation, vision loss and potentially even neonatal death.10 Even among neonates initially asymptomatic for infection, approximately 10%–15% manifest long-term health issues related to the infection during childhood or adolescence.11
The ‘Healthy China 2030’ and China’s Three-Child Policy were launched in 2016 and 2021, respectively. With advanced maternal age (AMA) pregnancies rising annually, good prenatal and postnatal care has received unprecedented attention. This trend heightens CMV transmission risks, as immunodeficiency and shortened interpregnancy intervals in AMA pregnancies may compromise gestational immunity, increasing maternal susceptibility. Concurrently, multiparous households amplify intrafamilial transmission, with children serving as key viral reservoirs and being the peak period for CMV acquisition. The interplay of rising multiparity rates and an expanding AMA population may perpetuate cyclical CMV transmission chains.12,15 As the prevalence of CMV in paediatric populations escalates, recurrent maternal exposure to the virus will likely drive a gradual but sustained increase in cCMV infection incidence. Therefore, it is imperative to explore effective strategies for controlling cCMV infection incidence and disrupting transmission chains.
Since CMV infection is often asymptomatic and difficult to detect early, vaccination may be one of the most effective prevention measures and disrupt the transmission chains.16 17 Given the significant disease burden associated with cCMV infection, the development of CMV vaccines has been a priority since 2000.18 Currently, four types of CMV vaccines are under investigation: attenuated vaccines, subunit vaccines, messenger RNA (mRNA) vaccines and DNA vaccines.19,22 The mRNA vaccine enables easy adaptation to different pathogens by simply altering the mRNA sequence encoding the desired antigen. Compared with the CMV attenuated vaccines (eg, V160 and Towne), mRNA vaccines only encode viral antigen proteins, do not contain the complete viral genome and do not pose a risk of infection or integration with host DNA.22 23 Compared with CMV subunit vaccines (eg, glycoprotein B (gB)/MF59), mRNA vaccines could encode the UI gH/gL/UL128/UL130/UL131A (Pentamer), while the sixth mRNA sequence encodes the full-length membrane-bound gB.20 24 25 Although none of these vaccines have received clinical approval yet, the recent reports suggest vaccine mRNA-1647 can achieve an efficacy of approximately 50% against CMV acquisition, for seropositive and seronegative individuals.20 26 To date, the majority of cost-effectiveness analyses regarding CMV vaccines have been conducted within the context of developed countries.27 28 The unique serological characteristics and national conditions of different populations result in these studies being inapplicable to China.29 30 Our team has previously examined various vaccination strategies through a Markov-decision tree model, concluding that vaccinating adults was cost-effective.31 However, there is a notable research gap in dynamic modelling studies of CMV vaccination strategies for Chinese women. Unlike static models, dynamic transmission models are particularly suited for CMV research as they can more accurately capture changes in force of infection (λ) over time.
This study aims to analyse the impact of CMV vaccination in Chinese young women of different age groups on the incidence of cCMV infection in newborns, in order to determine the most cost-effective strategy. The evaluation results will provide a theoretical basis for decision-making by health departments.
Methods
Study design and model development
This study presents a model-based analysis assessing the cost-effectiveness of CMV vaccination strategies for young women across different age groups in China, viewed from a societal perspective. The model was developed and implemented using Wolfram Mathematica V.13.3 (Wolfram Research, Champaign, Illinois, USA)
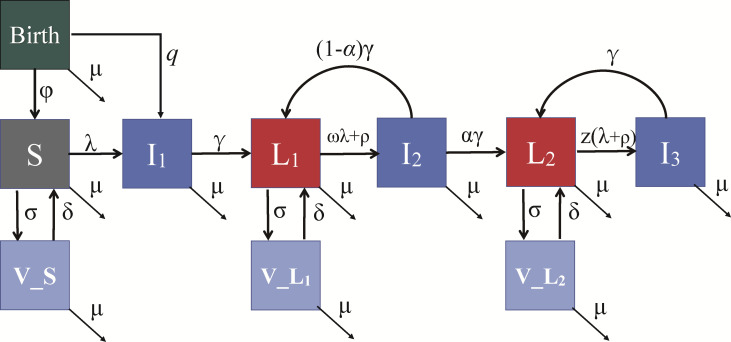
We developed a deterministic compartmental model that describes its spread across various infection states, age groups and genders (figure 1). This model is designed to incorporate the complex dynamics of primary infection, reinfection or reactivation and vertical transmission, offering a robust framework for analysis. Specifically, the population was divided into distinct compartments: susceptible individuals, latent infection individuals, high antibody titre latent infection individuals and acute infection individuals.
