ABSTRACT
Autism spectrum disorder (ASD) is a developmental disorder that is characterized by deficits in social communication and restricted, repetitive, and stereotyped behaviors. In addition to neurobehavioral symptoms, children with ASD often have gastrointestinal symptoms (e.g. constipation, diarrhea, gas, abdominal pain, reflux). Several studies have proposed the role of gut microbiota and metabolic disorders in gastrointestinal symptoms and neurodevelopmental dysfunction in ASD patients; these results offer promising avenues for novel treatments of this disorder. Interventions targeting the gut microbiota – such as fecal microbiota transplant (FMT), microbiota transplant therapy (MTT), probiotics, prebiotics, synbiotics, antibiotics, antifungals, and diet – promise to improve gut health and can potentially improve neurological symptoms. The modulation of the gut microbiota using MTT in ASD has shown beneficial and long-term effects on GI symptoms and core symptoms of autism. Also, the modulation of the gut microbiota to resemble that of typically developing individuals seems to be the most promising intervention. As most of the studies carried out with MTT are open-label studies, more extensive double-blinded randomized control trials are needed to confirm the efficacy of MTT as a therapeutic option for ASD. This review examines the current clinical research evidence for the use of interventions that target the microbiome – such as antibiotics, antifungals, probiotics/prebiotics, synbiotics, and MTT – and their effectiveness in changing the gut microbiota and improving gastrointestinal and neurobehavioral symptoms in ASD.
KEYWORDS: Autism, ASD, gut microbiome, fecal microbiota transplant, FMT, microbiota transplant therapy, MTT, prebiotics, probiotics, synbiotics
Introduction
Autism spectrum disorder (ASD) is a multifactorial neurodevelopmental disorder characterized by verbal communication deficiency, social interaction impairment, restricted interests, and repetitive behaviors.1 It affects about 1 in 36 children in the United States,2 impacting families and impeding the developmental progress of affected children.3 While ASD etiology is unclear, a combination of several factors, such as genetic and environmental,4,5 immune dysregulation, inflammation,6 and microbiome imbalances,7 may play a role in the onset and development of ASD. Individuals with ASD experience symptoms that vary in severity, and there are many common co-morbid symptoms in ASD,8 including gastrointestinal symptoms,9 such as abdominal pain, diarrhea, constipation, gas, reflux, bloating, and vomiting. Some studies have found an association between gastrointestinal (GI) symptoms and the severity of ASD-related symptoms.10–16 ASD and GI issues may be linked to dysbiosis in the gut microbiota through the microbiota-gut-brain axis, a bidirectional connection between the microbiota, the gut, and the brain.17,18 Many studies have reported differences in the composition of the gut microbiota between ASD patients and neurotypical individuals.19–36 Some of the differences can be attributed to (1) excessive use of antibiotics by ASD individuals,37,38 which can influence gut homeostasis by targeting pathogens and commensal bacteria;39,40 (2) dietary restrictions as children with ASD have unique dietary preferences;41 (3) host genetics;42 (4) mode of delivery;43 and (5) feeding patterns,44 including breastfeeding or formula feeding and/or supplementation.
The increasing rates of diagnoses and a lack of effective therapeutic options highlight the importance of investigating alternative treatments for ASD. A growing number of studies explored the potential impact of microbiota-based interventions in animal and human studies. ASD studies involving animal models that used probiotics45,46 and fecal microbiota transplant (FMT)47–51 showed positive effects by relieving the ASD-like behaviors in mouse models. For instance, the treatment with Bacteroides fragilis reduced gut permeability, altered gut microbiota composition, and decreased ASD-like behaviors in a mouse model of ASD.45 Some of the findings from these animal studies also support the evidence of human studies described in Tables 1 and 2. Gilbert et al. summarized a path for how interventions based on microbiome profiling can be used for neurodevelopmental disorders, such as ASD, moving the knowledge from pre-clinical animal models, such as with mice, to clinical trials with humans.52 However, this approach has not always been used, and it is often not needed.
Table 1.
Clinical trials of probiotic/prebiotics/synbiotic studies in ASD.
| Reference | Study design | Intervention | Findings |
|---|---|---|---|
| Probiotic Study | |||
| Kong et al.142 | Randomized double-blind placebo-controlled ASD Participants: 35 Treated: 18(3.6–18.50 yrs) Placebo:17(4.69–19.70 years) Diagnosis tool: ADOS-2 and DSM-5 |
Lactobacillus plantarum PS128 probiotic (6 × 1010 CFUs)/day was administered for 28 weeks. Intranasal oxytocin spray was given to the treated and placebo group at week 16. Primary and secondary outcome measures were assessed at 0, 16, 28 weeks. | Improvements in SRS and ABC(primary outcome) and Clinical Global Impression (CGI) (secondary outcome) in the treatment group that received the probiotics and intranasal oxytocin spray compared to the placebo group. Roseburia, Veillonella, and Streptococcus were higher in the probiotic-treated group |
| Guidetti et al.143 | A randomized, double-blind crossover study with a placebo ASD Participants: 61(24 months −16 yrs) Treated: 30 Placebo: 31 Diagnosis tool: ADOS-2 and ADI-R |
10 × 109 CFU of the probiotic mixture(Limosilactobacillus fermentum LF10, Ligilactobacillus salivarius LS03, Lactiplantibacillus plantarum LP0, Bifidobacterium longum) was administered to the treatment group per day for 3 months. The placebo group received 2.5 g of maltodextrin in powder form. | Improvements in GI symptoms, communication, and maladaptive behaviors. Streptococcus thermophilus, Bifidobacterium longum, Limosilactobacillus fermentum, and Ligilactobacillus salivarius species were increased in treatment group, and Alistipes finegoldii, Clostridium leptum, and Ruminococcus calldus were decreased |
| Sherman et al.144 | Randomized placebo-controlled ASD Participants: 35(3-20 yrs) Treated: 18 Placebo: 17 |
The treatment group received daily Lactobacillus plantarum probiotic (6 × 1010 CFUs), and the placebo group received microcrystalline cellulose for 16 weeks. | No change in ASD severity and GI symptoms. Increase in Lactobacillus. |
| Shaaban et al.145 | Prospective open-label study, Participants ASD: 30 (2-11 yrs) TD Controls: 30 typically developing age and gender match |
5 g of daily dose of 100 × 106CFU of probiotics (Lactobacillus acidophilus, Lactobacillus rhamnosus, and Bifidobacteria longum) 1 time/day for 3 months | Decrease in total ATEC scores (the primary outcome) and an improvement in the subscales of ATEC: speech, language, communication, sociability, and cognitive awareness. Improvements in GI symptoms (secondary outcome) measures: constipation, abdominal pain, flatulence. Increase in Bifidobacteria and Lactobacilli levels after supplementation |
| Prebiotic study | |||
| Inoue et al.155 | Open-label study, Participants ASD: 13 (4-9 yrs) TD Controls: 123 typically developing (2-75 years) Diagnosis tool: DSM-5 |
Prebiotic dietary supplement (partially hydrolyzed guar gum (PHGG) 6 g/day administered to ASD children and lasted 2 months or longer. | Reduction in constipation, behavioral irritability, and an increase in defecation per week in participants. Blautia and Acidaminococcus increased while Streptococcus, Odoribacter, and Eubacterium decreased after treatment |
| Grimaldi et al.156 | Randomized, double-blind, placebo-controlled, Participants ASD: 30 (4-11 yrs) TD Controls: No controls |
Children were randomly assigned to placebo and treated group using a random number system. The placebo group received maltodextrin-GLUCIDEX®; 1.8 g was given in powder form. The treated group received 1.8 g Bimuno galacto oligosaccharide (B-GOS) prebiotic supplementation during the 6-week feeding period. |
Improvement in abdominal pain, bowel movements, and ASD symptoms, such as social behavior. Increase in beneficial microbes, such as Coprococcus spp. and Dorea formicigenerans. |
| Synbiotic study | |||
| Li et al.146 | Open-label study Participants ASD: 53 (3-12 yrs) TD Controls: 45 typically developing age and gender match Diagnosis tool: DSM-5 |
Children received dry powder of probiotic Bifidobacterium animalis subsp. lactis Probio-M8 (Probio-M8)(1.0 × 1011CFU/g) daily for a period of 12 weeks, with a balanced diet (40% carbohydrates, 30% fats, and 30% proteins). | Improvement in CARS scores (the primary outcome) and GI symptoms (secondary outcome) and increased levels of Bifidobacterium animalis, Akkermansia muciniphila, Fusicatenibacter saccharivorans, and Sutterella sp. while also reducing Blautia obeum. |
| Phan et al.57 | Open-label Participants ASD: 170 (2-8 yrs) TD Control: 123 typically developing (2-75 years) Diagnosis tool: SRS2 |
ASD patients received a 3-month supplementation of personalized synbiotic. | No improvement in ASD related symptoms assessed using Social Responsiveness Scale (SRS2) (the primary outcome). However, there was an improvement in GI symptoms (secondary outcomes).The proportion of B. breve, L. reuteri, L. plantarum, and L.brevis increased in ASD patients after synbiotic treatment. |
| Wang et al.147 | A double-blind, placebo-controlled, Total ASD Participants: 26(2-8 yrs). Treated: 16 and Placebo:10 TD Controls: 24 typically developing (2-8 years) Diagnosis tool: DSM-5 and CCMD-3 |
1010CFU of four probiotics (B. infantis Bi26, L. rhamnosus HN001, B. Lactis BL-04, and L. Paracasei rhamnosus HN001) and fructo-oligosaccharide (FOS) were given to the treated group for 30 days. The placebo group received maltodextrin. | Reduction in the severity of autism assessed using ATEC (primary outcome) and improvements GI symptoms measured by GSRS (secondary outcome) compared to the placebo group. Increase in beneficial bacteria, such as Bifidobacteriales, and a decrease in Clostridium after intervention. |
| Sanctuary et al.148 | Randomized, double-blind crossover-controlled trial Participants ASD: 8 (2-11 yrs) |
20 billion CFU per day of Bifidobacterium infantis and colostrum were administered for 4 weeks, followed by wash out period for 2 weeks and 5 weeks of colostrum. | Reduced aberrant behaviors and GI symptoms. No differential abundant genera were observed after treatment. |
| Arnold et al.150 | Randomized placebo-controlled, crossover trial Total ASD Participants:10 (3-12 yrs) Treated: 6 Placebo: 4 Diagnosis tool: ADOS-2 and ADI-R |
900 billion bacteria per packet of probiotic mix (VISBIOME), consisting of L. casei, Lactobacillus plantarum, Lactobacillus acidophilus, and Lactobacillus delbrueckii subsp. Bulgaricus, B. longum, Bifidobacterium infantis, and Bifidobacterium breve, and S. thermophiles and starch were administered half packet twice daily for the first 4 weeks, then increased to an entire packet twice daily for the last 4 weeks if no effects were seen. | No improvement in pediatric quality of life inventory (PedsQL) (primary outcome), but they reported a moderate effect size compared to the placebo. Improvements in GI symptoms based on parents selected target symptoms (secondary outcome) with a large effect size. There were no significant alterations in the microbiome community. |
| Aldegheri et al.149 | Single patient case study Participant: 17-year-old adolescent male |
The patient received 2 tablets of antibiotics, Rifaximin, three times daily for 10 days, followed by a daily intake of 12.5 billion Bifidobacterium lactis Bi-07 and Lactobacillus acidophilus, as well as 250 mg of 2’-FL fucosylated HMO for 6 months. | Improvement in aggressiveness, increased mood stability and reduction in constipation. Decrease in Sutterella spp. after treatment. |
ADI-R: Autism Diagnostic Interview-Revised; ADOS-2: Autism Diagnostic Observation Schedule, Second Edition (ADOS-2); DSM-5: Diagnostic and Statistical Manual of Mental Disorders; CARS: Childhood Autism Rating Scale; ABC: Autism Behavior Checklist; SRS: Social Responsiveness Scale; CGI: Clinical Global Impression; CCMD-3: Chinese Classification of Mental Disorders; GSRS: Gastrointestinal Symptom Rating Scale; ATEC: Autism treatment evaluation checklist; TD: typically developing.
Table 2.
