ABSTRACT
Bacteria use chemotaxis to move to favourable ecological niches. For many pathogenic bacteria, chemotaxis is required for full virulence, particularly for the initiation of host colonisation. There do not appear to be limits to the type of compounds that attract bacteria, and we are just beginning to understand how chemotaxis adapts them to their lifestyles. Quantitative capillary assays for chemotaxis show that P. aeruginosa is strongly attracted to serotonin, dopamine, epinephrine and norepinephrine. Chemotaxis to these compounds is greatly decreased in a mutant lacking the TlpQ chemoreceptor, and complementation of this mutant with a plasmid harbouring the tlpQ gene restores wild‐type‐like chemotaxis. Microcalorimetric titrations of the TlpQ sensor domain with these four compounds indicate that they bind directly to TlpQ. All four compounds are hormones and neurotransmitters that control a variety of processes and are also important signal molecules involved in the virulence of P. aeruginosa . They modulate motility, biofilm formation, the production of virulence factors and serve as siderophores that chelate iron. Additionally, this is the first report of bacterial chemotaxis to serotonin. This study provides an incentive for research to define the contribution of chemotaxis to these host signalling molecules to the virulence of P. aeruginosa .
Keywords: catecholamine, chemoreceptor, chemotaxis, Pseudomonas aeruginosa
Pseudomonas aeruginosa is strongly attracted by the catecholamines serotonin, dopamine, epinephrine and norepinephrine. Chemotaxis is mediated by the TlpQ chemoreceptor that recognises these compounds at its dCache type ligand binding domain.

1. Introduction
Bacteria use chemotaxis to move to sites that are favourable for growth and survival. A chemotactic response is typically initiated by the binding of a chemoeffector to a chemoreceptor, stimulating chemosensory pathways and ultimately leading to chemotaxis (Parkinson et al. 2015). There do not appear to be any limits to the chemical properties or structure of chemoeffectors (Matilla et al. 2022a, 2022b). The chemosensory capacities of a bacterium are a reflection of its lifestyle (Colin et al. 2021). As the chemoeffectors recognised by most chemoreceptors are unknown, we are only beginning to understand the link between chemosensory repertoire, physiology and lifestyle. For pathogens with very different lifestyles, chemotaxis is often essential for full virulence, and particularly for the initiation of host colonisation (Matilla and Krell 2018). Pseudomonas aeruginosa is among the most serious human pathogens. It kills about half a million people annually (GBD 2019 Antimicrobial Resistance Collaborators 2022). The genome of the P. aeruginosa strain PAO1 encodes 26 predicted chemoreceptors, and the corresponding chemoeffectors have been identified for about half of them (Krell and Matilla 2024). Chemotaxis is required for the full virulence of P. aeruginosa (Schwarzer et al. 2016; Sheng et al. 2019).
The catecholamines dopamine, epinephrine and norepinephrine, and the indoleamine serotonin are signal molecules that act as hormones and neurotransmitters to control a variety of cellular processes in humans and animals (Savaliya and Goerge 2020). They also exert signalling functions in plants (Kulma and Szopa 2007) and bacteria (Boujnane et al. 2024), and they play major roles in inter‐kingdom signalling (Boukerb et al. 2021). Multiple studies show that these four compounds are central signal molecules in P. aeruginosa . Dopamine (Xiang et al. 2024) and serotonin (Knecht et al. 2016) act as exogenous quorum‐sensing molecules that regulate the production of a wide range of virulence factors, motility and biofilm formation. Epinephrine and norepinephrine increase pyoverdine and pyocyanine production (Medina Lopez et al. 2022) as well as adhesion and biofilm formation (Cambronel et al. 2019). Furthermore, these four compounds serve P. aeruginosa as siderophores (Perraud et al. 2022). Experimentation using different animal models shows that the four molecules modulate P. aeruginosa virulence (Knecht et al. 2016; Cambronel et al. 2019; Li et al. 2020; Ma et al. 2020).
