Abstract
Background
To improve the nutritional management of premature infants in neonatal intensive care units (NICUs), we developed and implemented a standardized parenteral nutrition (PN) protocol aimed at optimizing early macronutrient delivery. This study evaluated the impact of the new protocol on growth parameters and clinical outcomes in preterm neonates.
Methods
This prospective, non-randomized interventional cohort study included two groups of preterm infants born before 32 weeks of gestation or with birth weights under 1250 g. The PRE group received individualized PN formulations based on clinician discretion, while the POST group received PN guided by a newly introduced, stepwise algorithmic protocol aiming to optimize early protein and energy intake. Anthropometric data, daily energy and macronutrient intakes during the first 14 days, and weight at day 28 were collected. Clinical outcomes—including the incidence of sepsis, necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH) retinopathy of prematurity (ROP) and hospitalization duration—were compared between groups.
Results
A total of 139 infants were enrolled (69 PRE, 70 POST). While weight gain in the first 14 days was similar, the POST group showed significantly greater weight and weight velocity by day 28. These improvements paralleled higher mean daily energy and protein intakes during the early postnatal period. The incidence of bacterial sepsis was significantly reduced in the POST cohort, possibly reflecting better nutritional status and improved PN preparation practices. Although other complications did not differ significantly, fewer infants in the POST group required prolonged hospitalization (> 90 days).
Conclusion
Implementation of a standardized PN protocol improved early nutritional intake and was associated with better growth and reduced infection rates in premature infants. These findings support the use of structured PN strategies to enhance early neonatal outcomes in NICU settings.
Trial registration
The Iranian Registry of Clinical Trials (http//www.irct.ir) with the identification No. IRCT20240519061838N2. Registered 24 November 2024.
Keywords: Parenteral nutrition, Premature infants, Growth metrics, Neonatal outcomes, Standardized protocol
Introduction
The global incidence of preterm births continues to rise; however, advancements in neonatal care have significantly improved survival rates among premature infants [1]. Despite these advancements, preterm infants face significant obstacles in their growth and development [2]. These infants are at an increased risk of morbidity and mortality compared to their full-term counterparts, primarily due to complications associated with premature birth, such as respiratory distress syndrome, intraventricular hemorrhage (IVH), and necrotizing enterocolitis (NEC) [3, 4].
The management of preterm infants in neonatal intensive care units (NICUs) is a complex and multifaceted challenge. A critical aspect of their care is the provision of adequate nutrition, which is essential for promoting growth, development, and the preventing of complications. One of the most severe consequences of undernutrition in these infants is neuronal underdevelopment, and early nutrition intervention may mitigate deficits in cognitive function and brain development later in life [5–8]. Due to their immature physiological systems, preterm infants are particularly vulnerable to nutritional deficiencies. This vulnerability necessitates the implementation of specialized nutritional strategies, including parenteral nutrition (PN), which involves the intravenous delivery of nutrients including macronutrients and micronutrients, particularly for those who are unable to receive adequate enteral feeding [9, 10].
Studies have shown that the implementation of evidence-based PN guidelines in NICU may prevent extrauterine growth restriction, which is associated with long-term adverse effects [11, 12]. These guidelines typically outline recommendations for the composition and administration of PN, based on the latest research and expert consensus [9, 10]. Nonetheless, discrepancies are present among various intravenous nutrition protocols, and individual neonatologists often adopt distinct approaches to the nutritional management of preterm infants. Furthermore, there is a lack of consensus among NICUs concerning the optimal protocol for delivering postnatal nutrition to this vulnerable population. Consequently, it is essential for NICUs to develop local PN protocols through the adapting international guidelines to their specific resources, circumstances, and patient population.
In our country, the implementation of standardized PN guidelines in NICUs has been limited, often relying on one or more restricted intravenous fluid options. Additionally, these intravenous solutions are frequently prepared and mixed in hospital settings and non-sterile environments without adherence to standardized protocols.
This study aimed to evaluate the impact of implementing a standardized clinical guideline for intravenous nutrition within the NICU on growth and its associated early nutritional and clinical outcomes. The newly developed clinical guideline emphasizes the optimal delivery of energy and nutrients, including carbohydrates, proteins, lipids, and various micronutrients. Furthermore, efforts have been made to establish standard requirements for aseptic techniques in the preparation and administration of intravenous fluids.