MTT clinical trials in ASD.
| Reference | Study design | Pre-MTT treatment | Intervention | Findings |
|---|---|---|---|---|
| Kang et al.54 | Open-label Participants ASD: 18 (7-16 yrs) with GI symptoms Control: 20 neurotypicals (age and gender-matched) without GI symptoms Diagnosis tools: ADI-R |
Antibiotic treatment: oral vancomycin for 2 weeks Proton pump inhibitor Bowel cleansing |
MTT(SHGM) Initial high dose 2.5 × 1012cells/day for 2 days Maintenance dose 2.5 × 109 cells/day for 7–8 weeks Administration route: oral and rectal. Follow-up: 8 weeks and 2 years |
80% reduction in GI symptoms and 23% improvement in ASD symptoms. Improvement in GI and ASD was maintained after 2 years. Increased microbial diversity. Increased relative abundance of Prevotella, Desulfovibrio, and Bifidobacteria |
| Li et al.172 | Open-label Participants ASD: 40 (3-17 yrs) with GI symptoms Control: 16 neurotypical (age and gender match) without GI symptoms. Diagnosis tool: ADI-R |
Bowel Cleanse: Participants received 2 L Golytely (polyethylene glycol) the night before the transplantation |
2×1014CFU once a week for 4 weeks Administration route: Oral and rectal. Follow-up: 8 weeks |
35% decrease in GI symptoms and 10% decrease in CARS scores after four weeks of treatment Eubacterium Coprostanoligene was reduced in abundance after MTT treatment |
| Hu et al.176 | Single patient case study Participant: 7-year-old female. Diagnosis tool: ADOS-2; DSM-5 |
Antibiotic treatment: oral vancomycin for 14 days Bowel preparation: fasting for 8 hours, water prohibition for 4hrs, and administered polyethylene glycol electrolyte powder |
MTT fluid 80 ml of bacterial solution was administered five times, separated by 1 week through colonoscopy. No follow up |
ATEC, SRS, and CARS scores decreased after vancomycin treatment and further decreased after MTT treatment. Gastrointestinal symptoms also improved. Bacteroides and Ruminococcus increased, and Bifidobacterium, Anaerostipes, Streptococcus, and Faecalibacterium decreased after MTT |
| Li et al.174 | Open-label Participants ASD: 38 (3-14 yrs) 31 with GI symptoms and 7 with no GI symptoms Control: 30 neurotypical (age and gender match) without GI symptoms. Diagnosis tool: DSM-5 |
No antibiotic treatment was done | Lyophilized MTT capsules were administered orally consisting of 1 g of donor stool per 1 kg of recipient body weight, once every 4 weeks for a total of 12 weeks 8 weeks follow-up |
At the end of treatment, there was decrease in GSRS (51%), ABC (20%), CARS (10%) from baseline. At the end of follow-up, there was a decrease in GSRS (32%), ABC (23%), CARS (10%) and SRS (6%) from baseline. Increase in the abundance of Eubacterium_hallii_group, Anaerostipes, Fusicatenibacter, Collinsella, Ruminococcus_torques_group , and Dorea, and decrease in the abundance of Blautia Prevotella and Sellimonas |
| Chen et al.175 | Open-label Participants ASD: 29 (2-11 yrs) with GI symptoms Control: 36 neurotypical (age and gender match) Diagnosis tool: DSM-5 |
No antibiotic treatment or bowel cleanse was done | 2 capsules of freeze-dried microbiota (bacterial cells were equivalent to 200 g of fresh stool) was administered orally for 12 days per month for 4-months | Improvement in ABC, CARS, and GI symptoms after treatment A decrease in Collinsella |
| Hazan et al.177 | Single patient case study Participant: 19-year-old male Diagnosis tool: Not stated |
Antibiotic treatment: 500 mg of vancomycin three times daily Deep colonic wash prior to colonoscopy |
A single dose of MTT (300 mL) was infused directly into the cecum via colonoscopy. Follow-up: 15 months | Improvement in CARS, ATEC, and GI symptoms. Bifidobacterium increased, and Lactobacillus animalis decreased after treatment |
ADI-R: Autism Diagnostic Interview-Revised; DSM-5: Diagnostic and Statistical Manual of Mental Disorders; CARS: Childhood Autism Rating Scale; ABC: Autism Behavior Checklist; SRS: Social Responsiveness Scale; GSRS: Gastrointestinal Symptom Rating Scale; SHGM: standardized human gut microbiota; ATEC: Autism treatment evaluation checklist.
The above-mentioned strategies are important to test specific hypotheses for mechanistic research. However, clinical studies of microbiota-based interventions in ASD patients, although less mechanistic, provide translational evidence of the importance of the microbiota-gut-brain axis in ASD. Some of the clinical trials’ interventions – such as FMT,53 microbiota transplant therapy (MTT),54 antibiotics,55 probiotics,56 and synbiotics57 as novel strategies to modify the microbiome – demonstrated improved GI symptoms and core symptoms in individuals with ASD, enhancing their health and quality of life.
In this review, we focus on studies of interventions that target the microbiota to treat gastrointestinal and ASD-related symptoms and that reported changes in the microbiota of study participants after the intervention. We start with a brief description of the gut microbiota, metabolites, and immune system dysregulation associated with ASD, followed by clinical studies on interventions that target the microbiota, including antibiotics, antifungals, probiotics/synbiotics, diet/prebiotics, and FMT/MTT.
Gut microbiota and metabolites associated with ASD
Increasing reports have pointed to the possible role of the gut microbiota in ASD pathogenicity.21,23,25,26,32,34–36,58–68 Despite several reports on the gut microbial imbalance in ASD, there is no defined microbial signature for ASD diagnosis.7,69 However, multiple studies have found a significantly lower microbial diversity26,28,30,57 and a greater abundance of microbes, including some in the Clostridium and Bacteroides genera, in the gut microbiota of children with ASD.24,30,53,70–74 Also, some studies found a lack of other fiber fermenters, such as Prevotella.19,26,27,58 Two recent meta-analyses noted a reduced presence of Bifidobacterium at the genus level in the gut microbiota of children with ASD.24,69 A meta-analysis of multiple cohorts found 591 microbes that were more common in the gut microbiome of children with ASD and 169 microbes that were less common in their control counterparts.41 Recent multi-kingdom metagenomic analyses in a large cohort (n = 1627) revealed Virgibacillus species were enriched and Desulfovibrio vulgaris and Bacteroides species were depleted in ASD children.75 Overall, these studies reveal not only large variations in the microbiome of children with ASD but also some significant differences compared to typically developing (TD) children.
The gut microbiome is highly variable among individuals. The variations may be due to several factors, which were described in Krajmalnik-Brown et al. such as the use of siblings as controls or avoiding the use of relatives as controls, large heterogeneity in the microbiomes of individuals because of differences in geographic17 or ethnicities, and diet76,77 and children with ASD generally have more restricted diets.41 Also, there are variations in the findings from various studies, and these may be due to variety of protocols for sampling and techniques for characterizing the microbial ecology – such as workflows for specimen storage and processing, lack of standardization of experimental protocols, different analysis methods, and the extent to which other confounding factors were considered – could account for the inconsistencies in the findings from the studies.78–81
Several microbial-related mechanisms have been implicated in ASD. Microbial dysbiosis induces the breakdown of the gut integrity,82,83 produces toxins,58 and results in immunological84 and metabolic abnormalities. Alteration in intestinal permeability (referred to as “leaky gut”) can result in the release of proinflammatory substances, such as Lipopolysaccharide (LPS), which can modulate the central nervous system by increasing activity in brain areas, such as the amygdala that controls emotions and behavior.85 For instance, a microbial shift within the gut of mice yielded changes in serum metabolites and induced ASD-like phenotypes.45
The gut microbiota is a crucial component of the gut-brain axis and plays an important role in influencing the brain through endocrine, immunological, metabolic, and neurological systems.86,87 The gut microbiota influences brain functions via the secretion of active metabolites – such as neurotransmitters (e.g., serotonin), short chain fatty acids (SCFA), and immune modulators – which can cross the blood-gut and the blood-brain barriers.9,33,88 Dysregulation in the production or consumption of these compounds can induce ASD-like behaviors by affecting the host’s immune and neural system and modulating the host’s development.45,89,90 The level of an excitatory neurotransmitter, glutamate, has been reported to be higher in the feces and blood of individuals with autism.60,91 Also, an increased ratio of glutamate to GABA is known to be a signature of neuroinflammation linked to sensory processing, and the ratio between these two major neurotransmitters was reported to be different in blood samples from children with ASD compared to those from TD children.92 Altered levels of other neurotransmitters (e.g., serotonin and dopamine) and amino acids (e.g., tryptophan and indoles) have also been linked to autism and regulated by the gut microbiome.51
Pre-clinical studies have also shown that microbial metabolites, such as para-cresol93 and 4-ethylphenol sulfate,94 can cause ASD-like symptoms in mice. Similarly, high levels of urinary p-cresol sulfate have been correlated with ASD-related symptoms in humans.95,96 Microbes, such as Clostridium difficile (now reclassified as Clostridioides difficile),97 Blautia hydrogenotrophica, Romboutsia lituseburensis, and Anaerostipes hadrus, produce p-cresol.98 Although these microbes are known to produce p-cresol, most of them have not been reported in higher abundance in ASD individuals. Only a few studies have documented a higher abundance of Clostridium ,21,99–102 and a single study has reported an association with Anaerostipes 103 in the ASD population. P-cresol has many toxic properties and it is known to affect gamma-aminobutyric acid (GABA) and glutamate transport, causing neurological and physiological changes in patients with ASD104,105 and reducing glutathione (GSH), which acts as an antioxidant.106
This suggests that harmful microbial metabolites contribute to ASD symptoms in some children with ASD. Reviews by Peralta-Marzal et al. and Siracusano et al. reported differences in several metabolomic profiles in fecal, urine, and blood samples from individuals with ASD and typically developing controls.107,108 In addition, microbial species that colonize the gut in children with ASD can alter the synthesis of beneficial microbial products, such as short chain fatty acids (SCFA), vitamins, metabolites, and neurotransmitters, essential for human health and communication with the brain (Figure 1).109,110 For example, a meta-analysis by Morton et al. found that amino acid metabolism, carbohydrate metabolism, and lipid metabolism encoded by microbial species in the genera Prevotella, Bifidobacterium, Desulfovibrio, and Bacteroides correlated with changes in brain gene expression.41 Thus, abnormal gut microbiota in children with ASD provides a suitable target for microbiota-targeting interventions.
Figure 1.
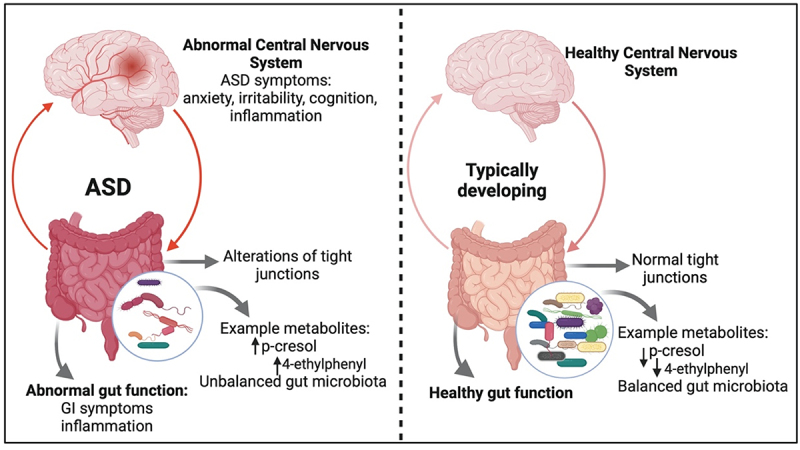
An overview of the gut microbiome effects on gastrointestinal symptoms and possible metabolite-based gut-brain connection in ASD and typically developing individuals.
Gut microbiota and immune system dysregulation in ASD
One important connection with the gut microbiome is the immune system. The microbiota plays a role in immune system maturation, and microbial dysbiosis in autism can lead to immune system dysregulation.45,84,111 The activated immune system, as a result of microbiota dysbiosis, releases chemokines and cytokines – such as interleukin-1β (IL-1β), interleukin-6 (IL-6), interferon-γ (INF-γ), tumor necrosis factor-α (TNF-α) – which cross the blood brain barrier (BBB) and these mediators can bind to endothelial cells in the brain and induce immune responses in the brain.112–114 A review by Critchfield et al. reported abnormal immune system function in ASD.115 Ashwood et al. found that proinflammatory cells such as Cluster of Differentiation 3 tumor necrosis factor-alpha (CD3+ TNFα+), Cluster of Differentiation 3 Interleukin-2 (CD3+ IL-2+), and Cluster of Differentiation 3 Interferon‐gamma(CD3+ IFNγ+), were higher in participants with ASD compared to typically developing controls.116,117 In contrast, mucosal Cluster of Differentiation 3 Interleukin-2 (CD3+ IL-10+) cells were lower in ASD children compared to typically developing controls. Enstrom et al. also found that immunoglobulin (IgG4) levels were higher in ASD compared to typically developing controls.118 A meta-analysis by Morton et al. showed that bacteria, such as Bacteroides thetaiotaomicron, abundance was correlated with lower transforming growth factor beta (TGF-β) levels.41 This bacteria is known to modulate maternal immune activation-dependent metabolites that are linked to behavioral symptoms.45 The authors also found that higher abundances of Bifidobacterium longum and Prevotella copri were associated with lower interleukin-6 (IL-6) (proinflammatory) levels.41
Interventions to modify the microbiome
Many studies have explored the potential impact of microbiota-based approaches to restore the ecological balance in the gut affected by dysbiosis and improve GI and core symptoms in individuals with ASD. The most common ways to change the gut microbiome are antibiotics, antifungals, probiotics, synbiotics, prebiotics, diet, fecal microbiota transplant, and microbiota transplant therapy.119,120 Some of these approaches are promising not only in the treatment of GI symptoms among people with ASD but also in alleviating other ASD-related symptoms.
Antibiotics
Antibiotics alter the gut microbial balance by suppressing the growth of many gut bacteria, including beneficial ones.121,122 Vargason et al. found that early exposure to antibiotics may result in a rise in recurring GI symptoms,16 suggesting that they may contribute to GI and possibly ASD symptoms by disrupting the gut microbiota. Many other studies have reported increased antibiotic use in children with autism versus typically developing children, especially for treating ear infections.38,123,124 Studies have shown that long-term antibiotic exposure during pregnancy or early infancy has been associated with ASD.125,126 The increased use likely increased their risk of developing gut microbiota imbalances, and possible enrichment of antibiotic-resistance genes in the microbiota, which may increase the individual’s vulnerability to infections127 leading to recurring GI disorders.37
A survey study of over 27,000 autism families reported that antibiotics were much more likely to result in the worsening of symptoms (33%) than improving symptoms (18%), with 2507 families reported.128 However, a clinical trial by Sandler et al.55 showed that oral vancomycin treatment for 8 weeks (much longer than the standard 10 days) resulted in temporary beneficial changes in GI and ASD symptoms. However, most improvements were lost within a few weeks when the treatment stopped. Oral vancomycin acts only on gut bacteria because it has minimal absorption into the rest of the body, therefore, strongly suggesting that alterations in the gut bacteria were the primary cause of the GI symptoms and contributed substantially to ASD symptoms. However, the temporary nature of the benefits suggests that harmful bacteria quickly regrew after treatment stopped. Similarly, a case series reported by Kuhn et al. showed that the administration of amoxicillin for six months to five children diagnosed with ASD and Lyme disease improved their speech, eye contact, and sleep behaviors and led to a reduction in repetitive behaviors.129 A possible explanation for the differences in the findings of Sandler et al. and Kuhn et al. can be attributed to the selection of antibiotics, length of treatment and dosages, and differences in the assessment tools used to evaluate outcomes. Limitations to these studies include the sample size used for the trials, lack of control groups, lack of microbiota analysis, and the lack of prior knowledge on the mechanism of action of the selected antibiotics on the microbiota-gut-brain connection.