2. Results and Discussion
Considering the central role of these four compounds in modulating virulence, we wanted to establish whether P. aeruginosa performs chemotaxis to these compounds. To this end, we conducted quantitative capillary chemotaxis assays using strain PAO1 that were performed as reported in (Matilla et al. 2022a, 2022b), with the exception that assays were conducted under dim light conditions. In this assay, microcapillaries filled with chemoeffector solutions are submerged at their open end into a bacterial suspension. After 30 min, the microcapillaries are emptied and the cells that swan into the capillary are quantified by plating serial dilutions. These assays revealed strong chemoattractant responses for all compounds (Figure 1a).
FIGURE 1.
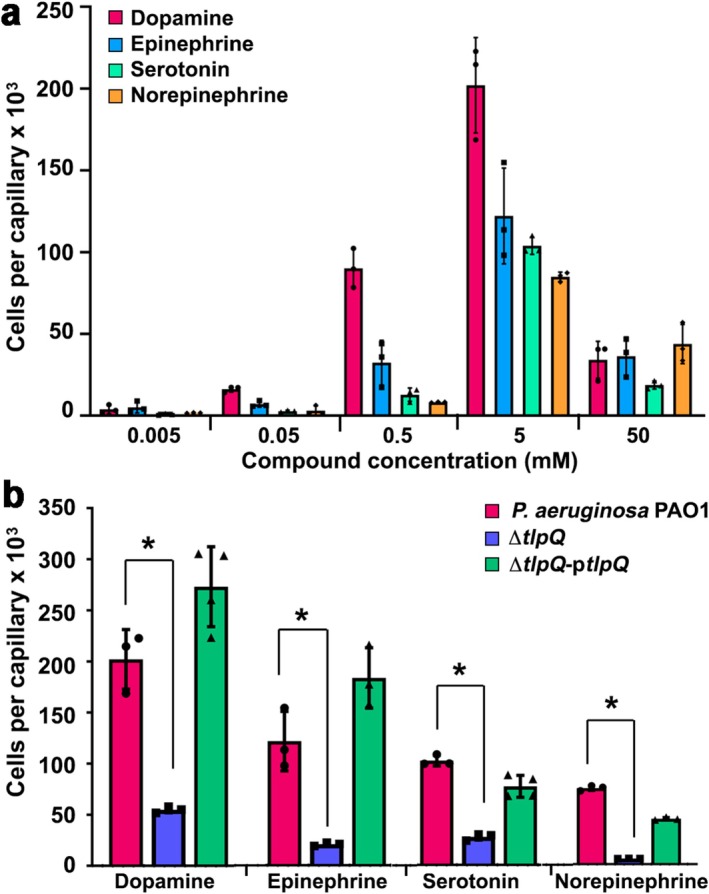
Chemotactic responses to dopamine, epinephrine, serotonin and norepinephrine of Pseudomonas aeruginosa PAO1. (a) Quantitative capillary chemotaxis assays of the wild‐type strain to different chemoeffector concentrations. Data were corrected for the number of bacteria that swam into buffer‐containing capillaries (3107 ± 821). (b) Responses to 5 mM of these four neurotransmitters by the wild‐type strain, the ΔtlpQ mutant and the ΔtlpQ mutant complemented with the ptlpQ plasmid. Data were corrected for the number of bacteria that swam into buffer‐containing capillaries (4071 ± 1536 for wt; 4143 ± 604 for ΔtlpQ and 2071 ± 371 for ΔtlpQ‐ptlpQ). Data are the means and standard deviations from at least three biological replicates conducted in triplicate. *Student's t‐test p < 0.01. The construction of the tlpQ deletion mutant and the plasmid ptlpQ for mutant complementation have been reported in (Corral‐Lugo et al. 2018) and (Kim et al. 2007), respectively.