Methods and materials
Study design
This was a prospective, non-randomized interventional cohort study conducted in the NICU of Alzahra Hospital, Tabriz, Iran—a major tertiary referral center for neonatal care in northwestern Iran. The study population included preterm infants born at < 32 weeks of gestation and/or weighing < 1250 g, admitted to the NICU before and after the implementation of a standardized intravenous nutrition protocol.
The PRE group (control cohort) comprised 70 preterm infants admitted between December 1, 2023, and February 31, 2024. Data were collected using a structured checklist.
A clinical guideline for intravenous nutrition was collaboratively developed by neonatologists, pediatric nutritionists, and pharmacists. Following a three-day expert panel review, the guideline was approved and officially endorsed by the Ministry of Health and Education of Iran under the title “Clinical Guide for Intravenous Infant Nutrition Care.” Following national endorsement, a dedicated Total Parenteral Nutrition (TPN) Unit was established at Alzahra Hospital, equipped with a clean room and specialized infrastructure—one of the first of its kind in the country.
NICU and pharmacy staff underwent training to ensure consistent implementation of the new protocol, which was supervised by clinical pharmacists and neonatologists.
After a three-month transition period, the POST group (intervention cohort) was recruited between July 1, 2024, and October 30, 2024. In this group, intravenous nutrition was administered using computerized prescriptions and automated aseptic compounding systems.
Infants with major congenital anomalies, chromosomal abnormalities, inborn errors of metabolism, cyanotic congenital heart disease, severe perinatal asphyxia (5-minute Apgar < 4), or those transferred or deceased within the first week of life were excluded from both cohorts.
Ethical approval was granted by the Ethics Committee of Tabriz University of Medical Sciences (Ethics Code: IR.TBZMED.REC.1397.940). The study was supported by the Pediatric Health Research Center.
Sample size
Based on the prevalence of neonatal sepsis reported by Phillips et al. [13], and calculated using the Poochak formula with a significance level (α) of 0.05 and power (1 − β) of 90%, the estimated required sample size was 67 participants per group. Accounting for a potential dropout rate of 20%, the final sample size was adjusted to 80 participants in each cohort.
Nutrition management
In the PRE group, intravenous nutrition was initiated from day two of life based on clinical discretion. In contrast, the POST group received PN starting on day one of life, in accordance with standardized guidelines. Both groups were provided with the following:
Dextrose: 4–8 mg/kg/min.
Amino acids: 1–4 g/kg/day (Aminoven Infant 10%, Fresenius Kabi, Austria).
Lipids: 1–4 g/kg/day (SMOF Lipid 20%, Fresenius Kabi, Austria).
Prior to the implementation of the protocol, there was considerable reluctance to initiate intravenous lipid administration in preterm infants due to concerns about an increased risk of sepsis. In the POST group, lipid administration followed the standardized protocol outlined in Table 1.
Table 1.
Summary of total parenteral nutrition (TPN) guideline that was implemented in neonatal intensive care unite
| Day 1 | Day 2 | Day 3 & then | ||||
|---|---|---|---|---|---|---|
| A (< 1000 g) |
B (1000–1500 g) |
A (< 1000 g) |
B (1000–1500 g) |
A (< 1000 g) |
B (1000–1500 g) |
|
| Glucose (g/kg/day) | 5.8–10 | 5.8–10 | 8–11 | 8–11 | 11–15 (Max = 17) | 11–15 (Max = 17) |
|
Amino Acid (g/kg/day) |
1.5-2 | 1.5-2 | 2–3 | 2–3 | 3.5-4 | 3.5 |
|
Fat (g/kg/day) |
- | - | 2 | 2 | 3–4 | 3–4 |
|
Na (mEq/kg/day) |
- | - | - | - | 2–5 | 2–5 |
|
K (mEq/kg/day) |
- | - | - | - | 1–3 | 1–3 |
| Calcium gluconate 10% (ml/kg/day) | 4–5 | 4–5 | 6–7 | 6–7 | 6–7 | 6–7 |
| Glycophose (ml/kg/day) | 1 | 1 | 1.5 | 1.5 | 1.5 | 1.5 |
| Mg sulfate (mg/kg/day) | - | - | 5 | 5 | 5 | 5 |
| Solovit (ml/kg/day) | 1 | 1 | 1 | 1 | 1 | 1 |
| Vitalipid infant (ml/kg/day) | - | - | 4 | 4 | 4 | 4 |
| Pediatrace (ml/kg/day) | - | - | - | - | 1 | 1 |
Note. Na; sodium, K; potassium, Mg; magnesium
In the PRE group, PN adjustments—including dose changes, tapering, and discontinuation—were determined by the attending physician. In the POST group, PN was gradually weaned as enteral feeding reached 50–70% of full volume.