Vancomycin, a glycopeptide antibiotic primarily targeting gram-positive bacteria, can alter the gut microbiota significantly by affecting major bacterial phyla while sparing certain taxa linked to clinically relevant infections. In the seminal study by Sandler et al.,55 vancomycin showed temporary improvements in children with ASD, highlighting the potential role of gut microbial modulation. However, antibiotics alone cannot reverse dysbiosis or sustain improvements long term. Vancomycin has also been used as a pre-treatment for microbiota transplants54 with the hope that the benefits of vancomycin recorded by Sandler et al.55 are preserved while the disruption of indigenous microbiota by vancomycin enhances the engraftment of donor bacteria. Long-term use of vancomycin may induce undesirable shifts in microbial composition, such as an increase in Proteobacteria (gram-negative bacteria), potentially posing additional risks.
Future studies of antibiotics with a narrower spectrum that target primarily harmful bacteria with less effect on beneficial bacteria may be very useful. Also, research with randomized control trials, larger sample sizes, designed follow-up points, microbiota, and metabolites analyses might help identify the mechanism of action of specific antibiotics on the gut-brain axis and could provide physiological insights into their efficacy.
Antifungals
In six studies, increased abundance of fungi or yeast (primarily C. albicans) has been reported in 25–58% of children with ASD at rates substantially higher than in typically developing controls.10,25,34,130–132 The overgrowth of fungi in ASD can be attributed to the increased use of antibiotics and lower bacterial diversity,133 which may allow opportunistic fungi/yeast like Candida to grow and dominate in the GI tract. Candida colonization is associated with inflammation and increased levels of inflammatory markers, such as cytokine IL-17.134 It has been reported that an increased abundance of C. albicans in ASD children correlates with a decreased abundance of beneficial bacteria Faecalibacterium prausnitzii and fiber consumer commensal Prevotella copri.135 Additionally, C. albicans have been observed with a bimodal distribution, and a higher relative abundance of Candida was associated with worse ASD symptoms.136 A national survey of over 27,000 autism families reported that two antifungals, Nystatin and Diflucan, had the highest reported benefit of any medication for ASD, with 62% and 55% reporting improvements in ASD symptoms and only 5% reporting worsening of symptoms.128 A small open-label clinical trial found that treating intestinal yeast overgrowth in children with ASD with antifungals led to some clinical improvements and reduced yeast metabolites.137 One of the limitations of these studies is the lack of data on the effect of the treatment on the bacterial community and the characterization of the fungal community to identify the changes in the fungal community. Data on long-term follow-up after treatment is also lacking in these studies. Overall, these studies suggest that intestinal fungi/yeast are common in children with ASD and may contribute to their ASD symptoms, and treatment with antifungals may be beneficial. However, future research with more rigorous antifungal studies is needed to determine which fungal species and subtypes of yeast or fungi are elevated in autism and how antifungal drugs can be used or modified to produce results that have a greater therapeutic action and also help to understand the mechanism of action of these antifungals. Also, future research should focus on the possibility of including dietary interventions to enhance the effect of antifungal drug therapy.
Probiotics and synbiotics
Probiotics are microorganisms considered beneficial for gut health.138 Probiotics have emerged as a candidate microbiota-targeting intervention due to their safety and widespread acceptance.139,140 Synbiotics are probiotics combined with prebiotics;141 prebiotics are compounds that feed beneficial gut bacteria or probiotics. The results of clinical trials performed with probiotic and synbiotic supplementation interventions in ASD, that included microbiome analysis, are summarized in Table 1. The studies in the table are focused on trials that reported the effect on GI symptoms, ASD symptoms, and changes to gut microbiota communities. Also, all the studies reported in Table 1 are preliminary; they used different formulations of probiotic strains, synbiotics, and dosing regimens.
In a randomized, double-blind, placebo-controlled pilot trial, 35 individuals with ASD aged 3–20 years were randomly assigned to a treatment or placebo group. The trial consisted of two stages. In the first stage, the treatment group received the oral probiotic L. plantarum PS128 (probiotic only), while the placebo group received an oral placebo (placebo only) for 16 weeks. In the second stage, both groups received intranasal oxytocin (probiotic + intranasal oxytocin and placebo + intranasal oxytocin), and the trial proceeded for 28 weeks. The treatment group showed significantly more improvements in ASD symptoms after receiving L. plantarum PS128 and intranasal oxytocin compared to the placebo group. The authors also reported a greater increase in Roseburia, Veillonella, and Streptococcus bacteria.142 In another randomized double-blinded control trial carried out by Guidetti et al. consisting of 61 ASD participants (age 24 months–16 years), the authors reported improvement in GI symptoms, communication, and maladaptive behaviors, as well as an increase in beneficial bacteria, such as Streptococcus thermophilus and Bifidobacterium longum, and a decrease in species, such as Alistipes finegoldii, Clostridium leptum, and Ruminococcus calldus .143 It’s important to clarify that increased fecal abundance of some probiotic strains, like Streptococcus thermophilus and Bifidobacterium longum, come directly from the probiotic supplement and likely represent only a transient alteration in the native gut microbiota. However, a randomized control trial by Sherman et al. found no change in ASD symptom severity and GI symptoms but reported a significant increase in Lactobacillus.144 An open-label study by Shaaban et al. involved 30 ASD children found improvement in GI symptoms and ASD symptoms in addition to an increase in Bifidobacterium and Lactobacillus determined using quantitative PCR.145 Overall, these probiotic studies suggest several probiotic species may benefit ASD. However, most probiotics do not colonize and need to be taken daily.
Interventions with synbiotic supplementation are reported in Table 1. Li et al. recently published an open-label study consisting of 53 (age 3–12 years) children with ASD and 45 age-matched typically developing children.146 The authors reported significant improvement in ASD symptoms measured by Childhood Autism Rating Scale (CARS) scores and GI symptoms measured by Gastrointestinal Symptom Rating Scale (GSRS), and an increased abundance of Bifidobacterium animalis, Akkermansia muciniphila, and Fusicatenibacter saccharivorans, as well as a decrease in Blautia obeum. Another open-label synbiotic study by Phan et al. showed no improvement in the Social Responsiveness Scale (SRS2). However, there was an improvement in GI symptoms and increase in beneficial microbes, such as Bifidobacterium and Lactobacillus species.57 Wang et al. also observed a reduction in the severity of autism and improvements in GI symptoms compared to the placebo group, a significant increase in beneficial bacteria Bifidobacteriales and a decrease in Clostridium after probiotic intervention after synbiotic trial.147 Sanctuary et al. used Bifidobacterium infantis and colostrum, involving 8 ASD participants (2–11 years), and reported improvement in GI symptoms and aberrant behaviors.148 Arnold et al. also enrolled 10 ASD participants (3–12 years) and found improvement in GI symptoms and quality of life of the participants. Sanctuary et al. and Arnold et al. found no significant changes in the microbiome. A recently published case study by Aldegheri et al. about a 17-year-old male who received probiotics and 250 mg of human milk oligosaccharides (HMO) for 6 months saw improvement in behavior, such as aggressiveness and mood stability, and a decrease in the bacteria Sutterella species.149
Overall, these studies used different probiotics and synbiotics and generally found good tolerance and some benefit in GI and ASD symptoms compared to the baseline, with the randomized control trials (RCT) studies being more conclusive. In addition to improvement in ASD and GI symptoms, there was an increase in beneficial bacteria, such as Bifidobacteria and Lactobacilli, in some of the studies,57,143,145–147 which could potentially be attributed to their presence in the probiotic supplementation. Other genera, such as Coprococcus and Roseburia, were increased, and a decrease in bacteria, such as Clostridium, in some of the studies. One of the studies found an increase in specific bacteria, such as Ligilactobacillus salivarius and Bifidobacterium longum, which were part of the probiotics mixture used in the study.143
The current evidence for the efficacy of probiotics and synbiotics in ASD is inconsistent and complex because treatment across these studies is variable, with different formulations, dosages, lengths of treatment, and administration methods. Also, as seen in Table 1, only a few studies clearly stated their primary and secondary outcome measures,57,142,145–147,150 and only one study reported effect size150 after treatment. None of the studies investigated attempts to optimize dosage. Also, none of the studies presented in Table 1 involved post-treatment follow-up assessments, which makes it unclear if there were lasting benefits. However, it is likely that most of these effects were temporary and would require constant treatment to maintain benefits. Because different studies used different evaluation tools, which treatments were most/least effective is also unclear. To enhance improvement, future research should focus on normalizing ASD assessments for better inter-study comparisons, optimizing dosages, and identifying gut commensals linked to improved symptoms for developing future probiotics or microbial biomarker-based assessment metrics.
Some drawbacks of studies looking at probiotics/synbiotics as an intervention targeting the gut microbiota include the following: (1) It is unclear how one or a few bacterial strains can alter significantly dense gut microbial communities consisting of approximately 500 bacterial strains. Moreover, considering the microbiome variability among individual patients, it is difficult to expect consistent benefits. Microbial communities are very complex, and this complexity highlights the need for further research into the mechanisms through which probiotics influence microbial ecosystems and their potential to provide consistent therapeutic outcomes. (2) Long-term effects of repeated treatment on the gut microbiome are unknown. (3) the studies did not report on whether the content of the probiotics was verified to match the label prior to use in the clinical trials, which may be of concern when dealing with live bacteria, whose viability can be greatly affected by shipping and storage conditions. And (4) there was a lack of reports on participants who were unresponsive to the probiotics/synbiotics interventions; because most of these interventions are often supported by the manufacturers, and due to the industry interest, participants with negative results may remain unreported.
Diet and prebiotics
Dietary interventions are major modulating factors of the intestinal microbiota,151 and dietary changes are commonly used for treating ASD-related symptoms.152 However, the many clinical trials of dietary interventions for ASD have generally not evaluated the effect on the microbiome, so we do not discuss them here. Yap et al. looked at the microbiome of 247 children, 99 with ASD, 51 related controls (siblings), and 97 unrelated controls.153 One of their conclusions was that dietary preferences drive differences between the gut microbiota of children with ASD and typically developing ones. This publication adds important knowledge concerning diet and the microbiome. However, we disagree with the main conclusion of this paper, which states that most or all the differences in microbiome composition reported previously are only because of diet and that there is no actual link between the gut microbiome and ASD. Diet is one of the factors that could differentiate the microbiome of ASD and TD due to the unique preferences in diets that are common in the ASD population; however, other factors, such as C-section birth, shorter duration of breastfeeding, excessive use of antibiotics,39,40 and host genetics,42 have also been identified to explain differences in the microbiome. A meta-analysis by Morton et al. showed that diet explained 3% of the variation in the microbiome of ASD and TD controls.41
Diet is known to affect the composition of gut microbiota. However, the responsiveness of individuals to different diets is expected to be heterogeneous, as how diets effect the microbiome depends on various factors, such as the existing composition of the microbiota and its metabolic activity, and extrinsic (lifestyle, medication) and intrinsic (immune and metabolic regulations) factors. Short-term dietary interventions can alter microbial diversity154; however, these alterations are transient, and whether long, prolonged dietary changes can induce permanent changes in the microbiota is unknown due to the lack of long-term follow-ups of short-term dietary interventions or lack of long-term human dietary interventions. Interventions with dietary approaches, such as organic substances (e.g., prebiotics), can selectively promote the metabolism and proliferation of beneficial microorganisms, thereby improving host health.
Regarding clinical trials of prebiotics in ASD described in Table 1, Inoue et al. found a significant reduction in constipation, behavioral irritability, and an increase in defecation per week, with a significant increase in Blautia and Acidaminococcus bacteria, while Streptococcus, Odoribacter, and Eubacterium decreased after treatment.155 A randomized, double-blind, placebo-controlled trial by Grimaldi et al. found that participants who received Bimuno galactooligosaccharide (B-GOS) treatment compared to placebo resulted in significant improvement in GI symptoms (abdominal pain and bowel movements), ASD symptoms (social behavior) and significant increase in beneficial microbes, such as Coprococcus spp., and Dorea formicigenerans.156 These two studies suggest that different prebiotics may benefit ASD, with the randomized control trial (RCT) on B-GOS being more conclusive. Despite these two studies demonstrating their benefits in improving ASD and GI symptoms, the results of their impact on specific microbes differed. This could be due to differences in the prebiotic products, heterogeneity in the ASD population, and their microbiota composition. As prebiotics are meant to enhance the proliferation of beneficial microbes, their impact on the microbiota also depends on the individual existing microbiota composition. This could also explain the differences observed in the impact of prebiotics on the microbiome.