The assay used is a reference method to detect and quantify chemotactic responses. Responses with more than 50,000 cells per capillary can be considered strong. The threshold for chemotaxis was at capillary concentrations of 50 μM for dopamine and epinephrine and 500 μM for serotonin and norepinephrine (Figure 1a). The maximum responses were seen at a capillary concentration of 5 mM and ranged from 65,000 to 200,000 cells per capillary. These responses are similar to those seen with other chemoattractants like nitrate (Martín‐Mora et al. 2019), and superior to those seen with malate (Martín‐Mora et al. 2018) or α‐ketoglutarate (Martín‐Mora et al. 2016). P. aeruginosa is a universal pathogen that infects almost all human tissues (Krell and Matilla 2024). Catecholamine/indoleamine concentrations in the human body are in the chemotactic response range. For example, dopamine is found to up to 100 mM in the carotid body, 1 mM in the adrenal gland and dorsal striatum, or 100 μM in the kidney and colon (Matt and Gaskill 2020). These are mean tissue concentrations, and local concentrations, such as in the synapsis, are likely to be higher. Furthermore, chemotaxis thresholds derived are based on the capillary concentrations. However, the real thresholds will be lower since this assay monitors bacteria that respond to compounds that diffuse from the capillary into the medium. Whereas bacterial chemotaxis to epinephrine, norepinephrine and dopamine has been observed previously (Pasupuleti et al. 2014; Lopes and Sourjik 2018), this is the first report of chemotaxis to serotonin (Brunet et al. 2025).
To identify the chemoreceptor(s) that detect these four molecules, we conducted chemotaxis assays using a quadruple mutant in the pctA, pctB, pctC and tlpQ chemoreceptor genes. As shown in Figure S1, chemotactic responses to all four compounds were lower than in the wild‐type strain. Since PctA, PctB and PctC are known to be chemoreceptors that recognise primarily amino acids (McKellar et al. 2015; Gavira et al. 2020), we performed chemotaxis assays using a tlpQ mutant. TlpQ is a chemoreceptor that binds and mediates chemoattraction to a number of polyamines, like spermidine, cadaverine and putrescine and histidine (Corral‐Lugo et al. 2018). Responses to all four molecules were significantly decreased, suggesting that TlpQ is their primary chemoreceptor (Figure 1b). Complementation of the mutant with a plasmid harbouring the tlpQ gene resulted in wild‐type‐like chemotaxis to all four compounds (Figure 1b). Control experiments showed that the responses of the wild‐type strain, the tlpQ mutant and the complemented tlpQ mutant to casamino acids are comparable (Figure S2).
Chemoreceptors can be activated by the binding of chemoeffectors or chemoeffector–loaded solute binding proteins to the chemoreceptor ligand binding domain (LBD) (Matilla et al. 2021). To determine the mode of TlpQ activation, we purified the TlpQ‐LBD as reported in (Corral‐Lugo et al. 2018). The purified protein was then subjected to Isothermal Titration Calorimetry binding studies with these four compounds. In these experiments, binding heats are measured that result from the injection of compound aliquots into the protein. Data analyses permit the calculation of the complete set of thermodynamic binding parameters, including the dissociation constant (K D). Whereas the titration of buffer with dopamine resulted in small and uniform peaks, representing dilution heats, titration of TlpQ‐LBD resulted in exothermic heat changes that diminished as protein saturation advanced (Figure 2a).
FIGURE 2.

Microcalorimetric titrations of dopamine, epinephrine, serotonin and norepinephrine to the TlpQ‐LBD. (a) Titration of 80 μM TlpQ‐LBD with aliquots of 10 mM dopamine. (b) Titration of 40 μM TlpQ‐LBD with aliquots of 10 mM epinephrine. (c) Competition assays: Titration of 18 μM TlpQ‐LBD with aliquots of 250 μM spermidine (Spe) in the absence and presence of 10 mM serotonin (Ser) or norepinephrine (Noe). Protein and ligand solutions were in 3 mM Tris, 3 mM PIPES, 3 mM MES, 150 mM NaCl, 10% (v/v) glycerol, pH 7.0. Experiments were conducted on a VP‐microcalorimeter (Microcal, Amherst, MA, USA) at 25°C (for dopamine) and at 10°C (for remaining compounds). Upper panels: Raw titration data. Lower panels: Integrated, concentration‐normalised and dilution‐heat‐corrected raw data fitted with the ‘one‐binding‐site’ model of the MicroCal version of ORIGIN (Microcal, Amherst, MA, USA). The corresponding binding parameters are shown in Table S1. (d) Structure of the TlpQ ligands identified.