Enteral feeds were initiated with maternal milk once infants demonstrated clinical and gastrointestinal stability. Trophic feeding started at 5–10 ml/kg/day and advanced to 150–180 ml/kg/day. Donor human milk from the Central Milk Bank was used if maternal milk was unavailable. Preterm formula was considered only when neither maternal nor donor milk was accessible.
Human milk fortifier (HMF) was added once enteral intake reached 100 ml/kg/day to meet increased nutrient demands. Fortification was maintained until full feeds and satisfactory growth were achieved. Medium-chain triglyceride (MCT) oil was not used in either group.
In the PRE group, PN prescriptions were handwritten and manually prepared in the intravenous fluid unit. In the POST group, fluid compositions were calculated based on a standardized table and computerized protocol aligned with weight and age. These were verified by a clinical pharmacist and compounded using an automated system in a Class 100 clean room with positive-pressure laminar airflow.
All TPN solutions were prepared under strict aseptic conditions by trained personnel using sterile attire and equipment. Final solutions were labeled with patient details and administered as 24-hour infusions.
Data collection
Demographic and clinical data were recorded using a structured questionnaire. Variables included gestational age, sex, delivery mode, and Apgar scores. Maternal risk factors such as diabetes, preeclampsia, hypertension, and PROM were also noted.
Anthropometric data—birth weight, length, and head circumference—were measured at birth. Weight was monitored daily for the first 14 days, and again at 28 days and discharge. Weight velocity (g/kg/day) was calculated for days 14 and 28.
Nutritional intake over the first 14 days was tracked, including daily energy and macronutrient (carbohydrates, proteins, lipids) intake. Caloric values were calculated using standard conversion factors: 3.7 kcal/g for dextrose, 4 kcal/g for amino acids, and 9 kcal/g for lipids. Human milk was assumed to provide 67 kcal per 100 ml.
Clinical outcomes during hospitalization were documented, including: sepsis; defined by positive blood or urine cultures, NEC; diagnosed using Bell’s criteria (Stages I–III), IVH; diagnosed via transfontanelle ultrasonography (Grade II or higher), Respiratory support; need for synchronized intermittent mandatory ventilation (SIMV), nasal continuous positive airway pressure (NCPAP) or high-flow nasal cannula (HFNC).
Biochemical markers—including glucose, blood urea nitrogen (BUN), creatinine, and total bilirubin—were measured at NICU admission and TPN initiation, and on day 14 of life.
Statistical methods
The primary outcome was energy and nutrient intake under TPN and the associated weight gain during hospitalization. Secondary outcomes included demographic characteristics and early clinical outcomes.
Continuous variables (e.g., birth weight, gestational age, nutrient intake) were compared using independent t-tests and presented as means ± SD. Categorical variables (e.g., sex, clinical complications) were analyzed using the Chi-square test and reported as counts and percentages. Statistical analysis was performed using SPSS version 18, with p <.05 considered statistically significant.
Results
Demographic and clinical characteristics
Before the implementation of the standardized PN protocol for preterm infants, a total of 87 infants born at < 32 weeks of gestation and weighing < 1250 g were assessed. Eighteen infants were excluded due to congenital anomalies (n = 3), early death within the first 7 days of life (n = 4), incomplete documentation (n = 7), or transfer to another facility (n = 4), resulting in 69 infants included in the PRE group. After implementation of the protocol, 79 eligible infants were assessed, with 9 excluded (congenital anomalies: n = 2; early death: n = 3; incomplete documentation: n = 4), leaving 70 infants in the POST group (Fig. 1).
Fig. 1.
Study flowchart
Demographic and clinical characteristics are summarized in Table 2. The sex distribution was balanced across both groups (~ 49% male). Mean gestational age was marginally higher in the POST group (28.1 ± 2.07 weeks) versus the PRE group (27.5 ± 3.61 weeks), though this difference was not statistically significant. Cesarean delivery occurred in over 70% of cases across both cohorts. APGAR scores at 1 and 5 min, as well as maternal conditions such as diabetes and preeclampsia/hypertension, were comparable. However, premature rupture of membranes (PROM) occurred significantly more often in the PRE group.
Table 2.