Fecal microbiota transplantation (FMT) and microbiota transplant therapy (MTT)
Fecal microbiota transplantation (FMT) is the most aggressive approach to modifying the microbiome. FMT is transplanted into patients to restore a healthy microbiota composition and function.157,158 FMT involves transplanting a consortium of gut microbiota from a well-screened healthy donor into a patient to modify their gut microbiota.159 Certain microbial species contribute to unfavorable gut microbiota and are linked to disease, and FMT aims to replace those unfavorable resident gut microbiota with favorable microbiota from a healthy donor. In brief, the preparation of FMT material typically involves screening donors for past and current GI issues, chronic illnesses, and infectious diseases, such as HIV and hepatitis. The microbiota can be delivered to the recipient through various techniques, such as enema, colonoscopy, nasal-gastric tubes, and orally (liquid, powder, or capsules).160 Currently, FMT is primarily used to treat Clostridioides difficile infections (CDI),161–164 where a patient is repeatedly treated with antibiotics to deplete their indigenous intestinal microbiota to clear the CDI infection. It mostly involves 1–2 administrations of intestinal microbiota from a healthy donor to restore the microbiota and offer resistance to CDI infection.157,158,165,166
Microbiota transplant therapy (MTT), a treatment derived from FMT, involves more intensive protocols to achieve donor engraftment and clinical benefit. Different elements of MTT use in ASD include pre-conditioning with antibiotics, bowel cleansing, and repeated administration of donor microbiota.167,168 Microbiota transfer therapy was used initially by Kang et al.54 However, this term is being replaced with microbiota transplant therapy as this term transplant recognizes the need for human donors and the engraftment of donor microbiota into recipients. From this point forward, we will refer to FMT studies that include antibiotic pre-treatment or multiple microbiota administrations as microbiota transplant therapy (MTT), rather than the general term FMT. Research into the effectiveness of MTT in treating individuals with ASD is still in its early stages. However, there is evidence that shows that MTT could aid in alleviating ASD and GI symptoms.
MTT studies for autism are summarized in Table 2. The first MTT study for ASD was an open-label clinical trial by Kang et al.54 with 18 children aged 7–16 years with ASD and gastrointestinal symptoms. The study found an initial 80% reduction in GI symptoms and a 23% improvement in ASD symptoms. These improvements were retained eight weeks after MTT treatment. A follow-up study at 2 years post-treatment found that most of the improvement in GI symptoms remained (59% reduction compared to baseline), and autism symptoms (Childhood Autism Rating Scale [CARS]) had continued to improve, with a 47% reduction compared to baseline.169 The study also found an increase in gut microbiota diversity, beneficial microbes (e.g., increased relative abundance of Prevotella, Desulfovibrio, and Bifidobacteria), functional genes, and a shift of microbial balance toward the microbiota composition of neurotypical children.31,54,169 The authors also showed a shift after MTT in plasma metabolite profiles, which became more similar to TD controls. In feces, levels of p-cresol sulfate decreased and became similar to levels in TD controls.31,73,170,171 This study demonstrated the potential of MTT as a therapeutic with long-term benefits for children with GI disorders.
Li et al. investigated the effects of a 4-week MTT protocol (administered 1×/week) with an eight-week follow-up involving 40 children with ASD (age 3–17 years) with GI symptoms and 16 age- and sex-matched TD children without GI problems in an open-label trial.172 Just as in Kang et al.54 the treatment involved a bowel cleanse where participants received polyethylene glycol before the donor microbiota capsules administration, but no antibiotics were supplied before treatment. Li et al. found that after MTT, children showed noticeable improvement in GI symptoms (35% decrease) and a 10% decrease in CARS scores after four weeks of treatment. They reported a shift in the bacterial community of ASD patients toward that of the TD controls, a significant decrease in Eubacterium coprostanoligenes bacteria abundance after MTT treatment, and a significant change in serum levels of neurotransmitters, such as GABA and 5-hydroxytryptamine (5-HT), which decreased after 4 weeks of MTT treatment but did not change further during the eighth week follow-up. GI and ASD improvements mostly continued at 8 weeks post-treatment, with some partial loss of benefit.172 The authors suggested that MTT may improve GI and ASD symptoms with extended treatment. A recently published study by Wang et al. also found significant improvement in GI and ASD symptoms in addition to a decrease in 5-hydroxyindoleacetic acid (5-HIAA) levels after MTT treatment.173
An open-label study by Li et al. investigated the effects of lyophilized donor microbiota (administered once every 4 weeks for 12 weeks) on 38 ASD children (age 3-14 years) with GI issues and 30 sex-matched TD children without GI problems – with an eight-week follow-up.174 There was no antibiotic treatment or a bowel cleanse before treatment. After MTT, children showed improvement in GI symptoms (51% decrease), a 10% decrease in CARS, and a 20% decrease in ABC scores. The authors reported a significant increase in Eubacterium_hallii_group, Anaerostipes, Fusicatenibacter, Collinsella, Ruminococcus_torques_group, and Dorea, and a decrease in the abundance of Blautia, Prevotella, and Sellimonas.
Chen et al. recently published an open-label study consisting of 29 ASD patients (aged 2–11 years) with GI symptoms and 36 TD children.175 The ASD children received two capsules of freeze-dried microbiota orally for 12 days per month for 4 months. The authors reported improved ABC, CARS, and GI symptoms and decreased Collinsella after treatment.
A case study by Hu et al. reported on a 7-year-old female with ASD treated with MTT.176 The child was pretreated with vancomycin antibiotic treatment for 2 weeks and a bowel cleanse prior to fecal donor microbiota (Table 2). The child received 80 ml of donor microbiota five times, each time separated by 1 week via colonoscopy. The core symptoms of ASD (assessed by ABC, CARS, SRS, ATEC) decreased after vancomycin treatment and further decreased after donor microbiota administration. The child’s gastrointestinal (GI) symptoms also significantly improved. After treatment, they also found that the child’s microbial diversity significantly increased with a significant increase in Bacteroides and Ruminococcus while Bifidobacterium, Anaerostipes, Streptococcus, and Faecalibacterium decreased. After treatment, the study showed a significant increase in SCFA, such as butyric acid.
Another case study was recently published by Hazan et al. about a 19-year-old male who received MTT from his typically developing female sibling.177 The patient received vancomycin treatment for ten days and a deep colonic wash prior to treatment, followed by a single dose of donor microbiota (300 mL) infused directly via colonoscopy (Table 2). After MTT treatment and during the follow-up, the patient experienced a significant improvement in behavioral and GI symptoms. The patient’s microbiome diversity also significantly increased after MTT, with an increase in Bifidobacterium and a decrease in Lactobacillus animals after treatment; these changes were maintained for at least during the 15-months.
The MTT studies described above suggest they can relieve not only gastrointestinal symptoms (e.g., constipation, diarrhea, indigestion, abdominal pain, and reflux) but also behavioral symptoms. However, these studies were open-label. Rigorous randomized, double-blind, placebo-controlled studies are needed to validate these findings. The Kang et al.54,169 studies showed short- and long-term improvements in ASD and GI symptoms, and Hazan et al. also found short and long-term benefits.177 The study by Li et al. found that benefits mostly continued at 8 weeks post-treatment but with some modest loss of benefit.172 A possible explanation for these different findings could be the different doses and treatment regimens used in each study (Table 2); for example, the Li et al. study did not involve pre-treatment with vancomycin,172 unlike the Kang et al.54,169 and Hazan et al.177 studies. The studies also showed that MTT changed intestinal microbiota composition. MTT led to an increase in microbial diversity,31,54,169,176,177 which is important because higher gut microbiota diversity is considered healthier in the context of the human gut. However, this is in contrast with Li et al., who did not find a significant change in microbial diversity.172 In addition to the increase in microbial diversity, Kang et al.54,169 and Hu et al.176 found that the treatment led to a shift in the microbial composition of the ASD participants to resemble that of the TD controls. Comparing a MTT study by Hazan et al., where a single dose of donor microbiota administration\was used,177 and Kang et al.,54 where multiple administrations of donor microbiota was used, changes in the microbiota were observed in both studies. However, the specific microbes that change in abundance differ, and Kang et al. reported a longer follow-up.
Variations in the findings of specific differences in the gut microbes after MTT treatment were also observed, which may be attributed to differences in donors, recipients, antibiotics use, cohort/geography, diet, treatment regimens, and donor microbiota preparation procedures. In the case of differences in the donors, studies have suggested that microbiome engraftment and clinical success depend on donor factors, such as the microbial composition of donor samples and successful engraftment of the donor microbes,178,179 and recipient factors, such as host genetics and diet. A recent paper by Chen et al. showed that a donor-recipient match indicated a likelihood of strain transfer and interactions between the donor and recipient microbes in ASD, which is important for species transfer and positive clinical outcomes in ASD.175 This evidence suggests the possibility of engraftment of donor microbiota as a factor that might have enhanced the treatment success for ASD. However, in the studies described above, engraftment of donor microbiota after MTT treatment were not reported. More precise engraftment measurements need to be incorporated into MTT investigations in ASD. Potential risks associated with MTT include the possibility of donors transferring opportunistic pathogens or multidrug-resistant organisms to recipients or causing infections. However, this can be avoided by carefully selecting and screening donors and their feces. Kang et al. found beneficial impacts of MTT continuing 2 years after completion of MTT treatment.169 However, more studies are needed to understand the long-term effects of MTT on ASD and the gut microbiome.
Furthermore, identifying an ideal “best personalized donor” whose microbiome produces metabolites tailored to meet the specific needs of a recipient based on the recipient health condition, genetics, existing microbiome composition, and donor-recipient compatibility could pave the way for MTT guided by precision medicine. Such an approach would enable interventions that are customized to individual differences, maximizing therapeutic benefits for ASD. Despite the promising effect of MTT intervention from these open-label studies, further research studies with randomized, double-blind, placebo-controlled studies are needed with a larger cohort to elucidate further their effect on ASD.180–183 Four randomized, double-blind, placebo-controlled clinical trials are expected to be completed in 2024 and 2025: (1) Trial no.: NCT03408886 and NCT04182633: these trials, expected to be completed in 2024, involve (a) adults (age 18–60 years) with ASD and GI issues and (b) children with ASD and GI issues. The treatment includes a 2-week vancomycin treatment, followed by 1-day bowel cleanse, 8-week MTT treatment for adults, and 12-week MTT treatment for children. Follow-up evaluations at 6, 12, and 18 months will be conducted. (2) Trial no.: ChiCTR2100043906 has started in China, involving 318 children (age 3–6 years) with ASD and GI issues, with results anticipated by the end of 2024. Participants will receive a 12-day treatment repeated every month for 4 months, followed by a 2-month follow-up.184 (3) Trial no.: ACTRN12622000015741 is expected to be completed by the end of 2025 in Australia. It includes 100 ASD adolescents and adults (aged 16–45 years) with mild to severe GI symptoms. The treatment involves an overnight bowel cleanse, 1–2 days of MTT treatment, and follow-up assessments at 6, 12, and 26 weeks post-treatment.185 (4) Trial no.: ChiCTR2200058459 involving 42 ASD children (age 3–9 years) in China. Treatment involves a bowel cleanse, a 12-week treatment period followed by a 12-week follow-up.186
Although research on MTT trials in ASD has been conducted, the available evidence is still insufficient, as all of the published studies to date are open-label.
The microbiota includes about several hundred bacterial species, as well as fungi and bacteriophages, and thus can reconstruct the gut microbial ecology,187 which regulates the bacterial community and also impacts the host immune system.188 As summarized in Figure 2, Findings from the reviewed studies suggest that interventions targeting the microbiome have some potential to address ASD symptoms and GI issues and have an effect on the microbiome composition in ASD. MTT seem the most promising of all the microbiome targeting interventions. MTT provide an entire set of gut microbiota from well-screened healthy donors. Probiotics may offer some health benefits but are limited to only introducing a small number of bacterial species most often cultured from non-human origins. Prebiotics may influence the growth of specific types of microbes, but some prebiotics may enhance the growth of harmful and beneficial bacteria. On the other hand, the disadvantages of the MTT approaches are the high costs of screening and production and the possibility of disease transmission with the transplanted material; the latter is minimized with careful screening.
Figure 2.
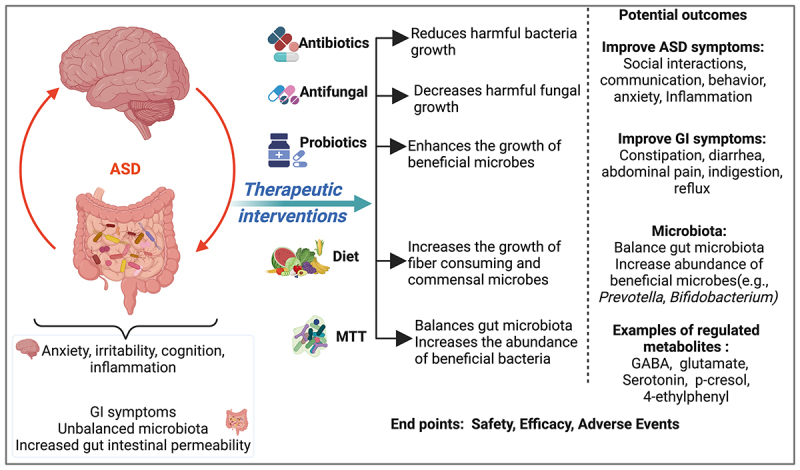
Summary of microbiota interventions targeting the gut microbiome and possibly GI and ASD symptoms outcomes.