The dissociation constant for dopamine was 137 μM. Titration with epinephrine also showed binding (K D = 363 μM, Figure 2b). Initial titrations with serotonin and norepinephrine gave indications of binding, but with an affinity lower than that for dopamine and epinephrine. The interaction of serotonin and norepinephrine with the TlpQ‐LBD could be better visualised using competition assays. Previous studies have shown that TlpQ‐LBD also binds polyamines like spermidine (Corral‐Lugo et al. 2018). The titration of TlpQ‐LBD with spermidine resulted in a K D of 120 nM (Figure 2c). When the TlpQ‐LBD was titrated with spermidine in the presence of 10 mM serotonin or norepinephrine, a significant reduction in binding was observed (Figure 2c), with lower changes in binding enthalpy (ΔH app) and increased dissociation constants (K Dapp) in the presence of either serotonin or norepinephrine compared to titration without competitor. Thus, serotonin and norepinephrine compete with spermidine for binding to the TlpQ‐LBD. Dissociation constants of above 1 mM were estimated for serotonin and norepinephrine. We conclude that TlpQ is activated by direct binding of dopamine, epinephrine, serotonin and norepinephrine. Our study also shows that the sensing mechanisms of catecholamine/indoleamine chemotaxis in P. aeruginosa and E. coli are different. Whereas in P. aeruginosa these compounds bind directly to the TlpQ‐LBD that belongs to the dCache family of sensor domains, E. coli has no chemoreceptors that contain a dCache domain (Parkinson et al. 2015). Data indicate that in E. coli these compounds are sensed by the Tsr and Tar chemoreceptors (Pasupuleti et al. 2018) (Lopes and Sourjik 2018) that possess 4‐helix bundle type LBDs.
This study thus forms the basis for exploring the effect of chemotaxis to catecholamines/indoleamines on P. aeruginosa virulence. It also suggests that other motile bacterial pathogens should be tested for chemotaxis to these compounds.
Author Contributions
Elizabet Monteagudo‐Cascales: conceptualization, investigation, supervision, visualization, writing – review and editing, formal analysis. Andrea Lozano‐Montoya: investigation. Tino Krell: conceptualization, writing – original draft, writing – review and editing, funding acquisition, supervision, project administration, visualization.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Appendix S1.
Acknowledgements
We are indebted to Dr. Michael Manson for critical reading and editing the manuscript. This study was supported by grants from the Spanish Ministry for Science and Innovation/Agencia Estatal de Investigación 10.13039/501100011033 (grants PID2020‐112612GB‐I00 and PID2023‐146216NB‐I00 to TK) and the Consejo Superior de Investigaciones Científicas (grant 2024AEP062 to TK).
Funding: This study was supported by grants from the Spanish Ministry for Science and Innovation/Agencia Estatal de Investigación 10.13039/501100011033 (grants PID2020‐112612GB‐I00 and PID2023‐146216NB‐I00 to TK) and the Consejo Superior de Investigaciones Científicas (grant 2024AEP062 to TK).
Contributor Information
Elizabet Monteagudo‐Cascales, Email: elizabet.monteagudo@eez.csic.es.