Demographic and clinical characteristics of study groups
| Variables # | PRE group (n = 69) |
POST group (n = 70) | P-value* |
|---|---|---|---|
| Gender (male) | 34 (49.3%) | 34 (48.6%) | 0.934 |
| Gestational age (weeks) | 27.5 ± 3.61 | 28.1 ± 2.07 | 0.251 |
| Caesarian section | 50 (74.6%) | 54 (77.1%) | 0.731 |
| APGAR Score | |||
|
- Min1 - Min5 |
5.66 ± 2.60 7.70 ± 2.12 |
5.75 ± 2.18 7.62 ± 1.60 |
0.823 0.797 |
| Maternal Diabetes | 5 (7.2%) | 6 (8.6%) | 0.772 |
| Preeclampsia/Hypertension | 25 (36.2%) | 24 (34.3%) | 0.810 |
| PROM | 14 (20.3%) | 3 (4.3%) | 0.004 |
Note. PROM = Premature rupture of membranes; APGAR = Appearance, pulse, grimace, activity and respiration
# Mean ± SD for numeric variables and number (percent) for categorical variables
*Independent samples t test for numeric variables and Pearson chi-square test for categorical variables
Anthropometric characteristics
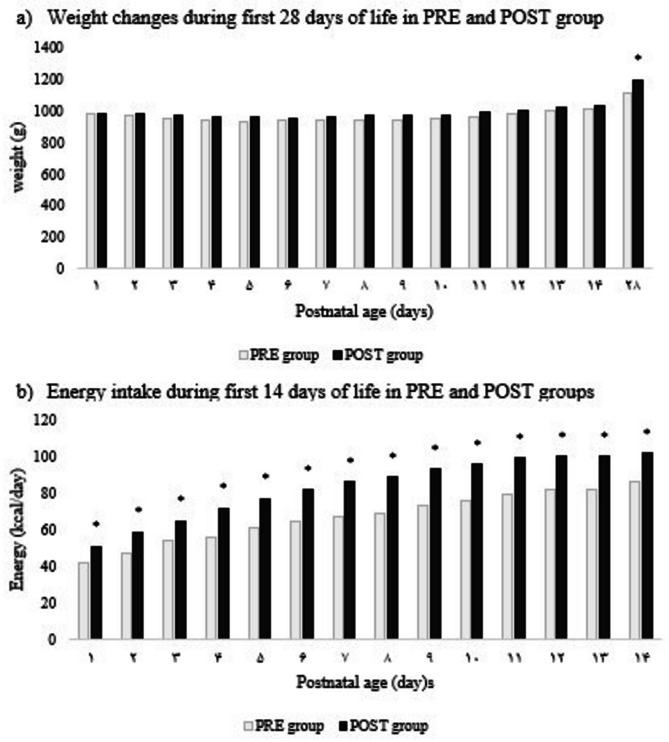
At birth, mean weight, length, and head circumference were similar between groups (PRE: 969.7 ± 188.7 g, 25.6 ± 1.8 cm, 35.9 ± 4.5 cm; POST: 983.8 ± 175 g, 26.0 ± 2 cm, 36.6 ± 7.5 cm). Weight at day 14 did not differ significantly. By day 28, however, infants in the POST group showed significantly greater weight (p <.05). While daily weight gain over the first 14 days was comparable, weight velocity at day 28 was significantly higher in the POST group (p <.001) (Table 3). Weight trajectories are shown in Fig. 2a, with a significant difference favoring the POST group noted only at day 28.
Table 3.
Anthropometric characteristics in the pre and post- implantation cohorts
| Variables # | PRE group (n = 69) |
POST group (n = 70) | P-value* |
|---|---|---|---|
| Birth weight (g) | 969.7 ± 188.7 | 983.8 ± 175.0 | 0.647 |
| Birth head circumference (cm) | 25.6 ± 1.8 | 26.0 ± 2.0 | 0.250 |
| Birth length (cm) | 35.9 ± 4.5 | 36.6 ± 7.5 | 0.515 |
| Weight at 14 days postpartum (g) | 1005.1 ± 193.4 | 1027.7 ± 180.4 | 0.481 |
| Weight at 28 days postpartum (g) | 1107.6 ± 217.0 | 1193.1 ± 212.2 | 0.033 |
| Weight velocity at 14 days (g/d) | 1.7 ± 7.5 | 3.4 ± 8.9 | 0.221 |
| Weight velocity at 28 days (g/d) | 5.1 ± 4.3 | 8.2 ± 5.0 | < 0.001 |
# Mean ± SD
*Independent samples t test
Fig. 2.