Figure 1. The transmission model of CMV. The model includes seronegative individuals (susceptible, S) and primary infection (I1) which occurs vertically from seropositive mothers (with probability q) or horizontally (with the force of infection λ) via contact with acutely infected individuals (I1,I2,I3). Following the primary infection stage (duration 1/γ year), individuals enter a latent stage characterised by low antibody concentrations (L1). L1 can be reinfected at a rate ωλ, where ω represents the reduction in susceptibility to reinfection compared with seronegative individuals and reactivation occurs at a rate ρ. After the reactivation/reinfection stage (I2), individuals transition to a second latent stage with high antibody concentrations (L2) with probability α. In this stage, susceptibility to reinfection or reactivation is significantly reduced, and the reinfection/reactivation (I3) rate is expressed as z(λ+ρ). The target population for vaccination includes S, L1 and L2, where σ denotes the effective vaccination rate and δ represents the waning rate. The population is stratified by sex and age, φ denotes the fertility rate and μ represents the mortality rate (online supplemental table S1). CMV, cytomegalovirus; q, parameter to represent the probability of vertical transmission during pregnancy.
λ was expressed as a weighted sum of the proportions of the population within these three infection categories, representing a nuanced representation of transmission potential. Moreover, latent individuals could experience reinfection at a rate of ωλ, where ω signifies the reduction in susceptibility to reinfection when compared with seronegative individuals. Additionally, the parameter (q) was used to represent the probability of vertical transmission during pregnancy, encompassing both primary, reinfection and reactivation to capture the full spectrum of potential outcomes. This comprehensive approach ensures that our model can accurately simulate CMV transmission (online supplemental table S1).
Data source
The health-related parameter inputs in the model were sourced from previous studies and State Statistics Bureau of China (online supplemental tables S2–S4). The model’s population structure was constructed using comprehensive demographic data from China, covering the years 2000–2050. This data set included critical elements such as population size, mortality rates and fertility rates, all sourced from United Nations statistics. This detailed demographic foundation ensured that the model accurately reflected population dynamics over time (online supplemental figure S1 and tables S5–S7).
Building on insights from our previous research, several key parameters were integrated into the model to enhance its realism and applicability, including the seroprevalence of CMV in China, the prevalence of primary CMV infection among pregnant women and the incidence rates of hearing loss, mental retardation, vision impairment in newborns affected by cCMV infection and the mortality rate among neonates with symptomatic cCMV infection. First, the model was designed to accommodate multiple reinfection and reactivation events over an individual’s lifetime, thus capturing the recurrent nature of CMV transmission. Second, based on current trends in vaccine research, a single vaccine type was incorporated, providing coverage for both seronegative and seropositive individuals. Third, rather than assuming an abrupt change of efficacy, the model featured a gradually decreasing vaccine waning rate, to more accurately simulate real-world immunological decay. Finally, the model took into account dynamic variations in population infectiousness by leveraging contact matrices and proportional population changes over time. These enhancements were crucial for illustrating the complex interactions of demographic changes, infection dynamics and vaccination strategies, leading to a model that could more effectively guide public health decisions.
Intervention strategies and health outcomes
To identify effective approaches for reducing cases of cCMV infection, we evaluated various intervention strategies. Our previous research has elucidated the limitations of neonatal vaccination strategies.31 As the efficacy of vaccines gradually diminishes over time, neonatal vaccination may result in a loss of vaccine protection by the time individuals reach reproductive age. This situation also reduces the naturally acquired immunity within the population, leading to an increased proportion of seronegative women of reproductive age, who are more susceptible to CMV infection.31 However, CMV infection during pregnancy poses substantial risks to fetal health, including cCMV syndrome. Recognising that vaccinating women of reproductive age can significantly lower the probability of vertical transmission, we specifically targeted young women aged 20–40 years, consistent with the focus of recent clinical vaccine trials.26
TORCH screening, including Toxoplasma gondii, Treponema pallidum, rubella virus, CMV and herpes virus, is a crucial component of pre-marriage health checks aimed at preventing infections during pregnancy.32 According to the ‘China Health Statistical Yearbook 2023’, the average rate of pre-marriage health check-ups in China was 74.8%, indicating a consistent annual increase.33 Considering the sociocultural context in China, the widely implemented pre-marriage health examination system offers a practical pathway for vaccination by leveraging existing healthcare infrastructure to enhance vaccine coverage. Consequently, we have further narrowed the reproductive-age population to identify the pre-marriage group as a crucial target for CMV vaccination. Targeting women before marriage can also ensure the establishment of an immune barrier prior to their peak fertility period, thereby maximising the preventive impact of the vaccine. According to Article 1047 of the Civil Code of the People’s Republic of China, enacted in 2021, the legal marriage age is defined as 22 years for men and 20 years for women. Furthermore, the average age of first marriage in China in 2020 was 29.4 years for men and 28 years for women.34 Therefore, the pre-marriage group for women was defined as those aged 20–28 years.
In our model, the vaccine was assumed to provide ‘degree/leaky’ protection. We assumed a vaccine coverage of 50%, with an initial efficacy of 50% for the first 5 years and gradually waning over the next 15 years, to reflect the natural decline in immunity. Our assumptions regarding vaccine efficacy and waning rates closely align with real-world scenarios, enhancing the reliability of our findings.