Tino Krell, Email: tino.krell@eez.csic.es.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Boujnane, M. , Boukerb A. M., and Connil N.. 2024. “Bacterial Gene Expression in Response to Catecholamine Stress Hormones.” Current Opinion in Endocrine and Metabolic Research 36: 100543. [Google Scholar]
- Boukerb, A. M. , Cambronel M., Rodrigues S., et al. 2021. “Inter‐Kingdom Signaling of Stress Hormones: Sensing, Transport and Modulation of Bacterial Physiology.” Frontiers in Microbiology 12: 690942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet, M. , Amin S. A., Bodachivskyi I., Kuzhiumparambil U., Seymour J. R., and Raina J.‐B.. 2025. “An Atlas of Metabolites Driving Chemotaxis in Prokaryotes.” Nature Communications 16: 1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambronel, M. , Tortuel D., Biaggini K., et al. 2019. “Epinephrine Affects Motility, and Increases Adhesion, Biofilm and Virulence of Pseudomonas aeruginosa H103.” Scientific Reports 9: 20203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin, R. , Ni B., Laganenka L., and Sourjik V.. 2021. “Multiple Functions of Flagellar Motility and Chemotaxis in Bacterial Physiology.” FEMS Microbiology Reviews 45: fuab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral‐Lugo, A. , Matilla M. A., Martín‐Mora D., et al. 2018. “High‐Affinity Chemotaxis to Histamine Mediated by the TlpQ Chemoreceptor of the Human Pathogen Pseudomonas Aeruginosa .” Molecular Biology & Microbiology 9, no. 6: e01894‐18. 10.1128/mBio.01894-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavira, J. A. , Gumerov V. M., Rico‐Jiménez M., et al. 2020. “How Bacterial Chemoreceptors Evolve Novel Ligand Specificities.” Molecular Biology & Microbiology 11, no. 1: e03066‐19. 10.1128/mBio.03066-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2019 Antimicrobial Resistance Collaborators . 2022. “Global Mortality Associated With 33 Bacterial Pathogens in 2019: A Systematic Analysis for the Global Burden of Disease Study 2019.” Lancet 400: 2221–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. E. , Shitashiro M., Kuroda A., Takiguchi N., and Kato J.. 2007. “Ethylene Chemotaxis in Pseudomonas Aeruginosa and Other Pseudomonas Species.” Microbes and Environments 22: 186–189. [Google Scholar]
- Knecht, L. D. , O'Connor G., Mittal R., et al. 2016. “Serotonin Activates Bacterial Quorum Sensing and Enhances the Virulence of Pseudomonas aeruginosa in the Host.” eBioMedicine 9: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krell, T. , and Matilla M. A.. 2024. “ Pseudomonas aeruginosa .” Trends in Microbiology 32: 216–218. [DOI] [PubMed] [Google Scholar]
- Kulma, A. , and Szopa J.. 2007. “Catecholamines Are Active Compounds in Plants.” Plant Science 172: 433–440. [Google Scholar]
- Li, J. , Ma X., Zhao L., Li Y., Zhou Q., and Du X.. 2020. “Extended Contact Lens Wear Promotes Corneal Norepinephrine Secretion and Pseudomonas aeruginosa Infection in Mice.” Investigative Ophthalmology & Visual Science 61: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes, J. G. , and Sourjik V.. 2018. “Chemotaxis of Escherichia coli to Major Hormones and Polyamines Present in Human Gut.” ISME Journal 12: 2736–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X. , Wang Q., Song F., et al. 2020. “Corneal Epithelial Injury‐Induced Norepinephrine Promotes Pseudomonas aeruginosa Keratitis.” Experimental Eye Research 195: 108048. [DOI] [PubMed] [Google Scholar]
- Martín‐Mora, D. , Ortega Á., Matilla M. A., Martínez‐Rodríguez S., Gavira J. A., and Krell T.. 2019. “The Molecular Mechanism of Nitrate Chemotaxis via Direct Ligand Binding to the PilJ Domain of McpN.” MBio 10: e02334‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín‐Mora, D. , Ortega Á., Pérez‐Maldonado F. J., Krell T., and Matilla M. A.. 2018. “The Activity of the C4‐Dicarboxylic Acid Chemoreceptor of Pseudomonas aeruginosa Is Controlled by Chemoattractants and Antagonists.” Scientific Reports 8: 2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín‐Mora, D. , Ortega A., Reyes‐Darias J. A., et al. 2016. “Identification of a Chemoreceptor in Pseudomonas aeruginosa That Specifically Mediates Chemotaxis Toward α‐Ketoglutarate.” Frontiers in Microbiology 7: 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilla, M. A. , and Krell T.. 2018. “The Effect of Bacterial Chemotaxis on Host Infection and Pathogenicity.” FEMS Microbiology Reviews 42: fux052. [DOI] [PubMed] [Google Scholar]