Weight and energy intake (kcal/day) of infants in PRE and POST groups
*p <.05, ** p <.001
Energy and macronutrient intake
The mean total daily energy intake, including both parenteral and transitional enteral nutrition, during the first 14 days of life was significantly higher in the POST group compared to the PRE group (85.3 ± 17.9 kcal/kg/day vs. 67.4 ± 14.8 kcal/kg/day, p <.001). In addition, the POST group received significantly greater average daily amounts of macronutrients—dextrose, amino acids, and intravenous lipids (p <.001) (Table 4). Figure 2b illustrates the trend in total daily energy intake over the 14-day period, demonstrating consistently higher values in the POST group.
Table 4.
Energy, and macronutrients intake in 14 days after birth in the pre and post- implantation cohorts
| Variables | PRE group (n = 69) |
POST group (n = 70) | P– value |
|---|---|---|---|
| Calorie (kcal/kg/day) | 67.4 ± 14.7 | 85.2 ± 17.8 | < 0.001 |
| Dextrose (g/kg/day) | 10.1 ± 2.0 | 11.6 ± 2.2 | < 0.001 |
| Protein (g/kg/day) | 1.9 ± 0.5 | 2.5 ± 0.5 | < 0.001 |
| Lipid (g/kg/day) | 0.003 ± 0.02 | 0.3 ± 0.1 | < 0.001 |
# Mean ± SD
*Independent samples t test
Biochemical and clinical outcomes
No significant differences were found in serum BUN, creatinine, or total bilirubin levels at day 14. Incidences of hypoglycemia and hyperglycemia were also similar between groups.
The mean NICU stay was 43.1 ± 22.9 days for the PRE group and 40.2 ± 18.2 days for the POST group. Total hospital stays were likewise comparable (PRE: 53.0 ± 25.6 days; POST: 52.0 ± 20.4 days). However, fewer infants in the POST group remained hospitalized beyond 90 days (p <.05).
There was a significant reduction in culture-confirmed bacterial sepsis in the POST group (7 vs. 16 episodes; p <.05), while rates of urine-culture-confirmed sepsis remained unchanged. No differences were observed in the need for respiratory support (SIMV, NCPAP, HFNC). Major neonatal morbidities, including NEC (Stage II), IVH, and retinopathy of prematurity (ROP), were similar between groups. Mortality, transfer rates, and discharge to home also did not differ significantly (Table 5).
Table 5.
Biochemical and clinical outcomes in the pre and post- implantation cohorts
| Variables # | PRE group (n = 69) |
POST group (n = 70) | P-value* |
|---|---|---|---|
| BUN | 54.8 ± 26.2 | 53.3 ± 24 | 0.713 |
| Cr | 0.88 ± 0.35 | 0.80 ± 0.33 | 0.188 |
| Total bilirubin | 8.0 ± 1.9 | 8.2 ± 2.0 | 0.607 |
| Hypoglycemia | 4 (5.8%) | 7 (10%) | 0.359 |
| Hyperglycemia | 23 (33.3%) | 16 (22.9%) | 0.169 |
| Length of NICU stay (day) | 43.1 ± 22.9 | 40.2 ± 18.2 | 0.106 |
| Length of NICU stay (day) | 0.311 | ||
|
- 40 - 40–60 - > 60 |
31 (50%) 24 (38.7%) 7 (11.3%) |
29 (46.8%) 30 (48.4%) 3 (4.8%) |
|
| Length of hospital stay (day) | 53.0 ± 25.6 | 52.0 ± 20.4 | 0.082 |
| Length of hospital stay (day) | 0.040 | ||
|
- 60 - 60–90 - > 90 |
39 (62.9%) 15 (24.2%) 8 (12.9%) |
40 (64.5%) 21 (33.9%) 1 (1.6%) |
|
| Sepsis | |||
|
- Positive blood culture - Positive urine culture |
16 (23.2%) 2 (2.9%) |
7 (10%) 4 (5.7%) |
0.036 0.414 |
| Respiratory support | |||
|
- SIMV - NCPAP - HFNC |
28 (40.6%) 68 (98.6%) 64 (92.8%) |
34 (48.6%) 70 (100%) 69 (98.6%) |
0.343 0.312 0.092 |
| NEC (Stage II) | 4 (5.8%) | 4 (6.1%) | 0.948 |
| IVH | 16 (23.5%) | 13 (18.8%) | 0.502 |
| ROP | 6 (8.8%) | 2 (2.9%) | 0.145 |
| Discharge to home | 59 (85.8%) | 61 (87.1%) | 0.779 |
| Referred | 3 (4.3%) | 1 (1.4%) | 0.303 |
| Expired | 7 (10.1%) | 8 (11.4%) | 0.807 |
Note. SIMV = Synchronized intermittent mandatory ventilation; NCPAP = Nasal continuous positive airway pressure; HFNC = High-flow nasal cannula; Hypoglycemia = Blood glucose < 50 mg/dl; Hyperglycemia = Blood glucose > 150 mg/dl; BUN = Blood urea nutrition; Cr = Creatinine; NEC = Necrotizing enterocolitis; IVH = Intraventricular hemorrhage; ROP = retinopathy of prematurity
# Mean ± SD for numeric variables and number (percent) for categorical variables
*Independent samples t test for numeric variables and Pearson chi-square test for categorical variables
Discussion
This prospective, non-randomized interventional cohort study evaluated the effect of a standardized PN protocol on early growth, nutritional intake, and short-term clinical outcomes in preterm infants. Comparisons were made between two cohorts—before and after protocol implementation.