To systematically explore the impact of different age-specific vaccination approaches, we categorised three strategies : (1) no vaccination, (2) a pre-marriage strategy targeting women aged 20–28 years and (3) a reproductive-age strategy encompassing women aged 20–40 years. Each strategy was designed to model the administration of vaccines as each cohort reached the specified age range, employing a combined approach of campaign-based and routine vaccinations to reach the baseline population within these groups.
The primary outcomes were the number of prevented cCMV infection cases relative to the no vaccination strategy (status quo). In addition, secondary outcomes included the incidence rate reduction (IRR), mortality rate reduction (MRR) and the cumulative number needed to vaccinate to prevent one case (NNV), compared with the status quo.
Cost-effectiveness analysis
The analysis was performed from a societal perspective, encompassing a wide range of costs. These included direct medical expenses (eg, healthcare costs), direct non-medical expenses (eg, special education costs) and indirect costs such as productivity losses and family support. To accurately reflect cost distribution, expenses were stratified based on the affected population groups, differentiating between costs associated with pregnant women and those linked to newborns. For pregnant women, the main costs included prenatal examination fees, such as ultrasound screenings and CMV serological testing. For children, the main expenses include medical costs, such as hearing loss, vision loss and mental retardation. The clinical trials for the vaccine mRNA-1647 primarily targeted women aged 18–40 (reproductive age), a demographic that closely aligns with the primary population for human papillomavirus (HPV) vaccination. Furthermore, epidemiological data indicate that the prevalence of cervical intraepithelial neoplasia grade 2 or worse lesions caused by HPV infection among Chinese women ranges from 1% to 2%, which is comparable with the incidence of cCMV infection. Given these parallels in both target populations and disease burden, the pricing strategy of the HPV vaccine provides a relevant reference. Consequently, the CMV vaccine price was set at US$300, mirroring the cost of the quadrivalent HPV vaccine.35 All financial figures were adjusted to 2023 US$ values, using an exchange rate of US$1 to ¥7.05, to ensure consistency and relevance in economic evaluation.
First of all, our study used disability-adjusted life years (DALYs) as the primary health utility measure to capture the long-term sequelae and premature mortality associated with cCMV infection in children. We calculated the DALY required to prevent each case of cCMV infection as well as its associated sequelae, using data from the most recent Global Burden of Disease Study.36 These sequelae included hearing loss, vision impairment and cognitive disabilities, reflecting the significant health burden imposed by cCMV infection.
Second, we computed the cost under various vaccination strategies, which consisted of vaccine cost, direct medical expenses, direct non-medical expenses and indirect expenses. Both costs and health utilities were discounted at a 3% annual rate to align with standard economic evaluation practices. According to WHO guidelines, the willingness-to-pay (WTP) threshold should be set at the per capita gross domestic product (GDP). In this context, a vaccination strategy was deemed cost-effective if the estimated incremental cost-effectiveness ratio (ICER) was below China’s per capita GDP for 2023 (US$12 675).
Sensitivity analyses and scenario analysis
We used both deterministic and probabilistic sensitivity analyses to assess the robustness of the results. Initially, comprehensive one-way sensitivity analyses were conducted for each model parameter and scenario. Subsequently, two-way sensitivity analyses were performed for selected variables, and probabilistic sensitivity analysis was carried out using Monte Carlo simulations with 1000 iterations. Finally, scenario analyses incorporated key parameters to evaluate potential epidemiological impacts, with vaccine protection duration modelled across a 10-year to lifelong protection.
Results
Epidemiological impacts
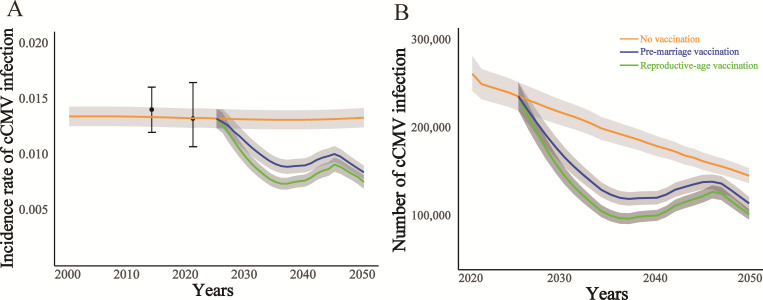
The model aligns closely with demographic and epidemiological data for the overall and age-stratified population in China. Our projections indicate that, without intervention, the incidence of cCMV infection will remain high from 2025 to 2050, likely due to the persistently elevated seroprevalence rate within China’s general population.