Regarding safety and adverse effects, most intervention studies reported few to no adverse effects, suggesting that they may be safe and tolerable. For instance, MTT treatment has generally been associated with few adverse reactions, suggesting it is safe and well-tolerated in most cases. Kang et al.54 found that 5%, 39%, and 28% of participants suffered from mild to moderate rashes, hyperactivity, and tantrums/aggression, respectively, at the start of vancomycin pre-treatment, and lasting only a few days, but the microbiota was well-tolerated. In the study by Li et al.,172 short-term adverse effects were noted, including fever (3.7%), hyperactivity (11.4%), and tantrums/aggression (3.7%). In the probiotic studies, Shaaban et al. reported that all adverse effects were mild and transient during the probiotic intervention.145 In general, probiotics, prebiotics, synbiotics, and MTT appear relatively safe and seem to be a helpful approach for the alleviation of GI discomfort in children with ASD for the short and long term, in addition to shifting gut microbiota features toward that of neurotypicals and improving patient quality of life. However, antibiotics appear to have only short-term benefits, and overuse of antibiotics may have contributed to worse GI and ASD symptoms.
Future perspectives of MTT studies in ASD
Emerging research into therapeutic interventions for the treatment of ASD symptoms is centered on microbial-based therapies, such as MTT, which have demonstrated potential in safely reducing ASD and GI symptoms and improving the microbiota. FMT therapy from a healthy donor to a patient is a proven treatment for C. difficile, and to enhance its efficacy, the American Gastroenterological Association (AGA) has developed guidelines.189 FMT is a well-established treatment for recurrent C. difficile infections (rCDI), often requiring just one or two doses to restore microbial balance. Given its success in reestablishing gut microbiota in rCDI, researchers have explored its potential in other conditions, including ASD. However, ASD is a more complex and clinically distinct condition with different microbiota alterations. As a result, this requires the use of MTT. MTT is a more sophisticated treatment that requires a pre-treatment with antibiotics, multiple doses and/or extended treatment durations to achieve lasting effects.
MTT hold promise as effective interventions for ASD. However, their application has many challenges, including donor selection criteria and factors, such as the microbial composition of donor samples, successful engraftment of the donor microbes, age of donor and donor-recipient compatibility, and host response.175,190 Other challenges include a lack of standardized assessment tools, comprehensive safety evaluations, an ideal dosing regimen, and a lack of standardization in the preparation and delivery.191
Some of the MTT studies described here did not include longer follow-ups. It is appropriate to incorporate longer follow-ups of clinical courses to further determine the interventions’ efficacy and safety. Further research and clinical studies are needed to identify the optimal treatment, dosing, and duration of MTT therapies, in addition to an in-depth understanding of the types of gut microbiota that may ameliorate ASD symptoms.
Future strategies to enhance the efficacy and long-term success of MTT in ASD treatment should focus on several key areas and for that, possible recommendations are: 1) Optimizing donor-recipient matching through microbiome-metabolomic profiling, host genetics, immune system compatibility, and machine learning methods may improve treatment outcomes by ensuring that the introduced microbial communities are more likely to engraft and persist. 2) Identifying reliable biomarkers for assessing intervention success is essential for tracking microbiota restoration and clinical improvements in ASD-related symptoms. 3) Incorporating dietary interventions or prebiotics alongside MTT may enhance the colonization and stability of beneficial microbes in the gut. Future trials should integrate dietary strategies to support sustained microbial and metabolic improvements before, during and after MTT administration. 4) Developing standardized ASD assessment tools is critical for ensuring consistency and comparability across clinical studies. The heterogeneity in ASD symptomatology underscores the need for validated, universally accepted outcome measures that accurately capture both GI and ASD symptoms improvement following MTT interventions. 5) Establishing standardized protocols for MTT preparation and administration in ASD-related research will enhance reproducibility and clinical translation. While some studies, such as Kang et al., have utilized standardized donor microbiota preparations under Good Manufacturing Practice (GMP) guidelines, the field would benefit from more comprehensive standardization, including dosage optimization, duration of treatment, and pre/post-treatment short and long-term monitoring. Establishing consensus guidelines will facilitate more rigorous clinical trial designs and help define best practices for future research in this area.
As MTT research in autism advances, it is essential to evaluate its safety and effectiveness thoroughly, and some of these trials are already underway.
Summary
The microbiota-gut-brain axis is important in gastrointestinal symptoms and neurodevelopmental dysfunctions in ASD patients. Meta-analyses of gut microbiota in ASD have revealed substantial differences between ASD and TD children, with great heterogeneity in the ASD group. Antibiotic therapies may have only temporary effects, and overuse of antibiotics may have contributed to worse GI and ASD symptoms. In contrast, antifungals, probiotics, prebiotics, synbiotics, and MTT appear safe and possibly beneficial, with MTT leading to long-term improvements in some studies. More research is needed to determine if optimal probiotics, prebiotics, and synbiotics and their dosage can have better and longer-lasting effects on the gut microbiome and ASD symptoms. Randomized clinical trials of antifungals (with pre-screening to select participants with high levels of fungi) are needed to determine the effect of treatment on ASD-related symptoms. Future research is also needed on randomized double-blinded control trials of MTT to evaluate its safety and efficacy fully. Additionally, personalized treatment plans that consider individual microbial profiles, diet, and genetic backgrounds could enhance the effectiveness of these interventions. The potential of these interventions to offer a holistic approach to managing ASD symptoms underscores the importance of continued research and development in this field.
Funding Statement
This work was supported by ASU Biodesign Center for Health Through Microbiomes, Flinn Foundation, AZ [grant #23-12139] and Autism Research Institute, CA.
Disclosure statement
K.N., J.B.A. and R.K.-B. have pending/approved patents for autism biomarkers and the use of FMT for various conditions, including autism. J.B.A. and R.K.-B are co-founders of Autism Diagnostics LLC and Gut-Brain Axis Therapeutics. E.T declares no competing interests.
Data availability statement
Data sharing is not applicable for this study.
References
- 1.Young S, Hollingdale J, Absoud M, Bolton P, Branney P, Colley W, Craze E, Dave M, Deeley Q, Farrag E.. Guidance for identification and treatment of individuals with attention deficit/hyperactivity disorder and autism spectrum disorder based upon expert consensus. BMC Med. 2020;18(1):146. doi: 10.1186/s12916-020-01585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC . Data and statistics on autism spectrum disorder | CDC. Centers for disease control and prevention. 2023. May 12 [accessed 2023 Nov 2]. https://www.cdc.gov/ncbddd/autism/data.html.
- 3.Hirota T, King BH. Autism spectrum disorder: a review. JAMA. 2023;329(2):157. doi: 10.1001/jama.2022.23661. [DOI] [PubMed] [Google Scholar]
- 4.Cheroni C, Caporale N, Testa G. Autism spectrum disorder at the crossroad between genes and environment: contributions, convergences, and interactions in ASD developmental pathophysiology. Mol Autism. 2020;11(1):69. doi: 10.1186/s13229-020-00370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masini E, Loi E, Vega-Benedetti AF, Carta M, Doneddu G, Fadda R, Zavattari P. An overview of the main genetic, epigenetic and environmental factors involved in autism spectrum disorder focusing on synaptic activity. Int J Mol Sci. 2020;21(21):8290. doi: 10.3390/ijms21218290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han VX, Patel S, Jones HF, Dale RC. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat Rev Neurol. 2021;17(9):564–25. doi: 10.1038/s41582-021-00530-8. [DOI] [PubMed] [Google Scholar]
- 7.Fattorusso A, Di Genova L, Dell’isola G, Mencaroni E, Esposito S. Autism spectrum disorders and the gut microbiota. Nutrients. 2019;11(3):521. doi: 10.3390/nu11030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Micai M, Fatta LM, Gila L, Caruso A, Salvitti T, Fulceri F, Ciaramella A, D’Amico R, Del Giovane C, Bertelli M, et al. Prevalence of co-occurring conditions in children and adults with autism spectrum disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2023;155:105436. doi: 10.1016/j.neubiorev.2023.105436. [DOI] [PubMed] [Google Scholar]
- 9.Leader G, Abberton C, Cunningham S, Gilmartin K, Grudzien M, Higgins E, Joshi L, Whelan S, Mannion A. Gastrointestinal symptoms in autism spectrum disorder: a systematic review. Nutrients. 2022;14(7):1471. doi: 10.3390/nu14071471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism – comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11(1):22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bresnahan M, Hornig M, Schultz AF, Gunnes N, Hirtz D, Lie KK, Magnus P, Reichborn-Kjennerud T, Roth C, Schjølberg S, et al. Association of maternal report of infant and toddler gastrointestinal symptoms with autism: evidence from a prospective birth cohort. JAMA Psychiarty. 2015;72(5):466. doi: 10.1001/jamapsychiatry.2014.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord. 2014;44(5):1117–1127. doi: 10.1007/s10803-013-1973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies C, Mishra D, Eshraghi RS, Mittal J, Sinha R, Bulut E, Mittal R, Eshraghi AA. Altering the gut microbiome to potentially modulate behavioral manifestations in autism spectrum disorders: a systematic review. Neurosci Biobehav Rev. 2021;128:549–557. doi: 10.1016/j.neubiorev.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson BJ, Dovgan K, Takahashi N, Beversdorf DQ. The relationship among gastrointestinal symptoms, problem behaviors, and internalizing symptoms in children and adolescents with autism spectrum disorder. Front Psychiatry. 2019;10:194. doi: 10.3389/fpsyt.2019.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Restrepo B, Angkustsiri K, Taylor SL, Rogers SJ, Cabral J, Heath B, Hechtman A, Solomon M, Ashwood P, Amaral DG, et al. Developmental–behavioral profiles in children with autism spectrum disorder and co-occurring gastrointestinal symptoms. Autism Res. 2020;13(10):1778–1789. doi: 10.1002/aur.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vargason T, McGuinness DL, Hahn J. Gastrointestinal symptoms and oral antibiotic use in children with autism spectrum disorder: retrospective analysis of a privately insured U.S. population. J Autism Dev Disord. 2019;49(2):647–659. doi: 10.1007/s10803-018-3743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krajmalnik-Brown R, Lozupone C, Kang DW, Adams JB. Gut bacteria in children with autism spectrum disorders: challenges and promise of studying how a complex community influences a complex disease. Microb Ecol Health Dis. 2015;26(8). doi: 10.3402/mehd.v26.26914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Zhou JM. The microbiota–gut–brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience. 2016;324:131–139. doi: 10.1016/j.neuroscience.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Dan Z, Mao X, Liu Q, Guo M, Zhuang Y, Liu Z, Chen K, Chen J, Xu R, Tang J, et al. Altered gut microbial profile is associated with abnormal metabolism activity of autism spectrum disorder. Gut Microbes. 2020;11(5):1246–1267. doi: 10.1080/19490976.2020.1747329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng P, Zhang Y, Zhao Y, Zhao P, Li E. Combined repetitive transcranial magnetic stimulation and gut microbiota modulation through the gut–brain axis for prevention and treatment of autism spectrum disorder. Front Immunol. 2024;15:1341404. doi: 10.3389/fimmu.2024.1341404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16(4):444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Hazan S, Spradling-Reeves KD, Papoutsis A, Walker SJ. Shotgun metagenomic sequencing identifies dysbiosis in triplet sibling with gastrointestinal symptoms and ASD. Children. 2020;7(12):255. doi: 10.3390/children7120255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho LKH, Tong VJW, Syn N, Nagarajan N, Tham EH, Tay SK, Shorey S, Tambyah PA, Law EC. Gut microbiota changes in children with autism spectrum disorder: a systematic review. Gut Pathog. 2020;12(1):6. doi: 10.1186/s13099-020-0346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iglesias-Vázquez L, Van Ginkel Riba G, Arija V, Canals J. Composition of gut microbiota in children with autism spectrum disorder: a systematic review and meta-analysis. Nutrients. 2020;12(3):792. doi: 10.3390/nu12030792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iovene MR, Bombace F, Maresca R, Sapone A, Iardino P, Picardi A, Marotta R, Schiraldi C, Siniscalco D, Serra N, et al. Intestinal dysbiosis and yeast isolation in stool of subjects with autism spectrum disorders. Mycopathologia. 2017;182(3–4):349–363. doi: 10.1007/s11046-016-0068-6. [DOI] [PubMed] [Google Scholar]