By day 28 of life, infants in the POST cohort demonstrated significantly greater weight gain and higher daily weight velocity compared to those in the PRE group, despite similar birth weights. This enhancement in growth coincided with significantly higher energy and macronutrient intakes during the initial 14 days. These findings are consistent with previous literature underscoring the critical importance of early nutritional support in promoting postnatal growth in preterm neonates [12, 14–19]. Rogger et al. and Johnson et al. reported improved weight velocity and nutrient intake following guideline implementation [14, 15]. Furthermore, another study indicated that the introduction of a strict nutritional protocol resulted in improved weight gain velocity and mitigated postnatal growth restriction in very preterm infants, although this effect was constrained by low adherence to the protocol [17]. Similarly, a systematic review suggest that early PN may reduce the time to regain birth weight and mitigate early weight loss [17]. However, not all studies corroborate these findings. Phillips et al., for instance, found no significant differences in growth parameters following PN guideline updates, potentially due to similar caloric delivery between cohorts and limitations in retrospective caloric assessment [13].
In our study, weight trajectories between groups remained largely similar during the first two postnatal weeks. Despite protocol standardization, energy intake did not exceed 85 kcal/kg/day by day 14, which remains below the ESPGHAN/ESPEN/ESPR/CSPEN recommendation of 90–100 kcal/kg/day for parenteral nutrition in neonates [20]. The comparable BUN levels between groups may reflect this suboptimal protein-energy intake.
Another factor potentially contributing to modest early weight gain was the lack of comprehensive data on enteral feeding practices. While PN constituted the primary source of early nutrition, the transition to and tolerance of enteral feeds substantially influence overall energy balance and growth [21]. Although data were collected on enteral feed volumes (mL/kg/day), type of milk (human vs. formula), and timing of initiation, critical variables such as feeding interruptions and episodes of intolerance—key barriers to achieving nutritional targets in preterm infants—were not recorded. This limitation highlights the importance of capturing detailed enteral nutrition data to contextualize neonatal growth outcomes accurately [22].
A significant reduction in the incidence of culture-proven sepsis was observed in the POST cohort. This improvement may be attributable to both enhanced nutritional status and improved infection control practices, particularly the preparation of PN in a clean room environment post-implementation. In contrast, no significant differences were observed in other clinical outcomes, including respiratory support needs, NEC, IVH, ROP, and mortality. These findings align with meta-analyses indicating that early or enhanced PN does not inherently increase adverse neonatal outcomes [16]. Conversely, other studies have reported a reduction in bronchopulmonary dysplasia (BPD) and ROP associated with higher energy intake in extremely preterm infants, without affecting sepsis or NEC incidence [23]. One interventional study introducing standardized PN formulations observed improvements in growth, biochemical parameters, and reduced respiratory support requirements in the post-consensus cohort [24]. Likewise, Costa et al. (2015) reported that early PN was associated with decreased rates of NEC and sepsis [18]. Such discrepancies may stem from differences in sample size, study duration, and the composition of PN regimens before and after protocol implementation. The modest sample size and limited follow-up duration in the present study may have reduced statistical power to detect differences in less frequent outcomes such as NEC, IVH, or ROP. Additionally, the observed reduction in sepsis may, in part, reflect the introduction of sterile PN preparation in a dedicated clean room, replacing preparation within the NICU during the pre-implementation phase.