By targeting women before marriage for vaccination, a substantial reduction in cumulative cases and deaths can be achieved (table 1). Under this pre-marriage strategy, an estimated 236 443 372 (95% uncertainty interval(UI), 233 842 495 to 238 571 362) individuals would receive the vaccine, resulting in the prevention of approximately 1 039 579 (9 71 958 to 1 107 198) cases of cCMV infection. Additionally, this strategy could prevent an estimated 25 389 (24 475 to 27 900) cases of hearing loss, 7405 (7255 to 7571) cases of mental retardation, 22 214 (21 666 to 23 913) cases of vision loss and 6050 (5631 to 6347) cases of infant death. Although a rebound in cCMV infection rates was observed between 2040 and 2045 due to waning vaccine efficacy, the long-term impact of the strategy remains highly favourable. By 2050, the pre-marriage strategy is projected to achieve an IRR of 38.8% (34.7% to 41.5%) and an MRR of 36.3% (33.5% to 40.2%) compared with the status quo. By 2050, the pre-marriage vaccination strategy is expected to reduce the percentage of cCMV infection to 8.4% (8.09% to 8.95%), cause a substantial improvement compared with the projected 13.73% (12.76% to 14.69%) under the status quo and reduce the number of reactivation/reinfections to 4 144 384 (4 124 213 to 4 171 536) compared with the status quo of 4 978 972 (4 952 513 to 4 998 279).
Table 1. Health impacts of CMV vaccination strategies.
| Vaccination strategy | Number of vaccinations | Number of cCMV infection | Number of infant deaths | Number of HL | Number of MR | Number of VL |
|---|---|---|---|---|---|---|
| No vaccination | – | 4 886 197(4 570 522–5 201 871) | 28 254 | 117 269 | 34 203 | 102 610 |
| (26 431–30 078) | (109 692–124 844) | (31 993–36 413) | (95 980–109 239) |
| Number of vaccinations | Number of cCMV infection averted | NNV per cCMV infection averted | Number of infant deaths averted | Number of HL averted | Number of MR averted | Number of VL averted | |
|---|---|---|---|---|---|---|---|
| Reproductive-age vaccination | 456 273 200 | 1 475 325 | 309 | 8678 | 36 264 | 10 577 | 31 731 |
| (453 079 288–458 554 566) | (1 379 009–1 571 639) | (300–318) | (8245–9023) | (35 102–37 951) | (9 851–11 296) | (30 526–32 488) | |
| Pre-marriage vaccination | 236 443 372 | 1 039 579 | 227 | 6050 | 25 389 | 7405 | 22 214 |
| (233 842 495–238 571 362) | (971 958-–1 107 198) | (218–235) | (5631–6347) | (24 475–27 900) | (7255–7571) | (21666–23913) |
Data are presented as medium and 95% uncertainty interval (UI).
cCMV, congenital cytomegalovirus; CMV, cytomegalovirus; HL, hearing loss; MR, mental retardation; NVV, number needed to vaccinate; VL, vision loss.
Including women of reproductive age (aged 20–40) in the vaccination strategy could enhance outcomes but would necessitate many more courses. This reproductive-age strategy would involve approximately 456 273 200 (453 079 288 to 458 554 566) recipients, leading to the prevention of 1,475,325 (1 379 009 to 1 571 639) cases of cCMV infection. Furthermore, this strategy is projected to prevent 36 264 (35 102 to 37 951) cases of hearing loss, 10 577 (9851 to 11 296) cases of mental retardation, 31 731 (30 526 to 32 488) cases of vision loss, and 8678 (8245 to 9023) cases of infant death. By 2050, this strategy would achieve an IRR of 43.3% (38.3% to 47.1%) and an MRR of 44.7% (40.4% to 49.1%) relative to the status quo. By 2050, implementing a broader vaccination strategy for women of reproductive age is projected to further reduce the percentage of cCMV infection to 7.79% (7.20% to 8.42%), and reduce the number of reactivation/re-infections to 4 023 212 (4 003 421 to 4 046 862) (figure 2 and online supplemental figure S2).
Figure 2. Projection of CMV epidemiology under different vaccination strategies. (A) Calibration of the incidence rate of cCMV infection and projected cCMV infection incidence rates from 2000 to 2050. (B) Projected cases of cCMV infection, in response to age-specific vaccination strategies. cCMV infection, congenital cytomegalovirus infection; CMV, cytomegalovirus.
Based on these findings, we calculated the cumulative NNV to prevent one cCMV infection for each strategy. The pre-marriage strategy demonstrated the highest efficiency, requiring an estimated 227 (218–235) courses per case prevented, whereas the reproductive-age strategy required approximately 309 (300–318) courses per case (online supplemental table S8).
Cost-effectiveness
In table 2, the vaccination strategies are ranked according to their cost per DALY averted, arranged in descending order of cost-effectiveness. This table provides a comprehensive comparison of costs and health outcomes associated with different vaccination approaches, all based on a vaccine with an assumed 50% efficacy and a waning rate over time. Each strategy’s performance is evaluated relative to a no-vaccination baseline and to the next most effective strategy.
Table 2. Cost-effectiveness of CMV vaccination strategies.
| Vaccination strategy | Cost (million US$) | DALY | ICER (US$/DALY averted) versus status quo | ICER (US$/DALY averted) versus next best |
|---|---|---|---|---|
| No vaccination | 4 26 696 (4 25 979–4 27 414) | 9 346 456 | – | – |
| (9 328 596–9 364 316) | ||||
| pre-marriage vaccination | 435 686 | 7 371 399 | 4751 | 4751 |
| (4 34 777–4 36 596) | (7 356 764–7 386 033) | (4124–5378) | (4124–5378) | |
| Reproductive-age vaccination | 457 021 | 6 549 424 | 10 814 | 25 553 |
| (4 54 882–4 57 470) | (6 536 887–6 561 960) | (10 290–11 338) | (12 566–36 126) |
Costs and DALYs were discounted at 3%. Data are presented as medium and 95% uncertainty interval (Ul).
cCMV, congenital cytomegalovirus; CMV, cytomegalovirus; DALY, disability-adjusted life year; ICER, incremental cost-effectiveness ratio.