- 26.Kang DW, Park JG, Ilhan ZE, Wallstrom G, LaBaer J, Adams JB, Krajmalnik-Brown R. Reduced incidence of prevotella and other fermenters in intestinal microflora of autistic children. PLOS ONE. 2013;8(7):e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kushak RI, Winter HS, Buie TM, Cox SB, Phillips CD, Ward NL. Analysis of the duodenal microbiome in autistic individuals: association with carbohydrate digestion. J Pediatr Gastroenterol Nutr. 2017;64(5):e110. doi: 10.1097/MPG.0000000000001458. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Guo W, Li S, Sun B, Li N, Xie D, Dong Z, Luo D, Chen W, Fu W, et al. Alteration of the gut microbiota profile in children with autism spectrum disorder in China. Front Microbiol. 2024;14:1326870. doi: 10.3389/fmicb.2023.1326870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luna RA, Oezguen N, Balderas M, Venkatachalam A, Runge JK, Versalovic J, Veenstra-VanderWeele J, Anderson GM, Savidge T, Williams KC. Distinct microbiome-neuroimmune signatures correlate with functional abdominal pain in children with autism spectrum disorder. Cellular Mol Gastroenterol Hepatol. 2017;3(2):218–230. doi: 10.1016/j.jcmgh.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma B, Liang J, Dai M, Wang J, Luo J, Zhang Z, Jing J. Altered gut microbiota in Chinese children with autism spectrum Disorders. Front Cell Infect Microbiol. 2019;9:40. doi: 10.3389/fcimb.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nirmalkar K, Qureshi F, Kang DW, Hahn J, Adams JB, Krajmalnik-Brown R. Shotgun metagenomics study suggests alteration an sulfur metabolism and oxidative stress in children with autism and improvement after microbiota transfer therapy. Int J Mol Sci. 2022;23(21):13481. doi: 10.3390/ijms232113481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose DR, Yang H, Serena G, Sturgeon C, Ma B, Careaga M, Hughes HK, Angkustsiri K, Rose M, Hertz-Picciotto I, et al. Differential immune responses and microbiota profiles in children with autism spectrum disorders and co-morbid gastrointestinal symptoms. Brain Behav Immun. 2018;70:354–368. doi: 10.1016/j.bbi.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanlier N, Kocabas Ş. The effect of probiotic, prebiotic and gut microbiota on ASD: a review and future perspectives. Crit Rev Food Sci Nutr. 2023;63(15):2319–2330. doi: 10.1080/10408398.2021.1973957. [DOI] [PubMed] [Google Scholar]
- 34.Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, Jousson O, Leoncini S, Renzi D, Calabrò A, et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 2017;5(1):24. doi: 10.1186/s40168-017-0242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, Ostatnikova D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. 2015;138:179–187. doi: 10.1016/j.physbeh.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 36.Zhang M, Ma W, Zhang J, He Y, Wang J. Analysis of gut microbiota profiles and microbe-disease associations in children with autism spectrum disorders in China. Sci Rep. 2018;8(1):13981. doi: 10.1038/s41598-018-32219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fjalstad JW, Esaiassen E, Juvet LK, Van Den Anker JN, Klingenberg C. Antibiotic therapy in neonates and impact on gut microbiota and antibiotic resistance development: a systematic review. J Antimicrob Chemother. 2018;73(3):569–580. doi: 10.1093/jac/dkx426. [DOI] [PubMed] [Google Scholar]
- 38.Niehus R, Lord C. Early medical history of children with autism spectrum disorders. J Dev Behav Pediatr. 2006;27(Supplement 2):S120–S127. doi: 10.1097/00004703-200604002-00010. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Gutierrez E, Narbad A, Rodríguez JM. Autism spectrum disorder associated with gut microbiota at immune, metabolomic, and neuroactive level. Front Neurosci. 2020;14:578666. doi: 10.3389/fnins.2020.578666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yassour M, Vatanen T, Siljander H, Hämäläinen A-M, Härkönen T, Ryhänen SJ, Franzosa EA, Vlamakis H, Huttenhower C, Gevers D, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8(343). doi: 10.1126/scitranslmed.aad0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morton JT, Jin DM, Mills RH, Shao Y, Rahman G, McDonald D, Zhu Q, Balaban M, Jiang Y, Cantrell K, et al. Multi-level analysis of the gut–brain axis shows autism spectrum disorder-associated molecular and microbial profiles. Nat Neurosci. 2023;26(7):1208–1217. doi: 10.1038/s41593-023-01361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu S, da Cunha AP, Rezende RM, Cialic R, Wei Z, Bry L, Comstock L, Gandhi R, Weiner H. The host shapes the gut microbiota via fecal MicroRNA. Cell Host & Microbe. 2016;19(1):32–43. doi: 10.1016/j.chom.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reyman M, Van Houten MA, Van Baarle D, Bosch AATM, Man WH, Chu MLJN, Arp K, Watson RL, Sanders EAM, Fuentes S, et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat Commun. 2019;10(1):4997. doi: 10.1038/s41467-019-13014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang I, Corwin EJ, Brennan PA, Jordan S, Murphy JR, Dunlop A. The infant microbiome: implications for infant health and neurocognitive development. Nurs Res. 2016;65(1):76–88. doi: 10.1097/NNR.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde E, McCue T, Codelli J, Chow J, Reisman S, Petrosino J, et al. The microbiota modulates gut physiology and behavioral abnormalities associated with autism. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Yang J, Zhang H, Yu J, Yao Z. Oral probiotic administration during pregnancy prevents autism‐related behaviors in offspring induced by maternal immune activation via anti‐inflammation in mice. Autism Res. 2019;12(4):576–588. doi: 10.1002/aur.2079. [DOI] [PubMed] [Google Scholar]
- 47.Abuaish S, Al-Otaibi NM, Abujamel TS, Alzahrani SA, Alotaibi SM, AlShawakir YA, Aabed K, El-Ansary A. Fecal transplant and bifidobacterium treatments modulate gut clostridium bacteria and rescue social impairment and hippocampal BDNF expression in a rodent model of autism. Brain Sci. 2021;11(8):1038. doi: 10.3390/brainsci11081038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avolio E, Olivito I, Rosina E, Romano L, Angelone T, De Bartolo A, Scimeca M, Bellizzi D, D’Aquila P, Passarino G, et al. Modifications of behavior and inflammation in mice following transplant with fecal microbiota from children with autism. Neuroscience. 2022;498:174–189. doi: 10.1016/j.neuroscience.2022.06.038. [DOI] [PubMed] [Google Scholar]
- 49.Goo N, Bae HJ, Park K, Kim J, Jeong Y, Cai M, Cho K, Jung SY, Kim D-H, Ryu JH. The effect of fecal microbiota transplantation on autistic-like behaviors in Fmr1 KO mice. Life Sci. 2020;262:118497. doi: 10.1016/j.lfs.2020.118497. [DOI] [PubMed] [Google Scholar]
- 50.Sharon G, Cruz NJ, Kang DW, Gandal MJ, Wang B, Kim Y-M, Zink EM, Casey CP, Taylor BC, Lane CJ, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177(6):1600–1618.e17. doi: 10.1016/j.cell.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao L, Yan J, Yang T, Zhu J, Li T, Wei H, Chen J. Fecal microbiome transplantation from children with autism spectrum disorder modulates tryptophan and serotonergic synapse metabolism and induces altered behaviors in germ-free mice. mSystems. 2021;6(2). doi: 10.1128/msystems.01343-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilbert JA, Krajmalnik-Brown R, Porazinska DL, Weiss SJ, Knight R. Toward effective probiotics for autism and other neurodevelopmental disorders. Cell. 2013;155(7):1446–1448. doi: 10.1016/j.cell.2013.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen CC, Chiu CH. Current and future applications of fecal microbiota transplantation for children. Biomed J. 2022;45(1):11–18. doi: 10.1016/j.bj.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1):10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Väisänen ML, Nelson MN, Wexler HM. Short-Term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15(7):429–435. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- 56.Abdellatif B, McVeigh C, Bendriss G, Chaari A. The promising role of probiotics in managing the altered gut in autism spectrum disorders. Int J Mol Sci. 2020;21(11):4159. doi: 10.3390/ijms21114159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phan J, Calvo DC, Nair D, Jain S, Montagne T, Dietsche S, Blanchard K, Treadwell S, Adams J, Krajmalnik-Brown R, et al. Precision synbiotics increase gut microbiome diversity and improve gastrointestinal symptoms in a pilot open-label study for autism spectrum disorder. mSystems. 2024;9(5):e00503–24. doi: 10.1128/msystems.00503-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Angelis MD, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, Cristofori F, Guerzoni ME, Gobbetti M, Francavilla R, et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLOS ONE. 2013;8(10):e76993. doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20(2):145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang DW, Ilhan ZE, Isern NG, Hoyt DW, Howsmon DP, Shaffer M, Lozupone CA, Hahn J, Adams JB, Krajmalnik-Brown R. Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe. 2018;49:121–131. doi: 10.1016/j.anaerobe.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Lou M, Cao A, Jin C, Mi K, Xiong X, Zeng Z, Pan X, Qie J, Qiu S, Niu Y, et al. Deviated and early unsustainable stunted development of gut microbiota in children with autism spectrum disorder. Gut. Published online 2021. Dec 20:gutjnl–325115. doi: 10.1136/gutjnl-2021-325115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Srikantha P, Mohajeri MH. The possible role of the microbiota-gut-brain-axis in autism spectrum disorder. Int J Mol Sci. 2019;20(9):2115. doi: 10.3390/ijms20092115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun H, You Z, Jia L, Wang F. Autism spectrum disorder is associated with gut microbiota disorder in children. BMC Pediatrics. 2019;19(1):516. doi: 10.1186/s12887-019-1896-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wan Y, Zuo T, Xu Z, Zhang F, Zhan H, Chan D, Leung T-F, Yeoh YK, Chan FKL, Chan R, et al. Underdevelopment of the gut microbiota and bacteria species as non-invasive markers of prediction in children with autism spectrum disorder. Gut. 2022;71(5):910–918. doi: 10.1136/gutjnl-2020-324015. [DOI] [PubMed] [Google Scholar]
- 65.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of sutterella spp. and ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism. 2013;4(1):42. doi: 10.1186/2040-2392-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie X, Li L, Wu X, Hou F, Chen Y, Shi L, Liu Q, Zhu K, Jiang Q, Feng Y, et al. Alteration of the fecal microbiota in Chinese children with autism spectrum disorder. Autism Res. 2022;15(6):996–1007. doi: 10.1002/aur.2718. [DOI] [PubMed] [Google Scholar]
- 67.Ye F, Gao X, Wang Z, Cao S, Liang G, He D, Lv Z, Wang L, Xu P, Zhang Q. Comparison of gut microbiota in autism spectrum disorders and neurotypical boys in China: a case-control study. Synth Syst Biotechnol. 2021;6(2):120–126. doi: 10.1016/j.synbio.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou R, Xu F, Wang Y, Duan M, Guo M, Zhang Q, Zhao H, Zheng H. Changes in the gut microbiota of children with autism spectrum disorder. Autism Res. 2020;13(9):1614–1625. doi: 10.1002/aur.2358. [DOI] [PubMed] [Google Scholar]
- 69.Andreo‐Martínez P, García‐Martínez N, Sánchez‐Samper EP, Martínez‐González AE. An approach to gut microbiota profile in children with autism spectrum disorder. Environ Microbiol Rep. 2020;12(2):115–135. doi: 10.1111/1758-2229.12810. [DOI] [PubMed] [Google Scholar]
- 70.Borsom EM, Lee K, Cope EK. Do the bugs in your gut eat your memories? Relationship between gut microbiota and alzheimer’s disease. Brain Sci. 2020;10(11):814. doi: 10.3390/brainsci10110814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coretti L, Paparo L, Riccio MP, Amato F, Cuomo M, Natale A, Borrelli L, Corrado G, De Caro C, Comegna M, et al. Gut microbiota features in young children with autism spectrum disorders. Front Microbiol. 2018;9:3146. doi: 10.3389/fmicb.2018.03146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liptak R, Gromova B, Gardlik R. Fecal microbiota transplantation as a tool for therapeutic modulation of non-gastrointestinal disorders. Front Med. 2021;8:665520. doi: 10.3389/fmed.2021.665520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qureshi F, Adams J, Hanagan K, Kang DW, Krajmalnik-Brown R, Hahn J. Multivariate analysis of fecal metabolites from children with autism spectrum disorder and gastrointestinal symptoms before and after microbiota transfer therapy. J Personalized Med. 2020;10(4):152. doi: 10.3390/jpm10040152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu M, Xu X, Li J, Li F. Association between gut microbiota and autism spectrum disorder: a systematic review and meta-analysis. Front Psychiatry. 2019;10:473. doi: 10.3389/fpsyt.2019.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su Q, Wong OWH, Lu W, Wan Y, Zhang L, Xu W, Li MKT, Liu C, Cheung CP, Ching JYL, et al. Multikingdom and functional gut microbiota markers for autism spectrum disorder. Nat Microbiol. [Published online 2024 Jul 8]. 9(9):2344–2355. doi: 10.1038/s41564-024-01739-1. [DOI] [PubMed] [Google Scholar]