Importantly, eight infants (four in each group) were diagnosed with Stage II NEC, based on Bell’s criteria, ensuring consistency in diagnosis. The lack of a significant difference in NEC incidence between cohorts may be explained by the small number of cases, limiting statistical power, and reinforces the complex, multifactorial pathogenesis of NEC that extends beyond nutritional factors alone [25].
Although the average length of NICU stay did not differ significantly between groups, fewer infants in the POST group required hospitalization beyond 90 days. This may indicate improved clinical stability; however, a slightly higher mean gestational age in the POST group may have influenced this observation. Future analyses should control for gestational age and stratify infants by categories such as very preterm (VPT) and extremely low birth weight (ELBW), as nutritional tolerance and clinical outcomes are known to vary by maturity level [26]. A similar trend was reported by Jeong et al. (2016), who observed a significant reduction in NICU stay following the establishment of a multidisciplinary nutrition support team [19].
Strengths and limitations
A notable strength of this study is its pragmatic implementation of a structured PN protocol in a resource-limited setting, yielding measurable improvements in early growth and sepsis reduction. Nevertheless, several limitations must be acknowledged. These include the single-center design, moderate sample size, and lack of granular data on enteral feeding practices. Furthermore, the absence of long-term neurodevelopmental follow-up restricts the ability to assess the sustained impact of the intervention. Variability in the nutritional composition of human milk versus formula may also have introduced uncertainty in caloric intake estimations [27].
Conclusion
The implementation of a standardized PN protocol in a preterm neonatal population was associated with improved early nutritional intake, enhanced weight gain by day 28 of life, and a reduction in the incidence of bacterial sepsis. While total caloric intake remained below ESPGHAN recommendations, the protocol facilitated more structured and consistent early nutrition delivery. These findings support the adoption of standardized PN protocols in preterm care and underscore the importance of optimizing and integrating enteral feeding strategies to enhance long-term outcomes.
Acknowledgements
The authors of the article extend their gratitude to the professors and staff of the NICU at Al-Zahra Hospital for their contributions to the development of the intravenous nutrition guidelines at the institution. They also wish to convey their sincere appreciation to Ms. Zakieh Salimi Rad for her ongoing dedication and efforts within the intravenous nutrition unit of the hospital. We would like to thank the Clinical Research Development Unit of Zahra Mardani Azari Children Educational and Treatment Center, Tabriz University of Medical Sciences, Tabriz, Iran for their assistance in this research.
Author contributions
MM conceptualized and designed the trial, reviewed and revised the manuscript. LG coordinated data acquisition, as well as conducted data curation and provided study administration and reviewed the manuscript. MBH, ES, BM contributed to the conceptualization of the study, supervised data collection, and reviewed the manuscript. KR designed the trial, carried out the data analysis, drafted the initial manuscript, and reviewed the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of study.
Funding
This research was supported by funding from Pediatric Health Research Center, Tabriz University of Medical Sciences.
Data availability
The data are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The trial has been approved in the Iranian Registry of Clinical Trials (http://www.irct.ir) with the identification No. IRCT20240519061838N2. The study was found to be compatible with the Declaration of Helsinki on ethical principles for medical research and were approved by the Ethics Committee of Tabriz University of Medical Science with reference number 1397.940. Written informed consent was obtained from all eligible parents of infants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Denne SC. Parenteral nutrition for the high-risk neonate, in Avery’s Diseases of the Newborn. Elsevier; 2018. pp. 1023–31. e2.