Using a WTP of US$12 675 per DALY averted (equivalent to 2023 China’s per capita GDP), the pre-marriage vaccination strategy is identified as the most cost-effective approach among the options assessed. This strategy successfully averted 1 960 275 DALYs (1 936 092–1 984 458) at an additional cost of US$8990 (US$7852–10 128) million. The resulting ICER is US$4751 per DALY averted (US$4124–5378), which is well within the cost-effectiveness threshold.
In comparison, the reproductive-age strategy demonstrates moderate cost-effectiveness, preventing 2 797 032 DALYs (2 775 851 to 2 818 213) at an additional cost of US$29 968 (US$28 531–31 405) million. This results in an ICER of US$10 814 (US$10 290–11 338) per DALY averted when compared with the status quo. However, when analysed against the pre-marriage strategy, the reproductive-age strategy is not cost-effective, with an ICER of US$25 553 (US$12 566–36 126) per DALY averted. These results are vividly shown in the cost-effectiveness frontier (figure 3A), underscoring the robustness and favourable cost-effectiveness of the pre-marriage strategy under the WTP level.
Figure 3. Cost-effectiveness frontier, deterministic sensitivity analyses and probabilistic sensitivity analyses of CMV vaccination strategies. (A) Decremental DALYs and incremental costs of CMV vaccination strategies compared with the status quo. The strategies on the upper left of the frontier are dominated by the strategies on the lower right of them. (B) The two-way sensitivity analysis of vaccine efficacy and duration of protection. The colour regions represent the most cost-effective vaccination strategy in the range. The small squares represent the baseline values. (C) Scatter plots of CMV vaccination strategies. (D) Cost-effectiveness acceptability curve. CE, cost effective; CMV, cytomegalovirus; DALY, disability-adjusted life year; GDP, gross domestic product; ICER, incremental cost-effectiveness ratio; WTP, willingness-to-pay.
Sensitivity and scenario analyses
We conducted a one-way sensitivity analysis by varying the values of individual model parameters, to assess how each influenced the cost-effectiveness of the vaccination strategies (online supplemental figure S3). The vaccine price and duration were the most important parameters. The reproductive-age strategy remained cost-effective when the vaccine price was at or below US$320 per course. For vaccine prices between US$320 and US$375, the pre-marriage strategy became the preferred option. However, if the vaccine price exceeded US$375, neither vaccination strategy was considered cost-effective. The pre-marriage vaccination strategy was cost-effective when the vaccine duration lasted 15 years or longer, while the WTP threshold for the reproductive-age vaccination strategy required a vaccine duration of at least 17 years to be cost-effective.
Additionally, we performed a two-way sensitivity analysis to explore the combined effects of variations in both vaccine efficacy and the duration of vaccine protection, with all other parameters held constant. The results, depicted in figure 3B, reveal that vaccination strategies are cost-effective only when vaccine efficacy reaches a minimum threshold of 25%. As both efficacy and the duration of protection increase, the optimal approach shifts from no vaccination to the pre-marriage strategy and ultimately to the reproductive-age strategy.
Figure 3C displays a scatter plot of cost-effectiveness outcomes from 1000 Monte Carlo simulations, illustrating the relationship between costs and DALYs across different strategies. As previously noted, the reproductive-age strategy achieves the most substantial reduction in DALYs, positioning it to the far left on the plot. To the right, green points represent the pre-marriage strategy, which also demonstrates significant DALY reductions while maintaining lower costs than the reproductive-age approach.
To further evaluate the preferred vaccination strategies across different WTP thresholds, we employed the cost-effectiveness acceptability curve, spanning a WTP range of US$0–20 000. As shown in figure 3D, when the WTP threshold aligns with China’s per capita disposable GDP (US$12 675), the pre-marriage strategy emerges as the most cost-effective option. For WTP values below US$5000, the no-vaccination strategy is favoured, while the pre-marriage vaccination strategy becomes optimal for WTP values above US$5000.
Assuming a protection duration of 10 years in the pessimistic scenario and lifetime protection in the optimistic scenario, as outlined in online supplemental table S9, the results demonstrate significant variation in the cost-effectiveness of the vaccination strategies. Under the pessimistic scenario, where vaccine protection wanes over a 10-year period, none of the vaccination strategies were deemed cost-effective when evaluated against the WTP threshold of US$12 675 per DALY averted. Under the optimistic scenario, the reproductive-age vaccination strategy demonstrated an ICER of US$1431 per DALY averted (-862 to 1611), significantly below the WTP threshold, indicating that it is a cost-effective intervention compared with no vaccination. However, its ICER remains above the WTP threshold when compared with the pre-marriage vaccination strategy, which proves to be more advantageous.