- 76.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8(1):51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 78.Fouquier J, Moreno Huizar N, Donnelly J, Glickman C, Kang D-W, Maldonado J, Jones RA, Johnson K, Adams JB, Krajmalnik-Brown R, et al. The gut microbiome in autism: study-site effects and longitudinal analysis of behavior change. mSystems. 2021;6(2). doi: 10.1128/msystems.00848-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nearing JT, Douglas GM, Hayes MG, MacDonald J, Desai DK, Allward N, Jones CMA, Wright RJ, Dhanani AS, Comeau AM, et al. Microbiome differential abundance methods produce different results across 38 datasets. Nat Commun. 2022;13(1):342. doi: 10.1038/s41467-022-28034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sarkar A, Harty S, Lehto SM, Moeller AH, Dinan TG, Dunbar RIM, Cryan JF, Burnet PWJ. The microbiome in psychology and cognitive neuroscience. Trends Cogn Sci. 2018;22(7):611–636. doi: 10.1016/j.tics.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 81.Sinha R, Abnet CC, White O, Knight R, Huttenhower C. The microbiome quality control project: baseline study design and future directions. Genome Biology. 2015;16(1):276. doi: 10.1186/s13059-015-0841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Navarro F, Liu Y, Rhoads JM. Can probiotics benefit children with autism spectrum disorders? World J Gastroenterol. 2016;22(46):10093. doi: 10.3748/wjg.v22.i46.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, Bennett A, Jabado O, Hirschberg DL, Lipkin WI, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLOS ONE. 2011;6(9):e24585. doi: 10.1371/journal.pone.0024585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26(3):383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haba R, Shintani N, Onaka Y, Wang H, Takenaga R, Hayata A, Baba A, Hashimoto H. Lipopolysaccharide affects exploratory behaviors toward novel objects by impairing cognition and/or motivation in mice: possible role of activation of the central amygdala. Behav Brain Res. 2012;228(2):423–431. doi: 10.1016/j.bbr.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 86.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 87.Montiel-Castro AJ, González-Cervantes RM, Bravo-Ruiseco G, Pacheco-López G. The microbiota-gut-brain axis: neurobehavioral correlates, health and sociality. Front Integr Neurosci. 2013;7:7. doi: 10.3389/fnint.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tran SMS, Mohajeri MH. The role of gut bacterial metabolites in brain development, aging and disease. Nutrients. 2021;13(3):732. doi: 10.3390/nu13030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee E, Lee J, Kim E. Excitation/Inhibition imbalance in animal models of autism spectrum disorders. Biol Psychiatry. 2017;81(10):838–847. doi: 10.1016/j.biopsych.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 90.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 91.Shinohe A, Hashimoto K, Nakamura K, Tsujii M, Iwata Y, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara G-I, et al. Increased serum levels of glutamate in adult patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(8):1472–1477. doi: 10.1016/j.pnpbp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 92.El-Ansary A, Al-Ayadhi L. GABAergic/Glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J Neuroinflammation. 2014;11(1):189. doi: 10.1186/s12974-014-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bermudez-Martin P, Becker JAJ, Caramello N, Fernandez SP, Costa-Campos R, Canaguier J, Barbosa S, Martinez-Gili L, Myridakis A, Dumas M-E, et al. The microbial metabolite p-Cresol induces autistic-like behaviors in mice by remodeling the gut microbiota. Microbiome. 2021;9(1):157. doi: 10.1186/s40168-021-01103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Needham BD, Funabashi M, Adame MD, Wang Z, Boktor JC, Haney J, Wu W-L, Rabut C, Ladinsky MS, Hwang S-J, et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature. 2022;602(7898):647–653. doi: 10.1038/s41586-022-04396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Altieri L, Neri C, Sacco R, Curatolo P, Benvenuto A, Muratori F, Santocchi E, Bravaccio C, Lenti C, Saccani M, et al. Urinary p -cresol is elevated in small children with severe autism spectrum disorder. Biomarkers. 2011;16(3):252–260. doi: 10.3109/1354750X.2010.548010. [DOI] [PubMed] [Google Scholar]
- 96.Gabriele S, Sacco R, Cerullo S, Neri C, Urbani A, Tripi G, Malvy J, Barthelemy C, Bonnet-Brihault F, Persico AM. Urinary p-cresol is elevated in young French children with autism spectrum disorder: a replication study. Biomarkers. 2014;19(6):463–470. doi: 10.3109/1354750X.2014.936911. [DOI] [PubMed] [Google Scholar]
- 97.Lawson PA, Citron DM, Tyrrell KL, Finegold SM. Reclassification of clostridium difficile as clostridioides difficile (Hall and O’Toole 1935) Prévot 1938. Anaerobe. 2016;40:95–99. doi: 10.1016/j.anaerobe.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 98.Saito Y, Sato T, Nomoto K, Tsuji H. Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol Ecol. 2018;94(9):fiy125. doi: 10.1093/femsec/fiy125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zuffa S, Schimmel P, Gonzalez-Santana A. Early-life differences in the gut microbiota composition and functionality of infants at elevated likelihood of developing autism spectrum disorder. Transl Psychiatry. 2023;13(1):257. doi: 10.1038/s41398-023-02556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu P, Wu L, Peng G, Han Y, Tang R, Ge J, Zhang L, Jia L, Yue S, Zhou K, et al. Altered microbiomes distinguish alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun. 2019;80:633–643. doi: 10.1016/j.bbi.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 101.Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen M-L, Bolte E, McTeague M, Sandler R, Wexler H, Marlowe E, et al. Gastrointestinal microflora studies in late‐onset autism. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2002;35(s1):S6–S16. doi: 10.1086/341914. [DOI] [PubMed] [Google Scholar]
- 102.Song Y, Liu C, Finegold SM. Real-Time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol. 2004;70(11):6459–6465. doi: 10.1128/AEM.70.11.6459-6465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wong SH, Ying J, Xu X. Gut microbiota in young adults with high-functioning autism spectrum disorder and its performance as diagnostic biomarkers. [Published online 2025 Jan 13.] doi: 10.21203/rs.3.rs-5753373/v1. [DOI] [Google Scholar]
- 104.Gevi F, Belardo A, Zolla L. A metabolomics approach to investigate urine levels of neurotransmitters and related metabolites in autistic children. Biochim Biophys Acta BBA - Mol Basis Dis. 2020;1866(10):165859. doi: 10.1016/j.bbadis.2020.165859. [DOI] [PubMed] [Google Scholar]
- 105.Roussin L, Prince N, Perez-Pardo P, Kraneveld AD, Rabot S, Naudon L. Role of the gut microbiota in the pathophysiology of autism spectrum disorder: clinical and preclinical evidence. Microorganisms. 2020;8(9):1369. doi: 10.3390/microorganisms8091369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu S, Rong Y, Kiang TKL. Effects of p-Cresol on oxidative stress, glutathione depletion, and necrosis in HepaRG cells: comparisons to other uremic toxins and the role of p-Cresol glucuronide formation. Pharmaceutics. 2021;13(6):857. doi: 10.3390/pharmaceutics13060857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peralta-Marzal LN, Prince N, Bajic D, Roussin L, Naudon L, Rabot S, Garssen J, Kraneveld AD, Perez-Pardo P. The impact of gut microbiota-derived metabolites in autism spectrum disorders. Int J Mol Sci. 2021;22(18):10052. doi: 10.3390/ijms221810052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Siracusano M, Arturi L, Riccioni A, Noto A, Mussap M, Mazzone L. Metabolomics: perspectives on clinical employment in autism spectrum disorder. Int J Mol Sci. 2023;24(17):13404. doi: 10.3390/ijms241713404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Magnúsdóttir S, Ravcheev D, De Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet. 2015;6:6. doi: 10.3389/fgene.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-Gut microbiota metabolic interactions. Science. 2012;336(6086):1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 111.Doenyas C. Gut microbiota, inflammation, and probiotics on neural development in autism spectrum disorder. Neuroscience. 2018;374:271–286. doi: 10.1016/j.neuroscience.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 112.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van De Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25(1):40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Theije CGM, Wu J, Da Silva SL, Kamphuis PJ, Garssen J, Korte SM, Kraneveld AD. Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur J Pharmacol. 2011;668:S70–S80. doi: 10.1016/j.ejphar.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 114.Li W, Dowd SE, Scurlock B, Acosta-Martinez V, Lyte M. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol Behav. 2009;96(4–5):557–567. doi: 10.1016/j.physbeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 115.Critchfield JW, Van Hemert S, Ash M, Mulder L, Ashwood P. The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterol Res Pract. 2011;2011:1–8. doi: 10.1155/2011/161358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ashwood P, Anthony A, Torrente F, Wakefield AJ. Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: mucosal immune activation and reduced counter regulatory Interleukin-10. J Clin Immunol. 2004;24(6):664–673. doi: 10.1007/s10875-004-6241-6. [DOI] [PubMed] [Google Scholar]
- 117.Ashwood P, Wakefield AJ. Immune activation of peripheral blood and mucosal CD3+ lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. J Neuroimmunol. 2006;173(1–2):126–134. doi: 10.1016/j.jneuroim.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 118.Enstrom A, Krakowiak P, Onore C, Pessah IN, Hertz-Picciotto I, Hansen RL, Van de Water JA, Ashwood P. Increased IgG4 levels in children with autism disorder. Brain Behav Immun. 2009;23(3):389–395. doi: 10.1016/j.bbi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Anixt JS, Ehrhardt J, Duncan A. Evidence-Based interventions in autism. Pediatr Clin North Am. 2024;71(2):199–221. doi: 10.1016/j.pcl.2024.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baribeau D, Vorstman J, Anagnostou E. Novel treatments in autism spectrum disorder. Curr Opin Psychiatry. 2022;35(2):101–110. doi: 10.1097/YCO.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 121.Angelucci F, Cechova K, Amlerova J, Hort J. Antibiotics, gut microbiota, and Alzheimer’s disease. J Neuroinflammation. 2019;16(1):108. doi: 10.1186/s12974-019-1494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palleja A, Mikkelsen KH, Forslund SK, Kashani A, Allin KH, Nielsen T, Hansen TH, Liang S, Feng Q, Zhang C, et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat Microbiol. 2018;3(11):1255–1265. doi: 10.1038/s41564-018-0257-9. [DOI] [PubMed] [Google Scholar]
- 123.Adams JB, Romdalvik J, Ramanujam VMS, Legator MS. Mercury, lead, and zinc in baby teeth of children with autism versus controls. J Toxicol Environ Health A. 2007;70(12):1046–1051. doi: 10.1080/15287390601172080. [DOI] [PubMed] [Google Scholar]
- 124.House SA, Goodman DC, Weinstein SJ, Chang CH, Wasserman JR, Morden NE. Prescription use among children with autism spectrum disorders in Northern New England: intensity and small area variation. J Pediatr. 2016;169:277–283.e2. doi: 10.1016/j.jpeds.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 125.Choi A, Lee H, Jeong HE, Lee S-Y, Kwon JS, Han JY, Choe YJ, Shin J-Y. Association between exposure to antibiotics during pregnancy or early infancy and risk of autism spectrum disorder, intellectual disorder, language disorder, and epilepsy in children: population based cohort study. BMJ. Published online 2024. May 22;e076885. doi: 10.1136/bmj-2023-076885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Slob EMA, Brew BK, Vijverberg SJH, Dijs T, van Beijsterveldt CEM, Koppelman GH, Bartels M, Dolan CV, Larsson H, Lundström S, et al. Early-life antibiotic use and risk of attention-deficit hyperactivity disorder and autism spectrum disorder: results of a discordant twin study. Int J Epidemiol. 2021;50(2):475–484. doi: 10.1093/ije/dyaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kovtun AS, Averina OV, Alekseeva MG, Danilenko VN. Antibiotic resistance genes in the gut microbiota of children with autistic spectrum disorder as possible predictors of the disease. Microb Drug Resist. 2020;26(11):1307–1320. doi: 10.1089/mdr.2019.0325. [DOI] [PubMed] [Google Scholar]
- 128.Autism Research Institute . 2009. Parent Ratings of Behavioral Effects of Biomedical Interventions. https://autism.org/treatment-ratings-for-autism/. Accessed 24 September 2024
- 129.Kuhn M, Grave S, Bransfield R, Harris S. Long term antibiotic therapy may be an effective treatment for children co-morbid with lyme disease and autism spectrum disorder. Med Hypotheses. 2012;78(5):606–615. doi: 10.1016/j.mehy.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 130.Alookaran J, Liu Y, Auchtung TA, Tahanan A, Hessabi M, Asgarisabet P, Rahbar MH, Fatheree NY, Pearson DA, Mansour R, et al. Fungi: friend or foe? A mycobiome evaluation in children with autism and gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. 2022;74(3):377–382. doi: 10.1097/MPG.0000000000003349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kantarcioglu AS, Kiraz N, Aydin A. Microbiota–Gut–Brain axis: yeast species isolated from stool samples of children with suspected or diagnosed autism spectrum disorders and in vitro susceptibility against nystatin and fluconazole. Mycopathologia. 2016;181(1–2):1–7. doi: 10.1007/s11046-015-9949-3. [DOI] [PubMed] [Google Scholar]
- 132.Zou R, Wang Y, Duan M, Guo M, Zhang Q, Zheng H. Dysbiosis of gut fungal microbiota in children with autism spectrum disorders. J Autism Dev Disord. 2021;51(1):267–275. doi: 10.1007/s10803-020-04543-y. [DOI] [PubMed] [Google Scholar]
- 133.Marsaux B, Moens F, Marzorati M, Van De Wiele T. The intricate connection between bacterial α-diversity and fungal engraftment in the human gut of healthy and impaired individuals as studied using the in vitro SHIME® model. J Fungi. 2023;9(9):877. doi: 10.3390/jof9090877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kumamoto CA. Inflammation and gastrointestinal candida colonization. Curr Opin Microbiol. 2011;14(4):386–391. doi: 10.1016/j.mib.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Retuerto M, Al-Shakhshir H, Herrada J, McCormick TS, Ghannoum MA. Analysis of gut bacterial and fungal microbiota in children with autism spectrum disorder and their non-autistic siblings. Nutrients. 2024;16(17):3004. doi: 10.3390/nu16173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nirmalkar K, Patel J, Kang DW, Bellinghiere A, Bowes DA, Qureshi F, Adams JB, Krajmalnik-Brown R. Bimodal distribution of intestinal candida in children with autism and its potential link with worse ASD symptoms. Gut Microbes Rep. 2024;1(1):2358324. doi: 10.1080/29933935.2024.2358324. [DOI] [Google Scholar]
- 137.Shaw W, Kassen E, Chaves E. Assessment of antifungal drug therapy in autism by measurement of suspected microbial metabolites in urine with gas chromatography-mass spectrometry clinical practice of alternative. Medicine (Baltimore). 2000;1(1):15–26. [Google Scholar]
- 138.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 139.Golbaghi N, Naeimi S, Darvishi A, Najari N, Cussotto S. Probiotics in autism spectrum disorder: recent insights from animal models. Autism. 2024;28(11):2722–2737. doi: 10.1177/13623613241246911. [DOI] [PubMed] [Google Scholar]