- Matilla, M. A. , Ortega Á., and Krell T.. 2021. “The Role of Solute Binding Proteins in Signal Transduction.” Computational and Structural Biotechnology Journal 19: 1786–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilla, M. A. , Velando F., Martín‐Mora D., Monteagudo‐Cascales E., and Krell T.. 2022a. “A Catalogue of Signal Molecules That Interact With Sensor Kinases, Chemoreceptors and Transcriptional Regulators.” FEMS Microbiology Reviews 46: fuab043. [DOI] [PubMed] [Google Scholar]
- Matilla, M. A. , Velando F., Tajuelo A., et al. 2022b. “Chemotaxis of the Human Pathogen Pseudomonas aeruginosa to the Neurotransmitter Acetylcholine.” MBio 13: e0345821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt, S. M. , and Gaskill P. J.. 2020. “Where Is Dopamine and How Do Immune Cells See It?: Dopamine‐Mediated Immune Cell Function in Health and Disease.” Journal of Neuroimmune Pharmacology 15: 114–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar, J. L. , Minnell J. J., and Gerth M. L.. 2015. “A High‐Throughput Screen for Ligand Binding Reveals the Specificities of Three Amino Acid Chemoreceptors From Pseudomonas Syringae Pv. Actinidiae .” Molecular Microbiology 96: 694–707. [DOI] [PubMed] [Google Scholar]
- Medina Lopez, A. I. , Fregoso D. R., Gallegos A., et al. 2022. “Beta Adrenergic Receptor Antagonist Can Modify Pseudomonas Aeruginosa Biofilm Formation In Vitro: Implications for Chronic Wounds.” FASEB Journal 36: e22057. [DOI] [PubMed] [Google Scholar]
- Parkinson, J. S. , Hazelbauer G. L., and Falke J. J.. 2015. “Signaling and Sensory Adaptation in Escherichia coli Chemoreceptors: 2015 Update.” Trends in Microbiology 23: 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupuleti, S. , Sule N., Cohn W. B., MacKenzie D. S., Jayaraman A., and Manson M. D.. 2014. “Chemotaxis of Escherichia coli to Norepinephrine (NE) Requires Conversion of NE to 3,4‐Dihydroxymandelic Acid.” Journal of Bacteriology 196: 3992–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupuleti, S. , Sule N., Manson M. D., and Jayaraman A.. 2018. “Conversion of Norepinephrine to 3,4‐Dihdroxymandelic Acid in Escherichia Coli Requires the QseBC Quorum‐Sensing System and the FeaR Transcription Factor.” Journal of Bacteriology 200, no. 1: e00564‐17. 10.1128/JB.00564-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraud, Q. , Kuhn L., Fritsch S., et al. 2022. “Opportunistic Use of Catecholamine Neurotransmitters as Siderophores to Access Iron by Pseudomonas aeruginosa .” Environmental Microbiology 24: 878–893. [DOI] [PubMed] [Google Scholar]
- Savaliya, M. , and Goerge J. J.. 2020. “The Monoaminergic System in Humans.” In Recent Trends in Science and Technology, 190–203. Department of Bioinformatics, Christ College, International Organization of Omics and Matics. [Google Scholar]
- Schwarzer, C. , Fischer H., and Machen T. E.. 2016. “Chemotaxis and Binding of Pseudomonas aeruginosa to Scratch‐Wounded Human Cystic Fibrosis Airway Epithelial Cells.” PLoS One 11: e0150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng, S. , Xin L., Yam J. K. H., et al. 2019. “The MapZ‐Mediated Methylation of Chemoreceptors Contributes to Pathogenicity of Pseudomonas aeruginosa .” Frontiers in Microbiology 10: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, S.‐L. , Xu K.‐Z., Yin L.‐J., Rao Y., Wang B., and Jia A.‐Q.. 2024. “Dopamine, an Exogenous Quorum Sensing Signaling Molecule or a Modulating Factor in Pseudomonas aeruginosa ?” Biofilms 8: 100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