Discussion
Congenital CMV infection is a common disease worldwide. In China, the seropositivity rate for CMV among women of childbearing age exceeds 95%, meaning that the vast majority of these women have been infected with CMV at least once before childbirth.6 Research indicates that seropositive women are more likely to transmit CMV to their newborns compared with seronegative women.3 The comprehensive implementation of China’s Three-Child Policy has led to a significant increase in the population of childbearing-age women and infants. Close maternal contact with infected children or exposure to their bodily fluids considerably heightens the risk of seroconversion, thereby increasing the incidence of cCMV infection in newborns. Vaccination could serve as an effective intervention to disrupt the transmission chain, particularly between infants, immunocompromised pregnant women and children during peak periods of CMV infection. By implementing the CMV vaccination programme, the incidence of cCMV infections can be significantly reduced, thereby improving maternal and child health outcomes and contributing to the goals of ‘Healthy China 2030’.
Our study is the first to use a dynamic model and gradually waning vaccine efficacy to assess the costs and health outcomes of CMV vaccination across different strategies in China. Based on the latest clinical trial designs and fertility surveys, our research indicates that the pre-marriage vaccination strategy is more effective. Administering vaccines to young women before marriage can significantly decrease the incidence of cCMV infection. We also assessed the reproductive-age vaccination strategy; while it proves effective, it is not economically viable compared with the pre-marriage strategy, primarily due to the larger size of the adult population and the relatively few individuals in their childbearing years. Our study demonstrates that, with a vaccine cost of US$300 per course, both the pre-marriage and reproductive-age strategies are cost-effective. Among these, the reproductive-age strategy offers the better healthy utility. However, its high cost makes it not cost-effective compared with the pre-marriage strategy. Regarding health outcomes, a rebound in both the cCMV incidence rate and the number of cCMV infections was observed during the period from 2040 to 2045. This phenomenon can be attributed to the gradual waning of vaccine-induced immunity over time. The impact of waning efficacy over time observed in other vaccines is worth referencing. Studies have shown that the protective efficacy of the varicella vaccine gradually declines over time. Specifically, the incidence of breakthrough varicella increases significantly with the duration since vaccination: The annual rate of breakthrough varicella significantly increased with the time since vaccination, from 1.6 cases per 1000 person-years (1.2–2.0) within 1 year after vaccination to 9.0 per 1000 person-years (6.9–11.7) at 5 years and 58.2 per 1000 person-years (36.0–94.0) at 9 years.37
There were some previous model studies and cost-effectiveness analyses on the CMV vaccine. The model studies in the US have demonstrated that universal vaccination of 6-month-old infants could reduce cCMV infection cases by 68%–97% over 50 years compared with no vaccination,38 and vaccinating 25-year-old women for 20 years could prevent 30% of cCMV infection cases in the Netherlands.39 However, these studies did not incorporate cost-effectiveness analyses based on dynamic modelling. Current economic evaluations of CMV vaccination predominantly use Markov-decision tree models. In a US-based study, it was demonstrated that a universal vaccination strategy targeting 100 000 adolescents aged 11 years would reduce healthcare costs by US$32.3 million compared with no vaccination and gain 1823 QALYs.27 In contrast, a French cost-effectiveness analysis revealed that the screening and vaccination strategy could achieve an ICER of €6000 per QALY, proving to be more cost-effective than both universal vaccination and no intervention.28 However, the seropositivity rate in developed countries is significantly lower than that in China, with less than 50% of the population being seropositive, and their population structure also differs.39 Therefore, previous research findings may not be applicable to China.
This study has several strengths. First, we developed the first dynamic model based on China’s actual gender, age and population-structured data. The model was calibrated to achieve a CMV seroprevalence of 95%–98% within the population, ensuring high realism. Second, our study is the first to develop a model study that establishes vaccination strategies based on marriage age, in contrast to previous studies that have used fixed age groups or specific ages. Third, vaccine parameters and target populations were defined using the latest clinical trial data for CMV vaccines. The vaccine efficacy was assumed to wane gradually. Finally, a cost-effectiveness analysis was conducted based on the dynamic model, using the WTP threshold to determine acceptable vaccine prices under different strategies and identify the optimal strategy for specific vaccine prices. Sensitivity analyses were performed on the model parameters and outcomes, enhancing the reliability of our findings.
Admittedly, this study has several limitations. First, since China lacks mandatory CMV reporting, we had to estimate critical parameters like vision loss and mortality rates using data from developed countries, which may introduce bias due to epidemiological differences. Second, our vaccine efficacy assumptions may be overly optimistic, as the actual effectiveness and duration of protection in real-world settings could be lower than modelled. Finally, the pre-marriage vaccination strategy’s projected impact relies on high coverage of women aged 20–28 years through health checkups. If some at-risk individuals are missed, residual transmission may persist, leading to potential overestimation of the strategy’s effectiveness.