- 140.Lewandowska-Pietruszka Z, Figlerowicz M, Mazur-Melewska K. Microbiota in autism spectrum disorder: a systematic review. Int J Mol Sci. 2023;24(23):16660. doi: 10.3390/ijms242316660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17(11):687–701. doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kong XJ, Liu J, Liu K, Koh M, Sherman H, Liu S, Tian R, Sukijthamapan P, Wang J, Fong M, et al. Probiotic and oxytocin combination therapy in patients with autism spectrum disorder: a randomized, double-blinded, placebo-controlled pilot trial. Nutrients. 2021;13(5):1552. doi: 10.3390/nu13051552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guidetti C, Salvini E, Viri M, Deidda F, Amoruso A, Visciglia A, Drago L, Calgaro M, Vitulo N, Pane M, et al. Randomized double-blind crossover study for evaluating a probiotic mixture on gastrointestinal and behavioral symptoms of autistic children. J Clin Med. 2022;11(18):5263. doi: 10.3390/jcm11185263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sherman HT, Liu K, Kwong K, Chan ST, Li AC, Kong XJ. Carbon monoxide (CO) correlates with symptom severity, autoimmunity, and responses to probiotics treatment in a cohort of children with autism spectrum disorder (ASD): a post-hoc analysis of a randomized controlled trial. BMC Psychiatry. 2022;22(1):536. doi: 10.1186/s12888-022-04151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shaaban SY, El Gendy YG, Mehanna NS, El-Senousy WM, El-Feki HSA, Saad K, El-Asheer OM. The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr Neurosci. 2018;21(9):676–681. doi: 10.1080/1028415X.2017.1347746. [DOI] [PubMed] [Google Scholar]
- 146.Li Y, Hu W, Lin B, Ma T, Zhang Z, Hu W, Zhou R, Kwok L-Y, Sun Z, Zhu C, et al. Omic characterizing and targeting gut dysbiosis in children with autism spectrum disorder: symptom alleviation through combined probiotic and medium-carbohydrate diet intervention - a pilot study. Gut Microbes. 2024;16(1):2434675. doi: 10.1080/19490976.2024.2434675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang Y, Li N, Yang JJ, Zhao D-M, Chen B, Zhang G-Q, Chen S, Cao R-F, Yu H, Zhao C-Y, et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol Res. 2020;157:104784. doi: 10.1016/j.phrs.2020.104784. [DOI] [PubMed] [Google Scholar]
- 148.Sanctuary MR, Kain JN, Chen SY, Kalanetra K, Lemay DG, Rose DR, Yang HT, Tancredi DJ, German JB, Slupsky CM, et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLOS ONE. 2019;14(1):e0210064. doi: 10.1371/journal.pone.0210064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Aldegheri L, Kharrat F, Conti A, Monica F, Busa F, Campisciano G, Zanotta N, Cason C, Comar M. Impact of human milk oligosaccharides and probiotics on gut microbiome and mood in autism: a case report. Microorganisms. 2024;12(8):1625. doi: 10.3390/microorganisms12081625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Arnold LE, Luna RA, Williams K, Chan J, Parker RA, Wu Q, Hollway JA, Jeffs A, Lu F, Coury DL, et al. Probiotics for gastrointestinal symptoms and quality of life in autism: a placebo-controlled pilot trial. J Child And Adolesc Psychopharmacol. 2019;29(9):659–669. doi: 10.1089/cap.2018.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jandhyala SM. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Matthews JS, Adams JB. Ratings of the effectiveness of 13 therapeutic diets for autism spectrum disorder: results of a national survey. J Personalized Med. 2023;13(10):1448. doi: 10.3390/jpm13101448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yap CX, Henders AK, Alvares GA, Wood DLA, Krause L, Tyson GW, Restuadi R, Wallace L, McLaren T, Hansell NK, et al. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell. 2021;184(24):5916–5931.e17. doi: 10.1016/j.cell.2021.10.015. [DOI] [PubMed] [Google Scholar]
- 154.David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, Erdman SE, Alm EJ. Host lifestyle affects human microbiota on daily timescales. Genome Biology. 2014;15(7):R89. doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Inoue R, Sakaue Y, Kawada Y, Tamaki R, Yasukawa Z, Ozeki M, Ueba S, Sawai C, Nonomura K, Tsukahara T, et al. Dietary supplementation with partially hydrolyzed guar gum helps improve constipation and gut dysbiosis symptoms and behavioral irritability in children with autism spectrum disorder. J Clin Biochem Nutr. 2019;64(3):217–223. doi: 10.3164/jcbn.18-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grimaldi R, Gibson GR, Vulevic J, Giallourou N, Castro-Mejía JL, Hansen LH, Leigh Gibson E, Nielsen DS, Costabile A. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome. 2018;6(1):133. doi: 10.1186/s40168-018-0523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Choi HH, Cho YS. Fecal microbiota transplantation: current applications, effectiveness, and future perspectives. Clin Endosc. 2016;49(3):257–265. doi: 10.5946/ce.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Staley C, Khoruts A, Sadowsky MJ. Contemporary applications of fecal microbiota transplantation to treat intestinal diseases in humans. Arch Med Res. 2017;48(8):766–773. doi: 10.1016/j.arcmed.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 159.National Health Service . Faecal microbiota transplantation. 2021. [Accessed 2024 Aug 5]. https://www.cuh.nhs.uk/patient-information/faecal-microbiota-transplantation-fmt/.
- 160.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent clostridium difficile infection. Am J Gastroenterol. 2012;107(5):761–767. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 161.Allegretti JR, Kelly CR, Grinspan A, Mullish BH, Hurtado J, Carrellas M, Marcus J, Marchesi JR, McDonald JAK, Gerardin Y, et al. Inflammatory bowel disease outcomes following fecal microbiota transplantation for recurrent C. difficile infection. Inflamm Bowel Dis. 2021;27(9):1371–1378. doi: 10.1093/ibd/izaa283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brandt LJ, Aroniadis OC, Mellow M, Kanatzar A, Kelly C, Park T, Stollman N, Rohlke F, Surawicz C. Long-Term follow-up of colonoscopic fecal microbiota transplant for recurrent clostridium difficile infection. Am J Gastroenterol. 2012;107(7):1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 163.Kelly CR, Khoruts A, Staley C, Sadowsky MJ, Abd M, Alani M, Bakow B, Curran P, McKenney J, Tisch A, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent clostridium difficile infection: a randomized trial. Ann Intern Med. 2016;165(9):609. doi: 10.7326/M16-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JFWM, Tijssen JGP, et al. Duodenal infusion of donor feces for recurrent clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 165.Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent clostridium difficile-associated diarrhea. J Clin Gastro. 2010;44(5):354–360. doi: 10.1097/MCG.0b013e3181c87e02. [DOI] [PubMed] [Google Scholar]
- 166.Khoruts A, Brandt LJ. Fecal microbiota transplant: a rose by any other name. Am J Gastroenterol. 2019;114(7):1176–1176. doi: 10.14309/ajg.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 167.Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding Q, Pagliano O, et al. Fecal microbiota transplant overcomes resistance to anti–PD-1 therapy in melanoma patients. Science. 2021;371(6529):595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Routy B, Lenehan JG, Miller WH. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: a phase I trial. Nat Med. 2023;29(8):2121–2132. doi: 10.1038/s41591-023-02453-x. [DOI] [PubMed] [Google Scholar]
- 169.Kang DW, Adams JB, Vargason T, Santiago M, Hahn J, Krajmalnik-Brown R, Marco ML. Distinct fecal and plasma metabolites in children with autism spectrum disorders and their modulation after microbiota transfer therapy. Marco ML, ed. mSphere. 2020;5(5):e00314–20. doi: 10.1128/mSphere.00314-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nirmalkar K, Qureshi F, Kang DW, Hahn J, Adams JB, Krajmalnik-Brown R. Reanalysis of metabolomics data reveals that microbiota transfer therapy modulates important fecal and plasma metabolite profiles in children with autism spectrum disorders. [Published online 2024 Dec 18.] doi: 10.1101/2024.12.16.628826. [DOI] [Google Scholar]
- 171.Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, Caporaso JG, Krajmalnik-Brown R. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci Rep. 2019;9(1):5821. doi: 10.1038/s41598-019-42183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li N, Chen H, Cheng Y, Xu F, Ruan G, Ying S, Tang W, Chen L, Chen M, Lv L, et al. Fecal microbiota transplantation relieves gastrointestinal and autism symptoms by improving the gut microbiota in an open-label study. Front Cell Infect Microbiol. 2021;11:759435. doi: 10.3389/fcimb.2021.759435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang L, Yu L, Liu Z, Che C, Wang Y, Zhao Y, Zhu M, Yang G, Cao A. FMT intervention decreases urine 5-HIAA levels: a randomized double-blind controlled study. Front Med. 2024;11:1411089. doi: 10.3389/fmed.2024.1411089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li Y, Xiao P, Cao R, Le J, Xu Q, Xiao F, Ye L, Wang X, Wang Y, Zhang T. Effects and microbiota changes following oral lyophilized fecal microbiota transplantation in children with autism spectrum disorder. Front Pediatr. 2024;12:1369823. doi: 10.3389/fped.2024.1369823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen Q, Wu C, Xu J, Ye C, Chen X, Tian H, Zong N, Zhang S, Li L, Gao Y, et al. Donor-recipient intermicrobial interactions impact transfer of subspecies and fecal microbiota transplantation outcome. Cell Host & Microbe. 2024;32(3):349–365.e4. doi: 10.1016/j.chom.2024.01.013. [DOI] [PubMed] [Google Scholar]
- 176.Hu C, He T, Zou B, Li H, Zhao J, Hu C, Cui J, Huang Z, Shu S, Hao Y. Fecal microbiota transplantation in a child with severe ASD comorbidities of gastrointestinal dysfunctions—a case report. Front Psychiatry. 2023;14:1219104. doi: 10.3389/fpsyt.2023.1219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hazan S, Haroon J, Jordan S, Walker SJ. Improvements in gut microbiome composition and clinical symptoms following familial fecal microbiota transplantation in a nineteen-year-old adolescent with severe autism. J Med Cases. 2024;15(4–5):82–91. doi: 10.14740/jmc4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Duvallet C, Zellmer C, Panchal P, Budree S, Osman M, Alm EJ, Carbonero F. Framework for rational donor selection in fecal microbiota transplant clinical trials. Carbonero F, ed. PLOS ONE. 2019;14(10):e0222881. doi: 10.1371/journal.pone.0222881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kump P, Wurm P, Gröchenig HP, Wenzl H, Petritsch W, Halwachs B, Wagner M, Stadlbauer V, Eherer A, Hoffmann KM, et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Alimentary Pharmacol & Ther. 2018;47(1):67–77. doi: 10.1111/apt.14387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chinna Meyyappan A, Forth E, Wallace CJK, Milev R. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: a systematic review. BMC Psychiatry. 2020;20(1):299. doi: 10.1186/s12888-020-02654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rosenfeld CS. Microbiome disturbances and autism spectrum disorders. Drug Metab Dispos. 2015;43(10):1557–1571. doi: 10.1124/dmd.115.063826. [DOI] [PubMed] [Google Scholar]
- 182.Tan Q, Orsso CE, Deehan EC, Kung JY, Tun HM, Wine E, Madsen KL, Zwaigenbaum L, Haqq AM. Probiotics, prebiotics, synbiotics, and fecal microbiota transplantation in the treatment of behavioral symptoms of autism spectrum disorder: a systematic review. Autism Res. 2021;14(9):1820–1836. doi: 10.1002/aur.2560. [DOI] [PubMed] [Google Scholar]
- 183.Vendrik KEW, Ooijevaar RE, De Jong PRC, Laman JD, van Oosten BW, van Hilten JJ, Ducarmon QR, Keller JJ, Kuijper EJ, Contarino MF. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol. 2020;10:98. doi: 10.3389/fcimb.2020.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen Y, Xueying Z, Jiaqu C, Qiyi C, Huanlong Q, Ning L, Yasong D, Xiaoxin Z, Rong Y, Jubao L, et al. FTACMT study protocol: a multicentre, double-blind, randomised, placebo-controlled trial of faecal microbiota transplantation for autism spectrum disorder. BMJ Open. 2022;12(1):e051613. doi: 10.1136/bmjopen-2021-051613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tweedie-Cullen RY, Leong K, Wilson BC, Derraik JGB, Albert BB, Monk R, Vatanen T, Creagh C, Depczynski M, Edwards T, et al. Protocol for the gut nugs in autism trial: a double-blind randomised placebo-controlled trial of faecal microbiome transfer for the treatment of gastrointestinal symptoms in autistic adolescents and adults. BMJ Open. 2024;14(2):e074625. doi: 10.1136/bmjopen-2023-074625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wei J, Chen J, Fang X, Liu T, Yuan Y, Zhang J. Protocol for the safety and efficacy of fecal microbiota transplantation liquid in children with autism spectrum disorder: a randomized controlled study. Front Microbiol. 2023;14:1236904. doi: 10.3389/fmicb.2023.1236904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. 2012;70:S38–S44. doi: 10.1111/j.1753-4887.2012.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Van Belleghem JD, Dąbrowska K, Vaneechoutte M, Barr JJ, Bollyky PL. Interactions between bacteriophage, bacteria, and the mammalian immune system. Viruses. 2018;11(1):10. doi: 10.3390/v11010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Peery AF, Kelly CR, Kao D, Vaughn BP, Lebwohl B, Singh S, Imdad A, Altayar O. AGA clinical practice guideline on fecal microbiota–based therapies for select gastrointestinal diseases. Gastroenterology. 2024;166(3):409–434. doi: 10.1053/j.gastro.2024.01.008. [DOI] [PubMed] [Google Scholar]
- 190.Wu Q, Boonma P, Badu S, Yalcinkaya N, So SY, Garey KW, Williams K, Arnold LE, Shulman RJ, Kellermayer R, et al. Donor-recipient specificity and age-dependency in fecal microbiota therapy and probiotic resolution of gastrointestinal symptoms. NPJ Biofilms And Microbiomes. 2023;9(1):54. doi: 10.1038/s41522-023-00421-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Porcari S, Benech N, Valles-Colomer M, Segata N, Gasbarrini A, Cammarota G, Sokol H, Ianiro G. Key determinants of success in fecal microbiota transplantation: from microbiome to clinic. Cell Host & Microbe. 2023;31(5):712–733. doi: 10.1016/j.chom.2023.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable for this study.