- 2.Fanaroff AA, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196(2):147. e1-147. e8. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics. 2001;107(2):270–3. [DOI] [PubMed] [Google Scholar]
- 5.Ehrenkranz RA, et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117(4):1253–61. [DOI] [PubMed] [Google Scholar]
- 6.Georgieff MK, et al. Effect of neonatal caloric deprivation on head growth and 1-year developmental status in preterm infants. J Pediatr. 1985;107(4):581–7. [DOI] [PubMed] [Google Scholar]
- 7.Ghods E, et al. Head circumference catch-up growth among preterm very low birth weight infants: effect on neurodevelopmental outcome. J Perinat Med. 2011;39(5):579–86. [DOI] [PubMed] [Google Scholar]
- 8.Hack M, et al. Effect of very low birth weight and subnormal head size on cognitive abilities at school age. N Engl J Med. 1991;325(4):231–7. [DOI] [PubMed] [Google Scholar]
- 9.Agostoni C, et al. Enteral nutrient supply for preterm infants: commentary from the European society of paediatric gastroenterology, hepatology and nutrition committee on nutrition. J Pediatr Gastroenterol Nutr. 2010;50(1):85–91. [DOI] [PubMed] [Google Scholar]
- 10.Koletzko B, et al. 1. Guidelines on paediatric parenteral nutrition of the European society of paediatric gastroenterology, hepatology and nutrition (ESPGHAN) and the European society for clinical nutrition and metabolism (ESPEN), supported by the European society of paediatric research (ESPR). J Pediatr Gastroenterol Nutr. 2005;41:S1–4. [DOI] [PubMed] [Google Scholar]
- 11.Kiss CM, et al. The impact of implementation of a nutrition support algorithm on nutrition care outcomes in an intensive care unit. Nutr Clin Pract. 2012;27(6):793–801. [DOI] [PubMed] [Google Scholar]
- 12.Yu VY. Extrauterine growth restriction in preterm infants: importance of optimizing nutrition in neonatal intensive care units. Croat Med J. 2005;46(5):737–43. [PubMed] [Google Scholar]
- 13.Phillips JL, et al. Impact of parenteral nutrition guideline implementation on growth of very low-birth‐weight infants in a neonatal intensive care unit. J Parenter Enter Nutr. 2022;46(4):836–41. [DOI] [PubMed] [Google Scholar]
- 14.Roggero P, et al. Implementation of nutritional strategies decreases postnatal growth restriction in preterm infants. PLoS ONE. 2012;7(12):e51166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson MJ, et al. Successfully implementing and embedding guidelines to improve the nutrition and growth of preterm infants in neonatal intensive care: a prospective interventional study. BMJ Open. 2017;7(12):e017727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moyses HE, et al. Early parenteral nutrition and growth outcomes in preterm infants: a systematic review and meta-analysis. Am J Clin Nutr. 2013;97(4):816–26. [DOI] [PubMed] [Google Scholar]
- 17.Wittwer A, Hascoët J-M. Impact of introducing a standardized nutrition protocol on very premature infants’ growth and morbidity. PLoS ONE. 2020;15(5):e0232659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa C, Torres T, Teles A. Neonatal nutrition and later outcomes of very low birth weight and preterm infants < 32 gestational age at a tertiary care hospital of Portugal. Open J Pediatr. 2015;5(3):190–8. [Google Scholar]
- 19.Jeong E, et al. The successful accomplishment of nutritional and clinical outcomes via the implementation of a multidisciplinary nutrition support team in the neonatal intensive care unit. BMC Pediatr. 2016;16:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joosten K, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: energy. Clin Nutr. 2018;37(6):2309–14. [DOI] [PubMed] [Google Scholar]
- 21.Miller M, et al. From parenteral to enteral nutrition: a nutrition-based approach for evaluating postnatal growth failure in preterm infants. J Parenter Enter Nutr. 2014;38(4):489–97. [DOI] [PubMed] [Google Scholar]
- 22.Terrin G, et al. Energy-enhanced parenteral nutrition and neurodevelopment of preterm newborns: A cohort study. Nutrition. 2021;89:111219. [DOI] [PubMed] [Google Scholar]
- 23.Klevebro S, et al. Early energy and protein intakes and associations with growth, BPD, and ROP in extremely preterm infants. Clin Nutr. 2019;38(3):1289–95. [DOI] [PubMed] [Google Scholar]
- 24.Bolisetty S, et al. Improved nutrient intake following implementation of the consensus standardised parenteral nutrition formulations in preterm neonates–a before-after intervention study. BMC Pediatr. 2014;14:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Battersby C, et al. Incidence of neonatal necrotising Enterocolitis in high-income countries: a systematic review. Archives Disease Childhood-Fetal Neonatal Ed. 2018;103(2):F182–9. [DOI] [PubMed] [Google Scholar]
- 26.Lapillonne A, Griffin IJ. Feeding preterm infants today for later metabolic and cardiovascular outcomes. J Pediatr. 2013;162(3):S7–16. [DOI] [PubMed] [Google Scholar]
- 27.Arslanoglu S, et al. Fortification of human milk for preterm infants: update and recommendations of the European milk bank association (EMBA) working group on human milk fortification. Front Pead. 2019;7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author upon reasonable request.