Conclusion
Our study demonstrates that prioritising CMV vaccination for pre-marriage young women could enhance the ability to control cCMV infection in China. The epidemiological and economic results underscore its potential to reduce the burden of cCMV infection. These findings provide essential evidence to support the development of CMV vaccination strategies and offer a scientific foundation to inform public health policymaking.
Supplementary material
Acknowledgements
We sincerely thank Prof. Ganna Rozhnova from the University Medical Center Utrecht, Utrecht University and Prof. Michiel van Boven from the Center for Infectious Disease Control, National Institute for Public Health and the Environment, The Netherlands, for email communication and sharing code associated with model development.
Footnotes
Funding: This study is supported in part by the Jiangsu Provincial Research Hospital (grant number YJXYY202204-YSA03 to GQ) and the Nantong Science and Technology Bureau, China (grant number MS22022086 to Y-HJ). The corresponding authors had full access to all study data and materials and had final responsibility for the decision to submit for publication.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Griffiths P, Reeves M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat Rev Microbiol. 2021;19:759–73. doi: 10.1038/s41579-021-00582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y, Li T, Yu H, et al. Maternal CMV seroprevalence rate in early gestation and congenital cytomegalovirus infection in a Chinese population. Emerg Microbes Infect. 2021;10:1824–31. doi: 10.1080/22221751.2021.1969290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawlinson WD, Boppana SB, Fowler KB, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis. 2017;17:e177–88. doi: 10.1016/S1473-3099(17)30143-3. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y, Tang J, Wang H, et al. Pre-existing maternal IgG antibodies as a protective factor against congenital cytomegalovirus infection: A mother-child prospective cohort study. EBioMedicine. 2022;77:103885. doi: 10.1016/j.ebiom.2022.103885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S, Hu L, Chen J, et al. Cytomegalovirus Seroprevalence in Pregnant Women and Association with Adverse Pregnancy/Neonatal Outcomes in Jiangsu Province, China. PLoS ONE. 2014;9:e107645. doi: 10.1371/journal.pone.0107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuhair M, Smit GSA, Wallis G, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev Med Virol. 2019;29:e2034. doi: 10.1002/rmv.2034. [DOI] [PubMed] [Google Scholar]
- 7.Weimer KED, Kelly MS, Permar SR, et al. Association of Adverse Hearing, Growth, and Discharge Age Outcomes With Postnatal Cytomegalovirus Infection in Infants With Very Low Birth Weight. JAMA Pediatr. 2020;174:133–40. doi: 10.1001/jamapediatrics.2019.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto AY, Anastasio ART, Massuda ET, et al. Contribution of Congenital Cytomegalovirus Infection to Permanent Hearing Loss in a Highly Seropositive Population: The Brazilian Cytomegalovirus Hearing and Maternal Secondary Infection Study. Clin Infect Dis. 2020;70:1379–84. doi: 10.1093/cid/ciz413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Q, Wang Q, Shen H, et al. Seroprevalence of Cytomegalovirus and Associated Factors Among Preconception Women: A Cross-Sectional Nationwide Study in China. Front Public Health. 2021;9:631411. doi: 10.3389/fpubh.2021.631411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57 Suppl 4:S178–81. doi: 10.1093/cid/cit629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross SA, Kimberlin D. Clinical outcome and the role of antivirals in congenital cytomegalovirus infection. Antiviral Res. 2021;191:105083. doi: 10.1016/j.antiviral.2021.105083. [DOI] [PubMed] [Google Scholar]
- 12.Britt WJ. Congenital Human Cytomegalovirus Infection and the Enigma of Maternal Immunity. J Virol. 2017;91:e02392-16. doi: 10.1128/JVI.02392-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannon MJ, Griffiths PD, Aston V, et al. Universal newborn screening for congenital CMV infection: what is the evidence of potential benefit? Rev Med Virol. 2014;24:291–307. doi: 10.1002/rmv.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leruez-Ville M, Foulon I, Pass R, et al. Cytomegalovirus infection during pregnancy: state of the science. Am J Obstet Gynecol. 2020;223:330–49. doi: 10.1016/j.ajog.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Leruez-Ville M, Ville Y. Fetal cytomegalovirus infection. Best Pract Res Clin Obstet Gynaecol. 2017;38:97–107. doi: 10.1016/j.bpobgyn.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Luisi K, Sharma M, Yu D. Development of a vaccine against cytomegalovirus infection and disease. Curr Opin Virol. 2017;23:23–9. doi: 10.1016/j.coviro.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Costa B, Gouveia MJ, Vale N. Safety and Efficacy of Antiviral Drugs and Vaccines in Pregnant Women: Insights from Physiologically Based Pharmacokinetic Modeling and Integration of Viral Infection Dynamics. Vaccines (Basel) 2024;12:782. doi: 10.3390/vaccines12070782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arvin AM, Fast P, Myers M, et al. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;39:233–9. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- 19.Das R, Blázquez-Gamero D, Bernstein DI, et al. Safety, efficacy, and immunogenicity of a replication-defective human cytomegalovirus vaccine, V160, in cytomegalovirus-seronegative women: a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Infect Dis. 2023;23:1383–94. doi: 10.1016/S1473-3099(23)00343-2. [DOI] [PubMed] [Google Scholar]
- 20.Fierro C, Brune D, Shaw M, et al. Safety and Immunogenicity of a Messenger RNA-Based Cytomegalovirus Vaccine in Healthy Adults: Results From a Phase 1 Randomized Clinical Trial. J Infect Dis. 2024;230:e668–78. doi: 10.1093/infdis/jiae114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin Infect Dis. 2017;64:87–91. doi: 10.1093/cid/ciw668. [DOI] [PubMed] [Google Scholar]
- 22.Plotkin SA, Smiley ML, Friedman HM, et al. Towne-vaccine-induced prevention of cytomegalovirus disease after renal transplants. Lancet. 1984;1:528–30. doi: 10.1016/s0140-6736(84)90930-9. [DOI] [PubMed] [Google Scholar]
- 23.Plotkin SA, Higgins R, Kurtz JB, et al. Multicenter trial of Towne strain attenuated virus vaccine in seronegative renal transplant recipients. Transplantation. 1994;58:1176–8. [PubMed] [Google Scholar]
- 24.Bernstein DI, Munoz FM, Callahan ST, et al. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: A randomized clinical trial. Vaccine (Auckl) 2016;34:313–9. doi: 10.1016/j.vaccine.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–79. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu X, Karthigeyan KP, Herbek S, et al. Human Cytomegalovirus mRNA-1647 Vaccine Candidate Elicits Potent and Broad Neutralization and Higher Antibody-Dependent Cellular Cytotoxicity Responses Than the gB/MF59 Vaccine. J Infect Dis. 2024;230:455–66. doi: 10.1093/infdis/jiad593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dempsey AF, Pangborn HM, Prosser LA. Cost-effectiveness of routine vaccination of adolescent females against cytomegalovirus. Vaccine (Auckl) 2012;30:4060–6. doi: 10.1016/j.vaccine.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 28.N’Diaye DS, Launay O, Picone O, et al. Cost-effectiveness of vaccination against cytomegalovirus (CMV) in adolescent girls to prevent infections in pregnant women living in France. Vaccine (Auckl) 2018;36:1285–96. doi: 10.1016/j.vaccine.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 29.Lanzieri TM, Gastañaduy PA, Gambhir M, et al. Review of Mathematical Models of Vaccination for Preventing Congenital Cytomegalovirus Infection. J Infect Dis. 2020;221:S86–93. doi: 10.1093/infdis/jiz402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto AY, Mussi-Pinhata MM, Boppana SB, et al. Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol. 2010;202:297. doi: 10.1016/j.ajog.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin M-Z, Gu Y-Y, Shu J-T, et al. Cost-effectiveness of cytomegalovirus vaccination for females in China: A decision-analytical Markov study. Vaccine (Auckl) 2023;41:5825–33. doi: 10.1016/j.vaccine.2023.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Wang XH, Li DL. Guidelines for preconception and prenatal care [in Chinese] Chinese Journal of Obstetrics and Gynecology. 2018;53:361–8. doi: 10.3760/cma.j.issn.0529-567x.2018.01.003. [DOI] [Google Scholar]
- 33.National Health Commission of the People’s Repubilc of China China health statistical yearbook. 2023. http://www.nhc.gov.cn/mohwsbwstjxxzx/tjtjnj/202501/b8d57baa95834269b5b3562bfec801a7/files/8d84a833f400454aa0a392856c02d558.pdf Available.
- 34.Yu J, Xie Y. Is there a Chinese pattern of the second demographic transition? China Popul Dev Stud . 2022;6:237–66. doi: 10.1007/s42379-022-00113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu X, Wang Y, Lv Z, et al. Prevalence and genotype distribution of high-risk HPV infection among women in Beijing, China. J Med Virol. 2021;93:5103–9. doi: 10.1002/jmv.27013. [DOI] [PubMed] [Google Scholar]
- 36.Naghavi M, Ong KL, Aali A, et al. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet. 2024;403:2100–32. doi: 10.1016/S0140-6736(24)00367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaves SS, Gargiullo P, Zhang JX, et al. Loss of vaccine-induced immunity to varicella over time. N Engl J Med. 2007;356:1121–9. doi: 10.1056/NEJMoa064040. [DOI] [PubMed] [Google Scholar]
- 38.Hogea C, Dieussaert I, Van Effelterre T, et al. A dynamic transmission model with age-dependent infectiousness and reactivation for cytomegalovirus in the United States: Potential impact of vaccination strategies on congenital infection. Hum Vaccin Immunother. 2015;11:1788–802. doi: 10.1080/21645515.2015.1016665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rozhnova G, E Kretzschmar M, van der Klis F, et al. Short- and long-term impact of vaccination against cytomegalovirus: a modeling study. BMC Med. 2020;18:174. doi: 10.1186/s12916-020-01629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.